Abstract
Background and Aims
Previous evidence has mainly supported transient changes in cardiac function during interictal or peri-ictal phases in people with epilepsy, but the long-term risk of cardiac arrhythmias is poorly described. This study aimed to assess the long-term association of epilepsy with cardiac arrhythmias, considering the potential role of genetic predisposition and antiseizure medications (ASMs) in any associations observed.
Methods
This population-based study evaluated UK Biobank data for individuals recruited between 2006 and 2010. Cox proportional hazards models and competing risk models were used to examine the association of epilepsy history with the long-term incidence risk of cardiac arrhythmias and arrhythmias subtypes. Polygenic risk scores (PRS) were calculated to investigate the effect of genetic susceptibility. The role of ASMs was also evaluated by integrating observational and drug target Mendelian randomization (MR) evidence.
Results
The study included 329 432 individuals, including 2699 people with epilepsy. Compared with those without epilepsy, people with epilepsy experienced an increased risk of all cardiac arrhythmias [hazard ratio (HR) 1.36, 95% confidence interval (CI) 1.21–1.53], atrial fibrillation (HR 1.26, 95% CI 1.08–1.46), and other cardiac arrhythmias (HR 1.56, 95% CI 1.34–1.81). The associations were not modified by genetic predisposition as indicated by PRS. Competing and sensitivity analyses corroborated these results. Individuals with epilepsy using ASMs, especially carbamazepine and valproic acid, were at a higher risk for cardiac arrhythmias. This observation was further supported by drug target MR results (PSMR < .05 and PHEIDI > .05).
Conclusion
This study revealed the higher risk of cardiac arrhythmias persists long term in people with epilepsy, especially among those using carbamazepine and valproic acid. These findings highlight the need for regular heart rhythm monitoring and management in people with epilepsy in order to reduce the risk of further cardiovascular complications.
Keywords: Epilepsy, Cardiac arrhythmias, Carbamazepine, Valproic acid, Atrial fibrillation
Structured Graphical Abstract
Structured Graphical Abstract.
Through observational data of UK Biobank and genetic summary data, this study implies that epilepsy and ASMs were associated with significant long-term cardiac arrhythmias risks, independent of genetic predisposition. ASM, antiseizure medication; HR, hazard ratio; CI, confidence interval; PRS, polygenic risk score.
See the editorial comment for this article ‘Long-term risk for atrial and ventricular arrhythmias: a cardinal manifestation of ‘the epileptic heart'’, by R.L. Verrier et al., https://doi.org/10.1093/eurheartj/ehad524.
Introduction
Epilepsy is one of the most common serious brain diseases, affecting 70 million individuals worldwide.1–3 Epidemiological studies consistently indicate that people with epilepsy have a higher prevalence of cardiac comorbidities than the general population.4,5 Over the past decades, the fundamental pathophysiology of the heart–brain axis has been elucidated, expanding our understanding of the subtle effects of epilepsy on the cardiovascular system. Specifically, epilepsy can lead to autonomic alterations and culminate in cardioinhibition through sympathetic and parasympathetic outflow pathways and the release of catecholamines by adrenal glands.1,6,7 In addition to autonomic alterations, repeated hypoxaemia and myocardial ischaemia during or after seizures can lead to electrical and mechanical dysfunction.8–10 Collectively, these mechanisms suggest that epilepsy may lead to deterioration in cardiac function and increased susceptibility to cardiac dysfunction.11,12
The concept of ‘epileptic heart’, defined as ‘chronic epilepsy-induced cardiac and coronary vascular damage resulting from repeated increases in catecholamines and hypoxaemia leading to electrical and mechanical dysfunction’,9,10 primarily stems from case reports or epidemiological studies on transient effects of epilepsy, such as myocardial infarction and sudden death in epilepsy. However, the long-term risk of cardiac arrhythmias among people with epilepsy, which may result in heart failure, acute coronary syndrome, and cardiovascular mortality,13,14 should not be disregarded.15 Besides, variants in arrhythmogenic genes may interact with epilepsy to significantly increase sudden unexpected death risk,16,17 and several antiseizure medications (ASMs) can trigger cardiac abnormalities by blocking sodium channel or leading to metabolic abnormalities.5,18 A deeper understanding of the relationship between epilepsy and the long-term risk of cardiac arrhythmias, as well as the potential role of relevant factors such as genetic susceptibility and ASMs, can help improve cardiac care for people with epilepsy.
In this study, we utilized the UK Biobank (UKB) database of detailed participant information including records of epilepsy, cardiac arrhythmias, ASM use, and genotype data. We first performed Cox proportional hazards regression and competing analyses to estimate the association between epilepsy and the incidence of cardiac arrhythmias, followed by a range of sensitivity analyses to test the robustness of the results. Then, we applied polygenic risk scores (PRS) to explore the relevance of genetic susceptibility in the epilepsy–arrhythmia association. Finally, we further explored the relationship between ASM use and arrhythmia risk through both conventional observational and drug target Mendelian randomization (MR) analyses.
Methods
Participants
This prospective cohort study was conducted using data from the UKB, which recruited more than 500 000 participants aged 40–69 years from 2006 to 2010 and collected a wide range of phenotype and genotype data. A detailed description of the UKB data has been provided elsewhere.19 All participants provided written informed consent, and the North West Multi-centre Ethics Committee granted ethical approval to UKB.
The study design is shown in Supplementary data online, Figure S1. In the present study, the analysis was restricted to a subset of participants with complete data available at baseline (enrolment date). Participants with the following criteria were excluded: (i) participants with the following cardiac diseases before baseline, including rheumatic heart disease, ischaemic heart disease, pericardial disease, endocarditis, valvular heart disease, myocarditis, cardiomyopathy, arrhythmias, and heart failure (identified by ICD-10 codes linked to the diagnosis; Supplementary data online, Table S1); (ii) participants with missing data on the covariates; (iii) participants with a diagnosis of epilepsy or self-reported epilepsy during follow-up; and (iv) participants with the use of ASMs but without diagnosed or self-reported epilepsy.
