Abstract
Objective.
Understanding the cognitive determinants of healthcare worker (HCW) behavior is important for improving the use of infection prevention and control (IPC) practices. Given a patient requiring only standard precautions, we examined the dimensions along which different populations of HCWs cognitively organize patient care tasks (i.e., their mental models).
Design.
HCWs read a description of a patient and then rated the similarities of 25 patient care tasks from an infection prevention perspective. Using multidimensional scaling, we identified the dimensions (i.e., characteristics of tasks) underlying these ratings, and the importance of each to HCWs.
Setting.
Adult inpatient hospitals across an academic hospital network.
Participants.
40 HCWs, comprising infection preventionists and nurses from intensive care units, emergency departments, and medical/surgical floors rated the similarity of tasks. To identify the meaning of each dimension, another 6 nurses rated the tasks in terms of specific characteristics of tasks.
Results.
Each population of HCWs perceived patient care tasks varying in similar ways along three dimensions simultaneously; most salient was the perceived magnitude of infection risk to the patient in a task, followed by the perceived dirtiness and risk of HCW exposure to body fluids, and lastly, the relative importance of a task for preventing versus controlling an infection in a patient.
Conclusion.
For a patient requiring only standard precautions, different populations of HCWs have similar mental models of how various patient care tasks relate to IPC. Techniques for eliciting mental models open new avenues for understanding and ultimately modifying the cognitive determinants of IPC behaviors.
INTRODUCTION
Preventing the transmission of pathogens during patient care depends, in part, on healthcare workers (HCWs) routinely practicing infection prevention procedures, such as hand hygiene and wearing personal protective equipment (PPE). These procedures are major components of standard precautions, which should be practiced regardless of whether a patient is known to be infectious. Although a variety of external constraints influence the use of such procedures (e.g., time pressure and inconvenient access to supplies1), so too do cognitive determinants of behavior (e.g., HCW motivation and social norms2).
Understanding the cognitive determinants of IPC behaviors, particularly in different populations of HCWs,2 is argued to be important for designing interventions to modify these behaviors.2–4 However, there is a need to better understand whether and how populations of HCWs differ, particularly in regard to an important, but under-studied,5 cognitive determinant of IPC behaviors - “mental models.”6,7 A mental model is an organized collection of individual beliefs that one uses to understand, explain, and predict events in the world around them8 in order to select appropriate courses of action. For example, HCWs often perform multiple tasks per patient room visit, which can require sequencing and recognizing transitions between tasks to minimize cross-contamination (e.g., from “dirty” to “clean” tasks9,10); how effectively HCWs organize their workflow depends, in part, on how HCWs believe different tasks are related.
This study assessed HCWs’ mental models of patient care tasks for a patient requiring only standard precautions in four populations of HCWs: infection preventionists (IPs) and registered nurses (RNs) from intensive care units (ICUs), emergency departments (EDs), and medical/surgical floors. Specifically, we determined whether these populations cognitively organize tasks along the same dimensions and whether the weight given to each dimension (i.e., its subjective importance) differs between populations. We chose these populations as it is reasonable to expect such differences to emerge; for example, IPs and RNs may organize tasks along different dimensions because IPs are formally trained in IPC. These populations may also organize tasks along some common dimensions, but weight those dimensions differently (e.g., risk to the patient or risk to the HCW); for example, RNs in ICUs work with acutely ill patients who are at greater risk of acquiring infections whereas RNs in the ED are at risk of prolonged contact with body fluids from patients whose infection status is often unknown.4
METHODS
Identifying Tasks.
To identify a set of patient care tasks, we surveyed four critical care RNs trained to care for patients with serious communicable diseases, three infectious disease physicians, and two IPs, who were each asked, “What patient care tasks do you (or others) do where it is important to protect you or your patient from a contagious disease?” Respondents produced 148 tasks in total. To reduce the number of tasks, we printed tasks on separate cards, which two critical care RN subject matter experts (SMEs) sorted into groups of redundant tasks or single tasks that could not be grouped with another task(s). The SMEs reviewed their groupings until they reached a consensus. We then carried forward tasks that RNs in the ICU, ED, and medical/surgical floors perform routinely, which resulted in a final list of 25 tasks (Supplemental material). Tasks were rephrased as needed to minimize ambiguity.
Recruitment.
We recruited IPs and RNs from EDs, ICUs, and medical/surgical floors from adult inpatient hospitals across a large academic hospital network. Participants were invited to take an hour-long online survey about infection prevention.
Rating Task-Pairs.
Participants reviewed the 25 tasks and then judged the similarity of all possible pairs of tasks using a scale from 1 (“Not at all similar,”) to 9 (“Extremely similar,”). When rating each task-pair, participants were told to take an “…infection prevention perspective (i.e., preventing the spread of pathogens)” and to assume that the patient is “…average-sized, not bed-bound, able to cooperate (e.g., understand and follow commands), but is weak. No known special precautions are required.” The order of the 300 task-pairs was randomly generated for each participant. After rating all task-pairs, participants described the reasoning behind their ratings. Each reason given by a participant was later printed on a separate card and then sorted into groups of recurring reasons.
Multidimensional Scaling.
