Abstract
Seasonal influenza virus vaccines are effective when they are well matched to circulating strains. Because of antigenic drift/change in the immunodominant hemagglutinin (HA) head domain, annual vaccine reformulations are necessary to maintain a match with circulating strains. In addition, seasonal vaccines provide little to no protection against newly emerging pandemic strains. Sequential vaccination with chimeric HA (cHA) constructs has been proven to direct the immune response toward the immunosubdominant but more conserved HA stalk domain. In this study, we show that immunization with group 2 cHA split vaccines in combination with the CpG 1018 adjuvant elicits broadly cross-reactive antibodies against all group 2 HAs, as well as systemic and local antigen-specific T cell responses. Antibodies elicited after sequential vaccination are directed to conserved regions of the HA such as the stalk and the trimer interface and also to the N2 neuraminidase (NA). Immunized mice were fully protected from challenge with a broad panel of influenza A viruses.
A group 2 HA universal influenza virus vaccine candidate protects mice with pre-existing immunity broadly from virus challenge.
INTRODUCTION
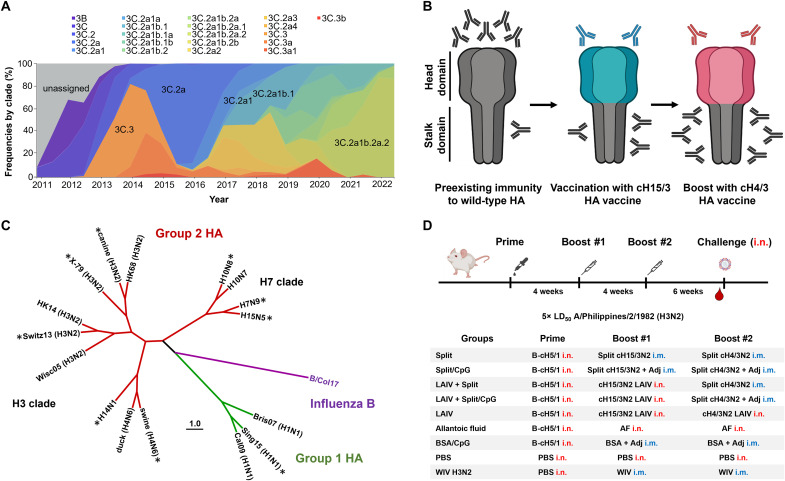
Seasonal influenza viruses represent a major public health burden every year. The vaccine effectiveness (VE) of commercially available influenza vaccines is in the range of 10 to 60%, being generally lower for H3N2 in comparison to H1N1 and influenza B viruses (1, 2). The main reasons for the lower VE for these vaccines appear to be the rapid antigenic evolution of H3N2 viruses (Fig. 1A) (3), the acquisition of N-linked glycans in the immunodominant hemagglutinin (HA) head domain (4), and egg-adaptive mutations (5). In addition, the risk of swine H3N2 influenza virus transmission to humans (6), the existence of other avian and mammalian animal reservoirs for viruses such as H3N8 (7), and sporadic interspecies transmission of other group 2 HA viruses like H7NX (8) and H10NX (9, 10) pose a potential pandemic threat (Fig. 1).
Fig. 1. Group 2 cHA vaccination strategy and experimental design.
(A) Timeline of global evolutionary frequencies of H3N2 viruses (02 December 2010 to 03 June 2022). The graph was adapted from nextstrain/flu/seasonal/h3n2/ha/12y (accessed on 10 November 2022) (78). (B) Schematic of sequential vaccination with group 2 cHA constructs to induce antibodies that target the immunosubdominant HA stalk domain as envisioned in humans with preexisting immunity to influenza virus. (C) Cladogram of HAs from the influenza viruses used in this study for challenge and of recombinant HA proteins used for serology analysis. HAs from challenge viruses are marked with an asterisk. The tree was constructed using amino acid sequences aligned in Clustal Omega (79) and visualized with the FigTree software (tree.bio.ed.ac.uk/software/figtree/). Strain designations can be found in Materials and Methods. (D) Different groups of BALB/c mice (n = 10) were primed i.n. with either a sublethal infection of the B-cH5/1 virus [105 plaque-forming units (PFU) per mouse] or PBS. After 4 weeks, mice were vaccinated with cH15/3HK14N2HK14 split vaccine (1 μg HA per mouse), BSA, or whole inactivated A/Philippines/2/1982 (H3N2, X-79) or A/Hunan/02285/2017 (H7N1) virus (WIV, positive control) via the i.m. route. Five mice per group were used for WIV control groups. Alternatively, mice were also infected with cH15/3HK14N2HK14 LAIV (105 PFU per mouse) i.n., or with allantoic fluid (AF), or PBS delivered i.n. After four additional weeks, mice were vaccinated/infected in the same manner but with cH4/3HK14N2HK14 split vaccine or LAIV. Groups coadministered with the CpG 1018 adjuvant (Adj) received a dose of 30 μg CpG 1018 per mouse. Six weeks after the second boost, mice were bled and challenged with 5× LD50 of the heterologous A/Philippines/2/1982 (H3N2, X-79) virus. This vaccination experiment was performed in two sets of independent mice (n = 5 mice per set) for serum analysis. Only one set of mice was challenged.
Current influenza virus vaccines are composed of H1N1 (group 1), H3N2 (group 2), and influenza B virus circulating strains. The immune response elicited by these vaccines mainly targets the immunodominant head domain of the most abundant influenza virus glycoprotein, the HA. The head domain of the HA is subject to strong antigenic drift (Fig. 1A) and can accommodate mutations that facilitate escape from preexisting immunity, hence annual revaccinations are required. Moreover, seasonal influenza virus vaccines would offer little to no protection against pandemic influenza viruses. The development of broadly protective vaccines is therefore of high importance (11, 12). One of the main approaches to develop this type of vaccine is to target conserved regions in the HA such as the receptor binding site and the trimer interface of the head domain (13), and the stalk domain (14). Several strategies that target the immunosubdominant HA stalk domain are currently under evaluation, including the use of hyperglycosylated HA heads, which direct the immune responses toward the stalk (15), the use of stabilized headless HA immunogens (16–19) and of chimeric HAs (cHAs) (20). cHAs are generated by swapping the HA head domains of seasonal influenza viruses with those of other influenza virus subtypes that do not circulate in humans (Fig. 1B) (20–22). Sequential vaccination with different cHA constructs has been proven to elicit broadly cross-reactive HA stalk antibodies in different animals models (23–25) and in humans (26–28). Most of these studies have been conducted with group 1 cHA constructs as recombinant proteins, live-attenuated influenza vaccine (LAIV), vectored vaccines, or as inactivated split vaccines in combination with the AS03 adjuvant. However, group 1 cHA vaccines induce little cross-reactivity to group 2 HAs. Moreover, there is less information available on the protective efficacy mediated by group 2 cHA constructs (29, 30). In this study, in preparation for clinical trials, we have developed group 2 cHA vaccine candidates, cH15/3HK14N2HK14 and cH4/3HK14N2HK14, and tested them as LAIVs, inactivated split vaccines in combination with the Toll-like receptor 9 agonist adjuvant, CpG 1018 (31), and combinations of these two platforms in the mouse model. We observed that sequential vaccination with these vaccines elicits broadly cross- reactive and protective responses against a variety of influenza A viruses (Fig. 1C). These results represent a step forward in the development of a universal influenza vaccine and will guide clinical development in humans.
RESULTS
Sequential vaccination with cHA constructs elicits broadly cross-reactive HA stalk antibodies and induces protective immune responses against challenge with a heterologous influenza virus
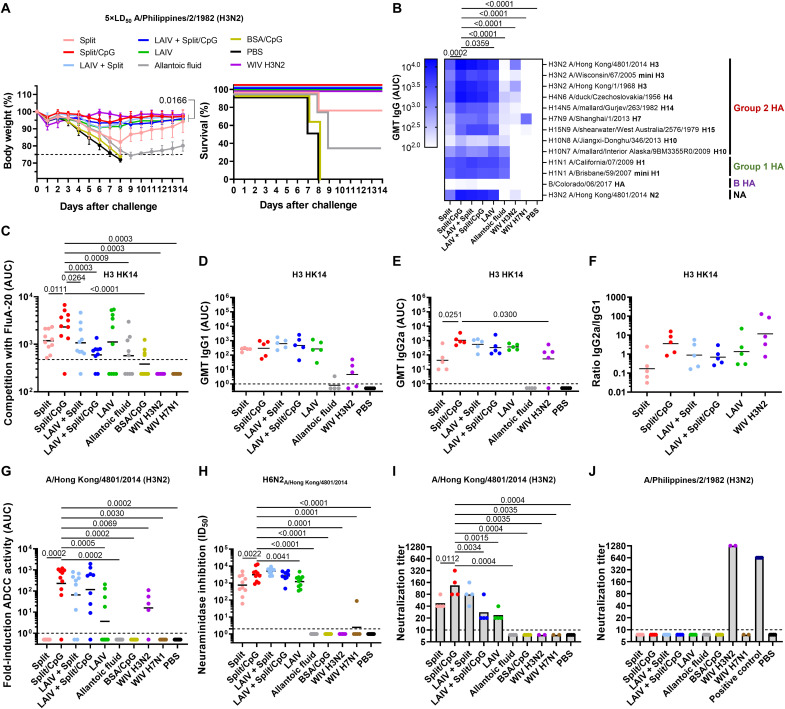
Inactivated split vaccine and LAIVs were evaluated as platforms to sequentially deliver our cH15/3HK14N2HK14 and cH4/3HK14N2HK14 virus constructs (Fig. 1D). These chimeric viruses contain the HA stalk domain and the neuraminidase (NA) of the A/Hong Kong/4801/2014 (H3N2) seasonal influenza virus, while the HA head domains are derived from exotic influenza viruses that do not circulate in humans. Split vaccines were tested in combination with the human-approved Toll-like receptor 9 agonist adjuvant CpG 1018 via the intramuscular (i.m.) route, whereas LAIV were administered intranasally (i.n.). Before vaccination, mice were primed with a sublethal infection (i.n.) of the B-cH5/1 virus to mimic preexisting immunity against the HA stalk domain in humans. Preexisting immunity to the group 1/H1 stalk is higher in humans than to the group 2/H3 stalk and therefore this is a realistic priming scenario (32, 33). It has also been hypothesized that priming with group 1/H1 HA may negatively affect group 2/H3 stalk immunity (34, 35). Seeing protection against group 2 viruses despite this “negative” imprinting would demonstrate the real-world potential of the tested vaccine candidates. Mice sequentially vaccinated with nonadjuvanted split vaccines were almost completely protected (80% survival) from challenge with a lethal dose of the heterologous A/Philippines/2/1982 (H3N2, X-79) virus (Fig. 2A, the X-79 HA stalk domain is 94.8% identical to A/Hong Kong/4801/2014 stalk in amino acid sequence). The combination of cHA split vaccines with the CpG 1018 adjuvant provided full protection from mortality with little to no morbidity, in the same range as LAIV, hybrid vaccination strategies (LAIV + split vaccine), and the matched inactivated H3N2 virus control.
Fig. 2. Vaccination with group 2 cHA constructs protects mice from lethal challenge and elicits cross-reactive antibodies.
(A) Weight loss and survival plots of mice (n = 5) challenged with 5× LD50 of A/Philippines/2/1982 (H3N2, X-79) (average and SD). Lines in the survival graphs are nudged to allow for distinction between overlapping groups. (B) ELISA geometric mean titers (GMT) of total serum IgG responses of the two sets of mice (n = 10) against a variety of influenza A and B virus HA and vaccine-matched NA protein. Comparisons were performed by considering all IgG responses to all tested influenza virus antigens in the split/CpG group and comparing these to the other groups. (C) Serum competition ELISA for two sets of vaccinated mice (n = 10) with the trimer interface mAb FluA-20 against the H3 HK14 protein. (D and E) GMT and ratio (F) of individual serum IgG1 and IgG2a responses against the vaccine matched H3 HK14 protein (n = 5). (G) Serum from the two sets of vaccinated mice (n = 10) was analyzed for ADCC reporter activity against the HK14 virus (fold induction over blank). (H) NAI assay of sera from the two sets of mice (n = 10) against an H6N2 reassortant virus expressing the N2 of HK14. NI activity was plotted as ID50. (I and J) Neutralizing activity of mouse sera against A/Philippines/2/1982 (H3N2, X-79) and HK14. Mouse sera from each one of the sets of vaccinated mice (n = 5) were pooled within each group from each set and analyzed in technical duplicate. The X-79 H3 head mAb 1F12 was used as a positive control (30 µg/mL). GMTs are shown as black line. Limit of detection (LoD): ELISA/ADCC = 1, competition ELISA = 476 (GMT of PBS group), NAI = 2, microneutralization = 10. Half the value of the LoD was assigned to negative samples. Statistical analyses were performed using one-way analysis of variance (ANOVA) corrected for Dunnett’s multiple comparisons test. Only statistically significant P values (<0.05) are shown. Experiments were conducted once.
