Abstract
Background and Objectives
Few data are available regarding the use of anesthetic infusions for refractory status epilepticus (RSE) in children and neonates, and ketamine use is increasing despite limited data. We aimed to describe the impact of ketamine for RSE in children and neonates.
Methods
Retrospective single-center cohort study of consecutive patients admitted to the intensive care units of a quaternary care children's hospital treated with ketamine infusion for RSE.
Results
Sixty-nine patients were treated with a ketamine infusion for RSE. The median age at onset of RSE was 0.7 years (interquartile range 0.15–7.2), and the cohort included 13 (19%) neonates. Three patients (4%) had adverse events requiring intervention during or within 12 hours of ketamine administration, including hypertension in 2 patients and delirium in 1 patient. Ketamine infusion was followed by seizure termination in 32 patients (46%), seizure reduction in 19 patients (28%), and no change in 18 patients (26%).
Discussion
Ketamine administration was associated with few adverse events, and seizures often terminated or improved after ketamine administration. Further data are needed comparing first-line and subsequent anesthetic medications for treatment of pediatric and neonatal RSE.
Classification of Evidence
This study provides Class IV evidence on the therapeutic utility of ketamine for treatment of RSE in children and neonates.
Many children with status epilepticus (SE) have persistent seizures despite administration of at least 2 appropriately dosed antiseizure medications, referred to as refractory SE (RSE).1 Continuous anesthetic infusions are often required,2 but few data are available regarding their safety or efficacy.3-6 Midazolam and pentobarbital are commonly administered anesthetics,4,7 but they have systemic side effects including respiratory failure and hypotension.5 Propofol is infrequently used in children given the risk of propofol infusion syndrome.2
Ketamine, a noncompetitive NMDA glutamate receptor antagonist, may be a beneficial alternative anesthetic. Animal models of SE indicate ketamine modulate cytokines and interacts with muscarinic, monoaminergic, nicotinic, and opioid receptor channels, thereby decreasing neuroinflammation which may contribute to SE refractoriness and acting as a neuroprotectant against glutamate-induced neuronal necrosis.3,8 In humans, ketamine is often administered for procedural sedation2,3,9 with rapid onset of a dissociative state9 and unconsciousness at high doses.9 Ketamine is less likely to cause cardiorespiratory compromise than other commonly used anesthetics.10 In addition, although ketamine is a direct myocardial depressant, it induces the release of endogenous catecholamines which can manifest as hypertension.10 A review of convulsive RSE treatment by the American Epilepsy Society concluded that insufficient evidence exists on the effectiveness of ketamine in adults and children.3,11 Some data indicate ketamine is safe and effective for RSE in adults, but pediatric data are very limited5,12-15 and neonatal data consist of only several case reports.16-18 Despite limited data, the use of ketamine for children with RSE is increasing, although often late in the course and in conjunction with pentobarbital.2 Given the knowledge gap regarding RSE management options,19 we studied consecutive neonates and children with RSE administered ketamine. We hypothesized that ketamine administration would be associated with few short-term adverse events and would often be followed by seizure termination.
Primary Research Questions
Is ketamine associated with adverse effects in children and neonates with RSE?
How often is ketamine administration followed by seizure termination or reduction in children and neonates with RSE?
Methods
Study Design and Data Collection
We performed a single-center retrospective study of consecutive patients who received a ketamine infusion for treatment of SE in the neonatal (NICU), cardiac (CICU), or pediatric intensive care units (PICU) at the Children's Hospital of Philadelphia.
Patients received care from Critical Care Medicine or Neonatology Services, along with the Neurology Critical Care Consultation Service. Continuous electroencephalographic monitoring (CEEG) interpretation was performed by the Critical Care Electroencephalography Service using report templates derived from published standardized critical care EEG terminology.20-22
To identify potential patients, we queried the electronic medical record (EMR) for all patients admitted to an intensive care unit (ICU) who were administered a ketamine infusion and had an order for CEEG using an Epic Clarity database (Verona, WI) using a custom SQL Oracle pipeline from EMR adoption in January 2011 to September 15, 2021. The first identified patient was admitted in 2018. All patients undergoing seizure management with anesthetic medications underwent clinically indicated CEEG based on an institutional pathway adherent to published guidelines.23-25 Thus, including CEEG in the query excluded patients receiving ketamine for indications other than seizure management.
Two neurocritical care nurse practitioners performed chart review of all potential patients to confirm the inclusion criteria: (1) ICU admission and (2) administration of ketamine infusion for seizures. Patients were excluded if the ketamine infusion was ordered but not initiated because the patient died or seizures terminated. We analyzed admission notes, consultation notes, daily progress notes, and any other documentation by nursing and medical staff. Data were entered into a Research Electronic Data Capture database.26
All patients underwent CEEG during ketamine administration. We classified the seizure burden on CEEG at ketamine initiation as acute repetitive seizures (seizure burden <5 minutes) or SE. SE was defined as >5 minutes of continuous and/or electrographic seizure activity or recurrent seizure activity without recovery between seizures, consistent with recent guideline definitions.11,24,27 SE was subclassified as brief seizures totaling >5 minutes in an hour, seizure(s) > 5 minutes, or both. We further classified RSE subtypes, new-onset RSE, and febrile infection–related epilepsy syndrome (FIRES) using consensus definitions.1 Patients fulfilling criteria of multiple categories were classified as the most severe category. CEEG background was categorized as continuous, discontinuous, burst suppression, or suppression. We assessed changes in seizures (termination, reduction, or no change), ictal-interictal continuum (IIC) patterns (improvement or no change), and the CEEG background category after ketamine administration. These changes after ketamine administration were assessed during clinical care and documented in Neurology Critical Care Consultation notes and CEEG reports.
