Abstract
Aim: Patients with end-stage kidney disease (ESKD) have an unparalleled risk of left ventricular hypertrophy (LVH) and vascular calcification (VC), both of which introduce excessive cardiovascular risk. However, it remains unclear whether LVH geometry co-modulates cardiovascular outcomes with VC in this population.
Methods: A retrospective cohort study was conducted. Patients with ESKD requiring chronic hemodialysis were identified from Shin Kong Wu Ho-Su Memorial Hospital between October and December 2018, with echocardiographic LVH geometry and aortic arch calcification (AoAC) determined. They were divided into four groups according to AoAC severity and eccentric or concentric LVH. We used Kaplan–Meier analysis and Cox proportional hazard regression to analyze their cardiovascular and all-cause mortality after multivariate adjustment.
Results: Overall, 223 patients with ESKD with LVH were analyzed, among whom 29.1%, 23.3%, 25.1%, and 22.4% had non-to-mild AoAC with eccentric and concentric LVH and moderate-to-severe AoAC with eccentric and concentric LVH, respectively. After 3.5 years of follow-up, patients with ESKD with moderate-to-severe AoAC and concentric LVH had a significantly higher risk of cardiovascular mortality than those with non-to-mild AoAC and eccentric LVH (hazard ratio 3.35,p=0.002). However, those with moderate-to-severe AoAC but eccentric LVH did not have higher cardiovascular mortality. Similarly, patients with ESKD with moderate-to-severe AoAC and concentric LVH had a significantly higher all-cause mortality than those with non-to-mild AoAC and eccentric LVH, whereas the other two groups did not have higher risk.
Conclusion: LVH geometry could help stratify the risk of patients with ESKD when they had severe VC, and co-existing severe VC and concentric LVH aggravated cardiovascular risk.
Keywords: Aortic calcification, Cardiac geometry, Echocardiography, End-stage kidney disease, Left ventricular hypertrophy, Vascular calcification
List of abbreviations: ANOVA, analysis of variance; AoAC, aortic arch calcification; ASE, American Society of Echocardiography; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; ESKD, end-stage kidney disease; HD, hemodialysis; HR, hazard ratio; IVC, inferior vena cava; IVS, interventricular septum; LVEF, left ventricular ejection fraction; left ventricular end-diastolic volume; LVESD, left ventricular end-systolic diameter; LVH, left ventricular hypertrophy; LVMI, left ventricular mass index; LVPW, left ventricular posterior wall; MRI, magnetic resonance imaging; PAD, peripheral artery disease; RWT, relative wall thickness; VC, vascular calcification
Introduction
Vascular calcification (VC) is an important outcome-modifying complication with a high prevalence in patients with chronic kidney disease (CKD) and end-stage kidney disease (ESKD). A meta-analysis involving 38 studies reported a 60% prevalence of coronary artery calcification in patients with CKD 1) . In the population with renal impairment, VC presumably involves both medial and intimal layers, whereas in the general population, intimal calcification accounts for the majority of VC due to its close association with atherosclerosis 2) . It was once believed that vascular medial dystrophic calcification was responsible for the pathologic picture for CKD-associated VC, but vascular smooth muscle cell (VSMC) transdifferentiation assuming an osteoblast-like phenotype with osteoid deposition has now been established as the primary mechanism 3) . This is supported by the fact that CKD-associated VC is characterized by nontraditional risk factors, including hyperphosphatemia and uremic toxins, chronic inflammation, and vascular senescence 4) . VSMCs, the main constituent of medial layer, exposed to high phosphate or uremic toxin have been shown to develop biomineralization resembling VC 5 , 6) . The presence of VC increases vascular stiffness, elevates cardiac afterload, and paves the way toward heart failure initiation and exacerbation in patients with CKD, thereby correlating with a higher cardiovascular mortality 7) .
Patients with CKD are also at risk for developing altered left ventricular (LV) remodeling, especially left ventricular hypertrophy (LVH). Epidemiological studies estimated that 87% of patients with ESKD had at least one structural abnormality and 49%–71% had echocardiographically determined LVH 8) . LVH, defined by an increased LV mass, exhibits an increasingly higher prevalence among those with lower renal function; the prevalence of LVH has been estimated at 30%–50% in patients with early CKD but rises to 50%–80% in those with advanced CKD and ESKD 9) . Furthermore, the presence of LVH significantly increases the risk of adverse outcomes in the general population, those with hypertension, diabetes mellitus, or CKD 10 , 11) . The associations between LV mass, LVH, and renal function are independent of confounders, including demographic profiles and blood pressure measurements, suggesting that pathophysiologic changes related to uremia likely play an important role in LVH development 12) . A prior study further disclosed that different pathologic machineries contributed to diverging types of LVH as a cardiac maladaptive response; a progressively rising afterload (pressure overload) tends to induce concentric hypertrophy, whereas increasing preload (volume overload) alternatively places one at risk for eccentric hypertrophy 13) . There can also be potential outcome differences between patients with concentric and eccentric LVH, as shown by an anecdotal study involving patients with ESKD 14) .
Based on the above arguments, it is clear that VC and LVH both worsen outcomes in patients with CKD. A previous report suggested that LVH might occur earlier than arterial remodeling in this population 15) , and it is very likely that ventricular remodeling can be further aggravated by arterial remodeling and rising vascular stiffness from VC, supporting the link between VC and LVH. Existing literature further showed that VC modified the outcome influences posed by LVH in older adults 16) , but it remains unclear whether LVH subtypes influence the cardiovascular risk introduced by VC in patients harboring a high risk of developing LVH, especially those with CKD. In the present study, we assembled a cohort of patients with ESKD with different types of LVH and follow-up to analyze the adverse outcome influence caused by VC and LVH types alone or in combination.
Methods
Ethics Statement
The protocol of this study adhered to the Declaration of Helsinki and has been approved by the institutional review board of Shin Kong Wu Ho-Su Memorial Hospital (no. 20211205R). The Institutional Review Board waived the need for informed consent, since this was a retrospective analysis of clinical data.
Participant Recruitment and Data Collection
This was a single center, retrospective cohort study. Patients with ESKD, or having an estimated glomerular filtration rate (eGFR) lower than 15 mL/min/1.73 m2 for more than 3 months requiring chronic hemodialysis, were retrospectively identified from the hemodialysis unit of Shin Kong Wu Ho-Su Memorial Hospital between October 1, 2018 and December 31, 2018. All of them were outpatients. Inclusion criteria were those who received chronic hemodialysis treatment in our institution during the case identification period, and participants should have received posteroanterior chest radiography and echocardiography with procedures described below. We enrolled those with echocardiographic LVH and subdivided them based on geometry subtypes (eccentric vs. concentric). Exclusion criteria were those who used a tunneled cuffed catheter to facilitate hemodialysis, since the use of such vascular access type introduced an excess risk of infection and future heart failure 17) .
