Abstract
The 8-oxoguanine DNA glycosylase (OGG1) repairs DNA by removing 8-hydroxyguanine, a highly mutagenic oxidative DNA adduct. Recently, the gene for OGG1 was cloned and several polymorphisms have been reported. Because environmental carcinogens produce 8-hydroxyguanine residues that potentially cause oncogenic mutations by mismatching to this modified base, the capacity to repair these lesions can be involved in cancer susceptibility. This study investigated the association between OGG1 Ser326Cys polymorphism and risk of the lung adenocarcinoma for Japanese by a prevalent case-control study in Japan. The subjects comprised 138 cases and 241 non-cancer outpatients as controls. OGG1 gene polymorphism was genotyped by a PCR-CTPP (polymerase chain reaction with confronting two-pair primers) method. The distribution of OGG1 Ser326Cys genotype among controls (Ser/Ser, 28.3%; Ser/Cys, 49.2%; and Cys/Cys, 22.5%) was not different from that among cases (Ser/Ser, 29.0%; Ser/Cys, 51.4%; and Cys/Cys, 24.0%). The sex-age adjusted odds ratio (OR) was 1.06 with 95% confidence interval (CI) 0.64-1.76 for Ser/Cys genotype and 0.81 with 0.44-1.52 for Cys/Cys genotype. The ORs according to the interval between diagnosis and study enrollment were also examined because the polymorphism was a potential prognostic factor of lung cancer. The ORs of Ser/Cys and Cys/Cys genotypes in the cases less than 3 years after diagnosis were higher than overall ORs; 1.86 (95%CI, 0.91 -3.77), and 1.46 (0.64-3.35), respectively. The OR for smoking was not statistically different among genotype, though the sample size was too small to detect even a moderate interaction. This study supported the first study by Sugimura et al (Cancer Epidemiol Biomarkers Prev, 1999; 8: 669-674), that the association of OGG1 Ser326Cys polymorphism was limited for the risk of lung adenocarcinoma.
Keywords: OGG1 polymorphism, lung adenocarcinoma, PCR-CTPP
INTRODUCTION
Oxidative DNA damage is thought to cause mutations, which can activate oncogenes or inactivate tumor suppresser genes finally leading to cancer1,2). To protect the damage, organisms have developed highly efficient DNA repair machineries3,4). Interindividual variation in the repair capacity has been implicated as a cancer susceptibility factor. 8-hydroxyguanine is one of the major forms of oxidative DNA damage produced by reactive oxygen species (ROS), and causes mutagenic / carcinogenic DNA misreading through G:C to T:A transversion5,6). G:C to T:A transversion are widely seen in tumors e. g. in lung tumors where these transversion are very frequently found in the p53 gene and compared with other tumors such as those of colon or breast7,8). Several kinds of human cancer tissues, including lung cancer, showed higher levels of 8-hydroxyguanine compared with their non-cancerous counterparts9-11). These findings indicate that this kind of oxidative DNA damage causes human cancer development.
The human 8-oxoguanine DNA glycosylase 1 is one of 8-hydroxyguanine repair enzymes12-16). OGG1 is the gene encoding the glycosylase, which belongs to the base excision repair gene family17). Studies on genetic structure have revealed the presence of several polymorphisms within the OGG1 locus18,19). Among them, Ser326Cys polymorphism has been shown to be very common in Japanese and Chinese populations19-21), but less common in Caucasian populations20,22). The Cys326 protein has been shown to have about 7 times weaker 8-hydroxyguanine-repair capacity than Ser326 protein in complementation assay using Escherichia coli mutant strain deficient in 8-hydroxyguanine19). However, Ser326Cys polymorphism did not correlate with the mean 8-hydroxyguanine levels in peripheral lymphocytes19) and nor with the adduct levels in human lung samples23). In addition, recent studies reported no difference in the catalytic activities between Ser326 and Cys326 alleles24,25). Therefore, the difference in the function in vivo between the two alleles remains unclear.
The association between OGG1 Ser326Cys polymorphism and the risk of lung cancer has been investigated in 3 studies19,20,22). In all studies, the frequencies of Cys/Cys genotype were slightly higher among cases than among controls, but not significant. For other cancers, a significant association with the risk of esophageal cancer risk has been reported in China21).
