Abstract
There are still many patients suffering from ischemic stroke and related disabilities worldwide. To develop a treatment that promotes functional recovery after acute ischemic stroke, we need to elucidate endogenous tissue repair mechanisms. The concept of a neurovascular unit (NVU) indicates the importance of a complex orchestration of cell–cell interactions and their microenvironment in the physiology and pathophysiology of various central nervous system diseases, particularly ischemic stroke. In this concept, microvascular pericytes play a crucial role in regulating the blood–brain barrier integrity, cerebral blood flow (CBF), and vascular stability. Recent evidence suggests that pericytes are also involved in the tissue repair leading to functional recovery following acute ischemic stroke through the interaction with other cell types constituting the NVU; pericytes may organize CBF recovery, macrophage-mediated clearance of myelin debris, intrainfarct fibrosis, and periinfarct astrogliosis and remyelination. In this review, we will discuss the physiological and pathophysiological functions of pericytes, their involvement in the molecular mechanisms underlying tissue repair and functional recovery after ischemic stroke, and a therapeutic strategy to promote endogenous regeneration.
Keywords: Acute ischemic stroke, Pericytes, Blood–brain barrier, Extracellular matrix, Functional recovery
1. Introduction
Ischemic stroke is a leading cause of mortality and disability worldwide. It is characterized by the obstruction of blood flow to the brain, which leads to brain damage and loss of neurological function. Recanalization therapy, such as intravenous thrombolysis and endovascular thrombectomy, in the acute phase of ischemic stroke has made remarkable progress. However, the number of patients who can benefit from it is limited, and there have been no established treatments except rehabilitation that bring functional recovery. Therefore, our urgent task is to elucidate the endogenous mechanisms promoting functional recovery and develop novel treatments to enhance recovery during the subacute phase.
Over the past decades, many neuroprotective agents, such as NMDA antagonists 1) , AMPA receptor antagonists 2) , and antiICAM1 antibodies 3) , have been tried in clinical trials but have failed to show their efficacy. One of the causes of the failure may be that only neuroprotective effects targeting the inhibition of excitotoxicity and apoptosis have been investigated. Then, the concept of a neurovascular unit (NVU) gradually permeated, and the progress of research focused on this has increasingly suggested the importance of a complex orchestration of cell–cell interactions and their microenvironment to repair the damaged tissue and promote functional recovery 4 , 5) . One of the cell types that has gained attention in the NVU is pericytes. Pericytes are perivascular mural cells that wrap around the endothelial cells of small vessels. They play a crucial role in regulating the blood–brain barrier (BBB) integrity, cerebral blood flow (CBF), and vascular stability. In addition, pericytes have emerged as key players not only in the pathophysiology of various central nervous system (CNS) diseases 6) but also in the repair process after ischemic stroke through the interaction among cell types in the NVU. In this review, we will discuss the physiological and pathophysiological functions of pericytes, their involvement in the molecular mechanisms underlying tissue repair and functional recovery after ischemic stroke, and a potential therapeutic strategy to promote endogenous regeneration.
2. Physiological and Pathophysiological Functions of Pericytes
Pericytes are located at the abluminal side of endothelial cells of precapillary arterioles, capillaries, and postcapillary venules and are embedded in the basement membrane (BM) ( Fig.1 ) . One of the important roles of brain pericytes is to maintain BBB integrity through direct interaction with endothelial cells 7) and the regulation of CBF. The BBB is formed by tight junctions between adjacent endothelial cells, functioning as a highly selective barrier that regulates the exchange of substances between the blood and the brain parenchyma to maintain CNS homeostasis. Although pericytes exist throughout the body at different covering ratios against endothelial tubes, they are most abundantly present in the brain and retina, where a high level of blood–tissue barrier is formed 8) . An important signaling pathway for pericyte recruitment around endothelial tubes is PDGF-BB–PDGFRβ. Pericyte recruitment is essential for the maturation of newly formed vessels harboring the BBB during development; the lack of pericyte recruitment results in embryonic lethality due to increased cerebral vascular permeability and rupture of microaneurysms leading to cerebral hemorrhage in Pdgfb- or Pdgfrb-deficient mice 9 , 10) .
Fig. 1. Schematic diagram showing the NVU.
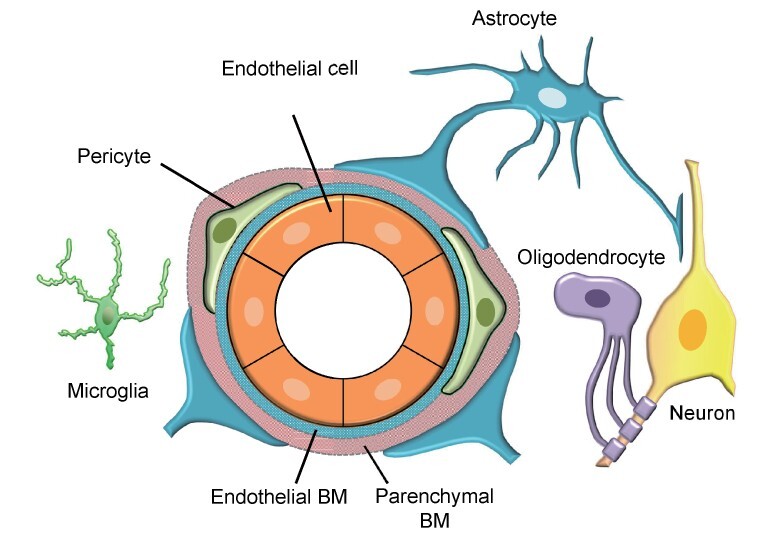
The NVU is a concept in which neurons, cerebrovascular endothelial cells, pericytes, astrocytes, oligodendrocytes, microglia, and ECM are considered one structural unit. BM surrounds blood vessels with two layers of endothelial BM and parenchymal BM and regulates barrier function and interactions between cells.
