Abstract
Background
The mobilization quantification score (MQS) provides an opportunity to quantify the duration and intensity of mobilization therapy in the intensive care unit (ICU) and predict functional outcomes in ICU patients after surgery and stroke. MQS is a numerical measurement of early mobilization dose in the ICU, and its relationship with activities of daily living (ADL) dependence has been shown. We created and validated the Japanese version of the MQS using the endpoint ADL in a mixed population of patients in the ICU.
Materials and methods
In this prospective study, consecutive patients who were admitted to one of three ICUs of a tertiary care hospital in Japan, aged ≥18 years, and who received mechanical ventilation for >48 hours were enrolled. The Japanese version of the MQS was applied twice daily by an ICU physiotherapist and data recorded for analysis. The primary outcome was ADL dependence at hospital discharge, defined as a Barthel index (BI) of <70 or in-hospital death. The reliability among assessors was verified by calculating the interclass correlation coefficient (ICC) (2.1) for the average daily MQS. We performed a multiple logistic regression analysis to examine and identify a binary cutoff point for high-/low-dose rehabilitation.
Results
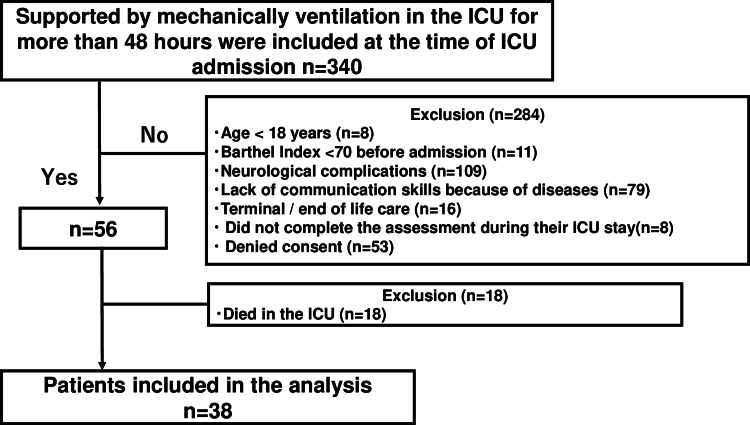
Of the 340 target patients, eight were aged <18 years, 109 had neurological complications, 11 had a BI <70 before admission, 79 had a lack of communication skills, 16 were terminally ill, eight did not complete the assessment during their ICU stay, 18 died in the ICU, and 53 denied consent. After 302 patients were excluded, 38 were included in the study. Six assessors, two at each hospital, measured the MQS in 38 patients. The ICC (2.1) for the MQS mean value was 0.98 (0.96-0.99) during the ICU stay. Logistic regression analysis using the mean MQS on admission to ICUs as an explanatory variable showed a significant association between increased MQS and decreased ADL dependence at discharge (odds ratio (OR): 0.76, confidence interval (CI): 0.61-0.96, adjusted p = 0.009). Logistic regression analysis using a high MQS on admission to ICUs as an explanatory variable showed a significant association between increased MQS and decreased ADL dependence at hospital discharge (OR: 0.14, CI: 0.03-0.66, adjusted p = 0.013).
Conclusions
We present a validated version of the Japanese MQS with a high inter-rater reliability that predicts ADL dependence at hospital discharge. The instrument can be used in future clinical trials in the ICU to control for the mobilization level in the ICU. The increased utilization of mobilization acutely in the ICU setting as quantified by the MQS may improve patient outcomes.
Keywords: reliability, rehabilitation, mechanical ventilation, activity of daily living (adl), early mobilization
Introduction
Post-intensive care syndrome (PICS), a general term for motor and cognitive dysfunction that becomes apparent after the acute phase of intensive care, has attracted attention in recent years [1]. PICS causes physical disability, cognitive dysfunction, and psychiatric disorders that develop or worsen in patients after recovery from severe acute conditions, such as severe sepsis and acute respiratory distress syndrome [2,3]. Early rehabilitation is one of the preventive methods; however, further investigation into the specific clinical strength and activity time is needed [4-6]. In addition, in Japan, variations in early rehabilitation programs based on the hospital environment and the experience of the therapist still exist, and many hospitals have not created evidence-based protocols [7].
Physical activity (PA) in patients with respiratory failure is a significant prognostic factor for mortality due to severe exacerbation [8,9]. In particular, PA in patients with severe respiratory failure tends to decrease due to disease management [10]. In addition, PA is often difficult to improve because of severe shortness of breath and impaired exercise tolerance. However, some patients are unable to take the step of mobilization to standing and walking while in the intensive care unit (ICU), and even minimal exercise activity can reduce weakness and wasting [11].