The protection conferred by sequential vaccination with cHA vaccines correlated with the presence of cross-reactive immunoglobulin G (IgG) antibodies against a broad panel of group 2 HAs (Fig. 1C), and these antibodies also targeted group 1 and 2 HA stalks (mini H1 and mini H3) (Fig. 2B). The level of cross-reactive antibodies was substantially lower for whole inactivated virus (WIV) controls. The combination of the split vaccine with the CpG 1018 adjuvant increased the level of HA cross-reactive and vaccine-matched N2 antibodies between 2- and 10-fold and to the same range as for LAIV and hybrid vaccinations. No cross-reactive antibodies were elicited against the influenza B virus HA (Fig. 2B), but, as expected, priming with the B-cH5/1 virus resulted in detectable antibody titers against the influenza B virus NA (fig. S1). In addition, all vaccine types were able to induce cross-reactive antibodies targeting the HA head trimer interface. HA head trimer interface antibodies were also detected after priming only with the B-cH5/1 virus, although at lower levels compared to mice sequentially vaccinated with cHA vaccines (Fig. 2C). Analysis of the type of immune response elicited by these different vaccine platforms revealed that combining cHA split vaccines with CpG 1018 boosted the production of IgG2a antibodies in comparison to unadjuvanted vaccines and to similar levels as LAIV and hybrid vaccinations (Fig. 2, D to F).
Analysis of functional antibody responses revealed that mice vaccinated with split vaccine + CpG 1018 elicited antibodies that were active in an antibody-dependent cellular cytotoxicity (ADCC) reporter assay against homologous and heterologous H3N2 viruses (Fig. 2G and fig. S2). Similarly, the antibodies elicited in these groups of vaccinated mice could inhibit the NA activity of the vaccine-matched N2 in the reassortant H6N2A/Hong Kong/4801/2014 virus, which contains an H6 glycoprotein to avoid H3-directed NAI activity (Fig. 2H) (36). Notably, no measurable neutralizing antibody titers could be detected against the heterologous A/Philippines/2/1982 (H3N2, X-79) virus, while some level of neutralizing antibodies against the vaccine-matched A/Hong Kong/4801/2014 (H3N2) virus were induced by these vaccines (Fig. 2, I and J). Overall, the different group 2 cHA vaccine platforms elicited potent humoral immune responses. However, the LAIV or hybrid vaccination strategies with group 1 cHA constructs showed lower immunogenicity in humans in comparison to split vaccines, likely because the cHA-based LAIVs are hyper-attenuated in humans (26, 28). Split vaccines in combination with the CpG 1018 adjuvant were therefore selected as the platform for further group 2 cHA vaccine development.
Antibodies elicited after vaccination with cHA split vaccines reduce viral lung titers and protect against influenza virus challenge in a serum passive transfer experiment
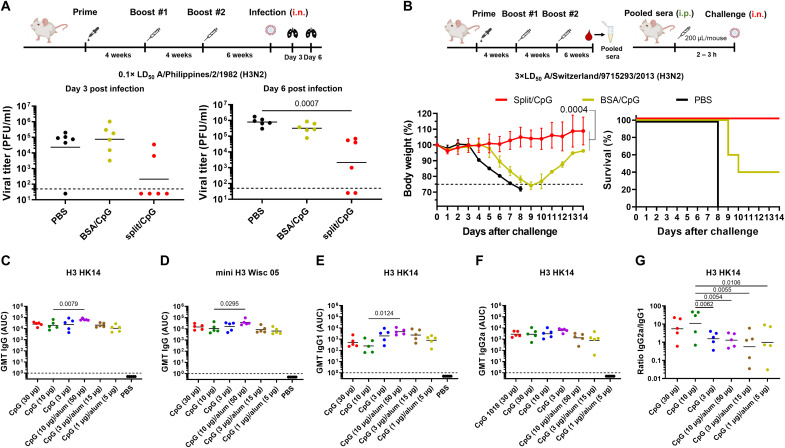
Sequential vaccination with split vaccines adjuvanted with CpG 1018 showed excellent immunogenicity results similar to levels observed following LAIV or hybrid vaccinations. To assess the control of virus replication in the lungs, mice were sequentially immunized with adjuvanted split vaccines and challenged with the X-79 virus. High levels of viral titers were found in the lung homogenates of mice vaccinated with phosphate-buffered saline (PBS; negative control), and mice primed with the B-cH5/1 virus and vaccinated with the irrelevant protein bovine serum albumin (BSA) + CpG 1018 in comparison to mice vaccinated with split vaccine + CpG 1018 (Fig. 3A).
Fig. 3. Lung viral loads and serum passive transfer and dose–de-escalation experiments.
(A) Day 3 and day 6 lung viral load of BALB/c mice (n = 6) vaccinated following the same vaccination regimen as shown in the previous experiment, and challenged with A/Philippines/2/1982 (H3N2, X-79). LoD was 50 PFU/ml, negative samples were assigned a titer of 25 PFU/ml. The GMT of viral titers are also shown. (B) Sera from sequentially vaccinated DBA/2J mice were pooled and transferred into naïve DBA/2J mice (n = 5), which were challenged with the heterologous A/Switzerland/9715293/2013 (H3N2) virus and monitored for weight loss and survival. Average and SD are shown. Statistical comparison of Split and Split/CpG weight loss curves is also indicated. Lines in the survival graphs are nudged to allow for distinction between overlapping groups. (C and D) GMT of total serum IgG responses from the dose–de-escalation experiment of CpG 1018 and combination with alum against the H3 HK14 protein and a stabilized headless H3 protein (mini H3) from the A/Wisconsin/67/2005 (H3N2) virus, respectively. (E and F) Serum IgG1 and IgG2a responses from the dose–de-escalation experiment of CpG 1018 and combination with alum against the H3 HK14 protein. (G) Ratio of IgG2a to IgG1 responses against the H3 HK14 protein. The GMTs are shown as black line. The LoD for ELISA was defined as 1, and 0.5 was assigned to negative samples. In all cases, mice vaccinated with split vaccines and BSA were initially primed with the B-cH5/1 virus. Statistical analyses were performed using one-way ANOVA corrected with the nonparametric Dunn’s test for viral titers and one-way ANOVA corrected for Dunnett’s multiple comparisons test for other assays. Only statistically significant P values (<0.05) are shown. Experiments were conducted once except for the lung titer experiments which were conducted twice (pooled data are shown). i.p., intraperitoneal.
A passive transfer experiment was also conducted to determine whether antibodies induced by split vaccine + CpG 1018 could reduce morbidity and protect following infection with a heterologous influenza virus. To this end, sera from mice sequentially vaccinated with PBS, BSA + CpG1018, and split vaccine + CpG 1018 were collected, pooled, and administered intraperitoneally to naïve DBA/2J mice. After 2 to 3 hours, mice were challenged with the A/Switzerland/9715293/2013 (H3N2) virus. Mice receiving serum from PBS- and BSA + CpG 1018–vaccinated mice exhibited a pronounced weight loss or succumbed to the challenge, while mice receiving serum from split vaccine + CpG 1018–vaccinated mice were fully protected without any sign of morbidity (Fig. 3B).
Lower doses of cHA split vaccines and CpG 1018 adjuvant protect mice against challenge with a heterologous influenza virus
After observing that adjuvanted split vaccines elicited broad protection at a 1-μg dose against heterologous influenza virus infection, we next assessed protection conferred by a range of different split vaccine and CpG 1018 doses. To this end, different groups of mice were vaccinated with different doses of split vaccine ranging from 0.1 to 3 μg of HA per mouse. All groups of mice vaccinated with split vaccine + CpG 1018 exhibited full protection against lethal challenge with the X-79 virus independently of the dose used (fig. S3A).
Different doses of CpG 1018 adjuvant in combination with the split vaccine were also evaluated (3, 10, and 30 μg of CpG 1018 per mouse) to determine whether the dose of adjuvant could be lowered. In addition, the combination of the CpG 1018 adjuvant with alum at different doses (10:50, 3:15, and 1:5 μg of CpG 1018:alum per mouse) was evaluated as a potential combination to increase the protection elicited by split vaccines since the combination of CpG 1018 with alum has been shown to be superior to CpG 1018 alone for some coronavirus disease 2019 vaccines. In all cases, complete protection from lethal challenge with the X-79 virus was achieved (fig. S3B). Complete protection was also observed when combining CpG 1018 and alum, but this combination did not provide an additional benefit in reducing weight loss in mice. A trend to more weight loss was detected in all groups of mice vaccinated with CpG 1018 + alum compared to the CpG 1018 only groups. Analysis of the antibody response elicited in mice vaccinated with split vaccine adjuvanted with different doses of CpG 1018 (with or without alum) showed a statistically significant increase in total IgG and HA stalk antibody levels in the highest dose of CpG 1018 + alum (10:50 μg per mouse) tested in comparison with CpG 1018 alone (10 μg per mouse) (Fig. 3, C and D). Higher levels of IgG1 antibodies were detected with low amounts of CpG 1018 and with the combination of CpG 1018 + alum, whereas the IgG2a levels remained the same in all the conditions tested (Fig. 3, E and F). Therefore, a significant decrease in the ratio of IgG2a/IgG1 antibodies was detected in the groups of mice vaccinated with CpG 1018 + alum (Fig. 3G).
The combination of split vaccines and CpG 1018 provided 100% protection, without an additional benefit of combining cHA split vaccines with CpG 1018 + alum. Moreover, different doses of split vaccine and CpG 1018 provided similar levels of protection. The split vaccine + CpG 1018 condition selected for further evaluation in the mouse model was 1 μg of HA and 10 μg of CpG 1018 per mouse. Notably, minor differences in weight loss in the corresponding groups in fig. S3 and Fig. 2A are likely the result of experimental variation.
Vaccination with cHA split vaccines elicits HA stalk and NA-specific cellular immune responses
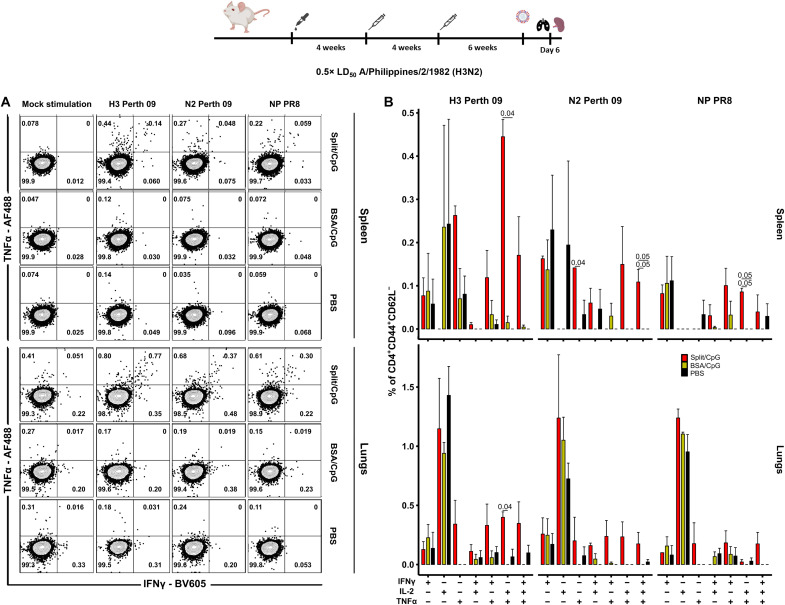
To assess antigen-specific T-cellular immune responses elicited by sequential vaccination with cHA split vaccines + CpG 1018, lymphocytes from the lungs and spleens of immunized mice were stimulated with HA, NA, and nucleoprotein (NP) overlapping peptide pools 6 days after challenge with the X-79 virus and measured via fluorescent activated cell sorting (Fig. 4A and fig. S4). No significant differences in the frequency of HA-, NA-, or NP-specific CD8+ T effector memory (EM) cells were observed between groups of mice (fig. S5). In the same line, no remarkable differences were detected in the frequency of monofunctional HA-, NA-, NP-specific CD4+ T EM cells between the different groups of vaccinated mice. However, the frequency of polyfunctional HA-, NA-, and NP-specific CD4+ EM T cells increased in the split vaccine group, especially in the spleens. Polyfunctional T cells have been positively associated with immune protection (37). The most prominent T-cellular immune response was observed in the spleens of vaccinated mice after H3 peptide pool stimulation, and was associated with the formation of polyfunctional TNFα+IL-2+ (tumor necrosis factor–α–positive–interleukin-2–positive) and, to a lesser extent, TNFα+IFNγ+ (interferon-γ–positive) and TNFα+IL-2+IFNγ+ cell subpopulations (Fig. 4B). Both NA and NP peptide pools also induced the activation of a specific T-cellular immune response but with a lower magnitude compared to HA-stimulated cells. The T-cellular immune response in the lungs of mice vaccinated with split vaccine was also primarily mediated by polyfunctional HA-specific CD4+ EM T cells. In PBS- and BSA-vaccinated mice, the majority of cytokine-secreting T cells were represented by monofunctional TNFα−IL-2+IFNγ− cells. The number of T lymphocytes of this phenotype was similar in all three groups. However, almost no polyfunctional cells were detected in PBS- and BSA-vaccinated mice after virus challenge (Fig. 4B).
