Abstract
The oral involvement in the Hematopoietic Stem Cell Transplantation is well described in the literature. The goal of the dental treatment and management of the oral lesions related to the HSCT is to reduce the harm caused by preexisting oral infection or even the worsening of oral acute/chronic GVHD and late effects. The aim of this guideline was to discuss the dental management of patients subjected to HSCT, considering three phases of the HSCT: pre-HSCT, acute phase, and late phase. The literature published from 2010 to 2020 was reviewed in order to identify dental interventions in this patient population. The selected papers were divided into three groups: pre-HSCT, acute and late, and were reviewed by the SBTMO Dental Committee's members. When necessary, an expertise opinion was considered for better translating the guideline recommendations to our population dental characteristics. This manuscript focused on the pre-HSCT dental management. The objective of the pre-HSCT dental management is to identify possible dental situations that can worsening during the acute phase after the HSCT. Each guideline recommendations were made considering the Dentistry Specialties. The clinical consensus on dental management prior to HSCT provides professional health caregivers with clinical setting-specific information to help with the management of dental problems in patients to be subjected to HSCT.
Keyword: Hematopoietic stem cell transplantation, Dental management, Consensus, Dentistry, Brazilian guideline
Introduction
HSCT-related oral complications is recognized in the literature and is estimated to be present in about 80% of patients undergoing this therapeutic modality.1, 2, 3, 4 These oral complications can directly impact complications and the patient's quality of life during HSCT.4, 5, 6, 7, 8, 9
The management of the oral cavity in patients undergoing HSCT is of utmost importance.5,10 This management can be subdivided into three stages; pre-HSCT; during HSCT, and late phase of HSCT.5 Each phase has specific characteristics in terms of oral complications and oral management. Thus, the importance of pre-existing oral infections in the pre-HSCT phase, the oral assessment during oral complications in the initial phase, and the diagnosis and management of chronic oral oral/dental complications in the late phase have been discussed.5,10
In the light of the reports, a group of oral medicine experts was invited by the Brazilian Society of Cellular Therapy and Bone Marrow Transplantation (SBTMO) to develop a guideline for HSCT patients’ dental care, taking into consideration the oral health characteristics of the Brazilian population.
Therefore, this is the first in a series of 3 articles that will discuss the dental management of the HSCT population. These manuscripts intend to provide continuing education to fellow dental surgeons on this specific area of care and, consequently, to facilitate HSCT patient's access to dental care.
Objective
The aim of this positioning manuscript was to provide recommendations by the Dental Committee of the Brazilian Society of Bone Cell Transplantation (SBTMO) for basic oral healthcare and dental treatment for patients undergoing the HSCT.
This article intends to detail the specific management guideline of various dental conditions that should be considered before the HSCT.
Review methods
A narrative review was conducted on the database MEDLINE/PubMed and Embase. The primary outcome was to retrieve original data on relevant articles containing dental protocols in patients undergoing the HSCT. All original studies in English, Spanish or Portuguese assessing oral health, oral complications and dental procedures in adult and pediatric patients subjected to HSCT were reviewed by the group of oral medicine experts. Manuscripts written in different languages other than the ones mentioned above, conference abstracts, case reports, and articles including solid tumors were excluded.
The panel of experts reviewed the selected articles and then discussed the most important aspects involving oral health through virtual meetings. Thus, the recommendations in these 3 steps guide was obtained based on literature data and on the experts’ clinical experience.
The articles and the experts were divided into three groups:1. Oral care before HSCT (oral preparation for HSCT); 2. Oral care during HSCT (until neutrophil engraftment) and 3. Special topics on oral health.
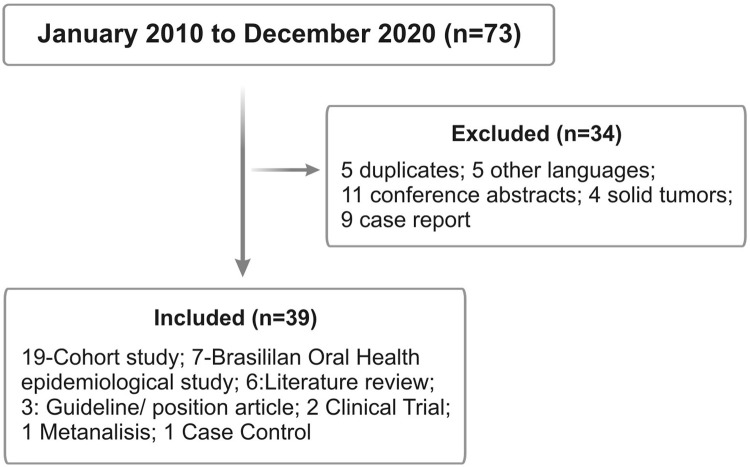
For this first article, the search strategy combined all key terms for HSCT, including the key terms separately or in combination: management, dental, oral, tooth, procedures, prophylaxis and surgery, health, Brazil, infection, submucosal scaling, periodontal and dentoalveolar surgery, endodontic, restorative, and orthodontic treatment published from the period of January 2010 to December of 2020 (Figure 1).