After participant identification, we collected their clinical features, including demographic profile, comorbidities, ESKD causes, laboratory parameters (hemogram, serum biochemistry, and electrolyte panels), dialysis efficiency, and medication regimens (antihypertensives, antidiabetics, statins, antiplatelets, and anticoagulants). They were divided according to the severity of VC in the form of aortic arch calcification (AoAC) and the geometric morphology of LVH, namely, eccentric or concentric type, with four groups (non-to-mild AoAC with eccentric LVH, non-to-mild AoAC with concentric LVH, moderate-to-severe AoAC with eccentric LVH, and moderate-to-severe AoAC with concentric LVH). Participants were followed up until outcome occurrences or December 31, 2021, whichever came first.
Chest Radiography and Echocardiography Performance
All participants received transthoracic echocardiography (TTE) for assessing cardiac geometry, with principles generally conforming to the updated protocols outlined by the American Society of Echocardiography (ASE) 18) , during the non-dialysis mid-week day. All echocardiographers were board-certified cardiologists with expertise in performing TTE and were blinded to our study design. We obtained measurements of left ventricular end-diastolic diameter (LVEDD) and end-systolic diameter (LVESD), posterior wall (LVPW) thickness, left atrium diameter, interventricular septum (IVS) thickness, aortic root diameter, and inferior vena cava diameter, using M-mode tracings. LV mass was determined based on the Devereux formula and normalized to body surface area to generate LV mass index using the Mosteller equation. LV ejection fraction (LVEF) was calculated according to recommendations made by the ASE 19) . Concentric LVH was defined if participants had an elevated LV mass (>95 or 115 g/m2 in females or males, respectively) and a relative wall thickness (RWT) ≥ 0.42, whereas eccentric LVH was defined if they had an elevated LV mass with an RWT <0.42.
For chest radiography, participants received posteroanterior imaging on the non-dialysis day during mid-week, with films retrieved for interpretation. VC was categorized according to AoAC severity, with none (score 0), mild (score 1), moderate (score 2), and severe (score 3) based on a validated classification scheme 20) . This severity-stratification approach has also been tested and verified for consistency in domestic cohorts and our prior studies 21 , 22) . We merged participants into those with non-to-mild (scores 0 and 1) and moderate-to-severe (scores 2 and 3) AoAC due to case number limitations.
Outcome Definition
The primary endpoint of this study was mortality due to cardiovascular origins, as adjudicated by physicians in charge of care of the index patient(s) without awareness of our study design. Cardiovascular mortality was defined as death attributable to myocardial infarction, heart failure, fatal cardiac arrhythmia, sudden cardiac death, aortic dissection, aortic aneurysm rupture, cardiac tamponade, pulmonary embolism, and ischemic or hemorrhagic cerebrovascular accident. The secondary outcome was all-cause mortality.
Statistical Analysis
Continuous data are expressed as means with standard deviations, whereas categorical data are presented as numbers with percentages in parentheses. We compared continuous data between the four groups with different AoAC severities and LVH subtypes using one-way analysis of variance (if normally distributed) or the Kruskal–Wallis test (if not normally distributed), respectively. We compared categorical variables using the Chi-square test. We first examined the intergroup differences with regard to demographic data, comorbidities, laboratory parameters, and concurrent medications. This was followed by an investigation of differences in all echocardiographic features between the four groups of participants. We then analyzed the incidence of primary and secondary outcomes between groups, using the Kaplan–Meier technique, and compared results using the log rank test. Finally, we used Cox proportional hazard regression to evaluate the risk of primary and secondary outcomes according to AoAC severities and LVH subtypes, adjusting for variables with significant differences between groups with different LVH subtypes and AoAC severities. In all analyses, a two-sided p value less than 0.05 was considered statistically significant.
Results
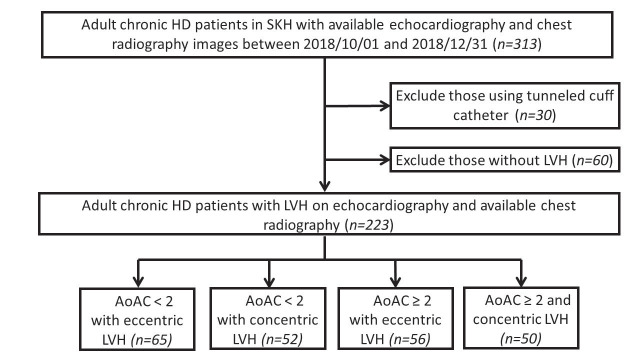
Overall, 313 patients with ESKD receiving echocardiography and chest radiography were identified during the study period, among whom 223 (71.2%) had LVH ( Fig.1 ) . They were subdivided according to their AoAC severities and LVH types, into those with non-to-mild AoAC and eccentric LVH (n=52, 23.3%), non-to-mild AoAC and concentric LVH (n=65, 29.1%), moderate-to-severe AoAC and eccentric LVH (n=50, 22.4%), and moderate-to-severe AoAC and concentric LVH (n=56, 25.1%).
Fig.1. Flowchart of participant selection in this study.
AoAC, aortic calcification; HD, hemodialysis; LVH, left ventricular hypertrophy; SKH, Shin Kong Wu Ho-Su Memorial Hospital
Regarding demographic and comorbidity data, patients with ESKD with moderate-to-severe AoAC and concentric LVH had significantly higher age (p<0.001) and a higher prevalence of female (p=0.008) and peripheral artery disease (PAD) (p=0.002) than those in the other three groups ( Table 1 ) . Those with non-to-mild AoAC and eccentric LVH had a lower prevalence of female and PAD than those in the other three groups, whereas those with non-to-mild AoAC and concentric LVH had the lowest age ( Table 1 ) . No difference was observed between groups regarding the cause of ESKD. Among the laboratory profile, patients with ESKD with moderate-to-severe AoAC and concentric LVH had the lowest serum albumin levels, and those with non-to-mild AoAC and eccentric LVH had the highest albumin (p=0.005) ( Table 1 ) . Parameters such as hemogram, lipid profile, fasting glucose, and liver function did not differ between the four groups, nor was the electrolyte panel. The prevalence of antihypertensive, antidiabetic, statin, antiplatelet, and anticoagulant use was similar between groups ( Table 1 ) .
