Abstract
Background
Ginseng has been used as a traditional medicine and functional cosmetic ingredients for many years. Recent studies have focused on the potential biological effects of the ginseng berry and its ingredients. (+)-Syringaresinol (SYR) is enriched in ginseng berry and its beneficial effects on the skin have been recently reported. However, little is known about the its effects on the wound healing process of skin.
Methods
Here, we evaluated the skin wound healing effect of (+)-SYR using the human fibroblast Hs68 cell and ex vivo pig and human skin tissue model. Scratch wound test and hydrogen peroxide (HPO) induce chemical wound model were employed.
Results
(+)-SYR promoted the migration and proliferation of Hs68 cells without significant cytotoxicity at the tested concentrations. Especially, in ex vivo pig and human skin tissue, HPO-induced chemical wound was recovered almost completely by (+)-SYR. In line with the finding in Hs68, the protein expression levels of TGF-β and PCNA, a proliferation marker were increased, demonstrating the beneficial effects of (+)-SYR on skin wound repair.
Conclusion
Collectively, we demonstrated that (+)-SYR from ginseng berry, can enhance the wound healing effect by accelerating cell proliferation and skin regeneration, suggesting the potential utility of (+)-SYR for skin wound repair.
Keywords: (+)-Syringaresinol, Panax ginseng, Ginseng berry, Wound healing
Graphical abstract
1. Introduction
Panax ginseng is a traditional herbal medicine with a variety of therapeutic and pharmacological effects [36]. Various biochemical and pharmacological effects of ginseng have been demonstrated, that include anti-cancer [50], anti-inflammatory [34], anti-fatigue [12], anti-oxidative [31], and anti-diabetic effects [59]. The different parts of ginseng such as roots, stems, leaves, flowers, berries, and seeds have different amount of bioactive components with different pharmacological activities [2,49]. Among other parts, ginseng berry has come into focus since many studies proved that ginseng berry has higher levels of ginsenosides and significantly stronger antioxidant activity than other parts of ginseng [16,30,47]. In addition, it contains larger amounts of vitamin E, vitamin K, folic acid, and potassium than other parts of ginseng.
Recently, (+)-syringaresinol (SYR), isolated from Panax ginseng, was shown to have anti-inflammatory [3], anti-fungal, and anti-aging effects [37]. Interestingly SYR is highly enriched in ginseng berry [11]. SYR activates SIRT1 gene expression which plays a critical role in age-related metabolic disorders [32,38]. According to Kim et al [33], SYR significantly increased skin thicknesses and reduced the levels of oxidative damage and reactive oxygen species in the skin. Also, the treatment of SYR activated FoxO3a expression in skin cells, which resulted in down-regulation of matrix metalloproteinase-2 (MMP-2) and activation of collagen synthesis, suggesting the utility of SYR for the improvement of various skin problems.
The skin is a principal barrier protecting the body from the external environment against physical stimulus, pathogens, and water loss and regulate homeostasis [43]. Accordingly, the skin is easy to get injured and wounded by various obnoxious stimuli. Upon injury, skin wound healing process is activated to repair and restructure the damaged tissue. Skin wound process includes cell migration, proliferation, and synthesis of extracellular matrix which are orchestrated by various growth factors [58]. The wound healing process can be divided into overlapping phases of inflammation, proliferation, and remodeling [24]. Inflammatory starts with hemostasis which prevents the loss of blood at the wound. Inflammatory cells, such as neutrophils and monocytes, are activated at the site of wound by cytokines and growth factors [46,51]. During the proliferative phase, keratinocytes, fibroblasts and endothelial cells migrate and proliferate, of which process is activated by the release of growth factors like TGF-β, transforming growth factor-β and VEGF, vascular endothelial growth factor [6]. The skin wound process is completed by the remodeling phase, which reorganizes the wounded tissue and substitutes the immature extracellular matrix [7]. Myofibroblasts induced by TGF-β initially secrete type Ⅲ collagen, which is degraded later and replaced by type Ⅰ collagen, ultimately providing proper strength to the extracellular matrix [28,29]. Angiogenesis, blood vessel growth [14], is also important for the integrity of repaired tissue, which is governed by angiogenic factors, VEGF, TGF- β, and eNOS [20,41].
Here, we investigated the effects of SYR derived from ginseng berry on the skin wound healing using a dermal fibroblast cell line, Hs68 and ex vivo human skin model in an effort to illuminate the utility of ginseng berry and its ingredients for the improvement of skin health.
2. Materials and methods
2.1. Materials
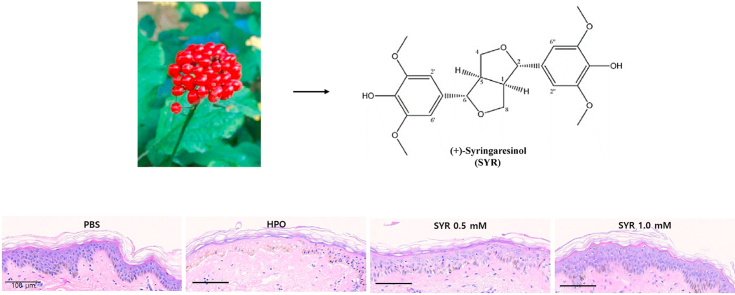
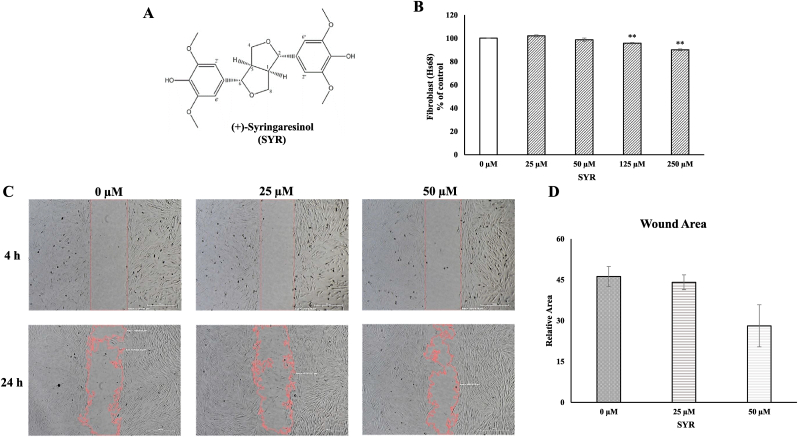
(+)-Syringaresinol (purity > 98%) purchased from Q-mine (Seoul, Korea) (Fig. 1A). A stock solution was prepared with DMSO purchased from Sigma-Aldrich (St Louis, MO, USA).
