ABSTRACT
Motility is essential for apicomplexan parasites to infect their hosts. In a three-dimensional (3D) environment, the apicomplexan parasite Toxoplasma gondii moves along a helical path. The cortical microtubules, which are ultra-stable and spirally arranged, have been considered to be a structure that guides the long-distance movement of the parasite. Here, we address the role of the cortical microtubules in parasite motility, invasion and egress by utilizing a previously generated mutant (dubbed ‘TKO’) in which these microtubules are destabilized in mature parasites. We found that the cortical microtubules in ∼80% of the non-dividing (i.e. daughter-free) TKO parasites are much shorter than normal. The extent of depolymerization was further exacerbated upon commencement of daughter formation or cold treatment, but parasite replication was not affected. In a 3D Matrigel matrix, the TKO mutant moved directionally over long distances, but along trajectories that were significantly more linear (i.e. less helical) than those of wild-type parasites. Interestingly, this change in trajectory did not impact either movement speed in the matrix or the speed and behavior of the parasite during entry into and egress from the host cell.
Keywords: Apicomplexa, Toxoplasma, Cortical microtubules, Invasion, Motility
Highlighted Article: Motility is essential for apicomplexan parasites to infect their hosts. The spirally arranged cortical microtubules underlie the helicity of movement of the human parasite Toxoplasma gondii.
INTRODUCTION
Apicomplexans are obligate intracellular parasites, many of which are important pathogens. For example, the malaria parasites, Plasmodium spp., kill more than half a million people each year (World Health Organization, 2022; https://www.who.int/publications/i/item/9789240064898). Toxoplasma gondii permanently infects ∼20% of the global human population and can infect any warm-blooded animal (Dubey, 2008). As obligate intracellular organisms, a successful infection requires the parasite to travel over a long distance through tissues with various biochemical and biophysical properties (Hopp et al., 2015), as well as to enter into host cells. The latter requires a shorter travel distance, but perhaps more finesse, as the host cell plasma membrane needs to remain intact to allow the parasite to use host cell resources for proliferation.
T. gondii moves along an irregular helical path in tissues and in a three-dimensional (3D) matrix over tens of microns (Leung et al., 2014). Chemical inhibition, as well as genetic manipulations, have shown that actin and a series of apical and cortical myosin motors provide the underlying force for the parasite motility (Dobrowolski et al., 1997; Gaskins et al., 2004; Graindorge et al., 2016; Heaslip et al., 2011; Meissner et al., 2002). The current model posits that the internal force is transmitted to the parasite surface through a coupling between the actomyosin complex and the secreted transmembrane adhesins (Opitz and Soldati, 2002). The characterization of these factors has significantly advanced the understanding of parasite motility (Dobrowolski et al., 1997; Frenal et al., 2010; Gaskins et al., 2004; Graindorge et al., 2016; Heaslip et al., 2011; Huynh and Carruthers, 2006; Huynh et al., 2003; Jacot et al., 2016; Meissner et al., 2002; Tosetti et al., 2019). However, although actin polymerization is critical for parasite movement, no native actin filaments have been detected, suggesting that the polymerization is likely to occur dynamically (Dobrowolski and Sibley, 1996; Mehta and Sibley, 2011; Periz et al., 2017; Sahoo et al., 2006; Schatten et al., 2003; Wetzel et al., 2003). Thus, it is still not known how the parasite is capable of traveling over long distances along a helical path. Intuitively, a stable, inherently helical cortical structure would be required for guiding continuous parasite movement over a long distance.
In Toxoplasma, one of the main cytoskeletal structures with a roughly helical arrangement is the set of cortex-associating (denoted as ‘subpellicular’ or ‘cortical’) microtubules that are anchored at one end in a ring-like structure (the apical polar ring) at the parasite apex (Leung et al., 2017; Morrissette and Sibley, 2002a; Nichols and Chiappino, 1987). Among the apicomplexans, the microtubule cytoskeleton displays intriguing species and life-stage specific variations that coincide with substantial differences in physiology. The mature Plasmodium ookinete has an intricate cage of ∼60 cortical microtubules (Bounkeua et al., 2010; Garnham et al., 1962; Wang et al., 2020), but the asexual blood-stage parasites only have a narrow band of two to four cortical microtubules that extends down one side of the parasite (Bannister and Mitchell, 1995; Morrissette and Sibley, 2002a). The Toxoplasma tachyzoite consistently has 22 cortical microtubules that are arranged in a spiral pattern. The parasite invests a considerable amount of resources in building and maintaining these polymers. Aside from the core building blocks, the tubulins, there is a suite of proteins that associate with the cortical microtubules in a region-specific manner (Leung et al., 2017; Liu et al., 2015, 2013; Tran et al., 2012). In mature parasites, these microtubules form an ultra-stable ‘rib cage’ that extensively interacts with the inner membrane complex (IMC). They remain intact under treatments that depolymerize microtubules in mammalian cells within minutes (Hu et al., 2002; Morrissette et al., 1997). This extraordinary stability makes it difficult to assess the function of these polymers in the mature parasite.
Previously, a suite of proteins has been found to associate with the cortical microtubules. Some of them do not bind along the entire length of the microtubule, but are restricted to distinct regions (Liu et al., 2015, 2013; Tran et al., 2012). We discovered that, in mature parasites, TLAP2 and SPM1 together stabilize the portion of the microtubules distal to the apical section, which contains TLAP3 (Liu et al., 2015). In the absence of TLAP2 and SPM1, TLAP3 stabilizes the apical section of the cortical microtubules (Liu et al., 2015). Thus, in the mutant that lacks TLAP2, SPM1 and TLAP3 (Δtlap2Δspm1Δtlap3, denoted ‘TKO’ for succinctness) the stability of the cortical microtubules in mature parasites is dramatically reduced. In the present work, we quantified the proportion of parasites with various levels of defects in the microtubule array under different conditions and found that ∼80% of the TKO parasites that are not forming daughters (i.e. ‘non-dividing’ in our terminology) have severely curtailed cortical microtubules. Depolymerization in mature TKO parasites becomes even more pronounced upon the initiation of daughter construction or cold treatment, with the latter effect being partly reversible. Although microtubule polymerization is essential for generating viable daughter cells, parasite replication is not affected by the destabilization of the cortical microtubules in the mature parasite. In a 3D Matrigel matrix, the TKO mutant parasite moves as persistently as the wild-type parasite, with a similar velocity and net displacement. However, it travels along a path that is significantly more linear than the wild-type parasite. Interestingly, even though the cytolytic efficiency of the TKO parasite is significantly lower, live-cell imaging revealed that the behaviors of the parasite during entry into and egress from the host cell are indistinguishable from those of the wild-type. This indicates that host cell entry and egress, which involve short-distance travel, is less sensitive to structural changes in the parasite than overall infection efficiency.
RESULTS
In TKO parasites, the daughter microtubule array is intact, and replication proceeds normally
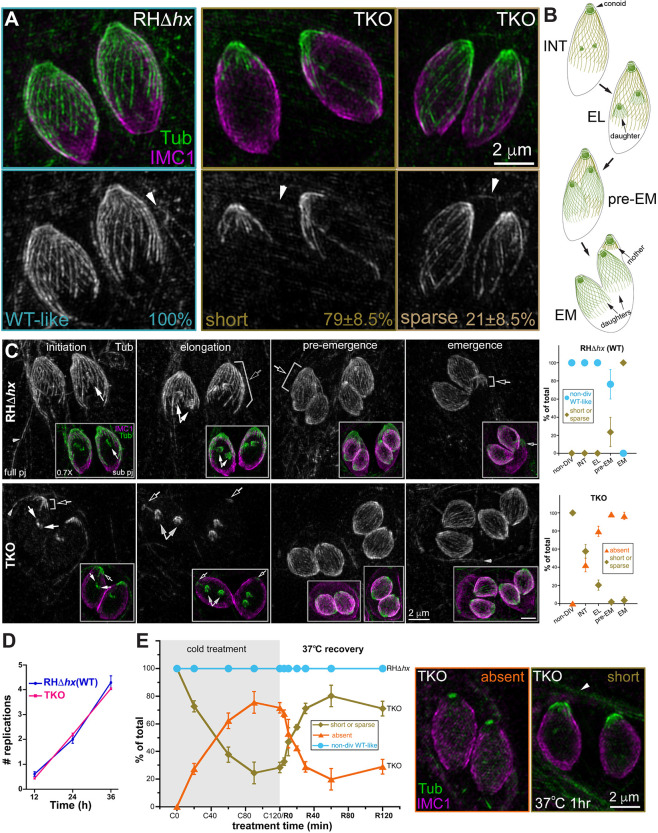
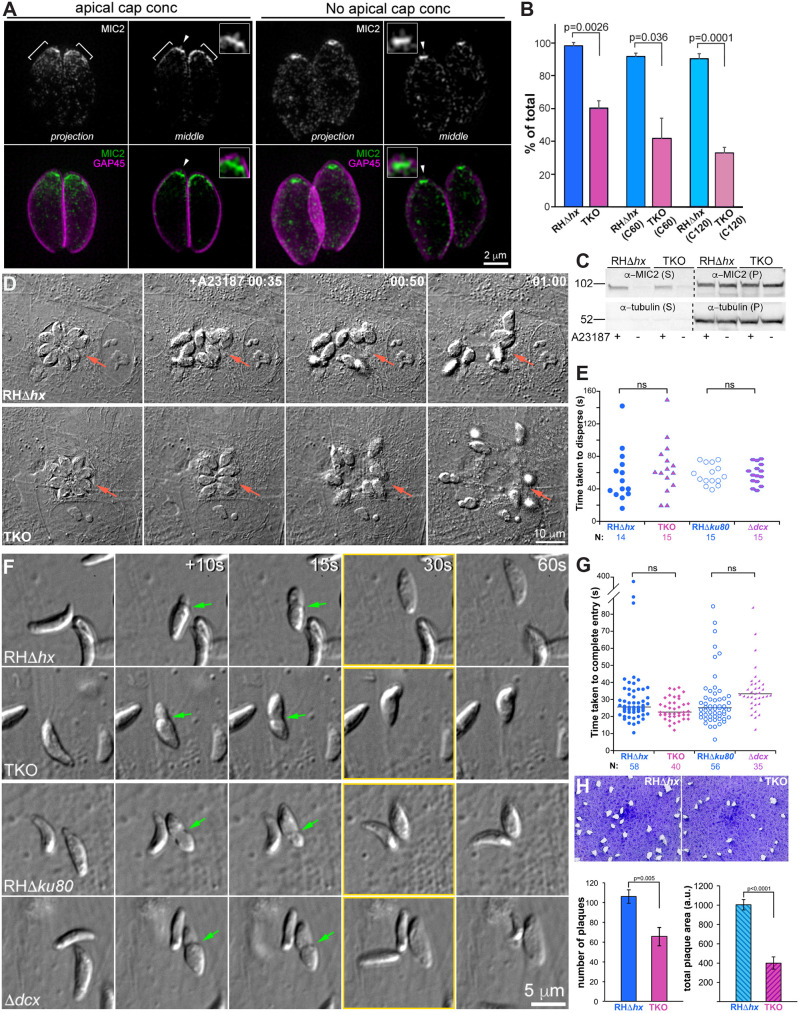
Previously, we showed qualitatively that the cortical microtubules in mature TKO parasites are destabilized compared with those in the wild-type parasites (Liu et al., 2015). Here, to quantify the extent of destabilization, we determined the proportions of TKO and wild-type parental (RHΔhx; Donald and Roos, 1994) parasites that display various characteristics in the microtubule array. We first examined wild-type and TKO parasites that did not contain visible daughters based on anti-tubulin labeling (denoted as ‘non-dividing’ in what follows). We found that whereas 100% of the wild-type parasites had a full, regularly arranged array, the cortical microtubules were severely curtailed in 79±8.5% (mean±s.d.) of TKO parasites (‘short’ phenotype) and sparse in the rest (Fig. 1A; three independent experiments, total 490 parasites analyzed for the TKO and 246 parasites analyzed for the wild-type). The ‘short’ and ‘sparse’ phenotypes are defined as follows: short, microtubules at approximately normal density extending to less than one-third of the parasite body length and very few microtubules are longer than half of the parasite body length; sparse, more microtubules are longer than one-half of the parasite body length compared to short, but there are obvious gaps in the array. The extent of depolymerization in mature TKO parasites was more pronounced during cell division (Fig. 1B,C). The proportion of parasites with nearly fully depolymerized cortical microtubules (the ‘absent’ category) was ∼42% at the initiation stage of daughter construction and increased to ∼98% prior to daughter emergence from the mother parasite (Fig. 1C). For wild-type parasites, the cortical microtubules of the mother parasite in the initiation and elongation stages of daughter construction were similar to those of the non-dividing stage (Fig. 1B,C). However, ∼24% of mature parasites at the pre-emergence stage and 100% during daughter emergence had short microtubules.