Fig. 4. Systemic and local CD4+ EM T cell responses are induced by split vaccines.
(A) Representative flow cytometry plots showing the percentage of IFNγ- and TNFα-producing CD4+ EM T lymphocytes in mouse spleens and lungs 6 days after challenge with A/Philippines/2/1982 (H3N2, X-79) virus. T cells were stimulated with overlapping peptide libraries covering the H3 protein sequence from A/Perth/16/2009 (H3N2) virus, the N2 protein from A/Perth/16/2009 (H3N2) virus, and the NP protein from A/Puerto Rico/8/1934 (H1N1) virus. Different groups of BALB/c mice were sequentially vaccinated with PBS, BSA + 10 μg per mouse of CpG 1018, or 1 μg of HA per mouse of split vaccine +10 μg per mouse of CpG 1018 following the vaccination regimen as shown for previous experiments. (B) Percentage of different cytokine-producing cell populations within the total CD4+ EM T cell subset (n = 3) after background subtraction. Average and SD values are shown. In all cases, mice vaccinated with split vaccines and BSA were initially primed with the B-cH5/1 virus. Statistical analyses were performed using one-way ANOVA corrected for Dunn’s multiple comparisons test. Only statistically significant P values (<0.05) are shown. Experiments were conducted once.
Mice vaccinated with cHA split vaccines are protected from challenge with a broad panel of influenza A viruses
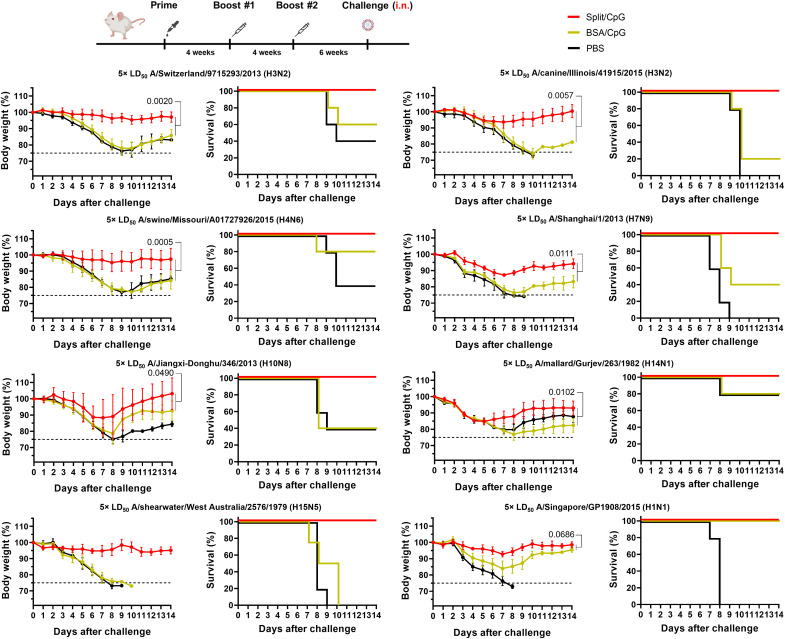
Our previous experiments showed that sequential vaccination with split vaccines + CpG 1018 adjuvant provided protection against lethal challenge with heterologous influenza viruses. We next evaluated the protective efficacy of these vaccines against direct challenge with other heterologous H3N2 viruses including the A/Switzerland/9715293/2013 (H3N2) virus and the more phylogenetically distant A/canine/Illinois/41915/2015 (H3N2) virus. Mice vaccinated with split vaccine + CpG 1018 were fully protected against challenge with these viruses, with little to no weight loss (Fig. 5).
Fig. 5. Sequential vaccination with group 2 cHA split vaccines protects mice from challenge with a broad spectrum of influenza A viruses.
Different groups of mice (n = 5) were sequentially vaccinated with 1 μg of HA per mouse of split vaccine adjuvanted with 10 μg per mouse of CpG 1018 as shown for previous experiments, and challenged with the heterologous A/Switzerland/9715293/2013 (H3N2) and A/canine/Illinois/41915/2015 (H3N2) viruses, the heterosubtypic group 2 A/swine/Missouri/A01727926/2015 (H4N6), A/Shanghai/1/2013 (H7N9), A/Jiangxi-Donghu/346/2013 (H10N8), A/mallard/Gurjev/263/1982 (H14N1), A/wedge-tailed shearwater/Western Australia/2576/1979 (H15N5) viruses, and the group 1 A/Singapore/GP1908/2015 (H1N1) virus. Body weight and survival were monitored over the course of 14 days after the challenge. Vaccination and challenge experiments with H3N2, H4N6, and H14N1 viruses were conducted in DBA/2J mice, whereas H7N9, H10N8, H15N5, and H1N1 challenge experiments were performed in BALB/c mice. The average weight values and SD are shown. In all cases, mice vaccinated with split vaccines and BSA were initially primed with the B-cH5/1 virus. Comparison of Split/CpG and BSA/CpG weight loss curves is shown. Statistical analyses were performed using one-way ANOVA corrected for Dunnett’s multiple comparisons test. Lines in the survival graphs are nudged to allow for distinction between the lines, which would otherwise overlap. Experiments were conducted once.
To further test the breadth of protection elicited by split vaccines, we next challenged mice with different heterosubtypic viruses containing all the other group 2 HAs, including the A/swine/Missouri/A01727926/2015 (H4N6), A/Shanghai/1/2013 (H7N9), A/Jiangxi-Donghu/346/2013 (H10N8), A/mallard/Gurjev/263/1982 (H14N1), and A/wedge-tailed shearwater/Western Australia/2576/1979 (H15N5) viruses (Fig. 1C). All mice vaccinated with split vaccine + CpG 1018 were fully protected against challenge with these viruses, with substantial weight loss differences between the split vaccine group and the PBS- and BSA-vaccinated groups (Fig. 5). As for H4N6 and H15N5 virus challenges, contribution of the heterologous H4 head of the cH4/3HK14 vaccine strain and the homologous H15 head of the cH15/3HK14 vaccine strain might have also contributed to the protection against challenge.
To test the possibility of intergroup influenza virus protection, mice vaccinated with split vaccine were also challenged with the group 1 HA A/Singapore/GP1908/2015 (H1N1) virus. Split vaccines provided complete protection from mortality in this case as well (Fig. 5).
DISCUSSION
The development of a universal influenza virus vaccine that protects against different H3N2 viruses as well as other phylogenetically distant group 2 influenza viruses is of paramount importance. Sequential vaccination with group 1 cHA constructs was proven successful to induce cross-group 1 HA immunity in different animal models (23, 25, 38) and more recently in phase I/II clinical trials in humans (26–28). Initial preclinical studies with other group 2 cHA constructs using the recombinant protein or viral vector platforms have shown promising results as well (29, 30, 39). Compared with the recombinant protein platform, cHA inactivated split vaccines can provide additional protection mediated by other viral components, such as the NA. Recently, we have developed a bioprocess to manufacture group 2 cHA split vaccines with correctly folded HA and enzymatically active NA (40). In this study, we aimed at evaluating the protective efficacy and the humoral and cellular immune responses elicited by our group 2 cHA inactivated split vaccines in the mouse model in the context of the more prevalent preexisting immunity against the H1 stalk (32). The CpG 1018 adjuvant was selected because of its extensive and excellent safety track record as part of the HEPLISAV-B vaccine (41) and because it is available for preclinical and clinical development in Good Manufacturing Practice (GMP) quality.
Sequential vaccination with split vaccines adjuvanted with CpG 1018 provided full protection against challenge with heterologous influenza viruses to levels similar to those observed for LAIV or hybrid vaccinations. The presence of broadly cross-reactive IgG antibodies against all group 2 HAs was detected in the sera of mice vaccinated with all cHA vaccine platforms. The level of broadly cross-reactive antibodies was higher in cHA split vaccines adjuvanted with CpG 1018 and hybrid vaccinations in comparison to unadjuvanted split vaccines, indicating that the combination with this adjuvant is key to elicit a potent antibody response. These antibodies target the HA stalk domain, which might explain the high level of cross-reactivity to heterologous H3 HAs and heterosubtypic group 2 HAs observed. Intergroup cross-reactive antibodies targeting the H1 (group 1) stalk were also detected, possibly indicating that some level of FI6-like antibodies was elicited (42). Detectable neutralization activity was measured but only against the stalk-homologous H3N2 strain, which agrees with the low neutralizing activity of HA stalk antibodies observed for group 1 cHAs (25). The mechanism of protection mediated by these HA stalk binding antibodies could be through the engagement of innate immune cells via Fc receptors since mice sera were active in an ADCC reporter assay (43). Previous work has shown that effector functions are a major contributor to stalk antibody-based protection (43, 44). Antibodies that targeted the conserved HA head trimer interface similarly to the FluA-20 monoclonal antibody (mAb) were also detected after vaccination with cHA constructs. These antibodies have been proven to mediate in vivo protection by disrupting the integrity of native HA trimers and limiting virus spread (45). The intact HA stalk and the more open HA head conformation of cH15/3HK14 and cH4/3HK14 HAs in comparison to wild-type influenza virus HAs might facilitate the accessibility of this epitope leading to the induction of antibodies targeting this conserved site (22). Antibodies binding to the vaccine matched N2 were also detected in mice vaccinated with the different vaccine platforms and were able to inhibit the NA activity, a mechanism that has been correlated with a reduction in viral shedding (46) and protection from morbidity and mortality by influenza virus challenge (47). The sera of mice containing these different classes of antibodies fully protected naïve mice from lethal challenge with the heterologous A/Switzerland/9715293/2013 (H3N2) virus in a serum passive transfer experiment. This strain has been chosen because it is a recent, complete H3N2 strain that was mouse adapted and represents current human H3N2 strains better than “old” H3N2 reassortant strains. However, it may be possible that the same experiment with a more aggressive historic reassortant strain may lead to less protection.
LAIV are known to induce all arms of the adaptive immune system and a strong mucosal response (48). Thus, LAIV-LAIV or LAIV-inactivated vaccine regimens generally result in a better protection against virus challenge in animal models (24, 25, 38, 49). However, preexisting immunity or hyperattenuation might limit the efficacy of the LAIV in adults as recently observed with group 1 cHA LAIV constructs (26, 28). For this reason, because of the robust immunogenicity results obtained with adjuvanted cHA split vaccines, and the fact that split vaccines are the most widely used platform for influenza vaccine production, we are moving cHA inactivated split vaccines forward for future clinical development.
The combination of CpG 1018 with our split vaccines allowed for dose sparing. Moreover, we also showed that the amount of adjuvant could be reduced. The CpG 1018 adjuvant is regarded as favoring a T cell helper 1 (TH1) immune response profile (31, 50). This was observed in the induction of similar IgG1 antibody titers between unadjuvanted and CpG 1018 adjuvanted vaccines, but with a higher level of IgG2a antibodies induced in the latter. In addition, the combination of CpG 1018 with alum did not increase protection in mice despite an increase in total IgG titers. Alum adjuvants are known to induce a TH2-skewed immune response, but their combination with CpG 1018 can TH1-bias the immune response as recently seen in different recombinant protein-based vaccines (51, 52). However, the combination of CpG 1018 + alum with our split vaccines resulted in a more TH2-biased response in comparison to CpG 1018 alone. This could be associated with the different nature of our immunogens, being larger and also more heterogeneous in size, structure, and composition than a defined soluble recombinant protein vaccine (40, 53).