A previous study by Scheffenbichler et al. created a new tool for assessing mobilization doses based on the level and duration of rehabilitation (mobilization quantification score, MQS) [12]. The MQS was developed based on expert opinions, and its reproducibility has been verified at multiple health centers. In addition, the MQS is a numerical measure of the dose of early mobilization in the ICU and has been shown to predict mortality and adverse discharge [12,13].
There are several benefits associated with using the MQS to assess PA for the early rehabilitation of patients in the ICU. Owing to the peculiarities of the ICU environment, it is difficult to evaluate the amount of PA using a PA meter or metabolic measuring instrument, and it is important to develop a simple evaluation scale, such as the MQS [12,13]. The MQS has excellent concurrency, inter-racial consensus, and no caps and provides consistent results among professionals. The MQS is available in English; however, Japanese versions are not currently available. Therefore, this study aimed to develop a Japanese version of the MQS and confirm its reliability and effectiveness. This study evaluated whether the MQS could predict activity of daily living (ADL) dependency at hospital discharge in patients with mechanical ventilation in mixed ICUs in Japan. We also evaluated the inter-rater reliability of the Japanese version of the MQS.
Materials and methods
Methods
The reliability and sensitivity of the translated MQS were assessed by two evaluators at each hospital. For each patient, two evaluators measured the two MQS scorings daily from the time the patient was admitted to the ICU to the time the patient was discharged from the ICU. The measurement values obtained by the two assessors for each patient were blinded to those obtained by the other assessors during the testing period. The researcher obtained permission to use the version of the MQS that was developed in English and translated it into the Japanese version of the MQS. The MQS was forward-translated by two native Japanese speakers in accordance with the guidelines for the process of cross-cultural adaptation of self-report measures [14]. One of the translators was a physical therapist with several years of experience, and the other was a person with no medical knowledge or education. The two translated Japanese versions were integrated into a consensus version after the parts with vague interpretations were discussed at a consensus meeting. Backward translation was performed by two translators who were bilingual and fluent in both Japanese and English but did not have any medical knowledge. The consensus version of the forward translation was then translated back into English. The final Japanese version was completed by comparing and revising all versions of the MQS by a committee of experts, including two doctors, two nurses, and two physiotherapists (see Appendix).
Patients
The study was performed over six months, from September 1, 2022 to March 31, 2023. The study included patients who were recently admitted to the medical ICU of Nagoya Medical Center, Naha City Hospital, and Steel Memorial Yawata Hospital, Japan. All patients were on mechanical ventilation for 48 hours or longer, and physiotherapy was ordered on weekdays. The exclusion criteria were patients under 18 years of age, those with a Barthel index (BI) <70 before admission, those with neurological complications or a lack of communication skills due to pre-existing mental diseases, and those in a terminal stage. We also excluded patients who died and those who did not complete the assessment during their ICU stay, as well as those who never met the criteria for physiological stability. All patients were managed according to the early mobilization expert consensus [7].
Ethical considerations
In accordance with the Declaration of Helsinki, written informed consent was obtained from all the participants prior to the ICU discharge after explaining the purpose, expected benefits, and potential harms of the study. This study was approved by the Ethics Committee of the Gifu University of Health Sciences (approval number: 2022-04).
Assessment
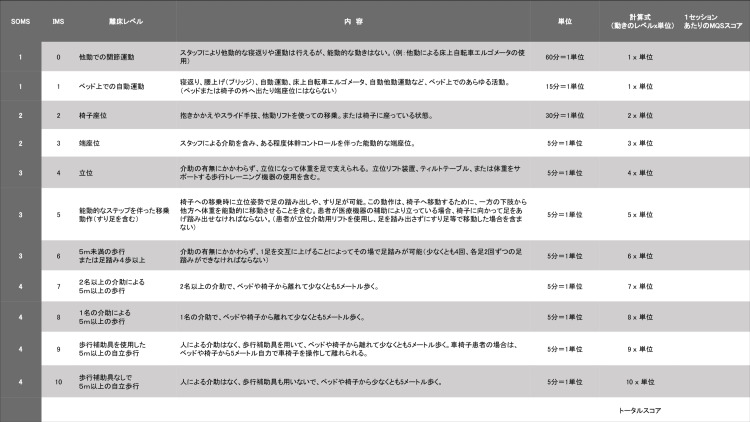
The rehabilitation levels were quantified based on the ICU mobility scale (levels 1-10) [15]. To calculate the rehabilitation dose (intensity × activity time), each rehabilitation level was assigned a duration to define one unit of the MQS. The daily MQS obtained from the nursing and physiotherapy data was totaled throughout the ICU stay and then divided by the duration of the ICU stay to obtain the average daily MQS (average daily MQS = total MQS during the ICU/ICU length of stay). In addition, the mean MQS was bisected to determine the cutoff points for high-/low-dose rehabilitation as the exposure variable. The median served as the cut-off for high-/low-dose rehabilitation recruitment as a binary variable.