Participants were classified as people with epilepsy if they met either of the following criteria: (i) participants with a diagnosis of epilepsy or (ii) participants who self-reported having epilepsy.
Outcome definition
The outcomes included all cardiac arrhythmias, atrial fibrillation (AF), other cardiac arrhythmias (refer to cardiac arrhythmias except for AF), bradyarrhythmias, and ventricular arrhythmias (identified by ICD-10 codes linked to the diagnosis; Supplementary data online, Table S1), followed up to 30 September 2021. The endpoint for each participant was censored at the date of outcome diagnosis, the date of death, or 30 September 2021, whichever occurred first.
Use of antiseizure medications
Antiseizure medication records in UKB were classified into seven classes according to the Anatomical Therapeutic Chemical (ATC) classification, with the code N03. The seven main classes included (i) barbiturates and derivatives (methylphenobarbital, phenobarbital, and primidone); (ii) hydantoin derivatives (phenytoin); (iii) succinimide derivatives (ethosuximide); (iv) benzodiazepine derivatives (clonazepam); (v) carboxamide derivatives (carbamazepine and oxcarbazepine); (vi) fatty acid derivatives (valproic acid, vigabatrin, and tiagabine); and (vii) other antiepileptics (lamotrigine, topiramate, gabapentin, levetiracetam, pregabalin, fenfluramine, and beclamide). Considering the reported association of sodium channel blocker use with increased cardiac arrhythmia risk, we added a category by combining phenytoin, carbamazepine, oxcarbazepine, valproic acid, lamotrigine, and topiramate into sodium channel blockers.20
Assessment of covariates
Through the touchscreen questionnaire and verbal interview at baseline, participants provided personal information for demographic and lifestyle behaviours, including age, sex, ethnicity, current smoking status, alcohol drinking status, income level, physical activity, and comorbidities. Body mass index (BMI) was calculated by weight in kilograms (BC-418MA body composition analyzer) divided by the square of height in meters (Seca 202 stadiometer). The income level was divided into five categories according to the total household income before tax (level 1, <₤18 000; level 2, ₤18 000–£30 999; level 3, ₤31 000–£51 999; level 4, ₤52 000–£100 000; and level 5, >₤100 000). Physical activity was assessed using a validated International Physical Activity Questionnaire (IPAQ).21 Based on these questions, metabolic equivalent-minutes per week (MET-min/week) were calculated and divided into three categories (low, medium, and high) to reflect the total physical activity volume. The comorbidities were identified according to ICD-10 at baseline: hypertension, stroke, dementia, head injury, obstructive sleep apnoea syndrome (OSA), hyperthyroidism, and diabetes (detailed ICD-10 codes are presented in Supplementary data online, Table S1).
Additional and sensitivity analyses
Several additional and sensitivity analyses were performed to test the robustness of our results. (i) Concerning the presence of prior diagnoses of comorbidities that might affect cardiac arrhythmia, we conducted secondary analyses by excluding participants with some relevant comorbidities, such as hypertension, stroke, head injury, OSA, hyperthyroidism, and diabetes. (ii) To account for the long-term relation between epilepsy and cardiac arrhythmias, we conducted the analysis after excluding participants with <2 or <5 years of follow-up. (iii) To minimize the misclassification bias by self-reporting, we restricted the definition of people with epilepsy by considering diagnosis records only. (iv) To minimize the bias from potential confounders, we performed the propensity score matching (PSM) to generate a matched cohort and replicated the main analysis. The details of PSM are shown in Supplementary data online, Methods. (v) To avoid the selection bias from missing data, we employed multiple imputation for participants with missing data,22 with 10 imputations drawn. The estimates of the results were then averaged across the 10 imputed data sets according to Rubin's rules.23
Statistical analyses
Characteristics of participants were presented as median (interquartile range, IQR) for continuous variables and absolute (percentage) for categorical variables. Mann–Whitney U test was used to compare differences between groups for continuous variables and the χ2 test for categorical variables.
The cumulative incidence of cardiac arrhythmia was calculated using Kaplan–Meier method, with the log-rank test used to determine the difference between people with epilepsy and without epilepsy. Cox proportional hazards regression models were applied to evaluate the associations between epilepsy and the study outcomes. Three models were used in the analysis: Model 1 was adjusted for age, sex, BMI, and ethnicity; Model 2 was further adjusted for alcohol drinking status, current smoking status, physical activity, and income level; Model 3 (the fully adjusted model) was further adjusted for hypertension, stroke, dementia, head injury, OSA, hyperthyroidism, and diabetes. In these cases, death may hinder the observation of cardiac arrhythmia. Therefore, the Fine and Grey competing risk models were used to extend the proportional hazards model, with death caused by all other conditions as the competing risk. Additionally, the above analyses were also performed in the subgroup stratified by age (<60 years and ≥60 years), sex, ethnicity, BMI (<25 kg/m2 and ≥25 kg/m2), current smoking status, alcohol drinking status, physical activity, and income levels to identify potential effect modifications.
To evaluate the role of genetic susceptibility in the association between epilepsy and long-term risk of arrhythmias, we calculated the PRS for cardiac arrhythmias and explored the role in the epilepsy–arrhythmia relationship. Details of PRS calculation and analysis are provided in Supplementary data online, Methods.
To assess the potential role of ASMs on cardiac arrhythmias, we performed analysis using Cox proportional hazards regression models, with people without epilepsy as the reference group. In the analysis for each class of medication, people with epilepsy were divided into two groups: those without the specific ASM class under study and those using that ASM class. To provide further support for the association identified in observational analyses, summary data–based Mendelian randomization (SMR) analysis was performed to explore the effect of ASMs on cardiac arrhythmias risk. Details of drug target MR analysis are shown in Supplementary data online, Methods.