Multidimensional scaling (MDS) takes a measure of psychological similarity (e.g., ratings) between all possible pairs of a set of “objects” (e.g., patient care tasks), places each object as a point in an n-dimensional space, and then iteratively configures the objects in space until the distance between each pair of objects reflects the corresponding measure of psychological similarity as closely as possible. Objects perceived as similar are close together in space whereas objects perceived as dissimilar are far apart. The overall congruence between the distances between objects in the multidimensional space and their corresponding measure of similarity is quantified as “stress,” which ranges from 0 to 1 with lower values indicating better fit. The dimensionality of the space is set by the analyst, who strives to find the smallest number of dimensions that best reproduces the psychological similarity of the objects. Each dimension may then be interpreted in terms of known characteristics of the objects, which are reflected in how objects are ordered along a dimension. We used a variant of MDS called Individual Differences Scaling (INDSCAL),11 which also measures how salient (i.e., important) each dimension is to each participant, called a “weight.” If a participant(s) does not perceive a certain dimension, then that dimension will have a weight of zero for that participant(s). The higher a weight is above zero, the more salient that dimension is to that participant(s). We implemented the INDSCAL model using the PROXSCAL algorithm, treating similarity ratings as ordinal data and using the primary approach to ties.12 We scaled data from all raters in 1 to 6 dimensions. For each dimensionality, we retained the best-fitting final configuration from 1,000 randomly generated starting configurations,15,16 according to recommended convergence criteria.15
Identifying Dimensionality.
We identified the dimensions underlying the task-pair ratings using three criteria: model fit 11–14and the interpretability11–14 and replicability12,14 of dimensions. Regarding model fit, we plotted the stress of the best-fitting final configuration as a function of dimensionality to identify the point at which positing additional dimensions failed to improve fit substantially.11–14
We took a multiple regression approach to interpret dimensions,12,13 in which we first hypothesized 12 characteristics of patient care tasks based on the input of a SME panel (three infectious disease physicians and four critical care RNs) and recurring reasons participants gave for their similarity judgments. We translated each characteristic into a statement that could be rated on a 5-point scale (“None,” “Low,” “Moderate,” “High,” and “Very High”). A new sample of HCWs independently rated the 25 tasks in terms of the hypothesized characteristics. For each HCW, the order of the 25 tasks and the order of the characteristics for each task were randomly generated. One hypothesized characteristic of tasks, the “Relative importance for preventing versus controlling (e.g., diagnosing or investigating) an infection in a patient,” was calculated from two characteristics as the arithmetic difference between the “Relevance for preventing an infection in a patient,” and the “Relevance for controlling (e.g., diagnosing or investigating) an infection in a patient.” For each of the final 11 hypothesized characteristics (Table 2), we calculated the mean rating for each task, which were predicted from tasks’ coordinate value on each dimension using multiple regression.12,13 Interpretation of dimensions followed recommended guidelines13; as a minimal requirement, the characteristic should have a multiple correlation that is significant at the α = 0.01 level. Additionally, the characteristic should have a high multiple correlation (R ≥ 0.70) and a high regression weight on one dimension only.
Table 2.
Standardized regression coefficients for each dimension predicting the 11 hypothesized characteristics.
| Hypothesized Characteristic: | Dim. 1 | Dim. 2 | Dim. 3 | R |
|---|---|---|---|---|
| Infection risk to patient. | 0.70*** | 0.16 | 0.19 | 0.74*** |
| Infection risk to patient if task performed improperly. | 0.71*** | 0.15 | 0.12 | 0.74*** |
| “Dirtiness” of task. | −0.03 | 0.83*** | 0.05 | 0.83*** |
| Risk of healthcare worker exposure to body fluids. | −0.03 | 0.71*** | −0.05 | 0.71*** |
| Amount that task varies. | 0.04 | 0.56** | 0.31 | 0.64** |
| Relative importance for preventing versus controlling (e.g., diagnosing or investigating) an infection in a patient. | 0.28 | −0.18 | 0.60** | 0.69** |
| Infection risk to healthcare worker. | −0.04 | 0.54** | −0.29 | 0.61* |
| Infection risk to healthcare worker if task performed improperly. | 0.03 | 0.48* | −0.37* | 0.60* |
| Amount of personal protective equipment needed. | 0.31 | 0.53** | 0.02 | 0.61* |
| Degree of invasiveness. | 0.48* | −0.02 | 0.18 | 0.52 |
| Amount of physical contact with patient. | −0.36 | 0.20 | 0.35 | 0.54 |
Note: Dim. = Dimension; R = multiple correlation coefficient.
p < 0.05
p < 0.01
p < 0.001.
To assess the replicability of the best-fitting and most interpretable configuration, we scaled each HCW population’s rating data separately.14 For each population, we used the coordinate values of the 25 tasks from the full-sample analysis as a reasonable starting configuration for the PROXSCAL algorithm to optimize.12 We assessed the similarity of dimensions between populations by calculating Pearson correlation coefficients between task coordinates on each pair of dimensions. We then averaged correlations between corresponding dimensions across populations using Fisher’s r to z transformation.
To determine whether the dimensions differed in their salience, and whether such patterns differed between populations of HCWs, we analyzed weights using a Greenhouse-Geisser corrected mixed model analysis of variance (ANOVA) with Dimension as a within-subjects factor and HCW Population as a between-subjects factor.11 Post-hoc pairwise comparisons were performed using Bonferroni’s correction for multiple comparisons. A power analysis in G*Power 3.1.9.215 suggested a total sample size of 40 HCWs was required to detect a large interaction (f = 0.4)16 between Dimension and HCW Population with at least 80% power and α = 0.05 across a range of dimensions (2 – 6). All statistical analyses were performed in SPSS version 26. A p-value less than 0.05 was considered significant.
