Abstract
Background:
The objective of this study is to provide a structured protocol for the treatment of verrucous carcinoma (VC) based on size, bone invasion, recurrence and whether neck dissection is necessary or not. In addition, the study evaluates the probability of a wrong histopathological diagnosis.
Data Sources:
A search was conducted in the Cochrane Library, PubMed and Google from January 1962 to October 2022 by using MeSH terms and keywords. Studies reporting treatment modalities for VC and different histopathological diagnoses after excision of the lesion were selected except case reports and review articles.
Study Eligibility Criteria:
Thirteen articles were selected. Six hundred and thirty cases of VC were treated by surgery, surgery + neck dissection, radiotherapy, chemotherapy and combination therapy. Statistical analysis revealed surgical treatment as a preferred option. Despite being enlarged, the lymph node was negative for metastasis. So, in OVC cases neck dissection adds only unnecessary morbidity to patients.
Participants and Interventions:
Radiotherapy or chemotherapy can be used to downstage the disease. 23.3% of cases reported wrong histopathology diagnosis.
Study Appraisal and Synthesis Methods:
Patients treated for squamous cell carcinoma (SCC) will only experience unnecessary morbidity unless the correct diagnosis is made between VC and hybrid VC. Irrespective of size VC does not metastasise until there are no foci of SCC.
Conclusions:
Surgical excision of T1- and T2-sized lesions can be performed under local anaesthetic as a biopsy procedure. T3 or T4 lesion can be resected with a safe margin. If it comes as hybrid VC or VC with close margin (0.5 cm, <0.5 cm), neck dissection and further margin should be excised as a second procedure respectively.
Keywords: Hybrid verrucous carcinoma, treatment modality, verrucous carcinoma
INTRODUCTION
Oral verrucous carcinoma (OVC) is a low-grade, locally aggressive variant of squamous cell carcinoma (SCC) with a low propensity to metastasise locally or distantly. It was also known as the Ackerman tumour after its discoverer in 1948. Clinically, VC is proliferative and warty with a cauliflower-like appearance and histologically pushing epithelial pegs deep into connective tissue with an intact basement membrane. However, sometimes this membrane can also be disrupted.[1] Since then, the efficacy of various treatment options such as surgery, radiotherapy and chemotherapy have been documented, but there was no structured protocol described in the literature. Various questions are unanswered in literature, like, as VC is a low-grade variant of SCC, neck dissection and radiotherapy can or cannot be included in the line of treatment, whether VC should be treated as well- differentiated SCC and how VC can be misleading to both pathologists and surgeons. This review article proposes answers to all questions in an orderly manner.
MATERIALS AND METHODS
Protocol and registration
This systematic review and meta-analysis was registered as a protocol on the International Prospective Register of Systematic Reviews (PROSPERO) platform (CRD42022331286), and reporting was carried out following the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses [Figure 1].
Figure 1.
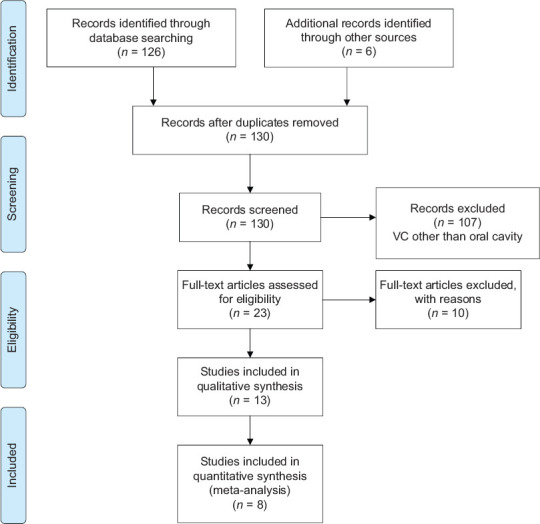
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flowchart
Focused question
This review was designed to answer the following questions:
Probability of histopathological evaluation of OVC being wrong
Protocol for treating VC
Neck dissection is necessary or not.