Table 1. Baseline characteristics of patients with ESKD according to AoAc severity and LVH type.
| Variable | Non-to-mild AoAC with eccentric LVH (n=52) | Non-to-mild AoAC with concentric LVH (n=65) | Moderate-to-severe AoAC with eccentric LVH (n=50) | Moderate-to-severe AoAC with concentric LVH (n=56) | p |
|---|---|---|---|---|---|
| Demographic data | |||||
| Age (years)** | 65.21±12.53 | 63.69±12.48 | 73.38±9.78 | 75.25±9.60 | <0.001 |
| Female (%)† | 19 (36.5) | 26 (40) | 24 (48) | 37 (66.1) | 0.008 |
| Weight (kg)** | 64.28±14.21 | 60.02±12.65 | 57.85±14.06 | 58.14±13.07 | 0.134 |
| Vintage (years)* | 4.94±5.53 | 7.65±7.59 | 6.27±6.76 | 7.40±7.08 | 0.074 |
| Comorbidities | |||||
| Type 2 DM (%)† | 33 (63.5) | 26 (40) | 21 (42) | 27 (48.2) | 0.061 |
| Hypertension (%)† | 44 (84.6) | 54 (83.1) | 38 (76) | 49 (87.5) | 0.452 |
| Hyperlipidemia (%)† | 33 (63.5) | 35 (53.8) | 30 (60) | 31 (55.4) | 0.721 |
| CAD (%)† | 26 (50) | 23 (35.4) | 21 (42) | 29 (51.8) | 0.245 |
| PAD (%)† | 5 (9.6) | 21 (32.3) | 12 (24) | 23 (41.1) | 0.002 |
| Heart failure (%)† | 12 (23.1) | 13 (20) | 8 (16) | 18 (32.1) | 0.224 |
| COPD (%)‡ | 3 (5.8) | 4 (6.2) | 3 (6) | 7 (12.5) | 0.519 |
| Malignancy (%)† | 4 (7.7) | 10 (15.4) | 5 (10) | 7 (12.5) | 0.606 |
| Causes of ESKD | 0.201 | ||||
| ADPKD | 1 (1.9) | 1 (1.5) | 1 (2.0) | 0 (0) | |
| CGN | 22 (42.3) | 26 (40.0) | 18 (36.0) | 23 (41.1) | |
| Diabetic nephropathy | 21 (40.4) | 33 (50.8) | 20 (40.0) | 28 (50.0) | |
| Hypertension | 1 (1.9) | 3 (4.6) | 3 (6.0) | 1 (1.8) | |
| Others | 7 (13.5) | 2 (3.1) | 8 (16.0) | 4 (7.1) | |
| Laboratory parameters | |||||
| Hemogram | |||||
| Hb (g/dL)* | 10.54±1.30 | 10.33±1.58 | 10.30±1.10 | 9.94±1.29 | 0.134 |
| Platelet (K/μL)* | 182.63±57.50 | 183.56±57.02 | 186.83±53.93 | 191.43±58.06 | 0.666 |
| Ferritin (ng/mL)* | 536.85±386.57 | 548.74±245.34 | 555.91±209.93 | 528.80±261.12 | 0.461 |
| TSAT (%)* | 32.17±12.26 | 34.69±16.14 | 28.91±10.44 | 29.22±11.12 | 0.219 |
| Biochemistry | |||||
| Albumin (g/dL)* | 4.00±0.31 | 3.95±0.32 | 3.89±0.34 | 3.76±0.43 | 0.005 |
| Total cholesterol (mg/dL)* | 151.46±37.52 | 158.73±43.49 | 154.98±39.54 | 160.98±44.17 | 0.803 |
| Triglyceride (mg/dL)* | 160.35±159.50 | 133.00±101.43 | 138.56±96.15 | 157.18±102.60 | 0.293 |
| Fasting glucose (mg/dL)* | 107.42±48.61 | 111.11±48.25 | 118.33±65.38 | 122.45±56.03 | 0.434 |
| Uric acid (mg/dL)* | 5.96±1.73 | 6.05±1.64 | 6.23±1.93 | 5.99±1.49 | 0.613 |
| AST (IU/L)* | 16.38±5.56 | 17.02±5.88 | 15.79±6.22 | 17.91±21.27 | 0.533 |
| Alkaline-P (IU/L)* | 67.54±27.63 | 75.63±45.67 | 80.89±46.06 | 83.89±46.06 | 0.157 |
| PTH (pg/mL)* | 276.14±241.18 | 243.74±189.03 | 286.28±337.78 | 330.58±311.38 | 0.671 |
| Electrolyte panels | |||||
| Na (meq/L)* | 138.25±2.71 | 137.94±3.24 | 137.90±2.74 | 137.39±3.26 | 0.446 |
| K (meq/L)* | 4.67±0.61 | 4.75±0.68 | 4.54±0.56 | 4.64±0.65 | 0.407 |
| iCa (mg/dL)* | 4.63±0.38 | 4.51±0.47 | 4.64±0.50 | 4.64±0.57 | 0.414 |
| P (mg/dL)* | 5.28±1.24 | 5.21±1.36 | 4.85±1.10 | 4.93±1.10 | 0.197 |
| Al (ng/mL)* | 5.62±2.11 | 6.87±3.53 | 6.95±3.89 | 7.29±4.73 | 0.144 |
| Dialysis efficiency | |||||
| Kt/V (Gotch)* | 1.33±0.22 | 1.38±0.18 | 1.37±0.19 | 1.43±0.18 | 0.090 |
| Medication regimen | |||||
| Anti-HTN drugs | |||||
| ACEI/ARB (%)† | 31 (59.6) | 44 (67.7) | 28 (56) | 33 (58.9) | 0.595 |
| β-Blockers (%)† | 30 (57.7) | 41 (63.1) | 23 (46) | 32 (57.1) | 0.330 |
| CCB (%)† | 33 (63.5) | 44 (67.7) | 32 (64) | 34 (60.7) | 0.884 |
| Statins (%)† | 27 (51.9) | 20 (30.8) | 21 (42) | 20 (35.7) | 0.117 |
| Antidiabetic agents | |||||
| OAD (%)† | 22 (42.3) | 15 (23.1) | 19 (38) | 22 (39.3) | 0.115 |
| Insulin and analogs (%)† | 13 (25) | 10 (15.4) | 10 (20) | 11 (19.6) | 0.639 |
| Antiplatelets (%)† | 26 (50) | 30 (46.2) | 28 (56) | 33 (58.9) | 0.502 |
| Anticoagulants (%)‡ | 1 (1.9) | 6 (9.2) | 2 (4) | 2 (3.6) | 0.275 |
Data are expressed as n (%) for categorical data and as mean±standard deviation for continuous data.
*Kruskal–Wallis test **One-way ANOVA †Chi-square test ‡Fisher’s exact test
ACEI, angiotensin-converting enzyme inhibitor; ADPKD, autosomal dominant polycystic kidney disease; Al, aluminum; Alkaline-P, alkaline phosphatase; ANOVA, analysis of variance; AoAC, aortic arch calcification; ARB, angiotensin receptor blocker; AST, aspartate transaminase; CAD, coronary artery disease; CCB, calcium channel blocker; CGN, chronic glomerulonephritis; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; ESKD, end-stage kidney disease; Hb, hemoglobin; HTN, hypertension; iCa, ionized calcium; LVH, left ventricular hypertrophy; OAD, oral antidiabetics; PAD, peripheral artery disease; PTH, parathyroid hormone; TSAT, transferrin saturation
We further examined the echocardiographic features in the four groups of participants. There was no significant difference in LV mass between the four groups ( Table 2 ) . Patients with ESKD with concentric LVH had significantly greater IVS (p=0.002), LVPW (p<0.001), and RWT (p<0.001) than those with eccentric LVH, whereas LVEDD (p<0.001) and LVESD (p=0.003) were significantly greater among those with eccentric LVH ( Table 2 ) . No significant difference in EF was noted between the four groups of participants (p=0.267).
