Abstract
A reference strain (Movár 33/63) of bovine herpesvirus type 4 (BHV-4) was inoculated into 14 different human cell lines and five primary cell cultures representing various human tissues. BHV-4 replicated in two embryonic lung cell lines, MRC-5 and Wistar-38, and in a giant-cell glioblastoma cell culture. Cytopathic effect and intranuclear inclusion bodies were observed in these cells. PCR detected a 10,000-times-higher level of BHV-4 DNA. Titration of the supernatant indicated a 100-fold increase of infectious particles. Since this is the first bovine (human herpesvirus 8 and Epstein-Barr virus related) herpesvirus which replicates on human cells in vitro, the danger of possible human BHV-4 infection should not be ignored.
In contrast to other beta- and gammaherpesviruses, bovine herpesvirus type 4 (BHV-4) replicates in a wide variety of cell cultures, i.e., established primary cell cultures of cattle, sheep, goats, dogs, cats, rabbits, pigs, and primary chicken kidneys (3). Various cell lines of these species are susceptible to BHV-4. The American reference strain (DN-599) was found to grow to high titers in mink lung and ferret kidney cells (14). Crandell feline kidney (CRFK) cells also support replication of BHV-4 (8). Several species are susceptible to BHV-4 infection. The virus was previously isolated from cattle (3), American bison (Bison bison) (18). African buffalo (Syncerus caffer) (15), goats (10), and nonruminant species such as lions (2a) and a cat suffering from urolithiasis (6). Simian herpesvirus aotus type 2, isolated from the kidney of an apparently normal owl monkey (Aotus trivirgatus), was proven to be a BHV-4 strain (4). This monkey isolate replicated in four monkey cell lines (owl monkey kidney [OMK]; squirrel monkey kidney, intestines, and lung [SMC]; cebus monkey kidney [CMK]; and African green monkey kidney [Vero]), rabbit kidney (RK) cells, and goat cells (GC), where cytopathic effect (CPE) and inclusion bodies were observed. No CPE was seen in a primary culture of whole human embryo cells (2).
To examine the susceptibility of various human cell lines to BHV-4, 105 cells from each type were added to wells of 24-well tissue culture plates (Greiner, Frickenhausen, Germany). The cells were maintained in minimal essential medium (Serva, Heidelberg, Germany) containing NaHCO3 supplemented with 10% fetal calf serum, 0.34 g of l-glutamine (Sigma, St. Louis, Mo.) per liter, 500,000 IU of penicillin per liter, and 0.5 g of streptomycin sulfate per liter. Two milliliters of cell culture fluid was added to each well, and the cells were inoculated with 50 μl of tissue culture fluid containing 105 PFU of the European reference strain of BHV-4 (Movár 33/63).
One hundred microliters from one well without cells was immediately titrated. The rest of the liquid was left to serve as the negative control for PCR studies, to determine the number of the inoculated virus particles.
The plates were incubated in a humidified 5% CO2 atmosphere at 37°C for a week, and then the cell-free supernatant was examined by titration and the whole culture with cells was assayed by PCR. The cell cultures were monitored daily for CPE.
For the 14 human cell lines, the five primary cell cultures, and the positive control Madin-Darby bovine kidney (MDBK) cell line, see the data in Table 1.
TABLE 1.