Fig. 1.
Cortical microtubules in intracellular mature TKO and wild-type parasites. (A) Projections of image stacks acquired by three-dimensional structured illumination microscopy (3D-SIM) of the non-dividing wild-type (WT; RHΔhx) and TKO (Δtlap2Δspm1Δtlap3) parasites. Whereas 100% of the wild-type parasites have the typical spirally arranged full microtubule array, ∼79±8.5% (mean±s.d.; three independent experiments) of the TKO parasites have significantly curtailed/short microtubules. The cortical microtubules are sparse in the other 21%. Green and grayscale, tubulin (Tub) labeling by a mouse anti-acetylated tubulin antibody, which also labels the microtubules in the host cell (arrowhead). Magenta, cortex labeling by a rabbit anti-IMC1 antibody. Arrowheads, host cell microtubules. (B) Diagrams of wild-type parasites at the initiation (INT), elongation (EL), pre-emergence (pre-EM) and emergence (EM) stages of daughter construction. Cortical microtubules and the conoid are shown for the mother and daughter parasites. (C) Left, projections of 3D-SIM images of TKO and wild-type parasites at the initiation, elongation, pre-emergence, and emergence stage of daughter construction. Black arrows, cortical microtubules in the mother parasite. White arrows, daughter parasites. Arrowheads, host-cell microtubules. Insets (0.7×) are projections that include a subset of the image stack (sub pj) to better display the structure of the daughter parasites, which are obscured in the full projections (full pj) for the wild-type parasites due to the cortical microtubules in the mother. Note that for the TKO parasite, due to the shortness or complete absence of the maternal microtubules, the daughters are as fully visible in the full projection as in the subset projection. Right, quantification of the proportions of wild-type (top) and TKO (bottom) parasites that display various characteristics in the maternal cortical microtubule array at different stages, including the non-dividing (non-DIV) stage or the initiation (INT), elongation (EL), pre-emergence (pre-EM), and emergence (EM) stages of daughter construction. For the TKO parasites, the two patterns observed are ‘short or sparse’ (i.e. similar to non-dividing TKO parasites) and ‘absent’. For the wild-type parasites, the two patterns observed are ‘non-dividing wild-type-like’ or ‘short or sparse’. Error bars are s.e.m. from three independent experiments. Total number of parasites analyzed were: 88, 68, 50 and 36 for TKO, and 52, 62, 54 and 49 for wild type at the initiation, elongation, pre-emergence and emergence stage, respectively. The data for the non-dividing stage is the same as shown in Fig. 1A. (D) Average number of cell divisions at 12, 24 or 36 h after infection in four independent experiments for RHΔhx (WT) and TKO parasites. Error bars show the s.d. (E) Left, quantification of the percentage of non-dividing wild-type (RHΔhx) and TKO parasites that display various characteristics in the array of the cortical microtubules after being placed at ∼7°C for 0, 20, 60, 90 or 120 min (C0–C120) as well as after recovery at 37°C for 5, 10, 20, 30, 60 or 120 min after 120 min of∼7°C incubation (R5–R120). For the TKO parasites, the two patterns observed are ‘short or sparse’ or ‘absent’. For wild-type parasites, the cortical microtubules remain similar to those in untreated parasites under all conditions tested here (after cold treatment and cold treatment plus 37°C recovery). Error bars are s.e.m. from three independent experiments. Middle, projection of 3D-SIM images of TKO parasites in which cortical microtubules are absent. Right, projection of 3D-SIM images of TKO parasites after 1 h of 37°C (R60) recovery, in which short cortical microtubules at the apical end are seen. Arrowhead, host-cell microtubules.
We examined the average number of rounds of replication at 12, 24, and 36 h after infection and found that the rate of replication of the TKO parasite was very similar to that of the wild-type parasite (Fig. 1D). At first glance, it is surprising that a mutant that has destabilized cortical microtubules does not have a replication defect, because microtubule polymerization is coupled with cortex formation, and thus is required for daughter growth (Hu, 2008; Hu et al., 2006; Morrissette and Sibley, 2002b; Stokkermans et al., 1996). However, as we observed previously (Liu et al., 2015), in the same cell in which the cortical microtubules of the mother TKO parasite are depolymerized, microtubules do polymerize in growing daughters, which develop normally. These results reveal a cell-stage-dependent microtubule-depolymerizing activity that acts on the cortical microtubules in mature parasites but not the polymerizing microtubules in growing daughters. The depolymerization of cortical microtubules in mature TKO parasites becomes apparent at a much earlier stage of daughter formation and is much more complete than in the wild-type parasites, likely because the former are already destabilized due to the lack of TLAP2, TLAP3 and SPM1.
In our previous work, we showed that incubation at ∼8°C can further destabilize the cortical microtubules of the TKO parasite (Liu et al., 2015). Here, we characterize the time course of cold-induced disassembly of cortical MTs in mature TKO parasites and found that, as expected, the extent of depolymerization increased with the time of cold-treatment (Fig. 1E). After ∼2 h of cold treatment (denoted C120), cortical MTs in ∼72% of the parasites were nearly completely depolymerized with no visible MTs (‘absent’ category) and only a small tubulin-positive ‘cap’ at the apical end. By contrast, the array of cortical microtubules in wild-type parasites was not affected by the cold treatment. The cold-induced depolymerization in the TKO parasites was partially reversible (Fig. 1E). When TKO cultures cold-treated for 2 h were returned to 37°C, the cortical MTs were significantly restored after 20 min of the temperature shift. Upon 1 h of 37°C incubation (R60), ∼80% of the parasites showed patterns (‘short or sparse’) similar to those of untreated parasites, with the microtubules concentrated at the apical end of the parasite (Fig. 1E), suggesting a strong preference for regrowth from pre-existing nucleation sites.
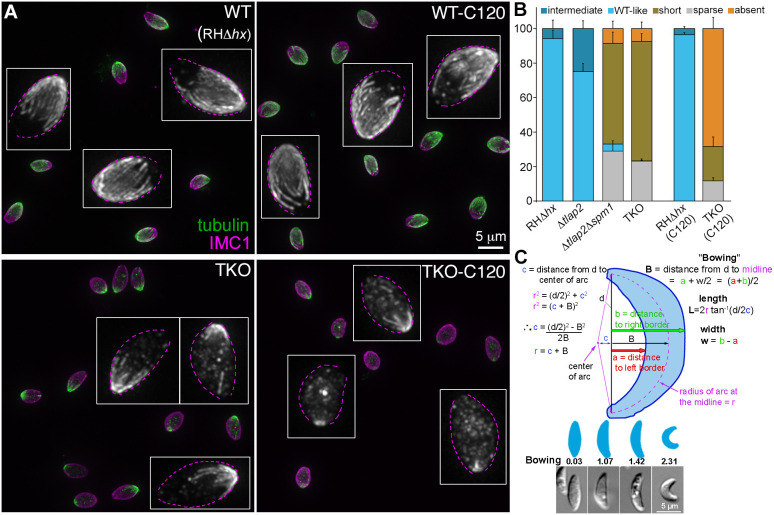
The preceding experiments were carried out with intracellular parasites residing in host cells. As the difference between intra and extracellular environment might affect subcellular structures in the parasite, we next examined the microtubule array in extracellular wild-type parental (RHΔhx) and TKO (Δtlap2Δspm1Δtlap3) parasites. We found that the distribution of different array morphologies was similar to what was observed in intracellular mature parasites (Fig. 2A,B). More than 94% of the parental parasites have a full, regularly arranged array with and without cold treatment. In contrast, the cortical microtubules were short in ∼69±4.4% (mean±s.e.m.), sparse in ∼23% and absent in ∼7.5% of the untreated TKO parasites. The cortical MTs were absent in ∼68±6.4% of the extracellular parasites harvested after ∼2 h of cold treatment (C120). Confirming the synergistic effect of the associated proteins, the cortical microtubules in 75±4.5% of the single knockout mutant Δtlap2 had wild-type-like arrangement. In contrast, only ∼4% of the double knockout mutant (Δtlap2Δspm1) had a wild-type-like microtubule array. The cortical microtubules were short in ∼58±6.7%, sparse in ∼29% and absent in ∼8.7% of this line.
Fig. 2.
Comparison of cortical microtubule morphology and shape of extracellular wild-type parental, Δtlap2, Δtlap2Δspm1 and TKO parasites. (A) Projections of image stacks of wide-field deconvolved images of extracellular wild-type RHΔhx (WT) and TKO (Δtlap2Δspm1Δtlap3) parasites with (C120) and without cold-treatment. Green and grayscale, tubulin labeling by a mouse anti-acetylated tubulin antibody. Magenta, cortex labeling by a rabbit anti-IMC1 antibody. Insets: grayscale images of the tubulin labeling in a few parasites in each field displayed at 3× magnification. Dotted outlines were drawn based on IMC1 labeling. (B) Quantification of the proportions of extracellular RHΔhx, Δtlap2, Δtlap2Δspm1 and TKO parasites that display various characteristics in the cortical microtubule array. ‘Short’, ‘sparse’ and ‘absent’ phenotypes are as defined in Fig. 1. The ‘intermediate’ phenotype refers to arrays with detectable gaps but that have less severe phenotypes than the ‘sparse’ arrays. For RHΔhx and TKO parasites, measurements of parasites harvested after 2 h (C120) of cold treatment are also included. Error bars are s.e.m. from four independent experiments for Δtlap2, Δtlap2Δspm1 and TKO, and three independent experiments for RHΔhx. (C) Top, illustration for the calculation of length, width and bowing of extracellular parasites. Bottom, diagrams and sample images of parasites that have different bowing indices. Quantification of length, width, and bowing of extracellular RHΔhx, Δtlap2, Δtlap2Δspm1, and TKO (Δtlap2Δspm1Δtlap3) parasites are included in Table 1.
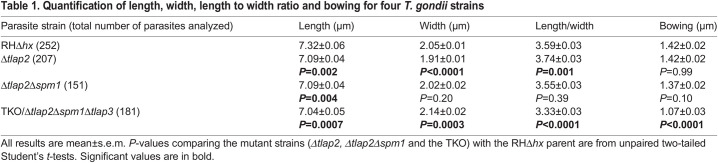
Because of their stable association with the cortex, it has been hypothesized that the cortical microtubules support the characteristic crescent shape of Toxoplasma. We took advantage of the panel of mutants with varying degrees of defects in the microtubule cytoskeleton and examined their length, width, length-width ratio and ‘bowing’. Length is defined as the distance from apical to basal tip measured along the arc that passes through both of them and through the center of the parasite, which is given by 2r tan−1(d/2C) in Fig. 2C. Bowing is a measurement related to curvature (see Fig. 2C and Materials and Methods for definition). Because apparently straight parasites might be curved but viewed from the wrong direction, measurements were performed on the most obviously bowed parasites for each line. The lengths of the microtubule mutants were slightly shorter than that of the wild-type parasite (Table 1). There was not a consistent correlation between microtubule defects and the width or length-width ratio of the parasite. The one parameter that stood out was the bowing index, which was significantly lower in the TKO parasite (1.07±0.03 μm; mean±s.e.m.) than in all the other lines (1.37–1.42 μm) (Table 1). Interestingly, whereas the Δtlap2Δspm1 parasite was much more similar in bowing to the RHΔhx parental (1.37 versus 1.42 μm) than to TKO parasites, the structural defect of the microtubule array in the Δtlap2Δspm1 was very pronounced and quite similar to that of the TKO (Fig. 2B). This indicates that the effect of the structure of the microtubule array on parasite shape is likely to be nonlinear.
Table 1.