Table 2. Echocardiographic features of patients with ESKD according to AoAc severity and LVH type.
| Features | Non-to-mild AoAC with eccentric LVH | Non-to-mild AoAC with concentric LVH | Moderate-to-severe AoAC with eccentric LVH | Moderate-to-severe AoAC with concentric LVH | p |
|---|---|---|---|---|---|
| Aortic root (mm)* | 32.42±3.85 | 31.88±4.43 | 32.40±4.34 | 31.82±4.95 | 0.666 |
| IVS (mm)* | 11.83±1.88 | 13.11±2.65 | 11.80±2.05 | 13.25±3.07 | 0.002 |
| LA diameter (mm)* | 44.46±7.89 | 43.46±8.26 | 43.86±6.31 | 42.70±7.11 | 0.617 |
| LVEDD (mm)* | 56.63±6.40 | 48.56±5.67 | 55.09±5.73 | 47.70±7.14 | <0.001 |
| LVESD (mm)* | 36.57±9.94 | 31.17±7.05 | 33.88±6.16 | 30.83±6.98 | 0.003 |
| LVPW (mm)* | 10.07±1.40 | 12.80±2.20 | 10.01±1.28 | 12.84±2.34 | <0.001 |
| LV mass (g)* | 264.07±75.92 | 254.36±58.44 | 249.49±67.28 | 257.81±100.66 | 0.570 |
| LVMI* | 155.18±38.92 | 155.95±33.07 | 155.83±34.51 | 162.28±58.16 | 0.922 |
| RWT (mm)* | 0.36±0.05 | 0.54±0.18 | 0.36±0.04 | 0.55±0.13 | <0.001 |
| IVC diameter (mm)** | 1.62±0.41 | 1.58±0.46 | 1.50±0.47 | 1.42±0.37 | 0.212 |
| EF (%)* | 63.50±15.32 | 66.66±10.01 | 67.90±11.13 | 64.72±10.90 | 0.267 |
Data are expressed as mean±standard deviation for continuous data.
*Kruskal–Wallis test **One-way ANOVA
ANOVA, analysis of variance; AoAC, aortic arch calcification; EF, ejection fraction; ESKD, end-stage kidney disease; IVC, inferior vena cava; IVS, interventricular septum; LA, left atrium; LV, left ventricle; LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic diameter; LVH, left ventricular hypertrophy; LVMI, left ventricular mass index; LVPW, left ventricular posterior wall; RWT, relative wall thickness
Outcome Analysis
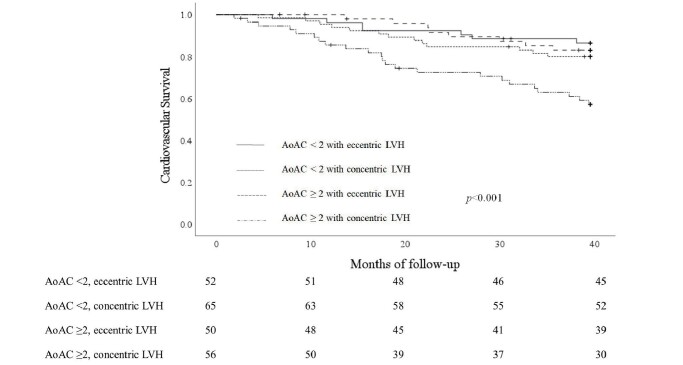
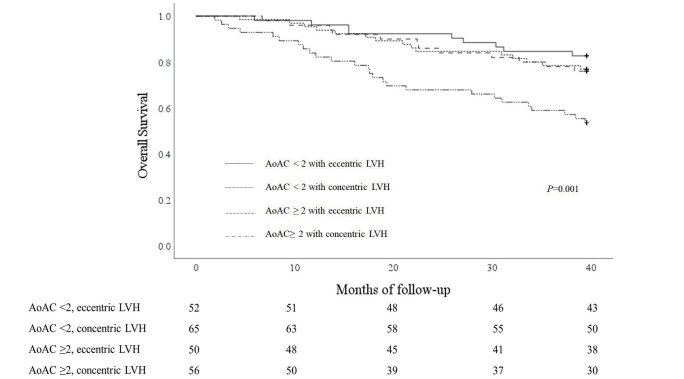
After 3.5 years of follow-up, 51 (22.9%) patients with ESKD died due to cardiovascular origins, and 62 (27.8%) died from any causes. Of all death events, 51 patients died from cardiovascular causes, 7 from infectious diseases, and 4 from malignancies. Of all cardiovascular death events, 6 patients died from myocardial infarction, 8 from heart failure, 9 from fatal cardiac arrhythmia, 20 from sudden cardiac death, 1 from aortic dissection, 1 from pulmonary embolism, and 6 from ischemic or hemorrhagic cerebrovascular accident. The cardiovascular mortality rates were 13.5%, 20%, 16%, and 41.1% among those with non-to-mild AoAC and eccentric LVH, non-to-mild AoAC with concentric LVH, moderate-to-severe AoAC with eccentric LVH, and moderate-to-severe AoAC with concentric LVH, respectively. Patients with ESKD with moderate-to-severe AoAC and concentric LVH had significantly higher cardiovascular mortality than those in the other three groups (log rank p<0.001) ( Fig.2 ) . Similar finding could be observed for all-cause mortality, with 17.3%, 23.1%, 24.0%, and 46.4% mortality among those with non-to-mild AoAC and eccentric LVH, non-to-mild AoAC with concentric LVH, moderate-to-severe AoAC with eccentric LVH, and moderate-to-severe AoAC with concentric LVH, respectively (p=0.001) ( Fig.3 ) .
Fig.2. Cardiovascular survival curves according to participants’ vascular calcification and LVH type.
AoAC, aortic arch calcification; LVH, left ventricular hypertrophy
Fig.3. Overall survival curves according to participants’ vascular calcification and LVH type.
AoAC, aortic arch calcification; LVH, left ventricular hypertrophy
Then, we analyzed the association between different AoAC severities, LVH types, and cardiovascular mortality among these patients. Patients with ESKD, moderate-to-severe AoAC and concentric LVH had more than three-fold higher risk of cardiovascular mortality than those with milder AoAC and eccentric LVH (hazard ratio (HR) 5.01, p=0.002) ( Table 3 ) . By contrast, those with moderate-to-severe AoAC but eccentric LVH did not have a higher cardiovascular mortality than those with milder AoAC regardless of LVH types ( Table 3 ) . After accounting for demographic data (age and sex; Table 3 , model 1) and additionally for comorbidity and serum albumin ( Table 3 , model 2), those with moderate-to-severe AoAC and concentric LVH still had a significantly higher cardiovascular mortality than those with non-to-mild AoAC and eccentric LVH (HR 4.07 and 3.68 in models 1 and 2, respectively). Similarly, patients with ESKD, moderate-to-severe AoAC and concentric LVH had a significantly higher all-cause mortality than those with non-to-mild AoAC and eccentric LVH (HR 3.22 and 2.78 in models 1 and 2, respectively) ( Table 3 ) . The results remained essentially unaltered after accounting for dialysis vintage ( Table 3 , model 3) and ESKD causes ( Table 3 , model 4). Sensitivity analyses using age- and vintage-matched patients with ESKD with non-to-mild AoAC and eccentric LVH and those with moderate-to-severe AoAC and concentric LVH yielded similar findings ( Supplementary Tables 1 and 2 ) .