In Japan, lung cancer is the leading cause of deaths among malignancies26). The cumulative incidence of lung adenocarcinoma has been steadily increasing for both males and females, while that for lung squamous cell carcinoma was almost constant from 1970s27). The reason is not clear, but the change in the kind of cigarettes from high-tar no-filtered to low-tar filtered is suspected to be one of the factors27). Although the association of old type cigarettes with the adenocarcinoma is weaker than with the squamous or small cell carcinoma, the adenocarcinoma may have a stronger association with low-tar filtered cigarettes. In order to detect high-risk individuals, studies on the interactions between genetic traits and smoking should be conducted for many tobacco-related polymorphisms. In this paper, we examined the association between Ser326Cys polymorphism and adenocarcinoma of the lung by a prevalent case-control study with hospital controls. Furthermore, the effect modification by this polymorphism was examined for smoking habit.
MAERIALS AND METHODS
Study subjects
Cases were lung cancer patients who visited Aichi Cancer center Hospital during 1999-2000. At the first stage, they were asked by their doctors in charge whether to participate in this study or not (not counted how many outpatients were asked by the doctors). At the second stage, the assentients at first stage were enrolled after written informed consent by staffs of Division of Epidemiology and Prevention. With a few exceptions all agree to participate in this study. A total of 138 Japanese patients with adenocarcinoma of the lung aged 26 to 80 years (68 males and 70 females) at diagnosis and 241 non-cancer controls aged 39-69 years (118 males and 123 females) were recruited. The cases had been diagnosed in the past 17 years at Aichi Cancer Center Hospital. The pathological distribution was as follows: 42 with well-differential adenocarcinoma, 73 with moderate-differential adenocarcinoma and 18 with poorly-differential adenocarcinoma. The controls were outpatients without a history of cancer who underwent gastroscopy, as described in our previous paper28). All subjects gave written informed consent for polymorphism genotyping, completed a self-administered questionnaire and provided a 7 ml of peripheral blood sample. Controls included 97 (40.2% out of 241) participants stated to be under medication for 107 disease (not confirmed by their medical records); 23 with gastric/duodenal ulcer, another 23 for so-called gastritis, 16 with hypertension, 8 for pain including arthritis and lumbargo, 7 with diabetes mellitus, 7 with hyperlipidemia, 3 with ischemic heart disease, 3 with thyroid disease, 2 with gynecological disease, 2 with hyperuricemia, 2 with Meniere disease, 2 with prostate disease, 1 with ulcerative colitis, 1 with pancreatitis, 1 with asthma, 1 with arrhythmia, 1 for epilepsy, 1 for neurosis, 1 with liver cirrhosis, 1 for ulticaria, and 1 after cerebral infarction. Smoking status at diagnosis for cases or at interview for controls was classified into three categories; current smokers including ex-smokers within one year after the cessation, never smokers including individuals who smoked less than 100 cigarettes in their lifetime, and former smokers for the rest who quitted smoking. The Institutional Review Board of Aichi Cancer Center approved this study before the study started.