Data on human cell lines and primary cultures included in the experiments
| Cell line | ATCC no. or reference | Cell type | BHV-4 growth at PID 7
|
||
|---|---|---|---|---|---|
| Increase of viral DNA compared to control (PCR) | Titer | Presence of inclusions | |||
| MRC-5 | CCL 171 | Embryonic diploid lungs | 104 | 102 | + |
| WI-38 | CCL 75 | Embryonic lung | 104 | 104 | + |
| U937 | CRL 1593 | Histiocytic lymphoma | |||
| THP-1 | TIB 202 | Monocytic leukemia | |||
| CEM-CM3 | TIB 195 | Lymphoblastic leukemia | |||
| UAC | 17 | Amniotic cell | |||
| Jurkat | 21 | T-cell leukemia | |||
| 293 | CRL 1573 | Embryonic kidney | |||
| CACO-2 | HTB 37 | Colonic adenocarcinoma | |||
| HeLa | CCL 2 | Cervical epithelioid carcinoma | |||
| HEp-2 | CCL 23 | Epidermoid carcinoma, larynx | |||
| Namalwa | CRL 1432 | Burkitt’s lymphoma, lymphoblastoid B cell | |||
| SK-N-SH | HTB 11 | Neuroblastoma | |||
| McCoya | CRL 1696 | Fibroblast | |||
| Brain endotheliumb | |||||
| Glioblastoma giant-cellb | 104 | 104 | + | ||
| Glioblastoma multiformeb3 | |||||
| MDBK | CCL 22 | Bovine kidney | 104 | 105 | + |
Mixed McCoy A (human) and B (mouse) cells.
Primary cell cultures.
To titrate the supernatant of the virus-infected cells, after the 7-day-long incubation period 10-fold dilutions of the supernatant were inoculated into dividing MDBK cells and were incubated as described above.
Replication of BHV-4 in human cells was examined by a thymidine kinase nested PCR (5). Limiting (10-fold) dilutions were examined in the PCR to detect the highest dilution which contained at least 1 to 10 particles. The dilutions yielding negative results were retested three times in order to confirm the absence of viral DNA. The plates were frozen and thawed three times, and the DNA was extracted by the phenol-chloroform method (16) with proteinase K (Sigma) digestion (100 μl of the suspension was incubated with 40 μg of proteinase K enzyme at 55°C for 1 h). Supernatants of all types of cell lines, primary cell cultures, and the cell-free control were examined by PCR.
Inoculation of cells was performed twice with all cell lines and cultures, even if the virus did not show any signs of replication, to verify that the cells were not permissive for BHV-4 infection. Where CPE was seen, the inoculation, titration of the supernatant, and PCR studies were repeated three times to confirm the positive result and prove its reproducibility.
CPE was detected on three cell lines, i.e., WI-38, MRC-5, and giant-cell glioblastoma cells (12). CPE appeared on postinoculation day (PID) 3, when scattered round, enlarged cells were observed. By PID 5, the round cells spread to all parts of the cell sheet, and at PID 7, the cells were floating in the supernatant and were not attached to the bottom of the flask.
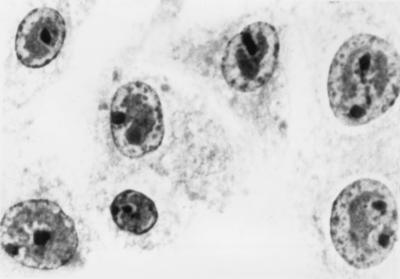
BHV-4-infected WI-38, MRC-5, and giant-cell glioblastoma cells, where CPE was observed, were stained with hematoxylin-eosin to detect BHV-4-specific intranuclear inclusion bodies (1) (Fig. 1).
FIG. 1.
Inclusion bodies of BHV-4 in nuclei of human embryonic lung cells (WI-38 cell line). Magnification, ca. ×1,000.
Titration of the supernatant of these cells showed a 100-fold increase in the number of infectious particles, since the titer rose from 102 to 104 in MRC-5, WI-38, and giant-cell glioblastoma cell lines and to 105 in MDBK cell culture.
The PCR revealed a 104-fold increase in the number of viral genomes after a weeklong incubation (1 μl of the cell-free control fluid gave a positive result in the PCR assay, while 10−4 μl of the WI-38, MRC-5, and giant-cell glioblastoma lines gave a positive result).
The data on BHV-4 replication in vitro on human cell lines may be interesting since only some primate herpesviruses, e.g., herpesvirus simiae (B virus), herpesvirus saimiri, and herpesvirus ateles, are known to replicate in human cells (HEp-2 and human foreskin fibroblasts) in vitro (7, 11). Replication of BHV-4 in lung cells is not unexpected, since the lungs play a key role in BHV-4 infections, the virus replicates in vivo and in vitro in tissues and cell lines of the respiratory tract (bovine lung and fetal calf turbinate [10, 13]), and the virus has often been isolated from animals with respiratory illnesses (3, 9).