RESULTS
Rating Task-Pairs.
We received task-pair rating data from 11 IPs and 10 ED, 10 medical/surgical, and 9 ICU (5 Medical ICU, 4 Surgical ICU) RNs. Most (83%) RNs worked in a large quaternary academic medical center. RNs had a median of 8 years (Range: 1 – 38 years) of nursing experience. 16 (40%) RNs reported working or having worked in a COVID-focused unit. 54% of IP respondents were from the same academic medical center. IPs had a median of 4.5 years (Range: 2 – 25 years) of experience as an IP. Nearly half (45%) of the IPs had a background in nursing.
An additional 6 ICU RNs with a median of 8.5 years (Range: 7 – 43 years) of nursing experience rated the 25 tasks on 12 scales. All 6 RNs were also trained in a hospital biocontainment unit, which involves annual exercises, skills training, and online course work related to IPC. Additionally, all 6 ICU RNs reported working or having worked in a COVID-focused.
Multidimensional Scaling.
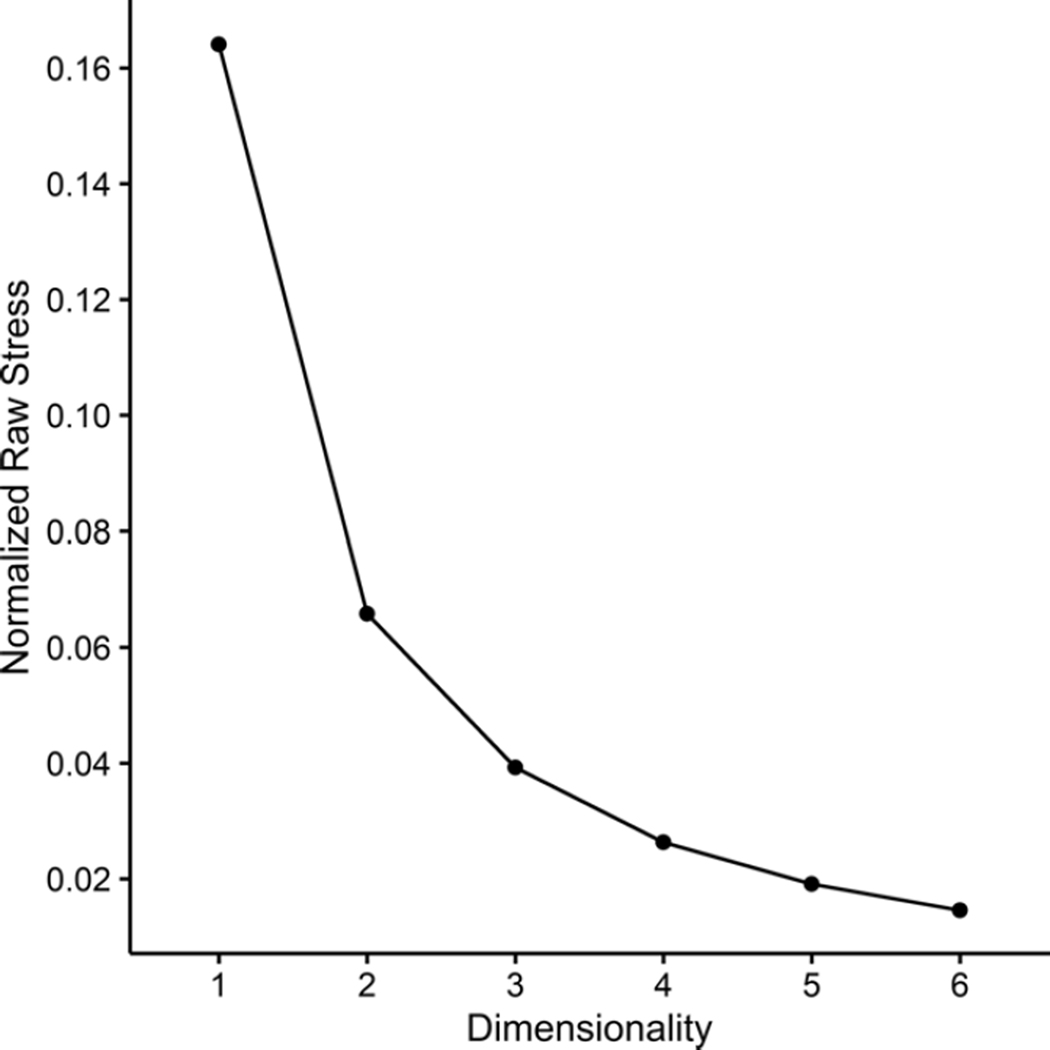
Figure 1 shows the stress of the best-fitting configuration obtained in 1 through 6 dimensions. Inspection of Figure 1 suggests that positing more than 4 dimensions does not substantially improve fit. However, the best-fitting configuration in 4 dimensions was only marginally better than that obtained in 3 dimensions. Using the multiple regression approach for interpreting dimensions described earlier,13 we found that only the 3-dimensional configuration was fully interpretable, which we then retained for further analyses.
Figure 1.
Stress of the best-fitting configuration obtained in 1 through 6 dimensions. Lower values of stress indicate a better fit to the rating data, with 0 corresponding to a perfect fit. The PROXSCAL algorithm minimizes a variant of stress called “normalized raw stress.”