Sequential vaccination of mice with split vaccine + CpG 1018 reduced viral replication in the lungs. Analysis of T cell responses after sequential vaccination and challenge did not show significant differences between the groups of mice in the induction of antigen-specific CD8+ T lymphocyte responses, which is in agreement with described immune responses to influenza virus split vaccines (54). Nevertheless, the formation of a HA-, NA-, or NP-specific polyfunctional CD4+ EM T-cellular immune response in the spleens and lungs of mice was observed, especially against the HA. The presence of antigen-specific polyfunctional T cells has been associated with an improved control of virus replication and a better promotion of virus clearance in comparison to monofunctional T cells (37, 55, 56). This indicates that adjuvanted split vaccines can induce a systemic immune response but can also recruit immune mediators that tackle the infection locally, resulting in a reduction of virus replication in the lungs. Notably, the combination of both humoral and cellular responses fully protected mice sequentially vaccinated with split vaccine + CpG 1018 from lethal challenge with a broad panel of influenza A viruses. Despite achieving complete protection, a higher level of morbidity was observed for the heterosubtypic A/Jiangxi-Donghu/346/2013 (H10N8) virus, which correlated with a lower level of cross-reactive IgG antibodies measured by enzyme-linked immunosorbent assay (ELISA) against this H10 subtype. However, a threefold increase in cross-reactive IgG antibody titers was measured against another H10 from the A/mallard/Interior Alaska/9BM3355R0/2009 (H10N7) virus. Because of lack of knowledge about antigenic/immunogenic differences between H10 HAs, it is currently not clear what drives these differences. Also, we cannot exclude the contribution of cross-reactive antibodies against the H15 (same head) and H4 (distant head) domains in the heterosubtypic H15N5 and H4N6 challenge experiments. Cross-protection against challenge with the intergroup A/Singapore/GP1908/2015 (H1N1) virus was also observed. Mice primed only with the B-cH5/1 virus were also fully protected from mortality but showed a substantially higher weight loss. This indicated that priming with the B-cH5/1 virus might have elicited cross-reactive antibodies to conserved epitopes in the HA such as the stalk or the trimer interface and that vaccination with split vaccines could have boosted these antibodies as seen by ELISA against an H1 and a stalk-only version of the H1 (mini H1). Notably, while the B-cH5/1 sublethal infection primes B cells to produce antibodies directed to the stalk region, this model does not take H1 or H3 head-specific priming into account which may influence the formation of FluA20-like antibodies.
Our study showed that sequential vaccination with group 2 cHA inactivated split vaccines in combination with CpG 1018 can elicit broadly cross-reactive immune responses that provide complete protection against challenge with any group 2 influenza virus in the mouse model and can boost intergroup HA cross-reactive antibodies. The study has also several limitations including the use of only one animal model, the lack of viral replication data in lungs of animals challenged with additional heterologous viruses, and the focus on parenteral immunization. Further studies in additional animal models are ongoing, and mucosal administration of this vaccine candidate will be tested in future studies. We think that our results warrant further research in other animal models and provide the basis for the upcoming clinical development of this vaccine platform. Ultimately, the group 2 constructs will be combined with group 1 cHA (23, 26) and mosaic HA influenza B (57) constructs into a trivalent vaccine for universal protection against all influenza A and B viruses.
MATERIALS AND METHODS
Study design
This study was designed for the preclinical evaluation of group 2 cHA constructs as universal influenza virus vaccines in the mouse model. The protective efficacy of this vaccine was assessed by comparing weight loss and survival profiles of sequentially immunized mice and controls against challenge with a broad range of heterologous and heterosubtypic influenza viruses. Different vaccine and adjuvant doses were tested. The analysis of serum cross-reactivity against different HAs and NAs, type of IgG response, the elicitation of HA stalk antibodies, competitive inhibition of HA head trimer interface antibodies, and screening of mouse sera for different functions including ADCC, neuraminidase inhibition (NAI), and microneutralization was performed. In addition, the presence of antigen-specific CD4+ and CD8+ T cell responses and the contribution of serum antibodies to protection were also evaluated. Randomization was achieved by randomly distributing mice into different cages upon arrival. No animal subjects were excluded from the sample collection or analysis unless serum was exhausted. No blinding was applied to the study design. This study was performed in preparation for clinical trials, and the combination of antigen with CpG 1018 adjuvant was chosen since CpG 1018 has an excellent safety profile in humans and since it is readily available in GMP quality.
Cell lines
Baculovirus generation and amplification were performed in Sf9 cells (CRL-1771, American Type Culture Collection), a clonal isolate of Spodoptera frugiperda Sf21 cells, and grown in Trichoplusia ni medium-formulation Hink insect cell medium (Gemini Bioproducts) supplemented with 10% (v/v) fetal bovine serum (FBS; Gibco), penicillin (100 U/ml)–streptomycin (100 μg/ml) solution (Gibco), and 0.1% (v/v) Pluronic F-68 (Gibco). High Five cells (BTI-TN-5B1-4, B85502, Thermo Fisher Scientific) were grown in Express Five SFM (Gibco) supplemented with 16 mM l-glutamine (Gibco) and used for recombinant HA and NA production (58). Both adherent cell lines were grown at 27°C.
Adherent Madin-Darby canine kidney (MDCK) and human embryonic kidney 293T cells were grown in modified Eagle’s medium (MEM) containing 10% (v/v) FBS and penicillin (100 U/ml)–streptomycin (100 μg/ml) solution in a humidified incubator at 37°C and 5% CO2.
Recombinant proteins and monoclonal antibodies
Recombinant HA and NA were produced in High Five cells and purified from cell culture supernatant using a Ni2+–nitrilotriacetic acid resin chromatography (58). The headless mini H1 protein was expressed in mammalian Expi293F cells (59). The A/Philippines/2/1982 H3 head murine mAb 1F12 was produced from hybridomas previously generated using a classical hybridoma fusion protocol (60).
Viruses
Group 2 cH15/3HK14N2HK14 and cH4/3HK14N2HK14 viruses expressing different cHA proteins were generated by reverse genetics (30). The H15 head domain of the cH15/3HK14N2HK14 virus was derived from the HA of A/wedge-tailed shearwater/Western Australia/2576/1979 (H15N9) virus, and the H4 head domain of the cH4/3HK14N2HK14 virus was derived from the A/duck/Czechoslovakia/1956 (H4N6) virus. The HA stalk domain and the NA of both viruses were derived from the A/Hong Kong/4801/2014 (H3N2) virus. As for inactivated split influenza virus vaccines, the internal genes were derived from the donor vaccine virus strain A/Puerto Rico/8/1934 (A/PR/8/34) (H1N1), whereas the internal genes for the LAIV were derived from the master donor A/Leningrad/134/17/1957 (H2N2) virus (61). The B-cH5/1 virus was also generated by reverse genetics (54, 62). The head domain of the chimeric B-cH5/1 virus was derived from the A/Vietnam/1203/2004 (H5N1) virus, and the HA stalk domain was derived from the A/PR/8/34 (H1N1) virus. Since the stalk domain was derived from the H1 HA, no polybasic cleavage site was included in this construct. In this case, the NA and the internal genes were derived from the B/Yamagata/16/1988 virus.
The influenza A viruses A/Hong Kong/4801/2014 (H3N2, 6:2 A/PR/8/34 H1N1 reassortant) (63), H6N2A/Hong Kong/4801/2014 (6:2 A/PR/8/34 reassortant) (64), A/Switzerland/9715293/2013 (H3N2) (mouse-adapted) (65), A/Philippines/2/1982 (H3N2, X-79 6:2 A/PR/8/34 reassortant, mouse-adapted) (66), A/canine/Illinois/41915/2015 (H3N2) (67), A/swine/Missouri/A01727926/2015 (H4N6, 6:2 A/PR/8/34 reassortant) (68), A/Hunan/02285/2017 (H7N1, 7:1 A/PR/8/34 reassortant) (69), A/Shanghai/1/2013 (H7N9, 6:2 A/PR/8/34 reassortant) (70), A/Jiangxi-Donghu/346/2013 (H10N8, 6:2 A/PR/8/34 reassortant) (71), A/mallard/Gurjev/263/1982 (H14N1, 7:1 A/PR/8/34 reassortant), A/wedge-tailed shearwater/Western Australia/2576/1979 (H15N5) (6:1:1 A/PR/8/34 reassortant), A/Singapore/GP1908/2015 (H1N1, 6:2 A/Texas/1/1977 H3N2 reassortant) (National Institute for Biological Standards and Control), as well as the cHA viruses previously mentioned were grown in 10-day old embryonated chicken eggs at 37°C for 48 hours and were cooled at 4°C overnight (O/N). Cell debris was removed by low-speed centrifugation (4000g, 4°C, 20 min). Viruses were aliquoted, stored at −80°C, and titrated by the plaque assay method on MDCK cells (72). The HA from H6N2A/Hong Kong/4801/2014 virus was derived from the A/turkey/Massachusetts/3740/1965 (H6N2) virus, and the NA from A/wedge-tailed shearwater/Western Australia/2576/1979 (H15N5) virus was derived from the A/mallard/Sweden/86/2003 (H12N5) virus.
All viruses described above were generated by reverse genetics except for A/Switzerland/9715293/2013 (H3N2), which was adapted to mice by serial passaging, and A/canine/Illinois/41915/2015 (H3N2), which was grown in MDCK cells after isolation.
Production of inactivated split influenza virus vaccines
Vaccine production was performed as previously described (40). Virus inactivation was performed with 0.05% (v/v) β-propiolactone (Millipore Sigma) prepared in ice-cold water for injection (Gibco) for 30 min after pH buffering with 0.01 M disodium hydrogen phosphate (Millipore Sigma), and stopped by incubation at 37°C for 2 hours. Then, the inactivated virus sample was centrifuged at 4000 rpm, 4°C for 30 min. Twenty-five to 30 ml of the supernatant was loaded on a 5 ml 30% (w/v) sucrose cushion prepared in 1× NTE buffer consisting of 1 M NaCl, 100 mM tris-HCl, and 10 mM EDTA in water for injection with the pH adjusted to 7.4. The supernatant containing the inactivated virus was concentrated by high-speed centrifugation (25000 rpm, 4°C for 2 hours), and the pelleted virus was resuspended in PBS (pH 7.4) (Gibco). The resuspended virus was split with 1% (v/v) Triton X-100 (Fisher Bioreagents), and the detergent was removed by incubation with 0.2 to 0.3 g of Bio-Beads SM-2 (Bio-Rad) per milliliter of inactivated split virus. The supernatant was collected, and the total protein concentration was adjusted to 0.5 to 1 mg/ml with PBS (pH 7.4) using the Bradford assay (Bio-Rad). Vaccine samples were aliquoted and stored at −80°C until use. The concentration of HA in the final cH15/3HK14N2HK14 and cH4/3HK14N2HK14 vaccine preparations was quantified in a nonreducing Western blot with the 12D1 murine mAb (73). Different dilutions of an H3 HK14 recombinant protein standard of known concentration were also included for absolute HA quantification.
Enzyme-linked immunosorbent assay
Immulon 4HBX 96-well plates (Thermo Fisher Scientific) were coated with recombinant protein (2 μg/ml; 50 μl per well) in PBS (pH 7.4) at 4°C O/N. The next day, plates were washed three times with PBS containing 0.1% (v/v) Tween 20 (PBS-T) and blocked in blocking solution [3% (v/v) goat serum and 0.5% (w/v) nonfat dry milk in PBS-T] for 1 hour at room temperature (RT). After blocking, mouse serum was added to the first well at a 1:30 dilution (150 μl per well) and serially diluted 1:3 in blocking solution and incubated for 2 hours at 20°C. Plates were washed three times with PBS-T before adding the secondary antibody (100 μl per well). For total IgG quantification, a 1:3000 dilution of sheep anti-mouse IgG (H&L) peroxidase conjugate (Rockland) in blocking solution was added. For IgG1 and IgG2a quantification, a 1:20,000 and 1:2000 dilution in blocking solution of rabbit anti-mouse IgG1 or rabbit anti-mouse IgG2a (Invitrogen) was added, respectively. Afterward, plates were incubated for 1 hour at 20°C and then washed four times with PBS-T with shaking. To develop plates, 100 μl of O-phenylenediamine dihydrochloride (OPD) substrate (SigmaFast OPD, Millipore Sigma) was added to each well. After a 10-min incubation, the reaction was stopped by adding 50 μl of 3 M hydrochloric acid (HCl) to each well. The optical density at 490 nm (OD490) was measured on a Synergy H1 microplate reader (BioTek). A cutoff value calculated as the average of the OD490 values of blank wells plus three times the SD was established for each plate and used for calculating the area under the curve (AUC). AUC values were determined using GraphPad Prism 9 software.