Pericytes regulate CBF by contracting and relaxing in response to neuronal activity (neurovascular coupling) through vasoactive substances, such as nitric oxide and prostaglandins 11 - 13) , whereas there is also a report that smooth muscle cells, not the capillary pericytes, primarily regulate CBF 14) . Compared with endothelial cells, pericytes are more easily injured or degenerated under stressed conditions, such as aging, diabetes mellitus, and ischemia. Pericyte loss, microaneurysms, and acellular capillaries, which are induced in Pdgfb-deficient mice, are characteristics of diabetic retinopathy 15 , 16) . Age-dependent chronic pericyte loss results in capillary rarefaction, leading to CBF reductions and BBB breakdown. It also disturbs the clearance of metabolic wastes that can cause vascular permeability increase and neurotoxicity in the brain, such as soluble Aβ, thereby leading to the development of Alzheimer disease-like pathology 17 , 18) . The “no-reflow” phenomenon, that is, impaired blood flow restoration even after successful recanalization of the proximal occluded artery, may be caused by inappropriate contraction of distal pericytes in the brain following ischemia–reperfusion 19) . Thus, pericytes are in the limelight in various pathological conditions, such as lifestyle-related diseases, cerebrovascular diseases, cerebral white matter lesions, and cognitive dysfunction.
3. Origin of Pericytes and Endogenous Stem Cells
Mesenchymal cells and neural crest cells are recognized as the main sources of pericytes; however, there have been some recent reports showing that macrophages or tissue-localized myeloid progenitors can be the origin of pericytes 20) . Kokovay et al. described that pericytes are recruited from peripheral bone marrow-derived cells and are involved in blood vessel stabilization during angiogenesis following ischemia 21) . It has also been elucidated that mature macrophages undergo transdifferentiation after attaching to microvessels during neurogenesis and contribute to vascular maturation as pericytes 22) . Therefore, pericytes may be a heterogeneous population, considering that the molecular markers of pericytes vary and change over time depending on the tissue and pathology. Pericyte-derived cells may behave like mesenchymal stem cells that produce neurotrophic and immunomodulatory factors 23) . Following brain infarction, pericytes in the pia mater can transdifferentiate into multipotent stem cells (ischemic-induced neural stem/progenitor cells) that have the potential to differentiate into both neural and vascular cells 24 , 25) .
It is known that neural stem cells, located in the subventricular zone even in human adults, migrate toward infarct areas. They differentiate primarily into reactive astrocytes in periinfarct areas and may contribute to functional recovery while harboring the potential to differentiate into neurons by forced expression of Ascl1 26) . Circulating endothelial progenitor cells adhere to ischemic vessels and participate in new vessel formation by supplying endothelial cells 27) , and their potential therapeutic effects in ischemic stroke have been gradually identified 28) .
4. Pericyte-Mediated Poststroke Tissue Repair and Functional Recovery
(1) CBF Recovery
Restoration of blood flow within infarct areas is the most important process for poststroke functional recovery through clearance of debris and tissue repair ( Fig.2 ) . According to the RESCUE-Japan registry, functional outcome at 3 months after stroke onset was better in patients with reperfusion by endovascular treatment, even in cases of similar neurological severity at 24 hours after the onset 29) . Basic research using mouse middle cerebral artery occlusion (MCAO) stroke models supports this clinical data; even though brain infarction is similarly produced in the perfusion areas on day 1, infarct volume became significantly smaller with enhanced intrainfarct fibrotic responses and better functional recovery on day 7 in mice with efficient reperfusion than without reperfusion 30) . The extent of tissue repair and functional recovery was better in mice with earlier reperfusion. An important pathological difference between transient MCAO (tMCAO) and permanent MCAO (pMCAO) was the extent of pericyte survival within infarct areas, which maintains blood flow within infarct areas, thereby promoting subsequent tissue repair even after the development of brain infarction. Unless the occluded artery is recanalized, remodeling of the pial collateral circulation and recruitment of pericytes around surviving microvascular endothelial cells are required to promote efficient reperfusion and subsequent repair within infarct areas. Because these processes following pMCAO were significantly suppressed in pericyte-deficient Pdgfrb heterozygous knockout (Pdgfrb+−) mice, pericytes play important roles in poststroke intrainfarct reperfusion, leading to tissue repair and functional recovery even in cases without recanalization 31) .
Fig. 2. Interaction of NVU components in tissue repair and functional recovery after ischemic stroke (modified from Shibahara et al. 31) ) .
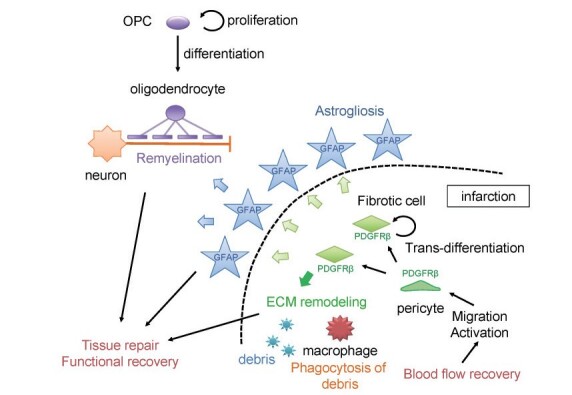
After cerebral ischemia, pericytes accumulate around the infarct lesions, differentiate into fibroblast-like cells, and produce ECM proteins such as fibronectin (fibrotic scar formation). Pericytes promote macrophage-mediated clearance of myelin debris within the infarct, periinfarct astrogliosis, and remyelination and thereby contribute to functional recovery by interacting with astrocytes and OPCs.