Summary data–based Mendelian randomization analysis was applied using the smr_linux software (version 1.3.1, https://yanglab.westlake.edu.cn/software/smr/), while all other statistical analyses were performed using Stata (version 16.0; StataCorp LLC, College Station, TX, USA). A two-sided P value <.05 was considered statistically significant.
Results
Participant baseline characteristics
A total of 329 432 participants were included in the study, consisting of 2699 people with epilepsy and 326 733 people without epilepsy at baseline (see Supplementary data online, Figure S2). As shown in Table 1, the median age at recruitment was 56 (IQR, 49–62) years, with 52.2% of the participants being female. The frequency of ASM use among the participants with epilepsy was 25.38% (n = 685) for carboxamide derivatives, 17.97% (n = 485) for fatty acid derivatives, and 17.23% (n = 465) for other antiepileptics. Additionally, 60.62% (n = 1636) of participants received sodium channel blockers (including phenytoin, carbamazepine, oxcarbazepine, valproic acid, lamotrigine, and topiramate).
Table 1.
Baseline characteristics of participants in the UK Biobank
| People with epilepsy | People without epilepsy | P value | ||
|---|---|---|---|---|
| n | 2699 | 326 733 | ||
| Age (years) | 56 (48–62) | 57 (49–62) | .007 | |
| Sex (%) | Female | 1316 (48.76) | 170 767 (52.26) | <.001 |
| Male | 1383 (51.24) | 155 966 (47.74) | ||
| Ethnicity (%) | Other | 75 (2.78) | 15 211 (4.66) | <.001 |
| White | 2644 (97.22) | 311 522 (95.34) | ||
| Body mass index (kg/m2) | 26.98 (24.21–30.26) | 26.54 (24.02–29.58) | <.001 | |
| Current smoking status (%) | Never | 2336 (86.55) | 293 677 (89.88) | <.001 |
| Usually | 292 (10.82) | 23 813 (7.29) | ||
| Occasionally | 71 (2.63) | 9243 (2.83) | ||
| Alcohol drinking status (%) | Never | 172 (6.37) | 11 400 (3.49) | <.001 |
| Previous | 241 (8.93) | 9912 (3.03) | ||
| Current | 2286 (84.70) | 305 421 (93.58) | ||
| Income level (%) | 1 | 879 (32.57) | 65 198 (19.95) | <.001 |
| 2 | 690 (25.57) | 80 676 (24.69) | ||
| 3 | 601 (22.27) | 88 158 (26.98) | ||
| 4 | 425 (15.75) | 72 683(22.25) | ||
| 5 | 104 (3.85) | 20 018 (6.13) | ||
| Physical activity (%) | Low | 610 (22.60) | 60 311(18.46) | <.001 |
| Medium | 1093 (40.50) | 134 205 (41.07) | ||
| High | 996 (36.90) | 132 217 (40.47) | ||
| Comorbidities | ||||
| Hypertension (yes, %) | 254 (9.41) | 16 143 (4.94) | <.001 | |
| Stroke (yes, %) | 120 (4.45) | 1481 (.45) | <.001 | |
| Dementia (yes, %) | 0 (0) | 28 (.01) | .631 | |
| Head injury (yes, %) | 155 (5.74) | 3044 (.93) | <.001 | |
| Obstructive sleep apnoea syndrome (yes, %) | 19 (.70) | 1325 (.45) | .015 | |
| Hyperthyroidism (yes, %) | 8 (.30) | 505 (.15) | .063 | |
| Diabetes (yes, %) | 79 (2.93) | 4400 (1.35) | <.001 | |
| ASMs | ||||
| All (yes, %) | 1790 (66.32) | |||
| Barbiturates and derivatives (yes, %) | 109 (4.04) | |||
| Hydantoin derivatives (yes, %) | 376 (13.93) | |||
| Succinimide derivatives (yes, %) | 8 (.30) | |||
| Benzodiazepine derivatives (yes, %) | 21 (.78) | |||
| Carboxamide derivatives (yes, %) | 685 (25.38) | |||
| Fatty acid derivatives (yes, %) | 485 (17.97) | |||
| Other antiepileptics (yes, %) | 465 (17.23) | |||
| Sodium channel blocker (yes, %) | 1636 (60.62) |
Continuous variables were presented as median (IQR); categorical variables were presented as absolute (percentage). ASMs, antiseizure medications.
Long-term incidence of cardiac arrhythmia is higher in people with epilepsy
The long-term incidence of cardiac arrhythmia in people with epilepsy was assessed compared with people without epilepsy. During the median follow-up period of 12.51 years (IQR 11.66–13.24), 297 (11.0%) participants with epilepsy and 25 687 (7.9%) participants without epilepsy developed cardiac arrhythmias. As shown in Figure 1, a higher cumulative incidence of all cardiac arrhythmias, AF, and other cardiac arrhythmias was observed in individuals with epilepsy than in those without (Plog-rank < .001 for all).
Figure 1.
Cumulative incidence of cardiac arrhythmias among groups with and without epilepsy. (A) all cardiac arrhythmias, (B) atrial fibrillation, and (C) other cardiac arrhythmias. Solid lines represent cumulative incidence; dotted lines show 95% confidence interval.
Epilepsy is associated with a higher risk of multiple arrhythmia subtypes
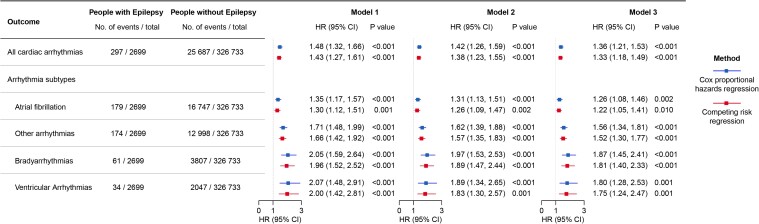
The results of Cox proportional hazard model analysis after full adjustment for covariates revealed a higher risk of all cardiac arrhythmia (HR 1.36, 95% CI 1.21–1.53) and subtypes of cardiac arrhythmia in people with epilepsy, including AF (HR 1.26, 95% CI 1.08–1.46), other types of arrhythmias (HR 1.56, 95% CI 1.34–1.81), bradyarrhythmias (HR 1.87, 95% CI 1.45–2.41), and ventricular arrhythmias (HR 1.80, 95% CI 1.28–2.53) (Figure 2). The observed associations persisted in the competing risk analysis (Figure 2). These findings indicate that epilepsy is associated with a higher long-term risk of cardiac arrhythmia as well as its subtypes.