To assess the replicability of the 3-dimensional configuration of the full sample, we scaled each HCW population’s data separately in 3 dimensions.14 The stress for IPs (Stress = 0.03) and ED (Stress = 0.03) and ICU (Stress = 0.03) RNs were comparable, whereas the stress for medical/surgical RNs (Stress = 0.05) was somewhat higher. Table 1 shows all pairwise correlations between each populations’ dimensions. Corresponding dimensions were strongly and significantly correlated between each population (Dimension 1: = 0.94, Dimension 2: = 0.85, and Dimension 3: = 0.79) whereas none of the cross-dimension correlations were significant. Thus, a replicable set of dimensions was found across populations.14
Table 1.
Pearson correlations between dimensions from populations.
| IPs | Medical/surgical RNs | ICU RNs | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dim. 1 | Dim. 2 | Dim. 3 | Dim. 1 | Dim. 2 | Dim. 3 | Dim. 1 | Dim. 2 | Dim. 3 | ||
| Medical/surgical RNs | Dim. 1 | 0.94*** | −0.09 | −0.05 | -- | -- | -- | -- | -- | -- |
| Dim. 2 | 0.01 | 0.84*** | 0.11 | -- | -- | -- | -- | -- | -- | |
| Dim. 3 | 0.06 | 0.01 | 0.79*** | -- | -- | -- | -- | -- | -- | |
| ICU RNs | Dim. 1 | 0.93*** | 0.22 | −0.03 | 0.88*** | 0.18 | 0.03 | -- | -- | -- |
| Dim. 2 | −0.39 | 0.80*** | −0.11 | −0.47 | 0.71** | −0.01 | -- | -- | -- | |
| Dim. 3 | 0.10 | 0.08 | 0.74** | 0.01 | 0.19 | 0.67* | -- | -- | -- | |
| ED RNs | Dim. 1 | 0.95*** | 0.14 | −0.11 | 0.91*** | 0.10 | −0.05 | 0.96*** | −0.24 | −0.05 |
| Dim. 2 | −0.17 | 0.96*** | 0.05 | −0.27 | 0.82*** | 0.06 | 0.04 | 0.86*** | 0.09 | |
| Dim. 3 | 0.30 | 0.02 | 0.89*** | 0.22 | 0.10 | 0.80*** | 0.26 | −0.21 | 0.80*** | |
Note: IP = Infection preventionist; RNs = Registered nurses; ICU = Intensive Care Unit; ED = Emergency Department; Dim. = Dimension. P-values are corrected for multiple comparisons using Bonferroni’s correction.
p < 0.05
p < 0.01
p < 0.001.
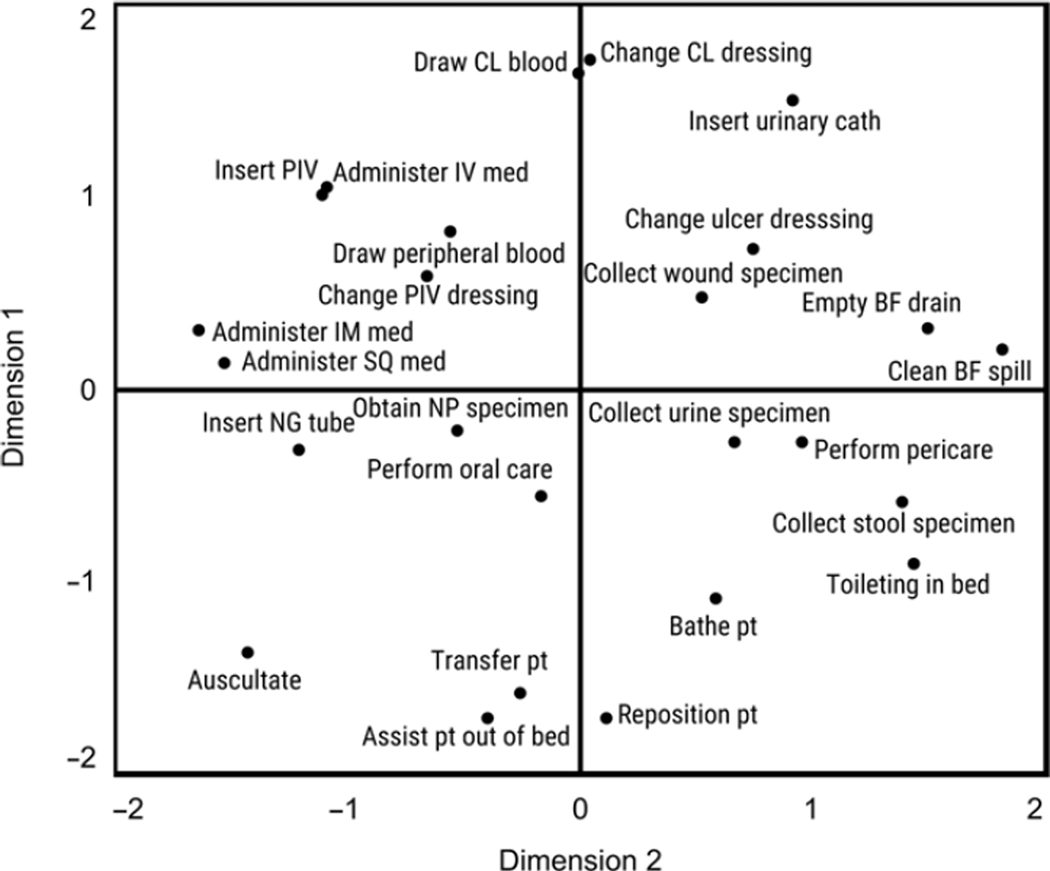
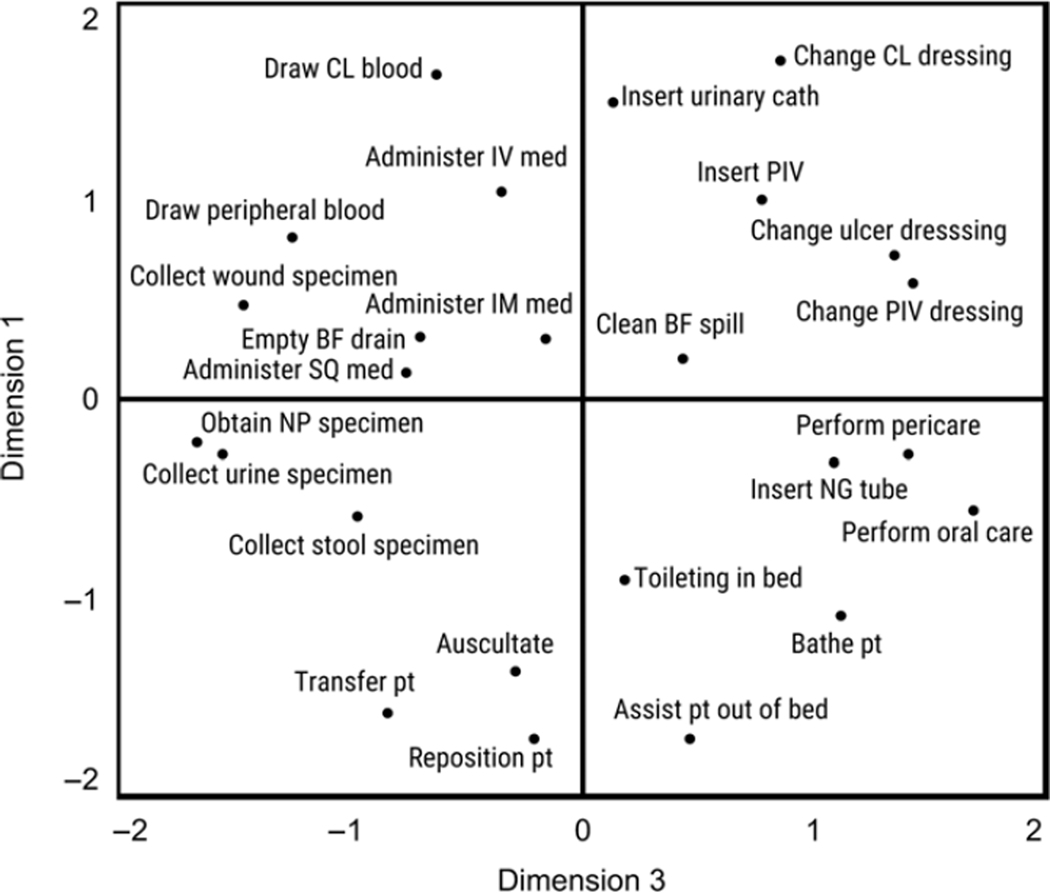