Genotyping procedure
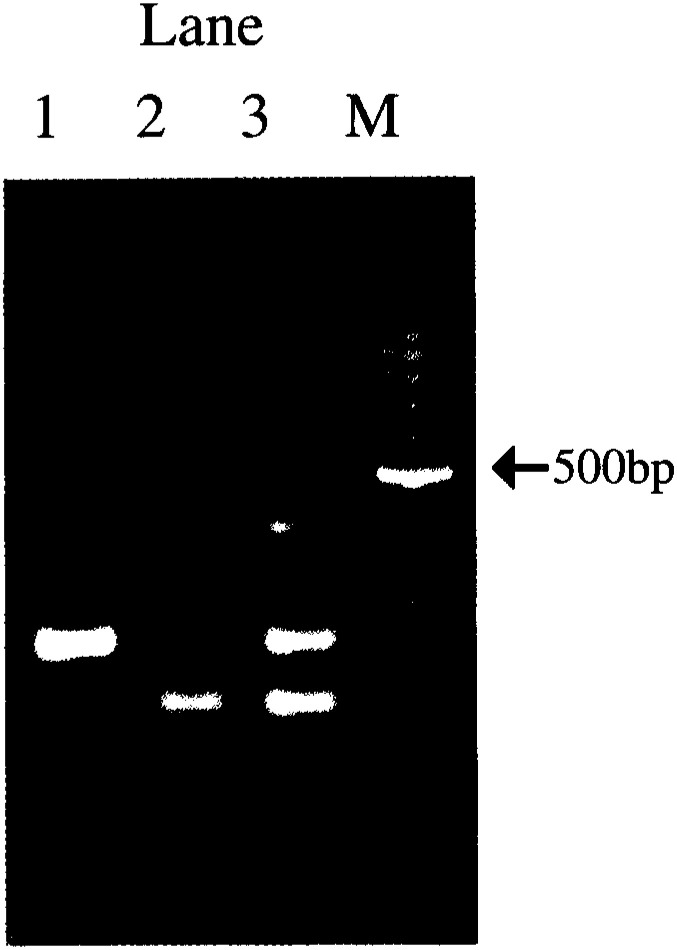
DNA was extracted from 200 μl buffy coat reserved -40°C by a QIAamp Blood Mini Kit (Qiagen, Valencia, CA) and OGG1 Ser326Cys (78435 C to G substitution) polymorphism was genotyped by a PCR-CTPP (polymerase chain reaction with confronting two-pair primers) method developed independently in our laboratory29), which is a similar method by Liu et al30). Each 25 μl reaction tube contained 30-100ng DNA, 0.18mM dNTPs, 12.5pmol each primer, 0.5 U AmpliTaq Gold (Perkin-Elmer, Foster City, CA) and 2.5 μl of 10 x PCR buffer including 15mM MgCl2. The four primers were F1, 5′-CAG CCC AGA CCC AGT GGA CTC-3′; R1, 5′-TGG CTC CTG AGC ATG GCG GG-3′; F2, 5′-CAG TGC CGA CCT GCG CCA ATG-3′; and R2, 5′-GGT AGT CAC AGG GAG GCC CC. Primer pair F1 & R1 for the C allele (Ser326) and F2 & R2 for the G allele (326Cys) produced allele-specific bands of 252bp and 194bp, respectively, as well as a common 406bp band between F1 and R2. The PCR was conducted as follows; 10 min of initial denaturation at 95°C, followed by 30 cycles of 1 min at 95°C, 1 min at 64°C and 1 min at 72°C, then 5 min extension at 72°C. Figure 1 shows the representative result of genotyping by the PCR-CTPP method.
Figure 1. Representative results for the OGG1 Ser326Cys polymorphism by PCR-CTPP method. DNA fragments stained with ethidium bromide are shown. Lane 1 for Ser/Ser, lane 2 for Ser/Cys, lane 3 for Cys/Cys, and lane M for a 100-bp DNA ladder.
Statistical Analysis
All statistical analyses were performed using STATA v.7.0 software (STATA, College Station, TX). Accordance with Hardy-Weinberg equilibrium, which indicates an absence of discrepancies between genotype and allele frequencies, was examined for controls with a χ2 test. Crude and sex-age adjusted odds ratios (ORs) and 95% confidence intervals (CIs) were calculated by an unconditional logistic regression model. Gene-environment interactions between OGG1 polymorphism and smoking habit was also estimated by the logistic model, which included an interaction term as well as variables for exposure (smoking), genotype (OGG1) and potential confounders (age and sex).
RESULTS
The characteristics among the cases and controls are shown in Table 1. The mean age was 60.7 years for cases and 56.8 years for controls. The median interval from diagnosis to study enrollment was 3.2 years, and the cases diagnosed in the past 2 years were 71 (51.4%). There was no difference in the percentage of smokers between cases (23.2%) and controls (22.8%), but the cases tended to be heavier smokers than controls, as shown in Table 1.
Table 1. Characteristics of cases and controls.