Figure 2.
Association between epilepsy and the risk of incident cardiac arrhythmias in the UK Biobank. Model 1: adjusted for age, sex, BMI, and ethnicity; Model 2: adjusted for age, sex, BMI, ethnicity, alcohol drinking status, current smoking status, physical activity, and income level; Model 3: adjusted for age, sex, BMI, ethnicity, alcohol drinking status, current smoking status, physical activity, income level, and diagnosis of hypertension, stroke, dementia, head injury, obstructive sleep apnoea syndrome, hyperthyroidism, and diabetes at baseline. HR, hazard ratio; CI, confidence interval.
Stratified analysis
As shown in Supplementary data online, Figure S3, the results of stratification analyses showed that the observed association between epilepsy and all cardiac arrhythmias was more evident in participants aged <60 years than in those aged ≥60 years (Pinteraction = .024 for the interaction between age and epilepsy). The associations between epilepsy and the outcomes of interest did not differ when stratified by the other socioeconomic and lifestyle factors including sex, ethnicity, BMI, current smoking status, alcohol drinking status, physical activity, and income level.
Sensitivity and additional analyses
After excluding individuals with hypertension, stroke, head injury, OSA, hyperthyroidism, or diabetes, all the aforementioned associations persisted (see Supplementary data online, Figure S4). In addition, no major changes were found in the results after excluding participants followed up for <2 or <5 years (see Supplementary data online, Figure S4). Results of sensitivity analyses using the restricted definition of people with epilepsy (n = 1230 under the new definition), PSM, and multiple imputation were consistent with our main findings (see Supplementary data online, Figures S5 and S6, and Supplementary data online, Tables S2 and S3).
After incorporating the PRS-z for cardiac arrhythmia into the fully adjusted model, the association between epilepsy and cardiac arrhythmias (HR 1.37, 95% CI 1.22–1.54) remained consistent with the main results, and there was not a clear trend observed across different strata for effect modification by PRS-z quartiles (see Supplementary data online, Figures S7–S9; Pinteraction = .07). Totally, these findings provided evidence that epilepsy was associated with an increased risk of cardiac arrhythmias, and this observed association might be independent of genetic susceptibility to cardiac arrhythmias.
Use of antiseizure medications is associated with an increased risk of cardiac arrhythmia
Compared with people without epilepsy, people with epilepsy but without medication still had an increased risk of cardiac arrhythmias (HR 1.28, 95% CI 1.04–1.58), while those with both epilepsy and the use of ASMs had an even higher risk (HR 1.40, 95% CI 1.22–1.60) (Figure 3). In the sub-analyses by ATC medication classes, the use of carboxamide derivatives, fatty acid derivatives, and other antiepileptics exhibited a more evident association with arrhythmia (HR 1.45, 95% CI 1.17–1.81 for carboxamide derivatives; HR 1.56, 95% CI 1.21–2.01 for fatty acid derivatives; and HR 1.48, 95% CI 1.12–1.95 for other antiepileptics) (Figure 3). Further analyses were conducted on the primary medications among these three medication classes, identifying stronger associations for carbamazepine (HR 1.46, 95% CI 1.17–1.82) and valproic acid (HR 1.55, 95% CI 1.20–2.00) (Figure 3). The use of barbiturates and derivatives, hydantoin derivatives, and benzodiazepine derivatives exhibited no significant association with cardiac arrhythmia risk among people with epilepsy (Figure 3; Supplementary data online, Table S4). As for sodium channel blockers, carbamazepine and valproic acid were associated with an increased risk of cardiac arrhythmia (see Supplementary data online, Figure S10).
Figure 3.
Association between antiseizure medications and incident cardiac arrhythmias. The model was adjusted for age, sex, ethnicity, BMI, alcohol drinking status, current smoking status, income level, physical activity, and diagnosis of hypertension, stroke, dementia, head injury, obstructive sleep apnoea syndrome, hyperthyroidism, and diabetes at baseline. Reference was defined as people without epilepsy. HR, hazard ratio; CI, confidence interval.
In drug target SMR analysis, the genetic variant related to the expression of SCN5A, which is a common specific genetic target of sodium channel blockers, was associated with an increased risk of cardiac arrhythmias, supporting our observational results for carbamazepine and valproic acid use (see Supplementary data online, Tables S5 and S6). Overall, ASMs, particularly carbamazepine and valproic acid, further elevated the long-term risk of cardiac arrhythmias in people with epilepsy.
Discussion
In this population-based cohort study with a follow-up period of over 10 years, we identified an increased risk of cardiac arrhythmias, as well as arrhythmia subtypes including AF, ventricular arrhythmias, and bradyarrhythmias, in people with epilepsy. These associations remained consistent across sensitivity and other additional analyses. Moreover, the higher risk of cardiac arrhythmias in people with epilepsy was independent of genetic predisposition to cardiac arrhythmias. Furthermore, these associations may be exacerbated by the use of ASMs, particularly carbamazepine and valproic acid, as indicated by observational and MR analyses (Structured graphical abstract).