Competition ELISA
Immulon 4HBX 96-well plates were coated with 2 μg/ml of H3 HK14 recombinant protein (50 μl per well) in PBS (pH = 7.4) O/N at 4°C. Plates were washed three times with PBS-T and blocked in blocking solution for 1.5 hours at RT. After discarding the blocking solution, mouse serum was added to the first well at a 1:50 dilution (100 μl per well) and serially diluted 1:3 in blocking solution and incubated for 2 hours at 20°C. Plates were then washed three times with PBS-T, biotinylated FluA-20 mAb (100 μl per well) (45) at a concentration of 0.05 μg/ml was added, and plates were incubated at 20°C for 1 hour. Plates were washed three times with PBS-T and subsequently incubated with a 1:3000 dilution of streptavidin conjugated to horseradish peroxidase (Thermo Fisher Scientific) in blocking solution (100 μl per well). After 1-hour incubation at 20°C, plates were washed four times with PBS-T with shaking and then developed with 100 μl of OPD substrate per well. After 10-min incubation, the reaction was stopped by adding 50 μl of 3 M HCl to each well. The OD490 was measured on a Synergy H1 microplate reader. Antibody competition was defined as the percentage ratio between sample signal and the signal of FluA-20 mAb binding diluted in PBS (no mouse serum) and calculated as [1 − (ODsample/ODno serum control)] × 100. The data were analyzed using GraphPad Prism 9 software, and values were expressed as AUC. The background of the assay was defined as the average of the AUC from the mouse serum of the PBS group.
ADCC reporter assay
ADCC activity in mouse sera was assessed using an FcγRIV cell–based ADCC reporter assay according to the manufacturer’s instructions (Promega) (57). Briefly, white 96-well plates (Corning) were seeded with 2 × 104 MDCK cells per well and incubated O/N at 37°C and 5% CO2. After 24 hours, MDCK cells were washed with PBS and infected with the A/Hong Kong/4801/2014 (H3N2) virus at a multiplicity of infection of 5 for a single cycle of virus replication and incubated O/N at 37°C and 5% CO2. The following day, the cell culture medium was removed, and 25 μl of assay buffer [RPMI 1640 medium supplemented with 4% (v/v) low IgG FBS, Gibco] was added to each well. Mouse sera previously heat-inactivated at 56°C for 1 hour were serially diluted twofold in RPMI 1640 medium and added to the infected MDCK cells (25 μl per well). The sera were incubated with MDCK cells at 37°C for 30 min. Then, 7.5 × 104 Jurkat cells expressing the mouse FcγRIV with a luciferase reporter gene under transcriptional control of the nuclear factor–activated T cell promoter (Promega) were added per well (25 μl per well) and incubated at 37°C for 6 hours. After incubation, 75 μl of Bio-Glo luciferase assay reagent (Promega) was added per well and incubated at RT in the dark for 10 min. The luminescence signal was measured using a Synergy H1 microplate reader. The fold induction was calculated as follows: (RLUinduced − RLUbackground)/(RLUno antibody control − RLUbackground), where RLU is relative luminescence units (57). The AUC values of the resulting fold induction values were calculated using the GraphPad Prism 9 software.
NAI assay
The NA activity of H6N2A/Hong Kong/4801/2014 virus was assessed by the NA assay on Immulon 4HBX 96-well plates coated with 100 μl of fetuin (Millipore Sigma) at 25 μg/ml in PBS at 4°C O/N. Fetuin-coated plates were washed three times with PBS-T and blocked with PBS + 5% (w/v) BSA (MP Biomedicals). On a separate plate, the virus was serially diluted 1:2 in PBS + 1% (w/v) BSA, and 75 μl of prediluted virus samples was added to fetuin-coated plates already containing 75 μl of PBS + 1% (w/v) BSA. The fetuin-coated plates were incubated at 37°C O/N. Afterward, plates were washed four times with PBS-T with shaking, and 100 μl per well of peroxidase-labeled lectin from Arachis hypogaea (peanut agglutinin, Millipore Sigma) at 5 μg/ml in PBS + 1% (w/v) BSA was added to the plates. Plates were incubated at 20°C for 1.5 hours before washing four times with PBS-T with shaking. To develop the plates, 100 μl of OPD substrate was added per well and incubated for 10 min at RT, and the reaction was stopped by adding 50 μl of 3 M HCl per well. The OD490 was measured on a Synergy H1 microplate reader, and the half maximal effective concentration (EC50) was determined using the GraphPad Prism 9 software.
For the NAI assay, Immulon 4HBX 96-well plates were coated with 100 μl of fetuin at 25 μg/ml in PBS at 4°C O/N. Fetuin-coated plates were washed three times with PBS-T and blocked with PBS + 5% (w/v) BSA. In parallel, heat-inactivated sera (56°C for 1 hour) were serially diluted 1:2 in PBS + 1% (w/v) BSA with a starting dilution of 1:30 in nonfetuin-coated 96-well plates (75 μl per well). Then, 75 μl of the H6N2A/Hong Kong/4801/2014 virus corresponding to 2× EC50 was added per well to the prediluted sera plates and incubated at 20°C for 1.5 hours. After incubation, 100 μl of the virus/serum mixture was transferred per well to fetuin-coated plates and incubated at 37°C O/N. After incubation, the rest of the assay was performed as described above for the NA assay. No serum (virus only) and background controls [PBS + 1% (w/v) BSA only] were also included to measure the NAI. The OD490 was measured on a Synergy H1 microplate reader, and the inhibition was calculated as (ODmeasured − ODbackground)/(ODno serum control − ODbackground) in the GraphPad Prism 9 software and expressed as 50% inhibitory dilution (ID50).
Microneutralization assay
Mouse sera were treated with receptor destroying enzyme (RDE) II (Denka Seiken) and incubated in a 37°C water bath for 18 to 20 hours. The same day, MDCK cells were seeded in 96-well cell culture–treated plates (Corning) at 1.8 × 104 cells per well (100 μl per well) and incubated at 37°C with 5% CO2 O/N. The following day, the RDE activity was stopped by the addition of a 2.5% (w/v) sodium citrate solution and incubation at 56°C for 1 hour (74). RDE-treated sera were serially diluted 1:2 in minimum essential medium (MEM, Gibco) with 10 mM 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid (HEPES) (Gibco), 2 mM l-glutamine (Gibco), 3.2% (w/v) sodium bicarbonate (Corning), 1.2% (w/v) BSA, penicillin (100 U/ml), and streptomycin (100 μg/ml; Gibco) (infection medium). Next, 120 μl of 100 × tissue culture infectious dose of virus prepared in infection medium and 120 μl of serially diluted sera were incubated on a shaker at RT for 1 hour. MDCK cells were washed with 220 μl of PBS and incubated with 100 μl of the incubated serum-virus mixture at 37°C with 5% CO2 for 1 hour. Afterward, the virus inoculum was aspirated, MDCK cells were washed with PBS, and 100 μl of the serially diluted sera containing N-tosyl-l-phenylalanine chloromethyl ketone (TPCK)–treated trypsin (1 μg/ml; Millipore Sigma) was added to the cells and incubated at 37°C with 5% CO2 for 48 hours. After 48 hours, the presence of virus was assessed by hemagglutination assay. Briefly, 50 μl of the cell supernatant was added to 96-well V-bottom plates (Nunc) and serially diluted 1:2. Then, 50 μl of 0.5% (v/v) turkey red blood cells (RBCs; Lampire Biological Laboratories) in PBS was added to each well, and plates were incubated on ice or at 4°C for 45 min. The HA titer was calculated as the endpoint titer at which no RBC tear drop formation (the absence is indicative of hemagglutination/presence of virus) after tilting the plate to 90° for 10 to 20 s could be detected.
Animal studies
All animal experiments were performed under protocols approved by the Icahn School of Medicine at Mount Sinai Institutional Animal Care and Use Committee. For all animal experiments conducted, 6- to 8-week-old female BALB/c and DBA/2J mice (Jackson Laboratories) were used unless otherwise mentioned. The CpG 1018 adjuvant used in this study, a Toll-like receptor 9 agonist, was provided by Dynavax Technologies. To simulate preexisting immunity to the H1 stalk, mice were anesthetized intraperitoneally with a ketamine/xylazine in water cocktail and i.n. inoculated with 105 plaque-forming units (PFU) of the chimeric B-cH5/1 virus (50 μl per mouse) (75). For i.m. vaccination, 1 μg HA of group 2 cHA split vaccine prepared in PBS with or without the CpG 1018 adjuvant was given per mouse (50 μl) unless otherwise indicated. A negative control (PBS), an irrelevant protein (BSA) + CpG 1018 adjuvant, and a positive control (matched inactivated virus) were prepared in PBS to 50 μl total volume. The amount of BSA was adjusted to the maximum amount of total protein in the vaccine administered to mice. When testing the combined effect of the CpG 1018 and aluminum hydroxide gel 2% (alum) adjuvants, vaccines were prepared in a saline solution composed of 20 mM tris and 100 mM NaCl (pH = 7.5). For the LAIV, mice were vaccinated with 105 PFU per mouse (50 μl) of group 2 cHA LAIV via the i.n. route. The vaccination regimen consisted of priming with the B-cH5/1 virus (i.n.) and sequential vaccination with the cH15/3HK14N2HK14 split vaccine or LAIV after 4 weeks and boosted with cH4/3HK14N2HK14 split vaccine or LAIV after four additional weeks. Combinations of split vaccine and LAIV were also tested. Six weeks after vaccination, mice were anesthetized and i.n. infected with 50 μl of influenza virus containing 0.1×, 0.5×, or 5× the 50% mouse lethal dose (LD50) depending on what readout was used. LD50 values were calculated by challenging 6- to 8-week-old female BALB/c or DBA/2J mice with different doses of the respective influenza A viruses. The calculated LD50 values were 316.2 PFU per mouse in DBA/2J mice for A/Switzerland/9715293/2013 (H3N2), 17.8 PFU per mouse in BALB/c mice for A/Philippines/2/1982 (H3N2, X-79), 63.1 PFU per mouse in DBA/2J mice for A/canine/Illinois/41915/2015 (H3N2), 177.8 PFU per mouse in DBA/2J mice for A/swine/Missouri/A01727926/2015 (H4N6), 1778.3 PFU per mouse in BALB/c mice for A/Shanghai/1/2013 (H7N9), 1778.3 PFU per mouse in BALB/c mice for A/Jiangxi-Donghu/346/2013 (H10N8), 56234.1 PFU per mouse in DBA/2J mice for A/mallard/Gurjev/263/1982 (H14N1), 562.3 PFU per mouse in BALB/c mice for A/wedge-tailed shearwater/Western Australia/2576/1979 (H15N5), and 56.2 PFU per mouse in BALB/c mice for A/Singapore/GP1908/2015 (H1N1). DBA/2J mice are more sensitive to influenza virus infection and were used in cases where viruses were nonlethal in BALB/c mice (76). The age of mice challenged after sequential immunization was in the range of 20 to 22 weeks old, meaning that they were older than the mice used for the LD50 experiments, and age can influence the lethal dose for a virus strain in the mouse model. In the past, we have performed studies to look at sex differences for HA stalk-based vaccination in mice in collaboration with Sabra Klein’s laboratory and found—in young mice—no differences in terms of protection (77). Therefore, since female mice are easier to handle, we have only used female mice here.
Mice were bled before virus challenge via submandibular bleeding for serological analysis. Blood was incubated at RT for 1 hour and centrifuged at 5000g for 30 min. Serum was separated from pellet and stored at 4°C until analysis. After virus challenge, weight loss was monitored for 14 days, and mice showing a weight loss of ≥25% as compared with their initial body weight were humanely euthanized.
For the serum passive transfer experiment, 6- to 8-week-old female DBA/2J mice were prime-boosted as previously described. Mice were anesthetized, and blood was collected by cardiac puncture, incubated at RT for 1 hour, and centrifuged at 5000g for 30 min. Serum was separated from the pellet and stored at 4°C until use. Naïve mice were administered 200 μl of pooled sera per mouse via the intraperitoneal route. After 2 to 3 hours, mice were challenged with 3 × LD50 of the A/Switzerland/9715293/2013 (H3N2) virus, and weight loss was monitored for the next 14 days.
For the duration of the experiments, mice were housed in individually ventilated cages on a 12-hour dark/light cycle with controlled temperature/humidity. Food and water were provided ad libitum.
