Abstract
Objective
While premenstrual dysphoric disorder (PMDD) as defined in DSM has become an established diagnosis, and a formal indication for drug treatment, the relative impact of the disparate symptoms named in the criteria, and to what extent they indeed constitute parts of one syndrome, remains insufficiently clarified. We have therefore explored the frequency, impact, and inter‐relationship of different PMDD symptoms.
Method
Using a web survey, 10,457 Swedish women of fertile age were asked to retrospectively assess if they experience reduced functioning due to symptoms clearly associated with the premenstrual phase. Those responding affirmatively reported presence, severity, and impact of each symptom named in the PMDD criteria.
Result
Nine percent reported impairing premenstrual symptoms. Whereas irritability was reported to cause impairment in 77% of those passing the gate questions, somatic symptoms were common but seldom causing impairment. A vast majority reported presence of at least 5 different symptoms, as required to meet the PMDD criteria, but few reported each of 5 different symptoms to be severe or impairing. An analysis of the association between symptoms revealed clear‐cut clustering of somatic and mood symptoms, respectively.
Conclusion
While retrospective account suggested irritability to be the clinically most important premenstrual symptom, some of the complaints named in the PMDD criteria were not or only weakly associated with mood symptoms and also reported to be of limited clinical significance. It is concluded that regarding all symptoms listed in the DSM criteria as clinically relevant manifestations of one and the same syndrome may be questioned.
Highlights
In women reporting significant premenstrual complaints, somatic symptoms are prevalent but causing much less impairment than irritability and other mood symptom.
The association between somatic symptoms and irritability is weak or – in the case of headache and joint and muscle pain – non‐existent.
Regarding all symptoms listed in the DSM criteria as clinically relevant manifestations of one and the same syndrome may be questioned.
That the days or week(s) prior to menstruation may be associated with distress in some women was recognized already by Trotula de Salerno in the 11th century, van Feuchtersleben in 1847, and Frank in 1931 (1). While these early writers usually focused solely or primarily on premenstrual changes in mood and behavior, Green and Dalton later put forward the view that the large variety of both somatic and mental symptoms that may occur premenstrually are all caused by progesterone deficiency and should be regarded as parts of one and the same syndrome (2).
Although the progesterone deficiency theory has subsequently been questioned (3, 4), most attempts to define a condition characterized by symptoms appearing premenstrually are still based on the assumption that any symptom displaying this temporal pattern should be regarded as part of the same disorder. In this vein, presence of one symptom of sufficient severity to cause impairment, be it a mood symptom, such as irritability, or a physical symptom, such as breast tenderness, is sufficient for the diagnosis of premenstrual tension syndrome as defined in ICD‐10 or for the diagnosis of premenstrual syndrome (PMS) as defined by the American College of Gynecologists (ACOG) (5). Moreover, in the criteria for these conditions, no symptom is named more important than the other. For the definition of the corresponding condition in the Diagnostic and Statistical Manual of Mental Disorders (DSM) editions IV and V – premenstrual dysphoric disorder (PMDD) – the American Psychiatric Association has taken a somewhat different stance in the sense that the presence of at least five different symptoms, one of which must be one of four key mood symptoms, is required (6). Somatic complaints, such as headache and bloating, are however included also among the PMDD criteria but are lumped together in the last of 11 items.
Epidemiological studies based on retrospective report are fairly consistent in suggesting that 5–9% of women of fertile age are afflicted by impairment‐ causing premenstrual complaints (6) and also reasonably unanimous with respect to which premenstrual symptoms that are most common. However, a symptom reported by many may yet be of limited importance in terms of impact; to clarify the clinical importance of the different symptoms one must hence address not only their frequency but also the self‐reported severity and impact of each symptom. This however remains an understudied issue.
Also unsettled is to what extent premenstrual symptoms that are disparate in nature, such as irritability and breast tenderness, should indeed be regarded as parts of one and the same syndrome (7, 8). That most or all premenstrual complaints have an underlying biological mechanism in common in the sense that they all seem to be triggered by fluctuations in sex hormone levels (9) cannot per se be sufficient for claiming that they should be regarded as one syndrome – for comparison, rheumatoid arthritis and asthma are both dependent on the immune system, and can both be effectively treated with anti‐inflammatory agents, but are still not regarded as one disorder. To justify regarding two premenstrual complaints as parts of the same disorder, one would instead have to show both to have a certain susceptibility factor in common, either by identifying such a factor, or by showing that a woman experiencing one of the symptoms is at enhanced risk of experiencing also the other.
The aim of this study was to shed light on the self‐reported impact of all different symptoms named in the DSM criteria for PMDD as well as on the possible interrelationship between them. Moreover, the relevance of the DSM requirement that 5 different symptoms should be present for the diagnosis to be made was addressed.
METHOD
Subjects and Recruitment
LifeGene is a large‐scale prospective cohort study combining biosampling with information on lifestyle factors and health (10). Participants are randomly selected from the general population (index persons) but members of the same household as these and subjects recruited by spontaneous sign‐up may also participate. When the current analyses were undertaken, 10,457 women of fertile age had been included, 4380 of which were index persons, approximately 20% of the contacted index persons having accepted to participate. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration.