Despite numerous studies focused on sudden cardiac events supporting the concept of ‘epileptic heart’, our study is the first comprehensive investigation into the association of epilepsy with the long-term risk of cardiac arrhythmias and arrhythmia subtypes. While there have been a limited number of studies indicating a higher risk of heart disease in people with epilepsy,24,25 these studies did not identify the long-term association between epilepsy and different subtypes of arrhythmias and could not establish whether the observed association was attributed to epilepsy per se or other factors such as genetic predisposition or ASM use.26 Furthermore, the mechanisms underlying the association between epilepsy and the long-term risk of cardiac arrhythmias remain unclear, with the potential involvement of sympathetic alterations, repeated hypoxaemia, and shared genetic factors.1,9,10 By utilizing a combination of observational and genetic data from a large population, our study contributes to the existing evidence that people with epilepsy tend to be at a higher long-term risk of cardiac arrhythmias, independent of genetic risk for arrhythmia. Future investigations should focus on elucidating the biological mechanisms underlying the potential long-term effect of epilepsy on incident arrhythmia.
Incorporating primary and several sensitivity analyses, our investigation showed increased risks of AF, bradyarrhythmias, and ventricular arrhythmias in people with epilepsy. In clinical practice, there have been several reported cases of AF in people with epilepsy.27–29 Large surveys have also shown that AF is the most common arrhythmia subtype in people with epilepsy.30 However, no studies have explored the long-term risk of AF in relation to epilepsy. Our findings have extended those of previous investigations and revealed that epilepsy is associated with increased AF risk in the long term. It has been hypothesized that the heightened sympathetic activity induced by epilepsy may be the trigger for AF.31 Regarding other subtypes of cardiac arrhythmias, our results also demonstrated a greater than one-fold increase in the risk of incident bradyarrhythmias among people with epilepsy, as observed in previous studies.15,32,33 The results of our main analyses have also revealed a significant association between epilepsy and ventricular arrhythmias, which has been reported previously.15,31,34 One study even showed a three-fold elevated risk of sudden cardiac death due to ventricular fibrillation associated with epilepsy.34 These findings extend the understanding of the incidence of different subtypes of cardiac arrhythmias and highlight the importance of preventive measures and effective management of arrhythmias in individuals with epilepsy to decrease cardiovascular events,35 such as routine monitors of 12-lead electrocardiogram and heart rate variability test.
Among individuals with epilepsy, the use of specific ASMs, especially carbamazepine and valproic acid, seemed to be associated with a higher risk of cardiac arrhythmia. Carbamazepine, exerting its anticonvulsant effects on voltage-gated sodium ion channels, is used as a first-line medication in patients with newly diagnosed focal epilepsy, but according to previous reports,36–40 it is notorious for inducing cardiac dysfunction and metabolism abnormality in people with epilepsy. Our research supports these findings and adds to the evidence for the potential adverse effect on cardiac outcomes, which can further raise the long-term risk of cardiac arrhythmias. Valproic acid, a kind of fatty acid derivative, is suitable for almost all types of epilepsy and is one of the most effective broad-spectrum ASMs.41 Valproic acid could lead to inhibitory activity in the brain by modulation of gamma-aminobutyric acid (GABA), voltage-gated sodium channels, and T-type calcium channels. Several small studies have explored the association between valproic acid and cardiac function, with inconsistent results.42–44 Of them, two studies have compared QT intervals between valproic acid users and non-users, consistent with our finding, and both showed that patients using valproic acid were prone to ventricular arrhythmia. Therefore, caution should be taken when selecting valproic acid for people with epilepsy. Besides, sodium channel blockers play a crucial role as a classification and primary mechanism of ASMs. Consistent with previous findings,18,45,46 sodium channel blockers are found to link to an elevated risk of cardiac arrhythmias in our study.
Using drug target MR analyses, we further validated the significance of carbamazepine and valproic acid in the development of cardiac arrhythmia from the genetic level. With validated drug target genes as appropriate MR instruments, we could overcome the potential bias of observational studies and explore the causality. Among the target genes of these two medications, SCN5A was proved to be associated with an increased risk of cardiac arrhythmias. As the encoding gene of the cardiac sodium channel protein subunit, SCN5A was the principal target gene for sodium channel blockers. Recent studies have also identified that SCN5A played a functional role in epilepsy, innate immune response, cardiac arrhythmia, and cardiomyopathy and further provide evidence of the association between epilepsy and cardiac arrhythmias.47 Together with observational and MR findings, our study emphasizes the importance of intensive cardiovascular monitoring in people with epilepsy receiving carbamazepine and valproic acid. The aforementioned studies, including our own, lacked information on drug dosages. Further large-scale studies are needed to confirm the effect of accumulated ASMs doses particularly carbamazepine and valproic acid.
There are several strengths in this study. First, it draws upon a large prospective cohort with over 500 000 individuals and assesses the association between epilepsy and long-term cardiac arrhythmias risks over a long follow-up period. Second, we further analysed the effect of the interaction between epilepsy and genetic predisposition on disease risk using PRS. Third, to confirm the robustness of our findings, we performed a series of sensitivity analyses. These included lagging the exposure by excluding incident cases of cardiac arrhythmias within 2 or 5 years of a seizure, implementing PSM, and performing multiple imputation, among other approaches. Finally, we explored the role of ASMs in cardiac arrhythmias and tested for causality through SMR analysis. This analysis provided further insights into the potential mechanisms underlying the observed associations, suggesting further exploration of the effect of ASMs on cardiac ion channels, and new structural analogues and derivatives with few cardiac adverse side effects were acquired.
We also acknowledge several limitations in our work. First, considering potential asymptomatic episodes of arrhythmias, the incidence of arrhythmias might have been underestimated. Second, due to the lack of detailed data, we were unable to obtain the duration and accumulated doses of the medication treatments. Therefore, although we observed an increased risk associated with carbamazepine and valproic acid, which were supported by MR results, further studies are still required to confirm our findings. Furthermore, due to the lack of information on the specific lobes associated with epilepsy occurrence in the participants in this study, we could not explore the influence of discharge within different lobes on cardiac arrhythmias. More large-scale studies are warranted to explore the association between different subtypes of epilepsy and cardiac arrhythmias. Also importantly, our analyses did not consider seizure frequency or severity or the duration of the individuals’ epileptic history, all of which seem likely to affect the magnitude of the effect on the heart. Therefore, further studies are required to clarify the correlation between the frequency of epileptic seizures and the occurrence of arrhythmia in the future. Despite these limitations, our study provides valuable insights into the need for cardiac care of people with epilepsy.