Search strategy
A search was conducted in PubMed, the Cochrane Library and Google Scholar from January 1962 to October 2022. We made a search engine in PubMed using the following terms (Mouth [MeSH Terms] OR ‘mouth’ [All Fields] OR ‘oral’ [All Fields]) AND ‘carcinoma,’ ‘verrucous’ (MeSH Terms) OR (‘carcinoma’ [All Fields] AND ‘verrucous’ [All Fields]) OR ‘verrucous carcinoma’ (All Fields) OR (‘verrucous’ [All Fields] AND ‘carcinoma’ [All Fields]) AND (treatment). Using keywords, additional articles were also searched.
Eligibility
Research question has been formulated by using the Population, Intervention, Control and Outcome format.
Problem – VC of oral cavity and false histopathological diagnosis
Intervention – Surgical or non-surgical
Control/non-control – Recurrence or no recurrence
Outcome – Treatment protocol by combining the results of author.
Inclusion criteria
Treatment modalities such as surgery, radiotherapy, chemotherapy and details of follow-up properly given
Details of initial and final histopathological diagnosis mentioned were included in the study.
Exclusion criteria
Case reports and review articles were excluded from the study.
Screening of studies, data extraction and selection
In the course of searching both electronically and manually for potential studies, two independent reviewers (MVS and HM) independently screened all potential studies. The title and abstract of each study have been screened by one of the authors (HM) to exclude case reports and irrelevant studies. After primary screening, two review authors (USP and VK) independently examined the full texts of potentially relevant studies, categorised them for inclusion and recorded the reasons for excluding non-eligible studies. Studies that met the inclusion criteria were further assessed by another author (SKY). Full text was studied and further selected by inclusion criteria. Author RS extracted the data as required for this systematic review and meta-analysis.
Data management
According to included studies, the following details were extracted: Treatment modalities such as surgical (excision or excision with neck dissection), chemotherapy, radiotherapy and combination therapy with type of study, author, publication year, number of patients and invasion of bone.
Data regarding discrepancies in diagnosis after histopathological examination of initial incisional biopsy specimen and final surgical treatment.
RESULTS
Electronic sources provided 126 records, and by manual searching, six were added. After removing duplicate or similar records, 130 abstracts were screened. There were 70 articles that did not meet our eligibility criteria because they were either case reports or reported verrucous lesions involving skin, larynx and other parts of the body. A total of 23 potentially full-text articles were thoroughly studied for eligibility. Out of 23 articles, 10 articles were excluded with Reason as below:
Peng et al.[2] – review article; Patel et al.[3] – some vocal cord lesions were also included; Batsakis et al.[4] – review article; Kraus et al.[5] – nothing was mentioned about size of lesion, recurrence cases were not clearly mentioned whether there were only excisions or excision with neck dissections; Oliveira et al.[6] – follow up time was not mentioned, table of clinical data was not clear; Mohammadi et al.[7] – recurrence rate data were not clear; Rath et al.[8] – clinical data were not clear; Wang et al.[9] – SEER based review article; Santosh et al. [10] – irrelevant to the present study; Thompson[11] – only histopathological features were mentioned.
Thirteen articles were selected for qualitative analysis, and out of these, 8[12,13,14,15,16,17,18,19] were included for meta-analysis to compare the efficacy of performed treatment. A total of 630 cases of VC were reported and all 630 cases were treatment by surgery. Surgery + neck dissection, radiotherapy, chemotherapy and combination therapy. The characteristics of included studies are reported in Table 1.
Table 1.