Quantification of length, width, length to width ratio and bowing for four T. gondii strains
The trajectory of movement of the TKO mutant is more linear than that of the wild-type parasite in 3D Matrigel
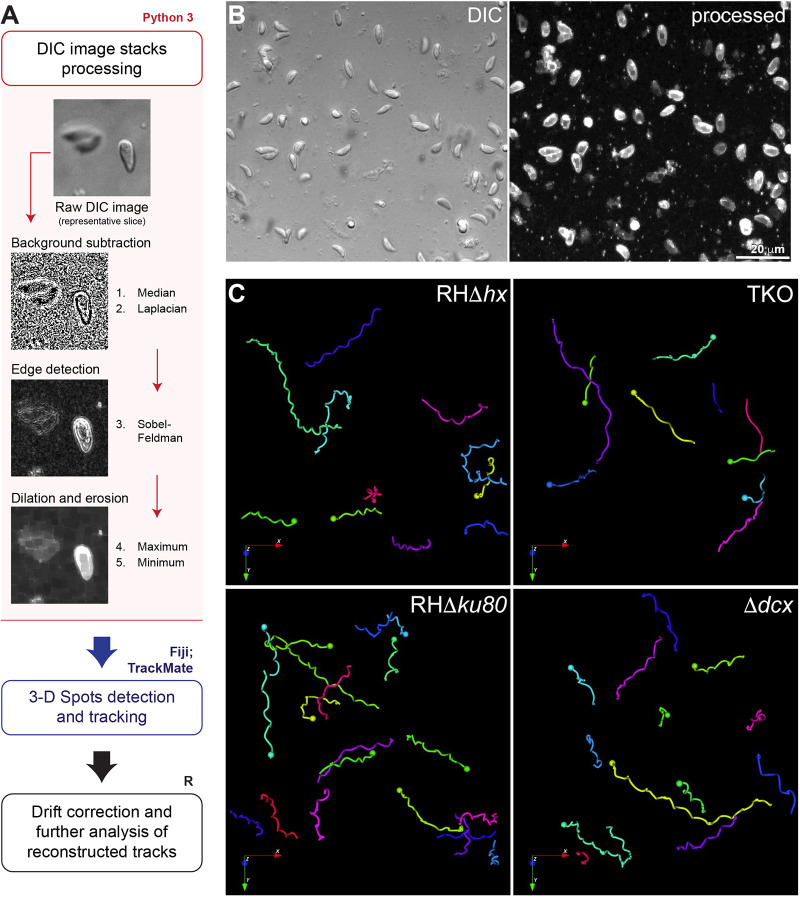
In wild-type parasites, the stable, spirally arranged cortical microtubules have been proposed to guide directionality of parasite movement (Stadler et al., 2017) or to function as a spring that transmits energy to drive gliding motility (Pavlou et al., 2020). We tested these hypotheses by comparing the 3D motile behaviors of the ‘wild-type’ parent (RHΔhx) with those of the TKO parasite, taking advantage of the destabilized and curtailed microtubule array in the TKO. By modifying an established Matrigel-based 3D motility assay (Leung et al., 2014), we obtained 3D time-lapse images of the moving parasites under a label-free condition through differential interference contrast (DIC) imaging. DIC imaging not only minimizes phototoxicity, but also enables the observation of parasite morphological changes as they move. However, whereas fluorescence images exhibit high contrast, which facilitates cell tracking, automated tracking of cell positions in DIC image stacks is not as straightforward. We applied a series of edge detection filters, followed by erosion and dilation filters on the DIC images to derive images with contrast similar to fluorescence images (Fig. 3A,B) that are amenable to 3D cell tracking. Detection and tracking were carried out using the TrackMate plugin in Fiji, which generates the 3D coordinates of the cell trajectories for further analysis and 3D visualization (Figs 3C and 4; Movies 1–4).
Fig. 3.
Workflow for image processing and automatic tracking of 3D motility data acquired by DIC imaging. (A) Schematic diagram of the image processing (pink outline and box), detection and tracking (blue outline) and tracks analysis (black outline) workflow for 3D motility data acquired by DIC imaging (pink). A representative area of a slice from a DIC image stack before processing and the resulting images corresponding to the filters applied on each processing step using Python3, showing the resulting high-contrast image. For details, see Materials and Methods. (B) Focused composite image of a DIC image stack (41 slices; 40 µm) of extracellular wild-type parasites in Matrigel and corresponding processed image (represented by its maximum projection) (see also Movie 1). (C) Examples of 3D tracks for motile RHΔhx parental, TKO, RHΔku80 parental and Δdcx parasites reconstructed in the Icy software (see also Movies 1–4). Length of arrows: 16 µm.
Fig. 4.
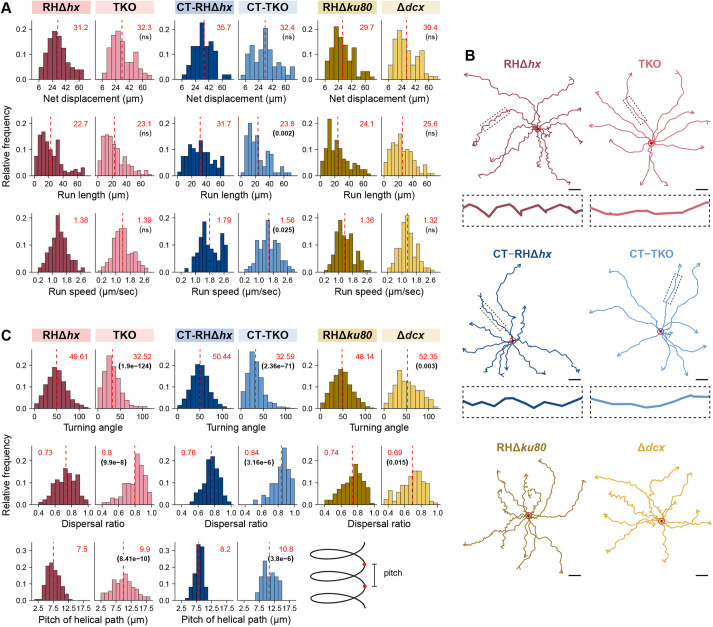
The 3D trajectories of TKO parasites are more linear than those of parental parasites. (A) Frequency distribution and statistical analysis of motion-related parameters, including net displacement, run length (distance between pauses >5.4 s), and run speed of RHΔhx parental and TKO parasites without or with cold treatment (CT), and RHΔku80 parental and Δdcx parasites. For cold-treated TKO parasites, intracellular parasite cultures were placed at 7°C for 2 hours before harvesting. For the other samples/conditions, the parasites were harvested as described previously in Munera Lopez et al. (2022). All motility assays were carried out at 37°C. (B) Representative tracks projected on the (x, y) plane with superimposed origins (red circles) for RHΔhx parental and TKO parasites without or with cold -treatment (CT), and RHΔku80 parental and Δdcx parasites. Enlarged insets (4×) include the segments indicated by the dashed boxes in the trajectories. Scale bars: 10 µm. (C) Frequency distribution and statistical analysis of helicity-related parameters including turning angle, dispersal ratio and the pitch of RHΔhx parent and TKO parasites without or with cold-treatment (CT). An illustration defining pitch is included. Data for all parameters are also displayed for RHΔku80 parental and Δdcx parasites except for the pitch. The mean values for individual histograms in A and C are shown at the top (in red) and also marked by the dashed line (see Table S1 for the number of data points for each analysis). P-values (in brackets under the mean values) were calculated by the Kruskal–Wallis test. ns, not significant.
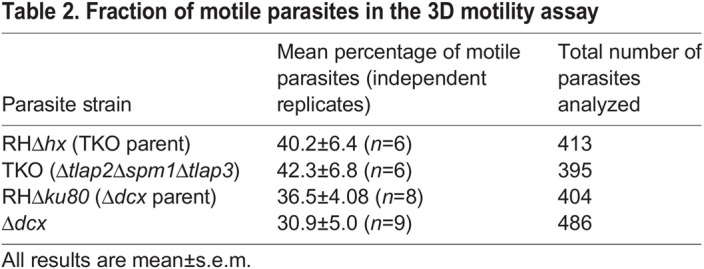
To compare the impact of different helically arranged cytoskeletal structures, we also analyzed the motile behavior of a conoid mutant (Δdcx; Nagayasu et al., 2016) and its ‘wild-type’ parent (RHΔhxΔku80, abbreviated as RHΔku80; Fox et al., 2009; Huynh and Carruthers, 2009) in parallel with those of TKO and its RHΔhx parent. The conoid is formed of helically arranged tubulin fibers (Hu et al., 2006, 2002; Nagayasu et al., 2016; Swedlow et al., 2002) (Fig. 1B). In the Δdcx mutant, a structural component (TgDCX) is removed, and the conoid structure becomes stunted and disorganized (Nagayasu et al., 2016). We found that on average, the motile percentage of the Δdcx was ∼31% (486 parasites from nine independent experiments analyzed), which was slightly lower than that of its wild-type parent (RHΔku80, 404 parasites from eight independent experiments analyzed) (Table 2). The average net displacement, run-length (i.e. distance between pauses of >5.4 s) and run speed (average speed during a run) of the Δdcx parasite were similar to those of the wild-type parasites (30.4 versus 29.7 µm, ∼25.6 versus 24.1 µm and 1.32 versus 1.36 µm/s, respectively, Fig. 4A). As previously reported (Leung et al., 2014), the wild-type parent moves along an irregular helical path. The average dispersal ratio (ratio of net displacement to total distance traveled of motile segments) of the trajectories was 0.74 (a value of 1 indicates a completely linear track). The average turning angle (relative angle of the parasite between two consecutive steps as defined in Beltman et al., 2009) was ∼48°. The trajectories of the Δdcx were helical in nature (Fig. 4B,C; Movies 3,4), with an average dispersal ratio of 0.69 and turning angle of ∼52°, indicating slightly tighter turns.
Table 2.
Fraction of motile parasites in the 3D motility assay
In contrast, the movement of the TKO parasite was visibly more linear than that of the wild-type parasite (Fig. 4B; Movies 1,2). Confirming this visual impression, we found that the dispersal ratio of the TKO parasites was significantly higher than that of the wild-type parent (RHΔhx) (0.8 versus 0.73, P<<0.0001, Fig. 4C). The turning angle of the TKO was significantly lower than the wild-type parasite (∼32.5° versus ∼50°, P<<0.0001). Both are consistent with the trajectories being more linear. For segments of trajectories during which the TKO parasite displays near helical movement, the average pitch of the helical turns was significantly longer than that of the wild-type parasite (9.9 µm versus 7.5 µm, P<<0.0001). Other motility parameters were similar between the two lines. 40.2% of the RHΔhx parasites were motile (413 parasites from 6 independent experiments analyzed) (Table 2) and ∼42.3% of the TKO parasites were motile (395 parasites from 6 independent experiments analyzed). The average net displacement, run-length and run speed were not significantly different between the TKO and RHΔhx parasite (32.3 versus 31.2 µm, ∼23.1 versus 22.7 µm and 1.39 versus 1.38 µm/s, respectively, Fig. 4A).
As shown in Fig. 1A, there were some remnant cortical microtubules in untreated TKO parasites. To determine whether these remaining microtubules influence the helicity of parasite movement, we cold-treated (CT) the parasites for 2 h, under which condition the cortical microtubules were nearly fully depolymerized in ∼70% of the TKO parasites but those in the wild-type parasites were not affected (Figs 1E and 2B). The parasites were then harvested and mixed with Matrigel for the 3D motility assay. The turning angle of the cold-treated TKO (CT-TKO) was nearly the same as that of the untreated TKO parasites (Fig. 4B,C). As observed in untreated parasites, the cold-treated TKO parasite moved much more linearly than the cold-treated wild-type parasite (CT-RHΔhx), as indicated by the significant differences in turning angle (∼32.6° versus ∼50°), dispersal ratio (0.84 versus 0.76) and pitch (10.8 µm versus 8.2 µm).
To determine the impact of individual microtubule-associated proteins, we examined the 3D motility of Δtlap2 and Δtlap2Δspm1, along with the TKO and the wild-type parent (Fig. 5). The loss of TLAP2 alone resulted in a mild defect in the microtubule array (Fig. 2B), and a very modest change in the turning angle and dispersal ratio of the movement. The loss of TLAP2 and SPM1 together led to pronounced defects in the microtubule array (Fig. 2B) as well as significantly more linear movement, as indicated by a turning angle and dispersal ratio that were significantly different from the wild-type parent (Fig. 5). The alteration in helicity of movement is therefore very much in line with the extent of perturbation of the microtubule array. We note that in this second set of 3D motility experiments, a new batch of Matrigel was used. It is well known that the mechanical properties of Matrigel can vary from batch to batch or change with prolonged storage (Aisenbrey and Murphy, 2020). In the second batch of Matrigel (Fig. 5), the turning angles of both wild-type and TKO parasites were much lower than those of the corresponding lines measured earlier (Fig. 4C). However, the differences in helicity (turning angle and dispersal ratio) remain highly significant between the mutant and the wild-type parent.
Fig. 5.