(2) Macrophage-Mediated Clearance of Debris Within Infarct Areas
A major purpose of blood flow restoration within infarct areas may be the recruitment of phagocytes, particularly macrophages, into infarct areas to remove cellular and myelin debris that encloses cytotoxic and proinflammatory molecules. It has been thought that infiltration of inflammatory cells is a detrimental factor to be targeted because it enhances vascular edema and cell death in periinfarct areas during the acute phase; however, recent evidence demonstrates that functional recovery may be worse without debris clearance or fibrotic repair during the subacute phase. Thus, proper macrophage-mediated debris clearance may be a prerequisite for promoting poststroke functional recovery (the clean-up hypothesis) 32 - 35) . Maintenance or immediate remodeling of the microcirculation by pericytes is important for macrophages to infiltrate into infarct areas through the blood. In addition to damage-associated molecular patterns (DAMPs), such as high mobility group box-1 and peroxiredoxin, which recruit macrophages and trigger inflammation 36) , PDGFRβ-positive pericytes around endothelial cells may recruit macrophages into infarct areas: 1) pericytes produced a chemokine CCL2 and a trophic factor CSF1 within infarct areas; 2) macrophages infiltrated around PDGFRβ-positive microvessels within infarct areas 37) ; and 3) macrophage infiltration and debris clearance were significantly suppressed in Pdgfrb knockout mice. Resident microglia are also activated immediately after brain ischemia. However, since microglia can neither survive within infarct areas nor infiltrate freely into infarct areas, blood-derived macrophages rather than microglia may be the predominant phagocytes within infarct areas. Macrophages removing intrainfarct debris can function as reparative ones (M2 type) 38) ; they can produce various trophic factors supporting intrainfarct fibrosis and periinfarct astrogliosis and oligodendrogenesis, leading to functional recovery, in contrast to proinflammatory ones (M1 type) 36 , 39) . Resident microglia may function as an eliminator of DAMPs and neuron-repairing cells primarily in periinfarct areas 38) .
Poststroke excessive inflammation and immune responses may have a downside that deteriorates periinfarct edema and expands infarct lesions. Macrophages produced IL-23, which induced IL-17 production from γδT cells around infarct areas and enhanced inflammation during the subacute phase 40) . Conversely, recent lines of evidence demonstrate that microglia- and macrophage-mediated immediate resolution of inflammation is a key to obtaining better functional recovery. Elimination of DAMPs via the upregulation of AIM or MSR1, efferocytosis, or accumulation of FOXP3-positive regulatory T cells may be involved in the mechanism 33 , 35 , 38 , 41 , 42) .
(3) Intrainfarct Fibrosis and Periinfarct Astrogliosis
Following macrophage-mediated phagocytosis of dead cells and debris, PDGFRβ-positive fibroblast-like cells gradually occupy and produce extracellular matrix (ECM) proteins within infarct areas with the aid of infiltrating macrophages. Göritz et al. demonstrated that type A pericytes leave the blood vessel and proliferate and differentiate into fibroblast-like cells after spinal cord or brain injury, completing wound healing 43 , 44) . Infiltrating macrophages phagocytosing debris effectively produce PDGF-B and bFGF, an upregulator of PDGFRβ, and prompt PDGFRβ-positive fibroblast-like cells to produce ECM proteins, such as fibronectin and collagen type I 45 - 47) . It is an important fact that fibrotic responses occur even in the brain, similar to other major organs such as the heart, lungs, and liver. Different from chronic fibrosis leading ultimately to organ failure, poststroke fibrosis may work beneficially for functional recovery following acute ischemic stroke because the suppression of pericyte-mediated fibrosis results in impaired functional recovery, as evidenced by a stroke model using Pdgfrb knockout mice 31) .
Periinfarct accumulation of GFAP-positive reactive astrocytes, that is, astrogliosis or glial scar, clearly demarcates infarct areas where pericyte-derived PDGFRβ-positive fibroblast-like cells and macrophages would occupy over subacute phases 46) . Because Pdgfrb-deficient mice had poor astrocyte accumulation 31 , 48) , PDGFRβ-positive cells may participate positively in astrocyte proliferation and migration in periinfarct areas. Indeed, PDGFRβ-positive cells can produce various trophic factors, such as NT-3, IL-6, and TGFβ, that can activate astrocytes 49) . Glial scar has traditionally been recognized to impede post-damage axonal regeneration in the CNS because astrocytes can produce chondroitin sulfate proteoglycans, potential inhibitors of axonal regeneration 50 , 51) . However, there are increasing lines of evidence that inhibition of astrogliosis attenuates axonal regrowth in CNS injury 52) . Thus, glial scar, positively regulated by intrainfarct fibrosis, does not necessarily inhibit functional recovery but may promote regeneration depending on the interaction with trophic factors and disease states.
(4) Periinfarct Oligodendrogenesis and Functional Recovery
Because oligodendrocytes are high-energy-demanding cells and are most vulnerable to energy deprivation among neural cells, demyelination easily occurs under stressed conditions, including ischemia. Oligodendrocyte precursor cells (OPCs) are widely distributed in the brain, mainly in the white matter, and function as a reservoir of oligodendrocytes to prepare for demyelination. Periinfarct OPC-mediated remyelination is a key factor promoting poststroke functional recovery 53) . OPCs can rapidly proliferate in periinfarct areas; however, clearance of myelin debris and an appropriate blood supply are absolutely required for their differentiation into mature oligodendrocytes accompanied by remyelination. Intrainfarct pericyte-derived cells and macrophages phagocytosing myelin debris can produce trophic factors, such as IGF-1 and BDNF, inducing periinfarct oligodendrogenesis 31) . Because pericyte-deficient mice showed white matter injury with fibrin(ogen) accumulation and blood flow reduction, pericytes alone may have the capacity to induce oligodendrogenesis 54) . It has been shown that AKAP12, a scaffolding protein organizing intracellular signal transduction expressed in pericytes, may play important roles in promoting OPC differentiation through the production of trophic factors, such as BDNF, LIF, GRO-α, and HGF 55 , 56) .