To investigate the agreement among the observers, the role of the evaluator was performed by two physiotherapists at each participating hospital (Nagoya Medical Center: SW, main assessor, and KK with 12 and seven years of experience working in the ICU, respectively; Naha City Hospital: DY and TT with 17 and 10 years of experience working in the ICU, respectively; Steel Memorial Yawata Hospital: KY and KG with 15 and seven years of experience working in the ICU, respectively). All the assessors were trained by the study members to apply the MQS in simulated cases and during routine care.
Data collection
The primary outcome was ADL dependence at hospital discharge. ADL dependence was defined as a BI of <70 (including hospital mortality). The following basic patient information was recorded at the time of ICU admission: age, sex, body mass index, acute physiologic assessment and chronic health evaluation (APACHE II) score, ICU admission diagnosis, ICU and hospital lengths of stay, duration of mechanical ventilation, and in-hospital mortality.
Statistical analyses
Continuous variables and categorical data were described using medians with interquartile ranges and numbers with percentages, respectively. The reliability among the assessors was verified by calculating the interclass correlation coefficient (ICC) (2.1) for the average daily MQS. ICC in the range of 0.75-0.90 was considered good, and >0.90 was excellent [16]. To examine the effectiveness, we performed a multiple logistic regression analysis with age and APACHE II scores as covariates. These factors have been shown in previous studies to be associated with adverse outcomes [17]. Logistic regression analysis was performed with ADL dependence at hospital discharge as the objective variable and the mean MQS score during all ICU admissions or the high dose of MQS as the explanatory variable. For effectiveness, we used data from the main assessor.
All analyses were performed using the JMP software (version 13.0; SAS Institute Inc., Cary, NC, USA). Statistical tests were two-sided, and p < 0.05 was considered statistically significant.
Results
Of the 340 target patients, eight were aged <18 years, 109 had neurological complications, 11 had a BI <70 before admission, 79 had a lack of communication skills, 16 were terminally ill, eight did not complete the assessment during their ICU stay, 18 died in the ICU, and 53 denied consent. After 302 patients were excluded, 38 were included in the study (Figure 1). The characteristics of these patients are presented in Table 1.
Table 1. Characteristics and results for the mobilization quantification score (n = 38).
Median (25th-75th percentile) or the number of patients (%)
IQR: interquartile range; BMI: body mass index; APACHE: acute physiologic assessment and chronic health evaluation; ICU: intensive care unit; MQS: mobilization quantification score
| Variable | n = 38 |
| Age (years), median (IQR) | 74.5 (63.8-82.0) |
| Sex (male), n (%) | 21 (55%) |
| BMI (kg/m2), median (IQR) | 19.0 (17.2-22.2) |
| ICU admission diagnosis, n (%) | |
| Acute respiratory failure | 9 (24%) |
| Cardiovascular disease | 5 (13%) |
| Gastric or colonic surgery | 20 (52%) |
| Other diagnoses | 4 (11%) |
| APACHE II score, median (IQR) | 22.5 (17.8-26.0) |
| Charlson comorbidity index, median (IQR) | 1 (0-3) |
| ICU length of stay, median (IQR) | 5.6 (4.1-8.2) |
| Duration of mechanical ventilation, median (IQR) | 3.7 (2.6-5.5) |
| Hospital length of stay (IQR) | 26.9 (17.7-43.1) |
| Mean MQS score (IQR) | 4.1 (2.4-7.3) |
| Hospital survival, n (%) | 34 (89%) |
| Barthel index at hospital discharge (IQR) | 85.0 (53.8-95.0) |
Figure 1. Flow chart of the patient selection process.
Neurological complications include cerebral infarction, cerebral hemorrhage, acute subdual hematoma, acute epidural hematoma, traumatic subarachnoid hemorrhage, and encephalitis.