Fig. 1.
Cell viability and migration effect of (+)-SYR in human skin cells
(A) The structure of (+)-SYR. (B) The toxicity of different concentrations of (+)-SYR in Hs68 by CCK-8 assay, establishing a range of concentration (0-250 μΜ) that did not affect their viability. (C, D) The effect of (+)-SYR (0-50 μM) on the migration of Hs68 was examined using an in vitro wound closure assay. Scale bar = 500 μm. N = 3 fields per a group. ∗p < 0.05 and ∗∗p < 0.01 vs control.
2.2. Cell culture
Human dermal fibroblast (Hs68) was obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA). The cell was cultured in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% Fetal Bovine Serum (FBS) and 1% antibiotics (penicillin and streptomycin). Cells were incubated at 37°C in a 5% CO2 atmosphere. Monolayers of 80% confluent cells were cultured with 0.05% trypsin (Hyclone, South Logan, UT, USA).
2.3. Ex vivo human and pig skin tissue
Ex vivo human skin model was prepared from the skin tissue discarded after surgery donated after agreement compliant to IRB (Institutional Review Board) approval of Seoul National University Bundang Hospital (Bundang-si, Gyeonggi-do, Korea). The donors had no history of skin disease and did not use immunosuppressants, corticosteroids or cytotoxic agents. Immediately after surgery, skin samples were transported to room temperature in a medium containing penicillin-streptomycin. The subcutaneous adipose tissue was removed from the skin tissue after multiple washes. Then the tissue was excised using a 10 mm biopsy punch (Acu-punch, Acuderm, Fort Lauderdale, FL, USA) and inserted into a cell culture insert so that the epidermal surface of the skin tissue was exposed to air. The gap between the skin tissue and the cell culture insert was sealed with a silicone ring. To confirm the integrity of the skin tissue, TEER (trans-epithelial electrical resistance) was measured (Millicell ERS-2 voltohmmeter, Merck, Germany) and only skin over 2,000 ohms was used for the experiment. The tissues were placed in medium-filled 6-well plates and pre-incubated for 20-24 h at 37°C in a humidified atmosphere containing 5% CO2. Medium was changed every 2 days.
Ex vivo pig skin were obtained from the ears of the pig in Apures Co. (Gyeonggi-do, South Korea), which was sacrificed for research on drug delivery. Skin tissue was removed off the subcutis layer and rinsed with 70% ethanol and PBS. Embedding onto 24 well cell culture insert was done similar as described above.
2.4. Cell and tissue viability assay
The cell viability was detected by Cell Counting Kit-8 (CCK-8) purchased from Dojindo Laboratories, Kumamoto, Japan. The Hs68 cells were plated at a density of 1.0 × 104 cells/well in 96-well plate. Briefly, the cells were treated with (+)-SYR at the doses of 25-250 μM for 24 h, and DMSO served as solvent control. After 24 h of incubation, 10 μl of CCK-8 solution was added to each well, and the 96-well plate was incubated at 37°C for 2 h, then absorbance was determined by microplate spectrophotometer readings at 450 nm (BioTek Instruments, Inc., Winooski, VT, USA).
Skin tissue viability was detected by the MTT (3-(4,5-dimethylthiazolyly-2)-2, 5-diphenyltetrazolium bromide) assay. Chemical wounds were induced by treating 30% HPO inside the skin tissue insert and exposing it for 45 minutes. After that, the tissue was washed with PBS and treated with (+)-SYR (0-1 mM) 3 times every other day. To evaluate viability, tissues were transferred to a 48-well plate that contained MTT (0.4 mg/mL) and incubated for 3 h at 37 °C and 5% CO2. Next, tissues were transferred to a new 6-well plate, prefilled with 2 mL of isopropanol. Formazan extraction was performed at room temperature for 3 h and 250 μL of formazan extract, per tissue was transferred to a 96 well plate. Optical density was measured at 570 nm with a multiplate reader (Infinite M200 Pro microplate reader, Tecan Group Ltd., Mannedorf, Switzerland), equipped at Ewha Drug Development Research Core Center. Tissue viability of the skin tissue was expressed as the ratio of the skin disk to its weight in milligrams.
2.5. Cell Migration assay
A wound closure assay was investigated using silicon culture inserts (Ibidi, LLC, Munchen, Germany) with two different parts of well for cell seeding. Each insert was placed in a 24-well plate, and 1.0 × 104 cells of Hs68 were seeded in each part of inserts. 24 h after cell seeding, the culture inserts were removed carefully to make a cell-free area, the “wound”, and washed with PBS twice. The cells with cell-free area were treated with (+)-SYR in 1% FBS-medium and incubated for 24 h. The cultures were photographed with a Nikon Eclipse Ts2 light microscope (Nikon Instech, Tokyo, Japan) to monitor the migration of cells into the wounded area, and the closure of the wounded area was calculated using the Nikon NIS Elements imaging software.
2.6. Cell proliferation assay
Cell proliferation assay was determined by the CCK-8 assay [52]. The Hs68 cells were treated with (+)-SYR. After incubation for 24 or 48h, comparing the number of cells at 24 and 48 h, cell proliferation was determined by CCK-8 assay.
Crystal violet staining was used to determine the proliferation of Hs68 cells after the treatment of (+)-SYR. Crystal violet assay kit (ab232855) was purchased from Abcam (USA). A total of 3.0 × 104 cells was seeded in 12 well at a confluency of 50% confluency and incubated overnight. Then, the cells were treated with the same concentrations of (+)-SYR in 5% FBS-medium. After 24 and 48 h, culture medium in each well was removed, and washed with 1X Washing Solution. After washing, crystal violet staining solution with methanol was added and incubated for 20 min in room temperature. Crystal violet solution was removed from the wells and the plates were washed four times. The cells were photographed with a Nikon Eclipse Ts2 light microscope.