Lung titers
Virus titers in the lungs of mice challenged with 0.1 × LD50 of the A/Philippines/2/1982 (H3N2, X-79) virus at days 3 and 6 after challenge were analyzed by the plaque assay method in 12-well plates (Corning). A sublethal challenge dose was used since it usually results in higher resolution between groups for lung titer experiments. Briefly, harvested lungs were homogenized in 2 disruption cycles (10 s/cycle) using tubes that contained high impact zirconium beads (Andwin Scientific) and 1 ml of PBS. Lung homogenates were serially diluted 1:10 in PBS. Samples were incubated for 1 hour with MDCK cells seeded at 3 × 105 cells per well (1 ml per well) the day before. After the 1-hour incubation, an agarose overlay containing a final concentration of 0.64% (w/v) agarose (Oxoid) in minimal essential medium (MEM) supplemented with 2 mM l-glutamine, 0.1% (w/v) of sodium bicarbonate, 10 mM HEPES, penicillin (100 U/ml), streptomycin (100 μg/ml), 0.2% (w/v) BSA, TPCK-treated trypsin (1 μg/ml), and 0.1% (w/v) diethylaminoethyl-dextran was added to the cells. The cells were incubated at 37°C for 48 hours, and visible plaques were counted after fixation with 3.7% (v/v) formaldehyde in PBS and visualization by immunostaining. All virus titers are presented as log10 PFU per milliliter.
Flow cytometry
For lung collection, mice were euthanized, the chest was opened, and the right ventricle of the heart was perfused with 10 ml of cold PBS. Lungs were minced and incubated in a collagenase I (150 U/ml; Gibco)/deoxyribonuclease I (50 U/ml; Millipore Sigma) solution in a 37°C water bath for 30 min. Following digestion, tissue was pushed several times through an 18G needle and filtered through a 70-μm cell strainer (BD Biosciences). Spleens were mechanically disrupted with pestle homogenizers for 1.5-ml tubes (Thermo Fisher Scientific) and filtered as described above. Erythrocytes were lysed with RBC lysis buffer (BioLegend) in accordance with the manufacturer’s instructions. Cells were washed with 2% (v/v) FBS in PBS and reconstituted in 5 ml of complete media [RPMI 1640 supplemented with 10% (v/v) FBS, penicillin (100 U/ml), and streptomycin (100 μg/ml)]. Cell count and viability were analyzed in a Countess II automated cell counter (Thermo Fisher Scientific) using a 0.4% (v/v) Trypan blue solution. Cells (2 × 106) per well were seeded in 96-well cell culture–treated plates. For antigen-specific T-cellular immune response analysis, cells were stimulated with peptide pool libraries (1 μl per well) containing 15 amino acid peptides with 11 amino acid overlap (5 μg/ml) covering the whole sequences of HA and NA proteins of the A/Perth/16/2009 (H3N2) influenza virus strain (NR-19266 for HA and NR-19267 for NA, BEI Resources), as well as the whole sequence of the NP protein of A/PR/8/34 (H1N1) virus (Miltenyi Biotec). Stimulation was performed at 37°C with 5% CO2 for 6 hours in the presence of Brefeldin A (5 μg/ml; BioLegend), 2 μM Monensin (BioLegend), and costimulatory anti-CD28 antibodies (25 μg/ml; BioLegend). Positive control samples were stimulated with Cell Activation Cocktail (BioLegend) in accordance with the manufacturer’s instructions. The negative control samples were incubated in the presence of all the aforementioned reagents, except the specific antigens.
Following stimulation, cells were stained with fluorescently labeled antibodies (BioLegend) targeting different surface markers for the discrimination of naïve, EM, and central memory T lymphocyte subpopulations (0.5 μl per sample of CD3-BV711, 0.125 μl per sample of CD4-PerCP/Cy5.5, 0.25 μl per sample of CD8-BV785, 0.125 μl per sample of CD62L-APC/Cy7, and 0.25 μl per sample of CD44-PE/Cy7). Intracellular staining of cytokines was performed with Cytofix/Cytoperm Fixation/Permeabilization Solution Kit (BD Biosciences) according to the manufacturer’s instructions. TNFα-AF488 (0.25 μl per sample), IFNγ-BV605 (1 μl per sample), and IL-2–phycoerythrin (0.5 μl per sample) antibodies (BioLegend) were used to identify the corresponding cytokines. Data were collected on an Attune flow cytometer (Thermo Fisher Scientific) and analyzed with the FlowJo_v10.8.1 software. The gating strategy is shown in fig. S4. To calculate statistical differences between groups, the percentage of cytokine-producing cells in nonstimulated samples was subtracted from the corresponding values for peptide-stimulated samples. Groups were compared using one-way analysis of variance (ANOVA) corrected for Dunn’s multiple comparisons test with the RStudio software (R version 4.2.0, RStudio Inc.).
Statistical analyses
The details about the type of statistical analyses performed for each experiment are listed in the respective figure legends. Briefly, GraphPad Prism 9 was used for statistical analysis of antibody titers, NAI, competition ELISA, and microneutralization assays using one-way ANOVA corrected for Dunnett’s multiple comparisons test. One-way ANOVA corrected for Dunn’s multiple comparisons test was used for the comparison of viral titers in lungs and T cell studies. Significance was considered with P values equal or less than 0.05. The chosen sample size in each experiment is sufficient to generate statistically significant results. The following are the strain designations for sequences used in Fig. 1C: Bris07 (H1N1): A/Brisbane/59/2007 (H1N1), Cal09 (H1N1): A/California/07/2009 (H1N1), Sing15 (H1N1): A/Singapore/GP1908/2015 (H1N1), canine (H3N2): A/canine/Illinois/41915/2015 (H3N2), HK14 (H3N2): A/Hong Kong/4801/2014 (H3N2), HK68 (H3N2): A/Hong Kong/1/1968 (H3N2), Switz13 (H3N2): A/Switzerland/9715293/2013 (H3N2), X-79 (H3N2): A/Philippines/2/1982 (H3N2, X-79), Wisc05 (H3N2): A/Wisconsin/67/2005 (H3N2), duck (H4N6): A/duck/Czechoslovakia/1956 (H4N6), swine (H4N6): A/swine/Missouri/A01727926/2915 (H4N6), H7N9: A/Shanghai/1/2013 (H7N9), H10N7: A/mallard/Interior Alaska/9BM3355R0/2009 (H10N7), H10N8: A/Jiangxi-Donghu/346/2013 (H10N8), H15N5: A/wedge-tailed shearwater/Western Australia/2576/1979 (H15N9), and B/Col17: B/Colorado/06/2017.
Acknowledgments
We thank Dynavax Technologies (Emeryville, CA, USA), especially D. Campbell, for providing the CpG 1018 adjuvant, L. Chang and M. Schotsaert for the alum adjuvant, I. Mena and A. García-Sastre for the A/canine/Illinois/41915/2015 (H3N2) virus, C. Marizzi for suggesting helpful language edits, R. Albrecht for help with further work on alternative animal models not included here (Department of Microbiology, Icahn School of Medicine at Mount Sinai, NY, USA), and Rebecca Gillespie and M. Kanekiyo for the mini H3 protein sequence (Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA). The following reagents were obtained through BEI Resources, NIAID, NIH: peptide array, influenza virus A/Perth/16/2009 (H3N2) hemagglutinin (NR-19266), and neuraminidase proteins (NR-19267). Some of the figures were created with BioRender.com.
Funding: This study was partially supported by a Department of Defense grant W81XWH-18-1-0488 (F.K.), the Collaborative Influenza Vaccine Innovation Centers (CIVIC) contract 75N93019C00051 (F.K., I.A.W., and P.P.), the Center for Excellence on Influenza Research and Response (CEIRR) contract 75N93021C00014 (P.P.), and NIH grants P01AI097092 (P.P.), R01AI145870 (P.P.), R01AI141226 (P.P.) and Dynavax Technologies. E.P.-M. was supported by a postdoctoral fellowship from Fundación Ramón Areces.
Author contributions: E.P.-M. and F.K. conceived and designed the study. E.P.-M., K.V., A.B., M.L., B.F., M.J.S., G.A.A., and I.G.-D. generated laboratory data. E.P.-M., K.V., and F.K. analyzed the data. X.Z., I.A.W., L.C., W.S., and P.P. provided monoclonal antibodies, proteins, and viruses. E.P.-M., K.V., and F.K. wrote the manuscript. All authors critically reviewed the paper and approved the final version of the paper for submission.
Competing interests: The Icahn School of Medicine at Mount Sinai has filed patent applications regarding influenza virus vaccines on which E.P.-M., F.K., and P.P. are listed as inventors. The Krammer laboratory has received support for influenza virus research in the past from GSK and is currently receiving support from Dynavax. F.K. is currently consulting for GSK, Third Rock Ventures, Pfizer, and Avimex. The authors declare that they have no other competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Reagents and antigens described in the manuscript can be provided by the Krammer laboratory pending scientific review and a completed material transfer agreement (and any required shipping/handling permits for viruses). Requests for the reagents and antigens should be submitted to florian.krammer@mssm.edu.