In conclusion, our study indicates that people with epilepsy are at an increased long-term risk of cardiac arrhythmias, particularly in individuals using carbamazepine and valproic acid. Our findings underscore the importance of incorporating cardiac care and implementing appropriate management measures for people with epilepsy to mitigate the risk of arrhythmias. Clinical management is warranted to promoting cardiovascular health and mitigating the risk of cardiac arrhythmias in people with epilepsy, including regular and long-term electrocardiogram monitoring and heart rate variability assessments along with vigilant monitoring of potential adverse effects of ASMs on cardiac function. Further studies could focus on clarifying the impact of seizure severity and frequency on the risk of cardiac arrhythmias, as well as the cumulative dose effect of ASMs, to better identify individuals at specifically elevated risk.
Supplementary Material
Acknowledgements
The authors would like to thank all the staff and study participants involved in this study or contributed to the UKB. The authors are also thankful for Prof. Bo Xiao from Xiangya Hospital for providing guidance in epilepsy on our research.
Contributor Information
Jie Wang, Clinical Research Center, The Third Xiangya Hospital, Central South University, 138 Tongzipo Road, Changsha, Hunan 410013, China; Department of Cardiology, The Third Xiangya Hospital, Central South University, 138 Tongzipo Road, Changsha, Hunan 410013, China.
Peiyuan Huang, MRC Integrative Epidemiology Unit (IEU), Bristol Medical School, University of Bristol, Oakfield House, Oakfield Grove, Bristol BS8 2BN, UK.
Qingwei Yu, Clinical Research Center, The Third Xiangya Hospital, Central South University, 138 Tongzipo Road, Changsha, Hunan 410013, China; Department of Neurosurgery, Xiangya Hospital, Central South University, 87 Xiangya Road, Changsha, Hunan 410008, China.
Jun Lu, Clinical Research Center, The Third Xiangya Hospital, Central South University, 138 Tongzipo Road, Changsha, Hunan 410013, China.
Pinbo Liu, Clinical Research Center, The Third Xiangya Hospital, Central South University, 138 Tongzipo Road, Changsha, Hunan 410013, China.
Yiping Yang, Clinical Research Center, The Third Xiangya Hospital, Central South University, 138 Tongzipo Road, Changsha, Hunan 410013, China.
Zeying Feng, Clinical Research Center, The Third Xiangya Hospital, Central South University, 138 Tongzipo Road, Changsha, Hunan 410013, China.
Jingjing Cai, Clinical Research Center, The Third Xiangya Hospital, Central South University, 138 Tongzipo Road, Changsha, Hunan 410013, China; Department of Cardiology, The Third Xiangya Hospital, Central South University, 138 Tongzipo Road, Changsha, Hunan 410013, China.
Guoping Yang, Clinical Research Center, The Third Xiangya Hospital, Central South University, 138 Tongzipo Road, Changsha, Hunan 410013, China.
Hong Yuan, Clinical Research Center, The Third Xiangya Hospital, Central South University, 138 Tongzipo Road, Changsha, Hunan 410013, China; Department of Cardiology, The Third Xiangya Hospital, Central South University, 138 Tongzipo Road, Changsha, Hunan 410013, China.
Haibo Tang, Department of Metabolic and Bariatric Surgery, The Third Xiangya Hospital, Central South University, Changsha, Hunan 410013, China.
Yao Lu, Clinical Research Center, The Third Xiangya Hospital, Central South University, 138 Tongzipo Road, Changsha, Hunan 410013, China; Department of Cardiology, The Third Xiangya Hospital, Central South University, 138 Tongzipo Road, Changsha, Hunan 410013, China; Faculty of Life Sciences & Medicine, King's College London, 150 Stamford Street, London SE1 9NH, UK.
Supplementary data
Supplementary data are available at European Heart Journal online.
Declarations
Disclosure of Interest
All authors declare no conflict of interest for this contribution.
Data Availability
The data are available from UKB Resource (www.ukbiobank.ac.uk/). This research has been conducted using the UKB Resource under Application Number 75283.
Ethical Approval
The UKB study protocol was approved by the North West Multi-centre Research Ethics Committee (11/NW/0382).
Funding
This work was supported by grants from National Key Research and Development Program (2022YFC3602400, 2022YFC3602401), National Natural Science Foundation of China (82170437, 81974054), Central South University Innovation-Driven Research Program (2023CXQD007), and Wellcome Trust PhD Studentship (224979/Z/22/Z).
Pre-registered Clinical Trial Number
None supplied.