| Characteristics | Cases (%) | Controls (%) | |
| (n=138) | (n=241) | ||
| Sex | Male | 68 (49.3) | 118 (49.0) |
| Female | 70 (50.7) | 123 (51.0) | |
| Age at diagnosis/interview | <= 50 | 15 (10.9) | 46 (19.1) |
| 51-60 | 43 (31.2) | 90 (37.3) | |
| 61-70 | 54 (39.1) | 105 (43.6) | |
| >= 71 | 26 (18.8) | 0 ( 0.0) | |
| Mean age ± SD | 60.7 ± 9.4 | 56.8 ±7.9 | |
| The intervals from diagnosis | < 3 years | 71 (51.4) | |
| >= 3 years | 67 (48.6) | ||
| Smoking status | Never smokers | 78 (56.5) | 140 (58.1) |
| Former smokers | 28 (20.3) | 46 (19.1) | |
| Current smokers | 32 (23.2) | 55 (22.8) | |
| Cigarette-years< 800 | 7 ( 8.8) | 26 (11.6) | |
| Cigarette-years>= 800 | 25 (18.1) | 27 (11.2) | |
| Unknown | 0 ( 0.0) | 2 ( 0.8) | |
The allele frequencies of OGG1 Ser326 for controls and cases were 0.55 and 0.53, respectively. The genotype was 28.3% for Ser/Ser, 49.2% for Ser/Cys and 22.5% for Cys/Cys genotype among controls (Table 2). The distribution of genotype among controls was in accordance with Hardy-Weinberg equilibrium (χ2=0.043, p=0.837). The distribution among cases did not differ from that among controls: 29.0% for Ser/Ser and 51.4% for Ser/Cys and 19.6% for Cys/Cys. There was a significant difference in the distribution of genotypes between the cases enrolled less than 3 years after diagnosis (recent cases) and those enrolled 3 years or longer after diagnosis (longer survivors): 19.7% for Ser/Ser, 56.3% for Ser/Cys, and 24.0% for Cys/Cys among the recent cases; 38.8%, 46.3%, and 14.9% among the longer survivors, respectively (Table 2).
Table 2. Distribution of genotype among cases and controls.
| Genotype | No. of cases (%) | No. of controls (%) † | ||
|
| ||||
| Total | The interval from diagnosis | |||
|
| ||||
| < 3 years* | >= 3 years* | |||
| Ser/Ser | 40 (29.0) | 14 (19.7) | 26 (38.8) | 68 (28.3) |
| Ser/Cys | 71 (51.4) | 40 (56.3) | 31 (46.3) | 118 (49.2) |
| Cys/Cys | 27 (19.6) | 17 (24.0) | 10 (14.9) | 54 (22.5) |
* Genotype distribution was significantly different; χ2 (2)= 6.45 and p=0.040.
† One subject could not genotyped.
As shown in Table 3, the crude and adjusted ORs for Ser/Cys or Cys/Cys genotypes compared with Ser/Ser were not significant. We also analyzed the ORs by the interval from diagnosis, because there was a significant difference in OGG1 genotype distribution between the recent cases and the longer survivors. The adjusted ORs compared with Ser/Ser genotype were 1.86 (95% CI, 0.83-3.24) for Ser/Cys and 1.53 (0.69-3.38) for Cys/Cys in the recent cases, and 0.69 (0.38-1.25) and 0.48 (0.22-1.09) in the longer survivors, respectively. The adjusted ORs for Ser/Cys and Cys/Cys genotypes combined were 1.72 (0.88-3.39) in the recent cases and 0.64 (0.36-1.14) in the longer survivors. The OR of the genotype among current smokers did not differ markedly from that among never smokers, though former smokers had a lower OR (Table 4).
Table 3. The overall ORs and that according to the interval from diagnosis.
| Genotype | Crude | Adjusted* | |||
|
|
|
||||
| OR | 95%CI | OR | 95%CI | ||
| Total | Ser/Ser | 1.00 | 1.00 | ||
| Ser/Cys | 1.02 | 0.63-1.67 | 1.06 | 0.64-1.76 | |
| Cys/Cys | 0.85 | 0.46-1.56 | 0.81 | 0.44-1.52 | |
| The interval from diagnosis | |||||
| < 3 years | Ser/Ser | 1.00 | 1.00 | ||
| Ser/Cys | 1.64 | 0.83-3.24 | 1.86 | 0.91-3.77 | |
| Cys/Cys | 1.53 | 0.69-3.38 | 1.46 | 0.64-3.35 | |
| >= 3 years | Ser/Ser | 1.00 | 1.00 | ||
| Ser/Cys | 0.69 | 0.38-1.25 | 0.71 | 0.39-1.31 | |
| Cys/Cys | 0.48 | 0.22-1.09 | 0.48 | 0.54-1.64 | |
*Adjusted for age and sex.
Table 4. Adjusted ORs according to smoking habit.
| Subjects | Genotype | No. of cases/controls | aOR* | 95%CI |
| Total | ||||
| Never smokers | Ser/Ser | 23/45 | 1.00 | |
| Ser/Cys | 41/65 | 1.36 | 0.71-2.62 | |
| Cys/Cys | 14/30 | 1.00 | 0.46-2.36 | |
| Former smokers | Ser/Ser | 8/11 | 1.00 | |
| Ser/Cys | 12/20 | 0.56 | 0.15-2.06 | |
| Cys/Cys | 8/14 | 0.35 | 0.08-1.60 | |
| Current smokers | Ser/Ser | 9/12 | 1.00 | |
| Ser/Cys | 18/33 | 0.76 | 0.25-1.63 | |
| Cys/Cys | 5/10 | 0.87 | 0.27-2.76 |
*Adjusted for age and sex.