Supplementary Materials
This PDF file includes:
Figs. S1 to S5
REFERENCES AND NOTES
- 1.E. A. Belongia, H. Q. McLean, Influenza Vaccine Effectiveness: Defining the H3N2 Problem. Clin. Infect. Dis. 69, 1817–1823 (2019). [DOI] [PubMed] [Google Scholar]
- 2.G. N. Okoli, F. Racovitan, T. Abdulwahid, C. H. Righolt, S. M. Mahmud, Variable seasonal influenza vaccine effectiveness across geographical regions, age groups and levels of vaccine antigenic similarity with circulating virus strains: A systematic review and meta-analysis of the evidence from test-negative design studies after the 2009/10 influenza pandemic. Vaccine 39, 1225–1240 (2021). [DOI] [PubMed] [Google Scholar]
- 3.R. A. Neher, T. Bedford, R. S. Daniels, C. A. Russell, B. I. Shraiman, Prediction, dynamics, and visualization of antigenic phenotypes of seasonal influenza viruses. Proc. Natl. Acad. Sci. U.S.A. 113, E1701–E1709 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Y. Suzuki, Positive selection for gains of N-linked glycosylation sites in hemagglutinin during evolution of H3N2 human influenza A virus. Genes Genet. Syst. 86, 287–294 (2011). [DOI] [PubMed] [Google Scholar]
- 5.S. Rajaram, R. Wojcik, C. Moore, R. Ortiz de Lejarazu, S. de Lusignan, E. Montomoli, A. Rossi, A. Pérez-Rubio, A. Trilla, V. Baldo, R. Jandhyala, G. Kassianos, The impact of candidate influenza virus and egg-based manufacture on vaccine effectiveness: Literature review and expert consensus. Vaccine 38, 6047–6056 (2020). [DOI] [PubMed] [Google Scholar]
- 6.L. K. Borkenhagen, G. L. Wang, R. A. Simmons, Z. Q. Bi, B. Lu, X. J. Wang, C. X. Wang, S. H. Chen, S. X. Song, M. Li, T. Zhao, M. N. Wu, L. P. Park, W. C. Cao, M. J. Ma, G. C. Gray, High risk of influenza virus infection among swine workers: Examining a dynamic cohort in China. Clin. Infect. Dis. 71, 622–629 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.R. Yang, H. Sun, F. Gao, K. Luo, Z. Huang, Q. Tong, H. Song, Q. Han, J. Liu, Y. Lan, J. Qi, H. Li, S. Chen, M. Xu, J. Qiu, G. Zeng, X. Zhang, C. Huang, R. Pei, Z. Zhan, B. Ye, Y. Guo, Y. Zhou, W. Ye, D. Yao, M. Ren, B. Li, J. Yang, Y. Wang, J. Pu, Y. Sun, Y. Shi, W. J. Liu, X. Ou, G. F. Gao, L. Gao, J. Liu, Human infection of avian influenza A H3N8 virus and the viral origins: A descriptive study. Lancet Microbe 3, e824–e834 (2022). [DOI] [PubMed] [Google Scholar]
- 8.C. Ke, C. K. P. Mok, W. Zhu, H. Zhou, J. He, W. Guan, J. Wu, W. Song, D. Wang, J. Liu, Q. Lin, D. K. W. Chu, L. Yang, N. Zhong, Z. Yang, Y. Shu, J. S. M. Peiris, Human Infection with Highly Pathogenic Avian Influenza A(H7N9) Virus, China. Emerg. Infect. Dis. 23, 1332–1340 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.H. Chen, H. Yuan, R. Gao, J. Zhang, D. Wang, Y. Xiong, G. Y. Fan, F. Yang, X. Li, J. Zhou, S. Zou, L. Yang, T. Chen, L. Dong, H. Bo, X. Zhao, Y. Zhang, Y. Lan, T. Bai, J. Dong, Q. Li, S. W. Wang, Y. P. Zhang, H. Li, T. Gong, Y. Shi, X. Ni, J. Li, J. Zhou, J. Fan, J. Wu, X. Zhou, M. Hu, J. Wan, W. Z. Yang, D. X. Li, G. Wu, Z. J. Feng, G. F. Gao, Y. Wang, Q. Jin, M. Liu, Y. Shu, Clinical and epidemiological characteristics of a fatal case of avian influenza A H10N8 virus infection: A descriptive study. Lancet 383, 714–721 (2014). [DOI] [PubMed] [Google Scholar]
- 10.J. Jing, L. Wang, G. Wang, Z. Dai, W. Ren, C. Yi, J. Wei, C. Xu, A human infection case with avian-origin H10N3 influenza virus. Quant. Imaging Med. Surg. 11, 4508–4510 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.M. Kanekiyo, B. S. Graham, Next-Generation Influenza Vaccines. Cold Spring Harb. Perspect. Med. 11, a038448 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.F. Krammer, A. Garcia-Sastre, P. Palese, Is It Possible to Develop a "Universal" Influenza Virus Vaccine? Potential Target Antigens and Critical Aspects for a Universal Influenza Vaccine. Cold Spring Harb. Perspect. Biol. 10, a028845 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.T. M. Caradonna, L. Ronsard, A. S. Yousif, I. W. Windsor, R. Hecht, T. Bracamonte-Moreno, A. A. Roffler, M. J. Maron, D. P. Maurer, J. Feldman, E. Marchiori, R. M. Barnes, D. Rohrer, N. Lonberg, T. H. Oguin III, G. D. Sempowski, T. B. Kepler, M. Kuraoka, D. Lingwood, A. G. Schmidt, An epitope-enriched immunogen expands responses to a conserved viral site. Cell Rep. 41, 111628 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.M. E. Ermler, E. Kirkpatrick, W. Sun, R. Hai, F. Amanat, V. Chromikova, P. Palese, F. Krammer, Chimeric hemagglutinin constructs induce broad protection against influenza B virus challenge in the mouse model. J. Virol. 91, e00286-17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D. Eggink, P. H. Goff, P. Palese, Guiding the immune response against influenza virus hemagglutinin toward the conserved stalk domain by hyperglycosylation of the globular head domain. J. Virol. 88, 699–704 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.S. M. Moin, J. C. Boyington, S. Boyoglu-Barnum, R. A. Gillespie, G. Cerutti, C. S. F. Cheung, A. Cagigi, J. R. Gallagher, J. Brand, M. Prabhakaran, Y. Tsybovsky, T. Stephens, B. E. Fisher, A. Creanga, S. Ataca, R. Rawi, K. S. Corbett, M. C. Crank, G. B. Karlsson Hedestam, J. Gorman, A. B. McDermott, A. K. Harris, T. Zhou, P. D. Kwong, L. Shapiro, J. R. Mascola, B. S. Graham, M. Kanekiyo, Co-immunization with hemagglutinin stem immunogens elicits cross-group neutralizing antibodies and broad protection against influenza A viruses. Immunity 55, 2405–2418.e7 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.M. McMahon, G. O’Dell, J. Tan, A. Sárközy, M. Vadovics, J. M. Carreño, E. Puente-Massaguer, H. Muramatsu, C. Bajusz, W. Rijnink, M. Beattie, Y. K. Tam, E. Kirkpatrick Roubidoux, I. Francisco, S. Strohmeier, M. Kanekiyo, B. S. Graham, F. Krammer, N. Pardi, Assessment of a quadrivalent nucleoside-modified mRNA vaccine that protects against group 2 influenza viruses. Proc. Natl. Acad. Sci. U.S.A. 119, e2206333119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.H. M. Yassine, J. C. Boyington, P. M. McTamney, C. J. Wei, M. Kanekiyo, W. P. Kong, J. R. Gallagher, L. Wang, Y. Zhang, M. G. Joyce, D. Lingwood, S. M. Moin, H. Andersen, Y. Okuno, S. S. Rao, A. K. Harris, P. D. Kwong, J. R. Mascola, G. J. Nabel, B. S. Graham, Hemagglutinin-stem nanoparticles generate heterosubtypic influenza protection. Nat. Med. 21, 1065–1070 (2015). [DOI] [PubMed] [Google Scholar]
- 19.A. W. Freyn, J. Ramos da Silva, V. C. Rosado, C. M. Bliss, M. Pine, B. L. Mui, Y. K. Tam, T. D. Madden, L. C. de Souza Ferreira, D. Weissman, F. Krammer, L. Coughlan, P. Palese, N. Pardi, R. Nachbagauer, A multi-targeting, nucleoside-modified mRNA influenza virus vaccine provides broad protection in mice. Mol. Ther. 28, 1569–1584 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.R. Hai, F. Krammer, G. S. Tan, N. Pica, D. Eggink, J. Maamary, I. Margine, R. A. Albrecht, P. Palese, Influenza viruses expressing chimeric hemagglutinins: Globular head and stalk domains derived from different subtypes. J. Virol. 86, 5774–5781 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.C.-J. Chen, M. E. Ermler, G. S. Tan, F. Krammer, P. Palese, R. Hai, Influenza A viruses expressing intra- or intergroup chimeric hemagglutinins. J. Virol. 90, 3789–3793 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.X. Zhu, J. Han, W. Sun, E. Puente-Massaguer, W. Yu, P. Palese, F. Krammer, A. B. Ward, I. A. Wilson, Influenza chimeric hemagglutinin structures in complex with broadly protective antibodies to the stem and trimer interface. Proc. Natl. Acad. Sci. U.S.A. 119, e2200821119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.R. Nachbagauer, D. Kinzler, A. Choi, A. Hirsh, E. Beaulieu, N. Lecrenier, B. L. Innis, P. Palese, C. P. Mallett, F. Krammer, A chimeric haemagglutinin-based influenza split virion vaccine adjuvanted with AS03 induces protective stalk-reactive antibodies in mice. npj Vaccines 1, 16015 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.W. C. Liu, R. Nachbagauer, D. Stadlbauer, S. Strohmeier, A. Solórzano, F. Berlanda-Scorza, B. L. Innis, A. García-Sastre, P. Palese, F. Krammer, R. A. Albrecht, Chimeric hemagglutinin-based live-attenuated vaccines confer durable protective immunity against influenza A viruses in a preclinical ferret model. Vaccines 9, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.R. Nachbagauer, W. C. Liu, A. Choi, T. J. Wohlbold, T. Atlas, M. Rajendran, A. Solórzano, F. Berlanda-Scorza, A. García-Sastre, P. Palese, R. A. Albrecht, F. Krammer, A universal influenza virus vaccine candidate confers protection against pandemic H1N1 infection in preclinical ferret studies. NPJ Vaccines 2, 26 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.R. Nachbagauer, J. Feser, A. Naficy, D. I. Bernstein, J. Guptill, E. B. Walter, F. Berlanda-Scorza, D. Stadlbauer, P. C. Wilson, T. Aydillo, M. A. Behzadi, D. Bhavsar, C. Bliss, C. Capuano, J. M. Carreño, V. Chromikova, C. Claeys, L. Coughlan, A. W. Freyn, C. Gast, A. Javier, K. Jiang, C. Mariottini, M. McMahon, M. McNeal, A. Solórzano, S. Strohmeier, W. Sun, M. van der Wielen, B. L. Innis, A. García-Sastre, P. Palese, F. Krammer, A chimeric hemagglutinin-based universal influenza virus vaccine approach induces broad and long-lasting immunity in a randomized, placebo-controlled phase I trial. Nat. Med. 27, 106–114 (2021). [DOI] [PubMed] [Google Scholar]
- 27.N. Folschweiller, C. vanden Abeele, L. Chu, P. van Damme, A. García-Sastre, F. Krammer, R. Nachbagauer, P. Palese, A. Solórzano, D. Bi, M. P. David, D. Friel, B. L. Innis, J. Koch, C. P. Mallett, R. N. Rouxel, B. Salaun, V. Vantomme, C. Verheust, F. Struyf, Reactogenicity, safety, and immunogenicity of chimeric haemagglutinin influenza split-virion vaccines, adjuvanted with AS01 or AS03 or non-adjuvanted: A phase 1-2 randomised controlled trial. Lancet Infect. Dis. 22, 1062–1075 (2022). [DOI] [PubMed] [Google Scholar]
- 28.D. I. Bernstein, J. Guptill, A. Naficy, R. Nachbagauer, F. Berlanda-Scorza, J. Feser, P. C. Wilson, A. Solórzano, M. van der Wielen, E. B. Walter, R. A. Albrecht, K. N. Buschle, Y. Q. Chen, C. Claeys, M. Dickey, H. L. Dugan, M. E. Ermler, D. Freeman, M. Gao, C. Gast, J. J. Guthmiller, R. Hai, C. Henry, L. Y. L. Lan, M. McNeal, A. K. E. Palm, D. G. Shaw, C. T. Stamper, W. Sun, V. Sutton, M. E. Tepora, R. Wahid, H. Wenzel, T. J. Wohlbold, B. L. Innis, A. García-Sastre, P. Palese, F. Krammer, Immunogenicity of chimeric haemagglutinin-based, universal influenza virus vaccine candidates: Interim results of a randomised, placebo-controlled, phase 1 clinical trial. Lancet Infect. Dis. 20, 80–91 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.F. Krammer, I. Margine, R. Hai, A. Flood, A. Hirsh, V. Tsvetnitsky, D. Chen, P. Palese, H3 stalk-based chimeric hemagglutinin influenza virus constructs protect mice from H7N9 challenge. J. Virol. 88, 2340–2343 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.I. Margine, F. Krammer, R. Hai, N. S. Heaton, G. S. Tan, S. A. Andrews, J. A. Runstadler, P. C. Wilson, R. A. Albrecht, A. García-Sastre, P. Palese, Hemagglutinin stalk-based universal vaccine constructs protect against group 2 influenza A viruses. J. Virol. 87, 10435–10446 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.J. D. Campbell, Development of the CpG adjuvant 1018: A case study. Methods Mol. Biol. 1494, 15–27 (2017). [DOI] [PubMed] [Google Scholar]