(5) Maintenance and Remodeling of ECM Proteins
ECMs are roughly divided into those existing in the BM or in the interstitial matrix 57) and regulate many cellular functions, such as cell survival, proliferation, differentiation, and migration, as the structural and functional core of the microenvironment around cells. ECM composition shows diversity across tissues under physiological conditions 58) . The vascular BM in the brain is composed of two layers, the endothelial BM and the parenchymal BM, separating the systemic circulation from the CNS to support the barrier function of the BBB 58 - 60) . Following brain ischemia, proteases such as matrix metalloproteinases (MMPs) and cathepsins degrade the ECMs of the BM, leading to the disruption of BBB integrity 58 , 61) . Then, surviving cells remodel ECMs dynamically within infarct areas ( Table 1 ) 61 , 62) . While the expression of perlecan and collagen IV was enhanced mainly in the vascular BM in intrainfarct areas, fibronectin and collagen I, produced primarily by PDGFRβ-positive cells, accumulated diffusely within infarct areas 46 , 62 , 63) . Although the BBB appeared to be intact under healthy conditions in mice lacking perlecan, it was severely disrupted after brain ischemia 63 , 64) . Macrophages adhere to fibronectin via integrins and enhance their phagocytic ability, thereby effectively removing debris within infarct areas 62) . Astrocyte-associated fibronectin also supports axonal regeneration in the CNS 65) . Vitronectin, a plasma glycoprotein similar to fibronectin, can also be produced by pericytes and may strengthen the interaction with endothelial cells via the integrin α5 receptor 62 , 66) . Laminin α2 is a major BM protein that limits the infiltration of inflammatory cells in the intact brain. It was degraded by MMPs within infarct areas and was redistributed to the infarct border 62) . Laminin α2 induced the differentiation of OPCs for remyelination, while it may limit the infiltration of inflammatory cells into the intact brain. Laminin γ1, derived from astrocytes, regulates pericyte differentiation and BBB integrity, and thus astrocytic laminin γ1-deficiency leads to severe BBB disruption and age-dependent intracerebral hemorrhage 67) . However, mural cell-derived laminin α5-deficient mice showed less severe vascular damage and attenuated ischemic injury in a tMCAO model, indicating a detrimental role of mural cell-derived laminin α5 in ischemic stroke 68) .
Table 1. Remodeling of extracellular matrix proteins after acute ischemic stroke.
| ECM proteins | Remodeling after ischemic stroke | Main cell source | Possible function in ischemic stroke |
|---|---|---|---|
| Collagen IV | vascular BM | endothelial cells | BBB maintenance 60) |
| Perlecan | vascular BM | endothelial cells | BBB maintenance pericyte recruitment 63) |
| Fibronectin | diffuse within infarction | PDGFRβ-positive fibroblast-like cells liver |
fibrosis 46) macrophage phagocytosis 62) |
| Collagen I | diffuse within infarction | PDGFRβ-positive fibroblast-like cells | fibrosis 46) |
| Vitronectin | diffuse within infarction |
PDGFRβ-positive fibroblast-like cells astrocytes liver |
BBB maintenance 66) |
| Laminin α2 | boundary of infarction | reactive astrocytes |
glial scar OPC differentiation 62) limiting inflammatory cells |
ECM: extracellular matrix, BM: basement membrane, BBB: blood-brain barrier, OPC: oligodendrocyte precursor cell.
5. Strategy for Pericyte Protection
As we mentioned above, based on basic research, pericytes may be a therapeutic target to promote poststroke functional recovery. Clinical studies support this concept because poststroke functional outcome at 3 months is significantly poorer in patients with aging, poor-controlled diabetes mellitus 69 - 71) , proteinuria 72) , and current smoking 73) , that are situations where pericyte dysfunction can be predicted 74) . Therefore, strategies to protect pericytes from these risk factors may improve functional outcomes after ischemic stroke. Furthermore, as a means of protecting and activating pericytes, it may be useful to focus on the intracellular metabolism, the acquisition of ischemic tolerance, and the functional activation of pericytes.
The expression of sodium glucose cotransporter 2 (SGLT2) was induced in periinfarct pericytes in a mouse stroke model. Preadministration of a low-dose SGLT2 inhibitor reduced pericyte/BBB injury during the development of brain infarction without affecting blood glucose levels, possibly by enhancing the ischemic tolerance of pericytes by increasing mitochondrial activity 75) . Although the clinical benefits of SGLT2 inhibitors are still unclear for the prevention of ischemic stroke, it is interesting that preconditioning of pericytes by a common clinical medication could result in a better outcome following ischemic stroke.
Administration of nanoparticle-mediated PDGF-BB, a growth factor promoting proliferation and migration of pericytes, may potentially reduce infarct volume following tMCAO through pericyte-mediated blood flow maintenance and neuroprotection 76) . However, it should be noted that administration of PDGF-CC, similar to VEGF 77 , 78) , may increase vascular permeability in mice treated with rt-PA following tMCAO.
Some of the ECM protein fragments have recently raised attention due to their various physiological activities, and the development of biomaterials targeting cell culture scaffolds and angiogenesis is progressing. Poststroke administration of perlecan domain V (endorepellin) increases the accumulation of pericytes around endothelial tubes and alleviates BBB breakdown within infarct areas through the cooperative functioning of PDGFRβ and integrin α5β1 63) . Moreover, perlecan domain V has been reported to have neuroprotective and proangiogenic effects after ischemic stroke through VEGF secretion by brain endothelial cells via integrin α5β1 and ERK-dependent signaling pathways 79 , 80) .
6. Future Directions and Conclusions
In summary, brain pericytes not only play a wide variety of functions in microvessels, such as regulation of microcirculation and maintenance of the BBB, but also play a crucial role in blood flow recovery and tissue repair after brain ischemia through their interaction with various cell types in the NVU. In particular, pericytes take command and systematically perform these tissue repair processes by regulating macrophage-mediated clearance of debris and the production of ECM proteins, providing the optimal microenvironment required for functional recovery ( Fig.2 ) . Based on this, it may be useful to focus on the intracellular metabolism and functionality of pericytes. However, there are still many unclear points about how pericytes accumulate around infarct areas and become transformed to play a wide variety of roles beyond mural cells. Further studies are expected to develop a new treatment targeting pericytes for functional recovery after ischemic stroke.
Acknowledgements
This study was supported in part by Grants-in-Aid for Scientific Research (B 16H05439 and B 20H03791 to T.K. and T.A.; C 20K09373 to T.A.; C 19K09511 and C 22K09236 to K.N.) from the Japan Society for the Promotion of Science (JSPS); a grant from Mochida Memorial Foundation for Medical and Pharmaceutical Research (K.N.); a grant from SENSHIN Medical Research Foundation, Japan (K.N., and T.A.); a grant from the Smoking Research Foundation (T.A.); and research grants from Astellas, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Eisai, MSD, Sanofi, Taisho and Takeda (T.K. and T.A.).