Adverse effects related to ketamine were identified through chart review based on clinical documentation (primary or consultation services), vital signs and medication logs, and any other available documentation. Hypertension, hypotension, arrhythmias, and delirium were considered adverse effects of ketamine if they occurred during ketamine administration or within 12 hours of the last ketamine administration (bolus or infusion) and required mitigation intervention(s). Hypotension was defined as systolic blood pressure ≤5th percentile for age and sex.28,29 Given the known risk of hypertension with ketamine, hypertension was also identified based on age-appropriate blood pressure guidelines defined as systolic blood pressure ≥95th percentile for age and sex.28,30,31 Concern for increased intracranial pressure (ICP) was considered an adverse effect if the medical team(s) initiated serial neuroimaging (defined as more than 1 CT or MRI within a 24-hour period) to monitor for signs of increased ICP or the patient had an invasive ICP monitor after ketamine initiation. Delirium was considered an adverse effect if identified by the medical team(s), often including assessments using the Cornell Assessment of Pediatric Delirium Scale.32 Withdrawal symptoms during weaning and after cessation of ketamine infusion were identified by the medical team(s), often including assessments using the Withdrawal Assessment Tool–Version 1.33
Standard Protocol Approvals, Registrations, and Patient Consents
The Institutional Review Board approved the study with a waiver of consent.
Data Analysis and Statistical Methods
Data were analyzed using STATA version 14.2 (StataCorp, College Station, TX). Most analyses used descriptive statistics. We compared differences between groups using Wilcoxon rank-sum tests. Statistical significance was considered a 2-tailed p value <0.05.
Data Availability
We will make the underlying data available to investigators with appropriate data transfer and Institutional Review Board approval.
Results
Subject Characteristics
The EMR query identified 97 patients, and chart review determined 69 patients received ketamine infusion for SE. Table 1 provides demographic and clinical characteristics. The median age at SE onset was 0.7 years (interquartile range [IQR] 0.15–7.2), and 13/69 patients (19%) were 0–30-days-old. Seventeen patients (25%) had preexisting epilepsy at presentation, and patients with preexisting epilepsy were on a median of 3 antiseizure medications at presentation (IQR 2–4), and 2/17 patients (12%) had a vagal nerve stimulator. SE occurred previously in 7/69 patients (10%), including 5/7 patients (71%) with prior RSE requiring anesthetic medications (super RSE or prolonged super RSE) which included 1 subject with prior FIRES. Preexisting neurologic problems were present in 21/69 patients (30%), and the most common was developmental delay and/or intellectual disability in 12/69 patients (17%). Preexisting medical problems were common, including congenital malformations in 24/69 (35%); of those, 21/24 (88%) with congenital heart malformations.
Table 1.
Demographics and Preadmission Clinical Data (N = 69)
Table 2 summarizes acute medical problems and management. Acute neurologic injury was present on admission in 22/69 patients (32%), including hypoxic-ischemic encephalopathy in 14/22 (64%), stroke in 4/22 (18%), and traumatic brain injury in 4/22 (18%). SE types included RSE in 25/69 (36%), super RSE in 29/69 (42%), and prolonged super RSE in 15/69 (22%). All patients received antiseizure therapies before ketamine infusion, including a median of 3 (IQR 2–4) antiseizure medications (eTable 1, links.lww.com/WNL/C192).
Table 2.
Clinical, Seizure, and Concomitant Therapy Data
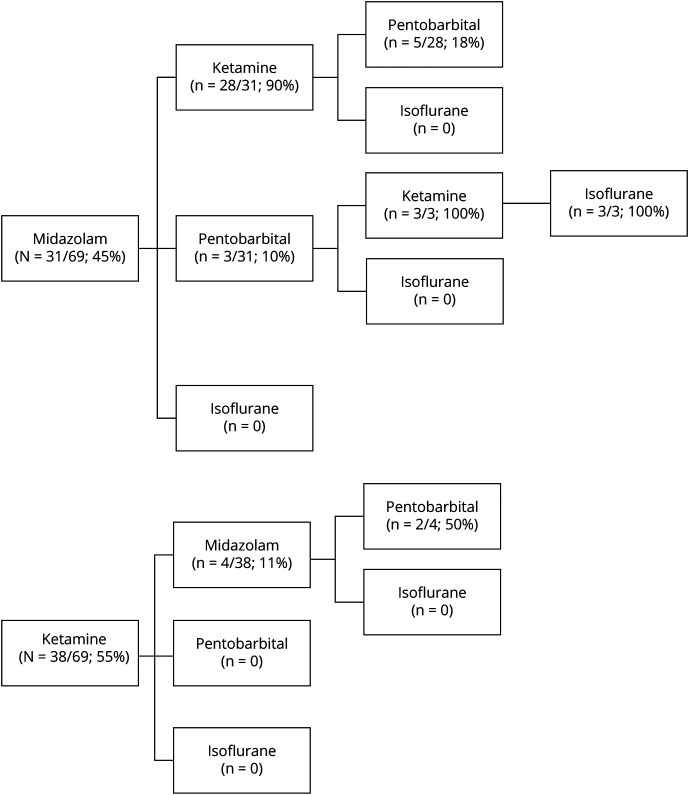
The median duration between SE onset and first anesthetic infusion was 0.5 days (IQR 0.2–1.2). The first anesthetic infusion was ketamine in 38/69 patients (55%) and midazolam in 31/69 patients (45%). No patients received pentobarbital or isoflurane as the first anesthetic (Figure 1). The median duration from SE onset to first anesthetic infusion initiation did not differ between midazolam (0.41 days, IQR 0.13–1.24) and ketamine (0.72 days, IQR 0.24–1.18) (p = 0.27). A second anesthetic infusion was administered to 35/69 patients (51%), including 28/35 (80%) administered ketamine, 4/35 (11%) administered midazolam, 3/35 (9%) administered pentobarbital, and none administered isoflurane. Among the patients requiring a second anesthetic infusion, 4/35 (11%) were due to seizures intractable to ketamine as a first-line anesthetic. A third anesthetic infusion was administered to 11/69 patients (16%), including 3/11 (27%) administered ketamine, 1/11 (11%) administered midazolam, 7/11 (64%) administered pentobarbital, and none administered isoflurane. A fourth anesthetic was administered to 3/69 patients (4%) and was always isoflurane (Figure 1).