Following recommended guidelines for interpreting dimensions using multiple regression (Table 2),13 we interpreted Dimension 1 (Figure 2) as the perceived magnitude of infection risk to the patient in a task. This dimension was strongly and exclusively related to the characteristics “Infection risk to patient,” and “Infection risk to patient if task performed improperly,” Dimension 2 (Figure 2) reflects the perceived “dirtiness” of a task and the perceived risk of HCW exposure to body fluids. This dimension was related to the “‘Dirtiness’ of task,” “Risk of healthcare worker exposure to body fluids,” and to a lesser extent, the “Amount that task varies.” Although failing to meet recommended guidelines,13 “Infection risk to healthcare worker,” “Infection risk to healthcare worker if task performed improperly,” and “Amount of PPE needed,” were weakly related to Dimension 2 as well. Lastly, Dimension 3 (Figure 3) reflects a task’s perceived relative importance for preventing versus controlling an infection in a patient. On this dimension, the arithmetic difference between “Relevance for preventing an infection in a patient,” and “Relevance for controlling (e.g., diagnosing or investigating) an infection in a patient,” was related.
Figure 2.
Plot of patient care tasks along Dimensions 1 and 2. See supplemental material for complete descriptions of the 25 tasks. Note: pt = Patient; NG = Nasogastric; NP = Nasopharyngeal; BF = Body fluid; SQ = Subcutaneous; IM = Intramuscular; IV = Intravenous line; PIV = Peripheral intravenous line; CL = Central line.
Figure 3.
Plot of patient care tasks along Dimensions 3 and 1. See supplemental material for complete descriptions of the 25 tasks. Note: pt = Patient; NG = Nasogastric; NP = Nasopharyngeal; BF = Body fluid; SQ = Subcutaneous; IM = Intramuscular; IV = Intravenous line; PIV = Peripheral intravenous line; CL = Central line.
Analysis of weights revealed a significant main effect of Dimension (p < .001), a non-significant main effect of HCW Population (p = .90), and a non-significant interaction between Dimension and HCW Population (p = .72). Post-hoc pairwise comparisons of the main effect of Dimension revealed that HCWs weighted Dimension 1 (M = 0.45, SEM = 0.01) significantly more heavily than Dimension 2 (M = 0.38, SEM = 0.01; p < .001) and Dimension 3 (M = 0.33, SEM = 0.01; p < .001). HCWs also weighted Dimension 2 significantly more heavily than Dimension 3 (p = .001).