Characteristics of study and treatment respective of depth of invasion and bone involvement
| Author/type of study | Number of patients | Surgery | Neck dissection | Follow up | Recurrence | Depth of invasion |
|---|---|---|---|---|---|---|
| Goethals et al./retrospective study[1] | 55 | Diathermy=28 Diathermy and radiation=9 Excision=7 Excision +neck dissection=10 Radiation=1 | Yes (negative for metastasis) | Minimum 5 years | Surgery=1 Radiation=7 | Skin of cheek=1 Palatal bone=1 Muscle=8 Submucosa=45 |
| Medina et al./retrospective study[12] | 102 | Surgery (n=90) Radiotherapy (n=12) | Yes, (negative for metastasis) | Minimum 2 years | Surgery (n=74) Radiotherapy (n=7) | Not mentioned |
| Tomes et al./retrospective review[13] | 16 | Surgery=11 Chemotherapy=1 Radiotherapy=4 | Not mentioned | 3 weeks–8 years | Sx=3 R=2 | Bony invasion=7 |
| Vidyasagar et al.[14] | 101 | Radiation | _ | Minimum 5 years | Recurrence in 52 | Mandibular bone involvement=19.6% |
| Yoshimura et al.[15] | 15 | Surgery=3 Chemotherapy=4 Radiotherapy=1 Combination therapy=6 | Yes (two positive for metastasis in SMLN | 6 months–7 years | 5 years DFS rate for surgery=78% | Yes |
| Ogawa et al./2004/Retrospective study[16] | 12 | Chemotherapy=3 Surgery=4 Combination therapy=5 | Yes (negative for metastasis) | 5 years survival rate 91.3% | Surgery=1 Chemotherapy=1 Combined therapy=1 | Mandibular bone involvement in 3 |
| Walvekar et al./2009/retrospective[20] | 101 | Surgery | Yes (negative for metastasis) | Bone invasion=6 Muscle=2 Skin=5 Soft tissue=3 | ||
| Rekha and Angadi 2010/retrospective[21] | 133 | Surgery + neck dissection and surgery + radiotherapy | Yes (negative for metastasis) | 1–4 years | 3 | Bone involvement=33 and up to muscle layer |
| Huang et al./retrospective[17] | 39 | Surgery + neck dissection | Yes (negative for metastasis) | Minimum 3 years | 1 | One patient |
| Sadasivan et al./retrospective[22] | 15 | Surgery + neck dissection | Yes, metastasis not mentioned | 1–39 months | Not mentioned | no |
| Candau-Alvarez et al.[18] | 14 | Surgery + neck dissection | Yes (negative for metastasis) | 6–53 months | 1 | Not mentioned |
| Franklyn et al./retrospective[19] | 30 | Surgery + neck dissection | Yes (negative for metastasis) | Median follow up of 24 months | 1 | Not mentioned |
DFS: Disease-free survival, SMLN: Sub mandibular Lymph node, Sx: Surgery, R: Radiotherapy
Six[14,16,18,19,22,23] studies shed light on wrong histopathological diagnosis due to differences in incisional (initial) and excisional (final) histopathological diagnosis (hybrid VC and invasive SCC). VCs were diagnosed in 262 cases after an incisional biopsy. Sixty-one cases reported a difference in diagnosis, such as hybrid VC and invasive carcinoma. Thus, the probability of wrong diagnosis in OVC is 23.3% (95% confidence interval [CI] for probability [18.16%–28.40%]).
Assessment of risk of bias
The symmetric funnel plot suggests no publication bias [Figure 2]. In addition, two review authors assessed the risk of bias for each study, resolving discrepancies with a third reviewer.
Figure 2.
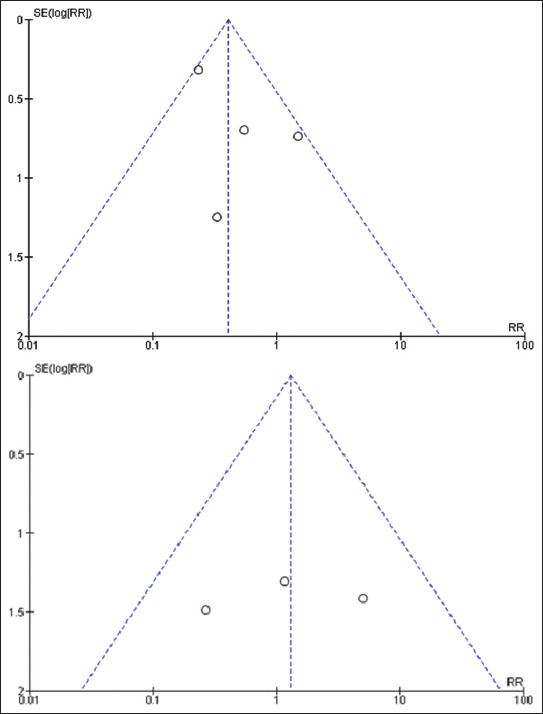
Funnel plot. SE: Standard error, RR: Risk ratio
Qualitative analysis
Efficacy of different treatment modalities
In this systematic review, the efficacy of the treatment has been decided by recurrence rate. Treatment options have been classified as surgical, radiotherapy, chemotherapy and combination therapy. For surgery, the recurrence rate is minimum (14.38%) and maximum for radiotherapy (50.85%). A significant association was found between recurrence of the verrucous lesion and treatment [P < 0.001, Table 2]. Surgery has been reported as the most effective and preferred treatment option. Treatment planning also changes by the extent and size of the lesion. Studies[12,13,14,15,16,17,18,19] mentioned treatment methods other than surgery, such as radiotherapy, chemotherapy or combination therapy, if there were multiple lesions or T4 sized. Bone involvement is an important deciding factor for change in treatment modality.[13,14,15,16,17,20,21] There is a significant relation between size (T) and treatment performed [P < 0.001, Table 3]. Rekha and Angadi[21] reported 33/133 bony lesions and mandibular resection was added to a treatment plan. Vidyasagar et al.[14] reported bone involvement in 19.6% of cases and used radiation as primary treatment modality. No significant association was found between size (T) and recurrence [P = 0.567, Table 4]. Studies[1,13,18,19,21,22] performed neck dissection as well, but no one reported nodal metastasis except one[15] in two patients.