ICU: intensive care unit
Inter-assessor reliability
Six assessors, two from each hospital, were able to measure the MQS in 38 patients. The mean ICC (2.1) for the MQS mean value was 0.84 (0.78-0.91) during the ICU stay.
Effectiveness
Logistic regression analysis using the mean MQS on admission to ICUs as an explanatory variable showed a significant association between increased MQS and decreased ADL dependence at discharge (odds ratio (OR): 0.76; confidence interval (CI): 0.61-0.96, adjusted p = 0.009) (Table 2). Logistic regression analysis using high MQS on admission to ICUs as an explanatory variable showed a significant association between increased MQS and decreased ADL dependence at discharge (OR: 0.14; CI: 0.03-0.66, adjusted p = 0.013).
Table 2. Multivariate logistic regression analysis of independent variables for activity of daily living independence at discharge, excluding fatal cases.
MQS: mobilization quantification score; ICU: intensive care unit; OR: odds ratio; CI: confidence interval; APACHE: acute physiology and chronic health evaluation
| Variable | Univariate | Model 1 adjusted for age and APACHE II | ||||
| OR | 95% CI | P value | OR | 95% CI | P-value | |
| Mean MQS during all ICU admissions | 0.75 | 0.59-0.95 | 0.004 | 0.76 | 0.61-0.96 | 0.009 |
| High dose of MQS (mean MQS >4.1) | 0.12 | 0.03-0.53 | 0.005 | 0.14 | 0.03-0.66 | 0.013 |
| Number of daily rehabilitations per person during ward | 0.95 | 1.02-1.05 | 0.095 | 0.08 | 0.01-0.50 | 0.007 |
Discussion
An examination of the reliability of an evaluation method is often based on how negligible the measurement error is when two assessors conduct the same test under the same conditions. Lamdis et al. [16] concluded that reproducibility was effective if the ICC was >0.8. Previous studies have confirmed the high inter-rater reliability of the MQS measurements. SOMS has also been reported to be good interrater reliability among excellent assessors [18]. In this study, the total and subtotal values of MQS was 0.8 or higher, demonstrating a high inter-rater reliability.
Patients admitted to the ICUs frequently develop PICS [19]. Insights into how intense PA should be targeted during the ICU stay for early recovery could help avoid increasing reliance on ADLs during hospital discharge for critically ill patients [20,21]. However, in many cases, it is difficult to perform PA assessment with a PA meter or a metabolic meter at an early stage in critically ill patients due to the effects of sedation and mechanical ventilation. In the cluster analysis of Fuest et al., the mobilization dose was determined by sessions per day, mean duration, early mobilization, and average and maximum level achieved, but it is difficult to use all these evaluations on the bedside [22]. The MQS has several benefits over other scales, including the combination of duration and intensity in a single score. This study included patients under mechanical ventilation, and high inter-rater reliability was observed even in critically ill patients. Therefore, high inter-rater reliability could be ensured, if the MQS measurement procedure is clarified and used, even in patients under mechanical ventilation.
In a previous study using the MQS, high-dose rehabilitation in the surgical ICU was an independent predictor of ADL dependence at hospital discharge [12]. Previous studies in stroke patients have similarly associated higher mobilization doses with a lower risk of losing the ability to live independently after hospital discharge [13]. In this study, we found a significant association between MQS and ADL independence, suggesting that MQS may be useful as a predictor of ADL independence. Our results highlight the need to quantify the rehabilitation activity time when investigating the effects of early rehabilitation in mechanically ventilated patients. However, in this study, MQS, which is a continuous variable, was transformed into a binary variable based on the median. For MQS above the median, a selection bias may have occurred, if all patients were included in the highly variable high-dose mobilization group.
This study has some limitations. This study lacked complete data and had a small sample size. Only 17% of the patients in the ICU were included during the study period, which is an important source of selection bias that may limit the generalizability of the findings to other ICUs. In addition, except for age and APACHE II score, factors assumed to be associated with ADL dependence at hospital discharge were not adjusted for in this study, such as the barriers to mobilization and medication. Furthermore, the reliability of the test-retest method, in which the MQS was measured twice, was not examined. In critically ill patients admitted to the ICU, the re-test method may not be highly reliable because of the effects of changes in pathology and sedation. In the future, verification of the reliability using the retest method will be required. In this study, both the assessors were physiotherapists, and it is necessary to examine whether similar results can be performed by multiple professionals, such as nurses involved in the ICU.
