Abstract
Coronavirus disease 2019 (COVID‐19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), can manifest itself in several ways, including coagulopathy and thrombosis. These complications can be the first and sometimes only manifestations of SARS‐CoV‐2 infection and can occur early or late in the course of the disease. However, these symptoms are more prevalent in hospitalized patients with venous thromboembolism, particularly those admitted to intensive care units. Moreover, various forms of arterial and venous thrombosis, or micro‐ or macro‐vasculature embolisms, have been reported during the current pandemic. They have led to harmful consequences, such as neurological and cardiac events, nearly all resulting from the hypercoagulable state caused by this viral infection. The severe hypercoagulability observed in patients with COVID‐19 accounts for most cases of the disease that become critical. Therefore, anticoagulants seem to be one of the most vital therapeutics for treating this potentially life‐threatening condition. In the current paper, we present a thorough review of the pathophysiology of COVID‐19‐induced hypercoagulable state and the use of anticoagulants to treat SARS‐CoV‐2 infections in different patient groups, as well as their pros and cons.
INTRODUCTION
The severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) that causes coronavirus disease 2019 (COVID‐19) can present itself in a variety of ways, including coagulopathy and thrombosis. 1 These symptoms might appear earlier or later in the course of the illness and occasionally serve as the only flag of the SARS‐CoV‐2 infection. Venous thromboembolism (VTE), which causes these symptoms, is more common in hospitalized patients, especially those who are hospitalized in intensive care units (ICUs). 2 , 3 Furthermore, there have been reports of different kinds of micro‐ or macro‐vasculature emboli in the present pandemic, including arterial and venous thrombosis. The hypercoagulable state brought on by this viral infection has almost always resulted in serious side effects, such as neurological and cardiac problems. 4 , 5 , 6
Decreased platelet counts, minor prolongation of prothrombin time, activated partial thromboplastin time (aPTT), and elevated serum D‐dimer and fibrinogen characterize the COVID‐19‐associated coagulopathy. 7 Moreover, complement cascade and antiphospholipid antibodies are also involved in the process. 8 , 9 Although other non‐COVID critical illnesses also predispose a patient to this hypercoagulable state, similar to SARS‐CoV‐2 infection, 10 there may be differences between the thrombotic condition in COVID‐19 and other severe cases of pneumonia, as the prothrombotic state may be more severe in patients with COVID‐19 than in patients with other infections. In severe SARS‐CoV‐2 infections, coagulopathy is usually accompanied by higher platelet counts and D‐dimer levels than would be found in severe non‐COVID‐19 cases of pneumonia. 11 Apart from arterial and venous thromboembolic events, SARS‐CoV‐2 infections can lead to diffuse microthrombi in the lung vasculature. The procoagulant and proinflammatory markers produced during severe illnesses, including COVID‐19, are responsible for hypercoagulability and thrombotic events.
The severe hypercoagulability observed in patients with COVID‐19 has been estimated to account for most cases of the disease that become critical. Therefore, anticoagulants are essential therapeutics for treating this potentially life‐threatening condition. 12 The specific aim of the current paper was to thoroughly review the pathophysiology of the COVID‐19‐induced hypercoagulable state and the clinical use of anticoagulants, including their pros and cons, in treating the infection with SARS‐CoV‐2.
THE MECHANISM OF COVID‐19‐INDUCED COAGULATIVE STATE
Different factors play a role in COVID‐19‐induced hypercoagulation, which requires more investigation to determine the underlying mechanism. However, in this section, we have tried to classify the primary factors and pathways participating in this event. The main pathways include (1) inflammatory state and elevated cytokines levels and (2) virus‐specific mechanisms, which have been discussed below. We have also explained the role of C‐type lectin domain family 4 member M (CLEC4M, also known as L‐SIGN or CD209L).
COVID‐19‐related cytokine storm syndrome (COVID‐CSS) is a life‐menacing condition caused by SARS‐CoV‐2‐induced immune dysregulation. It is a tremendous inflammatory state which is, in fact, due to the over‐secretion of proinflammatory and inflammatory cytokines. 13 Some cytokines like interleukin (IL)‐1β, IL‐6, IL‐10, and TNF‐α increase early in severely ill patients. 14 Along with IL‐8, GM‐CSF, and IFN‐γ, the abovementioned cytokines are the major proinflammatory cytokines released excessively in patients with severe conditions. 15 Although in vitro, many proinflammatory cytokines have been shown to play a role in the coagulation system activation, in vivo, elevated levels of IL‐1, IL‐6, and TNF were associated with acute inflammatory conditions (like sepsis) along with hypercoagulation, sometimes developing into severe conditions, like disseminated intravascular coagulation (DIC). 16 Previous studies suggest that IL‐6 is more likely to be the main mediator participating in the stimulation of cytokine‐induced coagulation compared to TNF‐α. 16 For example, Ruan et al. 17 proved that the non‐survivor cases of COVID‐19 had remarkably higher levels of IL‐6 compared to the surviving patients. Moreover, Bester and Pretorius' study affirmed that IL‐6 could cause more hypercoagulable clots than IL‐1β, probably due to notable changes in fibrin. 18 This cytokine exerts its impact in various ways. It can upregulate the expression of tissue factor (TF), which starts coagulation. IL‐6 can also trigger megakaryopoiesis. 19 Further, it has been reported that IL‐6 could enhance the expression of other coagulation factors, like factor VIII and fibrinogen. 20 , 21 Interestingly, some coagulation mediators (such as FIIa, FVIIa, and FXa) can induce the expression of proinflammatory cytokines through protease‐activated receptors, 22 which leads to a vicious cycle. On the other hand, IL‐1β can impair the coagulative state through the downregulation of thrombomodulin (exerts an anti‐inflammatory activity) and reducing the activation of protein C, which plays an essential role in the anti‐coagulation pathway. 18 After all, IL‐1, IL‐6, and TNF‐α all can lead to overexpression of TF and, in contrast, reduce the expression of important natural anticoagulants like protein C, antithrombin, and the inhibitors.
Angiotensin‐converting enzyme 2 (ACE2), an intermembrane receptor in various types of cells, such as the kidneys, bowels, heart, and lungs, is the leading site of engagement of S‐protein. This receptor counter‐regulates ACE function and decreases angiotensin II availability. Moreover, it has been demonstrated that viral engagement of ACE2 reduces its expression and activates the renin‐angiotensin system (RAS), which is responsible for promoting platelet adhesion and aggregation. 23 , 24 Further, the fibrinolytic system is also under the control of RAS. It appears that patients with systemic hypertension are highly prone to develop a condition of decreased fibrinolytic system activity and ischemic events. On the other hand, reduced angiotensin II activity positively impacts the frequency of cardiovascular events and fibrinolytic balance. 25
Initially, the SARS‐CoV‐2 virus enters the lungs and quickly binds to the ACE receptor in the alveoli of pneumocyte cells, triggering immune cells from the arteries and producing cytokines and chemokines. Following that, immune cells enter the alveoli from the blood and activate macrophages and neutrophils in the alveolar space. FXa converts prothrombin to thrombin. As a result, thrombin will convert fibrinogen to fibrin. Then, the accumulation of fibrin and blood cells causes thrombosis. The ATIII produced by the liver can inhibit the thrombosis process. Finally, thrombosis causes multiple organ failure, thrombotic heart damage includes DIC, myocardial infarction (MI), and VTE, and the lungs can develop acute respiratory distress syndrome (ARDS), acute lung injury, pulmonary embolism (PE), and pulmonary thromboendarterectomy (PTE), and kidney damage, including acute kidney injury (AKI) and end‐stage renal disease (ESRD). Stroke is also a complication of thrombosis in the brain. As shown in Figure 1, unfractionated heparin (UFH) with anti‐inflammatory properties can inhibit cytokines and chemokines. Moreover, aspirin and antithrombin can also prevent the aggregation of platelets and neutrophils by inhibiting IL‐6.
FIGURE 1.