Figure 1.
Flowchart of the study.
Review/results
General consideration
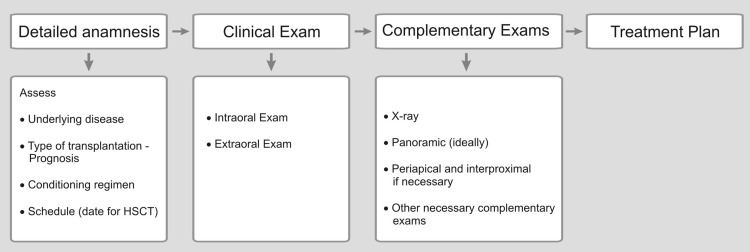
The objective of pre-HSCT dental management is to minimize risks of dental origin infections that may increase the patient's morbidity, during the first 100 days of HSCT.11, 12, 13 It is important to establish good communication between the transplant team and the dental group for the patient's best care. Dental treatment planning requires that the dentist (oral medicine) understands the basic principles of the diseases that lead to HSCT and the different treatment protocols involved in patient's care (Figure 2, Figure 3).5
Figure 2.
Flowchart for initial dental appointment.
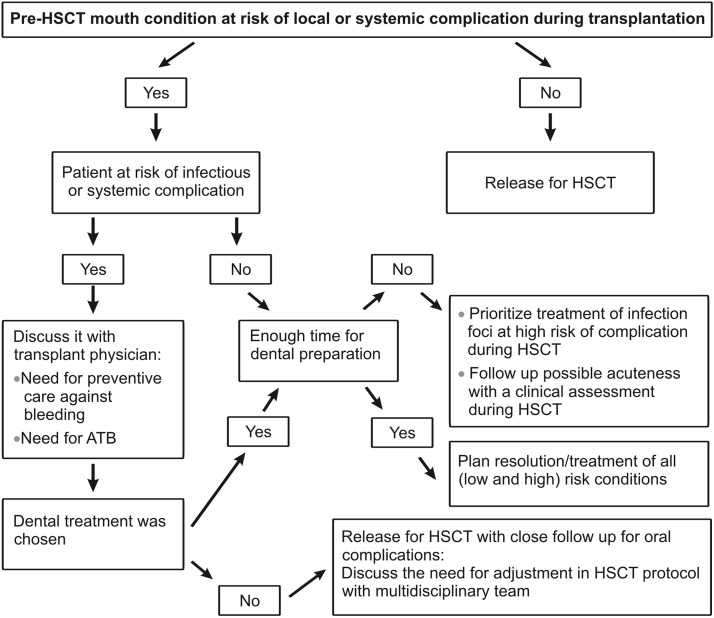
Figure 3.
Flowchart for proposing a dental treatment plan before HSCT.
Abbreviations: ATB- antibiotic.
Ideally, an initial dental appointment should be scheduled as sooner as possible to allow sufficient time for the dental treatment needed and to give time for tissues to heal after surgical procedure, preferably with a healing period of 2 to 3 weeks after the completion of the dental treatment (Figure 2).5,14 Previous dental assessment is indispensable regardless the time available for transplantation.
In mouth preparation, the resolution of infection foci must be prioritized. The indication of a surgical procedure out of the ideal time for healing should be discussed according to each case. Nevertheless, time constraints and patient´s medical condition may require modification of the dental treatment plan (Figure 3).5,15,16
Elective additional dental needs identified in pre-HSCT examination should be postponed to when the patient's overall health status allows it.5
Special considerations
Laboratory tests, such as blood count and platelets count, should be performed before dental treatment, mostly if the patient was underdoing any treatment that could increase the risk of bleeding or the risk of systemic and/or local infection. Other blood exam such coagulation screening should be performed in those patients which may present coagulation disorders.17
Antibiotic prophylaxis (AP), antibiotic treatment (AT), and the dental treatment plan need to be carefully discussed between the medical, dental, and multidisciplinary team before the dental treatment. The discussion should consider the underlying disease status, the current patient's immune status, and the risk of the dental procedure for local or systemic infection.13,17,18 (Table 1)
Table 1.
