Abstract
Until recently, group B streptococcus, serotype V (GBS-V), was an infrequent cause of disease. It is now recognized as a significant cause of infections in both children and adults. To determine if this increase was due to the recent introduction and spread of a single clone of GBS-V, we analyzed, by pulsed-field gel electrophoresis (PFGE), the SmaI chromosomal DNA digests of 45 bacteria: 41 isolated from human infections between 1986 and 1996 in the United States, 2 from human infections in Argentina, and 2 from naturally infected mice. Seventeen patterns were found and arbitrarily designated patterns A to Q. Pattern N constituted 24 (53%) of the isolates and was found in all of the years tested and from all surveillance areas, as well as in both isolates from Argentina, and was very similar to the GBS-V isolated from a mouse. Pattern P was found in three isolates, pattern F was found in two, and the remaining patterns were found in one isolate each. We concluded that the majority of isolates of GBS-V are of one PFGE subtype and that this subtype was predominate before the increase in disease caused by GBS-V and that GBS-V disease is caused by several different subtypes.
Group B streptococci (GBS) are a significant cause of morbidity and mortality in newborns and have recently been recognized as a cause of serious infections in adults, especially those who are immunocompromised or debilitated by underlying disease (1, 4, 5, 7). There are at least 13 different serotypes of GBS, based on combinations of carbohydrate and the c-protein antigens plus additional serotype combinations of the R and X protein antigens (6). Until recently, the predominate serotypes that were sent to the Centers for Disease Control and Prevention (CDC) were Ia, Ia/c, and III. The serotype distribution has changed in the past few years because of the dramatic increase in the percentage of cases caused by serotype V (3).
Serotype V GBS were first reported by Wilkinson in 1977 (8). In that report, the serotype was designated NT-1 for nontypeable (NT) type 1. Our first confirmed isolate of this serotype was obtained in 1975 and was discovered by retrospective analysis of GBS that were not typeable at the time of receipt. Prior to Wilkinson’s publication, and for the majority of the isolates tested after that publication, any isolates that could not be typed by our available antisera (Ia, Ib, Ic, II, and III) were reported as NT. These NT isolates, therefore, represent the maximum number of serotype V that we could have received. Of the nearly 3,000 GBS serotyped between 1973 and 1976, only 1.3% of the isolates were NT with our antisera. In the last multistate active surveillance project, conducted in 1986 and 1987, the percentage of NT isolates remained low, at 2.3%. A striking change, however, occurred in the 1990s, when the percentage of serotype V GBS went from 2.6% in 1992 to 14% in 1993 and then to 20% in 1994. To determine if this increase in the incidence of serotype V GBS infections was due to the spread of a new, more virulent subtype that was introduced into the United States in the early 1990s, we analyzed the SmaI chromosomal DNA digests of bacteria isolated from 1986 through 1996 by pulsed-field gel electrophoresis (PFGE) (2).
GBS identified as either NT, NT-1, or serotype V from 1986 to 1996 were retrieved from the CDC culture collection. Unfortunately, except for one isolate from 1975 and one from 1977, isolates from before October 1985 could not be recovered. The serotypes of these isolates were retested by using hot HCl extracts and rabbit antibodies to serotypes Ia, Ib, Ic, II, III, and V (3). Of the isolates found to be GBS-V, 45 were chosen based on the geographic locations and the dates on which they were isolated.
Type strains used for PFGE serotype pattern comparison were SS-617, serotype Ia; SS-1307, Ia/c; SS-885, Ia/c; SS-1304, Ib; SS-1034, Ib; SS-618, Ib; SS-1104, non-beta-hemolytic, NT/c; SS-1036, NT/c; SS-869, II; SS-619, II; SS-1033, III/c; SS-1030, III/c; SS-969, III; SS-620, III; SS-462, III; SS-1240, IV; SS-1354, VI; and SS-1476, VII.