Design and Study Procedure
All participants were subjected to an extensive web‐based questionnaire designed to collect information on physical, mental, and social well‐being (for details see (10)).
These questionnaires screen for a large number of somatic and mental disorders; the LifeGene project is hence not focused on premenstrual complaints but on health issues at large. In this report, however, only data directly related to premenstrual complaints will be presented. The responses to three gateway questions were used to identify women with severe premenstrual symptoms; for those not responding affirmatively to these, no more questions regarding premenstrual complaints were posed. The first question read: “Did you, for most menstrual cycles during the past year, experience any changes in mood and/or any physical symptoms during the week preceding menstruation, that is, what is commonly referred to as PMS?” Those responding affirmatively were then asked: “Have your PMS complaints been of sufficient severity to interfere with work or social activities?” Those responding affirmatively also to this question were finally asked: “Are you quite certain that these symptoms are restricted to the premenstrual period, that is, that you are always totally free from symptoms approximately 1 week after the onset of menstruation?” Finally, the participants were asked if they had taken any kind of medication for PMS at any time during the past year.
If the answers on the three gateway questions were affirmative, a follow‐up questionnaire was administered to assess severity and impact of all symptoms listed in the DSM IV criteria for PMDD (which are largely the same as those of the ACOG criteria for premenstrual syndrome) (see Table 1 for a list of all symptoms). Item number 11 was however split into five different symptoms: breast tenderness or swelling, headache, joint or muscle pain, a sensation of bloating, and weight gain. There were thus 15 different symptoms that the participants were to grade as “none”, mild”, “moderate”, or “severe”; in addition, there was an option to answer “I don't know”. For each symptom, the participants were also asked if it interfered with work, usual activities, or relationships with others (yes/no). The questionnaire bears resemblances to the Premenstrual Symptom Screening Tool (11), the major differences being i) that item 11 in the DSM list of PMDD symptoms was split into five different symptoms and ii) that the participants were asked to assess the possible impact of each individual symptom.
TABLE 1.
The percentage of subjects among those passing the screening questions that reported different symptoms to cause impairment. a
| Symptom | Percentage (%) | 95% CI |
|---|---|---|
| Irritability or anger | 77.4 | 74.3–80.3 |
| Affective lability | 52.3 | 48.7–55.8 |
| Depressed mood or feelings of hopelessness | 50.1 | 46.6–53.7 |
| Lack of energy, fatigue | 49.6 | 46.1–53.2 |
| Anxiety or tension | 39.3 | 35.9–42.8 |
| Decreased interest in usual | 24.4 | 21.4–27.5 |
| Headaches | 18.8 | 16.1–21.7 |
| Out of control or overwhelmed | 18.1 | 15.5–21.0 |
| Difficulty concentrating | 17.9 | 15.3–20.8 |
| Hypersomnia, insomnia | 16.9 | 14.3–19.7 |
| A sensation of bloating | 15.5 | 13.0–18.2 |
| Joint or muscle pain | 12.2 | 10.0–14.7 |
| Weight gain | 9.4 | 7.4–11.6 |
| Breast tenderness or swelling | 8.1 | 6.3–10.3 |
| Marked change in appetite, food cravings | 6.5 | 4.9–8.4 |
CI, confidence interval.
Exclusion Criteria and Missing Data
Elsewhere in the LifeGene questionnaire the women had been asked if they had menstruated for the previous 12 months and if they were pregnant when responding. Those answering no to the former and/or yes to the latter of these questions were excluded. When no data for a certain symptom was provided it was assumed that this symptom was not present to an impairment‐causing extent.
Statistical Analysis
Grouping of symptoms was performed using hierarchical clustering with complete linkage on euclidean distances of scaled variables. The fit of the clustering was evaluated using the cophenetic correlation coefficient (12). The best number of variable clusters was determined using the average silhouette method in the R package NbClust (13). Pearson's correlation analysis was used to evaluate associations among four‐graded symptoms and Fishers' exact test to evaluate significance of odds ratios for presence of symptoms as well as impairment of symptoms. SPSS version 20.0 (IBM Corp., Armonk, NY, USA) and R version 2.13.1 (R Foundation for Statistical Computing, Vienna, Austria) were used for statistical analysis and presentation of data.
RESULTS
Prevalence of Premenstrual Complaints Causing Impairment
10457 women responded to the questions related to premenstrual complaints. Of these, 1929 were omitted because they were not menstruating or pregnant. 4890 women (57.3%) responded affirmatively to the first screening question, hence confirming presence of premenstrual symptoms. 1145 of these (13.4% of the total population) confirmed that these were of sufficient severity to interfere with relationships, work or other activities. 797 of these (9.3% of the total sample) also confirmed that they were convinced that they were symptom‐free approximately one week after the onset of menstruation. Hence, 797 subjects (including 375 index persons) were asked to assess presence, severity and impact of all symptoms listed in the DSM criteria of PMDD. These subjects had an average age of 33 years (range 16 – 58 years). 19% of them reported that they had taken medication for their complaints, without further specification, at least at some time during the past year.
Frequency, Severity and Impact of Different Symptoms
There was a considerable variation between the 15 different symptoms with respect to frequency, severity, and possible impact (Figure 1). Irritability and affective lability were the most prevalent complaints and also those causing most impairment. Of note, many subjects rating maximal severity for a certain symptom did not report any impairment caused by the symptom in question, and, on the other hand, impairment was not uncommon for symptoms rated as less than maximally severe.