Figure 1. Anesthetic Administration.
Ketamine Dosing and Timing
Ketamine was administered in the PICU for 37/69 patients (53%), CICU for 27/69 (39%), and NICU for 5/69 (7%). The median duration of SE onset to ketamine infusion initiation was 20 hours (IQR 10.8–45.3). Ketamine was initiated within 0–12 hours of SE onset in 23/69 patients (33%), 12–24 hours in 18/69 (26%), 1–2 days in 14/69 (20%), 2–3 days in 4/69 (6%), 3–4 days in 1/69 (1%), and >4 days in 9/69 (13%). Ketamine was administered significantly sooner as the first anesthetic (17.4 hours, IQR 5.7–28.3) than as the second anesthetic after midazolam (34.9 hours, IQR 12.3–82.9) (p < 0.01).
At ketamine initiation, the CEEG demonstrated SE in 47/69 (68%) of patients (subdivided as brief seizures totaling >5 minutes in an hour in 18/47 [38%], individual seizures >5 minutes in 18/47 [38%], or both in 11/47 [23%]), acute repetitive seizures in 19/69 patients (28%), and IIC patterns (without seizures) in 3/69 patients (4%). IIC patterns were present in 14/69 patients (20%), including 3/14 (21%) without ongoing seizures and 11/14 (79%) with seizures at ketamine administration.
Sixty-five patients (94%) received a ketamine bolus at the time of ketamine infusion initiation, and a median of 4 boluses (IQR 2–7) were administered in the first 24 hours of infusion. Ketamine infusions were initiated at 1 mg/kg/h in 66/69 patients (96%), per institutional practice. In 2 patients (3%), the infusion was inadvertently initiated at 0.1 mg/kg/h (such as midazolam infusion dosing at our institution) and was rapidly corrected to 1 mg/kg/h. One subject (1%) had infusion dosing started at 0.5 mg/kg/h for undefined reasons. The maximum infusion doses were 7 mg/kg/h in 2/69 patients (3%), 6 mg/kg/h in 3/69 (4%), 5 mg/kg/h in 5/69 (7%), 4 mg/kg/h in 9/69 (13%), 3 mg/kg/h in 14/69 (20%), 2 mg/kg/h in 15/69 (22%), and 1 mg/kg/h in 20/69 (29%). The median duration from ketamine infusion initiation to maximum infusion dosing was 9.8 hours (IQR 3.2–29.9), and the median duration from ketamine infusion initiation to maximum infusion did not differ when ketamine was administered as the first anesthetic (8.6 hours, IQR 0–27.6) or after midazolam at any point in RSE treatment (13.5 hours, IQR 8.0–36.5) (p = 0.07). The median total duration of ketamine infusion was 85.7 hours (IQR 49.7–128.0), and the median total duration of ketamine administration was not different when ketamine was administered as the first anesthetic (74.6 hours, IQR 54.6–123.7) or after midazolam at any point in RSE treatment (87.0 hours, IQR 43.4–153.2) (p = 0.50).
Ketamine infusion was weaned in 61/69 patients (91%). Weaning data were unavailable for 8 patients in whom the ketamine infusion was stopped because of adequate seizure control at the starting infusion dose or death before weaning. Ketamine infusion weaning began at a median of 59.6 hours (IQR 43.8–76.6) from initiation of infusion, and ketamine infusion was weaned over a median of 22.9 hours (IQR 5.7–55.5).
Adverse Effects
Three patients had adverse effects that required intervention which resolved, and all 3 patients with adverse effects had improvement in seizures after ketamine administration. Delirium requiring quetiapine occurred in 1/69 subject (1%). Hypertension requiring intervention occurred in 2/69 patients (3%). The first subject had congenital heart disease (CHD), was on venoarterial extracorporeal membrane oxygenation (ECMO), and required a nicardipine infusion and decrease in ECMO flows to mitigate hypertension. The second subject had multiple preexisting medical comorbidities including chronic renal dysfunction resulting in hypertension and required 1 additional dose of antihypertensive medication during ketamine treatment. In both patients, the hypertension was transient and resolved when the ketamine infusion was weaned. A third subject had hypertension that worsened during ketamine administration but did not require any intervention.
Additional adverse effects occurred during RSE management which were not attributed to ketamine. Two patients (3%) were receiving treatment for preexisting arrhythmias that did not change after initiation of ketamine infusion. Sixty-six patients (96%) were intubated before ketamine initiation. Hypotension requiring treatment occurred in 24/69 patients (35%) during RSE management. Hypotension occurred before ketamine in 22/69 patients (32%), and it remained stable during ketamine infusion in 11/22 patients (50%) or improved during ketamine infusion in 11/22 (50%). Hypotension occurred after ketamine in 1/69 subject (1%). Hypotension occurred during ketamine treatment in 3/69 patients (4%), but none required intervention. There were no concerns regarding elevated ICP in any subject. No patients experienced withdrawal symptoms/signs during ketamine wean.