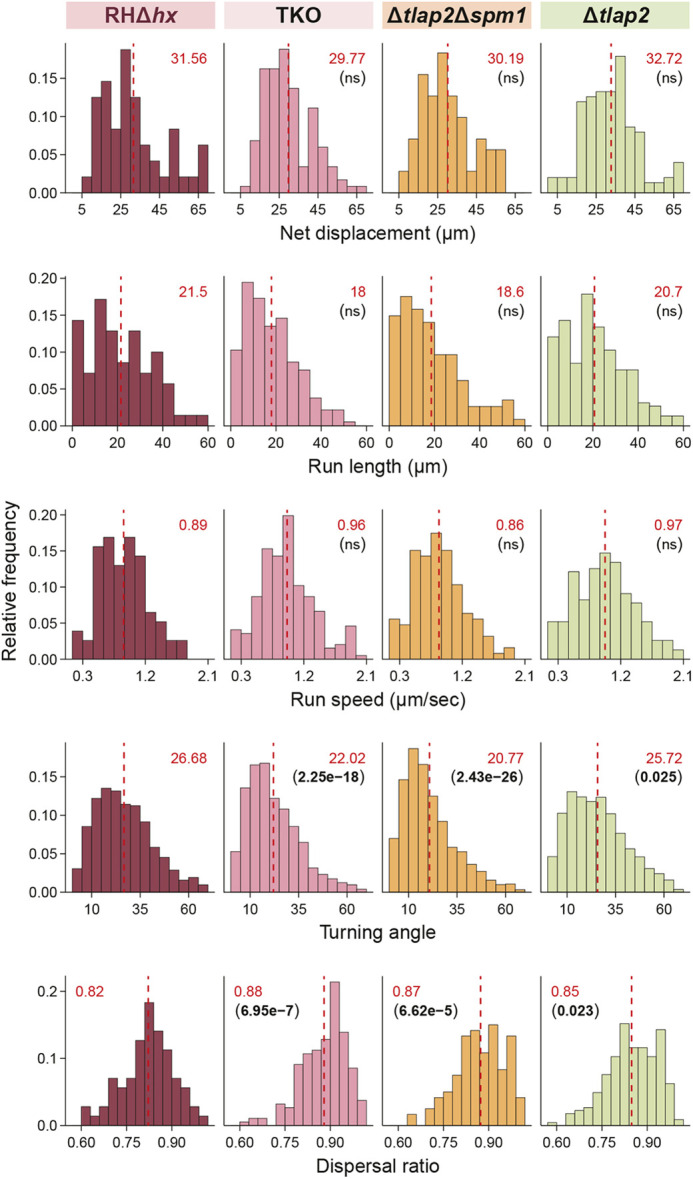
Comparison of 3D motility of wild-type parental, Δtlap2, Δtlap2Δspm1 and TKO parasites. Frequency distribution and statistical analysis of net displacement, run length, run speed, turning angle and dispersal ratio of parental RHΔhx, Δtlap2, Δtlap2Δspm1 and TKO (Δtlap2Δspm1Δtlap3) parasites. Mean values for individual histograms are shown at the top (in red) and also marked by the dashed line (see Table S1 for the number of data points for each analysis). P-values (in brackets under the mean values) were calculated by the Kruskal–Wallis test. ns, not significant.
The apical concentration of micronemal vesicles is partially disrupted in the TKO parasite
Transmembrane adhesins secreted from the membrane-bound vesicles (termed micronemes) are crucial for effective parasite movement (Carruthers et al., 2000; Carruthers and Sibley, 1997; Gras et al., 2017; Huynh and Carruthers, 2006; Rabenau et al., 2001). We have shown previously that micronemal vesicles co-align with the cortical microtubules (Leung et al., 2017). Here, we quantify the different patterns of microneme distribution in TKO and its parent (RHΔhx). Micronemal vesicles were labeled by immunofluorescence using an antibody that recognizes MIC2, a major adhesin protein (Carruthers et al., 2000; Carruthers and Sibley, 1997; Gras et al., 2017; Huynh and Carruthers, 2006). The micronemal vesicles were enriched in an apical cap region (Fig. 6A, ‘apical cap conc’) in 98% of the wild-type parasites, but only ∼60±4.3% (mean±s.e.m.) of the untreated TKO parasite (Fig. 6A,B). When cold-treated for 2 h (C120), this percentage further decreased to ∼33±3.5% in the TKO parasites. The cold-treatment also had a modest impact on the wild-type parent; parasites without the apical cap had an increase in frequency from ∼2% to ∼10% (Fig. 6B). In parasites that lost the microneme enrichment at the apical cap, an intense concentration of MIC2 labeling remained at the parasite apex (Fig. 6A, ‘no apical cap conc’). We note that a longitudinal bar was often observed at the parasite apex regardless of whether there was a prominent apical cap of microneme vesicles (Fig. 6A, insets). This signal is probably from micronemal vesicles congregating in the conoid as observed by electron microscopy (Aquilini et al., 2021). To investigate whether the secretion of MIC2 is affected, we examined the amount of MIC2 secreted into the supernatant by extracellular RHΔhx parental and TKO parasites treated with the Ca2+ ionophore A23187. We found that both lines responded robustly to the Ca2+ ionophore treatment (Fig. 6C; Fig. S1). The TKO parasite on average secreted MIC2 at ∼67% of the level of the RHΔhx parent (Fig. S1), but the difference between the two lines was not statistically significant.
Fig. 6.
The speeds of entry and egress of TKO parasites are similar to those of parental parasites despite altered localization of micronemal vesicles, and lower invasion and plaquing efficiency. (A) Projections and single sections of 3D-SIM images of representative intracellular parasites with and without an apical cap concentration of micronemal vesicles. Images of RHΔhx parasites and TKO parasites are used as examples for ‘apical cap concentration’ and ‘no apical cap concentration’, respectively. Green and grayscale, microneme labeling by a mouse anti-MIC2 antibody. Magenta, cortex labeling by a rabbit anti-GAP45 antibody. Insets (shown at 2×, contrast enhanced to display weaker signals) include apex regions of parasites indicated by arrowheads. A longitudinal bar of MIC2 labeling at the apex is observed. (B) Quantification (mean±s.e.m., three independent experiments) of the percentage of RHΔhx parental and TKO parasites that have an apical concentration of micronemal vesicles. Three conditions are included: no treatment, and treatment at ∼7°C for 60 (C60) or 120 min (C120) prior to the immunofluorescence labeling. P-values were calculated by unpaired two-tailed Student's t-tests. (C) Western blots of the secreted (supernatant, S) and unsecreted (pellet, P) fractions of RHΔhx and TKO parasites with (+) or without (−) A23187 treatment. The blot was probed by antibodies against MIC2 and tubulin. The sizes of molecular mass markers (M) are indicated in kDa on the left. The contrast for images of the western blot is enhanced and inverted to aid visualization of the bands. Please see Fig. S1 for the full blot; this blot is representative of three repeats. (D) Images selected from time-lapse experiments of intracellular RHΔhx parental and TKO parasites treated with 5 µM A23187 (also see Movie 5). Parasitophorous vacuoles are indicated by the arrows. The RHΔhx parental and TKO parasite cultures were placed at ∼7°C for 1 h prior to the egress experiment, which was conducted at 37°C. (E) Dot plots of time taken to disperse after treatment with 5 µM A23187 for RHΔhx parental, TKO, RHΔku80 parental and Δdcx parasites. P-values were calculated by unpaired two-tailed Student's t-tests. ns, not significant. (F) Images selected from time-lapse recording of RHΔhx parental, TKO, RHΔku80 parental and Δdcx parasites in the process of invasion (also see Movie 6). Frames marked in yellow, entry completed. Green arrows, constrictions formed during invasion. (G) Dot plots of time taken for entry (i.e. time from parasite contact with the host cell at its apical end to completion of entry) for RHΔhx parental, TKO, RHΔku80 parental and Δdcx parasites. P-values were calculated by unpaired two-tailed Student's t-tests. ns, not significant. (H) Top, plaques formed by the RHΔhx parental and TKO parasites. ‘Plaques’ are areas of the host cell culture lysed by repeated cycles of parasite invasion, replication, and egress. Bottom, bar graphs that compare the number of plaques (left) and total plaque area (right) of the RHΔhx parental and TKO parasites. Five independent experiments were conducted. Error bars are s.e.m. P-values were calculated by unpaired two-tailed Student's t-tests.
The infection efficiency of the TKO is lower than that of the wild-type parasite, whereas entry into and Ca2+-induced egress from the host cell is not affected
Parasite motility is critical for invasion into and egress from the host cell (Dobrowolski et al., 1997; Frenal et al., 2010; Gaskins et al., 2004; Graindorge et al., 2016; Heaslip et al., 2011; Jacot et al., 2016; Meissner et al., 2002; Munera Lopez et al., 2022; Tosetti et al., 2019). In addition, as the cortical microtubules are stable polymers that associate extensively with the parasite cortex, it is conceivable that they might provide mechanical support to the cortex when the parasites penetrate the membrane of the host cell during entry or exit. We therefore used video microscopy to determine whether the destabilization of the cortical microtubules in the TKO parasite might affect any of these processes. To examine parasite egress, the cultures infected by TKO or RHΔhx parental parasites were subjected to cold treatment for 1 h and then placed at 37°C for egress induced by A23187. Both TKO and wild-type parasites egressed actively and efficiently (Fig. 6D; Movie 5). Within 150 s, egress was completed in >93% of vacuoles for the wild-type parasites and in 100% of vacuoles for the TKO parasites. Of the egressed vacuoles, the average time taken to disperse was ∼56 s for the wild-type parasite (n=14) and ∼66 s for the TKO parasite (n=15; P-value not significant) (Fig. 6E). In parallel, we also analyzed the egress behaviors of the Δdcx and its RHΔku80 parent. We found that both lines egressed efficiently (Fig. 6E), with an average disperse time of ∼57.7 and ∼58.3 s for RHΔku80 (n=15) and Δdcx (n=15), respectively.
To assess parasite behavior during entry into the host cell, freshly harvested extracellular parasites were settled onto the host cell monolayer by a ∼10 min pre-incubation on ice and then imaged by DIC at 37°C for 15 min (Fig. 6F; Movie 6). The number of entry events was recorded for four parasite lines – wild-type RHΔhx parental, TKO, wild-type RHΔku80 parental and Δdcx parasite. We observed 58 events for RHΔhx in 10 videos, 40 for TKO in 13 videos, 56 for RHΔku80 in 9 videos, and 35 for Δdcx in 22 videos. The median speed of host cell entry was 25.5 s for RHΔhx, 22.5 s for the TKO, 25 s for the RHΔku80 and 33 s for the Δdcx parasites (Fig. 6G). We also did not observe any notable differences among these parasite lines regarding the morphological changes (e.g. the formation and resolution of constrictions) that accompany host cell entry.
The lower incidence of entry attempts observed in the time-lapse experiments for the TKO and Δdcx indicates lower overall invasion efficiency. Indeed, Δdcx parasites were shown in our previous work (Nagayasu et al., 2016) to invade at a much lower efficiency than the wild-type parasites when invasion was assessed by a two-color immunofluorescence assay that distinguishes invaded and uninvaded parasites based on different surface antigen accessibility (Carey et al., 2004; Mital et al., 2005). We determined the invasion efficiency of the TKO parasite using a similar approach and found that the TKO parasite invades at ∼47% of the level of its wild-type parent (P=0.026, unpaired Student's t-tests; mean±s.e.m. number of of parasites invaded/field: 25.2±3.2 for RHΔhx and 11.8±1.1 for TKO). Consistent with lower efficiency in invasion, the TKO parasite generated ∼60% the number of plaques compared to the wild-type parasite (P≈0.005) when examined by a plaque assay, where the parasites were incubated with the host cell monolayer unperturbed for 8 days (Fig. 6H). The total area of the host cell monolayer lysed by TKO was 40% of the area lysed by the wild-type parasite (P<0.0001), indicating significantly lower cytolytic efficiency. Thus, regarding the ability of the parasite to move helically or its conoid structure, host cell entry is less sensitive than overall infection efficiency.