FIGURE 1.
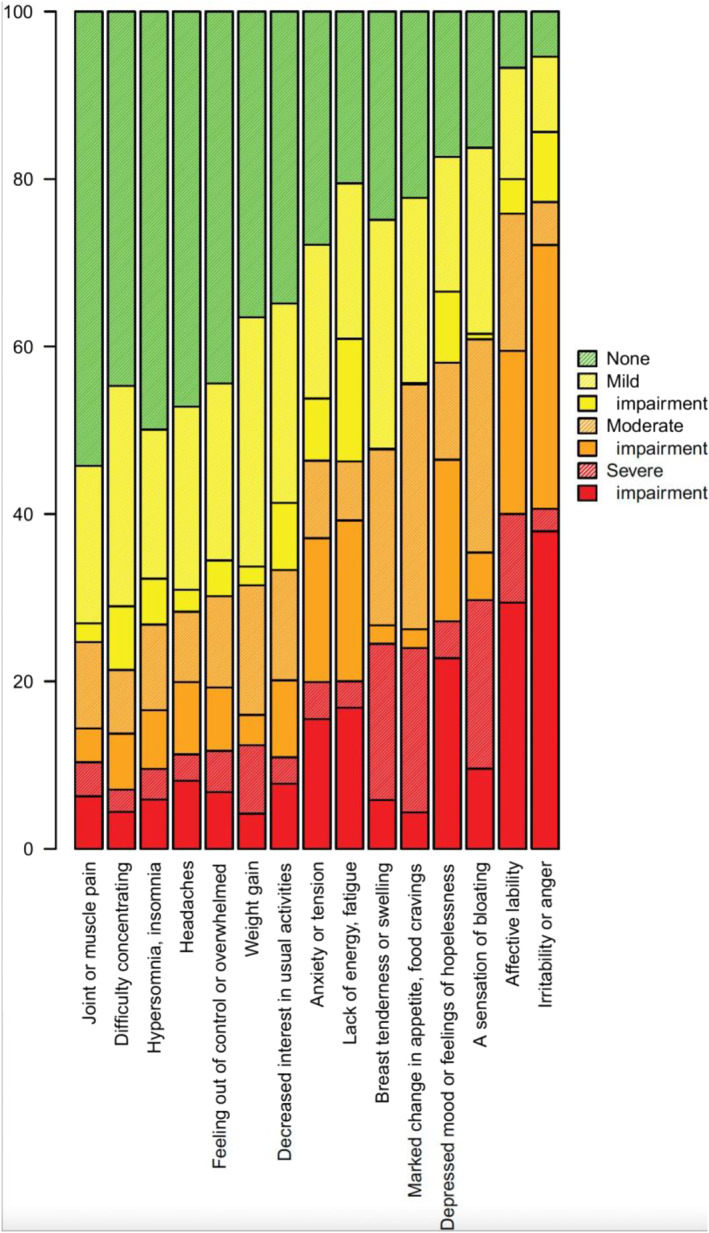
Frequency, severity and impairment for each symptom as reported by the sample passing the screening questions. Colors refer to severity. Shaded areas correspond to subjects within each severity level not reporting impairment. Filled areas correspond to subjects within each severity level reporting impairment.
Table 1 displays the percentage of subjects reporting impairment for each symptom. Whereas irritability was the most common reason for impairment (77%), no somatic symptom caused impairment in more than 20% of the subjects.
Number of Symptoms
To address the relevance of the DSM requirement of at least 5 different symptoms to be at hand for a provisional PMDD diagnosis to be made, the number of symptoms retrospectively reported by each subject was assessed. To this end, Figure 2 reveals A) percentage of subjects reporting any given number of symptoms, B) percentage of subjects rating any given number of symptoms as severe, and C) percentage of subjects reporting any given number of symptoms as causing impairment. In accordance with the DSM criteria, for these calculations the different somatic symptoms (i.e., breast tenderness or swelling, headache, joint or muscle pain, a sensation of bloating and weight gain) were lumped together and regarded as one item; a woman reporting at least one of these symptoms was hence regarded as confirming presence of DSM item 11, and one who reported at least one of these symptoms to be severe and/or causing impairment was regarded as having severe and/or impairing somatic complaints. Whereas a majority of the women passing the gateway question did report 5 or more symptoms to be present, only a small minority reported each of 5 or more symptoms to be severe and/or impairing.
FIGURE 2.