2.7. RNA extraction and real-time PCR
The Hs68 cells were washed twice with PBS after a 48 h exposure to (+)-SYR in 6-well plate and RNA was extracted from the cells using the RNeasy® mini kit (Qiagen). The RNA integrity was estimated by determining the optical density at 260 nm with a spectrophotometer (NanoDrop Technologies, INC., Wilmington, DE, USA). Relative expression levels of mRNA were measured by quantitative real-time PCR. cDNA was synthesized from 1,250 ng of total RNA with RT primers (Bioelpis, Seoul, Korea). A SYBR Green PCR master mix and a StepOnePlusTM Real-time PCR machine (Applied Biosystems, Warrington, UK) were used in each reaction. The sequence of primers was as follows:
Forward GAPDH, 5′-GCATCCTGGGCTACACTGAG-3’;
Reverse GAPDH, 5′-AAGTGGTCGTTGAGGGCAAT-3’;
Forward TGF-β 5′-CTTCAGCTCCACAGAGAAGAACTG-3’;
Reverse TGF-β 5′-CACGATCATGTTGCACACTGCTCC-3’;
Forward VEGF-c, 5′-CACGAGCTACCTCAGCAAGA-3’;
Reverse VEGF-c, 5′-GCTGCCTGACACTGTGGTA-3’;
Forward eNOS, 5′-CGCCTGATGAGGAGAAGCC-3’;
Reverse eNOS, 5′-TCTGTGGTCACCTGAAACCCT-3’;
Forward Collagen Ⅰ, 5′-GCTTCACCTACAGCGTCACT-3’;
Reverse Collagen Ⅰ, 5′-AAGCCGAATTCCTGGTCTGG-3’.
The mRNA levels of target genes were normalized to the levels of GAPDH as a reference gene.
2.8. Histological analysis in skin tissue
For the histological examination, biopsy skin samples were fixed in 10% neutral-buffered formalin. Preserved tissues from each group were paraffin wax-embedded, sectioned, stained with hematoxylin and eosin (H&E), and then examined microscopically under an Olympus DP71 microscope (Center Valley, PA, USA). All tissue images were obtained using the virtual slide system (Aperio Scanscope XT, Vista, CA, USA).
2.9. Immunohistochemistry (IHC) staining
For immunohistochemistry, skin samples were sequentially proceeded rehydration steps with descending graded series of ethanol. Next, pH 6.0 antigen retrieval (DAKO, S1699, Santa Clara, CA, USA) or pH 9.0 antigen retrieval (DAKO, S2367) was conducted using a high-pressure cooker for 15 min, followed the cooling phase over 1 h until the solution was fully transparent. After two washes in PBS, sections were incubated in 3% H2O2 for 30 min for blocking endogenous peroxidase. Another three washes in PBS, sections were incubated with protein block (DAKO, X0909) for 1–2 h at room temperature in a humidity-controlled chamber. Primary antibodies were incubated overnight at 4°C. After three washes in PBS, sections were incubated in HRP-labeled anti-rabbit antibody (DAKO, K4003) for 15 min at room temperature. For the development of HRP-labeled antibody on section, DAB (DAKO, K3468) was diluted and put it on each section for the identical time. Mayer's hematoxylin (DAKO, S3309) was used for counterstaining. The following primary antibodies were commercially purchased: Anti-VEGF (ab1316, 1:50), anti-eNOS (PA3-031A, 1:250), anti-PCNA (sc-56, 1:100), anti-TGF-β (GTX21279, 1:50), anti-Ki-67 (ab16667, 1:1000).
2.10. Quantitation of immunohistochemistry (IHC) staining
Qupath software [4] was utilized to quantify immunohistochemistry (IHC) images. To measure DAB-positive pixels representing the cells expressing the marker protein in IHC, we ran the ‘positive pixel count’ module built in the software and calculated the positive pixels above the threshold in the skin epidermis in 20X high power fields. The DAB positive pixel percentage was normalized with total stained pixel (hematoxylin + DAB) counts.
2.11. Statistics
Data are expressed as mean ± standard error of the mean (SEM) of three or more independent experiments. The statistical significance of differences between groups was assessed using a two-sided Student's t-test. p-values < 0.05 were considered significant.
3. Results
3.1. (+)-syringaresinol increases the migration of human fibroblast
Firstly, the cytotoxicity of (+)-SYR in human fibroblast Hs68 was examined over a wide range of concentrations by CCK-8 assay. (+)-SYR was not cytotoxic in Hs68 cells at any concentration tested from 25-250 μM. As the concentration increased, the cell viability tended to decrease but the overall viability was over 90% (Fig. 1B). In the whole phases of wound healing, fibroblasts play an important role in promoting cell migration/proliferation into wound margin and wound contraction [42,55]. The effect of (+)-SYR on the migration of Hs68 was examined using an in vitro wound closure assay. Fig. 1C shows that the closure of cell-free area was promoted by (+)-SYR in a dose-dependent manner. The treatment of 50 μM of (+)-SYR covered the scratch wound by approximately 71% ± 7.72 while the control group cells covered about 53% ± 3.68, demonstrating that (+)-SYR may enhance the migration or proliferation of fibroblasts (Fig. 1D).
3.2. (+)-syringaresinol promotes the proliferation of fibroblast Hs68 cells
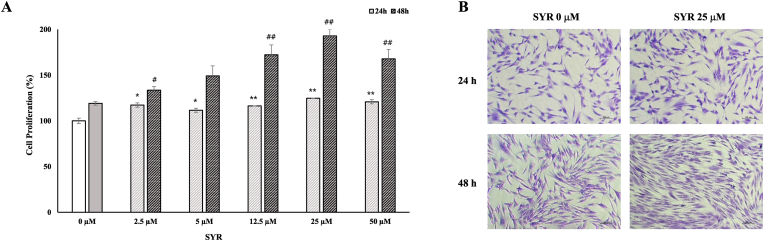
The effect of (+)-SYR on the proliferation of Hs68 cells was further investigated with different concentrations of (+)-SYR (2.5-50 μM) at 24 and 48 h. As a result, (+)-SYR promoted the proliferation of Hs68 in a dose-dependent manner of which effects plateaued at 25 μM (Fig. 2A). Increased cell proliferation by (+)-SYR could be also confirmed by crystal violet staining (Fig. 2B).
Fig. 2.
Cell proliferation of fibroblast
(A) Cell proliferation of Hs68 cells treated with (+)-SYR (0-50 μΜ) were measured by CCK-8 assay for 24 and 48 h. (B) Crystal violet staining was used to determine the proliferation of Hs68 cells after the treatment of (+)-SYR. N = 3 per a group. ∗ or #p < 0.05 and ∗∗ or ∗ or ##p < 0.01 vs the respective control.