DISCUSSION
The array of cortical microtubules is one of the major features of the cytoskeleton of Toxoplasma and other apicomplexan parasites. In Toxoplasma, the roots of the cortical microtubules are precisely located at evenly spaced intervals separated by cogwheel-like projections at the circumference of the apical polar ring (Leung et al., 2017; Morrissette and Sibley, 2002a; Nichols and Chiappino, 1987). The cortical microtubules are co-constructed and associated extensively with the inner membrane complex (IMC), guiding its formation during parasite replication (Hu et al., 2002; Morrissette et al., 1997). Inhibition of polymerization in replicating parasites blocks the elongation of daughter microtubules, and results in distorted daughter cortex and failed cell division (Hu, 2008; Hu et al., 2006; Morrissette and Sibley, 2002b; Stokkermans et al., 1996). In mature Toxoplasma, the cortical microtubules are extraordinarily stable and resistant to drug or cold temperature treatments that readily depolymerize mammalian microtubules (Hu, 2008; Hu et al., 2006; Morrissette and Sibley, 2002b). It has been proposed that the cortical microtubules might have various mechanical roles in facilitating parasite motility and invasion (Del Rosario et al., 2019; Pavlou et al., 2020; Stadler et al., 2017). However, the extraordinary stability of these polymers poses a major barrier against directly testing these hypotheses.
Previously, we generated a mutant (Δtlap2Δspm1Δtlap3, denoted TKO) in which the cortical microtubules are destabilized in the mature parasites while they continue to grow normally in developing daughters (Liu et al., 2015). Here, by quantifying the proportion of TKO parasites with different levels of defects in the array of cortical microtubules, we now have a more complete view of the extent of depolymerization in the non-dividing and dividing parasites. We found that (1) ∼80% of untreated non-dividing TKO parasites have severely shortened microtubules, and (2) the extent of depolymerization in the mature parasite increases markedly when the daughters start to form, but the development of daughters is not affected. Examining cortical microtubules in the wild type also revealed a small proportion of mature parasites with shortened cortical microtubules at the end of daughter construction. This suggests that microtubule depolymerization in the mother parasite might be a part of the normal preparation for disassembling maternal structures into component parts for possible recycling in the new generation. The cortical microtubules in mature TKO parasites are likely further sensitized to the depolymerizing activity due to the lack of TLAP2, SPM1 and TLAP3.
During division, the cortical microtubules of daughters grow continuously and do not display dynamic instability (Hu et al., 2002), indicating that these microtubules are stabilized by associated proteins. TLAP2, SPM1 and TLAP3 are recruited to the cortical microtubules of both mother and daughters (Liu et al., 2015; Tran et al., 2012) (Fig. S2), yet they have differential effects on the stability of these two sets of microtubules sharing the same cytoplasm. In the same TKO parasite, although the maternal microtubules are destabilized, the daughter microtubules grow normally. This apparent specific stabilizing activity of TLAP2, SPM1 and TLAP3 for mature cortical microtubules could be because daughter cortical microtubules acquire additional specific stabilizing factors. Of course, for those (hypothetical) factors, we would need to answer how they differentially act on the cortical microtubules of the mother and developing daughters, which coexist in the same cytoplasm.
When incubated at low temperature, the cortical microtubules in the TKO parasite can be fully depolymerized (Liu et al., 2015). Here, we show that this depolymerization is reversible – when the parasite was placed at 37°C after cold treatment, short cortical microtubules were observed concentrated at the apical end of the parasite. This is consistent with the current hypothesis that the apical polar ring is the nucleating center for the cortical microtubules (Leung et al., 2017; Morrissette and Sibley, 2002a; Nichols and Chiappino, 1987). It is tempting to suggest that the nucleation complexes in the apical polar ring in the mature parasite remain active and are capable of re-nucleating new cortical microtubules after complete depolymerization. However, we cannot exclude the possibility that the new growth occurred from short microtubule stubs that remained associated with the apical polar ring, as a small cap of tubulin antibody labeling remains at the apical end even after prolonged cold treatment. If the latter is true, it suggests differential regulation of stability along the length of the cortical microtubules.
In the 3D Matrigel, the TKO mutant moves much more linearly than the wild-type parasite, which travels along a helical path. The stable and spirally arranged cortical microtubules, with their extensive interactions with the cortex, could contribute to the helicity of the parasite movement by maintaining non-isotropic cortical properties (e.g. stiffness), and thus bias the orientation at which the parasite interacts with its environment. In plant cells, the transmembrane cellulose synthesis complex is dynamically coupled to the cortical microtubules via cellulose synthases interacting (CSI1) protein (Bringmann et al., 2012). This results in the co-alignment of the extracellular cellulose fibers with the cortical microtubules on the other side of the plasma membrane. One could imagine that a similar mechanism might be involved in the confinement of the force-generating actomyosin apparatus associated with the inner membrane complex in Toxoplasma, although currently a CSI1-like coupler is unknown. The trajectory of the parasite movement might also be affected by the shape of the parasite. For example, in Plasmodium berghei, the knockout of IMC1h, which changes the shape of the ookinete, results in more linear movement (Kan et al., 2014). However, in the IMC1h Plasmodium mutant, although the cortical microtubules are still present and have a recognizable twist, they are also disorganized, which makes it difficult to disentangle the individual contributions from changes in parasite shape versus in the organization of the microtubules. We do not think the change in parasite shape is the likely explanation for the change in movement trajectory of the Toxoplasma microtubule mutants, because there does not appear to be a clear correlation between the parasite shape and linearity of movement. For example, the Δtlap2Δspm1 parasite, which has a pronounced defect in the cortical microtubule array, moves much more linearly than the wild-type parasite even though their bowing indices are similar.
We note that although the net displacement, run length and run speed are similar in untreated parental and TKO parasites, these parameters are significantly higher for cold-treated wild-type parasites when compared with those for the cold-treated TKO parasite (Fig. 4A). This difference is due almost entirely to a response to the cold-treatment by the wild-type parasite, and the lack of response by the TKO parasites. This lack of response by the TKO parasite indicates that the residual microtubules in the TKO parasites under untreated conditions do not have a notable impact on motility. The increase in speed and persistence of wild-type parasite movement after cold treatment is not due to a direct effect of the microtubules on motility because (1) the cold treatment does not have a detectable effect on the microtubule array in the wild-type parasite, and (2) the untreated wild-type parasite, which has a full complement and normal arrangement of cortical microtubules, has the same speed and persistence as the TKO parasite, with few microtubules and a disordered array.
It has been proposed that the cortical microtubules act as a spring to store and release energy for helical gliding (Pavlou et al., 2020). However, our data suggest that these microtubules do not appear to act as a link in any energy relay vital for parasite movement. As discussed above, although the TKO mutant does not move helically, it is clearly capable of sustaining long-distance movement even under conditions where the TKO parasites were pre-treated with cold to nearly fully depolymerize the microtubules. Therefore, the cortical microtubules do not play a role in maintaining directional long-distance movement. This means other higher-order structures are needed to limit the direction of force generation by the actomyosin complex. Whatever these structures might be, they need to be regularly arranged and stably associated with the parasite cortex. One candidate is the array of inner membrane particles (IMPs), which form periodic rows of IMPs that run the length along the longitudinal axis of the parasite (Morrissette et al., 1997). A subset of the IMP rows co-align with the cortical microtubules. The depletion of a potential IMP component (GAPM1a) in mature parasites results in decreased parasite displacement in the 3D Matrigel as well as destabilized and disordered cortical microtubules (Harding et al., 2019). It is worth noting that, in contrast to the GAPM1a mutant, the net displacement of the TKO parasite is similar to that of the wild type (Fig. 4A). This separation of helicity from sustained parasite movement might be due to the cell-stage-dependent nature of the stability of the cortical microtubules in the TKO parasite. The IMC cortex of the parasite is built concomitantly with the cortical microtubules during daughter formation. Because the daughter cortical microtubules grow normally in the TKO parasite, the structures in the IMC also likely form largely normally. Thus, we think that, in the mature TKO parasites, we are mainly seeing the impact of the microtubule destabilization itself, which manifests solely in the change from helical to linear movement.
Given the precision by which the cortical microtubules are constructed, the extensive suite of associated proteins (Leung et al., 2017; Liu et al., 2015, 2013; Tran et al., 2012) and their extraordinary stability, we had expected that microtubules would provide important mechanical support for the cortex in the mature parasite. To our surprise, the shape of the TKO parasite is still crescent in nature, and there is also no detectable difference in the behavior of the parasite during host cell entry and egress, when the parasite experiences considerable mechanical stress pushing through the host cell cortex. This indicates that other cortical structures, such as the inner membrane complex and associated intermediate filament-like network, are more important as structural support in mature parasites. Notably, the conoid structure mutant, Δdcx, which invades with a much lower efficiency (Nagayasu et al., 2016), also showed wild-type-like behavior during entry. This suggests that host cell entry is considerably less sensitive to the change in the structure of the cortical microtubules and conoid compared with extracellular migration during infection.
Finally, TLAP2, SPM1 and TLAP3, the three proteins collectively responsible for the stability of cortical microtubules in mature Toxoplasma, have well-conserved orthologs in Plasmodium spp. (Liu et al., 2015). It would be intriguing to determine whether the microtubule-stabilizing function of these proteins, and the influence of cortical microtubule stability on parasite movement are also conserved among the apicomplexans. As the cortical microtubules in the developing daughter and the mature TKO parasites display distinct polymerization and depolymerization states, identifying the activities responsible for this maternal–filial distinction will generate insights into how the cortical microtubules of the mother and developing daughters (composed of the same polymer and less than half a micron apart) coexist in distinct biochemical states and how the switch from one state to the other occurs as the daughters become mature.
MATERIALS AND METHODS
T. gondii mutant strains and host cell cultures
Host cell and T. gondii tachyzoite parasite cultures were maintained as described previously (Leung et al., 2017; Liu et al., 2015; Munera Lopez et al., 2022; Roos et al., 1994). The TKO (Δtlap2Δspm1Δtlap3), Δtlap2Δspm1, Δtlap2 mutants and the mEmeraldFP-TLAP2 knock-in parasite were reported in Liu et al. (2015). The Δdcx parasite was reported in Nagayasu et al. (2016).
Temperature treatment of T. gondii for testing microtubule stability
Cultures of intracellular parasites growing in human foreskin fibroblast (HFF) cells plated in 3.5-cm glass-bottom dishes (MatTek Corporation, cat. no. P35G-1.5-14-C or cat. no. P35G-1.5-21-C) were incubated at 7°C for 20, 60, 90 or 120 min in L15 imaging medium, which is Leibovitz's L-15 (21083-027, Gibco-Life Technologies, Grand Island, NY, USA) supplemented with 1% (v/v) heat-inactivated cosmic calf serum (CCS; SH30087.3; Hyclone, Logan, UT, USA). For recovery experiments, the cultures were incubated at 7°C for 2 h and subsequently placed at 37°C for 5, 10, 20, 30, 60 or 120 min in L15 imaging medium. The samples were then processed for immunofluorescence as described below. The total number of intracellular parasites analyzed was: 444, 422, 468 and 423 of TKO and 246, 274, 300 and 390 of wild-type cold-treated at 7°C for 20, 60, 90 or 120 min, respectively; 440, 428, 456, 496, 396 and 360 of TKO, and 295, 168, 172, 166, 205 and 230 of wild-type recovered at 37°C for 5, 10, 20, 30, 60 or 120 min, respectively.
Immunofluorescence
All steps of the immunofluorescence labeling were performed at room temperature. For microtubule labeling of intracellular parasites (Fig. 1), T. gondii-infected HFF monolayers growing in glass-bottom dishes were fixed in 3% (v/v) formaldehyde and 50% (v/v) methanol in 0.5× PBS for 15 min, permeabilized in 0.5% (v/v) Triton X-100 (TX-100) in PBS for 15 min, washed with PBS twice, followed by incubating with the primary antibody (mouse monoclonal anti-acetylated tubulin, 6-11B-1, 1:1000, Sigma T6793) and then with the secondary antibody (goat anti-mouse IgG-Alexa Fluor 488, A11029, Molecular Probes) at 1:1000, for 1 h each. For labeling of the inner membrane complex protein 1 (IMC1), the samples were subsequently blocked with 1.5% BSA in PBS for 1 h, then incubated for 1 h with rabbit anti-IMC1 (a kind gift from Dr Con Beckers, William Carey University, Hattiesburg, MS, USA) at 1:1000, and finally with goat anti-rabbit IgG-Alexa Fluor 568 (A11036, Molecular Probes) at 1:1000 for 1 h. The antibodies were diluted in the blocking solution (i.e. 1.5% BSA in PBS).