Table 2.
Distribution recurrence according to treatment
| Treatment | Patients | Recurrence (%) | χ 2 | P |
|---|---|---|---|---|
| Surgery | 160 | 23 (14.38) | 43.5 | <0.001 |
| Radiotherapy | 118 | 60 (50.85) | ||
| Chemotherapy | 8 | 2 (25.00) | ||
| Combination therapy | 11 | 3 (27.77) |
Table 3.
Association of T stage with treatment
| Treatment | T1, n (%) | T2, n (%) | T3, n (%) | T4, n (%) | TX, n (%) |
|---|---|---|---|---|---|
| Surgical | 55 (93.2) | 64 (64.0) | 28 (40.6) | 18 (25.7) | 4 (100.0) |
| Radiation | 4 (6.8) | 35 (35.0) | 41 (59.4) | 52 (74.3) | 0 |
| Chemotherapy | 0 | 1 (1.0) | 0 | 0 | 0 |
| Total | 59 (100.0) | 100 (100.0) | 69 (100.0) | 70 (100.0) | 4 (100.0) |
| Significance (χ2, P) | 71.61, <0.001 | ||||
Table 4.
Distribution recurrence according to T stage
| T stage | Patients | Recurrence (%) | χ 2 | P |
|---|---|---|---|---|
| T1 | 33 | 6 (18.18) | 2.95 | 0.567 |
| T2 | 51 | 10 (19.61) | ||
| T3 | 20 | 5 (25.00) | ||
| T4 | 22 | 7 (31.82) |
Quantitative analysis
Surgery or radiotherapy
A meta-analysis was performed to compare surgical treatment and radiotherapy. Eight[12,13,14,15,16,17,18,19] studies were included. Meta-analysis results showed that the overall effect size (risk ratio) of the surgery group with respect to the radiotherapy group for recurrence was 0.41 (95% CI: 0.25–0.68). The value is <1, which shows that the surgery group had a relatively lower proportion of recurrence in comparison to the radiotherapy group [Table 5]. Further, the overall risk ratio was found to be statistically significant (P = 0.0005). As there was not sufficient data regarding combination therapy cases, quantitative analysis could not be performed.
Table 5.
Meta-analysis of comparison between surgery and radiotherapy for recurrence
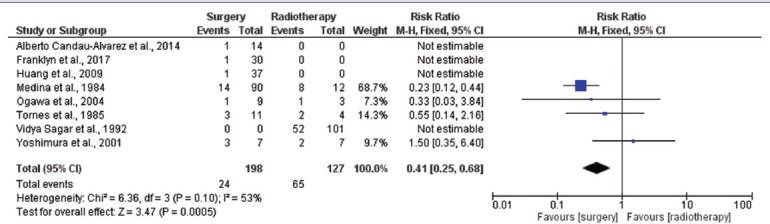
Surgery or chemotherapy
The meta-analysis of selected studies showed that the overall effect size (risk ratio) of the surgery group with respect to the chemotherapy group for recurrence was 1.30 (95% CI: 0.34–5.00). The value is more than 1, which shows that the surgery group had a relatively higher proportion of recurrence in comparison to the chemotherapy group. Further, the overall risk ratio was found to be statistically insignificant [P = 0.70, Table 6].