Efficacy
Ketamine administration was followed by CEEG determined seizure termination in 32/69 patients (46%), seizure reduction in 19/69 (28%), and no change in 18/69 (26%). Among the 51/69 patients (74%) with seizure termination or reduction after ketamine administration, the duration to the best achieved ketamine response occurred at 0–6 hours in 37/51 patients (73%), 6–12 hours in 10/51 (20%), 12–24 hours in 5/51 (10%), 24–36 hours in 6/51 (12%), 36–48 hours in 1/51 (2%), and >48 hours in 1/51 (2%) (Table 3). Ketamine was significantly more likely to be followed by seizure termination when administered as a first anesthetic (23/38, 61%) than after midazolam had been ineffective as a first-line anesthetic (9/31, 29%) (p < 0.01). When ketamine was administered as a first anesthetic, seizures terminated in 23/38 patients (61%), reduced in 7/38 (16%), or did not change in 9/39 (24%). When ketamine was administered after midazolam, seizures terminated in 9/31 patients (29%), reduced in 13/31 (42%), and did not change in 9/31 (29%).
Table 3.
Ketamine Data
There was no difference in seizure termination after ketamine administration between children (26/56, 46%) and neonates (6/13, 46%) (p = 0.99). Among neonates, ketamine was followed by seizure termination in 6/13 (46%), seizure reduction in 1/13 (8%), and no change in 6/13 (46%). Among children, ketamine was followed by seizure termination in 26/56 (46%), seizure reduction in 18/56 (32%), and no change in 12/56 (21%).
The best achieved seizure response occurred at a lower infusion dose when ketamine was administered as the first anesthetic (2 mg/kg/h, IQR 1–3) than when ketamine was administered after midazolam (3 mg/kg/h, IQR 2–5) (p < 0.01). Similarly, the maximum dose of ketamine infusion was lower when seizures terminated (2 mg/kg/h, IQR 1–3) than when seizures persisted (3 mg/kg/h, IQR 2–4) (p = 0.03).
Ketamine administration was followed by IIC improvement among 8/14 patients (57%) with IIC patterns. CEEG background changes after initiation of ketamine infusion included induction of burst suppression in 1/69 patients (1%), suppression in 2/69 (3%), and discontinuity in 0/69 (0%).
Outcome
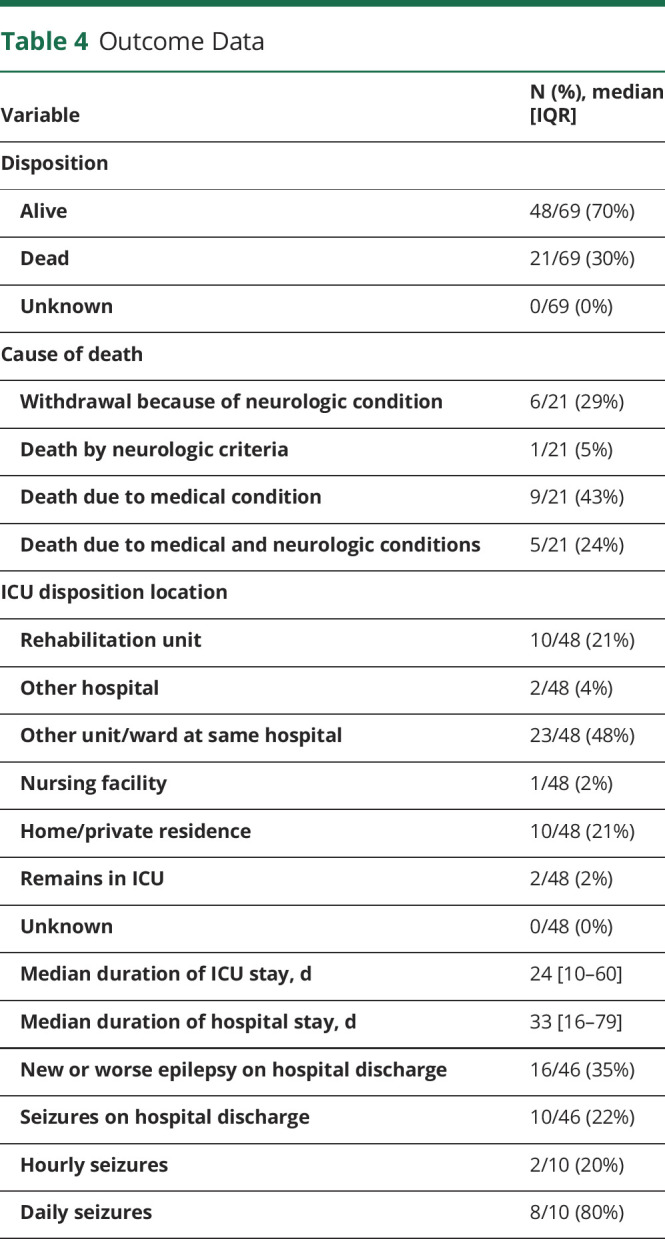
Table 4 summarizes outcome data. Twenty-one patients (30%) died before discharge, including 9/21 (43%) because of the systemic medical condition, 6/21 (29%) with withdrawal of technological support because of the neurologic condition, 1/21 (5%) with irreversible cessation of neurologic function, and 5/21 (24%) because of both systemic medical and neurologic conditions. The median duration of ICU and hospital stays were 24 days (IQR 10–60) and 33 days (IQR 16–79), respectively. ICU discharge disposition among the 48 survivors included transfer to a non-ICU ward in 23/48 (48%), transfer to rehabilitation in 10/48 (21%), discharge home in 10/48 (21%), transfer to a different hospital in 2/48 (4%), discharge to an extended living facility in 1/48 (2%), and 2/48 (4%) remained in an ICU. On discharge, 16/46 patients (35%) had new or worse epilepsy. Among survivors, 10/46 (22%) were still having seizures on discharge, including 2/10 (20%) with hourly seizures and 8/10 (80%) with daily seizures.
Table 4.
Outcome Data
Classification of Evidence
This study provides Class IV evidence on the therapeutic utility of ketamine for treatment of RSE in children and neonates.