For microtubule labeling of extracellular parasites (Fig. 2), parasites were harvested as described in Munera Lopez et al. (2022). To collect cold-treated parasites, cultures with intracellular parasites were placed at ∼7°C for 2 h as described above before harvesting. After harvesting, parasites were suspended in 3% (v/v) formaldehyde and 50% (v/v) methanol in 0.5× PBS and allowed to settle to the glass bottom of the MatTek dishes for 15 min. The samples were then processed as described above for the intracellular parasites.
Immunofluorescence labeling of intracellular parasites with a mouse anti-TgMIC2 antibody, 6D10 (Carruthers et al., 2000) (a kind gift from Dr Vern Carruthers, University of Michigan, Ann Arbor, MI, USA) was carried out as described in Munera Lopez et al. (2022). A rabbit anti-GAP45 antibody (a kind gift from Dr Con Beckers) at 1:2000, followed by a goat anti-rabbit IgG-Alexa Fluor 488, (A11034, Molecular Probes) at 1:1000, was used to label the parasite cortex in these experiments (Fig. 6A).
The labeling of the mEmeraldFP-TLAP2 knockin parasite with a mouse anti-ISP1 antibody (a kind gift from Dr Peter Bradley, University of California, Los Angeles, CA, USA; Beck et al., 2010) (Fig. S2) was carried out as described in Liu et al. (2015).
3D structured-illumination microscopy
3D structured-illumination microscopy (3D-SIM) image stacks were collected at z-increments of 0.125 μm using a DeltaVision OMX Flex imaging station (GE Healthcare-Applied Precision). A 60× oil immersion lens [numerical aperture (NA) 1.42] and immersion oil at refractive index 1.520 were used. Structured illumination image reconstructions and 3D projections were processed using the functions and software supplied by the manufacturer.
Replication assay
The intracellular replication assay was performed as described in Heaslip et al. (2011). Four independent experiments were performed. In each replicate, the number of parasites in ∼100 vacuoles was counted for each strain at each time point.
Shape analysis
Parasites were harvested from a heavily infected T12.5 flask of HFF by scraping, passing the suspension through a 23G needle, and filtering through a 3 μm pore size filter. The suspension was centrifuged at 1500 g for 3 min, and the pellet was suspended 1 ml of L15 medium containing 1% calf serum, and then centrifuged at 3500 g for 2.5 min, discarding all but ∼20 μl of the supernatant, which was used to resuspend the pellet. 10–15 μl of the concentrated parasite suspension was spread on a slide and covered with a coverslip. DIC images were collected as z-stacks of 7–19 slices in 0.8 μm z-steps, using a 60× NA 1.3 silicon immersion lens. A custom script in the image processing software Semper (Saxton, 1996) was used to display the image, choose the best-focus z-layer and pick parasites. The most obviously bowed parasites were selected for measurement because apparently straight parasites can be curved but viewed from the wrong direction. For each chosen parasite, shape parameters were calculated using the following manually identified features.
First, for each parasite image, a line was drawn from apical tip to basal tip. The coordinates of this line, with length denoted d, were used to redisplay an enlarged image of the chosen parasite with the drawn line vertical, apical tip upwards. On this enlarged, re-oriented image, first the left, then the right-hand edge of the parasite was marked. The distance from the apical-basal line to the left-hand border was denoted by a, and to the right-hand border by b. Distances to the right of the line are positive, to the left are negative. The width of the parasite at its midpoint is given by b−a, with a and b signed as defined. Length and bowing indices were then calculated from the coordinates of the marks as follows (see Fig. 2C).
The ‘bowing’, defined as the distance, B, from the drawn apical-basal line to the center (defined as half-way between the left-hand and right-hand edges) of the parasite is given by (a+b)/2, where a and b are signed numbers as defined above. Now consider an arc of an imaginary perfect circle that passes through the center of the parasite as well as through the endpoints of the previously drawn apical-basal line. Let C be the distance from the center of that circle to the midpoint of the apical-basal line, measured perpendicularly. Consider two radii, length r, of that circle, one from the center of the circle to the apical tip of the parasite, the other from the center of the circle to the midline of the parasite. Referring to the diagram in Fig. 2, it will be seen that for the first radius, r2=(d/2)2+C2, and for the second radius r2=(B+C)2. Solving for C gives C=[(d/2)2−B2]/2B, and r is then calculated as B+C. If the parasite were not bowed at all, then the parasite length would be exactly d.
We define the length (L) of the parasite to be the distance from apical to basal tip measured along the arc that passes through both of them and through the center of the parasite, which is given by 2r tan−1(d/2C).
3D motility assay and image acquisition
The 3D motility assay for Toxoplasma, originally developed in Leung et al. (2014), was carried out largely as described in Munera Lopez et al. (2022). The environmental controller of a DeltaVision OMX Flex imaging station (GE Healthcare-Applied Precision) was set to heat the stage and objective lens at 37°C and allowed to equilibrate for >30 min. A flow chamber was assembled as described in Munera Lopez et al. (2022), then placed onto the heated stage at least 10 min before imaging. For cold-treated parasites, cultures with intracellular parasites were placed at ∼7°C for 2 h, before harvesting as described in Munera Lopez et al. (2022). The harvested parasite suspension was kept on ice for ∼30 s, then mixed with an equal volume of Matrigel (Corning, 356237) on ice and pipetted into the pre-heated flow chamber. The chamber was then sealed with a polymerizable liquid silicone rubber (Nobilium Inc, Nobilsil; cat. nos 7453 and 7434) and image acquisition was started within ∼2–3 mins after the suspension had entered the 37°C chamber.
For each imaging experiment, 151–181 image stacks were acquired at 1.36 s intervals (7 ms exposure per optical section). Each stack consisted of 41 optical sections, spaced 1 μm apart in the z-plane (40 μm stacks), with field of view of 1500×1500 pixels, binning 2×2, with a final dimension of 750×750 pixels. Image stacks were acquired from at least ∽10 μm above the coverslip.
To represent the 3D DIC images in 2D format (Fig. 3 and Movies 1,3), we performed focus-stacking using Fiji by applying the Extended Depth of Field Sobel Projection plugin from Forster et al. (2004) and Schindelin et al. (2012).
DIC image stacks processing and cell tracking
DIC images were processed using our custom scripts extended from Munera Lopez et al. (2022). High-contrast images were generated by subjecting the DIC images to (1) a 3D median filter (kernel nx=ny=nz=2 px) to reduce noise, (2) a 2D Laplacian filter to attenuate out-of-focus portions of the cells, and (3) a 3D gradient filter of Sobel type for edge detection. A dilation and erosion process using a maximum filter followed by a minimum filter (kernel nz=3 px, nx=ny =13 px) was then applied. 2 pixels from all x and y edges were cropped before tracking to avoid edge artifacts. We implemented the imaging filters using Python 3 to enable GPU-acceleration using the CuPy plugin (Harris et al., 2020; https://cupy.dev/; Van Rossum and Fred, 2009). The TrackMate plugin in Fiji was then used to detect and track the parasites in the processed image stacks (Ershov et al., 2022; Schindelin et al., 2012; Tinevez et al., 2017). The Difference-of-Gaussian (DoG) detector was employed for spot detection with a diameter of 7 µm. To avoid artifacts associated with edge effects and out-of-focus signal, we excluded spots detected within the first and last 1 µm of the z-dimension and 3 µm of each of the x- and y-dimension. The Kalman filter tracker was used to reconstruct the cell tracks, and false reconstructions (i.e. ‘tracks’ between debris or random noise) were filtered out based on quality, track length and duration. The values for these parameters were determined empirically. The resulting tracks were manually verified and errors such as misdetections and gaps were corrected by adding or deleting individual spots. To refine spot positions, we used the TrackMate spot fitting feature to fit a 3D Gaussian with fixed radius on each spot. After conducting a second round of manual verification, we then separated the motile parasite tracks from the non-motile tracks by applying another set of filters based on displacement and speed. The 3D coordinates of both the non-motile and motile tracks were copied to different comma-separated value text files (.csv) for analysis in R version 4.1.2 in RStudio (https://www.rstudio.com/).
Trajectory analysis
We used the R package celltrackR v1.1.0 and our own custom functions to process and analyze the parasite trajectories (Wortel et al., 2021). The complete list of the rest of the R packages we used for our analysis is at the end of this section. First, the global drifts in the data were corrected according to the workflow developed by Wortel et al. (2021) and we calculated the drift velocity from the corresponding set of non-motile tracks. Then, non-motile ends (the very start or very end) of the parasite trajectories were trimmed to eliminate biases introduced by the asynchronous nature of the parasite motility. After trimming, only tracks with more than four steps (i.e. five tracking points) were included in the subsequent analysis.
In Figs 4 and 5, the net displacement is a simple Euclidean distance between the first point and the last point of the trajectories. The run length is the total distance traveled between two ‘pauses’, which we defined as an event when the parasite stops moving for more than 5.4 s (>=4 frames). ‘Stop moving’ means that the speed of a step (sub-track of length 1) is lower than a threshold. For each data set, the threshold value was calculated by taking the average step-speed of the non-motile tracks. We removed the track points that correspond to pause events, introducing gaps. The motile segments were then obtained by splitting the tracks around those gaps. The run speed was obtained by dividing the total distance of a motile segment (run length) by its total duration. For Figs 4C and 5, the dispersal ratio was calculated by taking the net displacement of the motile segments and dividing it by the run length, where a value of 1 indicates a completely linear track.
For analyzing the turning angle (Figs 4C and 5), any stops that were not filtered out before (i.e. stops that lasted less than 4 frames) and steps with displacement of less than 10% of the parasite body length (∼ 0.75 µm) were cut out from the motile segments before splitting into sub-segments. Sub-segments that are less than 3-frames long were excluded from the turning angle analysis, using the method described by Wortel et al. (2021).
The pitch length is the displacement associated with one complete helical turn, which was quantified as the straight-line distance between the starting and ending points of the turn. These points were identified through visual inspection of the trajectories and parasite orientation in 3D space for the pitch calculation in Fig. 4C.
The 3D reconstruction of the processed DIC image stacks, along with the tracks exported from TrackMate (Figs 3C and 4B, Movies 1–4), was done using the 3D viewer and Track Processor in Icy (de Chaumont et al., 2012). Gaussian blur with a sigma of 2 was applied to the processed stacks before Icy rendering.
Outliers were removed from the datasets before performing statistical analysis. For each measured variable (Figs 4A,C and 5), all the data points were combined to calculate the first (Q1), third (Q3) quartiles, and interquartile range (IQR). A data point is defined as an outlier if it is 1.5 times the IQR greater than the Q3 or 1.5 times the IQR less than the Q1.
The Kruskal–Wallis test was done to compare the groups for all of the track measurements in Figs 4A,C and 5. We used R version 4.1.2 (https://www.rstudio.com/) and the following R packages: data.table v. 1.14.6 (https://cran.r-project.org/web/packages/data.table/), gridExtra v. 2.3 (https://cran.r-project.org/web/packages/gridExtra/), onewaytests v. 2.7 (Dag et al., 2018), pracma v. 2.4.2 (https://cran.r-project.org/web/packages/pracma/), rmarkdown v. 2.20 (https://github.com/rstudio/rmarkdown), rstatix v. 0.7.2 (https://cran.r-project.org/web/packages/rstatix/), scales v. 1.2.1 (https://cran.r-project.org/web/packages/scales/) and tidyverse v. 2.0.0 (Wickham et al., 2019).
MIC2 secretion assay
To examine microneme secretion, freshly egressed parasites were harvested. A total of 4×107 parasites were resuspended in 90 µl DMEM with 1% bovine calf serum, incubated at 10 min at 37°C with or without 5 µM A23187, placed on ice for 5 min, and centrifuged for 10 min at ∼800 g to separate the secreted fraction (supernatant) from the parasites (pellet). The supernatant (40 µl) and pellet fractions were analyzed by western blotting as described in Leung et al. (2019) with a mouse 6D10 anti-TgMIC2 antibody (Carruthers et al., 2000) at 1:1000 dilution, a mouse anti-tubulin (Sigma, T6074) at 1:4000 dilution, and a donkey anti-mouse-IgG-IRDyE 680RD (Li-cor #926-68070) at 1:7000 as secondary antibody. To normalize A23187-induced MIC2 secretion, background-subtracted fluorescence of the MIC2 band from the supernatant sample was divided by background-subtracted fluorescence of the tubulin band in the corresponding pellet sample.
Egress assay
For the induced egress assay, parasites were added to MatTek dishes containing a confluent HFF or African green monkey renal epithelial cells (BS-C-1, ATCC# CCL-26) monolayer and grown for ∼35 h. Cultures were washed once and incubated in 2 ml of L15 imaging medium and placed at ∼7°C for 1 h, before being moved to the environmental chamber kept at 37°C. The medium was then replaced with ∼2 ml of 5 µM A23187 in L15 imaging medium. Images were collected at 37°C with 1 s interval for 10 min on an Applied Precision Delta Vision imaging station equipped with an environmental chamber.