The age-sex-adjusted OR relative to never smokers was slightly high for former smokers and current smokers 1.18 (0.59-2.34) and 1.29 (0.67-2.49), respectively (Table 5). The ORs for smoking status according to the OGG1 genotype show that the OR for current smokers was slightly high among individuals with Cys/Cys genotype (2.47, 0.50-12.24), though the interaction was not statistically significant.
Table 5. The ORs of smoking habit according to OGG1 Ser326Cys genotype.
| No. of cases/controls |
Adjusted for age and sex | ||
|
| |||
| OR | 95%CI | ||
| Total | |||
| Never smokers | 78/140 | 1.00 | |
| Former smokers | 28/46 | 1.18 | 0.59-2.34 |
| Current smokers | 32/55 | 1.29 | 0.67-2.49 |
| Ser/Ser | |||
| Never smokers | 23/45 | 1.00 | |
| Former smokers | 8/11 | 2.07 | 0.40-10.6 |
| Current smokers | 9/12 | 1.89 | 0.47-7.68 |
| Ser/Cys | |||
| Never smokers | 41/65 | 1.00 | |
| Former smokers | 12/20 | 0.84 | 0.31-2.26 |
| Current smokers | 18/33 | 0.92 | 0.38-2.25 |
| Cys/Cys | |||
| Never smokers | 14/30 | 1.00 | |
| Former smokers | 8/14 | 1.54 | 0.42-5.76 |
| Current smokers | 5/10 | 2.47 | 0.50-12.24 |
*Adjusted for age and sex.
DISCUSSION
This was the first study that OGG1 Ser326Cys polymorphism was genotyped by the PCR-CTPP method29), whose genotyping was quite clear as demonstrated in Figure 1. The method is easy, timesaving and inexpensive compared with PCR-SSCP. The genotype distribution was in accordance with Hardy-Weinberg law of equilibrium among controls (p=0.873), which partly supported appropriate control selection and correct genotyping. The genotype distribution (28.3% for Ser/Ser, 49.2% for Ser/Cys, and 22.5% for Cys/Cys) was similar to those previously reported by Sugimura et al. in Tokyo (27.7%, 57.4%, and 14.9%, respectively, n=94, p for Hardy-Weinberg equilibrium=0.103) and Okinawa populations (32.0%, 54.3%, and 13.7%, respectively, n=197, p for Hardy-Weinberg equilibrium=0.082)22), though the proportion for Cys/Cys was relatively high. The distribution of the Ser326Cys polymorphism was quite different among ethnic groups. The frequency of Cys326 allele in Japanese and Chinese populations was prevalent compared with other ethnic groups; Cys/Cys genotype was reported 3.6% for Australian Caucasian (n=138), 2.7% for Hungarian (n=149)22) and 1.9% for Caucasian in Germany (n=105)24).
In our study, compared with Ser/Ser genotype, the Ser/Cys and Cys/Cys genotypes were not significantly associated with the risk of adenocarcinoma of the lung. Because this was a prevalent case-control study, the estimated ORs indicated not only with disease occurrence but also with disease prognosis. In prevalent case-control studies, longer survivors are more likely to be sampled as cases31). Accordingly, the factors related to shorter survival are less frequent among prevalent cases than among incident cases, which include those with poor prognosis as well as long survivors. It produces a lower OR for the prevalent cases than that estimated for the incidence cases. We divided the case subjects into 2 groups (less than 3 years after diagnosis versus 3 years or longer) and estimated the OR according to the interval from diagnosis. Although the difference was not statistically significant, the ORs of Ser/Cys and Cys/Cys genotypes compared with Ser/Ser genotype were larger in the recent cases than in the longer survivors. This finding implies that OGG1 may influence prognosis of lung adenocarcinoma. To date, there have been no studies on the association between this polymorphism and cancer prognosis. The suspected prognostic effect should be examined prospectively in survival analysis adjusting for other prognostic factors.
In this study population, 70 (50.7%) of 138 patients with adenocarcinoma were women, and this proportion was similar to that for the whole patients with lung adenocarcinoma diagnosed at Aichi Cancer Center Hospital (46.8% of 828 patients between 1984 and 2000). The corresponding proportion was higher at Aichi Cancer Center than that reported in Japan32). Therefore, the high proportion of women with adenocarcinoma in this study was reflected in the sex distribution at Aichi Cancer Center Hospital.