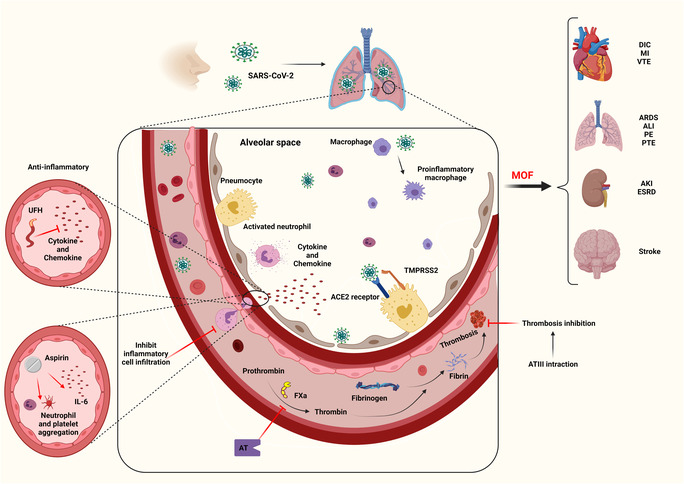
COVID‐19, thrombosis and anticoagulants. The SARS‐CoV‐2 virus enters the lungs and attaches to the ACE receptor in the alveoli of pneumocyte cells, triggering immune cells from the arteries and producing cytokines and chemokines. As a result, immune cells enter the alveoli from the blood and activate macrophages and neutrophils in the alveolar space. UFH, with anti‐inflammatory properties, can inhibit cytokines and chemokines. As a result, immune cells are prevented from entering the alveoli. Aspirin can also prevent the aggregation of platelets and neutrophils by inhibiting IL‐6. FXa converts prothrombin to thrombin, and AT can block this path. Thrombin can also convert fibrinogen to fibrin. Accumulation of fibrins and blood cells cause thrombosis ATIII can inhibit the thrombosis process. Finally, thrombosis causes MOF. Thrombotic heart damage includes DIC, MI, and VTE. The lungs can develop ARDS, ALI, PE, and PTE. Kidney damage includes AKI and ESRD. Stroke is a complication of thrombosis in the brain. ACE, angiotensin‐converting enzyme; AKI, acute kidney injury; ALI, acute lung injury; ARDS, acute respiratory distress syndrome; AT, antithrombin; COVID‐19, coronavirus disease 2019; DIC, disseminated intravascular coagulation; ESRD, end‐stage renal disease; FXa, Factor Xa; MI, myocardial infarction; MOF, multiple organ failure; PE, pulmonary embolism; PTE, pulmonary thromboendarterectomy; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2; UFH, unfractionated heparin; VTE, venous thromboembolism.
Both in vivo and in vitro studies demonstrated that the serum level and expression of plasminogen activator inhibitor‐1, the main inhibitor of the fibrinolytic system, rose after SARS‐CoV infection, compared to other pneumonia‐inducing infections, which shows a probable direct effect of infection on the expression of anti‐coagulant agents. 26 , 27
In severe cases, COVID‐19 could infect endothelial cells and lead to cell death, which causes the initiation of procoagulant interactions. 28 , 29 The endothelial cells' damage could also activate a principal defense mechanism called neutrophil extracellular traps (NETs) or NETosis. NETs are extracellular fibers of DNA. They are released due to chromatin decondensation and spread right after that. They are familiar for producing a link among inflammation, coagulation, and thrombosis on both localized and systemic levels. 30 Several pathways have been suggested for NET‐induced thrombosis. First, the von Willebrand factor (VWF), released by platelets and endothelium, interacts with NETs and results in the adhesion of platelets and fibrin formation. Second, histone proteins existing in NETS are recognized as powerful damage‐associated molecular patterns and can cause platelet aggregation by activating toll‐like receptors on platelets. Third, neutrophils release a serine protease, elastase, which could prevent the fibrinolytic system by degrading thrombomodulin and the tissue factor pathway inhibitor. 31 , 32
C‐type lectins, including CLEC4G/LESCtin, CD209/DC‐SIGN/CLEC4L, and CD209L/L‐SIGN/CLEC4M, are a group of receptors that work as pathogen receptors and adhesion molecules. 33 COVID‐19 can bind to various receptors, such as ACE2 receptors, CD209L/L‐SIGN/CLEC4M, CD209, and CD147. It has been shown that CLEC4M is associated with low levels of VWF. It functions as a receptor that binds and internalizes VWF and reduces the plasma level of VWF. Interestingly, it has been demonstrated that CLEC4M could also bind and internalize human FVIII in both VWF‐dependent and independent ways. 34 , 35 It could be possible that the engagement of SARS‐CoV‐2 with CLEC4M, preventing the clearance of VWF and FVIII, may form a pro‐coagulation state. Nevertheless, it is just a hypothesis and needs thorough investigations and more studies. 36
In patients with COVID‐19, the increasing levels of D‐dimer, a soluble fibrin breakdown product produced when the fibrinolytic system breaks down thrombi in an organized manner, can be related to several factors, such as the life cycle of the virus. 37 , 38 Another hypothesis in these patients with COVID‐19 is the inflammatory responses to viral infection and the dysfunction of endothelial cells with thromboembolic complications. We should also consider that a direct consequence of acute lung injury as seen in COVID‐19 infection. 39
It has been found that most patients with COVID‐19 have a prolonged aPTT. This laboratory abnormality reflects a clotting factor deficiency or the presence of an inhibitor, 40 meaning that using aPTT as a marker for initiating anticoagulation therapy seems unreasonable. On the other hand, D‐dimer would be a helpful indicator for identifying high‐risk patients in the early stages of the disease, making it helpful in predicting the prognosis of patients 41 and predicting the incidence of some of the disease's severe adverse events, such as ARDS and AKI. Thus, D‐dimer can be used to assess the risk of end‐organ damage, ICU admission, and mortality. Furthermore, as recommended by the International Society on Thrombosis and Hemostasis, D‐dimer levels can serve as a prognostic means for risk stratification 42 so that the hospitalization of those patients with COVID‐19 with significantly increased D‐dimers (3–4 fold than the upper limit of normal [ULN]) is indicated, even without other indicators of disease severity. 7 Nonetheless, the decision should be individualized. Moreover, although a rise in D‐dimer level indicates disease severity, administering anticoagulants cannot be based solely on this biomarker, and a thorough thromboembolic workup is warranted if clinical worsening is evident. 43
ANTICOAGULANTS USE IN THE SETTING OF COVID‐19
As far as we know, until now, there are no completed randomized clinical trials (RCTs) investigating the best anticoagulant choice and treatment timing, duration, and dosage in patients with COVID‐19. They are all in progress. 44 Plus, various guidelines regarding the management of patients with COVID‐19 have been proposed by various international organizations, yet most of them do not comment on anticoagulation strategies. These guidelines are based merely on consensus statements and expert views. They also have some limitations. For instance, they recognize SARs‐COV2 as a cause of microthrombi, which worsens the prognosis of patients; however, they cannot propose appropriate guidelines or consensus statements to address this issue. Moreover, they suggest therapeutic doses of heparin for diagnosed or highly suspected macro‐thrombotic events, like PE and deep vein thrombosis (DVT), although they miss the subject of undiagnosable micro‐thrombotic events. Finally, no distinct scoring system for measuring VTE risk on admission is specific to SARs‐CoV‐2. 44 , 45 For hospitalized patients with COVID‐19 with a more than normal upper limit D‐dimer level, in need of low‐flow oxygen, and with no elevated bleeding risk, heparin (in therapeutic doses), especially low‐molecular‐weight heparin (LMWH), has been recommended by the US National Institutes of Health (NIH) and many other trials 45 , 46 (Table 1).
TABLE 1.
Clinical recommendations for the use of anticoagulants in patients with COVID‐19.
| Patients | ASH 47 , 48 | NIH d | ISTH 49 |
|---|---|---|---|
| Non‐hospitalized | – | Did not recommend routine prophylaxis | Did not recommend routine prophylaxis |
| After discharge | – | Did not recommend prolonged prophylaxis | If there is a high risk of thrombosis and low risk of hemorrhage, apply LMWH or DOAC for 30 days |
| Critically ill | Prophylactic‐dose a anticoagulation over intermediate‐dose b or therapeutic‐dose c anticoagulation | Prophylactic‐dose anticoagulation |
|
| Non–critically ill | Therapeutic‐dose over prophylactic‐dose anticoagulation in acutely ill who do not have suspected or confirmed VTE or any other indication for anticoagulation | Prophylactic‐dose anticoagulation | Prophylactic‐dose anticoagulation with LMWH |
Abbreviations: ASH, American Society of Hematology; COVID‐19, coronavirus disease 2019; DOAC, direct‐acting oral anticoagulants; ISTH, International Society of Thrombosis and Hemostasis; LMWH, low‐molecular‐weight heparin; NIH, National Institute of Health; UFH, unfractionated heparin.
Enoxaparin (30–40 mg subcutaneous (s.c.) not exceeding <0.7 mg/kg/day or 30–40 mg s.c. not exceeding <0.4 mg/kg/12 h), UFH (5000 IU s.c. 3 times a day or 5000–7500 IU s.c. 3 times a day), or Fondaparinux (2.5 mg s.c. one time a day for 5–10 days).
Enoxaparin (≥0.4 but ≤0.7 mg/kg/12 h) or UFH (5000 IU s.c. 3 times a day or 5000–7500 IU s.c. 3 times a day).
Enoxaparin (≥0.7 mg/kg/12 h, ≥0.7 mg/kg/day in patients with chronic kidney disease, or >1.4 mg/kg/day), UFH (bolus intravenously followed by drip, with activated partial thromboplastin time guided dose adjustments), or Bivalirudin (bolus intravenously followed by drip, with activated clotting time guided dose adjustments).