Oral pre-HSCT conditions, classification of risk of local and systemic complications due to previous dental conditions and Dental Treatment recommendation.
| Dental Condition |
Specific Condition |
Classification for local or systemic complication related to previous oral condition | Treatment recommendation |
Recommendations for dental management |
|---|---|---|---|---|
| Chronic Periodontal Disease: PSR(Periodontal Screening & Recording) | 0 – (WHO periodontal color band): during probing the color band still visible at the gingival sulcus . No calculus or defective margin . Healthy gingival tissue with no bleeding at probing |
Low | Oral Hygiene Instructions (OHI) | No special recommendations *In the absence of the WHO periodontal prob it must be used another method that allows periodontal examination following the same principles presented in PSR index. |
| 1 - Color band still completely visible at the sextant deeper gingival sulcus . No calculus or defective margin with restoration . Bleeding at probing |
Low | OHI + Prophylaxis Removal of the bacterial plaque (including subgingival plaque) |
No special recommendations | |
| 2 - Color band still completely visible at the sextant deeper gingival sulcus . Supra- or subgingival calculus and/or . Defective margin with restoration |
Low | Root surface debridement (Removal of granulation tissue and supra- and subgingival calculus) . Removal of retentive areas . Ideal time of 1 week for transplantation |
In case of subgingival scraping and/or potential gingival lesion: . Check blood count (platelet count) . Check coagulogram if necessary . Discuss the need for prophylaxis/ATB therapy |
|
| 3 - Color band partially visible Pockets 3.5 – 5.5 mm |
Moderate | . Complete Periodontal Exam . OHI + Root surface debridement . Ideal time of 2 weeks for transplantation, at least 1 week * |
. Check blood count (platelet count) . Check coagulogram if necessary . Discuss the need for prophylaxis/ATB therapy |
|
| 4 – Color band completely invisible Pockets: > 5.5 mm – consider 6.0 mm |
High | . OHI + Root surface debridement . Complex treatment or difficulty of periodontal decontamination – indicate extraction at pre-transplantation . Ideal time of 2 weeks for transplantation, at least 1 week* |
. Check blood count (platelet count) . Check coagulogram if necessary . Discuss the need for prophylaxis/ATB therapy |
|
| Acute Periodontal Disease |
Periodontal abscess Symptoms like pain and sings like tenderness of the gingiva, swelling, presence of an ovoid elevation in the gingiva along the lateral part of the root, suppuration on probing, deep periodontal pocket (7.3–9.3 mm), bleeding on probing, and increased tooth mobility. Bone loss was normally observed in the radiographic examination. |
High | . This condition must be solved before transplantation. Subgingival irritant factor removal and antibiotic therapy. . Tooth extraction if the mobility was greater or equal than grade III and/or periodontal pocket was deeper than 6mm. . Ideal time of 2 weeks for transplantation, at least 1 week* |
. Check blood count (platelet count) . Check coagulogram if necessary . Discuss the need for prophylaxis/ATB therapy |
|
Necrotizing periodontal disease .Intraoral findings are necrosis and ulcer in the interdental papilla, gingival bleeding, pain, pseudomembrane formation, and halitosis. Extraoral signs included adenopathy and/or fever. |
High | . Debridement and antibiotic therapy before the transplantation. . Periodontal status must be observed for therapeutic decision . Ideal time of 2 weeks for transplantation, at least 1 week* |
. Check blood count (platelet count) . Check coagulogram if necessary . Discuss the need for prophylaxis/ATB therapy |
|
|
Endo-Perio . Presence of a periodontal pocket reaching or close to the apex combined with absence of pulp vitality. |
High | . Tooth extraction . Ideal time of 2 weeks for transplantation, at least 1 week |
. Check blood count (platelet count) . Check coagulogram if necessary . Discuss the need for prophylaxis/ATB therapy |
|
| Dental caries |
Caries with no pulp involvement Shallow cavity with no risk of pulp exposure during restorative treatment and with no symptomatology |
Low | . Definite restorative treatment whenever possible . Atraumatic restorative treatment if there is no time for definite treatment . Follow-up |
No special recommendations |
|
Caries with pulp involvement, and appropriate tooth structure Consider irreversible pulpitis and deep caries at risk of exposure during treatment |
High | . If there is enough time for HSCT: endodontic treatment and restoration or extraction. . If there is not enough time: consider provisional curative treatment or extraction . Follow-up |
In case of extraction: . Check blood count . Discuss the need for prophylaxis/ATB therapy |
|
|
Caries with pulp involvement, no appropriate tooth structure |
High | . Tooth extraction - If there is not enough time or no clinical condition for extraction -endodontic curative treatment - follow-up |
In case of extraction: . Check blood count (platelet count) . Check coagulogram if necessary . Discuss the need for prophylaxis/ATB therapy |
|
| Dental conditions that present risk for mucosal trauma | High for local complication | . Temporary or definite resolution of the condition depending on available time | . In case of bloody procedure: . check blood count (platelet count) . Check coagulogram if necessary . Discuss the need for prophylaxis/ATB therapy |
|
| Third molar |
Asymptomatic partially erupted tooth |
Low | . Periodontal assessment (see periodontal criteria) . Careful hygiene of the pericoronal hood. |
In case of bloody procedure: . Check blood count (platelet count) . Check coagulogram if necessary . Discuss the need for prophylaxis/ATB therapy |
|
Symptomatic partially erupted tooth |