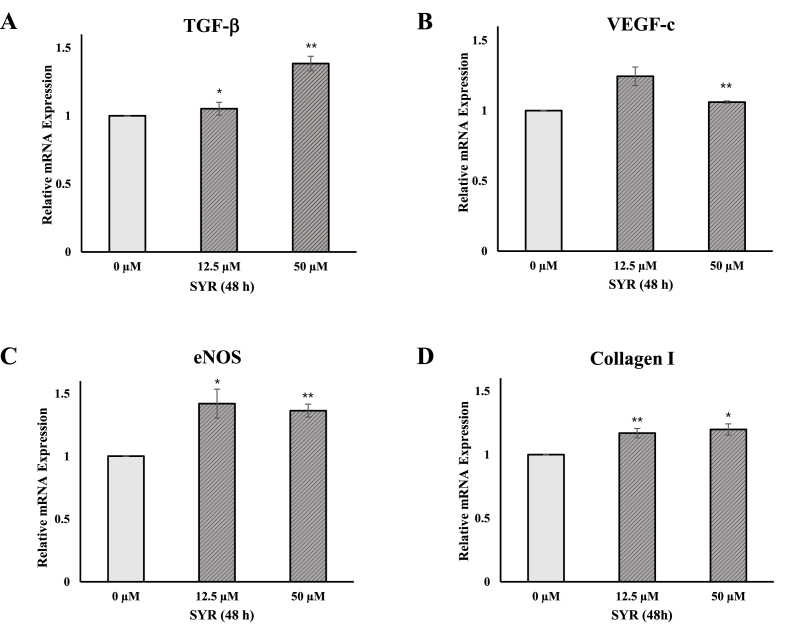
3.3. (+)-syringaresinol up-regulates the expression of TGF-β , VEGF-c, eNOS, and collagen Ⅰ in Hs68 cells
To investigate the expression of factors involved in the skin wound healing, qRT-PCR assay was performed. The expression of TGF-β, VEGF-c, eNOS, and Collagen type Ⅰ were determined in Hs68 after (+)-SYR was treated for 48 h. As a result, (+)-SYR increased the mRNA expression level of TGF-β, VEGF-c, eNOS and Collagen I, suggesting that (+)-SYR upregulates the factors involved in the skin wound healing process (Fig. 3).
Fig. 3.
Effects of (+)-SYR on mRNA level of Hs68 cells
mRNA expression levels of (A) TGF-β, (Β) VEGF-C, (C) eNOS and (D) Collagen I in Hs68 cells were determined by real-time PCR. The cells were treated with (+)-SYR at the indicated concentrations for 48 h (N = 3). ∗p < 0.05 and ∗∗p < 0.01 vs control (SYR 0 μM).
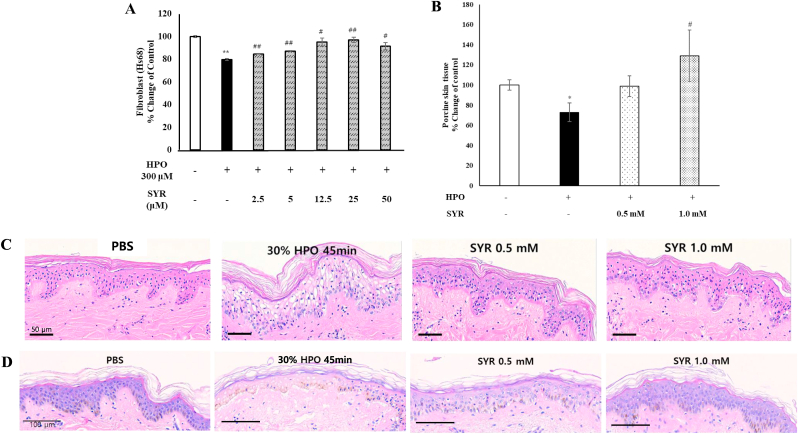
3.4. Effect of (+)-syringaresinol on cell viability against hydrogen peroxide induced Hs68 cells and ex vivo pig and human skin tissue
Hydrogen peroxide (HPO) is commonly used as an obnoxious stimulus to induce a skin injury model [8,25,48]. Exposure of Hs68 cells to HPO for 3 h decreased the viability to 80% but (+)-SYR treatment recovered them to as high as 97% at 25 μM (Fig. 4A). We also investigated the effects of (+)-SYR on HPO-induced skin injury in ex vivo pig skin model in which nearly all the skin components participating in the wound healing process exist. In a similar manner with Hs68 cells, tissue viability was determined using the MTT assay and the histology was done to examine the tissue integrity. When the ex vivo pig skin tissue was exposed to 30% HPO for 45 min, the tissue viability was reduced significantly (Fig. 4B). However, when 0.5 mM of (+)-SYR was treated 3 times every other day after HPO treatment, the chemical wound induced by HPO was repaired to an almost normal appearance. Compared to the negative control, 30% HPO induced vacuolization, spongiosis and pyknosis in some parts of the basal layer. However, these chemical-induced wounds were recovered almost to normal tissue after (+)-SYR treatment. In line with this, 1.0 mM of (+)-SYR restored the tissue viability almost to the level of intact untreated tissue (Fig. 4C). Similar pattern could be observed with the ex vivo human skin tissues (Fig. 4D)
Fig. 4.
Effect of (+)-SYR on cell viability for chemical wound
Effect of (+)-SYR on cell viability against HPO challenged (A) Hs68 (N = 3) and (B) ex vivo pig skin tissue (N = 4). P < 0.05 was considered significant (∗p < 0.05 vs control, #p < 0.05 and ##p < 0.01 vs HPO). (C) Treated ex vivo pig skin or (D) ex vivo human skin was processed and H&E stained. A representative microphotograph is shown. Scale bar = 100 μm. NC; negative control.