As part of Accelerating COVID‐19 Therapeutic Interventions and Vaccines (ACTIV‐4b), anticoagulant and antiplatelet therapy was used in 558 outpatients with symptomatic but stable COVID‐19 to prevent mortality from all causes, stroke, MI, venous or arterial thromboembolism, and hospitalization for cardiovascular or lung diseases. The trial was randomized, double‐blind, and placebo‐controlled. 50 According to ACTIV‐4b, the incidence of VTE was very low in patients with COVID‐19 treated as outpatients, suggesting no benefit from anticoagulation or antiplatelet therapy. It is possible, however, that earlier initiation of antithrombotic therapy may be beneficial, especially for patients at high risk of hospitalization, because patients in ACTIV‐4b had a long delay between diagnosis and study drug receipt; 3.3% of ACTIV‐4b patients were hospitalized between randomization and treatment. In ongoing trials, it may be uncovered that prophylactic anticoagulation may benefit a subset of outpatients with COVID‐19 at high cardiovascular event risk. 45 , 50
In addition to ACTIV‐4b, two other trials show that therapeutic‐dose anticoagulation does not benefit severely ill patients with COVID‐19 and may even harm them. Despite the fact that they are at high risk of thrombotic events, and higher doses of anticoagulation appear effective in preventing such events, they may already be too far along in their disease process to benefit from more intensive thromboprophylaxis. The benefits and risks of anticoagulation for severely ill patients with COVID‐19 may change as newly emerging coronavirus variants appear and care pathways mature. However, compared to critically ill patients, non‐critically ill hospitalized patients may benefit from therapeutic anticoagulation. 51
Several RCTs have been completed on anticoagulation regimens for patients with COVID‐19 that support these recommendations. Incorporating evidence from ongoing trials into evidence‐based guidelines will become necessary as the trials are completed and the results become available. A careful epidemiologic study will be essential to define thrombosis risk and determine whether new SARS‐CoV‐2 variants have a higher or lower intrinsic risk of thrombosis as population immunity waxes and wanes with infection and vaccination. For hospitalized patients with COVID‐19, conducting new RCTs to assess antithrombotic regimens will be crucial (Table 2).
TABLE 2.
Characteristic of publications on the antithrombotic and anticoagulant regimens for patients with COVID‐19.
| First author (year) | Location | Design | Type of anticoagulation and comparison | Results | Outcomes |
|---|---|---|---|---|---|
| Bikdeli et al. (2022) 52 | Iran | Multicenter randomized trial, 562 patients | Intermediate‐dose vs standard‐dose prophylactic anticoagulation | No difference was observed in venous or arterial thrombosis, ECMO treatment, or mortality rate following a 90‐day follow‐up. |
|
| Lawler et al. (2021) 53 | UK USA Canada Brazil Mexico Nepal Australia Netherlands Spain | An international, adaptive, multiplatform, randomized, controlled trial, 2219 patients | Therapeutic‐dose anticoagulation (heparin) vs usual‐care pharmacologic thromboprophylaxis | The probability of survival until hospital discharge was increased by therapeutic‐dose heparin. It also reduced the use of ICU‐level organ support. |
|
| Goligher et al. (2021) 54 | UK USA Canada Brazil Ireland Netherlands Australia Nepal Saudi Arabia Mexico | An international, adaptive, multiplatform, randomized, controlled trial, 1098 patients | Therapeutic‐dose anticoagulation vs usual‐care thromboprophylaxis | No difference was observed in the probability of survival to hospital discharge or a greater number of days free of cardiovascular or respiratory organ support. |
|
| Perepu et al. (2021) 55 | USA | Multicenter, open‐label, randomized controlled trial, 176 patients | Standard prophylactic dose vs intermediate dose enoxaparin | No difference was observed in mortality (p = 0.28) and thromboembolism. |
|
| Sadeghipour et al. (2021) 56 | Iran | Multicenter randomized trial, 562 patients | Intermediate‐dose vs standard‐dose prophylactic anticoagulation | No difference was observed in the risk of VTE (3.3% vs. 3.5%; p = 0.94) and mortality (within 30 days) (43.1% vs. 40.9%; p = 0.50). |
|
| Tang et al. (2020) 57 | China | Comparative, retrospective, 449 patients | Systemic anticoagulation with low‐molecular‐weight heparin vs. no anticoagulation | No difference was observed in mortality (30.3% vs. 29.7%; p = 0.91). |
|
| Paranjpe et al. (2020) 58 | USA | Comparative, retrospective, 2772 patients | Systemic anticoagulation vs. no anticoagulation | No difference in mortality (22.5% vs. 22.8%) or bleeding (1.9% vs. 3%; p = 0.2). Patients on anticoagulation required more mechanical ventilation (29.8% vs. 8.1%; p < 0.001). |
|
| Llitjos et al. (2020) 59 | France | Comparative, retrospective, 26 patients | Thromboprophylaxis vs therapeutic anticoagulation | Patients treated with thromboprophylaxis were at higher risk of VTE (100% vs 56%; p = 0.03). |
|
| Yin et al. (2020) 11 | China | Comparative, retrospective, 449 | Systemic anticoagulation with low‐molecular‐weight heparin vs no anticoagulation | No difference was observed in mortality (30.3% vs 29.7%; p = 0.91). In subgroup analysis of patients with D‐dimer >3.0 μg/mL, there was lower risk of mortality in the heparin group (32.8% vs. 52.4%; p = 0.02). |
|
| Klok et al. (2020) 60 | Netherland | Single‐arm, retrospective, 184 patients | Thromboprophylaxis | Any thromboembolic event, PE |
|
| Lodigiani et al. (2020) | Italy | Single‐arm, retrospective, 61 patients | Thromboprophylaxis | Thromboembolic events occurred in 16.7% of patients, VTE in 8.3%, PE in 4.2%, DVT in 2.1%, and stroke in 6.3% |
|
Note: To convert D‐dimer values to nmol/L, multiply by 5.476.
Abbreviations: COVID‐19, coronavirus disease 2019; DVT, deep venous thrombosis; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; PE, pulmonary embolism; SI, conversion factors; VTE, venous thromboembolism.
WHAT ARE THE BENEFITS OF ANTICOAGULANT THERAPY IN SARS‐COV‐2 INFECTIONS?
Initially, anticoagulants were used to treat confirmed or suspected DVT/PE in the course of COVID‐19. 52 Nevertheless, with the progression of the pandemic, much information about the necessity of these medications has been produced. It was initially believed that anticoagulant therapy could only improve in‐hospital survival for intubated patients with COVID‐19 and those with a high sepsis‐induced anticoagulant score or an elevated D‐dimer level. 53 , 54 However, it was later found that anticoagulants could help decrease the need for mechanical ventilation in patients moderately ill with SARS‐CoV‐2. It is essential to know that heparin derivatives also have anti‐arrhythmic, anti‐inflammatory, immunomodulatory, antiviral, and anti‐complement activity effects in addition to anticoagulatory effects. 55 , 56 These characteristics primarily occur in UFH rather than with LMWHs. 57 , 58 By neutralizing the chemokines and cytokines, the anti‐inflammatory effects of UFH/LMWH can prevent acute respiratory distress syndrome (ARDS) and consequently reduce mortality 52 (Figure 1).
Moreover, these agents are believed to limit viral cell entry. 59 Heparin derivatives can also prevent endothelial leakage, the pathophysiologic mechanism of ARDS, and AKI. 60 Complement activation, which triggers systemic thrombosis, can also be inhibited with these medications. These can diminish micro‐thrombus formation, resulting in a lower chance of end‐organ damage. 61 Anticoagulant therapy is also helpful in preventing and managing DIC, followed by septic shock in critically ill patients with COVID‐19. 62 In general, anticoagulants would improve the prognosis of these patients. Nonetheless, whether the risk of ARDS and mortality could be reduced in patients with severe forms of SARS‐CoV‐2, who have already been on chronic oral anticoagulants or antiplatelet therapy for other indications, is not yet well understood, and conflicting findings have been made. 63 , 64 , 65
WHEN SHOULD ANTICOAGULANT THERAPY BE INITIATED IN THE SARS‐COV‐2 INFECTION?
Due to the elevated risk of thrombosis in patients with COVID‐19, especially in severe cases, it is recommended that all patients, including severe and critical ones, should receive prophylactic doses of anticoagulants as soon as they are hospitalized, unless contraindicated (active bleeding and platelet counts <25 × 109/L). 49 It is well‐understood that hospitalized patients with COVID‐19 may also have multiple risk factors for thromboembolic events apart from their innate hypercoagulable state. Due to general weakness or disabilities, respiratory distress, intubation, and sedation, their immobility predisposes them to this complication. Moreover, most SARS‐CoV‐2‐infected patients have other comorbidities, including advanced age, pregnancy, cancer, cardiovascular diseases, and obesity, all of which are substantial risk factors for thromboembolism. COVID‐19‐related mortality has also been found to be higher in critically ill patients with high sepsis‐induced coagulopathy (SIC) scores or elevated D‐dimers. Therefore, thromboprophylaxis should be considered in any patient with COVID‐19 with significantly increased D‐dimers or a high SIC score. 54
WHAT IS THE BEST CHOICE OF ANTICOAGULANT?