For PFGE analysis, two or three colonies were picked from a fresh plate of overnight growth and incubated in 5.0 ml of Todd-Hewitt broth for 5 h at 35°C; 1.5 ml of this suspension was centrifuged at 2,000 × g for 10 min, and the pellet was resuspended in 0.6 ml of Tris-NaCl buffer (1.0 M NaCl in 10 mM Tris-HCl, pH 7.6). Then, 0.6 ml of 2% low-melting-temperature agarose (Bio-Rad Laboratories, Richmond, Calif.) in Tris NaCl buffer was added. The agarose-bacterium mixtures were poured into plug molds (Bio-Rad). After solidification, the plugs were removed and incubated overnight on a platform rocker at 35°C in lysis solution (6 mM Tris-HCl, 1.0 M NaCl, 100 mM sodium EDTA, 0.5% Brij 58, 0.2% sodium deoxycholate, 0.5% sodium lauroyl sarcosine, 1-mg/ml lysozyme, 5-U/ml mutanolysin, pH 7.6). This was followed by incubation for 24 h in a 50°C shaking water bath in EDTA-sarcosine-proteinase solution (0.5 M EDTA, 1% sodium lauroyl sarcosine, 0.1-mg/ml proteinase K, pH 8.0). The plugs were washed four times, for 2 h each time, in Tris-EDTA buffer (10 mM Tris-HCl, 0.1 mM EDTA, pH 7.6) and stored in the same buffer at 4°C. The plugs were incubated with 20 U of SmaI (Boehringer Mannheim Biochemicals, Indianapolis, Ind.) in the appropriate restriction buffer for 24 to 48 h at 25°C, heated for a few minutes at 72°C to melt the plugs, and dispensed into wells of a 1% pulsed-field grade agarose gel (Bio-Rad). The chromosomal digests were separated by PFGE with a switch time of 5 to 40 s for 25 h at a 120° angle with a voltage gradient of 6 V/cm at 10°C. The DNA size standard used was a lambda DNA ladder (Bio-Rad). Gels were stained with ethidium bromide and photographed with UV light.
Of nearly 3,000 GBS isolates that we received at CDC from the national surveillance during 1973 through 1977, only 1.3% were NT. This was before we could serotype these isolates with serotype V antisera and, although these NT isolates could not be recovered, this represents the maximum percentage of isolates that could have been serotype V during that time. As late as 1990 to 1991, the percentage of GBS-V isolates remained below 3%. The percentage of GBS-V isolates sharply increased in the next 4 years, and by 1993 to 1994 it equaled that of serotypes Ia and Ia/c combined. Type V is now found in about 20% of the GBS submitted to our laboratory.
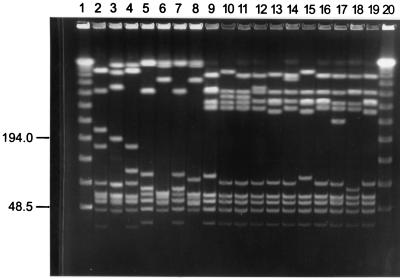
Figure 1 shows representative results of the SmaI digests of GBS-V from 1986 through 1996, and Table 1 shows isolate origins and PFGE pattern types. Diversity within GBS-V was found, with at least 17 different PFGE patterns identified among the 45 isolates tested. The most common pattern is shown in lane 16. This pattern was found with approximately 56% of the isolates tested. This pattern was also found in both isolates from Argentina (not shown) and shared many similarities with the serotype V isolated from a mouse in 1992 (lane 19). Most importantly, this PFGE pattern was also found with the GBS-V isolated in 1975 (not shown). Two facts indicate that the rapid increase was not the result of a sudden introduction of a new PFGE subtype of GBS-V into the U.S. population: this PFGE pattern was common several years before the rapid increase in the percentage of isolated GBS-V, and it was present in the United States since at least 1975. These serotype V PFGE patterns were compared with the PFGE patterns produced by type strains of other serotypes of GBS, and no similar patterns were found.
FIG. 1.
PFGE patterns of different serotype V GBS isolates.