- 32.R. Nachbagauer, A. Choi, R. Izikson, M. M. Cox, P. Palese, F. Krammer, Age dependence and isotype specificity of influenza virus hemagglutinin stalk-reactive antibodies in humans. MBio 7, e01996-15 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.R. Nachbagauer, A. Choi, A. Hirsh, I. Margine, S. Iida, A. Barrera, M. Ferres, R. A. Albrecht, A. García-Sastre, N. M. Bouvier, K. Ito, R. A. Medina, P. Palese, F. Krammer, Defining the antibody cross-reactome directed against the influenza virus surface glycoproteins. Nat. Immunol. 18, 464–473 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.K. M. Gostic, M. Ambrose, M. Worobey, J. O. Lloyd-Smith, Potent protection against H5N1 and H7N9 influenza via childhood hemagglutinin imprinting. Science 354, 722–726 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.M. Worobey, S. Plotkin, S. E. Hensley, Influenza vaccines delivered in early childhood could turn antigenic sin into antigenic blessings. Cold Spring Harb. Perspect. Med. 10, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Y. Q. Chen, L. Y. Lan, M. Huang, C. Henry, P. C. Wilson, Hemagglutinin stalk-reactive antibodies interfere with influenza virus neuraminidase activity by steric hindrance. J. Virol. 93, e01526-18 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.S. Kannanganat, C. Ibegbu, L. Chennareddi, H. L. Robinson, R. R. Amara, Multiple-cytokine-producing antiviral CD4 T cells are functionally superior to single-cytokine-producing cells. J. Virol. 81, 8468–8476 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.R. Nachbagauer, F. Krammer, R. A. Albrecht, A live-attenuated prime, inactivated boost vaccination strategy with chimeric hemagglutinin-based universal influenza virus vaccines provides protection in ferrets: A confirmatory study. Vaccine 6, 47 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.M. McMahon, G. Asthagiri Arunkumar, W. C. Liu, D. Stadlbauer, R. A. Albrecht, V. Pavot, M. Aramouni, T. Lambe, S. C. Gilbert, F. Krammer, Vaccination with viral vectors expressing chimeric hemagglutinin, NP and M1 antigens protects ferrets against influenza virus Challenge. Front. Immunol. 10, 2005 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.E. Puente-Massaguer, A. Beyer, M. Loganathan, I. Sapse, J. M. Carreño, G. Bajic, W. Sun, P. Palese, F. Krammer, Bioprocess development for universal influenza vaccines based on inactivated split chimeric and mosaic hemagglutinin viruses. Front. Bioeng. Biotechnol. 11, 1097349 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.G. H. Lee, S. G. Lim, CpG-adjuvanted hepatitis B vaccine (HEPLISAV-B®) update. Expert Rev. Vaccines 20, 487–495 (2021). [DOI] [PubMed] [Google Scholar]
- 42.D. Corti, J. Voss, S. J. Gamblin, G. Codoni, A. Macagno, D. Jarrossay, S. G. Vachieri, D. Pinna, A. Minola, F. Vanzetta, C. Silacci, B. M. Fernandez-Rodriguez, G. Agatic, S. Bianchi, I. Giacchetto-Sasselli, L. Calder, F. Sallusto, P. Collins, L. F. Haire, N. Temperton, J. P. M. Langedijk, J. J. Skehel, A. Lanzavecchia, A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza A hemagglutinins. Science 333, 850–856 (2011). [DOI] [PubMed] [Google Scholar]
- 43.D. J. Dilillo, G. S. Tan, P. Palese, J. V. Ravetch, Broadly neutralizing hemagglutinin stalk-specific antibodies require FcγR interactions for protection against influenza virus in vivo. Nat. Med. 20, 143–151 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.G. Asthagiri Arunkumar, A. Ioannou, T. J. Wohlbold, P. Meade, S. Aslam, F. Amanat, J. Ayllon, A. García-Sastre, F. Krammer, Broadly cross-reactive, nonneutralizing antibodies against influenza B virus hemagglutinin demonstrate effector function-dependent protection against lethal viral challenge in mice. J. Virol. 93, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.S. Bangaru, S. Lang, M. Schotsaert, H. A. Vanderven, X. Zhu, N. Kose, R. Bombardi, J. A. Finn, S. J. Kent, P. Gilchuk, I. Gilchuk, H. L. Turner, A. García-Sastre, S. Li, A. B. Ward, I. A. Wilson, J. E. Crowe Jr., A site of vulnerability on the influenza virus hemagglutinin head domain trimer interface. Cell 177, 1136–1152.e18 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.A. S. Monto, J. G. Petrie, R. T. Cross, E. Johnson, M. Liu, W. Zhong, M. Levine, J. M. Katz, S. E. Ohmit, Antibody to influenza virus neuraminidase: An independent correlate of protection. J Infect Dis 212, 1191–1199 (2015). [DOI] [PubMed] [Google Scholar]
- 47.T. J. Wohlbold, R. Nachbagauer, H. Xu, G. S. Tan, A. Hirsh, K. A. Brokstad, R. J. Cox, P. Palese, F. Krammer, Vaccination with adjuvanted recombinant neuraminidase induces broad heterologous, but not heterosubtypic, cross-protection against influenza virus infection in mice. mBio 6, e02556 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.K. G. Mohn, I. Smith, H. Sjursen, R. J. Cox, Immune responses after live attenuated influenza vaccination. Hum. Vaccin. Immunother. 14, 571–578 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.I. Isakova-Sivak, D. Korenkov, T. Smolonogina, T. Kotomina, S. Donina, V. Matyushenko, D. Mezhenskaya, F. Krammer, L. Rudenko, Broadly protective anti-hemagglutinin stalk antibodies induced by live attenuated influenza vaccine expressing chimeric hemagglutinin. Virology 518, 313–323 (2018). [DOI] [PubMed] [Google Scholar]
- 50.S. Shi, H. Zhu, X. Xia, Z. Liang, X. Ma, B. Sun, Vaccine adjuvants: Understanding the structure and mechanism of adjuvanticity. Vaccine 37, 3167–3178 (2019). [DOI] [PubMed] [Google Scholar]
- 51.T. Y. Kuo, M. Y. Lin, R. L. Coffman, J. D. Campbell, P. Traquina, Y. J. Lin, L. T. C. Liu, J. Cheng, Y. C. Wu, C. C. Wu, W. H. Tang, C. G. Huang, K. C. Tsao, C. Chen, Development of CpG-adjuvanted stable prefusion SARS-CoV-2 spike antigen as a subunit vaccine against COVID-19. Sci. Rep. 10, 20085 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.P. Richmond, L. Hatchuel, M. Dong, B. Ma, B. Hu, I. Smolenov, P. Li, P. Liang, H. H. Han, J. Liang, R. Clemens, Safety and immunogenicity of S-Trimer (SCB-2019), a protein subunit vaccine candidate for COVID-19 in healthy adults: A phase 1, randomised, double-blind, placebo-controlled trial. Lancet 397, 682–694 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.M. L. Myers, J. R. Gallagher, A. J. Kim, W. H. Payne, S. Maldonado-Puga, H. Assimakopoulos, K. W. Bock, U. Torian, I. N. Moore, A. K. Harris, Commercial influenza vaccines vary in both the structural arrangements of HA complexes and in induction of antibodies to cross-reactive HA epitopes. Nat. Commun. 14, 1763 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.F. Krammer, N. Pica, R. Hai, I. Margine, P. Palese, Chimeric hemagglutinin influenza virus vaccine constructs elicit broadly protective stalk-specific antibodies. J. Virol. 87, 6542–6550 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.R. A. Seder, P. A. Darrah, M. Roederer, T-cell quality in memory and protection: Implications for vaccine design. Nat. Rev. Immunol. 8, 247–258 (2008). [DOI] [PubMed] [Google Scholar]
- 56.A. Harari, V. Dutoit, C. Cellerai, P. A. Bart, R. A. du Pasquier, G. Pantaleo, Functional signatures of protective antiviral T-cell immunity in human virus infections. Immunol. Rev. 211, 236–254 (2006). [DOI] [PubMed] [Google Scholar]
- 57.Y. Liu, S. Strohmeier, I. González-Domínguez, J. Tan, V. Simon, F. Krammer, A. García-Sastre, P. Palese, W. Sun, Mosaic hemagglutinin-based whole inactivated virus vaccines induce broad protection against influenza B virus challenge in mice. Front. Immunol. 12, 746447 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.I. Margine, P. Palese, F. Krammer, Expression of functional recombinant hemagglutinin and neuraminidase proteins from the novel H7N9 influenza virus using the baculovirus expression system. J. Vis. Exp., e51112 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.C. M. Bliss, A. W. Freyn, T. G. Caniels, V. H. Leyva-Grado, R. Nachbagauer, W. Sun, G. S. Tan, V. L. Gillespie, M. McMahon, F. Krammer, A. V. S. Hill, P. Palese, L. Coughlan, A single-shot adenoviral vaccine provides hemagglutinin stalk-mediated protection against heterosubtypic influenza challenge in mice. Mol. Ther. 30, 2024–2047 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.M. Rajendran, W. Sun, P. Comella, R. Nachbagauer, T. J. Wohlbold, F. Amanat, E. Kirkpatrick, P. Palese, F. Krammer, An immuno-assay to quantify influenza virus hemagglutinin with correctly folded stalk domains in vaccine preparations. PLOS ONE 13, e0194830 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.I. Isakova-Sivak, L. M. Chen, M. Bourgeois, Y. Matsuoka, J. T. M. Voeten, J. G. M. Heldens, H. van den Bosch, A. Klimov, L. Rudenko, N. J. Cox, R. O. Donis, Characterization of reverse genetics-derived cold-adapted master donor virus A/Leningrad/134/17/57 (H2N2) and reassortants with H5N1 surface genes in a mouse model. Clin. Vaccine Immunol. 21, 722–731 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.R. Hai, A. Garcia-Sastre, D. E. Swayne, P. Palese, A reassortment-incompetent live attenuated influenza virus vaccine for protection against pandemic virus strains. J. Virol. 85, 6832–6843 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.F. Broecker, S. T. H. Liu, N. Suntronwong, W. Sun, M. J. Bailey, R. Nachbagauer, F. Krammer, P. Palese, A mosaic hemagglutinin-based influenza virus vaccine candidate protects mice from challenge with divergent H3N2 strains. npj Vaccines 4, 31 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.M. Rajendran, R. Nachbagauer, M. E. Ermler, P. Bunduc, F. Amanat, R. Izikson, M. Cox, P. Palese, M. Eichelberger, F. Krammer, Analysis of anti-influenza virus neuraminidase antibodies in children, adults, and the elderly by ELISA and enzyme inhibition: Evidence for original antigenic sin. mBio 8, e02281-16 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.E. Kirkpatrick Roubidoux, M. McMahon, J. M. Carreño, C. Capuano, K. Jiang, V. Simon, H. van Bakel, P. Wilson, F. Krammer, Identification and characterization of novel antibody epitopes on the N2 neuraminidase. mSphere 6, e00958-20 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.B. Z. Wang, F. S. Quan, S. M. Kang, J. Bozja, I. Skountzou, R. W. Compans, Incorporation of membrane-anchored flagellin into influenza virus-like particles enhances the breadth of immune responses. J. Virol. 82, 11813–11823 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.G. Wang, L. G. dos Anjos Borges, D. Stadlbauer, I. Ramos, M. C. Bermúdez González, J. He, Y. Ding, Z. Wei, K. Ouyang, W. Huang, V. Simon, A. Fernandez-Sesma, F. Krammer, M. I. Nelson, Y. Chen, A. García-Sastre, Characterization of swine-origin H1N1 canine influenza viruses. Emerg. Microbes Infect. 8, 1017–1026 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.F. Amanat, P. Meade, S. Strohmeier, F. Krammer, Cross-reactive antibodies binding to H4 hemagglutinin protect against a lethal H4N6 influenza virus challenge in the mouse model. Emerg. Microbes Infect. 8, 155–168 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.D. Stadlbauer, F. Amanat, S. Strohmeier, R. Nachbagauer, F. Krammer, Cross-reactive mouse monoclonal antibodies raised against the hemagglutinin of A/Shanghai/1/2013 (H7N9) protect against novel H7 virus isolates in the mouse model. Emerg. Microbes Infect. 7, 1–12 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.R. Hai, M. Schmolke, V. H. Leyva-Grado, R. R. Thangavel, I. Margine, E. L. Jaffe, F. Krammer, A. Solórzano, A. García-Sastre, P. Palese, N. M. Bouvier, Influenza A(H7N9) virus gains neuraminidase inhibitor resistance without loss of in vivo virulence or transmissibility. Nat. Commun. 4, 2854 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.T. J. Wohlbold, A. Hirsh, F. Krammer, An H10N8 influenza virus vaccine strain and mouse challenge model based on the human isolate A/Jiangxi-Donghu/346/13. Vaccine 33, 1102–1106 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.R. Hai, L. Martínez-Sobrido, K. A. Fraser, J. Ayllon, A. García-Sastre, P. Palese, Influenza B virus NS1-truncated mutants: Live-attenuated vaccine approach. J. Virol. 82, 10580–10590 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.T. T. Wang, G. S. Tan, R. Hai, N. Pica, E. Petersen, T. M. Moran, P. Palese, Broadly protective monoclonal antibodies against H3 influenza viruses following sequential immunization with different hemagglutinins. PLOS Pathog. 6, e1000796 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.F. Cuevas, H. Kawabata, F. Krammer, J. M. Carreno, An in vitro microneutralization assay for influenza virus serology. Curr. Protoc. 2, e465 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.J. M. Carreno, S. Strohmeier, E. K. Roubidoux, R. Hai, P. Palese, F. Krammer, H1 hemagglutinin priming provides long-lasting heterosubtypic immunity against H5N1 challenge in the mouse model. mBio 11, e02090-20 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.N. Pica, A. Iyer, I. Ramos, N. M. Bouvier, A. Fernandez-Sesma, A. García-Sastre, A. C. Lowen, P. Palese, J. Steel, The DBA.2 mouse is susceptible to disease following infection with a broad, but limited, range of influenza A and B viruses. J. Virol. 85, 12825–12829 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.S. Dhakal, S. Deshpande, M. McMahon, S. Strohmeier, F. Krammer, S. L. Klein, Female-biased effects of aging on a chimeric hemagglutinin stalk-based universal influenza virus vaccine in mice. Vaccine 40, 1624–1633 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.J. Hadfield, C. Megill, S. M. Bell, J. Huddleston, B. Potter, C. Callender, P. Sagulenko, T. Bedford, R. A. Neher, Nextstrain: Real-time tracking of pathogen evolution. Bioinformatics 34, 4121–4123 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.F. Sievers, A. Wilm, D. Dineen, T. J. Gibson, K. Karplus, W. Li, R. Lopez, H. McWilliam, M. Remmert, J. Söding, J. D. Thompson, D. G. Higgins, Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 7, 539 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figs. S1 to S5