Table 6.
Meta-analysis of comparison between surgery and chemotherapy for recurrence
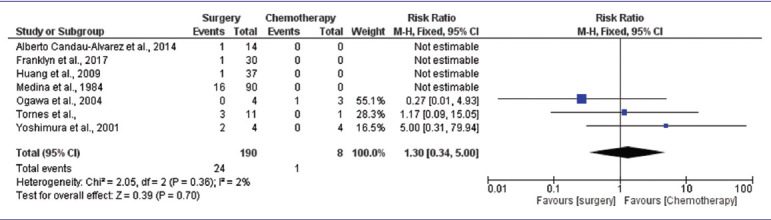
DISCUSSION
In this systematic review and meta-analysis, treatment options have been classified as surgical, radiotherapy, chemotherapy and combination therapy depending on tumour size, depth of invasion and underlying bone involvement. Surgical procedure was done mostly for T1 stage. Radiotherapy was administered for most of the T4-sized lesions. Few authors have discussed radiotherapy, chemotherapy and combination therapy for T3, T4 and multiple lesions.[12,14,15,16,22] Hence, if the lesion is unresectable or resection cannot be performed with a safe margin, other treatment options can be considered instead of surgery under regular follow-up. VC is a locally destructive lesion, involving the next anatomical barrier and eroding the cortical bone. In reference to studies by Rekha and Angadi and Vidyasagar et al., they are the largest reported studies with bone involvement.[14,21] Radiation therapy and surgical resection with or without neck dissection were the treatment options used in these two studies. VC is considered a notorious lesion for having a high recurrence rate. Every possible attempt should be made to achieve anatomically safe margins during surgical excision.[9] Maximal local control was reported in surgical treatment.
According to Table 6, the minimum recurrence of VC occurred in size T1 (18.18%) and the maximum with T4 (31.82%), yet this result was not statistically significant (P = 0.567). This indicates that the biology of the tumour is also a significant factor along with size. Authors of some studies also reported chemotherapy both as a single modality and in combination with other therapies.[13,15,16,24] Using the results of Tables 4-6 and meta-analysis, local excision and marginal or segmental resection for a tumour showing fixation to an alveolar portion of the bone or invasion of the underlying bone is the preferred treatment modality for pure VC with minimal recurrence.
In relation to surgical treatment, the question arises whether neck dissection should be carried out or not since VC is a low-grade variant of well-differentiated SCC. According to previous literature, authors treated VC similarly to SCC.[17] Few studies reported enlarged cervical lymph nodes in some cases of VC and performed surgical excision and neck dissection together. After histopathological examination, none of them reported metastatic lymph nodes. In all patients, lymphadenopathy was due to secondary infection. Walvekar et al. suggested a staged neck procedure if the surgeon suspects metastasis based on clinical experience.[20] Rekha and Angadi reported neck dissection in clinically large and aggressive-sized lesions, but lymphadenopathy was reported due to reactive hyperplasia after secondary infection or reported in very few cases.[7,21] Sadasivan et al. used criteria for neck dissection if tumour size was >4 cm, but after final histopathological report, all were locoregionally controlled.[22] Specifically, Franklyn et al. recommended omitting neck dissection in cases of confirmed VC or considering selective neck treatment such as supraomohyoid neck dissection in situations when there is uncertainty regarding the pathological diagnosis in clinically suspicious lymphadenopathy.[8,19] In conclusion, modified radical neck dissection is an overtreatment that can be avoided by staging neck dissection in patients with pure VC.
The probability of a wrong diagnosis in VC in this systematic review was 23.3% (95% CI), which is slightly higher than the reported data in the literature (20%).[2] Results were obtained by extracting the differences in incisional and excisional histopathology reports. Differential diagnoses of VC can be various as verrucous hyperplasia, verrucous keratosis or VCs may have a minor component of invasive SCC or conventional SCC, which can be easily missed in an incisional biopsy.[3,8] To avoid wrong diagnosis, deep biopsy has been advised because hyperplasia and keratosis remain superficial to normal epithelium.