Conclusions
The Japanese version of the MQS has been validated in this study. The reliability and effectiveness of the MQS have been studied. The inter-rater reliability of the MQS was high. In addition, the MQS was associated with ADL dependence at discharge, suggesting that it may be useful as a predictor of adverse physical outcomes. The MQS could ensure high inter-evaluator reproducibility if the measurement procedure was clarified and used, even under mechanical ventilation.
Further research is needed to identify and eliminate the confounding factors involved in MQS and ADL dependence.
Acknowledgments
We wish to sincerely thank the following persons who provided tremendous support in formulating the MQS for this study: Kaito Kochi and Mika Ono of the Nagoya Medical Center; Kawabata Shinya, Tonaki Takuya, Miyagi Yuuichi, Kamiya Taisuke, Touyama Hiroyuki, Miyata Yuuji, and Tomiyama Hiroshi of Naha City Hospital; Kei Goto, Shota Tanaka, and Tokuaki Shinya of Steel Memorial Yawata Hospital; and Keibun Liu of the Prince Charles Hospital. There are no prescribed conflicts of interest for any of the authors of this paper.
Appendices
Figure 2. Japanese version of the mobilization quantification score.
SOMS: surgical intensive care unit optimal mobilization score; IMS: ICU mobility scale
The authors have declared financial relationships, which are detailed in the next section.
Stefan J. Schaller declare(s) personal fees and non-financial support from GmbH (Munich, Germany), ASP GmbH (Attendorn, Germany), STIMIT AG (Biel, Switzerland), ESICM (Geneva, Switzerland), grants. SJS received grants and non-financial support from Reactive Robotics GmbH (Munich, Germany), ASP GmbH (Attendorn, Germany), STIMIT AG (Biel, Switzerland), ESICM (Geneva, Switzerland), grants, personal fees, and non-financial support from Fresenius Kabi Deutschland GmbH (Bad Homburg, Germany), grants from the Innovations fond of the Federal Joint Committee (G-BA), personal fees from Springer Verlag GmbH (Vienna, Austria) for educational purposes and Advanz Pharma GmbH (Bielefeld, Germany), non-financial support from national and international societies (and their congress organizers) in the field of anesthesiology and intensive care medicine, outside the submitted work.ny), ASP GmbH (Attendorn, Germany), STIMIT AG (Biel, Switzerland), ESICM (Geneva, Switzerland), grants, personal fees, and non-financial support from Fresenius Kabi Deutschland GmbH (Bad Homburg, Germany), grants from the Innovations fond of the Federal Joint Committee (G-BA), personal fees from Springer Verlag GmbH (Vienna, Austria) for educational purposes and Advanz Pharma GmbH (Bielefeld, Germany), non-financial support from national and international societies (and their congress organizers) in the field of anesthesiology and intensive care medicine, outside the submitted work. Dr. Schaller holds stocks in small amounts from Alphabeth Inc., Bayer AG, and Siemens AG; these holdings have not affected any decisions regarding his research or this study.
Human Ethics
Consent was obtained or waived by all participants in this study. Ethics Committee of the Gifu University of Health Sciences issued approval 2022-04. In accordance with the Declaration of Helsinki, written informed consent was obtained from all the participants prior to the ICU discharge after explaining the purpose, expected benefits, and potential harms of the study.