Discussion
We evaluated 69 consecutive neonates and children who received ketamine for SE management. Almost all patients (99%) had seizures refractory to multiple medications before ketamine administration, and approximately two-thirds experienced more severe forms of RSE (super RSE and prolonged super RSE). Ketamine was associated with adverse effects requiring intervention in 4% of patients, including hypertension requiring intervention in 2 patients and delirium requiring an antipsychotic medication in 1 subject. Ketamine administration was followed by seizure improvement in 74% of patients, including seizure termination in 46% and seizure reduction in 28% of patients. These findings were similar among children and neonates (seizure termination in 46% of each subgroup). Ketamine was more often followed by seizure termination when administered as a first-line anesthetic (61%) than as a subsequent anesthetic, although even 29% of patients with seizures refractory to midazolam had seizure termination after ketamine administration. Furthermore, the best seizure response after ketamine administration occurred rapidly (0–6 hours) in 73% of patients. These data suggest that ketamine may be beneficial for SE management in children and neonates.
Ketamine may have neuroprotective benefits when administered before or after SE onset,8,34 and ketamine use for RSE is increasing.2 However, systematic reviews indicated published literature focused mostly on adults and was limited to only small case series and case reports in children and neonates.13,15,35-38 There is an exception study2 which reported 48 children for RSE treated with ketamine. However, all the patients also received pentobarbital in conjunction with ketamine, and ketamine was generally administered late in the course of RSE, thereby limiting the generalizability of the findings especially during initial treatment. A multicenter retrospective review of 60 RSE episodes (46 adults, 12 children) from North America and Europe between 1999 and 2012 was performed. Ketamine was often administered as a loading bolus dose followed by a continuous infusion, and it was always part of a multidrug regimen involving 2–12 concurrent medications. RSE control occurred in 57% of RSE episodes, and ketamine contributed to the control of RSE in 32% of episodes. Ketamine was deemed ineffective in contributing to the control of RSE using continuous infusion rates lower than 0.9 mg/kg/h, when initiated ≥8 days after SE onset or if seizures persisted despite ≥7 drugs.12 Although adverse events were mostly attributed to concurrent anesthetics and other medications, ketamine was discontinued in 5 patients for possible adverse events. The authors concluded that ketamine was a safe and effective drug for the treatment of RSE and most effective when introduced early during the course.12 However, the study included only 12 children and did not include any neonates (the youngest subject was 7-months-old). Data regarding the use of ketamine for seizures in neonates have been limited to case reports.16-18
Given limited pediatric data, this study of 69 consecutive children and neonates treated with ketamine, including many with ketamine as the initial anesthetic, adds substantially to the available knowledge. Only 3 patients had adverse effects requiring intervention referable to ketamine, including 2 patients with hypertension requiring treatment and 1 subject with delirium requiring treatment. The adverse effects resolved after intervention, and all 3 patients with adverse effects had improvement in seizures after ketamine administration. Hypotension occurred during ketamine treatment in 13% of patients, but none required intervention. No patients had concerns for increased ICP during ketamine treatment. No patients experienced withdrawal during weaning or within 12 hours of last ketamine administration. These data indicate ketamine was safe in neonates and children with SE, and rare adverse effects were manageable. The lack of short-term adverse effects is consistent with other limited pediatric data. A case series of 18 children with RSE managed with ketamine reported increases in saliva secretion but no other adverse effects.13 A case series of 9 children with RSE managed with ketamine reported no adverse effects.15 These data are consistent with a study of 68 adults with super RSE managed with ketamine which noted a reduction in vasopressor requirements over time. Furthermore, among 11 patients who underwent multimodality monitoring, there was no impact on ICP, cerebral blood flow, or cerebral perfusion pressure.39 Similarly, a study of 60 children who received ketamine in the PICU (only 2 with SE) identified only rare adverse effects, including hypersalivation, hypertension, and delirium in 1 patient each.40 Although animal models of SE indicate ketamine may decrease neuroinflammation and serve as a neuroprotectant against glutamate-induced neuronal necrosis,3,8 some models indicate that prolonged ketamine exposure may cause neurodegeneration in the developing brain.41 We assessed for acute and clinically identifiable adverse effects and could not assess for neurodegeneration. Future study in humans is needed, although it will be challenging to differentiate between changes related to the SE etiology, SE itself, and the impact of medications such as ketamine.
We assessed for seizure changes after ketamine administration but had no comparison group to determine whether any seizure reductions were occurring along a usual trajectory or were attributable to ketamine. Ketamine administration was followed by seizure improvement in 74% of patients, including termination in 46% and reduction in 28%, and the favorable response often occurred rapidly (0–6 hours in 73% of patients). The median time from ketamine infusion initiation to maximum dosing was 9.8 hours. Weaning data were available for 91% of patients. Weaning started at a median of 59.6 hours from start of infusion, and ketamine was weaned over a median of 22.9 hours. These findings and the relatively wide interquartile ranges may reflect the lack of an institution standard for weaning, and we generally aimed for >24 hours of seizure control before weaning of anesthetic infusions. Seizure improvement after ketamine administration has been reported by other smaller studies. A case series described seizure control in 61% of 18 children with RSE when ketamine was administered for a median of 4 days at a median dose of 2.2 mg/kg/h, including all 7 children who received a ketamine bolus at a median dose of 1.5 mg/kg.13 A case series described RSE resolution in 66% of 9 children with RSE managed with ketamine which was administered after other anesthetic medications.15 A case series of ketamine use for 19 episodes of RSE in 13 children found that ketamine administration at 22 hours to 17 days with doses ranging from 7 to 60 µ/kg/min was followed by RSE resolution in 74% of 19 RSE episodes. Furthermore, use of ketamine before other anesthetics avoided intubation for 5 patients.38 A study of 68 adults with super RSE managed with ketamine reported seizure burden decreased by 50% within 24 hours in 81% of patients, including seizure cessation in 63% at an average dose of 2.2 mg/kg/h for a median duration of 2 days. Midazolam was used concurrently with ketamine (initiated a median of 0.4 days before ketamine) at an average dose of 1 mg/kg/h.39 Similarly, ketamine administration was followed by RSE termination in 100% of 12 dogs.42
Ketamine rarely affected the EEG background, indicating the goal of ketamine administration is seizure improvement and not induction of a change in EEG background. However, this is discordant with a recent pediatric case series of 13 patients with 19 RSE episodes in which a ketamine bolus (3 mg/kg) was followed by a suppression-burst pattern in 10/14 RSE episodes that responded to ketamine.38 Further study of the impact of ketamine administration on the EEG is needed. Periodic and rhythmic patterns, often constituting IIC patterns, occur in approximately 10% of critically ill children, but few data are available regarding management.43 In this cohort, IIC patterns improved in 57% of 14 patients after ketamine administration, suggesting ketamine may be an effective strategy for some patients.