The ideal thrombophylactic regimen in patients with COVID‐19 is not yet understood. During the SARS‐CoV‐2 infection, anticoagulation can be attained by intravenous (i.v.) or oral anticoagulants. Although some antiviral agents used to treat SARS‐CoV‐2 infections interact with oral anticoagulants, such as anti‐factor Xa (e.g., rivaroxaban and apixaban), heparin rarely demonstrates such interactions. 66 , 67 Moreover, these oral agents are highly susceptible to metabolic changes in the body during critical or acute phases of the disease because their metabolism and serum levels are affected, leading to uncontrolled bleeding or thrombosis. 68 Among the different types of I.V. anticoagulants, UFH is the only agent requiring frequent laboratory monitoring. However, UFH is more affordable, has a shorter half‐life, and can be easily excreted from the body, even in those with renal dysfunction, obesity, and other critical conditions. 69 In addition, the anti‐inflammatory effects of UFH can be beneficial in alleviating the inflammatory processes of COVID‐19, such as the associated COVID‐19 CSS. 70 Interestingly, LMWH has less direct antiviral activity due to the shorter duration of competitive spike protein binding than UFH. 54 Nevertheless, LMWH might be preferred to UFH due to several reports of heparin resistance in patients with COVID‐19, 71 the lower risk of bleeding and dose‐dependent plasma levels, and a longer half‐life. 72 , 73
In patients with COVID‐19 with a history of heparin‐induced thrombocytopenia (HIT), activated factor X (Xa) inhibitors, like fondaparinux, could be administered as an alternative. Due to the occurrence of coagulopathy in ARDS, which might be limited to the lungs, it has been hypothesized that nebulized anticoagulation might be the best approach. However, previous reports have not shown the clinical benefit of this route of administration, but there is a need for further studies. 74 , 75 Furthermore, although UFH is the drug of choice in any trimester of pregnancy, LMWH is the best option for postpartum patients. 76 In conditions where anticoagulants are contraindicated or unavailable, mechanical thromboprophylaxis, such as pneumatic compression devices, should be considered. However, applying mechanical and pharmacological thromboprophylaxis for critically ill patients with COVID‐19 might lead to better outcomes if not contraindicated. Conversely, anticoagulation therapy is not beneficial in outpatient settings, as it has not been associated with an improved prognosis. 77 Nonetheless, there are conditions where anticoagulation therapy would appear necessary, such as immediately following discharge from the hospital.
As shown in Figure 2, coagulation occurs due to tissue damage, and several pathways happen, such as contact activation (intrinsic), tissue factor (extrinsic), and common pathways. Anticoagulants such as warfarin inhibit FII, FIX, FX, protein‐S, and protein‐C. In addition to warfarin, tissue factor pathway inhibitors can inhibit the conversion pathway of FVII to FVIIa and generally prevent blood coagulation. FIIa inhibitors include DTIs, DFXal, and LMWH. DTIs are inhibitors that inhibit the polymerization and stabilization pathway of FIa.
FIGURE 2.
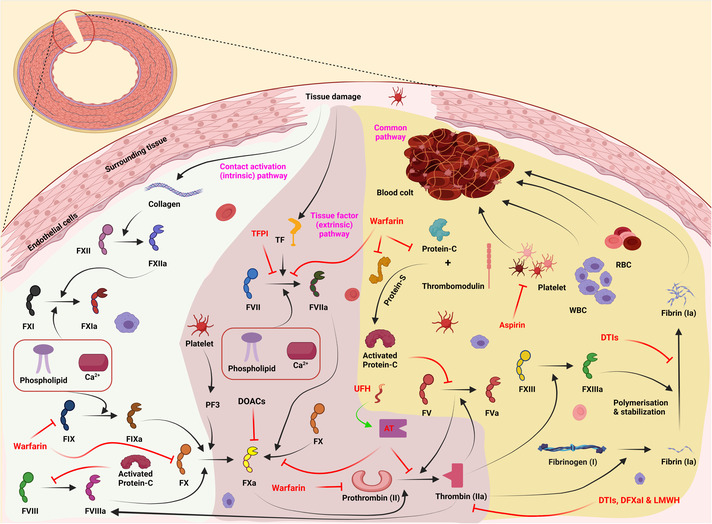
A summary of coagulation pathways. Coagulation occurs through contact activation (intrinsic), tissue factor (extrinsic), and common pathways when tissue damage occurs. The intrinsic pathway includes FVIII, FIX, FX, FX, FXI, and FXII. The extrinsic pathway contains coagulation factors FII, FVII, and FX. The common pathway also includes coagulation factors FI, FV, and FXIII. Each of these factors is activated by affecting the other factor. Anticoagulants are used to prevent blood clots in some diseases. Warfarin is one of these anticoagulants that inhibits FII, FIX, FX, protein‐S, and protein‐C. It can also block the conversion path of FVII to FVIIa. DOACs inhibit FXa. AT also blocks the conversion path of FII to FIIa and FX to FXa simultaneously. FIIa inhibitors include DTIs, DFXal, and LMWH. DTIs are inhibitors that inhibit the polymerization and stabilization pathway of FIa. UFH works to boost AT function. Aspirin inhibits the accumulation of active platelets next to each other. In addition to warfarin, TFPI can inhibit the conversion pathway of FVII to FVIIa. AT, antithrombin; DFXal, direct factor Xa inhibitors; DOACs, direct oral anticoagulants; DTIs, direct thrombin inhibitors; LMWH, low‐molecular‐weight heparin; RBC, red blood cells; TFPI, tissue factor pathway inhibitor; UFH, unfractionated heparin; WBC, white blood cells.
It is important to note that due to long half‐lives and unpredictable and unstable metabolic effects in those with acute diseases, direct oral anticoagulants (DOACs), such as dabigatran, apixaban, rivaroxaban, and edoxaban, are not appropriate in critically ill patients with COVID‐19. However, they may be ideal for patients with COVID‐19 who require continued or prolonged anticoagulation after hospital discharge. 78 , 79 The advantage of these agents is that they do not require regular international normalized ratio monitoring, which makes them preferred over vitamin K antagonists, such as warfarin. 80 The best available DOACs to administer are rivaroxaban (10 mg once a day), betrixaban (160 mg on the first day, followed by 80 mg once a day), and apixaban (2.5 mg twice a day). 81 It is crucial to consider the potential interference of dexamethasone with DOACs. Dexamethasone can potentially decrease the plasma levels of DOACs by inducing cytochrome P450 3A4 (CYP3A4) and P‐glycoprotein. This is another reason to avoid using oral anticoagulants in hospitalized patients, especially severe and critical patients, who are usually on corticosteroids. 82 Finally, thrombolytic therapy is usually not recommended in patients with COVID‐19 unless there is another clinical indication, such as ST‐elevation MI or massive PE with hemodynamic instability. 83
WHAT IS THE APPROPRIATE DOSE OF ANTICOAGULANTS?
Like any hospitalized patient, patients with COVID‐19 are recommended to be started on prophylactic doses of anticoagulants to prevent thromboembolic complications associated with immobility. 84 Previously, it was agreed that higher doses of anticoagulants should only be considered in those patients with indications for therapeutic anticoagulation, such as having a mechanical valve, VTE, or atrial fibrillation. 85 However, it has been reported that many patients with COVID‐19, particularly those admitted to the ICU, have developed thromboembolic events, even without the mentioned comorbidities and despite being on prophylactic anticoagulation doses, which have mostly been LMWH. 84 , 86 The reason for this might be the COVID‐19‐associated changes in circulating prothrombotic factors, resulting in increased hypercoagulability, despite being on thromboprophylaxis. 87 Therefore, it can be inferred that some conditions might require higher or therapeutic doses of anticoagulants. Intermediate doses of therapeutic anticoagulants are estimated to decrease mortality in critically ill patients. 88 , 89 It is recommended that all hospitalized patients with COVID‐19 should be started on prophylactic doses of UFH/LMWH unless contraindicated. However, some conditions necessitate therapeutic doses of anticoagulants, which include: (1) intubated patients with sudden clinical and laboratory findings suggestive of PE; (2) patients with any signs of thrombosis, such as acral ischemia and thrombophlebitis; and (3) patients with respiratory failure, ARDS, and suspected microthrombosis, especially those with elevated D‐dimer and fibrinogen levels. 12
In hospitalized patients with COVID‐19, therapeutic doses of anticoagulants may involve administering 0.5 mg/kg of enoxaparin subcutaneously (s.c.) twice daily, heparin 7500 units s.c. three times daily, or via continuous heparin infusion. It is better to de‐escalate the anticoagulant dose in patients when they get better and are transferred out of the ICU. In pregnancy, especially in the third trimester, intermediate doses of anticoagulants are often recommended by the American College of Obstetricians and Gynecologists. 90 Finally, whether to use prophylactic or therapeutic doses of anticoagulants should be made individually.