Competing Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1).Fix AS, Horn JW, Wightman KA, Johnson CA, Long GG, Storts RW, Farber N, Wozniak DF and Olney JW: Neuronal vacuolization and necrosis induced by the noncompetitive N-methyl-D-aspartate (NMDA) antagonist MK(+)801 (dizocilpine maleate): a light and electron microscopic evaluation of the rat retrosplenial cortex. Exp Neurol, 1993; 123: 204-215 [DOI] [PubMed] [Google Scholar]
- 2).Meden P, Overgaard K, Sereghy T and Boysen G: Enhancing the efficacy of thrombolysis by AMPA receptor blockade with NBQX in a rat embolic stroke model. J Neurol Sci, 1993; 119: 209-216 [DOI] [PubMed] [Google Scholar]
- 3).Enlimomab Acute Stroke Trial I: Use of anti-ICAM-1 therapy in ischemic stroke: results of the Enlimomab Acute Stroke Trial. Neurology, 2001; 57: 1428-1434 [DOI] [PubMed] [Google Scholar]
- 4).Lo EH, Dalkara T and Moskowitz MA: Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci, 2003; 4: 399-415 [DOI] [PubMed] [Google Scholar]
- 5).Abbott NJ: Blood-brain barrier structure and function and the challenges for CNS drug delivery. J Inherit Metab Dis, 2013; 36: 437-449 [DOI] [PubMed] [Google Scholar]
- 6).Zlokovic BV: The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron, 2008; 57: 178-201 [DOI] [PubMed] [Google Scholar]
- 7).Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR and Betsholtz C: Pericytes regulate the blood-brain barrier. Nature, 2010; 468: 557-561 [DOI] [PubMed] [Google Scholar]
- 8).Armulik A, Abramsson A and Betsholtz C: Endothelial/pericyte interactions. Circ Res, 2005; 97: 512-523 [DOI] [PubMed] [Google Scholar]
- 9).Lindahl P, Johansson BR, Leveen P and Betsholtz C: Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science (New York, NY, 1997; 277: 242-245 [DOI] [PubMed] [Google Scholar]
- 10).Soriano P: Abnormal kidney development and hematological disorders in PDGF beta-receptor mutant mice. Genes & development, 1994; 8: 1888-1896 [DOI] [PubMed] [Google Scholar]
- 11).Peppiatt CM, Howarth C, Mobbs P and Attwell D: Bidirectional control of CNS capillary diameter by pericytes. Nature, 2006; 443: 700-704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Hall CN, Reynell C, Gesslein B, Hamilton NB, Mishra A, Sutherland BA, O’Farrell FM, Buchan AM, Lauritzen M and Attwell D: Capillary pericytes regulate cerebral blood flow in health and disease. Nature, 2014; 508: 55-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Kisler K, Nelson AR, Rege SV, Ramanathan A, Wang Y, Ahuja A, Lazic D, Tsai PS, Zhao Z, Zhou Y, Boas DA, Sakadzic S and Zlokovic BV: Pericyte degeneration leads to neurovascular uncoupling and limits oxygen supply to brain. Nat Neurosci, 2017; 20: 406-416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Hill RA, Tong L, Yuan P, Murikinati S, Gupta S and Grutzendler J: Regional Blood Flow in the Normal and Ischemic Brain Is Controlled by Arteriolar Smooth Muscle Cell Contractility and Not by Capillary Pericytes. Neuron, 2015; 87: 95-110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Enge M, Bjarnegard M, Gerhardt H, Gustafsson E, Kalen M, Asker N, Hammes HP, Shani M, Fassler R and Betsholtz C: Endothelium-specific platelet-derived growth factor-B ablation mimics diabetic retinopathy. EMBO J, 2002; 21: 4307-4316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Wakisaka M and Nagao T: Sodium glucose cotransporter 2 in mesangial cells and retinal pericytes and its implications for diabetic nephropathy and retinopathy. Glycobiology, 2017; 27: 691-695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Sagare AP, Bell RD, Zhao Z, Ma Q, Winkler EA, Ramanathan A and Zlokovic BV: Pericyte loss influences Alzheimer-like neurodegeneration in mice. Nat Commun, 2013; 4: 2932 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18).Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R and Zlokovic BV: Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron, 2010; 68: 409-427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Yemisci M, Gursoy-Ozdemir Y, Vural A, Can A, Topalkara K and Dalkara T: Pericyte contraction induced by oxidative-nitrative stress impairs capillary reflow despite successful opening of an occluded cerebral artery. Nat Med, 2009; 15: 1031-1037 [DOI] [PubMed] [Google Scholar]
- 20).Yamazaki T, Nalbandian A, Uchida Y, Li W, Arnold TD, Kubota Y, Yamamoto S, Ema M and Mukouyama YS: Tissue Myeloid Progenitors Differentiate into Pericytes through TGF-beta Signaling in Developing Skin Vasculature. Cell Rep, 2017; 18: 2991-3004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Kokovay E, Li L and Cunningham LA: Angiogenic recruitment of pericytes from bone marrow after stroke. J Cereb Blood Flow Metab, 2006; 26: 545-555 [DOI] [PubMed] [Google Scholar]
- 22).Yamamoto S, Muramatsu M, Azuma E, Ikutani M, Nagai Y, Sagara H, Koo BN, Kita S, O’Donnell E, Osawa T, Takahashi H, Takano KI, Dohmoto M, Sugimori M, Usui I, Watanabe Y, Hatakeyama N, Iwamoto T, Komuro I, Takatsu K, Tobe K, Niida S, Matsuda N, Shibuya M and Sasahara M: A subset of cerebrovascular pericytes originates from mature macrophages in the very early phase of vascular development in CNS. Sci Rep-Uk, 2017; 7: 3855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Caplan AI and Correa D: The MSC: an injury drugstore. Cell stem cell, 2011; 9: 11-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Nakagomi T, Molnar Z, Nakano-Doi A, Taguchi A, Saino O, Kubo S, Clausen M, Yoshikawa H, Nakagomi N and Matsuyama T: Ischemia-induced neural stem/progenitor cells in the pia mater following cortical infarction. Stem Cells Dev, 2011; 20: 2037-2051 [DOI] [PubMed] [Google Scholar]