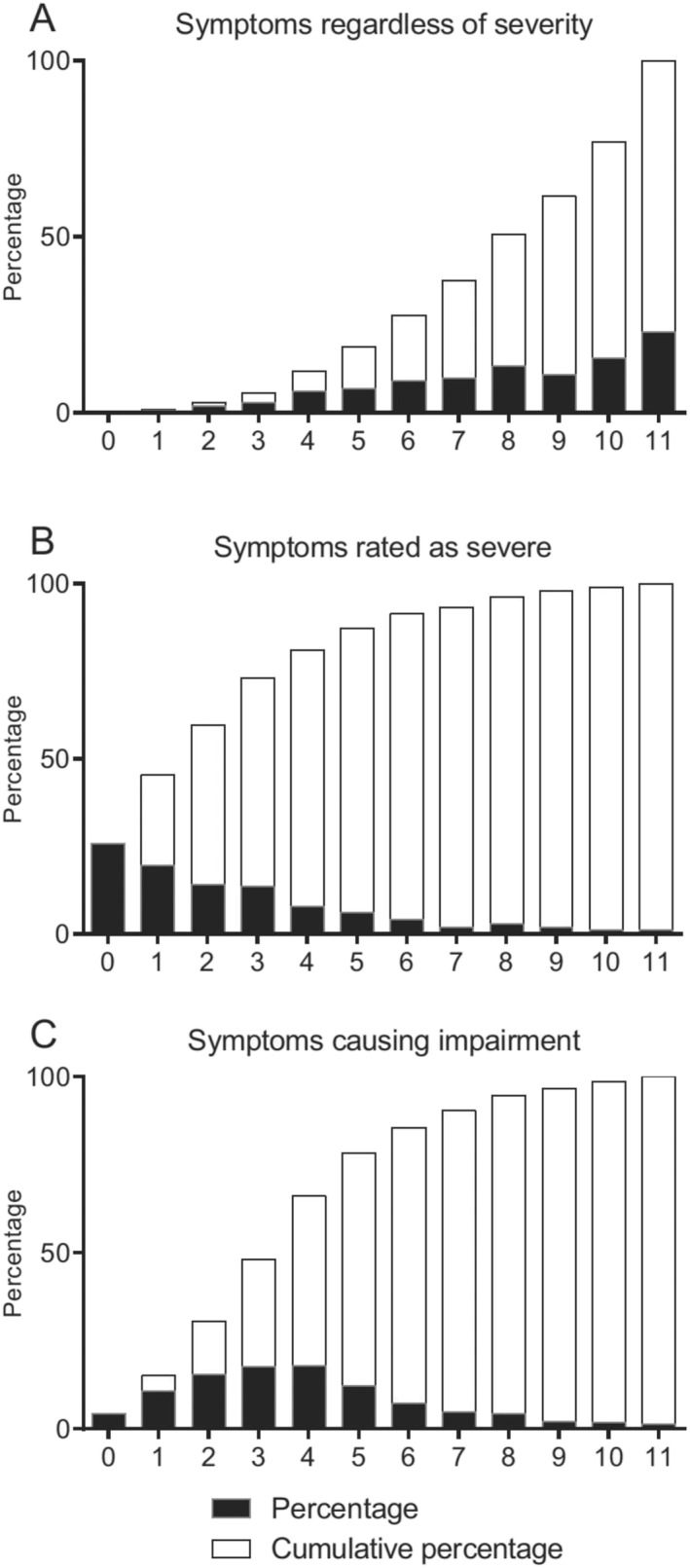
Symptoms, severe symptoms and impairing symptoms in subjects passing the screening questions. Data presented as percentage (black bars) of subjects that reported A. a certain number of symptoms, B. a certain number of symptoms to be severe, and C. a certain number of symptoms to cause impairment. Blank bars refer to cumulative percentage, that is, to the percentage reporting the indicated number of symptoms or fewer.
Association Between Symptoms
The associations between symptoms based on the 1 to 4 grading are displayed in Figure 3. Whereas two major clusters could be identified, one comprising mainly mood‐ and behavior‐related symptoms and one comprising mainly somatic symptoms, the NbClust‐based analysis suggested the existence of three clusters in the sense that headache and joint or muscle pain appeared to be separated from other somatic symptoms.
FIGURE 3.
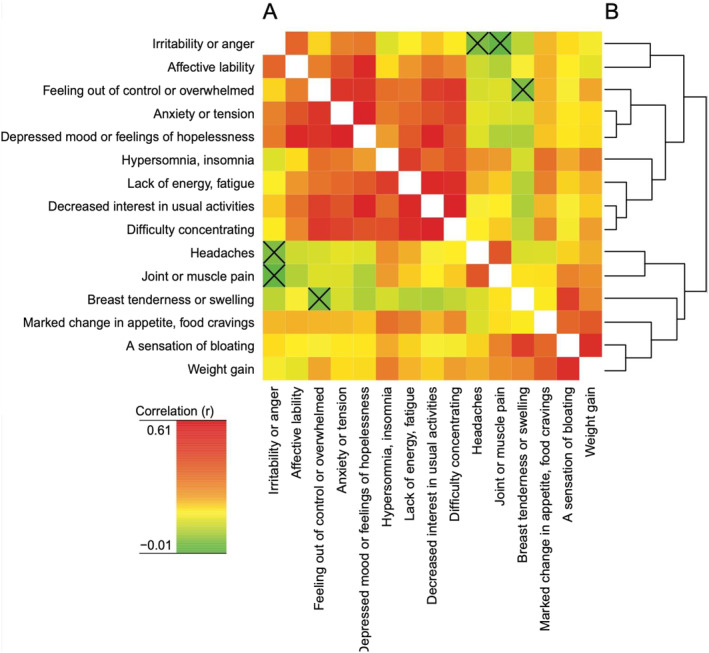
Heat maps and clustering of symptoms. A. Heat map displaying the relative strength of pair‐wise correlations with respect to symptom severity. Uncorrelated (p > 0.05) pairs of symptoms are indicated by crossed squares. B. Hierarchical clustering of symptoms based on correlations between pairs of symptoms.