TABLE 1.
Representative PFGE pattern types of serotype V GBS isolated from 1986 to 1996
| Lane no.a | Yr isolate/state or host | PFGE type |
|---|---|---|
| 1 | λ ladder | |
| 2 | 90/Ohio | A |
| 3 | 89/Tenn. | B |
| 4 | 89/Mo. | C |
| 5 | 87/Calif. | D |
| 6 | 86/Calif. | E |
| 7 | 86/N.J. | F |
| 8 | 86/N.J. | G |
| 9 | 96/S.C. | H |
| 10 | 95/Ga. | I |
| 11 | 96/S.C. | J |
| 12 | 96/Md. | K |
| 13 | 96/Md. | L |
| 14 | 86/Calif. | M |
| 15 | 94/Md. | N |
| 16 | 95/Ga. | O |
| 17 | 96/Md. | P |
| 18 | 96/Md. | Q |
| 19 | 92/mouse | L |
| 20 | λ ladder |
Lane numbers correspond to those in Fig. 1.
Our results indicate that the reported rapid increase in the previously rare serotype V GBS in the United States since 1992 has been due primarily to a strain with a particular PFGE subtype. The reasons for this increase are unclear, since serotype V GBS with this PFGE subtype has been present in the population since at least 1975. The emergence of the newly predominant serotype V GBS has prompted evaluation of GBS vaccines which include this capsular polysaccharide in addition to those previously considered important.
Acknowledgments
This research was supported in part by an appointment of K. D. Farmer to the Research Participation Program at the National Center for Infectious Diseases, Division of Bacterial and Mycotic Diseases, CDC. The program was administered by Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and CDC. The majority of the isolates tested for this report originated from surveillance projects supported by the FDA and the National Vaccine Program Office.
REFERENCES
- 1.Blumberg H M, Stephens D S, Modansky M, Erwin M, Elliott J, Facklam R R, Schuchat A, Baughman W, Farley M M. Invasive group B streptococcal disease: the emergence of serotype V. J Infect Dis. 1996;173:365–373. doi: 10.1093/infdis/173.2.365. [DOI] [PubMed] [Google Scholar]
- 2.Gordillo M E, Singh K V, Baker C J, Murray B E. Typing of group B streptococci: comparison of pulsed-field gel electrophoresis and conventional electrophoresis. J Clin Microbiol. 1993;31:1430–1434. doi: 10.1128/jcm.31.6.1430-1434.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harrison L H, Dwyer D M, Johnson J A. Emergence of serotype V group B streptococcal infection among infants and adults. J Infect Dis. 1995;171:513. doi: 10.1093/infdis/171.2.513. [DOI] [PubMed] [Google Scholar]
- 4.Harrison L H, Ali A, Dwyer D M, Libonati J P, Reeves M W, Elliott J A, Billmann L, Lashkerwala T, Johnson J A. Relapsing invasive group B streptococcal infection in adults. Ann Intern Med. 1995;123:421–427. doi: 10.7326/0003-4819-123-6-199509150-00004. [DOI] [PubMed] [Google Scholar]
- 5.Hussain S M, Luedtke G S, Baker C J, Schlievert P M, Leggiadro R J. Invasive group B streptococcal disease in children beyond early infancy. Pediatr Infect Dis J. 1995;14:278–281. doi: 10.1097/00006454-199504000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Jelinkova J, Motlova J. Worldwide distribution of two new serotypes of group B streptococci: type IV and provisional type V. J Clin Microbiol. 1985;21:361–362. doi: 10.1128/jcm.21.3.361-362.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wessels M R, Kasper D L. The changing spectrum of group B streptococcal disease. N Engl J Med. 1993;328:1843–1844. doi: 10.1056/NEJM199306243282510. [DOI] [PubMed] [Google Scholar]
- 8.Wilkinson H W. Nontypable group B streptococci isolated from human sources. J Clin Microbiol. 1977;6:183–184. doi: 10.1128/jcm.6.2.183-184.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]