Heparin-like moieties of the cell surface serve as the initial receptors for BHV-4 (19). Such receptors must be present on human embryonic lung cells, but the receptor may be missing or changed on cell lines originating from adult individuals. The giant-cell glioblastoma culture consisted of mostly undifferentiated cells.
Another factor which is in accordance with virus replication on embryonic tissues is the dependence of BHV-4 replication on S phase of the cell cycle (20). BHV-4 needs dividing cells for effective DNA replication, and all three BHV-4-permissive cell lines were fast growing. Even though this paper contains in vitro data on BHV-4 replication, the danger of possible human infection (especially by consuming raw bovine milk or beef) cannot be ignored.
Since BHV-4 is in the same herpesvirus subgroup as Kaposi’s sarcoma herpesvirus (human herpesvirus 8), identification of cells permissive and nonpermissive for BHV-4 infection may provide additional insight into the cell surface receptors utilized by this related human herpesvirus.
Acknowledgments
This work was supported by the Hungarian National Research Grant OTKA T 21183.
The cells were kindly provided by János Minárovits and György Berencsi, from the Johan Béla Institution for Public Health, Budapest; József Ongrádi, from the Virology Department of Semmelweis Medical University, Budapest; Ivett Mándy, from the Virology Department, and Judit Deák, from the Department of Clinical Microbiology, of Szentgyörgyi Medical University, Szeged; Zoltán Nagy, from The National Stroke Centre, Budapest; and Ilona Katona, from the National Institute of Neurosurgery, Budapest, Hungary. I am grateful to technician Szilvia Pap for her skilled and reliable assistance throughout the experiment.
REFERENCES
- 1.Augsburger H R, Metzler A E. In vitro growth characteristics of bovine herpesvirus 4 (BHV-4) as revealed by indirect immunofluorescence assay with monoclonal antibodies and polyvalent antisera. Arch Virol. 1989;104:309–321. doi: 10.1007/BF01315552. [DOI] [PubMed] [Google Scholar]
- 2.Barahona H H, Melendez L V, King N W, Daniel M D, Fraser C E O, Preville A C. Herpesvirus aotus type 2: a new viral agent from owl monkeys (Aotus trivirgatus) J Infect Dis. 1973;127:171–178. doi: 10.1093/infdis/127.2.171. [DOI] [PubMed] [Google Scholar]
- 2a.Bartha, A. Personal communication.
- 3.Bartha A, Juhász M, Liebermann H. Isolation of a bovine herpesvirus from calves with respiratory disease and keratoconjunctivitis. Acta Vet Acad Sci Hung. 1966;16:357–358. [PubMed] [Google Scholar]
- 4.Bublot M, Dubuisson J, Van Bressem M F, Danyi S, Pastoret P P, Thiry E. Antigenic and genomic identity between simian herpesvirus aotus type 2 and bovine herpesvirus type 4. J Gen Virol. 1991;72:715–719. doi: 10.1099/0022-1317-72-3-715. [DOI] [PubMed] [Google Scholar]
- 5.Egyed L, Ballagi-Pordány A, Bartha A, Belák S. Studies on the in vivo distribution of bovine herpesvirus type 4 in the natural host. J Clin Microbiol. 1996;34:1091–1095. doi: 10.1128/jcm.34.5.1091-1095.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fabricant C G, Gillespie J H, Krook L. Intracellular and extracellular mineral crystal formation induced by viral infection of cell cultures. Infect Immun. 1971;3:416–419. doi: 10.1128/iai.3.3.416-419.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hilliard J K, Eberle R, Lipper S L, Munoz R M, Weiss S A. Herpesvirus simiae (B virus): replication of the virus and identification of viral polypeptides in infected cells. Arch Virol. 1987;93:185–198. doi: 10.1007/BF01310973. [DOI] [PubMed] [Google Scholar]