Hierarchical clustering was undertaken also for the dichotomous variable “presence of a symptom”, with 1 (not present) defined as “no” and 2 (mild), 3 (moderate) and 4 (severe) defined as “yes” (Supplement Figure 1). Likewise, a corresponding analysis was performed for the dichotomous variable “impairment” (yes or no) (Supplement Figure 2). Whereas the cluster pattern was essentially the same for the two dichotomous analyses as for the one presented in Figure 3, p‐values were generally higher, rendering many associations between the somatic cluster and the mood cluster, and within the somatic cluster, non‐significant. In contrast, most associations within the mood cluster remained significant also for dichotomous analyses. The cophenetic correlation coefficient was around 0.8 for all hierarchical cluster analyses.
Number of Patients With Impairing Somatic and/or Mood Symptoms
Whereas 733 (93%) of women passing the screening questions reported at least one mood symptom to cause impairment, 325 (41%) named at least one somatic symptom to be impairing. Of those reporting impairment from a somatic symptom, the vast majority (99%) also reported impairment from a mood symptom.
Conversely, among those reporting impairment from a mood symptom, 41% also named at least one somatic symptom to be impairing. The most common causes for impairment in subjects not reporting impairment from any mood symptom were joint or muscle pain (1.3% of the women passing the screening questions and 0.1% of the total sample) and headache (1.4% and 0.1%, respectively).
There was no significant difference between those reporting that they had medicated for PMDD (with no further specification of medication) during the past year and those who had not with respect to percentage stating impairment with respect to somatic symptoms (medicated: 43%, non‐medicated: 41%, p = 0.4). Somewhat fewer in the medication group (88%) than in the non‐medicated group (95%) (p < 0.05) reported impairment from mood symptoms.
Discussion
Largely in line with previous studies (4), 9% of the women claimed to be suffering from premenstrual complaints of sufficient severity to cause reduced functioning. The main finding of our study is the dominating role played by mood symptoms in general, and irritability in particular, as the major reasons for self‐reported impairment within this group. In contrast, somatic symptoms such as bloating and breast tenderness, though being experienced by many, were regarded as causing impairment by merely a small minority (Table 1).
Our finding that somatic symptoms are perceived as less significant than mood symptoms is partly at odds with other reports (using retrospective or 1–2 months of prospective rating) suggesting somatic premenstrual symptoms to be as important as mood symptoms in population‐based cohorts (14, 15). One reason for this discrepancy may be that our analysis is based on a subset of women who initially had stated that they suffer from impact‐causing premenstrual complaints; thus, somatic symptoms may be as common as mood symptoms in the general population but regarded, in comparison, as less impairing in the most severely affected group. Also, while we specifically asked the participants to address the possible impact of each symptom, most previous studies have assessed the significance of individual symptoms on the basis of self‐rated severity rather than impact (14, 16). That self‐rated severity is not equivalent to impact is however demonstrated by our finding that only 17% of those reporting a severity of 3 or 4 (on a 1–4 scale) for breast tenderness found the symptom to exert an impact (the corresponding number for irritability being 90%).
Some women did report somatic symptoms to cause reduced functioning but most of these also reported impairing symptoms from the mood cluster. Our data hence argue against the existence of a large subgroup of women experiencing impairing somatic symptoms but no impairing mood symptoms. On the other hand, the possibility that many women not passing the screening questions would have reported breast tenderness or bloating as sole symptom, though not feeling impaired by its presence, cannot be excluded.
The possible association of somatic and mood symptoms was assessed by means of a cluster analysis suggesting mood symptoms to be separated from somatic symptoms (Figure 3 and Supplement). These findings are largely in line with earlier studies, regardless of whether these have been based on samples recruited from the general population without any pre‐selection of those with significant symptoms (17, 18) or from relatively small groups of PMS or PMDD patients (19, 20). While we hence identified clear‐cut symptom profiles, the correlation analysis however revealed that women rating high severity of mood symptoms tend to report high severity also of somatic symptoms; thus, correlations were observed not only within but also between clusters. It should however be noted that, for example, headache correlated neither with irritability nor bloating, and that joint and muscle pain correlated neither with irritability nor anxiety/tension; in this vein, the NbClust analysis suggested these symptoms to form a cluster of their own. We conclude that regarding these symptoms as parts of the same syndrome as the mood symptoms, and naming them as symptoms in the DSM criteria, may be questioned. Thus, while omitting headache from the symptoms listed in the DSM‐V criteria of PMDD hence seems justified, the decision to include joint and muscle pain may be questioned (6).