Ketamine may be particularly relevant in specific subgroups. In the cohort, 35% of patients had congenital malformations, including 88% with CHD. Furthermore, 19% of patients were neonates. The CHD population is at high risk for acute neurologic brain injury because of complex cardiac physiology with intracardiac shunts and tenuous medical status.44-48 Neonates with CHD requiring cardiopulmonary bypass are known to be at high risk of seizures.49,50 Only limited data are available to guide seizure management in these medically complex patients. Although neonatal seizures are often managed with phenobarbital, alternative strategies may be beneficial for neonates with CHD. These data support continued investigation of ketamine in neonates with CHD experiencing SE.
This study has several limitations. First, this was a single-center study. Generalizability will be enhanced by data from other institutions or multicenter studies. Second, data were obtained retrospectively. Although data collection was performed by 2 experienced neurocritical care nurse practitioners, some clinical data may not have been available in the chart, yielding misclassification bias. There may be side effects of ketamine that were not identified because of the retrospective review and definitions. Similarly, although CEEG reporting used standardized terminology and report templates,20 there may have been some differences between electroencephalographers. Seizure, IIC, and EEG background changes in response to ketamine were categorized based on documentation form treating clinicians and electroencephalographers and not based on reassessment of the CEEG tracing. Third, although this is one of the largest cohorts of pediatric patients treated with ketamine for SE, it still only evaluated 69 patients, yielding small subsets when subdivided by age, preexisting neurologic and medical problems, and SE type. Multicenter studies, potentially targeting specific subgroups, may be necessary to obtain larger cohorts and provide more certainty, especially when comparing ketamine with other available treatments. Fourth, this was an observational study. Thus, we cannot determine whether seizure changes after ketamine administration were following an expected trajectory or attributable to ketamine. Trials comparing ketamine with placebo or other anesthetics are required to better understand the efficacy of ketamine. Fifth, our institution classifies ketamine as anesthesia which necessitated transfer of patients from NICU to PICU for ketamine dose escalation, and this constraint may have yielded the small number of patients (5) located in the NICU despite more patients (13) being neonates. This institutional policy may limit the use of ketamine for SE in the NICU. If only patients with more RSE were treated with ketamine given this policy, then our data could underestimate the potential benefit of ketamine among neonates. Finally, we did not measure serum ketamine levels, precluding pharmacokinetic assessments or comparisons of ketamine levels in patients with or without improvement of SE after ketamine. Despite these limitations, our data indicate that adverse effects are rare, and seizures often terminate or improve after ketamine administration in neonates and children with SE, thereby motivating future larger studies regarding the comparative effectiveness of available anesthetic medications, including on short-term seizure control and long-term neurobehavioral outcomes.
In this large cohort of consecutive and contemporary children and neonates with SE, adverse effects were rare, and seizures often ceased after ketamine administration. Adverse effects requiring intervention were transient hypertension in 2 patients and delirium in 1 subject. These adverse effects resolved, and each of these patients had seizure improvement after ketamine administration. Ketamine infusion was followed by seizure termination in 46% of patients and seizure reduction in 28% of patients. These data suggest ketamine may be a beneficial anesthetic infusion for neonates and children with SE. Further study is needed to compare ketamine with other anesthetic medications and evaluate long-term outcomes.
Glossary
- CEEG
continuous electroencephalographic monitoring
- CHD
congenital heart disease
- CICU
SE in the cardiac
- ECMO
extracorporeal membrane oxygenation
- FIRES
febrile infection–related epilepsy syndrome
- ICP
intracranial pressure
- ICU
intensive care unit
- IQR
interquartile range
- IIC
ictal-interictal continuum
- NICU
SE in the neonatal
- PICU
SE in the pediatric
- RSE
refractory SE
- SE
status epilepticus
Appendix. Authors
Footnotes
Class of Evidence: NPub.org/coe
CME Course: NPub.org/cmelist
Study Funding
This work was supported by the National Institute for Neurological Disorders and Stroke (K02 NS112600), including support through the Center Without Walls on ion channel function in epilepsy (Channelopathy-associated Research Center, U54 NS108874), the Eunice Kennedy Shriver National Institute of Child Health and Human Development through the Intellectual and Developmental Disabilities Research Center (IDDRC) at Children's Hospital of Philadelphia and the University of Pennsylvania (U54 HD086984), and by intramural funds of the Children's Hospital of Philadelphia through the Epilepsy NeuroGenetics Initiative (ENGIN). Research reported in this publication was also supported by the National Center for Advancing Translational Sciences of the NIH under Award Number UL1TR001878. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. This project was also supported in part by the Institute for Translational Medicine and Therapeutics (ITMAT) Transdisciplinary Program in Translational Medicine and Therapeutics at the Perelman School of Medicine of the University of Pennsylvania.