After summarising the work of the authors of included studies and statistical analysis, focused questions are answered under the following headings:
Verrucous carcinoma versus hybrid verrucous carcinoma
Biologic behaviour of tumour predicts the anticipated response of treatment. Hence, correct diagnosis is the most important requirement for favourable outcome in VC patients. The hybrid VC term is used when conventional SCC is detected alongside VC in histopathology. In this systemic review, the probability of wrong diagnosis in VC is 23.3% rather than 20% mentioned in the literature.[23] VC is a thick, bulky and highly keratinised lesion. It is paramount that adequate material for biopsy be secured for proper diagnosis. Gokavarapu et al. reported incisional biopsy in 51% of cases failed to identify an invasive component.[23] Batsakis et al. reported that metastatic transformation in primary VC could be explained due to either incorrect histopathological diagnosis or an occult SCC component in the bulk of VC.[4,10,11,25] A literature review identified anaplastic transformation of VC after radiotherapy, and the explanation was that this was caused by missing components of conventional SCC occurring during incisional biopsy.[4,5,26]
Surgery versus surgery and neck dissection
Controversy exists as to whether neck dissection should be included in VC treatment. There has been no report of metastasis in enlarged regional lymph nodes in the current systematic review. For pure VC, lymphadenopathy results from bacterial superinfection or reactive hyperplasia.[6,27,28,29] Irrespective of size, VC does not metastasise until there are no foci of SCC. Treatment like SCC will only add additional risks such as shoulder dysfunction, pain, paresis/paralysis of the marginal branch of the facial nerve, scar and post-operative sensory deficits.
Treatment protocol
Surgical excision of T1- and T2-sized lesions can be performed under local anaesthetic as a biopsy procedure. If the defect size cannot be closed primarily, reconstruction should be done with local or locoregional flaps. If a histopathological report reveals hybrid VC (foci of SCC also present), neck dissection should be performed as a second procedure. Whenever a T3 or T4 lesion can be resected with a safe margin of 1 cm, it should be excised as primary surgery.[27] If it comes as hybrid VC or VC with close margin (0.5 cm or <0.5 cm), neck dissection and further margin should be excised as a second procedure, respectively. VC can be treated with radiation therapy or chemotherapy instead of a second operation. In cases where there is no hope of resecting a T4 lesion, multiple lesions or in cases where the patient’s condition does not permit an operation, radiotherapy or chemotherapy can be given to downstage the lesion with proper follow-up.[8,24,30] In addition, the surgeon should be more vigilant if there is any suspicion of field cancerisation or other pre-malignant lesions such as leucoplakia, oral submucous fibrosis or dysplasia in VC patients.[7]
Some limitations of this article are that it does not include other treatments such as laser, cryotherapy and diathermy. Meta-analysis of chemotherapy and combination therapy could not be performed due to a lack of sufficient data. These things can be included in future research.