Whereas the distinct symptom clustering suggests that PMDD should not be regarded as one syndrome, the observation that the two major clusters nevertheless did correlate could speak in favor of the existence of a common pathophysiological factor enhancing the propensity for both mood symptoms and somatic complaints. Two aspects however render it difficult to interpret these associations. On the one hand, it cannot be excluded that the apparent association between severity of somatic and mood symptoms partly be the result of personality‐related differences in proneness to use the extremes of the rating scale (21, 22), hence yielding artificially high associations, and it is also possible that mood symptoms may render somatic complaints less tolerable than they would otherwise have been. Supporting this possibility, the number of significant associations between symptoms within the somatic and mood clusters, respectively, or within the somatic cluster, was lower when the assessment of associations was based on a dichotomous variable, such as presence (yes/no) or impact (yes/no) of a certain symptom (see Supplement). On the other hand, the fact that we only included women reporting impairing premenstrual complaints may have led to a non‐representative accumulation of subjects experiencing impairing symptoms of one type or another, hence yielding negative correlations for non‐related symptoms and making associations between partly related symptoms appear weaker than they actually are. To address this issue, symptom assessment in the entire cohort, including also those not confirming the presence of impairing premenstrual complaints, would have been required.
Given the clear‐cut symptom clustering, one may suggest that future search for biological markers and new treatments would be facilitated by regarding, for example, premenstrual irritability, on the one hand, and premenstrual breast tenderness, on the other, as different conditions. Of note is that intermittent administration of selective serotonin reuptake inhibitors (SSRIs), generally regarded as first line of treatment for PMDD, exerts an impressive effect on mood and behavioral symptoms (23, 24) but is less effective in reducing somatic symptoms (23, 24, 25).
Whereas the PMDD criteria are not met unless 5 symptoms are present, it may be argued that individuals with fewer complaints should qualify for a diagnosis in case these are severe enough to cause impairment. In this vein, one impairment‐causing symptom is sufficient for the diagnosis of premenstrual syndrome as defined by ACOG (5). However, in line with previous reports (15), we note that a vast majority (>88%) of those responding affirmatively to the screening questions do confirm presence of at least 5 different symptoms. On the other hand, those reporting each of 5 or more symptoms to cause impairment were much fewer (<35%). To what extent the DSM criteria should be interpreted as requiring each of five symptoms to be of sufficient severity to cause impairment, or if it is sufficient that one of them (or all when combined) cause impairment, is unclear.
It should be noted that our questionnaire is based on the DSM‐IV criteria of PMDD, which, like the ACOG criteria for PMS, require interference with work, usual activities, or relationship with others. However, in DSM‐V this has been rephrased so that, if there is no interference with daily activities, significant distress would qualify for a diagnosis (6).
Of all symptoms reported to cause impairment, irritability and affect lability (mood swings) were the most common, and considerably more common than, for example, depressed mood or tension. This observation, which is well in line with previous reports also suggesting irritability and/or mood swings to be more common than depressed mood (4, 15, 26, 27), supports the notion that PMDD should not be regarded as a subtype of depression (28), and may, as elaborated elsewhere (29), explain why SSRIs exert symptom reduction with a much shorter onset in PMDD than when used for depression or anxiety disorders. Heightened irritability being the hallmark of PMDD and severe PMS also makes sense from an evolutionary point of view if one regards these conditions as a reminiscence of the oestrous cycle‐related changes in behavior (with the purpose of facilitating reproduction) seen in lower species (30, 31).
According to the DSM criteria of PMDD, the cyclicity of symptoms must be supported by two subsequent cycles of daily prospective symptom rating. It should hence be underlined that this study only provided a provisional diagnosis of PMDD or severe PMS, that is, one based on retrospective account, and that the results should be interpreted accordingly. However, since an important reason for requiring prospective symptom rating is that many women believing they suffer from PMDD or PMS in fact display symptoms also in the follicular phase, we included a screening question asking the women to consider if they were convinced of being entirely symptom‐free in at least parts of the postmenstrual phase; this maneuver led to the exclusion of 30% of those having responded affirmatively to the first two gateway questions. While this is not a validated technique to render retrospective symptom assessment of PMDD or severe PMS more reliable, we do believe the large percentage responding “no” to this question suggests that it may, to some extent, have served its purpose. Moreover, the outcome of this study with respect to the commonness of different symptoms being well in line with previous reports using prospective rating (27) does suggest that the studied population was largely the one aimed for. Whereas the feasibility of web questionnaires for the screening of women with premenstrual symptoms gains support from previous work (32), the fact that responses were not obtained by a face‐to‐face interview is clearly another important limitation.
It should be underlined that the LifeGene cohort cannot be regarded as a representative sample of the general population. In addition to being randomly sampled from the population, there are hence other routes of becoming part of this study including self‐recruitment (10). Also, given that the participants are requested to take part in many activities within the framework of this longitudinal study, including repeated biosampling, it is not surprising that only 20% of those invited to be index persons have accepted to participate. A population not being representative however does not mean that information on aspects such as risk factors, or (as in the present study) the association between different symptoms, are not generalizable; accordingly, the aspect of response rate has been de‐emphasized in many recent prospective projects (33). Also, it may be regarded as an important advantage that LifeGene is not focused on the issue of premenstrual complaints, which might have made women with a particular interest in this subject more likely than others to participate, hence introducing a bias that might be difficult to avoid, for example, in studies requiring prospective symptom rating. In the present study, questions regarding PMDD were instead imbedded within a large survey covering un‐related health issues.