DISCUSSION
For a patient requiring only standard precautions, IPs and RNs from ICUs, EDs, and medical/surgical floors cognitively organized patient care tasks along three common dimensions; the most salient dimension was the perceived magnitude of infection risk to the patient in a task.2,17–20 This finding underscores the importance of assessing HCWs’ perceptions of patient risk in a task, particularly if attempting to explain IPC behaviors.7 Regarding hand hygiene adherence, for example, attempts to relate HCW’s perception of risk to the patient in a task (versus the authors’ conceptualization of risk) and hand hygiene adherence are uncommon in the literature.19,20 Some authors have even concluded that HCWs fail to assess the risk that tasks pose to patients because HCWs did not behave according to the authors’ conceptualization of risk.18 However, failing to assess risk entirely and failing to assess risk accurately are separate issues. For the latter, ambiguity regarding adverse outcomes following unsafe behaviors (e.g., not performing hand hygiene when indicated) can prevent HCWs from developing accurate risk perceptions,21 which should not be confused with failing to assess risk.
Less salient to HCWs was the perceived dirtiness and risk of HCW exposure to body fluids during a task. These characteristics have been identified previously,4,17,22–29 particularly as strong cues for self-protective behaviors. For example, HCWs have reported that the perceived dirtiness of a task influences their use of hand hygiene17,28,30 and PPE.4,23,27,29,31 Our results suggest that the perceived dirtiness and risk of exposure to body fluids are more prominent characteristics of tasks to HCWs than the perceived infection risk to the HCW; the scales “Infection risk to healthcare worker,” “Infection risk to healthcare worker if task performed improperly,” and the “Amount of PPE needed,” during a task were only weakly related to this dimension. This is consistent with observations that the perceived dirtiness of a task and the personal risk of infection are not necessarily synonymous to HCWs.23 Moreover, that this dimension was less salient to HCWs than the infection risk to the patient may reflect a desensitization17 of HCWs to risks to themselves. For example, some HCWs perceive that exposure risk is “omnipresent” in clinical practice32 and that constant exposure to pathogens has rendered them either immune or already colonized.33
Least salient to HCWs appears to be the relative importance of a task for preventing versus controlling (e.g., diagnosing or investigating) an infection in a patient. This dimension reflects why certain tasks are performed, from an IPC perspective. Compared to the first two dimensions, the implications of this dimension tend to be removed more in time (e.g., changing a central line dressing to prevent an infection). The lower salience of this dimension may suggest that HCWs prioritize characteristics of tasks that have more immediate implications for their behavior, such as using sterile technique to prevent cross-contamination during a high-risk task or donning PPE for a task involving body fluids. Whether adverse outcomes are related to perceptions of this dimension, however, is an avenue for future research as this dimension has not, to the best of our knowledge, appeared previously in the literature.
Lastly, previous studies identified the expectation of patient or environmental contact during a task33–35 as a determinant for following infection prevention procedures (e.g., using PPE). In the present study, the amount of patient contact in a task was not related to any dimension, even though we included tasks that vary in this respect (e.g., “Performing perineal care,” versus “Cleaning up a body fluid spill on the floor,”). As previous studies concerned patients on isolation precautions,33–35 our findings suggest that the expectation of patient contact may not be a part of HCW’s mental models of tasks for patients requiring only standard precautions.
There are some limits to the generalizability of our findings. Similarity judgments can depend on the context in which they are elicited.36 In the present study, participants considered a single description of a fairly generic patient (e.g., average-sized and cooperative) who was not on isolation precautions. Given a description of a patient with other characteristics (e.g., isolation status), different dimensions or weightings may emerge. Lastly, most participants came from a large academic medical center for adult inpatients; it is possible that other dimensions or weightings may be obtained from HCWs in lower-resourced settings or from HCWs caring for other patient populations (e.g., pediatrics23). Thus, future research should vary patient descriptions and include more disparate HCW populations.
Mental model representation techniques not only reveal how individuals structure their beliefs but also allow the similarity of these structures to be measured. Consequently, future research on understanding or modifying IPC behaviors (e.g., via training) should consider incorporating mental model representation techniques. For example, assessing learner’s mental models before delivering training is beneficial as most learning involves incorporating new information into what is already known.10,37 Learning is facilitated when information to-be-learned is organized to be compatible with the learner’s existing mental models.37
Additionally, if a gold-standard referent structure exists, then the accuracy of mental models can be assessed (e.g., before and after training).5,38–40 Mental model accuracy can predict skill acquisition and skill-based performance,39 even beyond traditional paper-and-pencil tests of factual knowledge.40 However, a lack of established referents (e.g., for the actual “dirtiness” of tasks) 10,13,27 is a barrier to assessing the accuracy of HCWs’ mental models. Nonetheless, techniques for representing HCW mental models open new avenues for understanding, comparing, and ultimately modifying the cognitive determinants of IPC behaviors.
Supplementary Material
Acknowledgements.
We wish to thank Josia Mamora for input on selecting patient care tasks and Ellen Jordan for assistance in manuscript preparation.
Funding.
This work was supported by award number NU38CK000481 from the Centers for Disease Control and Prevention National Infection Control Strengthening Program. JHA was supported by the Antibacterial Resistance Leadership Group fellowship [National Institute of Allergy and Infectious Diseases UM1AI104681]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Centers for Disease Control and Prevention or National Institutes of Health.
Footnotes
Competing Interests. There are no competing interests for any author.