- 25).Nakagomi T, Kubo S, Nakano-Doi A, Sakuma R, Lu S, Narita A, Kawahara M, Taguchi A and Matsuyama T: Brain Vascular Pericytes following Ischemia have Multipotential Stem Cell Activity to Differentiate into Neural and Vascular Lineage Cells. Stem cells, 2015; [DOI] [PubMed] [Google Scholar]
- 26).Faiz M, Sachewsky N, Gascon S, Bang KW, Morshead CM and Nagy A: Adult Neural Stem Cells from the Subventricular Zone Give Rise to Reactive Astrocytes in the Cortex after Stroke. Cell stem cell, 2015; 17: 624-634 [DOI] [PubMed] [Google Scholar]
- 27).Custodia A, Ouro A, Sargento-Freitas J, Aramburu-Nunez M, Pias-Peleteiro JM, Hervella P, Rosell A, Ferreira L, Castillo J, Romaus-Sanjurjo D and Sobrino T: Unraveling the potential of endothelial progenitor cells as a treatment following ischemic stroke. Front Neurol, 2022; 13: 940682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).Li YF, Ren LN, Guo G, Cannella LA, Chernaya V, Samuel S, Liu SX, Wang H and Yang XF: Endothelial progenitor cells in ischemic stroke: an exploration from hypothesis to therapy. J Hematol Oncol, 2015; 8: 33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).Tatebayashi K, Yoshimura S, Sakai N, Uchida K, Kageyama H, Yamagami H, Morimoto T and investigators RE-JR: Relationship Between Acute Neurological Function and Long-Term Prognosis in Patients with Large Arterial Occlusions. J Stroke Cerebrovasc Dis, 2021; 30: 105625 [DOI] [PubMed] [Google Scholar]
- 30).Tachibana M, Ago T, Wakisaka Y, Kuroda J, Shijo M, Yoshikawa Y, Komori M, Nishimura A, Makihara N, Nakamura K and Kitazono T: Early Reperfusion After Brain Ischemia Has Beneficial Effects Beyond Rescuing Neurons. Stroke, 2017; 48: 2222-2230 [DOI] [PubMed] [Google Scholar]
- 31).Shibahara T, Ago T, Nakamura K, Tachibana M, Yoshikawa Y, Komori M, Yamanaka K, Wakisaka Y and Kitazono T: Pericyte-Mediated Tissue Repair through PDGFRbeta Promotes Peri-Infarct Astrogliosis, Oligodendrogenesis, and Functional Recovery after Acute Ischemic Stroke. eNeuro, 2020; 7: ENEURO.0474-0419.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32).McMahon EJ, Suzuki K and Matsushima GK: Peripheral macrophage recruitment in cuprizone-induced CNS demyelination despite an intact blood-brain barrier. J Neuroimmunol, 2002; 130: 32-45 [DOI] [PubMed] [Google Scholar]
- 33).Yu F, Wang Y, Stetler AR, Leak RK, Hu X and Chen J: Phagocytic microglia and macrophages in brain injury and repair. CNS Neurosci Ther, 2022; 28: 1279-1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34).Ting SM, Zhao X, Sun G, Obertas L, Ricote M and Aronowski J: Brain Cleanup as a Potential Target for Poststroke Recovery: The Role of RXR (Retinoic X Receptor) in Phagocytes. Stroke, 2020; 51: 958-966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).Zhang W, Zhao J, Wang R, Jiang M, Ye Q, Smith AD, Chen J and Shi Y: Macrophages reprogram after ischemic stroke and promote efferocytosis and inflammation resolution in the mouse brain. CNS Neurosci Ther, 2019; 25: 1329-1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36).Shichita T, Hasegawa E, Kimura A, Morita R, Sakaguchi R, Takada I, Sekiya T, Ooboshi H, Kitazono T, Yanagawa T, Ishii T, Takahashi H, Mori S, Nishibori M, Kuroda K, Akira S, Miyake K and Yoshimura A: Peroxiredoxin family proteins are key initiators of post-ischemic inflammation in the brain. Nat Med, 2012; 18: 911-917 [DOI] [PubMed] [Google Scholar]
- 37).Shibahara T, Ago T, Tachibana M, Nakamura K, Yamanaka K, Kuroda J, Wakisaka Y and Kitazono T: Reciprocal Interaction Between Pericytes and Macrophage in Poststroke Tissue Repair and Functional Recovery. Stroke, 2020; 51: 3095-3106 [DOI] [PubMed] [Google Scholar]
- 38).Shichita T, Ito M, Morita R, Komai K, Noguchi Y, Ooboshi H, Koshida R, Takahashi S, Kodama T and Yoshimura A: MAFB prevents excess inflammation after ischemic stroke by accelerating clearance of damage signals through MSR1. Nat Med, 2017; 23: 723-732 [DOI] [PubMed] [Google Scholar]
- 39).Hu X, Leak RK, Shi Y, Suenaga J, Gao Y, Zheng P and Chen J: Microglial and macrophage polarization-new prospects for brain repair. Nat Rev Neurol, 2015; 11: 56-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40).Shichita T, Sugiyama Y, Ooboshi H, Sugimori H, Nakagawa R, Takada I, Iwaki T, Okada Y, Iida M, Cua DJ, Iwakura Y and Yoshimura A: Pivotal role of cerebral interleukin-17-producing gammadeltaT cells in the delayed phase of ischemic brain injury. Nature medicine, 2009; 15: 946-950 [DOI] [PubMed] [Google Scholar]
- 41).Maehara N, Taniguchi K, Okuno A, Ando H, Hirota A, Li Z, Wang CT, Arai S and Miyazaki T: AIM/CD5L attenuates DAMPs in the injured brain and thereby ameliorates ischemic stroke. Cell Rep, 2021; 36: 109693 [DOI] [PubMed] [Google Scholar]