CONCLUSION
Whatever approach is to be used, a comprehensive clinical examination and proper histopathological examination are essential requirements in order to offer the best treatment. VCs, however, should be treated preferably by surgical excision. Even radiation therapy has shown promising results in local control but has not surpassed those obtainable with surgery. In addition, there is no need to subject the patient to the associated morbidity of neck dissection. If there is clinical uncertainty between VC and hybrid VC, the surgeon can undergo sentinel node biopsy or staged neck dissection without compromising oncologic outcome.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Goethals PL, Harrison EG, Devine KD. Verrucous squamous carcinoma of the oral cavity. Am J Surg. 1963;5:845–51. doi: 10.1016/0002-9610(63)90413-6. [DOI] [PubMed] [Google Scholar]
- 2.Peng Q, Wang Y, Quan H, Li Y, Tang Z. Oral verrucous carcinoma: From multifactorial etiology to diverse treatment regimens (Review) Int J Oncol. 2016;49:59–73. doi: 10.3892/ijo.2016.3501. [DOI] [PubMed] [Google Scholar]
- 3.Patel KR, Chernock RD, Sinha P, Müller S, El-Mofty SK, Lewis JS., Jr Verrucous carcinoma with dysplasia or minimal invasion: A variant of verrucous carcinoma with extremely favorable prognosis. Head Neck Pathol. 2015;9:65–73. doi: 10.1007/s12105-014-0551-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Batsakis JG, Hybels R, Crissman JD, Rice DH. The pathology of head and neck tumors: Verrucous carcinoma, part 15. Head Neck Surg. 1982;5:29–38. doi: 10.1002/hed.2890050107. [DOI] [PubMed] [Google Scholar]
- 5.Kraus FT, Perezmesa C. Verrucous carcinoma. Clinical and pathologic study of 105 cases involving oral cavity, larynx and genitalia. Cancer. 1966;19:26–38. doi: 10.1002/1097-0142(196601)19:1<26::aid-cncr2820190103>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 6.Oliveira DT, de Moraes RV, Fiamengui Filho JF, Fanton Neto J, Landman G, Kowalski LP. Oral verrucous carcinoma: A retrospective study in São Paulo region, Brazil. Clin Oral Investig. 2006;10:205–9. doi: 10.1007/s00784-006-0050-7. [DOI] [PubMed] [Google Scholar]
- 7.Mohammadi K, Mohiyuddin SM, Harshitha N, Suresh TN, Prasad CS, Sagayaraj A, et al. Outcome of treatment in verrucous carcinoma of oral cavity: A tertiary rural hospital experience. Indian J Otolaryngol Head Neck Surg. 2022;74:1768–72. doi: 10.1007/s12070-019-01782-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rath S, Gandhi AK, Rastogi M, Agarwal A, Singhal A, Sharma V, et al. Treatment pattern and outcomes in verrucous carcinoma of oral cavity: A single institutional retrospective analysis from a tertiary cancer center and review of literature. Indian J Otolaryngol Head Neck Surg. 2022;74:1790–6. doi: 10.1007/s12070-020-01798-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang N, Huang M, Lv H. Head and neck verrucous carcinoma: A population-based analysis of incidence, treatment, and prognosis. Medicine (Baltimore) 2020;99:e18660. doi: 10.1097/MD.0000000000018660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santosh HN, Nagaraj T, Saxena S, Biswas A, Pai SA. Verrucous carcinoma: A clinicopathological study. J Oral Maxillofac Pathol. 2019;23:303. doi: 10.4103/jomfp.JOMFP_59_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson LD. Verrucous squamous cell carcinoma. Ear Nose Throat J. 2021;100:540S–1S. doi: 10.1177/0145561319871712. [DOI] [PubMed] [Google Scholar]
- 12.Medina JE, Dichtel W, Luna MA. Verrucous-squamous carcinomas of the oral cavity. A clinicopathologic study of 104 cases. Arch Otolaryngol. 1984;110:437–40. doi: 10.1001/archotol.1984.00800330019003. [DOI] [PubMed] [Google Scholar]
- 13.Tomes K, Bang G, Koppang HS, Pedersen KN. Oral verrucous carcinoma. Int J Oral Surg. 1985;14:485–92. doi: 10.1016/s0300-9785(85)80054-5. [DOI] [PubMed] [Google Scholar]
- 14.Vidyasagar MS, Fernandes DJ, Kasturi DP, Akhileshwaran R, Rao K, Rao S, et al. Radiotherapy and verrucous carcinoma of the oral cavity. A study of 107 cases. Acta Oncol. 1992;31:43–7. doi: 10.3109/02841869209088264. [DOI] [PubMed] [Google Scholar]