ANTICOAGULATION IN SPECIAL POPULATIONS
Obesity
It is well‐recognized that obesity (body mass index [BMI] ≥30 kg/m2) is a significant risk factor for mortality in patients with COVID‐19. Apart from the difference in prophylactic and therapeutic doses of anticoagulants, it should be noted that in obese individuals, weight‐based dosing is the best strategy. 91 In hospitalized cases with obesity (BMI 30 kg/m2) or morbid obesity (BMI 40 kg/m2), intermediate‐dose or weight‐adjusted LMWH as a thromboprophylaxis regimen should be taken into consideration (Table 3). 97 However, DOACs with weight‐adjusted dosing are good options in outpatient settings. 98
TABLE 3.
Clinical recommendations for the use of anticoagulants in special populations infected with COVID‐19.
| Special population | Recommendations and dosage | Study or guideline (year) |
|---|---|---|
| Obesity (BMI ≥30 kg/m2) or morbid obesity (BMI ≥40 kg/m2) | When hospitalized, intermediate‐dose or weight‐adjusted LMWH as a thromboprophylaxis regimen should be considered. | Spyropoulos et al. (2020) 92 |
| Renal impairment | When CrCl <30 mL/min or acute kidney injury occurred, 5000 units of UFH subcutaneously two or three times a day or low‐dose LMWH should be considered. | OBE et al. (2020) 93 |
| Pregnant women |
Antepartum evaluation
Postpartum evaluation
|
RCOG (2022) 94 |
|
Antepartum evaluation
Postpartum evaluation
|
NIH (2022) 95 | |
| Pediatrics | When hospitalized with significantly increased D‐dimer or superimposed clinical risk factors for hospital‐related VTE, initiation of low‐dose LMWH subcutaneously twice a day should be considered. | Goldenberg et al. (2020) 96 |
Abbreviations: BMI, body mass index; COVID‐19, coronavirus disease 2019; CPAP, continuous positive airway pressure; CrCl, creatinine clearance; LMWH, low‐molecular‐weight heparin; NIH, National Institute of Health; RCOG, Royal College of Obstetricians and Gynecologists; UFH, unfractionated heparin; VTE, venous thromboembolism.
Renal impairment
In hospitalized patients with COVID‐19 with renal impairment, a lower dose of LMWH (50 IU/kg daily of anti‐Xa) can be administered to those with an estimated glomerular filtration rate (eGFR) less than 30 mL/min. However, in patients undergoing continuous veno‐venous hemofiltration or extracorporeal membrane oxygenation, UFH infusion is recommended. In brief, UFH is preferred over LMWH in patients with AKI or an eGFR of 15–30 mL/min. However, danaparoid, argatroban, or bivalirudin can be the UFH alternatives, with dose adjustment. 99 On the other hand, warfarin is preferred over DOACs in renal failure for outpatients with ongoing anticoagulation. Nonetheless, at an eGFR of 15–30 mL/min, apixaban may be harmless due to being partially excretable via dialysis, but it is better to avoid DOACs at an eGFR less than 15 mL/min or in patients with ESRD.
Pregnancy
Pregnancy per se is accompanied by an increased risk of thromboembolic complications, such as VTE and PE. Thus, SARS‐CoV‐2‐infected pregnant women should be started on anticoagulants unless the bleeding risk is high and labor is expected to occur within 12 h. 90 The clinical recommendations for the use of anticoagulants in this special population and the other ones have been stated in Table 3.
Pediatrics
Children and adolescents rarely develop severe forms of COVID‐19. Therefore, hospitalization and anticoagulation therapy are seldom necessary for this population. However, thromboprophylaxis seems reasonable in severe cases, such as multisystem inflammatory syndrome, due to the increased risk of thromboembolic complications. 100 In hospitalized children with significantly increased D‐dimers (≥5 times ULN), standard dose thromboprophylaxis with LMWH or UFH is recommended. 92 Nevertheless, in critically ill patients with high D‐dimers, ferritin, and CRP, intermediate to therapeutic doses of anticoagulants are favored, along with extending thromboprophylaxis to 30 days post‐discharge. 101
DURATION OF THE ANTICOAGULATION THERAPY
There has been a question of whether thromboprophylaxis should be continued after hospital discharge and, if yes, for how long. Not all SARS‐CoV‐2‐infected patients necessarily need thromboprophylaxis beyond the time of hospital discharge. However, continuing anticoagulation may be reasonable in patients with ongoing thromboembolic risk factors. In patients with moderate to severe SARS‐CoV‐2 infections and ongoing thromboembolic risk factors, prophylaxis is recommended for a limited period (e.g., for at least 10 days or upon complete recovery). Conversely, for critically ill patients with COVID‐19, prolonged thromboprophylaxis with UFH or LMWH is recommended until a full recovery has been made, or at least until they are fully mobile. 102 The NIH recommends using one of these criteria to recognize patients with an ongoing need for post‐discharge anticoagulation: (1) modified International Medical Prevention Registry on Venous Thromboembolism (IMPROVE) VTE (MIV) score (Table 4) greater than or equal to 4; (2) MIV greater than 2 with a D‐dimer greater than two times the ULN; (3) age greater than or equal to 75 years old; (4) age greater than 60 years, with a D‐dimer greater than two times the ULN, and (5) age 40–60 years with a D‐dimer greater than two times the ULN and a history of VTE or diagnosed malignancy. 44
TABLE 4.
Modified IMPROVE VTE (MIV) risk score. 103
| VTE risk factor | VTE risk score |
|---|---|
| Previous VTE | 3 |
| Known thrombophillia a | 2 |
| Current lower limb paralysis or paresis b | 2 |
| History of malignancy c | 2 |
| ICU/CCU admission | 1 |
| Complete immobilization d ≥1 day | 1 |
| Age ≥ 60 years | 1 |
Abbreviations: CCU, cardiac care unit; ICU, intensive care unit; IMPROVE, International Medical Prevention Registry on Venous Thromboembolism; NIH, National Institutes of Health; VTE, venous thromboembolism.
A congenital or acquired condition that causes the risk of excessive thrombosis (e.g., factor C/S deficiency, lupus anticoagulant, and Leiden Factor V).
Feet falls into bed 5 s but has an effort against gravity (taken from the NIH stroke scale).
Malignancy (not including non‐melanoma skin cancer) at any time in the past 5 years (malignancy must be in remission to meet eligibility criteria).
Immobilization is limited to beds or chairs with or without bathroom privileges.
In pregnant patients with COVID‐19 with severe manifestations but without thromboembolic complications, anticoagulation is recommended for at least 10–42 days post‐discharge. 76 In patients who have developed significant thromboembolic events, such as VTE or PTE, it is recommended to continue anticoagulation for at least 3 months following the discharge. 93 In significant thromboembolic events where extended anticoagulation is needed, the preference should be oral anticoagulant agents, such as rivaroxaban, or once‐daily i.v. agents, such as enoxaparin. 94
IS THERE A PROBABILITY OF THROMBOEMBOLIC CONSEQUENCES, DESPITE APPROPRIATELY DOSED THROMBOPROPHYLAXIS?