Disclosure
N. Abend was supported by the Wolfson Family Foundation. I. Helbig was supported by The Hartwell Foundation through an Individual Biomedical Research Award and by the NORSE Foundation. The other authors report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Hirsch LJ, Gaspard N, van Baalen A, et al. Proposed consensus definitions for new-onset refractory status epilepticus (NORSE), febrile infection-related epilepsy syndrome (FIRES), and related conditions. Epilepsia. 2018;59(4):739-744. [DOI] [PubMed] [Google Scholar]
- 2.Keros S, Buraniqi E, Alex B, et al. Increasing ketamine use for refractory status epilepticus in US pediatric hospitals. J Child Neurol. 2017;32(7):638-646. [DOI] [PubMed] [Google Scholar]
- 3.Vossler DG, Bainbridge JL, Boggs JG, et al. Treatment of refractory convulsive status epilepticus: a comprehensive review by the American Epilepsy Society Treatments Committee. Epilepsy Curr. 2020;20(5):245-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prisco L, Ganau M, Aurangzeb S, et al. A pragmatic approach to intravenous anaesthetics and electroencephalographic endpoints for the treatment of refractory and super-refractory status epilepticus in critical care. Seizure. 2020;75:153-164. [DOI] [PubMed] [Google Scholar]
- 5.Wilkes R, Tasker RC. Intensive care treatment of uncontrolled status epilepticus in children: systematic literature search of midazolam and anesthetic therapies. Pediatr Crit Care Med. 2014;15(7):632-639. [DOI] [PubMed] [Google Scholar]
- 6.Abend NS, Dlugos DJ. Treatment of refractory status epilepticus: literature review and a proposed protocol. Pediatr Neurol. 2008;38(6):377-390. [DOI] [PubMed] [Google Scholar]
- 7.Tasker RC, Goodkin HP, Sanchez Fernandez I, et al. , Pediatric Status Epilepticus Research Group. Refractory status epilepticus in children: intention to treat with continuous infusions of midazolam and pentobarbital. Pediatr Crit Care Med. 2016;17(10):968-975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujikawa DG. Neuroprotective effect of ketamine administered after status epilepticus onset. Epilepsia. 1995;36(2):186-195. [DOI] [PubMed] [Google Scholar]
- 9.Akeju O, Song AH, Hamilos AE, et al. Electroencephalogram signatures of ketamine anesthesia-induced unconsciousness. Clin Neurophysiol. 2016;127(6):2414-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hijazi Y, Bodonian C, Bolon M, Salord F, Boulieu R. Pharmacokinetics and haemodynamics of ketamine in intensive care patients with brain or spinal cord injury. Br J Anaesth. 2003;90(2):155-160. [DOI] [PubMed] [Google Scholar]
- 11.Glauser T, Shinnar S, Gloss D, et al. Evidence-based guideline: treatment of convulsive status epilepticus in children and adults: report of the guideline committee of the American Epilepsy Society. Epilepsy Curr. 2016;16(1):48-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaspard N, Foreman B, Judd LM, et al. Intravenous ketamine for the treatment of refractory status epilepticus: a retrospective multicenter study. Epilepsia. 2013;54(8):1498-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu J, Wang Q, Qian SY, et al. Effectiveness of ketamine in the treatment of refractory and super-refractory status epilepticus in children [in Chinese]. Zhonghua Er Ke Za Zhi. 2020;58(4):295-300. [DOI] [PubMed] [Google Scholar]
- 14.Samanta D. Ketamine infusion for super refractory status epilepticus in alternating hemiplegia of childhood. Neuropediatrics. 2020;51(3):225-228. [DOI] [PubMed] [Google Scholar]
- 15.Rosati A, L'Erario M, Ilvento L, et al. Efficacy and safety of ketamine in refractory status epilepticus in children. Neurology. 2012;79(24):2355-2358. [DOI] [PubMed] [Google Scholar]
- 16.Huntsman RJ, Strueby L, Bingham W. Are ketamine infusions a viable therapeutic option for refractory neonatal seizures? Pediatr Neurol. 2020;103:8-11. [DOI] [PubMed] [Google Scholar]
- 17.Tarocco A, Ballardini E, Garani G. Use of ketamine in a newborn with refractory status epilepticus: a case report. Pediatr Neurol. 2014;51(1):154-156. [DOI] [PubMed] [Google Scholar]
- 18.Freibauer A, Jones K. KCNQ2 mutation in an infant with encephalopathy of infancy with migrating focal seizures. Epileptic Disord. 2018;20(6):541-544. [DOI] [PubMed] [Google Scholar]
- 19.Sanchez Fernandez I, Abend NS, Agadi S, et al. , Pediatric Status Epilepticus Research Group pSERG. Gaps and opportunities in refractory status epilepticus research in children: a multi-center approach by the Pediatric Status Epilepticus Research Group (pSERG). Seizure. 2014;23(2):87-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Witzman S, Massey SL, Kessler S, et al. Acceptability of standardized EEG reporting in an electronic Health record. J Clin Neurophysiol. 2020;37(5):455-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirsch LJ, LaRoche SM, Gaspard N, et al. American clinical neurophysiology society's standardized critical care EEG terminology: 2012 version. J Clin Neurophysiol. 2013;30:1-27. [DOI] [PubMed] [Google Scholar]
- 22.Hirsch LJ, Fong MWK, Leitinger M, et al. American clinical neurophysiology society's standardized critical care EEG terminology: 2021 version. J Clin Neurophysiol. 2021;38:1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fung FW, Wang Z, Parikh DS, et al. Electrographic seizures and outcome in critically ill children. Neurology. 2021;96(22):e2749-e2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brophy GM, Bell R, Claassen J, et al. , Neurocritical Care Society Status Epilepticus Guideline Writing Committee. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care. 2012;17(1):3-23. [DOI] [PubMed] [Google Scholar]