Because of the possible lack of representativity of the present population, and the lack of prospective symptom rating, it however deserves to be underlined that the purpose of this study was not to assess the prevalence of PMDD, but to explore the relative self‐rated impact of the different symptoms listed in the DSM criteria – as well as their interrelationship – in a large group of women for which a web‐based questionnaire was used to make a provisional PMDD/PMS diagnosis. We see no obvious reasons why this population should differ markedly in these regards from more properly diagnosed women with PMDD or severe PMDS; yet the results should be interpreted with this caveat in mind.
In summary, premenstrual mood and behavioral symptoms, and in particular irritability, were far more important as reasons for impairment than somatic symptoms in the studied cohort. Whereas it cannot be excluded that there is a common factor enhancing the susceptibility for both mood and somatic symptoms, the clear‐cut symptom clustering justifies the question if all premenstrual complaints should indeed be regarded as parts of one syndrome.
Supporting information
Supporting Information S1
This work was supported by the Swedish Science Council (grant number 2015‐02515), Söderberg's Foundation (MT30/09), Hållsten's Foundation, the Brain Foundation (FO2011‐0293) and the Sahlgrenska University Hospital.
None of the authors report any competing interests.
REFERENCES
- 1. O'Brien PMS. History of premenstrual disorders. In: O'Brien PMS, Schmidt PJ, editors. The premenstrual syndromes: PMS and PMDD. London: Informa Healthcare; 2007. p. 1–8. [Google Scholar]
- 2. Greene R, Dalton K. The premenstrual syndrome. Br Med J. 1953;1(4818):1007–1014. 10.1136/bmj.1.4818.1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Freeman E, Rickels K, Sondheimer SJ. Polansky: ineffectiveness of progesterone suppository treatment for premenstrual syndrome. JAMA. 1990;264(3):349–353. 10.1001/jama.1990.03450030073035 [DOI] [PubMed] [Google Scholar]
- 4. Yonkers KA, O'Brien PM, Eriksson E. Premenstrual syndrome. Lancet. 2008;371(9619):1200–1210. 10.1016/s0140-6736(08)60527-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. ACOG . ACOG practice bulletin: premenstrual syndrome. Int J Gynecol Obstet. 2001;73:183–191. [Google Scholar]
- 6. Epperson CN, Steiner M, Hartlage SA, Eriksson E, Schmidt PJ, Jones I, et al. Premenstrual dysphoric disorder: evidence for a new category for DSM‐5. Am J Psychiatry. 2012;169(5):465–475. 10.1176/appi.ajp.2012.11081302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Halbreich U, O'Brien PM, Eriksson E, et al. Are there differential symptom profiles that improve in response to different pharmacological treatments of premenstrual syndrome/premenstrual dysphoric disorder? CNS Drugs. 2006;20:523547. [DOI] [PubMed] [Google Scholar]
- 8. O'Brien PM, Backstrom T, Brown C, Dennerstein L, Endicott J, Epperson CN, et al. Towards a consensus on diagnostic criteria, measurement and trial design of the premenstrual disorders: the ISPMD Montreal consensus. Arch Womens Ment Health. 2011;14(1):13–21. 10.1007/s00737-010-0201-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hammarback S, Backstrom T. Induced anovulation as treatment of premenstrual tension syndrome. A double‐blind cross‐over study with GnRH‐agonist versus placebo. Acta Obstet Gynecol Scand. 1988;67(2):159–166. 10.3109/00016348809004191 [DOI] [PubMed] [Google Scholar]
- 10. Almqvist C, Adami HO, Franks PW, Groop L, Ingelsson E, Kere J, et al. LifeGene ‐ a large prospective population‐based study of global relevance. Eur J Epidemiol. 2011;26(1):67–77. 10.1007/s10654-010-9521-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Steiner M, Macdougall M, Brown E. The premenstrual symptoms screening tool (PSST) for clinicians. Arch Women´s Mental Health. 2003;6(3):203–209. 10.1007/s00737-003-0018-4 [DOI] [PubMed] [Google Scholar]
- 12. Sokal RR, Rohlf FJ. The comparison of dendograms by objective methods. Taxon XI; 1962. p. 33–40. [Google Scholar]
- 13. Charrad M, Ghazzali N, Boiteau V, et al. An R package for determining the relevant number of clusters in a data set. J Stat Softw. 2014;61:1–36. [Google Scholar]
- 14. Dennerstein L, Lehert P, Heinemann K. Global study of women's experiences of premenstrual symptoms and their effects on daily life. Menopause Int. 2011;17(3):8895–8895. 10.1258/mi.2011.011027 [DOI] [PubMed] [Google Scholar]