- 8.Kruger J M, Osborne C A, Goyal S M, Pomeroy K A, O’Brien T D. Clinicopathologic and pathologic findings of herpesvirus-induced urinary tract infection in conventionally reared cats. Am J Vet Res. 1990;51:1649–1655. [PubMed] [Google Scholar]
- 9.Mohanty S B, Hammond R C, Lillie M G. A new bovine herpesvirus and its effect on experimentally infected calves. Arch Gesamte Virusforsch. 1971;33:394–395. doi: 10.1007/BF01254696. [DOI] [PubMed] [Google Scholar]
- 10.Moreno-Lopez J, Goltz M, Rehbinder C, Valsala K V, Ludwig H. A bovine herpesvirus (BHV-4) as passenger virus in ethmoidal tumours in Indian cattle. J Vet Med Ser B. 1989;36:481–486. doi: 10.1111/j.1439-0450.1989.tb00633.x. [DOI] [PubMed] [Google Scholar]
- 11.Mou S W, Hilliard J K, Song C H, Eberle E. Comparison of the primate alphaherpesviruses. I. Characterization of two herpesviruses from spider monkeys and squirrel monkeys and viral polypeptides synthesized in infected cells. Arch Virol. 1986;91:117–133. doi: 10.1007/BF01316733. [DOI] [PubMed] [Google Scholar]
- 12.Müller W, Slowik F, Firsching R, Afra D, Sanker P. Contribution to the problem of giant cell astrocytomas. Neurosurg Rev. 1987;10:213–219. doi: 10.1007/BF01782050. [DOI] [PubMed] [Google Scholar]
- 13.Osorio F A, Rock D L, Reed D E. Studies on the pathogenesis of a bovine cytomegalo-like virus in an experimental host. J Gen Virol. 1985;66:1941–1951. doi: 10.1099/0022-1317-66-9-1941. [DOI] [PubMed] [Google Scholar]
- 14.Peterson R B, Goyal S M. Propagation and quantitation of animal herpesviruses in eight cell culture systems. Comp Immunol Microbiol Infect Dis. 1988;11:93–98. doi: 10.1016/0147-9571(88)90023-9. [DOI] [PubMed] [Google Scholar]
- 15.Rossiter P B, Gumm I D, Stagg D A, Conrad P A, Mukolwe S, Davies F G, White H. Isolation of bovine herpesvirus-3 from African buffaloes (Syncerus caffer) Res Vet Sci. 1984;46:337–343. [PubMed] [Google Scholar]
- 16.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 17.Sorrentino V, Di Francesco P, Soria M, Rossi G B. A human amniotic cell line yielding high titres of human fibroblast interferon. J Gen Virol. 1982;63:509–511. doi: 10.1099/0022-1317-63-2-509. [DOI] [PubMed] [Google Scholar]
- 18.Todd W J, Storz J. Morphogenesis of a cytomegalovirus from an American bison affected with malignant catarrhal fever. J Gen Virol. 1983;64:1025–1030. doi: 10.1099/0022-1317-64-5-1025. [DOI] [PubMed] [Google Scholar]
- 19.Vanderplasschen A, Bublot M, Dubuisson J, Pastoret P P, Thiry E. Attachment of the gammaherpesvirus bovine herpesvirus 4 is mediated by the interaction of gp8 glycoprotein with heparinlike moieties on the cell surface. Virology. 1993;196:232–240. doi: 10.1006/viro.1993.1471. [DOI] [PubMed] [Google Scholar]
- 20.Vanderplasschen A, Goltz M, Lyaku J, Benarafa C, Buhk H J, Thiry E, Pastoret P P. The replication in vitro of the gammaherpesvirus bovine herpesvirus 4 is restricted by its DNA synthesis dependence on the S phase of the cell cycle. Virology. 1995;213:328–340. doi: 10.1006/viro.1995.0006. [DOI] [PubMed] [Google Scholar]
- 21.Weiss A L, Wiskocil R L, Stobo J D. The role of T3 surface molecules in the activation of human T cells: a two-stimulus requirement for IL 2 production reflects events occurring at a pre-translational level. J Immunol. 1984;133:123–128. [PubMed] [Google Scholar]