Invasion assays
To analyze the behavior of live parasites during invasion, extracellular parasites were used to infect a MatTek dish containing a nearly confluent monolayer of HFF cells. The dishes were incubated on ice in L15 imaging medium for ∼10 min to facilitate parasite settling on the host cells. The culture was then imaged at 37°C with 1 s intervals for 15 min to capture invasion events.
Immunofluorescence-based invasion assays were carried out as described in Munera Lopez et al. (2022) with some modifications. ∼4×106 freshly egressed parasites were used to inoculate a MatTek dish of nearly confluent HFF cells. After a 10 min incubation on ice and then a 10 min incubation at 37°C, the samples were processed for immunofluorescence. Three biological replicates, each with three technical replicates, were performed. Parasites in 15 fields were counted for each strain per technical replicate. In total (intracellular+extracellular), 4852 parasites were counted for the RHΔhx parent and 2944 for the TKO parasite. P-values were calculated by performing unpaired two-tailed Student's t-tests for the biological replicates.
Plaque assays
A total of 50, 100 and 150 freshly harvested parasites were added to each well of six-well plates containing confluent HFF monolayers and were grown undisturbed for 8 days at 37°C, then fixed with 70% ethanol for 15 min and stained with 1% (w/v) Crystal Violet in 25% (v/v) methanol, rinsed with PBS, air-dried and imaged.
Supplementary Material
Acknowledgements
We are grateful to Dr. Owen Saxton (University of Cambridge, United Kingdom) for providing the source code for his ‘Semper’ image processing package, Dr. Con Beckers (William Carey University, Mississippi) for the rabbit anti-TgIMC1 and anti-TgGAP45 antibodies, Dr. Peter Bradley (University of California, Los Angeles) for the mouse anti-TgISP1 antibody, and Dr. Vern Carruthers (University of Michigan, Ann Arbor) for the mouse mAb 6D10 anti-TgMIC2 antibody. We thank Melissa Molina for tissue culture support.
Footnotes
Author contributions
Conceptualization: I.F.T., L.F.A.P., J.M.L., K.H.; Methodology: I.F.T., L.F.A.P., J.M.L., J.L., J.M.M.; Software: I.F.T., P.T.B.; Validation: I.F.T., L.F.A.P., J.M.L., K.H.; Formal analysis: I.F.T., L.F.A.P., J.M.L., J.M.M.; Investigation: I.F.T., L.F.A.P., J.M.L., J.L., J.M.M., K.H.; Resources: K.H.; Data curation: I.F.T., L.F.A.P., J.M.L., J.M.M.; Writing - original draft: I.F.T., L.F.A.P., J.M.L., K.H.; Writing - review & editing: I.F.T., L.F.A.P., J.M.L., J.L., P.T.B., J.M.M., K.H.; Visualization: I.F.T., L.F.A.P., J.M.L.; Supervision: K.H.; Project administration: K.H.; Funding acquisition: K.H.
Funding
This study was supported by funding from the National Institutes of Health (National Institute of Allergy and Infectious Diseases) (R01-AI132463) awarded to K.H. Deposited in PMC for release after 12 months.
Data availability
All relevant data can be found within the article and its supplementary information.
Peer review history
The peer review history is available online at https://journals.biologists.com/jcs/lookup/doi/10.1242/jcs.261270.reviewer-comments.pdf.
References
- Aisenbrey, E. A. and Murphy, W. L. (2020). Synthetic alternatives to Matrigel. Nat. Rev. Mater 5, 539-551. 10.1038/s41578-020-0199-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aquilini, E., Cova, M. M., Mageswaran, S. K., Dos Santos Pacheco, N., Sparvoli, D., Penarete-Vargas, D. M., Najm, R., Graindorge, A., Suarez, C., Maynadier, M.et al. (2021). An Alveolata secretory machinery adapted to parasite host cell invasion. Nat. Microbiol. 6, 425-434. 10.1038/s41564-020-00854-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannister, L. H. and Mitchell, G. H. (1995). The role of the cytoskeleton in Plasmodium falciparum merozoite biology: an electron-microscopic view. Ann. Trop. Med. Parasitol. 89, 105-111. 10.1080/00034983.1995.11812940 [DOI] [PubMed] [Google Scholar]
- Beck, J. R., Rodriguez-Fernandez, I. A., de Leon, J. C., Huynh, M.-H., Carruthers, V. B., Morrissette, N. S. and Bradley, P. J. (2010). A novel family of Toxoplasma IMC proteins displays a hierarchical organization and functions in coordinating parasite division. PLoS Pathog. 6, e1001094. 10.1371/journal.ppat.1001094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltman, J., Marée, A. and de Boer, R. (2009). Analysing immune cell migration. Nat. Rev. Immunol. 9, 789-798. 10.1038/nri2638 [DOI] [PubMed] [Google Scholar]
- Bounkeua, V., Li, F. and Vinetz, J. M. (2010). In vitro generation of Plasmodium falciparum ookinetes. Am. J. Trop. Med. Hyg. 83, 1187-1194. 10.4269/ajtmh.2010.10-0433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bringmann, M., Landrein, B., Schudoma, C., Hamant, O., Hauser, M. T. and Persson, S. (2012). Cracking the elusive alignment hypothesis: the microtubule-cellulose synthase nexus unraveled. Trends Plant Sci. 17, 666-674. 10.1016/j.tplants.2012.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey, K. L., Westwood, N. J., Mitchison, T. J. and Ward, G. E. (2004). A small-molecule approach to studying invasive mechanisms of Toxoplasma gondii. Proc. Natl. Acad. Sci. USA 101, 7433-7438. 10.1073/pnas.0307769101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carruthers, V. B., Sherman, G. D. and Sibley, L. D. (2000). The Toxoplasma adhesive protein MIC2 is proteolytically processed at multiple sites by two parasite-derived proteases. J. Biol. Chem. 275, 14346-14353. 10.1074/jbc.275.19.14346 [DOI] [PubMed] [Google Scholar]
- Carruthers, V. B. and Sibley, L. D. (1997). Sequential protein secretion from three distinct organelles of Toxoplasma gondii accompanies invasion of human fibroblasts. Eur. J.Cell Biol. 73, 114-123. [PubMed] [Google Scholar]
- Dag, O., Dolgun, A. and Konar, N. M. (2018). onewaytests: an R Package for one-way tests in independent groups designs. The R Journal 10, 175-199. 10.32614/RJ-2018-022 [DOI] [Google Scholar]
- de Chaumont, F., Dallongeville, S., Chenouard, N., Hervé, N., Pop, S., Provoost, T., Meas-Yedid, V., Pankajakshan, P., Lecomte, T., Le Montagner, Y.et al. (2012). Icy: an open bioimage informatics platform for extended reproducible research. Nat. Methods 9, 690-696. 10.1038/nmeth.2075 [DOI] [PubMed] [Google Scholar]
- Del Rosario, M., Periz, J., Pavlou, G., Lyth, O., Latorre-Barragan, F., Das, S., Pall, G. S., Stortz, J. F., Lemgruber, L., Whitelaw, J. A.et al. (2019). Apicomplexan F-actin is required for efficient nuclear entry during host cell invasion. EMBO Rep. 20, e48896. 10.15252/embr.201948896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrowolski, J. M., Carruthers, V. B. and Sibley, L. D. (1997). Participation of myosin in gliding motility and host cell invasion by Toxoplasma gondii. Mol. Microbiol. 26, 163-173. 10.1046/j.1365-2958.1997.5671913.x [DOI] [PubMed] [Google Scholar]
- Dobrowolski, J. M. and Sibley, L. D. (1996). Toxoplasma invasion of mammalian cells is powered by the actin cytoskeleton of the parasite. Cell 84, 933-939. 10.1016/S0092-8674(00)81071-5 [DOI] [PubMed] [Google Scholar]
- Donald, R. G. and Roos, D. S. (1994). Homologous recombination and gene replacement at the dihydrofolate reductase-thymidylate synthase locus in Toxoplasma gondii. Mol. Biochem. Parasitol. 63, 243-253. 10.1016/0166-6851(94)90060-4 [DOI] [PubMed] [Google Scholar]
- Dubey, J. P. (2008). The history of Toxoplasma gondii--the first 100 years. J. Eukaryot. Microbiol. 55, 467-475. 10.1111/j.1550-7408.2008.00345.x [DOI] [PubMed] [Google Scholar]
- Ershov, D., Phan, M.-S., Pylvänäinen, J. W., Rigaud, S. U., Le Blanc, L., Charles-Orszag, A., Conway, J. R. W., Laine, R. F., Roy, N. H., Bonazzi, D.et al. (2022). TrackMate 7: integrating state-of-the-art segmentation algorithms into tracking pipelines. Nat. Methods 19, 829-832. 10.1038/s41592-022-01507-1 [DOI] [PubMed] [Google Scholar]
- Forster, B., Van De Ville, D., Berent, J., Sage, D. and Unser, M. (2004). Complex wavelets for extended depth–of–field: A new method for the fusion of multichannel microscopy images. Microsc. Res. Tech. 65, 33-42. 10.1002/jemt.20092 [DOI] [PubMed] [Google Scholar]
- Fox, B. A., Ristuccia, J. G., Gigley, J. P. and Bzik, D. J. (2009). Efficient gene replacements in Toxoplasma gondii strains deficient for nonhomologous end joining. Eukaryot. Cell 8, 520-529. 10.1128/EC.00357-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenal, K., Polonais, V., Marq, J. B., Stratmann, R., Limenitakis, J. and Soldati-Favre, D. (2010). Functional dissection of the apicomplexan glideosome molecular architecture. Cell Host Microbe 8, 343-357. 10.1016/j.chom.2010.09.002 [DOI] [PubMed] [Google Scholar]
- Garnham, P. C., Bird, R. G. and Baker, J. R. (1962). Electron microscope studies of motile stages of malaria parasites. III. The ookinetes of Haemamoeba and Plasmodium. Trans. R. Soc. Trop. Med. Hyg. 56, 116-120. 10.1016/0035-9203(62)90137-2 [DOI] [PubMed] [Google Scholar]
- Gaskins, E., Gilk, S., DeVore, N., Mann, T., Ward, G. and Beckers, C. (2004). Identification of the membrane receptor of a class XIV myosin in Toxoplasma gondii. J. Cell Biol. 165, 383-393. 10.1083/jcb.200311137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graindorge, A., Frenal, K., Jacot, D., Salamun, J., Marq, J. B. and Soldati-Favre, D. (2016). The conoid associated motor MyoH is indispensable for Toxoplasma gondii entry and exit from host cells. PLoS Pathog. 12, e1005388. 10.1371/journal.ppat.1005388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gras, S., Jackson, A., Woods, S., Pall, G., Whitelaw, J., Leung, J. M., Ward, G. E., Roberts, C. W. and Meissner, M. (2017). Parasites lacking the micronemal protein MIC2 are deficient in surface attachment and host cell egress, but remain virulent in vivo. Wellcome Open Res. 2, 32. 10.12688/wellcomeopenres.11594.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding, C. R., Gow, M., Kang, J. H., Shortt, E., Manalis, S. R., Meissner, M. and Lourido, S. (2019). Alveolar proteins stabilize cortical microtubules in Toxoplasma gondii. Nat. Commun. 10, 401. 10.1038/s41467-019-08318-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, C. R., Millman, K. J., van der Walt, S. J., Gommers, R., Virtanen, P., Cournapeau, D., Wieser, E., Taylor, J., Berg, S., Smith, N. J.et al. (2020). Array programming with NumPy. Nature 585, 357-362. 10.1038/s41586-020-2649-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaslip, A. T., Nishi, M., Stein, B. and Hu, K. (2011). The motility of a human parasite, Toxoplasma gondii, is regulated by a novel lysine methyltransferase. PLoS Pathog. 7, e1002201. 10.1371/journal.ppat.1002201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp, C. S., Chiou, K., Ragheb, D. R. T., Salman, A. M., Khan, S. M., Liu, A. J. and Sinnis, P. (2015). Longitudinal analysis of Plasmodium sporozoite motility in the dermis reveals component of blood vessel recognition. Elife 4, e07789. 10.7554/eLife.07789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, K. (2008). Organizational changes of the daughter basal complex during the parasite replication of Toxoplasma gondii. PLoS Pathog. 4, 108-121. 10.1371/journal.ppat.0040010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, K., Johnson, J., Florens, L., Fraunholz, M., Suravajjala, S., DiLullo, C., Yates, J., Roos, D. S. and Murray, J. M. (2006). Cytoskeletal components of an invasion machine – the apical complex of Toxoplasma gondii. PLoS Pathog. 2, 0121-0139. 10.1371/journal.ppat.0020013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, K., Roos, D. S. and Murray, J. M. (2002). A novel polymer of tubulin forms the conoid of Toxoplasma gondii. J. Cell Biol. 156, 1039-1050. 10.1083/jcb.200112086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh, M.-H. and Carruthers, V. B. (2006). Toxoplasma MIC2 is a major determinant of invasion and virulence. PLoS Pathog. 2, e84. 10.1371/journal.ppat.0020084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh, M. H. and Carruthers, V. B. (2009). Tagging of endogenous genes in a Toxoplasma gondii strain lacking Ku80. Eukaryot. Cell 8, 530-539. 10.1128/EC.00358-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh, M. H., Rabenau, K. E., Harper, J. M., Beatty, W. L., Sibley, L. D. and Carruthers, V. B. (2003). Rapid invasion of host cells by Toxoplasma requires secretion of the MIC2-M2AP adhesive protein complex. EMBO J. 22, 2082-2090. 10.1093/emboj/cdg217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacot, D., Tosetti, N., Pires, I., Stock, J., Graindorge, A., Hung, Y. F., Han, H., Tewari, R., Kursula, I. and Soldati-Favre, D. (2016). An apicomplexan actin-binding protein serves as a connector and lipid sensor to coordinate motility and invasion. Cell Host Microbe 20, 731-743. 10.1016/j.chom.2016.10.020 [DOI] [PubMed] [Google Scholar]