- 42).Ito M, Komai K, Mise-Omata S, Iizuka-Koga M, Noguchi Y, Kondo T, Sakai R, Matsuo K, Nakayama T, Yoshie O, Nakatsukasa H, Chikuma S, Shichita T and Yoshimura A: Brain regulatory T cells suppress astrogliosis and potentiate neurological recovery. Nature, 2019; 565: 246-250 [DOI] [PubMed] [Google Scholar]
- 43).Goritz C, Dias DO, Tomilin N, Barbacid M, Shupliakov O and Frisen J: A pericyte origin of spinal cord scar tissue. Science (New York, NY, 2011; 333: 238-242 [DOI] [PubMed] [Google Scholar]
- 44).Dias DO, Kalkitsas J, Kelahmetoglu Y, Estrada CP, Tatarishvili J, Holl D, Jansson L, Banitalebi S, Amiry-Moghaddam M, Ernst A, Huttner HB, Kokaia Z, Lindvall O, Brundin L, Frisen J and Goritz C: Pericyte-derived fibrotic scarring is conserved across diverse central nervous system lesions. Nat Commun, 2021; 12: 5501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45).Arimura K, Ago T, Kamouchi M, Nakamura K, Ishitsuka K, Kuroda J, Sugimori H, Ooboshi H, Sasaki T and Kitazono T: PDGF receptor beta signaling in pericytes following ischemic brain injury. Current neurovascular research, 2012; 9: 1-9 [DOI] [PubMed] [Google Scholar]
- 46).Makihara N, Arimura K, Ago T, Tachibana M, Nishimura A, Nakamura K, Matsuo R, Wakisaka Y, Kuroda J, Sugimori H, Kamouchi M and Kitazono T: Involvement of platelet-derived growth factor receptor beta in fibrosis through extracellular matrix protein production after ischemic stroke. Exp Neurol, 2015; 264: 127-134 [DOI] [PubMed] [Google Scholar]
- 47).Nakamura K, Arimura K, Nishimura A, Tachibana M, Yoshikawa Y, Makihara N, Wakisaka Y, Kuroda J, Kamouchi M, Ooboshi H, Kitazono T and Ago T: Possible involvement of basic FGF in the upregulation of PDGFRbeta in pericytes after ischemic stroke. Brain Res, 2016; 1630: 98-108 [DOI] [PubMed] [Google Scholar]
- 48).Shen J, Ishii Y, Xu G, Dang TC, Hamashima T, Matsushima T, Yamamoto S, Hattori Y, Takatsuru Y, Nabekura J and Sasahara M: PDGFR-beta as a positive regulator of tissue repair in a mouse model of focal cerebral ischemia. J Cereb Blood Flow Metab, 2012; 32: 353-367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49).Ishitsuka K, Ago T, Arimura K, Nakamura K, Tokami H, Makihara N, Kuroda J, Kamouchi M and Kitazono T: Neurotrophin production in brain pericytes during hypoxia: a role of pericytes for neuroprotection. Microvascular research, 2012; 83: 352-359 [DOI] [PubMed] [Google Scholar]
- 50).Silver J and Miller JH: Regeneration beyond the glial scar. Nat Rev Neurosci, 2004; 5: 146-156 [DOI] [PubMed] [Google Scholar]
- 51).Hara M, Kobayakawa K, Ohkawa Y, Kumamaru H, Yokota K, Saito T, Kijima K, Yoshizaki S, Harimaya K, Nakashima Y and Okada S: Interaction of reactive astrocytes with type I collagen induces astrocytic scar formation through the integrin-N-cadherin pathway after spinal cord injury. Nat Med, 2017; 23: 818-828 [DOI] [PubMed] [Google Scholar]
- 52).Anderson MA, Burda JE, Ren Y, Ao Y, O’Shea TM, Kawaguchi R, Coppola G, Khakh BS, Deming TJ and Sofroniew MV: Astrocyte scar formation aids central nervous system axon regeneration. Nature, 2016; 532: 195-200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53).Shi H, Hu X, Leak RK, Shi Y, An C, Suenaga J, Chen J and Gao Y: Demyelination as a rational therapeutic target for ischemic or traumatic brain injury. Exp Neurol, 2015; 272: 17-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54).Montagne A, Nikolakopoulou AM, Zhao Z, Sagare AP, Si G, Lazic D, Barnes SR, Daianu M, Ramanathan A, Go A, Lawson EJ, Wang Y, Mack WJ, Thompson PM, Schneider JA, Varkey J, Langen R, Mullins E, Jacobs RE and Zlokovic BV: Pericyte degeneration causes white matter dysfunction in the mouse central nervous system. Nat Med, 2018; 24: 326-337 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 55).Maki T, Choi YK, Miyamoto N, Shindo A, Liang AC, Ahn BJ, Mandeville ET, Kaji S, Itoh K, Seo JH, Gelman IH, Lok J, Takahashi R, Kim KW, Lo EH and Arai K: A-Kinase Anchor Protein 12 Is Required for Oligodendrocyte Differentiation in Adult White Matter. Stem Cells, 2018; 36: 751-760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56).Maki T, Maeda M, Uemura M, Lo EK, Terasaki Y, Liang AC, Shindo A, Choi YK, Taguchi A, Matsuyama T, Takahashi R, Ihara M and Arai K: Potential interactions between pericytes and oligodendrocyte precursor cells in perivascular regions of cerebral white matter. Neurosci Lett, 2015; 597: 164-169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57).Watt FM and Huck WT: Role of the extracellular matrix in regulating stem cell fate. Nat Rev Mol Cell Biol, 2013; 14: 467-473 [DOI] [PubMed] [Google Scholar]
- 58).Baeten KM and Akassoglou K: Extracellular matrix and matrix receptors in blood-brain barrier formation and stroke. Dev Neurobiol, 2011; 71: 1018-1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59).Engelhardt B, Vajkoczy P and Weller RO: The movers and shapers in immune privilege of the CNS. Nat Immunol, 2017; 18: 123-131 [DOI] [PubMed] [Google Scholar]