In the current pandemic, it has become evident that even intermediate‐to‐high doses of thromboprophylaxis may not prevent thromboembolic events in some patients with COVID‐19, especially among critically ill patients. 95 Furthermore, laboratory reports indicate that critically ill patients with COVID‐19 might have hypercoagulable profiles, despite being on therapeutic doses of anticoagulants. 96 Many studies reported SARS‐CoV‐2‐infected patients developing thromboembolic events, despite being on adequate anticoagulant doses. 104 , 105 The reasons behind this condition might be the activation of different underlying prothrombotic and inflammatory pathways that cannot be inhibited entirely, despite high doses of anticoagulants. 106 , 107 Moreover, ethnicity could be another critical risk factor, as the African‐American population is more prone to thromboembolic events than other ethnicities. Therefore, the usual doses of anticoagulants may not suffice in these patients. 108 , 109 , 110
ADVERSE EVENTS FOLLOWING ANTICOAGULATION THERAPY
Compared with prophylactic doses, therapeutic‐dose of anticoagulants may have more adverse events, such as major bleeding. 51 This adverse event is more prevalent in older patients with a more severe infection, recent trauma or surgery history, and being hospitalized longer. 111 Hemorrhagic complications occur more commonly with DOACs because they have longer half‐lives, and their elimination takes longer. 112 HIT is another complication of heparin therapy, resulting from the production of antiplatelet factor 4 (PF4) antibodies. 113
LABORATORY MONITORING
The laboratory tests for anticoagulant monitoring include aPTT for UFH and anti‐Xa levels for LMWH. However, LMWH has an advantage over UFH among heparin derivatives because frequent laboratory monitoring is not required due to the low risk of bleeding or thrombosis. 114 Although it is clear that the dose of UFH should be monitored in patients on long‐term anticoagulant therapy, this coagulation index may not be reliable among patients with COVID‐19 because these patients may have baseline aPTT abnormalities. 115 Nevertheless, despite the unreliability in patients with COVID‐19, aPTT is often used as a monitoring tool because it is widely available. This raises the question of whether follow‐up strategies are required in hospitalized patients with COVID‐19 receiving UFH or LMWH. It is suggested that daily monitoring of anti‐Xa levels be performed in ICU‐admitted patients while monitoring one or two times a week seems sufficient for patients not admitted to the ICU. 102 Furthermore, in patients with documented heparin resistance (e.g., the need for >35,000 units of heparin per 24 h, according to their aPTT levels), anti‐Xa assay is preferred over aPTT for monitoring the therapeutic doses of UFH.
CONCLUSION
The severe hypercoagulability observed in patients with COVID‐19 has been estimated to account for most cases where the disease reaches a critical phase. Therefore, anticoagulants seem to remain one of the essential therapeutics for this potentially life‐threatening condition. Thus, choosing a suitable anticoagulant and the appropriate dosage is vitally important. The main pathways of COVID‐19‐induced hypercoagulation include (1) inflammatory state and elevated cytokines levels and (2) virus‐specific mechanisms. COVID‐19‐related CSS plays a role in the coagulation system activation and hypercoagulation. Moreover, cytokines like IL‐1, IL‐6, and TNF‐α all can lead to overexpression of TF and, in contrast, reduce the expression of important natural anticoagulants like protein C and antithrombin. It has been demonstrated that viral engagement of ACE2 reduces its expression and activates the RAS, which is responsible for promoting platelet adhesion and aggregation. PAI‐1 rose after SARS‐CoV‐2 infection, which shows a probable direct effect of infection on the expression of anticoagulant agents. Finally, the endothelial cells' damage could also activate NETosis, which produces a link between inflammation, coagulation and thrombosis on both localized and systemic levels.
CONFLICT OF INTEREST STATEMENT
T.T.S. reports that he provides strategic and scientific recommendations as a member of the Advisory Board and speaker for Novocure, Inc. and also as a member of the Advisory Board to Galera Therapeutics, which are not in any way associated with the content or disease site as presented in this manuscript. All other authors have no relevant financial interests to be declared.
ACKNOWLEDGMENTS
The authors would like to thank the clinical research development center of Imam Reza Hospital, Kermanshah University of Medical Sciences, for their kind support. All figures were created with BioRender.com.
Mohseni Afshar Z, Tavakoli Pirzaman A, Hosseinzadeh R, et al. Anticoagulant therapy in COVID‐19: A narrative review. Clin Transl Sci. 2023;16:1510‐1525. doi: 10.1111/cts.13569
Zeinab Mohseni Afshar and Ali Tavakoli Pirzaman contributed equally to this study and are jointly the first authors of this article.
Contributor Information
Mohammad Barary, Email: m.barary@mubabol.ac.ir.
Soheil Ebrahimpour, Email: drsoheil1503@yahoo.com.
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Javanian M, Bayani M, Shokri M, et al. Risk factors for mortality of 557 adult patients with COVID 19 in Babol, Northern Iran: a retrospective cohort study. Bratisl Lek Listy. 2021;122(1):34–38. [DOI] [PubMed] [Google Scholar]
- 2. Ten Cate H. Surviving Covid‐19 with heparin? N Engl J Med. 2021;385(9):845‐846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nopp S, Moik F, Jilma B, Pabinger I, Ay C. Risk of venous thromboembolism in patients with COVID‐19: a systematic review and meta‐analysis. Res Pract Thromb Haemost. 2020;4(7):1178‐1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ebrahimpour S, Mohseni Afshar Z, Mohseni S, et al. Neurologic manifestations in patients with COVID‐19: a case report. Caspian J Intern Med. 2020;11(Suppl 1):557‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Afshara ZM, Babazadehb A, Javanian M, et al. A review of cardiac involvement in COVID‐19 infection. Cor Vasa. 2020;62(6):610‐615. [Google Scholar]
- 6. Shahjouei S, Naderi S, Li J, et al. Risk of stroke in hospitalized SARS‐CoV‐2 infected patients: a multinational study. EBioMedicine. 2020;59:102939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barrett CD, Moore HB, Yaffe MB, Moore EE. ISTH interim guidance on recognition and management of coagulopathy in COVID‐19: a comment. J Thromb Haemost. 2020;18(8):2060‐2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS‐CoV‐2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089‐1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gralinski LE, Sheahan TP, Morrison TE, et al. Complement activation contributes to severe acute respiratory syndrome coronavirus pathogenesis. mBio. 2018;9(5):e01753‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. al‐Samkari H, Karp Leaf RS, Dzik WH, et al. COVID‐19 and coagulation: bleeding and thrombotic manifestations of SARS‐CoV‐2 infection. Blood. 2020;136(4):489‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yin S, Huang M, Li D, Tang N. Difference of coagulation features between severe pneumonia induced by SARS‐CoV2 and non‐SARS‐CoV2. J Thromb Thrombolysis. 2021;51(4):1107‐1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gozzo L, Viale P, Longo L, Vitale DC, Drago F. The potential role of heparin in patients with COVID‐19: beyond the anticoagulant effect. A review. Front Pharmacol. 2020;11:1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ragab D, Salah Eldin H, Taeimah M, Khattab R, Salem R. The COVID‐19 cytokine storm; what we know so far. Front Immunol. 2020;11:1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bergamaschi L, Mescia F, Turner L, et al. Longitudinal analysis reveals that delayed bystander CD8+ T cell activation and early immune pathology distinguish severe COVID‐19 from mild disease. Immunity. 2021;54(6):1257‐1275.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Conti P, Ronconi G, Caraffa A, et al. Induction of pro‐inflammatory cytokines (IL‐1 and IL‐6) and lung inflammation by Coronavirus‐19 (COVI‐19 or SARS‐CoV‐2): anti‐inflammatory strategies. J Biol Regul Homeost Agents. 2020;34(2):327‐331. [DOI] [PubMed] [Google Scholar]
- 16. Levi M, van der Poll T. Coagulation and sepsis. Thromb Res. 2017;149:38‐44. [DOI] [PubMed] [Google Scholar]
- 17. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID‐19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846‐848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bester J, Pretorius E. Effects of IL‐1beta, IL‐6 and IL‐8 on erythrocytes, platelets and clot viscoelasticity. Sci Rep. 2016;6:32188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Folman CC, Linthorst GE, van Mourik J, et al. Platelets release thrombopoietin (Tpo) upon activation: another regulatory loop in thrombocytopoiesis? Thromb Haemost. 2000;83(6):923‐930. [PubMed] [Google Scholar]
- 20. Stouthard JM, Levi M, Hack CE, et al. Interleukin‐6 stimulates coagulation, not fibrinolysis, in humans. Thromb Haemost. 1996;76(5):738‐742. [PubMed] [Google Scholar]
- 21. Stirling D, Hannant WA, Ludlam CA. Transcriptional activation of the factor VIII gene in liver cell lines by interleukin‐6. Thromb Haemost. 1998;79(1):74‐78. [PubMed] [Google Scholar]