- Kan, A., Tan, Y.-H., Angrisano, F., Hanssen, E., Rogers, K. L., Whitehead, L., Mollard, V. P., Cozijnsen, A., Delves, M. J., Crawford, S.et al. (2014). Quantitative analysis of Plasmodium ookinete motion in three dimensions suggests a critical role for cell shape in the biomechanics of malaria parasite gliding motility. Cell. Microbiol. 16, 734-750. 10.1111/cmi.12283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung, J. M., He, Y., Zhang, F., Hwang, Y. C., Nagayasu, E., Liu, J., Murray, J. M. and Hu, K. (2017). Stability and function of a putative microtubule organizing center in the human parasite Toxoplasma gondii. Mol. Biol. Cell 28, 1361-1378. 10.1091/mbc.e17-01-0045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung, J. M., Liu, J., Wetzel, L. A. and Hu, K. (2019). Centrin2 from the human parasite Toxoplasma gondii is required for its invasion and intracellular replication. J. Cell Sci. 132, jcs228791. 10.1242/jcs.228791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung, J. M., Rould, M. A., Konradt, C., Hunter, C. A. and Ward, G. E. (2014). Disruption of TgPHIL1 alters specific parameters of Toxoplasma gondii motility measured in a quantitative, three-dimensional live motility assay. Plos One 9, e85763. 10.1371/journal.pone.0085763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J., He, Y., Benmerzouga, I., Sullivan, W. J., Jr, Morrissette, N. S., Murray, J. M. and Hu, K. (2015). An ensemble of specifically targeted proteins stabilizes cortical microtubules in the human parasite Toxoplasma gondii. Mol. Biol. Cell 27, 549-571. 10.1091/mbc.e15-11-0754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J., Wetzel, L., Zhang, Y., Nagayasu, E., Ems-McClung, S., Florens, L. and Hu, K. (2013). Novel thioredoxin-like proteins are components of a protein complex coating the cortical microtubules of Toxoplasma gondii. Eukaryot. Cell 12, 1588-1599. 10.1128/EC.00082-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta, S. and Sibley, L. D. (2011). Actin depolymerizing factor controls actin turnover and gliding motility in Toxoplasma gondii. Mol. Biol. Cell 22, 1290-1299. 10.1091/mbc.e10-12-0939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner, M., Schluter, D. and Soldati, D. (2002). Role of Toxoplasma gondii myosin A in powering parasite gliding and host cell invasion. Science 298, 837-840. 10.1126/science.1074553 [DOI] [PubMed] [Google Scholar]
- Mital, J., Meissner, M., Soldati, D. and Ward, G. E. (2005). Conditional expression of Toxoplasma gondii apical membrane antigen-1 (TgAMA1) demonstrates that TgAMA1 plays a critical role in host cell invasion. Mol. Biol. Cell 16, 4341-4349. 10.1091/mbc.e05-04-0281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissette, N. S., Murray, J. M. and Roos, D. S. (1997). Subpellicular microtubules associate with an intramembranous particle lattice in the protozoan parasite Toxoplasma gondii. J. Cell Sci. 110, 35-42. 10.1242/jcs.110.1.35 [DOI] [PubMed] [Google Scholar]
- Morrissette, N. S. and Sibley, L. D. (2002a). Cytoskeleton of apicomplexan parasites. Microbiol. Mol. Biol. Rev. 66, 21-38. 10.1128/MMBR.66.1.21-38.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissette, N. S. and Sibley, L. D. (2002b). Disruption of microtubules uncouples budding and nuclear division in Toxoplasma gondii. J. Cell Sci. 115, 1017-1025. 10.1242/jcs.115.5.1017 [DOI] [PubMed] [Google Scholar]
- Munera Lopez, J., Tengganu, I. F., Liu, J., Murray, J. M., Arias Padilla, L. F., Zhang, Y., Brown, P. T., Florens, L. and Hu, K. (2022). An apical protein, Pcr2, is required for persistent movement by the human parasite Toxoplasma gondii. PLoS Pathog. 18, e1010776. 10.1371/journal.ppat.1010776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagayasu, E., Hwang, Y.-C., Liu, J., Murray, J. and Hu, K. (2016). Loss of a doublecortin (DCX) domain containing protein causes structural defects in a tubulin-based organelle of Toxoplasma gondii and impairs host cell invasion. Mol. Biol. Cell 28, 411-428. 10.1091/mbc.e16-08-0587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols, B. A. and Chiappino, M. L. (1987). Cytoskeleton of Toxoplasma gondii. J. Protozool 34, 217-226. 10.1111/j.1550-7408.1987.tb03162.x [DOI] [PubMed] [Google Scholar]
- Opitz, C. and Soldati, D. (2002). ‘The glideosome': a dynamic complex powering gliding motion and host cell invasion by Toxoplasma gondii. Mol. Microbiol. 45, 597-604. 10.1046/j.1365-2958.2002.03056.x [DOI] [PubMed] [Google Scholar]
- Pavlou, G., Touquet, B., Vigetti, L., Renesto, P., Bougdour, A., Debarre, D., Balland, M. and Tardieux, I. (2020). Coupling polar adhesion with traction, spring, and torque forces allows high-speed helical migration of the protozoan parasite Toxoplasma. ACS Nano 14, 7121-7139. 10.1021/acsnano.0c01893 [DOI] [PubMed] [Google Scholar]
- Periz, J., Whitelaw, J., Harding, C., Gras, S., Del Rosario Minina, M. I., Latorre-Barragan, F., Lemgruber, L., Reimer, M. A., Insall, R., Heaslip, A.et al. (2017). Toxoplasma gondii F-actin forms an extensive filamentous network required for material exchange and parasite maturation. Elife 6, e24119. 10.7554/eLife.24119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabenau, K. E., Sohrabi, A., Tripathy, A., Reitter, C., Ajioka, J. W., Tomley, F. M. and Carruthers, V. B. (2001). TgM2AP participates in Toxoplasma gondii invasion of host cells and is tightly associated with the adhesive protein TgMIC2. Mol. Microbiol. 41, 537-547. 10.1046/j.1365-2958.2001.02513.x [DOI] [PubMed] [Google Scholar]
- Roos, D. S., Donald, R. G., Morrissette, N. S. and Moulton, A. L. (1994). Molecular tools for genetic dissection of the protozoan parasite Toxoplasma gondii. Methods Cell Biol. 45, 27-78. 10.1016/S0091-679X(08)61845-2 [DOI] [PubMed] [Google Scholar]
- Sahoo, N., Beatty, W., Heuser, J., Sept, D. and Sibley, L. D. (2006). Unusual kinetic and structural properties control rapid assembly and turnover of actin in the parasite Toxoplasma gondii. Mol. Biol. Cell 17, 895-906. 10.1091/mbc.e05-06-0512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton, W. O. (1996). Semper: distortion compensation, selective averaging, 3-D reconstruction, and transfer function correction in a highly programmable system. J. Struct. Biol. 116, 230-236. 10.1006/jsbi.1996.0035 [DOI] [PubMed] [Google Scholar]
- Schatten, H., Sibley, L. D. and Ris, H. (2003). Structural evidence for actin-like filaments in Toxoplasma gondii using high-resolution low-voltage field emission scanning electron microscopy. Microsc. Microanal. 9, 330-335. 10.1017/S1431927603030095 [DOI] [PubMed] [Google Scholar]
- Schindelin, J., Arganda-Carreras, I., Frise, E., Kaynig, V., Longair, M., Pietzsch, T., Preibisch, S., Rueden, C., Saalfeld, S., Schmid, B.et al. (2012). Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676-682. 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler, R. V., White, L. A., Hu, K., Helmke, B. P. and Guilford, W. H. (2017). Direct measurement of cortical force generation and polarization in a living parasite. Mol. Biol. Cell 28, 1912-1923. 10.1091/mbc.e16-07-0518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokkermans, T. J., Schwartzman, J. D., Keenan, K., Morrissette, N. S., Tilney, L. G. and Roos, D. S. (1996). Inhibition of Toxoplasma gondii replication by dinitroaniline herbicides. Exp. Parasitol. 84, 355-370. 10.1006/expr.1996.0124 [DOI] [PubMed] [Google Scholar]
- Swedlow, J. R., Hu, K., Andrews, P. D., Roos, D. S. and Murray, J. M. (2002). Measuring tubulin content in Toxoplasma gondii: a comparison of laser-scanning confocal and wide-field fluorescence microscopy. Proc. Natl. Acad. Sci. USA 99, 2014-2019. 10.1073/pnas.022554999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinevez, J. Y., Perry, N., Schindelin, J., Hoopes, G. M., Reynolds, G. D., Laplantine, E., Bednarek, S. Y., Shorte, S. L. and Eliceiri, K. W. (2017). TrackMate: An open and extensible platform for single-particle tracking. Methods 115, 80-90. 10.1016/j.ymeth.2016.09.016 [DOI] [PubMed] [Google Scholar]
- Tosetti, N., Dos Santos Pacheco, N., Soldati-Favre, D. and Jacot, D. (2019). Three F-actin assembly centers regulate organelle inheritance, cell-cell communication and motility in Toxoplasma gondii. Elife 8, e42669. 10.7554/eLife.42669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran, J. Q., Li, C., Chyan, A., Chung, L. and Morrissette, N. S. (2012). SPM1 stabilizes subpellicular microtubules in Toxoplasma gondii. Eukaryot. Cell 11, 206-216. 10.1128/EC.05161-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rossum, G. A. D. and Fred, L. (2009). Python 3 Reference Manual. Scotts Valley, CA, USA: CreateSpace. [Google Scholar]
- Wang, X., Qian, P., Cui, H., Yao, L. and Yuan, J. (2020). A protein palmitoylation cascade regulates microtubule cytoskeleton integrity in Plasmodium. EMBO J. 39, e104168-e104168. 10.15252/embj.2019104168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzel, D. M., Hakansson, S., Hu, K., Roos, D. and Sibley, L. D. (2003). Actin filament polymerization regulates gliding motility by apicomplexan parasites. Mol. Biol. Cell 14, 396-406. 10.1091/mbc.e02-08-0458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham, H., Averick, M., Bryan, J., Chang, W., McGowan, L. D. A., François, R., Grolemund, G., Hayes, A., Henry, L., Hester, J.et al. (2019). Welcome to the Tidyverse. J. Open Source Softw. 4, 1686. 10.21105/joss.01686 [DOI] [Google Scholar]
- Wortel, I. M. N., Liu, A. Y., Dannenberg, K., Berry, J. C., Miller, M. J. and Textor, J. (2021). CelltrackR: An R package for fast and flexible analysis of immune cell migration data. ImmunoInformatics 1-2, 100003. 10.1016/j.immuno.2021.100003 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.