High | . Periodontal assessment (see periodontal criteria) .If there is time for healing (14 days): .Orpeculotomy or extraction . If there is no time for healing: local cleaning using chlorhexidine 0.12% + discuss antibiotic therapy * |
In case of bloody procedure: . Check blood count (platelet count) . Check coagulogram if necessary . Discuss the need for prophylaxis/ATB therapy |
|
| Impacted tooth with no radiographic lesion | Low | . Maintenance/ observation |
. No recommendations | |
| Endodontic Problem | Incomplete endodontic treatment with no symptoms or radiographic signs | Low | . Follow-up | . No recommendations |
| Acute periapical infection (periapical abscess) | High | . Consider the possibilities of periapical infection control: extraction or endodontic treatment |
. Check blood count (platelet count) . Check coagulogram if necessary . Discuss the need for prophylaxis/ATB therapy |
|
| Chronic periapical change (radiographic lesion with no symptoms during percussion) | Low | . Tooth with endodontic treatment: - clinical follow-up . Tooth with no endodontic treatment - depending on sufficient time until HSCT and on the possibility of resolution of the periapical picture: endodontic treatment; intracanal medication therapy, extraction *Important to exclude other changes that may simulate periapical lesion |
. In case of extraction: . Check blood count (platelet count) . Check coagulogram if necessary . Discuss the need for prophylaxis/ATB therapy |
|
| Residual roots | Asymptomatic roots and no periapical lesion | Low | -Extraction*, or clinical follow-up if there is no sufficient time until HSCT. | . Check blood count (platelet count) . Check coagulogram if necessary . Discuss the need for prophylaxis/ATB therapy |
| Roots with percussion symptoms and/or with periapical lesion | High | -Extraction | . Check blood count (platelet count) . Check coagulogram if necessary . Discuss the need for prophylaxis/ATB therapy |
|
| Orthodontic appliance | High (oral mucositis) | . Mandatory removal The maintenance of fixed retainers, away from the oral mucosa and in patients with good hygiene can be discussed |
. No recommendations | |
| Dental pediatrics |
Teeth in physiological exfoliation |
High (If thrombocytopenia otherwise moderate risk) |
-Deciduous teeth with more than 2/3 of reabsorbed root and grade 3 mobility must be extracted . If there is enough time: extraction . If there is not enough time: follow-up |
. Check blood count (platelet count) . Check coagulogram if necessary . Discuss the need for prophylaxis/ATB therapy |
| Deciduous teeth with endodontic treatment and symptomatic, or with mobility, or with radiographic lesions | High | - Extraction .If there is not enough time: treatment with local care and use of antibiotic therapy |
. Check blood count (platelet count) . Check coagulogram if necessary . Discuss the need for prophylaxis/ATB therapy |
|
| Deciduous teeth with pulpar and/or periapical involvement | High | -Extraction . If there is not enough time: treatment with local care and use of antibiotic therapy |
. Check blood count (platelet count) . Check coagulogram if necessary . Discuss the need for prophylaxis/ATB therapy |
|
| Permanent teeth with incomplete eruption and gingival hood, no symptoms | Low | . If there is enough time: Orpeculotomy . If there is not enough time: Optimization of oral hygiene and local cleaning using chlorhexidine |
-In case of Orpeculotomy: . Check blood count (platelet count) . Check coagulogram if necessary . Discuss the need for prophylaxis/ATB therapy |
|
| Permanent teeth with incomplete eruption and gingival hood, with inflammatory/infectious symptoms | High | . Orpeculotomy | . Check blood count (platelet count) . Check coagulogram if necessary . Discuss the need for prophylaxis/ATB therapy |
|
For mouth preparation, resolution of infectious foci must be prioritized. The indication of surgical procedure out of the ideal time for healing must be discussed according to each case.
⁎⁎ All surgical manipulations must consider the potential risks of bone necrosis of the maxillaries due to previous exposure to radiation therapy or induction drugs.
Dental management
1 – Basic oral care
The maintenance of oral hygiene, through tooth brushing, must be reinforced and maintained throughout the HSCT period. It has been reported the beneficial effect of chlorhexidine mouthwash use in association with tooth brushing on the reduction of gingival inflammation.19,20 Therefore, chlorhexidine mouth wash should be recommended as an auxiliar therapy in periodontal treatment.20,21 Chlorhexidine can also be recommended for biofilm control for those patients who present exposed necrotic bone.19,22
The anticariogenic benefits of fluoride therapy is well documented in the literature.23 Compromised oral health, high caries risk, alteration of caries-related factors, and dysbiosis of oral microbiota were related to cancer treatment in children24 and in patients who underwent HSCT.25, 26, 27 These findings may be critical to the development of strategies for personalized preventive management of dental caries for this patient population.24
2 – Dental assessment
2.1 – Periodontal treatment – the presence of periodontal disease has been considered a risk condition for local and systemic infection and local bleeding complications in the first 100 days of HSCT.28, 29, 30, 31 It is important to establish a routine periodontal screening in order to facilitate the treatment planning as well as the periodontal follow-up in this patient population.29 In this article, Periodontal Screening & Recording (PSR)32 was used as a guidance method for comprehensive periodontal assessment. The recommendations for periodontal management based on the PSR criteria, the risk for local or systemic complication of the previous periodontal condition to the initial phase of the HSCT, and the timing for its execution and tissue healing were detailed in Table 1.