In the study by Sugimura et al.22), the significant association between the Cys/Cys genotype of OGG1 and lung cancer risk was observed only for squamous cell carcinoma (n=l 18), and a slightly elevated insignificant OR for adenocarcinoma of the lung (n=78) was also documented. Confirming the findings for the adenocarcinoma was required. Our finding for the recent cases was very similar in the size of OR to their results. Our study was also too short to confirm the association, but added information to the finding observed by the previous study. Although different histological types of lung adenocarcinoma may be associated differently with this polymorphism, neither this study nor previous studies were large enough to examine the possible heterogeneous association.
Biological evidence supporting the association with lung carcinoma risk is still limited. Previous reports showed that loss of hetrozygosity (LOH) of 3p, which includes OGG1 gene, was very common in lung cancer tissues21,24,25). Although mean 8-hydroxyguanine levels in lung tissue was higher with LOH than without LOH, the genotype of OGG1 Ser326Cys was not associated with the level, but a polymorphism of glutathione peroxidase I gene also located in 3p was correlated33). Recently an association between lung adenocarcinoma risk and new OGG1 polymorphisms at exon 1 has been reported for Japanese20). Further biological studies on these OGG1 polymorphisms, especially in relation to environmental exposures, will be required to elucidate the biological roles in the carcinogenesis.
Currently, adenocarcinoma of the lung is considered to be associated with smoking, though the relative risk is lower than of squamous cell and small cell carcinoma34-36). Since high levels of 8-hydroxyguanine have been detected in smokers’ lung tissue and leukocytes37), the interaction with the OGG1 genotype was to be examined. In our study, the OR of the genotype was not different between never smokers and current smokers, and the difference in the OR of smoking was not large among the genotypes; 1.89 for Ser/Ser, 0.92 for Ser/Cys, and 2.47 for Cys/Cys. This suggests that OGG1 Ser326Cys polymorphism-smoking interaction was not marked in adenocarcinoma of the lung enough to be detected in our study. In the past studies, the possible interaction with smoking was reported for CYP1A1 and GSTM138,39). Our previous study found the interaction between L-myc L/S polymorphism and smoking for esophageal cancer40).
In conclusion, this study suggested that the association between OGG1 Ser326Cys polymorphism and the risk of lung adenocarcinoma was limited, if any, but that the Cys/Cys genotype might be associated with the poor prognosis. In this study, no difference in the OR of smoking among the genotypes was observed. The sample size was not enough for detailed analysis, but this was the largest study on the association with lung adenocarcinoma so far.
ACKNOWLEDGMENTS
The authors are grateful to Ms. Mayumi Kato for polymorphism genotyping. This work was supported in part by Grant-in-Aid from the Ministry of Education, Science, Sports, Culture, and Technology of Japan.
REFERENCES
- 1.Fearon ER. Human cancer syndromes: clues to the origin and nature of cancer. Science, 1997; 278: 1043-1050. [DOI] [PubMed] [Google Scholar]
- 2.Mamett LJ. Oxyradicals and DNA damage. Carcinogenesis, 2000; 21: 361-370. [DOI] [PubMed] [Google Scholar]
- 3.Laval J, Jurado J, Saprabaev M, Sidorkina O. Antimutagenic role of base-excision repair enzymes upon free radical-induced DNA damage. Mutat Res, 1998; 402: 93-102. [DOI] [PubMed] [Google Scholar]
- 4.Bohr VA, Dianov GL. Oxidative DNA damage processing in nuclear and mitochondrial DNA. Biochimie, 1999; 81: 155-160. [DOI] [PubMed] [Google Scholar]
- 5.Shibutani S, Takeshita M, Grollman AP. Insertion of specific bases during DNA synthesis past the oxidation-damaged base 8-oxodG. Nature, 1991; 349: 431-434. [DOI] [PubMed] [Google Scholar]