References
- 1. Thijs RD, Ryvlin P, Surges R. Autonomic manifestations of epilepsy: emerging pathways to sudden death? Nat Rev Neurol 2021;17:774–88. 10.1038/s41582-021-00574-w [DOI] [PubMed] [Google Scholar]
- 2. Fisher RS, Acevedo C, Arzimanoglou A, Bogacz A, Cross JH, Elger CE, et al. ILAE Official Report: a practical clinical definition of epilepsy. Epilepsia 2014;55:475–82. 10.1111/epi.12550 [DOI] [PubMed] [Google Scholar]
- 3. Thijs RD, Surges R, O'Brien TJ, Sander JW. Epilepsy in adults. Lancet 2019;393:689–701. 10.1016/s0140-6736(18)32596-0 [DOI] [PubMed] [Google Scholar]
- 4. Keezer MR, Sisodiya SM, Sander JW. Comorbidities of epilepsy: current concepts and future perspectives. Lancet Neurol 2016;15:106–15. 10.1016/s1474-4422(15)00225-2 [DOI] [PubMed] [Google Scholar]
- 5. Shmuely S, van der Lende M, Lamberts RJ, Sander JW, Thijs RD. The heart of epilepsy: current views and future concepts. Seizure 2017;44:176–83. 10.1016/j.seizure.2016.10.001 [DOI] [PubMed] [Google Scholar]
- 6. Devinsky O. Effects of seizures on autonomic and cardiovascular function. Epilepsy Curr 2004;4:43–6. 10.1111/j.1535-7597.2004.42001.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nass RD, Motloch LJ, Paar V, Lichtenauer M, Baumann J, Zur B, et al. Blood markers of cardiac stress after generalized convulsive seizures. Epilepsia 2019;60:201–10. 10.1111/epi.14637 [DOI] [PubMed] [Google Scholar]
- 8. Park KJ, Sharma G, Kennedy JD, Seyal M. Potentially high-risk cardiac arrhythmias with focal to bilateral tonic-clonic seizures and generalized tonic-clonic seizures are associated with the duration of periictal hypoxemia. Epilepsia 2017;58:2164–71. 10.1111/epi.13934 [DOI] [PubMed] [Google Scholar]
- 9. Verrier RL, Pang TD, Nearing BD, Schachter SC. Epileptic heart: a clinical syndromic approach. Epilepsia 2021;62:1780–9. 10.1111/epi.16966 [DOI] [PubMed] [Google Scholar]
- 10. Verrier RL, Pang TD, Nearing BD, Schachter SC. The epileptic heart: concept and clinical evidence. Epilepsy Behav 2020;105:106946. 10.1016/j.yebeh.2020.106946 [DOI] [PubMed] [Google Scholar]
- 11. Read MI, McCann DM, Millen RN, Harrison JC, Kerr DS, Sammut IA. Progressive development of cardiomyopathy following altered autonomic activity in status epilepticus. Am J Physiol Heart Circ Physiol 2015;309:H1554–64. 10.1152/ajpheart.00256.2015 [DOI] [PubMed] [Google Scholar]
- 12. Powell KL, Liu Z, Curl CL, Raaijmakers AJA, Sharma P, Braine EL, et al. Altered cardiac structure and function is related to seizure frequency in a rat model of chronic acquired temporal lobe epilepsy. Neurobiol Dis 2021;159:105505. 10.1016/j.nbd.2021.105505 [DOI] [PubMed] [Google Scholar]
- 13. Brundel B, Ai X, Hills MT, Kuipers MF, Lip GYH, de Groot NMS. Atrial fibrillation. Nat Rev Dis Primers 2022;8:21. 10.1038/s41572-022-00347-9 [DOI] [PubMed] [Google Scholar]
- 14. Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2018;72:e91–e220. 10.1016/j.jacc.2017.10.054 [DOI] [PubMed] [Google Scholar]
- 15. van der Lende M, Surges R, Sander JW, Thijs RD. Cardiac arrhythmias during or after epileptic seizures. J Neurol Neurosurg Psychiatry 2016;87:69–74. 10.1136/jnnp-2015-310559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bleakley LE, Soh MS, Bagnall RD, Sadleir LG, Gooley S, Semsarian C, et al. Are variants causing cardiac arrhythmia risk factors in sudden unexpected death in epilepsy? Front Neurol 2020;11:925. 10.3389/fneur.2020.00925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chahal CAA, Salloum MN, Alahdab F, Gottwald JA, Tester DJ, Anwer LA, et al. Systematic review of the genetics of sudden unexpected death in epilepsy: potential overlap with sudden cardiac death and arrhythmia-related genes. J Am Heart Assoc 2020;9:e012264. 10.1161/jaha.119.012264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bardai A, Blom MT, van Noord C, Verhamme KM, Sturkenboom MC, Tan HL. Sudden cardiac death is associated both with epilepsy and with use of antiepileptic medications. Heart 2015;101:17–22. 10.1136/heartjnl-2014-305664 [DOI] [PubMed] [Google Scholar]
- 19. Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, et al. UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med 2015;12:e1001779. 10.1371/journal.pmed.1001779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. White HS, Smith MD, Wilcox KS. Mechanisms of action of antiepileptic drugs. Int Rev Neurobiol 2007;81:85–110. 10.1016/s0074-7742(06)81006-8 [DOI] [PubMed] [Google Scholar]
- 21. Cassidy S, Chau JY, Catt M, Bauman A, Trenell MI. Cross-sectional study of diet, physical activity, television viewing and sleep duration in 233,110 adults from the UK Biobank; the behavioural phenotype of cardiovascular disease and type 2 diabetes. BMJ Open 2016;6:e010038. 10.1136/bmjopen-2015-010038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nguyen CD, Carlin JB, Lee KJ. Practical strategies for handling breakdown of multiple imputation procedures. Emerg Themes Epidemiol 2021;18:5. 10.1186/s12982-021-00095-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Toutenburg H, Rubin DB. Multiple imputation for nonresponse in surveys. Stat Pap 1990;31:180–180. 10.1007/BF02924688 [DOI] [Google Scholar]
- 24. Cheng CY, Hsu CY, Wang TC, Liu CY, Yang YH, Yang WH. Risk of cardiac morbidities and sudden death in patients with epilepsy and no history of cardiac disease: a population-based nationwide study. Mayo Clin Proc 2021;96:964–74. 10.1016/j.mayocp.2020.04.050 [DOI] [PubMed] [Google Scholar]