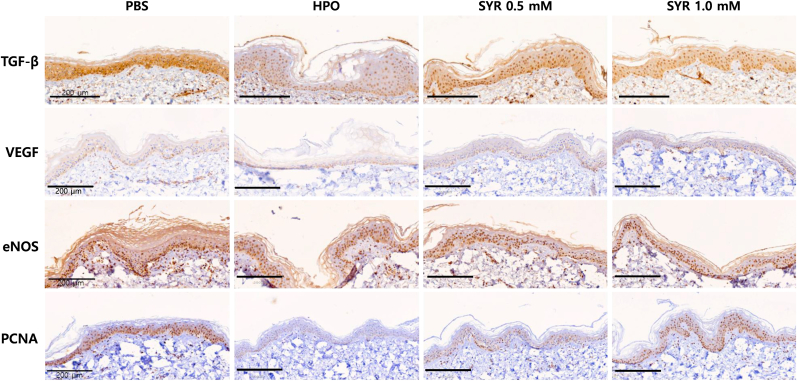
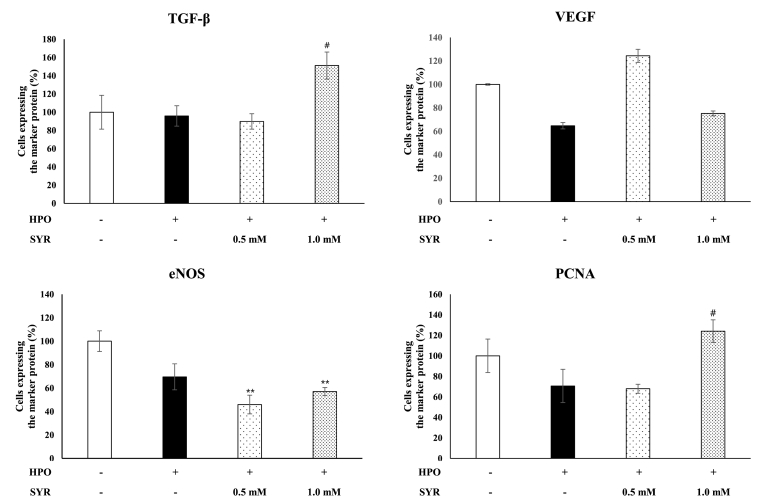
3.5. Immunohistochemical staining of key protein components of the wound healing in ex vivo human skin model
The wound healing effects of (+)-SYR with respect to TGF-β, VEGF and eNOS as confirmed at the cellular level in Hs68 were evaluated at the tissue level. Also, cellular proliferation was assessed by monitoring the expression of proliferating cell nuclear antigen (PCNA). The human ex vivo skin tissues treated with (+)-SYR were examined with IHC staining using antibodies against TGF-β, VEGF, eNOS and PCNA (Fig. 5). When exposed to HPO, TGF-β, and PCNA decreased compared to intact tissue, but (+)-SYR significantly recovered them. However, for VEGF and eNOS, it was confirmed that (+)-SYR treatment could not recover the HPO-induced changes (Fig. 6).
Fig. 5.
IHC staining of key protein components of the wound healing
IHC images of ex vivo human skin with antibodies against TGF-β, VEGF, eNOS and PCNA. The regions in which the expression of the specific protein is significantly higher than the negative control are indicated with red arrows. Scale bar = 200 μm. Representative images of tissue sections.
Fig. 6.
Quantification of the cells expressing marker proteins in IHC images
DAB-positive pixels were quantified in the epidermis using Qupath software. The DAB positive pixels were normalized with total stained pixel counts. Data are presented as the mean ± SEM (N = 4 fields per a group). ∗p < 0.05 vs control, #p < 0.05 and ##p < 0.01 vs HPO.
4. Discussion
This study was undertaken to examine the effects of (+)-SYR on the skin wound healing using human dermal fibroblast cell line and ex vivo human skin tissues. (+)-SYR promoted the migration and proliferation of fibroblasts after a scratch wound. A protective effect of (+)-SYR against chemical-induced wound was also confirmed in Hs68 cells as determined by increased survival of Hs68 cells. It appears that the wound healing effects of (+)-SYR were attributable to the upregulation of TGF-β, eNOS, VEGF-c and collagen-I in fibroblasts. Most importantly, the wound healing effects of (+)-SYR was confirmed in HPO induced skin injury model using ex vivo human and pig skin tissues, substantiating the clinical applicability of (+)-SYR for the human skin wound healing.
Wound healing requires the coordinated completion of different cellular activities, including phagocytosis, chemotaxis, mitogenesis, and synthesis of components of the extracellular matrix, and is carefully regulated [24]. These activities occur in a cascade with the appearance of a variety of cell types in the wound during different stages of the healing process. The primary cell types in the skin, fibroblasts, have long been known to respond to mechanical forces [21,57]. They have been extensively studied with respect to the extracellular matrix (ECM) modulation in response to their microenvironment changes [19,39,45]. In this study, (+)-SYR up-regulated the mRNA level of TGF-β and VEGF in fibroblast. TGF-β is a multifunctional growth factor that has pleiotropic effects on wound healing by regulating cell proliferation and migration, differentiation, ECM production, and immune modulation [40]. With the assistance of platelet released PDGF and VEGF, and fibroblast growth factor (FGF), endothelial cells proliferate and angiogenesis ensues. This process is essential for the synthesis, deposition, and organization of a new ECM [5,26].
Here, to evaluate the wound healing effect of (+)-SYR identified in human fibroblast, ex vivo human and pig skin model was used. The skin is an organ composed of epidermis, dermis, and subcutaneous tissue. The three layers of the skin form a barrier to effectively defend against the external environment, transmit sensory information, and play an important role in maintaining homeostasis. Traditionally, the wound repair model that has been used as a dermal route is an acute wounding in the mouse in vivo. Mice are very tractable for mechanistic studies and their effectiveness has been demonstrated, particularly in aging and diabetes [1]. However, species differences in skin structure and healing dynamics have not yet been resolved, despite the general similarities in wound healing between mouse and human. Recently, various skin equivalent systems have been developed for culturing skin in laboratory settings from artificial matrix and isolated skin cells [17,53]. Although these models are more capable of mimicking human skin compared to most in vitro cell-based approaches, they still cannot fully simulate the native tissue environment, and are generally too fragile to damage reproducibly. Furthermore, recent studies have demonstrated that ex vivo human and pig skin tissue retain resident immune cells that are obviously involved in its repair [18,27,56]
Of particular note in this study was that TGF-β was significantly increased when (+)-SYR was treated at the cellular and tissue level while eNOS and VEGF was not reproduced in ex vivo tissue models. The normal wound healing process depends on the appropriate levels of growth factors and cytokines to allow cellular responses to be mediated in a coordinated manner [22]. In addition, since TGF-β is known to be involved in the regulation of proliferation of fibroblasts, collagen synthesis in fibroblasts, generation of wound granulation tissue [13], and differentiation of fibroblasts into myofibroblasts in granulation tissue [15], the upregulation of TGF-β was evaluated to play a very important role in wound healing effects of SYR. Further corroborating this, SYR promoted the cell proliferation at cell and tissue levels.