- 22. Cugno M, Borghi A, Garcovich S, Marzano AV. Coagulation and skin autoimmunity. Front Immunol. 2019;10:1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guang C, Phillips RD, Jiang B, Milani F. Three key proteases‐angiotensin‐I‐converting enzyme (ACE), ACE2 and renin‐within and beyond the renin‐angiotensin system. Arch Cardiovasc Dis. 2012;105(6–7):373‐385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Marshall RP. The pulmonary renin‐angiotensin system. Curr Pharm Des. 2003;9(9):715‐722. [DOI] [PubMed] [Google Scholar]
- 25. Vaughan DE. Angiotensin, fibrinolysis, and vascular homeostasis. Am J Cardiol. 2001;87(8A):18C‐24C. [DOI] [PubMed] [Google Scholar]
- 26. Wu YP, Wei R, Liu ZH, et al. Analysis of thrombotic factors in severe acute respiratory syndrome (SARS) patients. Thromb Haemost. 2006;96(1):100‐101. [DOI] [PubMed] [Google Scholar]
- 27. Tang BS, Chan KH, Cheng VC, et al. Comparative host gene transcription by microarray analysis early after infection of the Huh7 cell line by severe acute respiratory syndrome coronavirus and human coronavirus 229E. J Virol. 2005;79(10):6180‐6193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID‐19. Lancet. 2020;395(10234):1417‐1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stern D, Nawroth P, Handley D, Kisiel W. An endothelial cell‐dependent pathway of coagulation. Proc Natl Acad Sci U S A. 1985;82(8):2523‐2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Duca ST, Costache AD, Miftode RŞ, Mitu O, PetriŞ A, Costache II. Hypercoagulability in COVID‐19: from an unknown beginning to future therapies. Med Pharm Rep. 2022;95(3):236‐242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Becker RC. COVID‐19 update: Covid‐19‐associated coagulopathy. J Thromb Thrombolysis. 2020;50(1):54‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Loo J, Spittle DA, Newnham M. COVID‐19, immunothrombosis and venous thromboembolism: biological mechanisms. Thorax. 2021;76(4):412‐420. [DOI] [PubMed] [Google Scholar]
- 33. Lim S, Zhang M, Chang TL. ACE2‐independent alternative receptors for SARS‐CoV‐2. Viruses. 2022;14(11):2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Flood VH, Garcia J, Haberichter SL. The role of genetics in the pathogenesis and diagnosis of type 1 Von Willebrand disease. Curr Opin Hematol. 2019;26(5):331‐335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Swystun LL, Notley C, Georgescu I, et al. The endothelial lectin clearance receptor CLEC4M binds and internalizes factor VIII in a VWF‐dependent and independent manner. J Thromb Haemost. 2019;17(4):681‐694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Alam W. Hypercoagulability in COVID‐19: a review of the potential mechanisms underlying clotting disorders. SAGE Open Med. 2021;9:20503121211002996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Guler N, Siddiqui F, Fareed J. Is the reason of increased D‐dimer levels in COVID‐19 because of ACE‐2‐induced apoptosis in endothelium? Clin Appl Thromb Hemost. 2020;26:1076029620935526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Weitz JI, Fredenburgh JC, Eikelboom JW. A test in context: D‐dimer. J Am Coll Cardiol. 2017;70(19):2411‐2420. [DOI] [PubMed] [Google Scholar]
- 39. Otifi HM, Adiga BK. Endothelial dysfunction in Covid‐19 infection. Am J Med Sci. 2022;363(4):281‐287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844‐847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhou F, Yu T, du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kollias A, Kyriakoulis KG, Dimakakos E, Poulakou G, Stergiou GS, Syrigos K. Thromboembolic risk and anticoagulant therapy in COVID‐19 patients: emerging evidence and call for action. Br J Haematol. 2020;189(5):846‐847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bruinstroop E, van de Ree MA, Huisman MV. The use of D‐dimer in specific clinical conditions: a narrative review. Eur J Intern Med. 2009;20(5):441‐446. [DOI] [PubMed] [Google Scholar]
- 44. Chandra A, Chakraborty U, Ghosh S, Dasgupta S. Anticoagulation in COVID‐19: current concepts and controversies. Postgrad Med J. 2022;98(1159):395‐402. [DOI] [PubMed] [Google Scholar]
- 45. Fanaroff AC, Lopes RD. COVID‐19 thrombotic complications and therapeutic strategies. Annu Rev Med. 2023;74:15‐30. [DOI] [PubMed] [Google Scholar]
- 46. National Institutes of Health . COVID‐19 Treatment Guidelines. National Institutes of Health: National Institutes of Health; 2022. [Google Scholar]
- 47. Cuker A, Tseng EK, Nieuwlaat R, et al. American Society of Hematology living guidelines on the use of anticoagulation for thromboprophylaxis in patients with COVID‐19: January 2022 update on the use of therapeutic‐intensity anticoagulation in acutely ill patients. Blood Adv. 2022;6(17):4915‐4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cuker A, Tseng EK, Nieuwlaat R, et al. American society of hematology 2021 guidelines on the use of anticoagulation for thromboprophylaxis in patients with COVID‐19. Blood Adv. 2021;5(3):872‐888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Thachil J, Tang N, Gando S, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID‐19. J Thromb Haemost. 2020;18(5):1023‐1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Connors JM, Brooks MM, Sciurba FC, et al. Effect of antithrombotic therapy on clinical outcomes in outpatients with clinically stable symptomatic COVID‐19: the ACTIV‐4B randomized clinical trial. JAMA. 2021;326(17):1703‐1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. The REMAP‐CAP, ACTIV‐4a, and ATTACC Investigators . Therapeutic anticoagulation with heparin in critically ill patients with Covid‐19. N Engl J Med. 2021;385(9):777‐789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Weeks LD, Sylvester KW, Connors JM, Connell NT. Management of therapeutic unfractionated heparin in COVID‐19 patients: a retrospective cohort study. Res Pract Thromb Haemost. 2021;5(4):e12521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Paranjpe I, Fuster V, Lala A, et al. Association of treatment dose anticoagulation with In‐hospital survival among hospitalized patients with COVID‐19. J Am Coll Cardiol. 2020;76(1):122‐124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094‐1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zang L, Zhu H, Wang K, Liu Y, Yu F, Zhao W. Not just anticoagulation‐new and old applications of heparin. Molecules. 2022;27(20):6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Baumann Kreuziger L, Sholzberg M, Cushman M. Anticoagulation in hospitalized patients with COVID‐19. Blood. 2022;140(8):809‐814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Davidson BL, Geerts WH, Lensing AW. Low‐dose heparin for severe sepsis. N Engl J Med. 2002;347(13):1036‐1037. [DOI] [PubMed] [Google Scholar]
- 58. Menezes‐Rodrigues FS, Padrão Tavares JG, Pires de Oliveira M, et al. Anticoagulant and antiarrhythmic effects of heparin in the treatment of COVID‐19 patients. J Thromb Haemost. 2020;18(8):2073‐2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Buijsers B, Yanginlar C, Maciej‐Hulme ML, de Mast Q, van der Vlag J. Beneficial non‐anticoagulant mechanisms underlying heparin treatment of COVID‐19 patients. EBioMedicine. 2020;59:102969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. LaRiviere WB, Schmidt EP. The pulmonary endothelial Glycocalyx in ARDS: a critical role for Heparan sulfate. Curr Top Membr. 2018;82:33‐52. [DOI] [PubMed] [Google Scholar]
- 61. Driggin E, Madhavan MV, Bikdeli B, et al. Cardiovascular considerations for patients, health care workers, and health systems during the COVID‐19 pandemic. J Am Coll Cardiol. 2020;75(18):2352‐2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Levi M, de Jonge E, van der Poll T. Rationale for restoration of physiological anticoagulant pathways in patients with sepsis and disseminated intravascular coagulation. Crit Care Med. 2001;29(7 Suppl):S90‐S94. [DOI] [PubMed] [Google Scholar]
- 63. Russo V, Bottino R, D'Andrea A, et al. Chronic oral anticoagulation and clinical outcome in hospitalized COVID‐19 patients. Cardiovasc Drugs Ther. 2022;36(4):705‐712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Russo V, di Maio M, Attena E, et al. Clinical impact of pre‐admission antithrombotic therapy in hospitalized patients with COVID‐19: a multicenter observational study. Pharmacol Res. 2020;159:104965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Iaccarino G, Grassi G, Borghi C, et al. Preexisting oral anticoagulant therapy ameliorates prognosis in hospitalized COVID‐19 patients. Front Cardiovasc Med. 2021;8:633878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China . JAMA. 2020;323(11):1061‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Testa S, Prandoni P, Paoletti O, et al. Direct oral anticoagulant plasma levels' striking increase in severe COVID‐19 respiratory syndrome patients treated with antiviral agents: the Cremona experience. J Thromb Haemost. 2020;18(6):1320‐1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gong IY, Kim RB. Importance of pharmacokinetic profile and variability as determinants of dose and response to dabigatran, rivaroxaban, and apixaban. Can J Cardiol. 2013;29(7 Suppl):S24‐S33. [DOI] [PubMed] [Google Scholar]
- 69. Chlebowski MM, Baltagi S, Carlson M, Levy JH, Spinella PC. Clinical controversies in anticoagulation monitoring and antithrombin supplementation for ECMO. Crit Care. 2020;24(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Shi C, Wang C, Wang H, et al. The potential of low molecular weight heparin to mitigate cytokine storm in severe COVID‐19 patients: a retrospective cohort study. Clin Transl Sci. 2020;13(6):1087‐1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Beun R, Kusadasi N, Sikma M, Westerink J, Huisman A. Thromboembolic events and apparent heparin resistance in patients infected with SARS‐CoV‐2. Int J Lab Hematol. 2020;42(Suppl 1):19‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hao C, Sun M, Wang H, Zhang L, Wang W. Low molecular weight heparins and their clinical applications. Prog Mol Biol Transl Sci. 2019;163:21‐39. [DOI] [PubMed] [Google Scholar]