Table 3. Analysis of event risk between the four groups according to AoAC severity and LVH type.
| Events | Crude | Model 1* | Model 2** | Model 3*** | Model 4**** | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |
| CV mortality | ||||||||||
| Non-to-mild AoAC with eccentric LVH | 1 | – | 1 | – | 1 | – | 1 | – | 1 | – |
| Non-to-mild AoAC with concentric LVH | 1.54 (0.62–3.86) | 0.36 | 1.71 (0.68–4.31) | 0.25 | 1.49 (0.58–3.85) | 0.41 | 1.47 (0.57–3.82) | 0.43 | 1.44 (0.56–3.71) | 0.45 |
| Moderate-to-severe AoAC with eccentric LVH | 1.23 (0.45–3.39) | 0.69 | 1.14 (0.41–3.17) | 0.81 | 1.16 (0.41–3.26) | 0.78 | 1.16 (0.41–3.27) | 0.78 | 1.19 (0.43–3.33) | 0.73 |
| Moderate-to-severe AoAC with concentric LVH | 5.01 (2.38–10.52) | 0.002 | 4.07 (1.68–9.84) | 0.002 | 3.68 (1.48–9.17) | 0.01 | 3.68 (1.47–9.18) | 0.005 | 4.08 (1.64–10.13) | 0.002 |
| Mortality | ||||||||||
| Non-to-mild AoAC with eccentric LVH | 1 | – | 1 | – | 1 | – | 1 | – | 1 | – |
| Non-to-mild AoAC with concentric LVH | 1.38 (0.60–3.15) | 0.45 | 1.56 (0.68–3.57) | 0.29 | 1.28 (0.54–3.01) | 0.58 | 1.26 (0.53–2.99) | 0.60 | 1.21 (0.51–2.83) | 0.67 |
| Moderate-to-severe AoAC with eccentric LVH | 1.43 (0.60–3.40) | 0.41 | 1.25 (0.52–2.99) | 0.62 | 1.12 (0.45–2.76) | 0.81 | 1.11 (0.45–2.75) | 0.82 | 1.21 (0.49–2.95) | 0.68 |
| Moderate-to-severe AoAC with concentric LVH | 3.35 (1.57–7.15) | 0.002 | 3.22 (1.46–7.10) | 0.004 | 2.78 (1.22–6.33) | 0.02 | 2.77 (1.21–6.31) | 0.02 | 3.31 (1.46–7.49) | 0.004 |
*Adjusted for age and sex
**Adjusted for age, sex, peripheral artery disease, and albumin
*** Adjusted for age, sex, peripheral artery disease, albumin, and dialysis vintage
**** Adjusted for age, sex, peripheral artery disease, albumin, dialysis vintage, and ESKD cause.
AoAC, aortic arch calcification; CI, confidence interval; CV, cardiovascular; ESKD, end-stage kidney disease; HR, hazard ratio; LVH, left ventricular hypertrophy
Supplementary Table 1. Baseline characteristics of ESKD patients according to AoAC severity and the type of LVH matched by age and vintage.
| Data | Non-to-mild AoAC with eccentric LVH (n=22) | Moderate-to-severe AoAC with concentric LVH (n=22) | P value |
|---|---|---|---|
| Demographic data | |||
| Age (years)* | 75.09±8.54 | 74.86±7.76 | 0.927 |
| Female (%)† | 9 (40.9) | 14 (63.6) | 0.131 |
| Weight (kg)* | 60.59±9.05 | 58.92±14.20 | 0.646 |
| Vintage (years)** | 6.05±5.48 | 6.14±5.51 | 0.953 |
| Comorbidities | |||
| Type 2 DM (%)† | 14 (63.6) | 13 (59.1) | 0.757 |
| Hypertension (%)‡ | 20 (90.9) | 17 (77.3) | 0.412 |
| Hyperlipidemia (%)† | 13 (59.1) | 10 (45.5) | 0.365 |
| CAD (%)† | 14 (63.6) | 11 (50) | 0.361 |
| PAD (%)† | 2 (9.1) | 8 (36.4) | 0.031 |
| Heart failure (%)† | 6 (27.3) | 6 (27.3) | 1.000 |
| COPD (%)‡ | 3 (13.6) | 1 (4.5) | 0.607 |
| Malignancy (%)‡ | 2 (9.1) | 2 (9.1) | 1.000 |
| Arrhythmia‡ | 3 (13.6) | 4 (18.2) | 1.000 |
| Laboratory parameters | |||
| Hemogram | |||
| Hb (g/dL)* | 10.53±1.29 | 9.61±1.23 | 0.020 |
| Platelet (K/μL)* | 163.86±41.15 | 186.64±64.76 | 0.171 |
| Ferritin (ng/mL)* | 443.19±254.95 | 485.26±174.89 | 0.527 |
| TSAT (%)* | 31.45±11.57 | 28.73±11.07 | 0.430 |
| Biochemistry | |||
| Albumin (g/dL)* | 3.89±0.30 | 3.80±0.40 | 0.427 |
| Total cholesterol (mg/dL)* | 156.95±36.25 | 155.95±38.56 | 0.930 |
| Triglyceride (mg/dL)** | 112.32±53.30 | 132.41±58.38 | 0.526 |
| Fasting glucose (mg/dL)** | 111.68±62.19 | 122.14±58.38 | 0.622 |
| Uric acid (mg/dL)* | 5.77±1.79 | 5.92±1.62 | 0.765 |
| AST (IU/L)* | 17.36±5.17 | 14.45±5.31 | 0.073 |
| Alkaline-P (IU/L)** | 64.32±23.86 | 83.50±63.24 | 0.557 |
| PTH (pg/mL)** | 210.39±230.04 | 249.64±253.71 | 0.439 |
| Electrolyte panels | |||
| Na (meq/L)* | 138.50±2.63 | 137.14±3.26 | 0.134 |
| K (meq/L)* | 4.71±0.62 | 4.62±0.74 | 0.675 |
| iCa (mg/dL)** | 4.62±0.43 | 4.66±0.63 | 0.944 |
| P (mg/dL)* | 5.09±1.11 | 4.88±1.14 | 0.532 |
| Al (ng/mL)* | 5.50±1.85 | 6.25±2.98 | 0.320 |
| Dialysis efficiency | |||
| Kt/V (Gotch)* | 1.36±0.18 | 1.41±0.17 | 0.290 |
| Medication regimen | |||
| Anti-HTN drugs | |||
| ACEI/ARB (%)† | 14 (63.6) | 15 (68.2) | 0.750 |
| β-blockers (%)† | 13 (59.1) | 12 (54.5) | 0.761 |
| CCB (%)† | 14 (63.6) | 14 (63.6) | 1.000 |
| Statins (%)† | 11 (50) | 5 (22.7) | 0.060 |
| Anti-diabetic agents | |||
| OAD (%)† | 9 (40.9) | 10 (45.5) | 0.761 |
| Insulin and analogues (%)† | 5 (22.7) | 7 (31.8) | 0.498 |
| Antiplatelets (%)† | 13 (59.1) | 14 (63.6) | 0.757 |
Data are expressed as n (%) for categorical data and as mean±standard deviation for continuous data.