- 15. Hartlage SA, Freels S, Gotman, et al. Criteria for premenstrual dysphoric disorder: secondary analyses of relevant data sets. Arch Gen Psychiatry. 2012;69(3):300–305. 10.1001/archgenpsychiatry.2011.1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bloch M, Schmidt PJ, Rubinow DR. Premenstrual syndrome: evidence for symptom stability across cycles. Am J Psychiatry. 1997;154(12):1741–1746. 10.1176/ajp.154.12.1741 [DOI] [PubMed] [Google Scholar]
- 17. Teng CT, Filho AH, Artes R, Gorenstein C, Andrade LH, Wang YP. Premenstrual dysphoric symptoms amongst Brazilian college students: factor structure and methodological appraisal. Eur Arch Psychiatr Clin Neurosci. 2005;255(1):51–56. 10.1007/s00406-004-0535-9 [DOI] [PubMed] [Google Scholar]
- 18. Wang YP, Teng CT, Vieira Filho AH, Gorenstein C, Andrade L. Dimensionality of the premenstrual syndrome: confirmatory factor analysis of premenstrual dysphoric symptoms among college students. Braz J Med Biol Res. 2007;40(5):639–647. 10.1590/s0100-879x2007000500006 [DOI] [PubMed] [Google Scholar]
- 19. Freeman EW, DeRubeis RJ, Rickels K. Reliability and validity of a daily diary for premenstrual syndrome. Psychiatry Res. 1996;65(2):97–106. 10.1016/s0165-1781(96)02929-0 [DOI] [PubMed] [Google Scholar]
- 20. Marr J, Niknian M, Shulman LP, Lynen R. Premenstrual dysphoric disorder symptom cluster improvement by cycle with the combined oral contraceptive ethinylestradiol 20 mcg plus drospirenone 3 mg administered in a 24/4 regimen. Contraception. 2011;84(1):81–86. 10.1016/j.contraception.2010.10.010 [DOI] [PubMed] [Google Scholar]
- 21. Naemi BD, Beal DJ, Payne SC. Personality predictors of extreme response style. J Pers. 2009;77(1):261–286. 10.1111/j.1467-6494.2008.00545.x [DOI] [PubMed] [Google Scholar]
- 22. Prior KN, Bond MJ. Patterns of ‘abnormal’ illness behavior among healthy individuals. Am J Health Behav. 2017;41(2):139–146. 10.5993/ajhb.41.2.4 [DOI] [PubMed] [Google Scholar]
- 23. Eriksson E, Ekman A, Sinclair S, Sörvik K, Ysander C, Mattson UB, et al. Escitalopram administered in the luteal phase exerts a marked and dose‐dependent effect in premenstrual dysphoric disorder. J Clin Psychopharmacol. 2008;28(2):195–202. 10.1097/jcp.0b013e3181678a28 [DOI] [PubMed] [Google Scholar]
- 24. Landen M, Nissbrandt H, Allgulander C, Sörvik K, Ysander C, Eriksson E. Placebo‐controlled trial comparing intermittent and continuous paroxetine in premenstrual dysphoric disorder. Neuropsychopharmacology. 2007;32(1):153–161. 10.1038/sj.npp.1301216 [DOI] [PubMed] [Google Scholar]
- 25. Sundblad C, Hedberg MA, Eriksson E. Clomipramine administered during the luteal phase reduces the symptoms of premenstrual syndrome: a placebo‐controlled trial. Neuropsychopharmacology. 1992;9(2):133–145. 10.1038/npp.1993.52 [DOI] [PubMed] [Google Scholar]
- 26. Angst J, Sellaro R, Merikangas KR, Endicott J. The epidemiology of perimenstrual psychological symptoms. Acta Psychiatr Scand. 2001;104(2):110–116. 10.1034/j.1600-0447.2001.00412.x [DOI] [PubMed] [Google Scholar]
- 27. Pearlstein T, Yonkers KA, Fayyad R, Gillespie JA. Pretreatment pattern of symptom expression in premenstrual dysphoric disorder. J Affect Disord. 2005;85(3):275–282. 10.1016/j.jad.2004.10.004 [DOI] [PubMed] [Google Scholar]
- 28. Endicott J, Amsterdam J, Eriksson E, Frank E, Freeman E, Hirschfeld R, et al. Is premenstrual dysphoric disorder a distinct clinical entity? J Womens Health Gend Based Med. 1999;8(5):663–679. 10.1089/jwh.1.1999.8.663 [DOI] [PubMed] [Google Scholar]
- 29. Landen M, Erlandsson H, Bengtsson F, Andersch B, Eriksson E. Short onset of action of a serotonin reuptake inhibitor when used to reduce premenstrual irritability. Neuropsychopharmacology. 2009;34(3):585–592. 10.1038/npp.2008.86 [DOI] [PubMed] [Google Scholar]
- 30. Ho H.‐P, Olsson M, Westberg L, et al. The serotonin reuptake inhibitor fluoxetine reduces sex steroid‐related aggression in female rats: an animal model of premenstrual irritability? Neuropsychopharmacology. 2001;24(5):502–510. 10.1016/s0893-133x(00)00219-0 [DOI] [PubMed] [Google Scholar]
- 31. Gillings MR. Were there evolutionary advantages to premenstrual syndrome? Evol Appl. 2014;7(8):897–904. 10.1111/eva.12190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wallenstein GV, Blaisdell‐Gross B, Gajria K, Guo A, Hagan M, Kornstein SG, et al. Development and validation of the Premenstrual Symptoms Impact Survey (PMSIS): a disease‐specific quality of life assessment tool. J Womens Health. 2008;17(3):439–450. 10.1089/jwh.2007.0377 [DOI] [PubMed] [Google Scholar]
- 33. Manolio TA, Weis BK, Cowie CC, Hoover RN, Hudson K, Kramer BS, et al. New models for large prospective studies: is there a better way? Am J Epidemiol. 2012;175(9):859–866. 10.1093/aje/kwr453 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1