- 73. Alquwaizani M, Buckley L, Adams C, Fanikos J. Anticoagulants: a review of the pharmacology, dosing, and complications. Curr Emerg Hosp Med Rep. 2013;1(2):83‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Camprubí‐Rimblas M, Tantinyà N, Guillamat‐Prats R, et al. Effects of nebulized antithrombin and heparin on inflammatory and coagulation alterations in an acute lung injury model in rats. J Thromb Haemost. 2020;18(3):571‐583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. van Haren FMP, Page C, Laffey JG, et al. Nebulized heparin as a treatment for COVID‐19: scientific rationale and a call for randomized evidence. Crit Care. 2020;24(1):454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. D'Souza R, Malhamé I, Teshler L, Acharya G, Hunt BJ, McLintock C. A critical review of the pathophysiology of thrombotic complications and clinical practice recommendations for thromboprophylaxis in pregnant patients with COVID‐19. Acta Obstet Gynecol Scand. 2020;99(9):1110‐1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Flam B, Wintzell V, Ludvigsson JF, Mårtensson J, Pasternak B. Direct oral anticoagulant use and risk of severe COVID‐19. J Intern Med. 2021;289(3):411‐419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Mazzeffi MA, Chow JH, Tanaka K. COVID‐19 associated hypercoagulability: manifestations, mechanisms, and management. Shock. 2021;55(4):465‐471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Schutgens RE. DOAC in COVID‐19: yes or No? Hema. 2021;5(1):e526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G. Oral anticoagulant therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e44S‐e88S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Spyropoulos AC, Lipardi C, Xu J, et al. Modified IMPROVE VTE risk score and elevated D‐dimer identify a high venous thromboembolism risk in acutely ill medical population for extended thromboprophylaxis. TH Open. 2020;4(1):e59‐e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Bosch FTM, Candeloro M, Potere N, Porreca E, di Nisio M, Kamphuisen PW. Effect of dexamethasone on direct Xa‐inhibitor oral anticoagulant plasma levels in patients with COVID‐19. Thromb Res. 2021;205:106‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Whyte CS, Morrow GB, Mitchell JL, Chowdary P, Mutch NJ. Fibrinolytic abnormalities in acute respiratory distress syndrome (ARDS) and versatility of thrombolytic drugs to treat COVID‐19. J Thromb Haemost. 2020;18(7):1548‐1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID‐19. Thromb Res. 2020;191:145‐147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Lillicrap D. Disseminated intravascular coagulation in patients with 2019‐nCoV pneumonia. J Thromb Haemost. 2020;18(4):786‐787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Hanify JM, Dupree LH, Johnson DW, Ferreira JA. Failure of chemical thromboprophylaxis in critically ill medical and surgical patients with sepsis. J Crit Care. 2017;37:206‐210. [DOI] [PubMed] [Google Scholar]
- 87. Panigada M, Bottino N, Tagliabue P, et al. Hypercoagulability of COVID‐19 patients in intensive care unit: a report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020;18(7):1738‐1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Talasaz AH, Sadeghipour P, Kakavand H, et al. Recent randomized trials of antithrombotic therapy for patients with COVID‐19: JACC state‐of‐the‐art review. J Am Coll Cardiol. 2021;77(15):1903‐1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Meizlish ML, Goshua G, Liu Y, et al. Intermediate‐dose anticoagulation, aspirin, and in‐hospital mortality in COVID‐19: a propensity score‐matched analysis. Am J Hematol. 2021;96(4):471‐479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. American College of Obstetricians and Gynecologists' Committee on Practice Bulletins–Obstetrics . ACOG practice bulletin No. 196: thromboembolism in pregnancy. Obstet Gynecol. 2018;132(1):e1‐e17. [DOI] [PubMed] [Google Scholar]
- 91. Thachil J. The versatile heparin in COVID‐19. J Thromb Haemost. 2020;18(5):1020‐1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Goldenberg NA, Sochet A, Albisetti M, et al. Consensus‐based clinical recommendations and research priorities for anticoagulant thromboprophylaxis in children hospitalized for COVID‐19‐related illness. J Thromb Haemost. 2020;18(11):3099‐3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Barnes GD, Burnett A, Allen A, et al. Thromboembolism and anticoagulant therapy during the COVID‐19 pandemic: interim clinical guidance from the anticoagulation forum. J Thromb Thrombolysis. 2020;50(1):72‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Cohoon KP, Mahé G, Tafur AJ, Spyropoulos AC. Emergence of institutional antithrombotic protocols for coronavirus 2019. Res Pract Thromb Haemost. 2020;4(4):510‐517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Middeldorp S, Coppens M, van Haaps TF, et al. Incidence of venous thromboembolism in hospitalized patients with COVID‐19. J Thromb Haemost. 2020;18(8):1995‐2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Tsantes AE, Frantzeskaki F, Tsantes AG, et al. The haemostatic profile in critically ill COVID‐19 patients receiving therapeutic anticoagulant therapy: an observational study. Medicine (Baltimore). 2020;99(47):e23365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Spyropoulos AC, Levy JH, Ageno W, et al. Scientific and standardization committee communication: clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID‐19. J Thromb Haemost. 2020;18(8):1859‐1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Wang TF, Carrier M, Fournier K, Siegal DM, le Gal G, Delluc A. Oral anticoagulant use in patients with morbid obesity: a systematic review and meta‐analysis. Thromb Haemost. 2022;122(5):830‐841. [DOI] [PubMed] [Google Scholar]
- 99. Garcia DA, Baglin TP, Weitz JI, Samama MM. Parenteral anticoagulants: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e24S‐e43S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383(4):334‐346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Karimi M, Bozorgi H, Zarei T, et al. Antithrombotic prophylaxis in children and adolescents' patients with SARS‐CoV‐2 (COVID‐19) infection: a practical guidance for clinicians. Acta Biomed. 2020;91(4):e2020170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Vandenbriele C, van Aelst L, Balthazar T, et al. Anticoagulant therapy in COVID‐19 critically ill: should we go for more. J Kardiol. 2020;57(5):168‐170. [Google Scholar]
- 103. Rachmi DA, Mulia EPB, Nugroho J. Possible mechanism and current recommendation of thromboembolism in COVID‐19. Open Access Macedonian J Med Sci. 2020;8(T1):66‐74. [Google Scholar]
- 104. di Tano G, Moschini L, Loffi M, Testa S, Danzi GB. Late pulmonary embolism after COVID‐19 pneumonia despite adequate rivaroxaban treatment. Eur J Case Rep Intern Med. 2020;7(7):001790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. le Jeune S, Suhl J, Benainous R, et al. High prevalence of early asymptomatic venous thromboembolism in anticoagulated COVID‐19 patients hospitalized in general wards. J Thromb Thrombolysis. 2021;51(3):637‐641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Iba T, Levy JH, Connors JM, Warkentin TE, Thachil J, Levi M. The unique characteristics of COVID‐19 coagulopathy. Crit Care. 2020;24(1):360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. REMAP‐CAP Investigators , ACTIV‐4a Investigators , ATTACC Investigators , et al. Therapeutic anticoagulation with heparin in noncritically ill patients with Covid‐19. N Engl J Med. 2021;385(9):790‐802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Zakai NA, McClure LA. Racial differences in venous thromboembolism. J Thromb Haemost. 2011;9(10):1877‐1882. [DOI] [PubMed] [Google Scholar]
- 109. Oudkerk M, Kuijpers D, Oudkerk SF, van Beek EJR. The vascular nature of COVID‐19. Br J Radiol. 2020;93(1113):20200718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Helms J, Severac F, Merdji H, et al. Higher anticoagulation targets and risk of thrombotic events in severe COVID‐19 patients: bi‐center cohort study. Ann Intensive Care. 2021;11(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Cossette B, Pelletier MÈ, Carrier N, et al. Evaluation of bleeding risk in patients exposed to therapeutic unfractionated or low‐molecular‐weight heparin: a cohort study in the context of a quality improvement initiative. Ann Pharmacother. 2010;44(6):994‐1002. [DOI] [PubMed] [Google Scholar]
- 112. Kamel MH, Yin W, Zavaro C, Francis JM, Chitalia VC. Hyperthrombotic milieu in COVID‐19 patients. Cell. 2020;9(11):2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Patell R, Khan AM, Bogue T, et al. Heparin induced thrombocytopenia antibodies in Covid‐19. Am J Hematol. 2020;95(10):E295‐E296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Schünemann HJ, Cushman M, Burnett AE, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv. 2018;2(22):3198‐3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Berkman SA, Tapson VF. COVID‐19 and its implications for thrombosis and anticoagulation. Semin Respir Crit Care Med. 2021;42(2):316‐326. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that supports the findings of this study are available from the corresponding author upon reasonable request.