Wound healing is an elaborate process including cell proliferation, cell cycle progression, cell migration, and the synthesis and secretion of ECM molecules involved in various signaling pathways [23]. There are many signaling pathways involved in skin cell proliferation and tissue repair. AKT/mTOR signaling has a critical effect on many aspects of tissue repair such as cell proliferation, protein translation, energy metabolism, and wound healing [44]. Also, β-catenin is elevated in mesenchymal cells during the proliferative phase [9] and is thought to regulate dermal fibroblast proliferation rate, motility and invasiveness [10]. Wnt5a signaling and Wnt/Ca2+ pathway are also known to control cell migration, although whether they promote or suppress cell motility seems to depend on cell types involved [35,54]. Therefore, it is necessary to investigate the effects of (+)SYR on these key mechanisms associated with cell proliferation and migration in the future to further explore the utility of (+)SYR for the skin regeneration
In conclusion, we demonstrated that (+)-SYR, derived from ginseng berry, can promote the skin wound healing process by accelerating cell proliferation and skin regeneration through inducing the expression levels of TGF-β. Considering the increasing public interest on ginseng and related phytochemicals, we believe that (+)-SYR can be a potential new cosmetic ingredient or dermatological drug for skin regeneration and wound management.
Declaration of competing interest
None
Acknowledgments
This work was supported by grants from National Research Foundation of Korea (Grant No. 2021R1A2C2013347 and MSIT 2018R1A5A2025286).
Contributor Information
Sang-Jip Nam, Email: sjnam@ewha.ac.kr.
Kyung-Min Lim, Email: kmlim@ewha.ac.kr.
References
- 1.Ansell D.M., Holden K.A., Hardman M. Animal models of wound repair: are they cutting it? Exp Dermatol. 2012;21(8):581–585. doi: 10.1111/j.1600-0625.2012.01540.x. [DOI] [PubMed] [Google Scholar]
- 2.Attele A.S., Wu J.A., Yuan C.-S. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58(11):1685–1693. doi: 10.1016/s0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 3.Bajpai V.K., Alam M.B., Quan K.T., Ju M.-K., Majumder R., Shukla S., Huh Y.S., Na M., Lee S.H., Han Y.-K. Attenuation of inflammatory responses by (+)-syringaresinol via MAP-Kinase-mediated suppression of NF-κB signaling in vitro and in vivo. Sci Rep. 2018;8(1):1–10. doi: 10.1038/s41598-018-27585-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bankhead P., Loughrey M.B., Fernández J.A., Dombrowski Y., McArt D.G., Dunne P.D., McQuaid S., Gray R.T., Murray L.J., Coleman H.G. QuPath: open source software for digital pathology image analysis. Sci Rep. 2017;7(1):1–7. doi: 10.1038/s41598-017-17204-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barrientos S., Stojadinovic O., Golinko M.S., Brem H., Mjwr Tomic-Canic, regeneration . Vol. 16. 2008. pp. 585–601. (Growth Factors and Cytokines in Wound Healing). 5. [DOI] [PubMed] [Google Scholar]
- 6.Bennett S., Griffiths G., Schor A., Leese G., Schor S. Growth factors in the treatment of diabetic foot ulcers. Br J Surg. 2003;90(2):133–146. doi: 10.1002/bjs.4019. [DOI] [PubMed] [Google Scholar]
- 7.Bielefeld K.A., Amini-Nik S., Alman B.A. Cutaneous wound healing: recruiting developmental pathways for regeneration. Cellular and Molecular Life Sciences. 2013;70(12):2059–2081. doi: 10.1007/s00018-012-1152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cai C., Guo Z., Yang Y., Geng Z., Tang L., Zhao M., Qiu Y., Chen Y., He P. Inhibition of hydrogen peroxide induced injuring on human skin fibroblast by Ulva prolifera polysaccharide. International Journal of Biological Macromolecules. 2016;91:241–247. doi: 10.1016/j.ijbiomac.2016.05.071. [DOI] [PubMed] [Google Scholar]
- 9.Cheon S., Poon R., Yu C., Khoury M., Shenker R., Fish J., Alman B.A. Prolonged β-catenin stabilization and tcf-dependent transcriptional activation in hyperplastic cutaneous wounds. Laboratory Investigation. 2005;85(3):416–425. doi: 10.1038/labinvest.3700237. [DOI] [PubMed] [Google Scholar]
- 10.Cheon S.S., Cheah A.Y., Turley S., Nadesan P., Poon R., Clevers H., Alman B.A. β-Catenin stabilization dysregulates mesenchymal cell proliferation, motility, and invasiveness and causes aggressive fibromatosis and hyperplastic cutaneous wounds. Proceedings of the National Academy of Sciences. 2002;99(10):6973–6978. doi: 10.1073/pnas.102657399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi W., Kim H.S., Park S.H., Kim D., Hong Y.D., Kim J.H., Cho J.Y. Syringaresinol derived from Panax ginseng berry attenuates oxidative stress-induced skin aging via autophagy. Journal of Ginseng Research. 2022;46(4):536–542. doi: 10.1016/j.jgr.2021.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christensen L.P. Ginsenosides: chemistry, biosynthesis, analysis, and potential health effects. Advances in Food and Nutrition Research. 2008;55:1–99. doi: 10.1016/S1043-4526(08)00401-4. [DOI] [PubMed] [Google Scholar]
- 13.Clark R., Nielsen L.D., Welch M.P., McPherson J.M. Collagen matrices attenuate the collagen-synthetic response of cultured fibroblasts to TGF-beta. Journal of Cell Science. 1995;108(3):1251–1261. doi: 10.1242/jcs.108.3.1251. [DOI] [PubMed] [Google Scholar]
- 14.Demidova-Rice T.N., Durham J.T., Herman I.M. Wound healing angiogenesis: innovations and challenges in acute and chronic wound healing. Advances in Wound Care. 2012;1(1):17–22. doi: 10.1089/wound.2011.0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Desmoulière A., Geinoz A., Gabbiani F., Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. The Journal of Cell Biology. 1993;122(1):103–111. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dey L., Xie J., Wang A., Wu J., Maleckar S., Yuan C.-S. Anti-hyperglycemic effects of ginseng: comparison between root and berry. Phytomedicine. 2003;10(6–7):600–605. doi: 10.1078/094471103322331908. [DOI] [PubMed] [Google Scholar]