*Independent t-test; **Mann-Whitney U test; †Chi-square test; ‡Fisher’s exact test
ACEI, angiotensin-converting enzyme inhibitor; Al, aluminum; Alkaline-P, alkaline phosphatase; AoAC, aortic arch calcification; ARB, angiotensin receptor blocker; AST, aspartate transaminase; CAD, coronary artery disease; CCB, calcium channel blocker; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; ESKD, end-stage kidney disease; Hb, hemoglobin; HTN, hypertension; iCa, ionized calcium; LVH, left ventricular hypertrophy; OAD, oral anti-diabetics; PAD, peripheral artery disease; PTH, parathyroid hormone; TSAT, transferrin saturation
Supplementary Table 2. Analysis of event risk between age- and vintage-matched patients with non-to-mild AoAC with eccentric LVH and those with moderate-to-severe AoAC with concentric LVH.
| Events | Crude | Model 1* | Model 2** | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |
| CV mortality | ||||||
| Non-to-mild AoAC with eccentric LVH | 1 | - | 1 | - | 1 | - |
| Moderate-to-severe AoAC with concentric LVH | 5.68 (1.60-20.22) | 0.007 | 8.66 (2.16-34.72) | 0.002 | 7.67 (1.75-33.63) | 0.007 |
| Mortality | ||||||
| Non-to-mild AoAC with eccentric LVH | 1 | - | 1 | - | 1 | - |
| Moderate-to-severe AoAC with concentric LVH | 4.05 (1.45-11.29) | 0.007 | 6.02 (1.93-18.81) | 0.002 | 4.65 (1.37-15.86) | 0.014 |
*Adjusted for age and sex **Adjusted for age, sex, PAD and Hb
AoAC, aortic arch calcification; CI, confidence interval; CV, cardiovascular; ESKD, end-stage kidney disease; HR, hazard ratio; LVH, left ventricular hypertrophy
Discussion
In the current study, we showed that patients with ESKD with concentric LVH and moderate-to-severe AoAC exhibited the highest risk of cardiovascular mortality compared to those with eccentric LVH and milder AoAC, followed by those with moderate-to-severe AoAC but with eccentric LVH, whereas there was no significant difference in cardiovascular risk between those with concentric and eccentric LVH if they had non-to-mild AoAC only. There was a tendency that AoAC posed worse cardiovascular influences than concentric LVH did, and the presence of greater VC severity and concentric LVH concurrently introduced the greatest risk. Similar phenomenon was observed if we focused on all-cause mortality. Our findings can potentially shed light on the prognostic importance of combining LV geometry and VC for estimating cardiovascular outcomes in patients with ESKD.
Although LV geometry alterations independently correlate with an increased myocardial work 23) , concentric and eccentric LVH can have different origins and clinical risk implications. Based on myocardial fiber stress simulation, a prior report demonstrated that valvular lesion types determined LV geometry alterations, with aortic stenosis more likely causing concentric LVH, whereas aortic and mitral regurgitation resulting in eccentric LVH 24) . Hemodynamically, concentric LVH frequently co-exists with systolic wall stress, myocardial fibrosis, and cardiomyocyte apoptosis signaling activation, whereas eccentric LVH is accompanied by diastolic wall stress and increasing angiogenesis 25) . Both LVH types correlate with increasing myocardial inflammatory cytokine expressions, although the severity of inflammation is milder in the eccentric form than in the concentric form, whereas fibrosis occurs as replacement instead of reaction in the former 26) . Interestingly, in animal models, mice with volume overload and eccentric LVH had better outcomes than those with pressure overload and concentric LVH 25) . Clinical differences in risk associates have also been reported between LVH types 27) . Eccentric LVH increases the risk for heart failure with reduced ejection fraction, whereas concentric LVH predisposes one to heart failure with preserved ejection fraction 28) . Our findings ( Table 3 ) are generally compatible with findings in other populations 29 , 30) .
Several reasons may be responsible for the differential influences posed by LVH types. First, as suggested above, concentric LVH frequently results from pressure overload, and the presence of aortic calcification certainly increases vascular stiffness and cardiac afterload. As both processes enhance myocardial workload through a similar mechanism, it is likely that the enhancement of cardiovascular risk observed from the co-existence of both becomes more prominent than that from eccentric LVH and aortic calcification. Second, it is also plausible that systolic function was already impaired in those with concentric LVH and AoAC, since they were also older and had a significantly higher prevalence of peripheral artery disease ( Table 1 ) , signifying greater vascular morbidity severity. However, the values of ejection fraction did not differ between groups with concentric and eccentric LVH ( Table 2 ) . Third, concentric LVH type is more frequently associated with chronic inflammation than eccentric type, and the severity tends to be more severe in the former 26 , 31) . In our participants, those with concentric LVH have significantly lower serum albumin than those with eccentric LVH ( Table 1 ) , hinting at the possibility that the former group had higher systemic inflammation severity, since hypoalbuminemia and anemia are long considered presentations of systemic inflammation in patients with ESKD. Although higher systemic inflammation potentially spread to involve individual organs, local myocardial inflammation can be difficult to measure. We propose that participants with concentric LVH and more severe AoAC might harbor both greater systemic and local myocardial inflammation, leading to a higher cardiovascular event risk than the others. This can be supported by our findings that the elevated cardiovascular risk associated with concentric LVH and more severe AoAC could be modestly attenuated by adjusting for serum albumin but remained significant ( Table 3 ) .
We did not identify a significant difference regarding cardiovascular and overall mortality between those with different LVH types and those with non-to-mild AoAC and between those with non-to-mild AoAC and those with moderate-to-severe AoAC and eccentric LVH ( Table 3 ) . We presumed that LVH types did not influence the degree of cardiovascular risk when VC severity was mild, whereas prominently assisted in stratifying patients’ risk when VC severity was higher. The compound influences related to aggravated pressure overload from greater vascular stiffness and pre-existing concentric myocardial maladaptation may accentuate cardiovascular risk more than the combination of volume and pressure overload to a milder degree. Our findings further shed light on the subgroup of patients that should be targeted for intensive risk factor reduction regarding VC and LVH related ones, and also for regular monitoring of adverse cardiac outcomes, especially sudden cardiac death and cardiovascular mortality, according to findings from prior studies in patients with ESKD 32 - 34) .