Disclaimer. The content is solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention or the National Institute of Allergy and Infectious Diseases.
Contributor Information
Mumma Joel Michael, Emory University School of Medicine, Division of Infectious Diseases, Department of Medicine, 1364 Clifton Road Northeast, GG17A, Atlanta, GA, 30322.
Howard-Anderson Jessica, Emory University School of Medicine, Division of Infectious Diseases, Department of Medicine Atlanta, GA.
Morgan Jill, Emory Healthcare Atlanta, GA.
Schink Kevin, Emory Healthcare Atlanta, GA.
Marissa J. Wheatley, Children’s Healthcare of Atlanta Atlanta, GA.
Kraft Colleen, Emory University School of Medicine Department of Pathology and Laboratory Medicine Division of Infectious Diseases, Department of Medicine Atlanta, GA.
Lane Morgan, Emory University School of Medicine Division of Infectious Diseases, Department of Medicine Atlanta, GA.
Kaufman Noah, Emory University School of Medicine Division of Infectious Diseases, Department of Medicine Atlanta, GA.
Ayeni Oluwateniola, Emory University School of Medicine Division of Infectious Diseases, Department of Medicine Atlanta, GA.
Erik A. Brownsword, Emory University School of Medicine Division of Infectious Diseases, Department of Medicine Atlanta, GA.
Jesse T. Jacob, Emory University School of Medicine Division of Infectious Diseases, Department of Medicine Atlanta, GA; Emory University Rollins School of Public Health Department of Epidemiology Atlanta, GA.
Data Availability Statement.
Data are available upon reasonable request.
REFERENCES
- 1.Smiddy MP, O’ Connell R, Creedon SA. Systematic qualitative literature review of health care workers’ compliance with hand hygiene guidelines. American Journal of Infection Control. 2015;43(3):269–274. [DOI] [PubMed] [Google Scholar]
- 2.Pittet D. The Lowbury lecture: behaviour in infection control. Journal of Hospital Infection. 2004;58(1):1–13. [DOI] [PubMed] [Google Scholar]
- 3.Edwards R, Charani E, Sevdalis N, et al. Optimisation of infection prevention and control in acute health care by use of behaviour change: a systematic review. The Lancet Infectious Diseases. 2012;12(4):318–329. [DOI] [PubMed] [Google Scholar]
- 4.Evanoff B, Kim L, Mutha S, et al. Compliance With Universal Precautions Among Emergency Department Personnel Caring for Trauma Patients. Annals of Emergency Medicine. 1999;33(2):160–165. [DOI] [PubMed] [Google Scholar]
- 5.Bogdanovic J, Petralito S, Passerini S, Sax H, Manser T, Clack L. Exploring healthcare providers’ mental models of the infection prevention “patient zone” - a concept mapping study. Antimicrobial Resistance & Infection Control. 2019;8(1):138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacob JT, Herwaldt LA, Durso FT, CDC Prevention Epicenters Program. Preventing healthcare-associated infections through human factors engineering. Curr Opin Infect Dis. 2018;31(4):353–358. [DOI] [PubMed] [Google Scholar]
- 7.Sax H, Clack L. Mental models: a basic concept for human factors design in infection prevention. J Hosp Infect. 2015;89(4):335–339. [DOI] [PubMed] [Google Scholar]
- 8.Rouse WB, Morris NM. On looking into the black box: Prospects and limits in the search for mental models. Psychological Bulletin. 1986;100(3):349–363. [Google Scholar]
- 9.Gregory L, Weston LE, Harrod M, Meddings J, Krein SL. Understanding nurses’ workflow: Batching care and potential opportunities for transmission of infectious organisms, a pilot study. American Journal of Infection Control. 2019;47(10):1213–1218. [DOI] [PubMed] [Google Scholar]
- 10.Chang N-C, Jones M, Reisinger HS, et al. Hand hygiene and the sequence of patient care. Infect Control Hosp Epidemiol. Published online April 6, 2021:1–6. [DOI] [PubMed] [Google Scholar]
- 11.Arabie P, Aldenderfer MS, Carroll D, DeSarbo WS. Three Way Scaling: A Guide to Multidimensional Scaling and Clustering. SAGE; 1987. [Google Scholar]
- 12.Borg I, Groenen PJ, Mair P. Applied Multidimensional Scaling. Springer Science & Business Media; 2012. [Google Scholar]
- 13.Kruskal J, Wish M. Multidimensional Scaling. SAGE Publications, Inc.; 1978. [Google Scholar]
- 14.Mair P, Borg I, Rusch T. Goodness-of-Fit Assessment in Multidimensional Scaling and Unfolding. Multivariate Behavioral Research. 2016;51(6):772–789. [DOI] [PubMed] [Google Scholar]
- 15.Faul F, Erdfelder E, Lang A-G, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–191. [DOI] [PubMed] [Google Scholar]
- 16.Cohen J. A power primer. Psychol Bull. 1992;112(1):155–159. [DOI] [PubMed] [Google Scholar]
- 17.Jang T-H, Wu S, Kirzner D, et al. Focus Group Study of Hand Hygiene Practice among Healthcare Workers in a Teaching Hospital in Toronto, Canada. Infection Control & Hospital Epidemiology. 2010;31(2):144–150. [DOI] [PubMed] [Google Scholar]
- 18.Jenner EA, Fletcher BC, Watson P, Jones FA, Miller L, Scott GM. Discrepancy between self-reported and observed hand hygiene behaviour in healthcare professionals. J Hosp Infect. 2006;63(4):418–422. [DOI] [PubMed] [Google Scholar]