- 6.Cheng KC, Cahill DS, Kasai H, Nishimura S, Loeb LA. 8-Hydroxyguanine, an abundant form of oxidative DNA damage, causes G-T and A-C substitutions. J Biol Chem, 1992; 267: 166-172. [PubMed] [Google Scholar]
- 7.Hussain SP, Harris CC. Molecular epidemiology of human cancer: contribution of mutation spectra studies of tumor suppressor genes. Cancer Res, 1998; 58: 4023-4037. [PubMed] [Google Scholar]
- 8.Hollstein M, Sidransky D, Vogelstein B, Harris CC. p53 mutations in human cancers. Science, 1991; 253: 49-53. [DOI] [PubMed] [Google Scholar]
- 9.Jaruga P, Zastawny TH, Skokowski J, Dizdaroglu M, Olinski R. Oxidative DNA base damage and antioxidant enzyme activities in human lung cancer. FEBS Lett, 1994; 341: 59-64. [DOI] [PubMed] [Google Scholar]
- 10.Kondo S, Toyokuni S, Iwasa Y, et al. Persistent oxidative stress in human colorectal carcinoma, but not on adenoma. Free Radic Biol Med, 1999; 27: 401-410. [DOI] [PubMed] [Google Scholar]
- 11.Musarrat J, Arezina-Wilson J, Wani AA. Prognostic and aetiological relevance of 8-hydroxyguanosine in human breast carcinogenesis. Eur J Cancer, 1996; 32A: 1209-1214. [DOI] [PubMed] [Google Scholar]
- 12.van der Kemp PA, Thomas D, Barbey R, de Oliveira R, Boiteux S. Cloning and expression in Escherichia coli of the OGG1 gene of Saccharomyces cerevisiae, which codes for a DNA glycosylase that excises 7,8-dihydro-8-oxoguanine and 2,6-diamino-4-hydroxy-5-N-methylformamidopyrimidine. Proc Natl Acad Sci U S A, 1996; 93: 5197-5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nash HM, Bruner SD, Scharer OD, et al. Cloning of a yeast 8-oxoguanine DNA glycosylase reveals the existence of a base-excision DNA-repair protein superfamily. Curr Biol, 1996; 6: 968-980. [DOI] [PubMed] [Google Scholar]
- 14.Aburatani H, Hippo Y, Ishida T, et al. Cloning and characterization of mammalian 8-hydroxyguanine-specific DNA glycosylase/apurinic, apyrimidinic lyase, a functional mutM homologue. Cancer Res, 1997; 57: 2151-2156. [PubMed] [Google Scholar]
- 15.Arai K, Morishita K, Shinmura K, et al. Cloning of a human homolog of the yeast OGG1 gene that is involved in the repair of oxidative DNA damage. Oncogene, 1997; 14: 2857-2861. [DOI] [PubMed] [Google Scholar]
- 16.Sakumi K, Furuichi M, Tsuzuki T, et al. Cloning and expression of cDNA for a human enzyme that hydrolyzes 8-oxo-dGTP, a mutagenic substrate for DNA synthesis. J Biol Chem, 1993; 268: 23524-23530. [PubMed] [Google Scholar]
- 17.Dianov G, Bischoff C, Piotrowski J, Bohr VA. Repair pathways for processing of 8-oxoguanine in DNA by mammalian cell extracts. J Biol Chem, 1998; 273: 33811-33816. [DOI] [PubMed] [Google Scholar]
- 18.Ishida T, Takashima R, Fukayama M, et al. New DNA polymorphisms of human MMH/OGG1 gene: prevalence of one polymorphism among lung-adenocarcinoma patients in Japanese. Int J Cancer, 1999; 80: 18-21. [DOI] [PubMed] [Google Scholar]
- 19.Kohno T, Shimura K, Tosaka M, et al. Genetic polymorphisms and alternative splicing of the hOGG1 gene, that is involved in the repair of 8-hydroxyguanine in damaged DNA. Oncogene, 1998; 16: 3219-3225. [DOI] [PubMed] [Google Scholar]
- 20.Sugimura H, Kohno T, Wakai K, et al. hOGG1 Ser326Cys polymorphism and lung cancer susceptibility. Cancer Epidemiol Biomarkers Prev, 1999; 8: 669-674. [PubMed] [Google Scholar]
- 21.Xing DY, Tan W, Song N, Lin DX. Ser326Cys polymorphism in hOGG1 gene and risk of esophageal cancer in a Chinese population. Int J Cancer, 2001; 95: 140-143. [DOI] [PubMed] [Google Scholar]
- 22.Wikman H, Risch A, Klimel F, et al. hOGG1 polymorphism and loss of heterozygosity (LOH): significance for lung cancer susceptibility in a Caucasian population. Int J Cancer, 2000; 88: 932-937. [DOI] [PubMed] [Google Scholar]