Our study has its strengths and limitations. The idea of this study has not been addressed before in patients with ESKD, and our results enrich the literature through demonstrating the prognosis-modulating effect of VC and LVH subtypes. However, several limitations still need to be taken into consideration. First, our cohort size is modest, and the statistical efficacy for detecting differences of smaller magnitude may be compromised. Our study was observational in nature, and the causality between LVH, AoAC, and cardiovascular risk in these patients could not be confirmed. However, the risk introduced by concentric LVH and VC remained significant and was independent of other confounders. Second, we did not employ other modalities to verify LVH geometry or evaluate for possible LVH etiology, such as cardiac magnetic resonance imaging (MRI) 35) . However, the accessibility of cardiac MRI could be limited, and echocardiography remained a useful option for LV morphological/functional assessment during clinical practice. For pathogenic mechanism investigation, it would be helpful to assess myocardial inflammation severity, but we did not perform such examination. Finally, residual interferences resulting from other outcome-influencing factors in patients with ESKD could not be excluded, such as frailty/sarcopenia 36) , genetic susceptibility 37) , or potential treatments 38) . Further studies are required to confirm our findings and test the applicability to other populations.
Conclusion
Based on a cohort of patients with ESKD and LVH, we discovered that those with more severe VC and concentric LVH had a significantly higher cardiovascular and overall mortality than those with milder VC or eccentric LVH. LVH types could help stratify patients’ risk when they had more severe VC, whereas VC severity introduced cardiovascular risk mostly during the co-existence of concentric LVH rather than eccentric LVH. Our findings are expected to assist in uncovering subgroups of patients with ESKD at particularly high cardiovascular risk that necessitates earlier provision of risk mitigation strategies.
Ethics, Consent, and Permission:
The institutional review board of Shin Kong Wu Ho-Su Memorial Hospital (NO. 20211205R) approved this study, whose protocol adhered to the Declaration of Helsinki, and waived the need for informed consent due to the retrospective nature of this study.
Consent for Publication:
not applicable
Availability of Data and Material:
The raw data for conducting this analysis are available upon reasonable request to the corresponding author.
Author Contributions:
Study design: CTC and CKW; Data analysis: CKW; Article drafting: CTC, MTL, and CKW; All authors approved the final version of the manuscript.
Funding Disclosure:
This study was supported by a grant from the Shin-Kong Wu Ho-Su Memorial Hospital Research Foundation (NO. 2021SKHADR004) and from the Ministry of Science and Technology, Taiwan (MOST 109–2314-B-341–003-MY3).
Acknowledgments:
We are grateful to the Second Core Laboratory, Department of Medical Research of National Taiwan University Hospital and the Genomic Research Center of National Taiwan University College of Medicine for their technical input.
Competing Interests:
The authors have no relevant financial or non-financial competing interests to declare regarding this manuscript.
References
- 1).Wang XR, Zhang JJ, Xu XX, Wu YG. Prevalence of coronary artery calcification and its association with mortality, cardiovascular events in patients with chronic kidney disease: a systematic review and meta-analysis. Ren Fail, 2019; 41: 244-256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Kim JS, Hwang HS. Vascular calcification in chronic kidney disease: distinct features of pathogenesis and clinical implication. Korean Circ J, 2021; 51: 961-982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Tsai YT, Yeh HY, Chao CT, Chiang CK. Superoxide dismutase 2 (SOD2) in vascular calcification: a focus on vascular smooth muscle cells, calcification pathogenesis, and therapeutic strategies. Oxid Med Cell Longev, 2021; 2021: 6675548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Nelson AJ, Raggi P, Wolf M, Gold AM, Chertow GM, Roe MT. Targeting vascular calcification in chronic kidney disease. JACC Basic Transl Sci, 2020; 5: 398-412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Voelkl J, Lang F, Eckardt KU, Amann K, Kuro-O M, Pasch A, Pieske B, Alesutan I. Signaling pathways involved in vascular smooth muscle cell calcification during hyperphosphatemia. Cell Mol Life Sci, 2019; 76: 2077-2091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Chao CT, Liu YP, Su SF, Yeh HY, Chen HY, Lee PJ, Chen WJ, Lee YM, Huang JW, Chiang CK, Hung KY, Chen HW. Circulating MicroRNA-125b predicts the presence and progression of uremic vascular calcification. Arterioscler Thromb Vasc Biol, 2017; 37: 1402-1414 [DOI] [PubMed] [Google Scholar]
- 7).Chung WS, Shih MP, Wu PY, Huang JC, Chen SC, Chiu YW, Chang JM, Chen HC. Progression of aortic arch calcification is associated with overall and cardiovascular mortality in hemodialysis. Dis Markers, 2020; 2020: 6293185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Hickson LJ, Negrotto SM, Onuigbo M, Scott CG, Rule AD, Norby SM, Albright RC, Casey ET, Dillon JJ, Pellikka PA, Pislaru SV, Best PJM, Villarraga HR, Lin G, Williams AW, Nkomo VT. Echocardiography criteria for structural heart disease in patients with end-stage renal disease initiating hemodialysis. J Am Coll Cardiol, 2016; 67: 1173-1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Paoletti E, Bellino D, Cassottana P, Rolla D, Cannella G. Left ventricular hypertrophy in nondiabetic predialysis CKD. Am J Kidney Dis, 2005; 46: 320-327 [DOI] [PubMed] [Google Scholar]
- 10).Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med, 1990; 322: 1561-1566 [DOI] [PubMed] [Google Scholar]
- 11).Ravera M, Noberasco G, Re M, Filippi A, Gallina AM, Weiss U, Cannavò R, Ravera G, Cricelli C, Deferrari G. Chronic kidney disease and cardiovascular risk in hypertensive type 2 diabetics: a primary care perspective. Nephrol Dial Transplant, 2009; 24: 1528-1533 [DOI] [PubMed] [Google Scholar]
- 12).Nardi E, Mulè G, Giammanco A, Mattina A, Geraci G, Nardi C, Averna M. Left ventricular hypertrophy in chronic kidney disease: a diagnostic criteria comparison. Nutr Metab Cardiovasc Dis, 2021; 31: 137-144 [DOI] [PubMed] [Google Scholar]
- 13).Amann K, Rychlík I, Miltenberger-Milteny G, Ritz E. Left ventricular hypertrophy in renal failure. Kidney Int Suppl, 1998; 68: S78-S85 [DOI] [PubMed] [Google Scholar]