- 60).Xu L, Nirwane A and Yao Y: Basement membrane and blood-brain barrier. Stroke Vasc Neurol, 2019; 4: 78-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61).Yao Y: Basement membrane and stroke. J Cereb Blood Flow Metab, 2019; 39: 3-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62).Shibahara T, Nakamura K, Wakisaka Y, Shijo M, Yamanaka K, Takashima M, Takaki H, Hidaka M, Kitazono T and Ago T: PDGFRbeta-positive cell-mediated post-stroke remodeling of fibronectin and laminin alpha2 for tissue repair and functional recovery. J Cereb Blood Flow Metab, 2023; 43: 518-530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63).Nakamura K, Ikeuchi T, Nara K, Rhodes CS, Zhang P, Chiba Y, Kazuno S, Miura Y, Ago T, Arikawa-Hirasawa E, Mukouyama YS and Yamada Y: Perlecan regulates pericyte dynamics in the maintenance and repair of the blood-brain barrier. J Cell Biol, 2019; 218: 3506-3525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64).Arikawa-Hirasawa E, Watanabe H, Takami H, Hassell JR and Yamada Y: Perlecan is essential for cartilage and cephalic development. Nat Genet, 1999; 23: 354-358 [DOI] [PubMed] [Google Scholar]
- 65).Tom VJ, Doller CM, Malouf AT and Silver J: Astrocyte-associated fibronectin is critical for axonal regeneration in adult white matter. J Neurosci, 2004; 24: 9282-9290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66).Ayloo S, Lazo CG, Sun S, Zhang W, Cui B and Gu C: Pericyte-to-endothelial cell signaling via vitronectin-integrin regulates blood-CNS barrier. Neuron, 2022; 110: 1641-1655 e1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67).Yao Y, Chen ZL, Norris EH and Strickland S: Astrocytic laminin regulates pericyte differentiation and maintains blood brain barrier integrity. Nat Commun, 2014; 5: 3413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68).Nirwane A, Johnson J, Nguyen B, Miner JH and Yao Y: Mural cell-derived laminin-alpha5 plays a detrimental role in ischemic stroke. Acta Neuropathol Commun, 2019; 7: 23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69).Kamouchi M, Matsuki T, Hata J, Kuwashiro T, Ago T, Sambongi Y, Fukushima Y, Sugimori H, Kitazono T and Investigators FSR: Prestroke glycemic control is associated with the functional outcome in acute ischemic stroke: the Fukuoka Stroke Registry. Stroke, 2011; 42: 2788-2794 [DOI] [PubMed] [Google Scholar]
- 70).Ago T, Matsuo R, Hata J, Wakisaka Y, Kuroda J, Kitazono T, Kamouchi M and Fukuoka Stroke Registry I: Insulin resistance and clinical outcomes after acute ischemic stroke. Neurology, 2018; 90: e1470-e1477 [DOI] [PubMed] [Google Scholar]
- 71).Kiyohara T, Matsuo R, Hata J, Nakamura K, Wakisaka Y, Kamouchi M, Kitazono T, Ago T and Investigators FSR: beta-Cell Function and Clinical Outcome in Nondiabetic Patients With Acute Ischemic Stroke. Stroke, 2021; 52: 2621-2628 [DOI] [PubMed] [Google Scholar]
- 72).Kumai Y, Kamouchi M, Hata J, Ago T, Kitayama J, Nakane H, Sugimori H, Kitazono T and Investigators FSR: Proteinuria and clinical outcomes after ischemic stroke. Neurology, 2012; 78: 1909-1915 [DOI] [PubMed] [Google Scholar]
- 73).Matsuo R, Ago T, Kiyuna F, Sato N, Nakamura K, Kuroda J, Wakisaka Y, Kitazono T and Fukuoka Stroke Registry I: Smoking Status and Functional Outcomes After Acute Ischemic Stroke. Stroke, 2020; 51: 846-852 [DOI] [PubMed] [Google Scholar]
- 74).Aldibbiat AM: Insulin Signaling Via Retinal Pericytes, New Insights and Potential Implications in Diabetic Retinopathy. Endocrinology, 2022; 163: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75).Takashima M, Nakamura K, Kiyohara T, Wakisaka Y, Hidaka M, Takaki H, Yamanaka K, Shibahara T, Wakisaka M, Ago T and Kitazono T: Low-dose sodium-glucose cotransporter 2 inhibitor ameliorates ischemic brain injury in mice through pericyte protection without glucose-lowering effects. Commun Biol, 2022; 5: 653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76).Takagishi S, Arimura K, Murata M, Iwaki K, Okuda T, Ido K, Nishimura A, Narahara S, Kawano T and Iihara K: Protein Nanoparticles Modified with PDGF-B as a Novel Therapy After Acute Cerebral Infarction. eNeuro, 2021; 8: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77).Su EJ, Fredriksson L, Geyer M, Folestad E, Cale J, Andrae J, Gao Y, Pietras K, Mann K, Yepes M, Strickland DK, Betsholtz C, Eriksson U and Lawrence DA: Activation of PDGF-CC by tissue plasminogen activator impairs blood-brain barrier integrity during ischemic stroke. Nat Med, 2008; 14: 731-737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78).Su EJ, Cao C, Fredriksson L, Nilsson I, Stefanitsch C, Stevenson TK, Zhao J, Ragsdale M, Sun YY, Yepes M, Kuan CY, Eriksson U, Strickland DK, Lawrence DA and Zhang L: Microglial-mediated PDGF-CC activation increases cerebrovascular permeability during ischemic stroke. Acta Neuropathol, 2017; 134: 585-604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79).Lee B, Clarke D, Al Ahmad A, Kahle M, Parham C, Auckland L, Shaw C, Fidanboylu M, Orr AW, Ogunshola O, Fertala A, Thomas SA and Bix GJ: Perlecan domain V is neuroprotective and proangiogenic following ischemic stroke in rodents. J Clin Invest, 2011; 121: 3005-3023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80).Biose IJ, Rutkai I, Clossen B, Gage G, Schechtman K, Adkisson HDt and Bix GJ: Recombinant Human Perlecan DV and Its LG3 Subdomain Are Neuroprotective and Acutely Functionally Restorative in Severe Experimental Ischemic Stroke. Transl Stroke Res, 2022; [DOI] [PMC free article] [PubMed] [Google Scholar]