- 19.Lee K, Burnett E, Morrison K, Ricketts I. Use of hand-held computers to determine the relative contribution of different cognitive, attitudinal, social, and organizational factors on health care workers’ decision to decontaminate hands. American Journal of Infection Control. 2014;42(2):133–138. [DOI] [PubMed] [Google Scholar]
- 20.Pittet D, Simon A, Hugonnet S, Pessoa-Silva CL, Sauvan V, Perneger TV. Hand Hygiene among Physicians: Performance, Beliefs, and Perceptions. Ann Intern Med. 2004;141(1):1. [DOI] [PubMed] [Google Scholar]
- 21.Clack L, Passerini S, Manser T, Sax H. Likelihood of Infectious Outcomes Following Infectious Risk Moments During Patient Care-An International Expert Consensus Study and Quantitative Risk Index. Infect Control Hosp Epidemiol. 2018;39(3):280–289. [DOI] [PubMed] [Google Scholar]
- 22.Erasmus V, Brouwer W, van Beeck EF, et al. A qualitative exploration of reasons for poor hand hygiene among hospital workers: lack of positive role models and of convincing evidence that hand hygiene prevents cross-infection. Infect Control Hosp Epidemiol. 2009;30(5):415–419. [DOI] [PubMed] [Google Scholar]
- 23.Jackson C, Griffiths P. Dirt and disgust as key drivers in nurses’ infection control behaviours: an interpretative, qualitative study. J Hosp Infect. 2014;87(2):71–76. [DOI] [PubMed] [Google Scholar]
- 24.Loveday HP, Lynam S, Singleton J, Wilson J. Clinical glove use: healthcare workers’ actions and perceptions. J Hosp Infect. 2014;86(2):110–116. [DOI] [PubMed] [Google Scholar]
- 25.Meengs MR, Giles BK, Chisholm CD, Cordell WH, Nelson DR. Hand washing frequency in an emergency department. Ann Emerg Med. 1994;23(6):1307–1312. [DOI] [PubMed] [Google Scholar]
- 26.Whitby M, McLaws M-L, Ross MW. Why healthcare workers don’t wash their hands: a behavioral explanation. Infect Control Hosp Epidemiol. 2006;27(5):484–492. [DOI] [PubMed] [Google Scholar]
- 27.Wilson J, Bak A, Loveday HP. Applying human factors and ergonomics to the misuse of nonsterile clinical gloves in acute care. American Journal of Infection Control. 2017;45(7):779–786. [DOI] [PubMed] [Google Scholar]
- 28.Raboud J, Saskin R, Wong K, et al. Patterns of handwashing behavior and visits to patients on a general medical ward of healthcare workers. Infect Control Hosp Epidemiol. 2004;25(3):198–202. [DOI] [PubMed] [Google Scholar]
- 29.Reid SM, Farion KJ, Suh KN, Audcent T, Barrowman NJ, Plint AC. Use of personal protective equipment in Canadian pediatric emergency departments. CJEM. 2011;13(2):71–78. [DOI] [PubMed] [Google Scholar]
- 30.Erasmus V, Daha TJ, Brug H, et al. Systematic review of studies on compliance with hand hygiene guidelines in hospital care. Infect Control Hosp Epidemiol. 2010;31(3):283–294. [DOI] [PubMed] [Google Scholar]
- 31.Flores A, Wrigley M, Askew P, et al. Use of non-sterile gloves in the ward environment: an evaluation of healthcare workers’ perception of risk and decision making. J Infect Prev. 2020;21(3):108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fix GM, Reisinger HS, Etchin A, et al. Health care workers’ perceptions and reported use of respiratory protective equipment: A qualitative analysis. Am J Infect Control. 2019;47(10):1162–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harrod M, Weston LE, Gregory L, et al. A qualitative study of factors affecting personal protective equipment use among health care personnel. Am J Infect Control. 2020;48(4):410–415. [DOI] [PubMed] [Google Scholar]
- 34.Gralton J, Rawlinson WD, McLaws M-L. Health care workers’ perceptions predicts uptake of personal protective equipment. American Journal of Infection Control. 2013;41(1):2–7. [DOI] [PubMed] [Google Scholar]
- 35.Krein SL, Mayer J, Harrod M, et al. Identification and Characterization of Failures in Infectious Agent Transmission Precaution Practices in Hospitals: A Qualitative Study. JAMA Intern Med. 2018;178(8):1016–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldstone RL, Medin DL, Halberstadt J. Similarity in context. Mem Cognit. 1997;25(2):237–255. [DOI] [PubMed] [Google Scholar]
- 37.Cooke NM, Durso FT, Schvaneveldt RW. Recall and measures of memory organization. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1986;12(4):538–549. [Google Scholar]
- 38.Gonzalvo P, Cañas JJ, Bajo M-T. Structural representations in knowledge acquisition. Journal of Educational Psychology. 1994;86(4):601–616. [Google Scholar]
- 39.Day EA, Arthur Jr. W, Gettman D. Knowledge structures and the acquisition of a complex skill. Journal of Applied Psychology. 2001;86(5):1022–1033. [DOI] [PubMed] [Google Scholar]
- 40.Schuelke MJ, Day EA, McEntire LE, et al. Relating indices of knowledge structure coherence and accuracy to skill-based performance: Is there utility in using a combination of indices? J Appl Psychol. 2009;94(4):1076–1085. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request.