- 25.Herman ST, Abend NS, Bleck TP, et al. , Critical Care Continuous EEG Task Force of the American Clinical Neurophysiology Society. Consensus statement on continuous EEG in critically ill adults and children, part I: indications. J Clin Neurophysiol. 2015;32(2):87-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trinka E, Cock H, Hesdorffer D, et al. A definition and classification of status epilepticus: report of the ILAE task force on classification of status epilepticus. Epilepsia. 2015;56(10):1515-1523. [DOI] [PubMed] [Google Scholar]
- 28.Banker A, Bell C, Gupta-Malhotra M, Samuels J. Blood pressure percentile charts to identify high or low blood pressure in children. BMC Pediatr. 2016;16:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Topjian AA, French B, Sutton RM, et al. Early postresuscitation hypotension is associated with increased mortality following pediatric cardiac arrest. Crit Care Med. 2014;42(6):1518-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flynn JT, Kaelber DC, Baker-Smith CM, et al. , Subcommittee on Screening and Management of High Blood Pressure in Children. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. 2017;140(3):e20171904. [DOI] [PubMed] [Google Scholar]
- 31.Falkner B, Daniels SR. Summary of the fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Hypertension. 2004;44(4):387-388. [DOI] [PubMed] [Google Scholar]
- 32.Traube C, Silver G, Kearney J, et al. Cornell Assessment of Pediatric Delirium: a valid, rapid, observational tool for screening delirium in the PICU. Crit Care Med. 2014;42(3):656-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Franck LS, Harris SK, Soetenga DJ, Amling JK, Curley MAQ. The Withdrawal Assessment Tool-1 (WAT-1): an assessment instrument for monitoring opioid and benzodiazepine withdrawal symptoms in pediatric patients. Pediatr Crit Care Med. 2008;9(6):573-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fujikawa DG. Starting ketamine for neuroprotection earlier than its current use as an anesthetic/antiepileptic drug late in refractory status epilepticus. Epilepsia. 2019;60(3):373-380. [DOI] [PubMed] [Google Scholar]
- 35.Golub D, Yanai A, Darzi K, Papadopoulos J, Kaufman B. Potential consequences of high-dose infusion of ketamine for refractory status epilepticus: case reports and systematic literature review. Anaesth Intensive Care. 2018;46(5):516-528. [DOI] [PubMed] [Google Scholar]
- 36.Rosati A, De Masi S, Guerrini R. Ketamine for refractory status epilepticus: a systematic review. CNS Drugs. 2018;32(11):997-1009. [DOI] [PubMed] [Google Scholar]
- 37.Hofler J, Trinka E. Intravenous ketamine in status epilepticus. Epilepsia. 2018;59(suppl 2):198-206. [DOI] [PubMed] [Google Scholar]
- 38.Ilvento L, Rosati A, Marini C, L'Erario M, Mirabile L, Guerrini R. Ketamine in refractory convulsive status epilepticus in children avoids endotracheal intubation. Epilepsy Behav. 2015;49:343-346. [DOI] [PubMed] [Google Scholar]
- 39.Alkhachroum A, Der-Nigoghossian CA, Mathews E, et al. Ketamine to treat super-refractory status epilepticus. Neurology. 2020;95(16):e2286-e2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sperotto F, Giaretta I, Mondardini MC, Pece F, Daverio M, Amigoni A. Ketamine prolonged infusions in the pediatric intensive care unit: a tertiary-care single-center analysis. J Pediatr Pharmacol Ther. 2021;26(1):73-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zou X, Patterson TA, Divine RL, et al. Prolonged exposure to ketamine increases neurodegeneration in the developing monkey brain. Int J Dev Neurosci. 2009;27(7):727-731. [DOI] [PubMed] [Google Scholar]
- 42.Roynard P, Bilderback A, Dewey CW. Intravenous ketamine bolus(es) for the treatment of status epilepticus, refractory status epilepticus, and cluster seizures: a retrospective study of 15 dogs. Front Vet Sci. 2021;8:547279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fung FW, Parikh DS, Massey SL, et al. Periodic and rhythmic patterns in critically ill children: incidence, interrater agreement, and seizures. Epilepsia. 2021;62(12):2955-2967. [DOI] [PubMed] [Google Scholar]
- 44.Marino BS, Lipkin PH, Newburger JW, et al. , American Heart Association Congenital Heart Defects Committee, Council on Cardiovascular Disease in the Young, Council on Cardiovascular Nursing, and Stroke Council. Neurodevelopmental outcomes in children with congenital heart disease: evaluation and management: a scientific statement from the American Heart Association. Circulation. 2012;126(9):1143-1172. [DOI] [PubMed] [Google Scholar]
- 45.Wernovsky G, Licht DJ. Neurodevelopmental outcomes in children with congenital heart disease-what can we impact?. Pediatr Crit Care Med. 2016;17(8 suppl 1):S232-S242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goff DA, Shera DM, Tang S, et al. Risk factors for preoperative periventricular leukomalacia in term neonates with hypoplastic left heart syndrome are patient related. J Thorac Cardiovasc Surg. 2014;147(4):1312-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lynch JM, Buckley EM, Schwab PJ, et al. Time to surgery and preoperative cerebral hemodynamics predict postoperative white matter injury in neonates with hypoplastic left heart syndrome. J Thorac Cardiovasc Surg. 2014;148(5):2181-2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Licht DJ, Shera DM, Clancy RR, et al. Brain maturation is delayed in infants with complex congenital heart defects. J Thorac Cardiovasc Surg. 2009;137(3):529-536; discussion 536-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Naim MY, Gaynor JW, Chen J, et al. Subclinical seizures identified by postoperative electroencephalographic monitoring are common after neonatal cardiac surgery. J Thorac Cardiovasc Surg. 2015;150(1):169-178; discussion 178-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Naim MY, Putt M, Abend NS, et al. Development and validation of a seizure prediction model in neonates after cardiac surgery. Ann Thorac Surg. 2021;111(6):2041-2048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
We will make the underlying data available to investigators with appropriate data transfer and Institutional Review Board approval.