- 14).de Roij van Zuijdewijn CL, Hansildaar R, Bots ML, Blankestijn PJ, van den Dorpel MA, Grooteman MP, Kamp O, ter Wee PM, Nubé MJ. Eccentric left ventricular hypertrophy and sudden death in patients with end-stage kidney disease. Am J Nephrol, 2015; 42: 126-133 [DOI] [PubMed] [Google Scholar]
- 15).Pluta A, Stróżecki P, Krintus M, Odrowąż-Sypniewska G, Manitius J. Left ventricular remodeling and arterial remodeling in patients with chronic kidney disease stage 1-3. Ren Fail, 2015; 37: 1105-1110 [DOI] [PubMed] [Google Scholar]
- 16).Cho IJ, Chang HJ, Lee SE, Shim CY, Hong GR, Chung N. Prognostic application of thoracic aortic calcium scoring for adverse clinical outcome risk in elderly patients with left ventricular hypertrophy. Korean Circ J, 2017; 47: 918-928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Castro V, Farber A, Zhang Y, Dicken Q, Mendez L, Levin SR, Cheng TW, Hasley RB, Siracuse JJ. Reasons for long-term tunneled dialysis catheter use and associated morbidity. J Vasc Surg, 2021; 73: 588-592 [DOI] [PubMed] [Google Scholar]
- 18).Mitchell C, Rahko PS, Blauwet LA, Canaday B, Finstuen JA, Foster MC, Horton K, Ogunyankin KO, Palma RA, Velazquez EJ. Guidelines for performing a comprehensive transthoracic echocardiographic examination in adults: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr, 2019; 32: 1-64 [DOI] [PubMed] [Google Scholar]
- 19).Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr, 2015; 28: 1-39.e14 [DOI] [PubMed] [Google Scholar]
- 20).Hashimoto H, Iijima K, Hashimoto M, Son BK, Ota H, Ogawa S, Eto M, Akishita M, Ouchi Y. Validity and usefulness of aortic arch calcification in chest X-ray. J Atheroscler Thromb, 2009; 16: 256-264 [DOI] [PubMed] [Google Scholar]
- 21).Lee SY, Chao CT, Huang JW, Huang KC. Vascular calcification as an underrecognized risk factor for frailty in 1783 community-dwelling elderly individuals. J Am Heart Assoc, 2020; 9: e017308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Chao CT, Yeh HY, Tsai YT, Chiang CK, Chen HW. A combined microRNA and target protein-based panel for predicting the probability and severity of uraemic vascular calcification: a translational study. Cardiovasc Res, 2021; 117: 1958-1973 [DOI] [PubMed] [Google Scholar]
- 23).Tadic M, Cuspidi C, Saeed S, Lazic JS, Vukomanovic V, Grassi G, Sala C, Celic V. The influence of left ventricular geometry on myocardial work in essential hypertension. J Hum Hypertens, 2022; 36: 524-530 [DOI] [PubMed] [Google Scholar]
- 24).Maksuti E, Westerhof BE, Ugander M, Donker DW, Carlsson M, Broomé M. Cardiac remodeling in aortic and mitral valve disease: a simulation study with clinical validation. J Appl Physiol, 2019; 126: 1377-1389 [DOI] [PubMed] [Google Scholar]
- 25).You J, Wu J, Zhang Q, Ye Y, Wang S, Huang J, Liu H, Wang X, Zhang W, Bu L, Li J, Lin L, Ge J, Zou Y. Differential cardiac hypertrophy and signaling pathways in pressure versus volume overload. Am J Physiol Heart Circ Physiol, 2018; 314: H552-H562 [DOI] [PubMed] [Google Scholar]
- 26).Anversa P, Ricci R, Olivetti G. Quantitative structural analysis of the myocardium during physiologic growth and induced cardiac hypertrophy: a review. J Am Coll Cardiol, 1986; 7: 1140-1149 [DOI] [PubMed] [Google Scholar]
- 27).Shah N, Badheka AO, Grover PM, Patel NJ, Chothani A, Mehta K, Hoosien M, Singh V, Savani GT, Deshmukh A, Rathod A, Patel N, Panaich SS, Arora S, Schwartz C, Blisker M, Coffey JO, Mitrani RD, Fuster V, Viles-Gonzalez JF. Influence of left ventricular remodeling on atrial fibrillation recurrence and cardiovascular hospitalizations in patients undergoing rhythm-control therapy. Int J Cardiol, 2014; 174: 288-292 [DOI] [PubMed] [Google Scholar]
- 28).Velagaleti RS, Gona P, Pencina MJ, Aragam J, Wang TJ, Levy D, D’Agostino RB, Lee DS, Kannel WB, Benjamin EJ, Vasan RS. Left ventricular hypertrophy patterns and incidence of heart failure with preserved versus reduced ejection fraction. Am J Cardiol, 2014; 113: 117-122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).Grossman C, Levin M, Koren-Morag N, Bornstein G, Leibowitz A, Ben-Zvi I, Shemesh J, Grossman E. Left ventricular hypertrophy predicts cardiovascular events in hypertensive patients with coronary artery calcifications. Am J Hypertens, 2018; 31: 313-320 [DOI] [PubMed] [Google Scholar]
- 30).Dykun I, Geisel MH, Kälsch H, Lehmann N, Bauer M, Moebus S, Jöckel KH, Möhlenkamp S, Erbel R, Mahabadi AA. Association of computed tomography-derived left ventricular size with major cardiovascular events in the general population: the Heinz Nixdorf recall study. Atherosclerosis, 2015; 240: 46-52 [DOI] [PubMed] [Google Scholar]
- 31).Velagaleti RS, Gona P, Levy D, Aragam J, Larson MG, Tofler GH, Lieb W, Wang TJ, Benjamin EJ, Vasan RS. Relations of biomarkers representing distinct biological pathways to left ventricular geometry. Circulation, 2008; 118: 2252-2258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32).Tanaka S, Nakano T, Hiyamuta H, Taniguchi M, Tokumoto M, Masutani K, Ooboshi H, Tsuruya K, Kitazono T. Impact of multivascular disease on cardiovascular mortality and morbidity in patients receiving hemodialysis: ten-year outcomes of the Q-cohort study. J Atheroscler Thromb, 2021; 28: 385-395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33).Hiyamuta H, Tanaka S, Taniguchi M, Tokumoto M, Fujisaki K, Nakano T, Tsuruya K, Kitazono T. The incidence and associated factors of sudden death in patients on hemodialysis: 10-year outcome of the Q-cohort study. J Atheroscler Thromb, 2020; 27: 306-318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34).Joki N, Tanaka Y, Hayashi T. Sudden death, A common cause of death in Japanese hemodialysis patients. J Atheroscler Thromb, 2020; 27: 303-305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).Grajewski KG, Stojanovska J, Ibrahim EH, Sayyouh M, Attili A. Left ventricular hypertrophy: evaluation with cardiac MRI. Curr Probl Diagn Radiol, 2020; 49: 460-475 [DOI] [PubMed] [Google Scholar]
- 36).Wu PY, Chao CT, Chan DC, Huang JW, Hung KY. Contributors, risk associates, and complications of frailty in patients with chronic kidney disease: a scoping review. Ther Adv Chronic Dis, 2019; 10: 2040622319880382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37).Chao CT, Huang JW, Chiang CK, Chen YC, Fang CC, Hu FC, Chang CC, Yen CJ. Diabetes mellitus, superoxide dismutase and peroxisome proliferator activated receptor gamma polymorphisms modify the outcome of end-stage renal disease patients of Han Chinese origin. Nephrology, 2018; 23: 117-125 [DOI] [PubMed] [Google Scholar]
- 38).Chao CT, Yeh HY, Tsai YT, Chuang PH, Yuan TH, Huang JW, Chen HW. Natural and nonnatural antioxidative compounds: potential candidates for treeatment of vascular calcification. Cell Death Discov, 2019; 5: 145 [DOI] [PMC free article] [PubMed] [Google Scholar]