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders' conference. Needham DM, Davidson J, Cohen H, et al. Crit Care Med. 2012;40:502–509. doi: 10.1097/CCM.0b013e318232da75. [DOI] [PubMed] [Google Scholar]
- 2.Instruments to measure outcomes of post-intensive care syndrome in outpatient care settings - Results of an expert consensus and feasibility field test. Spies CD, Krampe H, Paul N, et al. J Intensive Care Soc. 2021;22:159–174. doi: 10.1177/1751143720923597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prevalence of post-intensive care syndrome among Japanese intensive care unit patients: a prospective, multicenter, observational J-PICS study. Kawakami D, Fujitani S, Morimoto T, et al. Crit Care. 2021;25:69. doi: 10.1186/s13054-021-03501-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Determinants of gait independence after mechanical ventilation in the intensive care unit: a Japanese multicenter retrospective exploratory cohort study. Watanabe S, Kotani T, Taito S, et al. J Intensive Care. 2019;7:53. doi: 10.1186/s40560-019-0404-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Changes in barriers to implementing early mobilization in the intensive care unit: a single center retrospective cohort study. Watanabe S, Liu K, Morita Y, et al. Nagoya J Med Sci. 2021;83:443–464. doi: 10.18999/nagjms.83.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Association between early mobilization in the ICU and psychiatric symptoms after surviving a critical illness: A multi-center prospective cohort study. Watanabe S, Liu K, Nakamura K, et al. J Clin Med. 2022;11 doi: 10.3390/jcm11092587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The Japanese Society of Intensive Care Medicine: evidence based expert consensus for early rehabilitation in the intensive care unit [Article in Japanese] Ad Hoc Committee for Early Rehabilitation. Jpn J Int Care Med. 2017;24:255–303. [Google Scholar]
- 8.Prognostic value of the objective measurement of daily physical activity in patients with COPD. Garcia-Rio F, Rojo B, Casitas R, et al. Chest. 2012;142:338–346. doi: 10.1378/chest.11-2014. [DOI] [PubMed] [Google Scholar]
- 9.Factors associated with reduction of sedentary time following tiotropium/olodaterol therapy in treatment-naïve chronic obstructive pulmonary disease. Takahashi K, Tashiro H, Tajiri R, et al. Int J Chron Obstruct Pulmon Dis. 2021;16:3297–3307. doi: 10.2147/COPD.S338560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Physical inactivity is associated with a higher risk for severe COVID-19 outcomes: a study in 48 440 adult patients. Sallis R, Young DR, Tartof SY, et al. Br J Sports Med. 2021;55:1099–1105. doi: 10.1136/bjsports-2021-104080. [DOI] [PubMed] [Google Scholar]
- 11.Early rehabilitation in the intensive care unit: preventing physical and mental health impairments. Parker A, Sricharoenchai T, Needham DM. Curr Phys Med Rehabil Rep. 2013;1:307–314. doi: 10.1007/s40141-013-0027-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Effects of the level and duration of mobilization therapy in the surgical ICU on the loss of the ability to live independently: an international prospective cohort study. Scheffenbichler FT, Teja B, Wongtangman K, et al. Crit Care Med. 2021;49:0–57. doi: 10.1097/CCM.0000000000004808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Effects of mobility dose on discharge disposition in critically-ill stroke patients. Mazwi N, Lissak I, Wongtangman K, et al. PM R. 2023 doi: 10.1002/pmrj.13039. [DOI] [PubMed] [Google Scholar]
- 14.Guidelines for the process of cross-cultural adaptation of self-report measures. Beaton DE, Bombardier C, Guillemin F, Ferraz MB. Spine (Phila Pa 1976) 2000;25:3186–3191. doi: 10.1097/00007632-200012150-00014. [DOI] [PubMed] [Google Scholar]
- 15.Feasibility and inter-rater reliability of the ICU Mobility Scale. Hodgson C, Needham D, Haines K, et al. Heart Lung. 2014;43:19–24. doi: 10.1016/j.hrtlng.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 16.A guideline of selecting and reporting intraclass correlation coefficients for reliability research. Koo TK, Li MY. J Chiropr Med. 2016;15:155–163. doi: 10.1016/j.jcm.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Factors associated with discharge home among medical ICU patients in an early mobilization program. Kim RY, Murphy TE, Doyle M, et al. Crit Care Explor. 2019;1:0. doi: 10.1097/CCE.0000000000000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The German validation study of the surgical intensive care unit optimal mobility score. Schaller SJ, Stäuble CG, Suemasa M, et al. J Crit Care. 2016;32:201–206. doi: 10.1016/j.jcrc.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 19.Post-intensive care syndrome-10 years after its proposal and future directions. Inoue S, Nakanishi N, Nakamura K. J Clin Med. 2022;11 doi: 10.3390/jcm11154381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Association between intensive care unit-acquired weakness and early nutrition and rehabilitation intensity in mechanically ventilated patients: a multicenter retrospective observational study. Watanabe S, Hirasawa J, Naito Y, et al. Cureus. 2023;15:0. doi: 10.7759/cureus.37417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Association between the early mobilization of mechanically ventilated patients and independence in activities of daily living at hospital discharge. Watanabe S, Hirasawa J, Naito Y, et al. Sci Rep. 2023;13:4265. doi: 10.1038/s41598-023-31459-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clustering of critically ill patients using an individualized learning approach enables dose optimization of mobilization in the ICU. Fuest KE, Ulm B, Daum N, et al. Crit Care. 2023;27:1. doi: 10.1186/s13054-022-04291-8. [DOI] [PMC free article] [PubMed] [Google Scholar]