2.2 – Caries treatment –restorative treatment usually does not represent a risk of complication for these patients. Extensive dental caries with potential endodontic involvement can represent a risk for pulpitis and infection.13,33 Some dental conditions, such sharper broken dental crown, can represent risk for mucosal injury mostly during the mucositis period. These situations should be managed before the HSCT (Table 1).
2.3 – Exodontia – dental extraction represents risk of local/systemic infection and bleeding. As well as in all invasive dental treatment, special concern about the risk of infection and bleeding must be part of the dental assessment plan and must be discussed with the medical team. Proper attention should be devoted to those patients who present risk of developing osteonecrosis of the jaw regarding the use of antibiotics prophylaxis.34, 35, 36 Other clinical situations, such as residual root, which require invasive approaches are detailed in Table 1.
2.4 – Third Molar – third molar extraction can present a high incidence of complications37 that might impair the HSCT schedule. In this guideline, third molar extraction was recommended for symptomatic non-erupted and semi-erupted third molar or for those patients at high risk of developing chronic GVHD. It is mandatory to consider the timing for complete wound healing before the HSCT conditioning regimen initiation. If there is not available healing time, consider non-invasive management. (Table 1)
2.5 - Endodontic treatment – due to the different endodontic treatment needs and the uncertainty of the time required for apical sterilization, there is a discussion on the literature focusing on the risk of acute infection reactivation during the immunosuppression period of HSCT.13,33 In this guideline, the recommendation for endodontic treatment took into consideration the presence of signs or symptoms of infection and, within the required timeframe for the HSCT initiation. (Table 1)
2.6 - Orthodontic appliance – orthodontic appliances can impair the oral basic care and raise the risk of mucosal harm, mostly during the oral mucositis period.38 In this guideline, the recommendation is to avoid the use of removable appliances and withdraw the fixed orthodontic apparatus before the initiation of the conditioning regimen up to day 100 of the HSCT (Table 1).
2.7 – Children Patients – for the pediatric HSCT population, the dental planning should take into consideration the same rationale described above in terms of basic oral care, good relationship between the medical and dental team, and dental management strategies. However, the recommendation for oral hygiene, including toothbrushing technique and mouthwash, should be personalized based on the children age and the motor coordination.39, 40, 41, 42, 43 The dental approach for deciduous teeth must consider the degree of the deciduous root resorption and the degree of the permanent root growth (Table1).40
Discussion and conclusion
The development of a guideline of dental care addressed to patients to be subjected to HSCT, aligned with the population characteristics of oral health in our country, provides tools to general dentists and oral medicine professionals in providing a safe dental treatment to this particular group of patients.
The last oral health survey conducted by the Brazilian Ministry of Health44 showed that periodontal disease, as well as dental caries, are the oral infections of the highest prevalence and incidence in our population. These findings have been observed since childhood and are perpetuated into adulthood, probably being responsible for the high incidence of edentulous adults (>60 years) in Brazil.44
The impact of pre-HSCT dental treatment on the early phase events of HSCT, such as oral mucositis, is not well determined in the literature. However, it is common sense that oral cavity preparation, as well as the removal of infectious foci, should be performed in these patients to decrease the risk of developing local and/or systemic infections of odontogenic origin.5,6
A study carried out in a Brazilian tertiary service found a high incidence of periodontal (33%) and endodontic (15%) foci, and active caries (27%) in pre-transplant patients. In the same study, oral diseases and poor oral health indices were more frequent in children and young people with chronic onco-hematologic diseases. About 50% of these patients showed some necessity for dental intervention before HSCT.45 These findings have been corroborated by studies published in the literature.16,46,47
In Brazil, not every Bone Marrow Transplant Services have a dentist as a member of the multidisciplinary team. In these situations, patients need to be evaluated by dentists working in public dental services or in private practices, where the professionals may not be familiar with the particularities of dental care for this specific group of patients. This guideline was written taking into consideration the characteristics of oral health described in the Brazilian Oral Health Survey44 and the most frequent dental conditions observed in the clinical routine of the Dentistry services of the Transplant Centers in Brazil.
The details of the procedures described here considered the general health conditions of the patient and the time between the end of the dental treatment and the beginning of the HSCT emphasizing the importance of the close work between the dentist and the multidisciplinary team. We hope to facilitate the patient's access to safe and effective dental treatment.
Statement of clinical relevance
The complications in the oral cavity may increase mortality and morbidity in the different phases of the hematopoietic stem cell transplantation. There is not a Brazilian guideline that guides the work of dentists with patients subjected to HSCT. This first part of the consensus offers guidance towards pre-HSCT dental intervention.
Funding information
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Conflicts of interest
None.
Acknowledgements
We thank the Brazilian Bone Marrow Transplantation Society (SBTMO) for the support and trusting in our team. We also thank Nathalia Cristine André for her assistance.