- 25. Zack M, Luncheon C. Adults with an epilepsy history, notably those 45–64 years old or at the lowest income levels, more often report heart disease than adults without an epilepsy history. Epilepsy Behav 2018;86:208–10. 10.1016/j.yebeh.2018.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Surges R, Shmuely S, Dietze C, Ryvlin P, Thijs RD. Identifying patients with epilepsy at high risk of cardiac death: signs, risk factors and initial management of high risk of cardiac death. Epileptic Disord 2021;23:17–39. 10.1684/epd.2021.1254 [DOI] [PubMed] [Google Scholar]
- 27. Sethi NK. Atrial fibrillation associated with epileptic seizures. JAMA Neurol 2013;70:273–4. 10.1001/jamaneurol.2013.1265 [DOI] [PubMed] [Google Scholar]
- 28. Elnazeir M, Badugu P, Narayanan S, Hussain A, Bhagat R, Jones CM, et al. Generalized tonic-clonic seizures with post-ictal atrial fibrillation. Epilepsy Behav Rep 2020;13:100343. 10.1016/j.ebr.2019.100343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Herskovitz M, Schiller Y. Atrial fibrillation associated with epileptic seizures. Arch Neurol 2012;69:1197–9. 10.1001/archneurol.2011.3647 [DOI] [PubMed] [Google Scholar]
- 30. Desai R, Rupareliya C, Patel U, Naqvi S, Patel S, Lunagariya A, et al. Burden of arrhythmias in epilepsy patients: a nationwide inpatient analysis of 1.4 million hospitalizations in the United States. Cureus 2017;9:e1550. 10.7759/cureus.1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Volders PG. Novel insights into the role of the sympathetic nervous system in cardiac arrhythmogenesis. Heart Rhythm 2010;7:1900–6. 10.1016/j.hrthm.2010.06.003 [DOI] [PubMed] [Google Scholar]
- 32. Rugg-Gunn FJ, Simister RJ, Squirrell M, Holdright DR, Duncan JS. Cardiac arrhythmias in focal epilepsy: a prospective long-term study. Lancet 2004;364:2212–9. 10.1016/s0140-6736(04)17594-6 [DOI] [PubMed] [Google Scholar]
- 33. Tinuper P, Bisulli F, Cerullo A, Carcangiu R, Marini C, Pierangeli G, et al. Ictal bradycardia in partial epileptic seizures: autonomic investigation in three cases and literature review. Brain 2001;124:2361–71. 10.1093/brain/124.12.2361 [DOI] [PubMed] [Google Scholar]
- 34. Bardai A, Lamberts RJ, Blom MT, Spanjaart AM, Berdowski J, van der Staal SR, et al. Epilepsy is a risk factor for sudden cardiac arrest in the general population. PLoS One 2012;7:e42749. 10.1371/journal.pone.0042749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chahal CAA, Gottwald JA, St Louis EK, Xie J, Brady PA, Alhurani RE, et al. QT prolongation in patients with index evaluation for seizure or epilepsy is predictive of all-cause mortality. Heart Rhythm 2022;19:578–84. 10.1016/j.hrthm.2021.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Celik IE, Akyel A, Colgecen M, Ozeke O. A rare cause of 2:1 atrioventricular block: carbamazepine. Am J Emerg Med 2015;33:1541.e3-4. 10.1016/j.ajem.2015.07.055 [DOI] [PubMed] [Google Scholar]
- 37. Benassi E, Bo GP, Cocito L, Maffini M, Loeb C. Carbamazepine and cardiac conduction disturbances. Ann Neurol 1987;22:280–1. 10.1002/ana.410220217 [DOI] [PubMed] [Google Scholar]
- 38. Perucca P, Scheffer IE, Kiley M. The management of epilepsy in children and adults. Med J Aust 2018;208:226–33. 10.5694/mja17.00951 [DOI] [PubMed] [Google Scholar]
- 39. Jain S, Nair PP, Aghoram R, Wadwekar V, Wagh S, Balachandran M, et al. Interictal autonomic changes in persons with epilepsy (PWE) on carbamazepine (CBZ) versus other anti-seizure drug monotherapy: a cross-sectional study. Epilepsy Behav 2021;125:108396. 10.1016/j.yebeh.2021.108396 [DOI] [PubMed] [Google Scholar]
- 40. Mintzer S, Trinka E, Kraemer G, Chervoneva I, Werhahn KJ. Impact of carbamazepine, lamotrigine, and levetiracetam on vascular risk markers and lipid-lowering agents in the elderly. Epilepsia 2018;59:1899–907. 10.1111/epi.14554 [DOI] [PubMed] [Google Scholar]
- 41. Tomson T, Battino D, Perucca E. Valproic acid after five decades of use in epilepsy: time to reconsider the indications of a time-honoured drug. Lancet Neurol 2016;15:210–8. 10.1016/s1474-4422(15)00314-2 [DOI] [PubMed] [Google Scholar]
- 42. Radgoudarzi M, Vafaee Shahi M, Naderi F. Effect of sodium valproate treatment on the cardiac index in new cases with status epilepticus. Open Neurol J 2021;15:59–64. 10.2174/1874205X02115010059 [DOI] [Google Scholar]
- 43. Asoğlu R, Özdemir M, Aladağ N, Asoğlu E. Evaluation of cardiac repolarization indices in epilepsy patients treated with carbamazepine and valproic acid. Medicina (Kaunas) 2020;56:20. 10.3390/medicina56010020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Altun Y, Yasar E. Effects of valproate, carbamazepine and levetiracetam on Tp-e interval, Tp-e/QT and Tp-e/QTc ratio. Ideggyogy Sz 2020;73:121–7. 10.18071/isz.73.0121 [DOI] [PubMed] [Google Scholar]
- 45. Hookana E, Ansakorpi H, Kortelainen ML, Junttila MJ, Kaikkonen KS, Perkiömäki J, et al. Antiepileptic medications and the risk for sudden cardiac death caused by an acute coronary event: a prospective case-control study. Ann Med 2016;48:111–7. 10.3109/07853890.2016.1140225 [DOI] [PubMed] [Google Scholar]
- 46. Auerbach DS, Biton Y, Polonsky B, McNitt S, Gross RA, Dirksen RT, et al. Risk of cardiac events in long QT syndrome patients when taking antiseizure medications. Transl Res 2018;191:81–92.e7. 10.1016/j.trsl.2017.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Remme CA. SCN5A Channelopathy: arrhythmia, cardiomyopathy, epilepsy and beyond. Philos Trans R Soc Lond B Biol Sci 2023;378:20220164. 10.1098/rstb.2022.0164 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data are available from UKB Resource (www.ukbiobank.ac.uk/). This research has been conducted using the UKB Resource under Application Number 75283.