- 17.Diekmann J., Alili L., Scholz O., Giesen M., Holtkötter O., Brenneisen P. A three-dimensional skin equivalent reflecting some aspects of in vivo aged skin. Experimental Dermatology. 2016;25(1):56–61. doi: 10.1111/exd.12866. [DOI] [PubMed] [Google Scholar]
- 18.Dijkgraaf F.E., Matos T.R., Hoogenboezem M., Toebes M., Vredevoogd D.W., Mertz M., van den Broek B., Song J.-Y., Teunissen M., Luiten R.M. Tissue patrol by resident memory CD8+ T cells in human skin. Nature Immunology. 2019;20(6):756–764. doi: 10.1038/s41590-019-0404-3. [DOI] [PubMed] [Google Scholar]
- 19.Driskell R.R., Lichtenberger B.M., Hoste E., Kretzschmar K., Simons B.D., Charalambous M., Ferron S.R., Herault Y., Pavlovic G., Ferguson-Smith A.C.J.N. Vol. 504. 2013. pp. 277–281. (Distinct Fibroblast Lineages Determine Dermal Architecture in Skin Development and Repair). 7479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duda D.G., Fukumura D., Jain R.K. Role of eNOS in neovascularization: NO for endothelial progenitor cells. Trends in Molecular Medicine. 2004;10(4):143–145. doi: 10.1016/j.molmed.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Duscher D., Maan Z.N., Wong V.W., Rennert R.C., Januszyk M., Rodrigues M., Hu M., Whitmore A.J., Whittam A.J., Mtjjob Longaker. Vol. 47. 2014. pp. 1997–2005. (Mechanotransduction and Fibrosis). 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Finnson K.W., McLean S., Di Guglielmo G.M., Philip A. Dynamics of transforming growth factor beta signaling in wound healing and scarring. Advances in Wound Care. 2013;2(5):195–214. doi: 10.1089/wound.2013.0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gurtner G.C., Werner S., Barrandon Y., Longaker M. Wound repair and regeneration. Nature. 2008;453(7193):314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 24.Gurtner G.C., Werner S., Barrandon Y., Longaker M.T. Wound repair and regeneration. Nature. 2008;453(7193):314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 25.Hahn H.J., Kim K.B., An I.S., Ahn K.J., Han H.J. Protective effects of rosmarinic acid against hydrogen peroxide-induced cellular senescence and the inflammatory response in normal human dermal fibroblasts. Molecular Medicine Reports. 2017;16(6):9763–9769. doi: 10.3892/mmr.2017.7804. [DOI] [PubMed] [Google Scholar]
- 26.Hantash B.M., Zhao L., Knowles J.A., Lorenz H.P.J.F.B. Vol. 13. 2008. pp. 51–61. (Adult and Fetal Wound Healing). 1. [DOI] [PubMed] [Google Scholar]
- 27.He X., de Oliveira V.L., Keijsers R., Joosten I., Koenen H.J. Lymphocyte isolation from human skin for phenotypic analysis and ex vivo cell culture. JoVE. 2016;110 doi: 10.3791/52564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hinz B., Gabbiani G. Cell-matrix and cell-cell contacts of myofibroblasts: role in connective tissue remodeling. Thrombosis and Haemostasis. 2003;90(12):993–1002. doi: 10.1160/TH03-05-0328. [DOI] [PubMed] [Google Scholar]
- 29.Karppinen S.-M., Heljasvaara R., Gullberg D., Tasanen K., Pihlajaniemi T. Toward understanding scarless skin wound healing and pathological scarring. F1000Research. 2019;8 doi: 10.12688/f1000research.18293.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim C.-K., Cho D.H., Lee K.-S., Lee D.-K., Park C.-W., Kim W.G., Lee S.J., Ha K.-S., Goo Taeg O., Kwon Y.-G. Ginseng berry extract prevents atherogenesis via anti-inflammatory action by upregulating phase II gene expression. Evidence-based Complementary and Alternative Medicine 2012. 2012 doi: 10.1155/2012/490301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim H.-G., Cho J.-H., Yoo S.-R., Lee J.-S., Han J.-M., Lee N.-H., Ahn Y.-C., Son C.-G. Antifatigue effects of Panax ginseng CA Meyer: a randomised, double-blind, placebo-controlled trial. PloS One. 2013;8(4) doi: 10.1371/journal.pone.0061271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim J., Cho S.Y., Kim S.H., Kim S., Park C.-W., Cho D., Seo D.B., Shin S.S. Ginseng berry and its biological effects as a natural phytochemical. Natural Products Chemistry & Research. 2016 [Google Scholar]
- 33.Kim J., Toda T., Watanabe K., Shibuya S., Ozawa Y., Izuo N., Cho S., Seo D.B., Yokote K., Shimizu T. Syringaresinol reverses age-related skin atrophy by suppressing FoxO3a-mediated matrix Metalloproteinase–2 activation in copper/zinc superoxide Dismutase–Deficient mice. Journal of Investigative Dermatology. 2019;139(3):648–655. doi: 10.1016/j.jid.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 34.Kim J.H., Yi Y.-S., Kim M.-Y., Cho J.Y. Role of ginsenosides, the main active components of Panax ginseng, in inflammatory responses and diseases. Journal of Ginseng Research. 2017;41(4):435–443. doi: 10.1016/j.jgr.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kurayoshi M., Oue N., Yamamoto H., Kishida M., Inoue A., Asahara T., Yasui W., Kikuchi AJCr. Vol. 66. 2006. pp. 10439–10448. (Expression of Wnt-5a Is Correlated with Aggressiveness of Gastric Cancer by Stimulating Cell Migration and Invasion). 21. [DOI] [PubMed] [Google Scholar]