Footnotes
On behalf of the Dental Committee of the Brazilian Society of Gene Therapy and Bone Marrow Transplantation (SBTMO)
References
- 1.Angelo SN, Yamaguti GG, Lourenco GJ, Honma HN, Silva EF, Sagarra AF, et al. The role of the CYP1A1 gene defects in cancer risk in southeastern Brazil. J Clin Oncol. 2008;26(15) [Google Scholar]
- 2.Epstein JB, Thariat J, Bensadoun RJ, Barasch A, Murphy BA, Kolnick L, et al. Oral complications of cancer and cancer therapy: from cancer treatment to survivorship. CA Cancer J Clin. 2012;62(6):400–422. doi: 10.3322/caac.21157. [DOI] [PubMed] [Google Scholar]
- 3.Epstein JB, Raber-Durlacher JE, Raber-Drulacher JE, Wilkins A, Chavarria MG, Myint H. Advances in hematologic stem cell transplant: an update for oral health care providers. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107(3):301–312. doi: 10.1016/j.tripleo.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 4.Elting LS, Cooksley C, Chambers M, Cantor SB, Manzullo E, Rubenstein EB. The burdens of cancer therapy. Clinical and economic outcomes of chemotherapy-induced mucositis. Cancer. 2003;98(7):1531–1539. doi: 10.1002/cncr.11671. [DOI] [PubMed] [Google Scholar]
- 5.Elad S, Raber-Durlacher JE, Brennan MT, Saunders DP, Mank AP, Zadik Y, et al. Basic oral care for hematology-oncology patients and hematopoietic stem cell transplantation recipients: a position paper from the joint task force of the Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO) and the European Society for Blood and Marrow Transplantation (EBMT) Support Care Cancer. 2015;23(1):223–236. doi: 10.1007/s00520-014-2378-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mank A, Quinn B, Wallhult E, Raber-Durlacher J, Elad S, Brennan MT, et al. Basic oral care for hematology-oncology patients and hematopoietic stem cell transplantation recipients: a position paper from the joint task force of the Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO) and the European Society for Blood and Marrow Transplantation (EBMT) Bone Marrow Transplant. 2015;50:S501. doi: 10.1007/s00520-014-2378-x. -S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boer CC, Correa MEP, Tenuta LMA, Souza CA, Vigorito AC. Post-allogeneic Hematopoietic Stem Cell Transplantation (HSCT) changes in inorganic salivary components. Support Care Cancer. 2015;23(9):2561–2567. doi: 10.1007/s00520-015-2613-0. [DOI] [PubMed] [Google Scholar]
- 8.Alborghetti MR, Correa MEP, Adam RL, Metze K, Coracin FL, de Souza CA, et al. Late effects of chronic graft-vs.-host disease in minor salivary glands. J Oral Pathol Med. 2005;34(8):486–493. doi: 10.1111/j.1600-0714.2005.00347.x. [DOI] [PubMed] [Google Scholar]
- 9.Noce CW, Gomes A, Copello A, Barbosa RD, Sant'anna S, Moreira MC, et al. Oral involvement of chronic graft-versus-host disease in hematopoietic stem cell transplant recipients. Gen Dent. 2011;59(6):458–462. quiz 63-4. [PubMed] [Google Scholar]
- 10.Samim F, Ten Böhmer KL, Koppelmans RGA, Raber-Durlacher JE, Epstein JB. Oral care for hematopoietic stem cell transplantation patients: a narrative review. Oral Health Prev Dent. 2019;17(5):413–423. doi: 10.3290/j.ohpd.a43271. [DOI] [PubMed] [Google Scholar]
- 11.Mendes SR, Silva MES, Firmo JOA, de Abreu MHNG. What haematopoietic stem cell transplant patients think about health and oral care: a qualitative study in a Brazilian health service. Eur J Cancer Care. 2018;27(3):e12851. doi: 10.1111/ecc.12851. [DOI] [PubMed] [Google Scholar]
- 12.Santos PS, Coracin FL, Barros JC, Dulley FL, Nunes FD, Magalhães MG. Impact of oral care prior to HSCT on the severity and clinical outcomes of oral mucositis. Clin Transplant. 2011;25(2):325–328. doi: 10.1111/j.1399-0012.2010.01283.x. [DOI] [PubMed] [Google Scholar]
- 13.Seppänen L, Lemberg KK, Lauhio A, Lindqvist C, Rautemaa R. Is dental treatment of an infected tooth a risk factor for locally invasive spread of infection? J Oral Maxillofac Surg. 2011;69(4):986–993. doi: 10.1016/j.joms.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 14.Hansen HJ, Estilo C, Owosho A, Solano AK, Randazzo J, Huryn J, et al. Dental status and risk of odontogenic complication in patients undergoing hematopoietic stem cell transplant. Support Care Cancer. 2021;29(4):2231–2238. doi: 10.1007/s00520-020-05733-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Durey K, Patterson H, Gordon K. Dental assessment prior to stem cell transplant: treatment need and barriers to care. Br Dent J. 2009;206(9):E19. doi: 10.1038/sj.bdj.2009.304. discussion 478-9. [DOI] [PubMed] [Google Scholar]