- 23.Hardie LJ, Briggs JA, Davidson LA, et al. The effect of hOGG1 and glutathione peroxidase I genotypes and 3p chromosomal loss on 8-hidroxydeoxyguanosine levels in lung cancer. Carcinogenesis, 2000; 21: 167-172. [DOI] [PubMed] [Google Scholar]
- 24.Dherin C, Radicella JP, Dizdaroglu M, Boiteux S. Excision of oxidatively damaged DNA bases by the human alpha-hOgg1 protein and the polymorphic alpha-hOgg1 (Ser326Cys) protein which is frequently found in human populations. Nucleic Acids Res, 1999; 27: 4001-4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Janssen K, Schlink K, Gotte W, et al. DNA repair activity of 8-oxoguanine DNA glycosylase 1 (OGG1) in human lymphocytes is not dependent on genetic polymorphism Ser326/Cys326. Mutat Res, 2001; 486: 207-216. [DOI] [PubMed] [Google Scholar]
- 26.Statistics and Information Department, Ministry of Health and Welfare. Vital statistics of Japan 1998, Vol 3, 2000. Health and Welfare Statistics Association, Tokyo, (in Japanese)
- 27.Sobue T, Ajiki W, Tsukuma H, et al. Trends of lung cancer incidence by histologic type: a population-based study in Osaka, Japan. Jpn J Cancer Res, 1999; 90: 6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamajima N, Matsuo K, Saito T, et al. Interleukin 1 polymorphisms, lifestyle factors, and Helicobacter pylori infection. Jpn J Cancer Res, 2001; 92: 383-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamajima N, Saito H, Matsuo K, Kozaki K, Takahashi T, Tajima K. Polymerase chain reaction with confronting two-pair primers for polymorphism genotyping. Jpn J Cancer Res., 2000; 91: 865-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Q, Thorland EC, Heit JA, Sommer SS. Overlapping PCR for bidirectional PCR amplification of specific alleles: a rapid one-tube method for simultaneously differentiating homozygotes and heterozygotes. Genome Res, 1997; 7: 389-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamajima N, Matsuo K, Yuasa H. Adjustment of prognostic effects in prevalent case-control studies on genotype. J Epidemiol, 2001; 11: 204-210, Corrections, 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sekine I, Nagai K, Tsugane S, et al. Association between smoking and tumor progression in Japanese women with adenocarcinoma of the lung. Jpn J Cancer Res, 1999; 90: 129-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hibi K, Takahashi T, Yamakawa K, et al. Three distinct regions involved in 3p deletion in human lung cancer. Oncogene, 1992; 7: 445-449. [PubMed] [Google Scholar]
- 34.Asami S, Hirano T, Yamaguchi R, et al. Increase of a type of oxidative DNA damage, 8-hydroxyguanine, and its repair activity in human leukocytes by cigarette smoking. Cancer Res, 1996; 56: 2546-2549. [PubMed] [Google Scholar]
- 35.Barbone F, Bovenzi M, Cavalleri F, Stanta G. Cigarette smoking and histologic type of lung cancer in men. Chest, 1997; 112: 1474-1479. [DOI] [PubMed] [Google Scholar]
- 36.Kabet GC. Aspects of the epidemiology of the lung cancer in smokers and nonsmokers in the United States. Lung Cancer, 1996; 15: 1-20. [DOI] [PubMed] [Google Scholar]
- 37.Wei Q, Cheng L, Hong WK, Spitz MR. Reduced DNA repair capacity in lung cancer patients. Cancer Res, 1996; 56: 4103-4107. [PubMed] [Google Scholar]
- 38.Nakachi K, Hayashi S, Kawajiri K, Imai K. Association of cigarette smoking and CYP1A1 polymorphisms with adenocarcinoma of the lung by grades of differentiation. Carcinogenesis, 1995; 16: 2209-2213 [DOI] [PubMed] [Google Scholar]
- 39.Kawajiri K, Eguchi H, Nakachi K, Sekiya T, Yamamoto M. Association of CYP1A1 germ line polymorphisms with mutations of the p53 gene in lung cancer. Cancer Res, 1996; 56: 72-76 [PubMed] [Google Scholar]
- 40.Kumimoto H, Hamajima N, Nishizawa, et al. Different susceptibility of each L-myc genotype to esophageal cancer risk factors. Jpn J Cancer Res, 2001; 92: 735-739. [DOI] [PMC free article] [PubMed] [Google Scholar]