- 36.Lu J.-M., Yao Q., Chen C. Ginseng compounds: an update on their molecular mechanisms and medical applications. Current Vascular Pharmacology. 2009;7(3):293–302. doi: 10.2174/157016109788340767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oh J.H., Joo Y.H., Karadeniz F., Ko J., Kong C.-S. Syringaresinol inhibits UVA-induced MMP-1 expression by suppression of MAPK/AP-1 signaling in HaCaT keratinocytes and human dermal fibroblasts. International Journal of Molecular Sciences. 2020;21(11):3981. doi: 10.3390/ijms21113981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park H.W., Cho S.Y., Kim H.H., Yun B.S., Kim J.U., Lee S.J., Park J. Enantioselective induction of SIRT1 gene by syringaresinol from Panax ginseng berry and Acanthopanax senticosus Harms stem. Bioorganic & Medicinal Chemistry Letters. 2015;25(2):307–309. doi: 10.1016/j.bmcl.2014.11.045. [DOI] [PubMed] [Google Scholar]
- 39.Paterno J., Vial I.N., Wong V.W., Rustad K.C., Sorkin M., Shi Y., Bhatt K.A., Thangarajah H., Glotzbach J.P., Gurtner G.C.J.W.R., et al. Vol. 19. 2011. pp. 49–58. (Akt-mediated Mechanotransduction in Murine Fibroblasts During Hypertrophic Scar Formation). 1. [DOI] [PubMed] [Google Scholar]
- 40.Penn J.W., Grobbelaar A.O., Kjjijob Rolfe, trauma The Role of the TGF-β Family in Wound Healing. Burns and Scarring: A Review. 2012;2(1):18. [PMC free article] [PubMed] [Google Scholar]
- 41.Pettet G., Byrne H., McElwain D., Norbury J. A model of wound-healing angiogenesis in soft tissue. Mathematical Biosciences. 1996;136(1):35–63. doi: 10.1016/0025-5564(96)00044-2. [DOI] [PubMed] [Google Scholar]
- 42.Porter S. The role of the fibroblast in wound contraction and healing. WOUNDS UK. 2007;3(1):33. [Google Scholar]
- 43.Proksch E., Brandner J.M., Jensen J.M. The skin: an indispensable barrier. Experimental Dermatology. 2008;17(12):1063–1072. doi: 10.1111/j.1600-0625.2008.00786.x. [DOI] [PubMed] [Google Scholar]
- 44.Qing C. The molecular biology in wound healing & non-healing wound. Chinese Journal of Traumatology. 2017;20(4):189–193. doi: 10.1016/j.cjtee.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rinkevich Y., Walmsley G.G., Hu M.S., Maan Z.N., Newman A.M., Drukker M., Januszyk M., Krampitz G.W., Gurtner G.C., Lorenz H.P.J.S. Vol. 348. 2015. p. aaa2151. (Identification and Isolation of a Dermal Lineage with Intrinsic Fibrogenic Potential). 6232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schultz G.S., Davidson J.M., Kirsner R.S., Bornstein P., Herman I.M. Dynamic reciprocity in the wound microenvironment. Wound Repair and Regeneration. 2011;19(2):134–148. doi: 10.1111/j.1524-475X.2011.00673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shao Z.-H., Xie J.-T., Hoek T.L.V., Mehendale S., Aung H., Li C.-Q., Qin Y., Schumacker P.T., Becker L.B., Yuan C.-S. Antioxidant effects of American ginseng berry extract in cardiomyocytes exposed to acute oxidant stress. Biochimica et Biophysica Acta (BBA)-General Subjects. 2004;1670(3):165–171. doi: 10.1016/j.bbagen.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 48.Shen T., Duan C., Chen B., Li M., Ruan Y., Xu D., Shi D., Yu D., Li J., Wang C. Tremella fuciformis polysaccharide suppresses hydrogen peroxide-triggered injury of human skin fibroblasts via upregulation of SIRT1. Molecular Medicine Reports. 2017;16(2):1340–1346. doi: 10.3892/mmr.2017.6754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shi W., Wang Y., Li J., Zhang H., Ding L. Investigation of ginsenosides in different parts and ages of Panax ginseng. Food Chemistry. 2007;102(3):664–668. [Google Scholar]
- 50.Shin H.R., Kim J.Y., Yun T.K., Morgan G., Vainio H. The cancer-preventive potential of Panax ginseng: a review of human and experimental evidence. Cancer Causes & Control. 2000;11(6):565–576. doi: 10.1023/a:1008980200583. [DOI] [PubMed] [Google Scholar]
- 51.Singer A.J., Clark R.A. Cutaneous wound healing. New England Journal of Medicine. 1999;341(10):738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 52.Song J., Jung K.J., Yang M.J., Han S.C., Lee K. Assessment of acute and repeated pulmonary toxicities of oligo(2-(2-ethoxy)ethoxyethyl guanidium chloride in mice. Toxicol Res. 2021;37(1):99–113. doi: 10.1007/s43188-020-00058-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vidal Yucha S.E., Tamamoto K.A., Nguyen H., Cairns D.M., Kaplan D.L. Human skin equivalents demonstrate need for neuro-immuno-cutaneous system. Advanced Biosystems. 2019;3(1) doi: 10.1002/adbi.201800283. [DOI] [PubMed] [Google Scholar]
- 54.Weeraratna A.T., Jiang Y., Hostetter G., Rosenblatt K., Duray P., Bittner M., Trent J.M. Wnt5a signaling directly affects cell motility and invasion of metastatic melanoma. Cancer Cell. 2002;1(3):279–288. doi: 10.1016/s1535-6108(02)00045-4. [DOI] [PubMed] [Google Scholar]
- 55.Werner S., Krieg T., Smola H. Keratinocyte–fibroblast interactions in wound healing. Journal of Investigative Dermatology. 2007;127(5):998–1008. doi: 10.1038/sj.jid.5700786. [DOI] [PubMed] [Google Scholar]
- 56.Wilkinson H.N., Kidd A.S., Roberts E.R., Hardman M.J. Human ex vivo wound model and whole-mount staining approach to accurately evaluate skin repair. JoVE. 2021;168 doi: 10.3791/62326. [DOI] [PubMed] [Google Scholar]
- 57.Wong V.W., Longaker M.T. Gurtner GC Soft tissue mechanotransduction in wound healing and fibrosis. Seminars in Cell & Developmental Biology. 2012;9:981–986. doi: 10.1016/j.semcdb.2012.09.010. Elsevier. [DOI] [PubMed] [Google Scholar]
- 58.Woodley D.T., O’Keefe E.J., Prunieras M. Cutaneous wound healing: a model for cell-matrix interactions. Journal of the American Academy of Dermatology. 1985;12(2):420–433. doi: 10.1016/s0190-9622(85)80005-0. [DOI] [PubMed] [Google Scholar]
- 59.Yuan H.-D., Kim J.T., Kim S.H., Chung S.H. Ginseng and diabetes: the evidences from in vitro, animal and human studies. Journal of Ginseng Research. 2012;36(1):27. doi: 10.5142/jgr.2012.36.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]