- 15.Yoshimura Y, Mishima K, Obara S, Nariai Y, Yoshimura H, Mikami T. Treatment modalities for oral verrucous carcinomas and their outcomes: Contribution of radiotherapy and chemotherapy. Int J Clin Oncol. 2001;6:192–200. doi: 10.1007/pl00012104. [DOI] [PubMed] [Google Scholar]
- 16.Ogawa A, Fukuta Y, Nakajima T, Kanno SM, Obara A, Nakamura K, et al. Treatment results of oral verrucous carcinoma and its biological behavior. Oral Oncol. 2004;40:793–7. doi: 10.1016/j.oraloncology.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 17.Huang TT, Hsu LP, Hsu YH, Chen PR. Surgical outcome in patients with oral verrucous carcinoma: Long-term follow-up in an endemic betel quid chewing area. ORL J Otorhinolaryngol Relat Spec. 2009;71:323–8. doi: 10.1159/000267306. [DOI] [PubMed] [Google Scholar]
- 18.Candau-Alvarez A, Dean-Ferrer A, Alamillos-Granados FJ, Heredero-Jung S, García-García B, Ruiz-Masera JJ, et al. Verrucous carcinoma of the oral mucosa: An epidemiological and follow-up study of patients treated with surgery in 5 last years. Med Oral Patol Oral Cir Bucal. 2014;19:e506–11. doi: 10.4317/medoral.19683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franklyn J, Janakiraman R, Tirkey AJ, Thankachan C, Muthusami J. Oral verrucous carcinoma: Ten year experience from a tertiary care hospital in India. Indian J Med Paediatr Oncol. 2017;38:452–5. doi: 10.4103/ijmpo.ijmpo_153_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walvekar RR, Chaukar DA, Deshpande MS, Pai PS, Chaturvedi P, Kakade A, et al. Verrucous carcinoma of the oral cavity: A clinical and pathological study of 101 cases. Oral Oncol. 2009;45:47–51. doi: 10.1016/j.oraloncology.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 21.Rekha KP, Angadi PV. Verrucous carcinoma of the oral cavity: A clinico-pathologic appraisal of 133 cases in Indians. Oral Maxillofac Surg. 2010;14:211–8. doi: 10.1007/s10006-010-0222-0. [DOI] [PubMed] [Google Scholar]
- 22.Sadasivan A, Thankappan K, Rajapurkar M, Shetty S, Sreehari S, Iyer S. Verrucous lesions of the oral cavity treated with surgery: Analysis of clinico-pathologic features and outcome. Contemp Clin Dent. 2012;3:60–3. doi: 10.4103/0976-237X.94548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gokavarapu S, Chandrasekhara Rao LM, Patnaik SC, Parvataneni N, Raju KV, Chander R, et al. Reliability of incision biopsy for diagnosis of oral verrucous carcinoma: A multivariate clinicopathological study. J Maxillofac Oral Surg. 2015;14:599–604. doi: 10.1007/s12663-014-0715-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Keukeleire S, De Meulenaere A, Deron P, Huvenne W, Fréderic D, Bouckenooghe O, et al. Verrucous hyperplasia and verrucous carcinoma in head and neck: Use and benefit of methotrexate. Acta Clin Belg. 2021;76:487–91. doi: 10.1080/17843286.2020.1752455. [DOI] [PubMed] [Google Scholar]
- 25.Samah S, Samia A, Souha BB, Nour BM, Sameh S. SAS journal of surgery abbreviated key title: SAS j surg specificities of oral verrucous carcinoma: Case report and literature review. SAS J. 2021;7:542–8. [doi: 10.36347/sasjs.2021.v07i10.004] [Google Scholar]
- 26.Sreelatha SV, Krishnan S. Masquerading verrucous carcinoma: A pathologist's and surgeon's dilemma. Indian J Med Res. 2020;152:S189–90. doi: 10.4103/ijmr.IJMR_2292_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fawaz B, Vieira C, Decker A, Lawrence N. Surgical treatment of verrucous carcinoma: A review. J Dermatolog Treat. 2022;33:1811–5. doi: 10.1080/09546634.2021.1914312. [DOI] [PubMed] [Google Scholar]
- 28.da Silva ID, de Souza Tolentino E, Camarini C, Berlin EG, Veltrini VC, da Silva MC, et al. Oral verrucous carcinoma: A 24-year epidemiological study and case report. Arch Health Invest. 2021;10:975–8. [Google Scholar]
- 29.Ye Q, Hu L, Jia M, Deng LJ, Fang S. Cutaneous verrucous carcinoma: A clinicopathological study of 21 cases with long-term clinical follow-up. Front Oncol. 2022;12:953932. doi: 10.3389/fonc.2022.953932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kristofelc N, Zidar N, Strojan P. Oral verrucous carcinoma: A diagnostic and therapeutic challenge. Radiol Oncol. 2023;57:1–11. doi: 10.2478/raon-2023-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]