- 16.Elad S, Garfunkel AA, Or R, Michaeli E, Shapira MY, Galili D. Time limitations and the challenge of providing infection-preventing dental care to hematopoietic stem-cell transplantation patients. Support Care Cancer. 2003;11(10):674–677. doi: 10.1007/s00520-003-0499-8. [DOI] [PubMed] [Google Scholar]
- 17.Hupp WS, Firriolo FJ, De Rossi SS. Laboratory evaluation of chronic medical conditions for dental treatment: Part III. Hematology. Compend Contin Educ Dent. 2011;32(7) 10-2, 4-8; quiz 20, 32. [PubMed] [Google Scholar]
- 18.Morimoto Y, Niwa H, Imai Y, Kirita T. Dental management prior to hematopoietic stem cell transplantation. Spec Care Dentist. 2004;24(6):287–292. doi: 10.1111/j.1754-4505.2004.tb01707.x. [DOI] [PubMed] [Google Scholar]
- 19.James P, Worthington HV, Parnell C, Harding M, Lamont T, Cheung A, et al. Chlorhexidine mouthrinse as an adjunctive treatment for gingival health. Cochrane Database Syst Rev. 2017;3 doi: 10.1002/14651858.CD008676.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van der Weijden FA, Van der Sluijs E, Ciancio SG, Slot DE. Can chemical mouthwash agents achieve plaque/gingivitis control? Dent Clin North Am. 2015;59(4):799–829. doi: 10.1016/j.cden.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 21.Nashwan AJ. Use of chlorhexidine mouthwash in children receiving chemotherapy: a review of literature. J Pediatr Oncol Nurs. 2011;28(5):295–299. doi: 10.1177/1043454211408103. [DOI] [PubMed] [Google Scholar]
- 22.Teshome A. The efficacy of chlorhexidine gel in the prevention of alveolar osteitis after mandibular third molar extraction: a systematic review and meta-analysis. BMC Oral Health. 2017;17(1):82. doi: 10.1186/s12903-017-0376-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weyant RJ, Tracy SL, Anselmo TT, Beltrán-Aguilar ED, Donly KJ, Frese WA, et al. Topical fluoride for caries prevention: executive summary of the updated clinical recommendations and supporting systematic review. J Am Dent Assoc. 2013;144(11):1279–1291. doi: 10.14219/jada.archive.2013.0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y, Zeng X, Yang X, Que J, Du Q, Zhang Q, et al. Oral Health, Caries Risk Profiles, and Oral Microbiome of Pediatric Patients with Leukemia Submitted to Chemotherapy. Biomed Res Int. 2021;2021 doi: 10.1155/2021/6637503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saleh W, Katz J. Periodontal diseases, caries, and dental abscesses prevalence in hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 2021;56(3):720–722. doi: 10.1038/s41409-020-01057-0. [DOI] [PubMed] [Google Scholar]
- 26.Santos-Silva AR, PoS Feio, Vargas PA, Correa ME, Lopes MA. cGVHD-related caries and its shared features with other 'dry-mouth'-related caries. Braz Dent J. 2015;26(4):435–440. doi: 10.1590/0103-6440201300200. [DOI] [PubMed] [Google Scholar]
- 27.Castellarin P, Stevenson K, Biasotto M, Yuan A, Woo SB, Treister NS. Extensive dental caries in patients with oral chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2012;18(10):1573–1579. doi: 10.1016/j.bbmt.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 28.Wu Y, Shi X, Li Y, Gu Y, Qian Q, Hong Y. Hematopoietic and lymphatic cancers in patients with periodontitis: a systematic review and meta-analysis. Med Oral Patol Oral Cir Bucal. 2020;25(1):e21–ee8. doi: 10.4317/medoral.23166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raber-Durlacher JE, Laheij AM, Epstein JB, Epstein M, Geerligs GM, Wolffe GN, et al. Periodontal status and bacteremia with oral viridans streptococci and coagulase negative staphylococci in allogeneic hematopoietic stem cell transplantation recipients: a prospective observational study. Support Care Cancer. 2013;21(6):1621–1627. doi: 10.1007/s00520-012-1706-2. [DOI] [PubMed] [Google Scholar]
- 30.Hong CHL, Hu S, Haverman T, Stokman M, Napeñas JJ, Braber JB, et al. A systematic review of dental disease management in cancer patients. Support Care Cancer. 2018;26(1):155–174. doi: 10.1007/s00520-017-3829-y. [DOI] [PubMed] [Google Scholar]
- 31.Gürgan CA, Özcan M, Karakuş Ö, Zincircioğlu G, Arat M, Soydan E, et al. Periodontal status and post-transplantation complications following intensive periodontal treatment in patients underwent allogenic hematopoietic stem cell transplantation conditioned with myeloablative regimen. Int J Dent Hyg. 2013;11(2):84–90. doi: 10.1111/j.1601-5037.2012.00550.x. [DOI] [PubMed] [Google Scholar]
- 32.Landry RG, Jean M. Periodontal Screening and Recording (PSR) Index: precursors, utility and limitations in a clinical setting. Int Dent J. 2002;52(1):35–40. doi: 10.1111/j.1875-595x.2002.tb00595.x. [DOI] [PubMed] [Google Scholar]
- 33.Bordagaray MJ, Fernández A, Garrido M, Astorga J, Hoare A, Hernández M. Systemic and extraradicular bacterial translocation in apical periodontitis. Front Cell Infect Microbiol. 2021;11 doi: 10.3389/fcimb.2021.649925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Igoumenakis D, Giannakopoulos NN, Parara E, Mourouzis C, Rallis G. Effect of causative tooth extraction on clinical and biological parameters of odontogenic infection: a prospective clinical trial. J Oral Maxillofac Surg. 2015;73(7):1254–1258. doi: 10.1016/j.joms.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 35.Nicolatou-Galitis O, Kouri M, Papadopoulou E, Vardas E, Galiti D, Epstein JB, et al. Osteonecrosis of the jaw related to non-antiresorptive medications: a systematic review. Support Care Cancer. 2019;27(2):383–394. doi: 10.1007/s00520-018-4501-x. [DOI] [PubMed] [Google Scholar]
- 36.Nicolatou-Galitis O, Schiødt M, Mendes RA, Ripamonti C, Hope S, Drudge-Coates L, et al. Medication-related osteonecrosis of the jaw: definition and best practice for prevention, diagnosis, and treatment. Oral Surg Oral Med Oral Pathol Oral Radiol. 2019;127(2):117–135. doi: 10.1016/j.oooo.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 37.Ohman D, Björk Y, Bratel J, Kristiansson C, Johansson P, Johansson JE, et al. Partially erupted third molars as a potential source of infection in patients receiving peripheral stem cell transplantation for malignant diseases: a retrospective study. Eur J Oral Sci. 2010;118(1):53–58. doi: 10.1111/j.1600-0722.2009.00705.x. [DOI] [PubMed] [Google Scholar]
- 38.Marothiya S, Jain U, Bharti C, Polke P, Agrawal P, Shah R, et al. Evaluation of changes in microbiology and periodontal parameters during and after fixed orthodontic appliances. Mymensingh Med J. 2020;29(4):983–990. [PubMed] [Google Scholar]
- 39.Gibson F, Auld EM, Bryan G, Coulson S, Craig JV, Glenny AM. A systematic review of oral assessment instruments: what can we recommend to practitioners in children's and young people's cancer care? Cancer Nurs. 2010;33(4):E1–E19. doi: 10.1097/NCC.0b013e3181cb40c0. [DOI] [PubMed] [Google Scholar]
- 40.Glenny AM, Gibson F, Auld E, Coulson S, Clarkson JE, Craig JV, et al. The development of evidence-based guidelines on mouth care for children, teenagers and young adults treated for cancer. Eur J Cancer. 2010;46(8):1399–1412. doi: 10.1016/j.ejca.2010.01.023. [DOI] [PubMed] [Google Scholar]
- 41.Marinho VC, LY Chong, Worthington HV, Walsh T. Fluoride mouthrinses for preventing dental caries in children and adolescents. Cochrane Database Syst Rev. 2016;7 doi: 10.1002/14651858.CD002284.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hong CH, daFonseca M. Considerations in the pediatric population with cancer. Dent Clin North Am. 2008;52(1):155–181. doi: 10.1016/j.cden.2007.10.001. ix. [DOI] [PubMed] [Google Scholar]
- 43.Yamagata K, Onizawa K, Yoshida H, Kojima Y, Koike K, Tsuchida M. Dental management of pediatric patients undergoing hematopoietic stem cell transplant. Pediatr Hematol Oncol. 2006;23(7):541–548. doi: 10.1080/08880010600814187. [DOI] [PubMed] [Google Scholar]
- 44.Ministério da Saúde B. Secretaria de Vigilância em Saúde; SB Brasil: 2012. Pesquisa Nacional de Saúde Bucal: resultados principais. [Google Scholar]
- 45.Reis TC, Bortolotti F, Innocentini LMAR, Ferrari TC, Ricz HMA, Cunha RLG, et al. Assessment of oral health condition in recipients of allogeneic hematopoietic cell transplantation. Hematol Transfus Cell Ther. 2021 doi: 10.1016/j.htct.2021.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lucas VS, Roberts GJ, Beighton D. Oral health of children undergoing allogeneic bone marrow transplantation. Bone Marrow Transplant. 1998;22(8):801–808. doi: 10.1038/sj.bmt.1701415. [DOI] [PubMed] [Google Scholar]
- 47.Yamagata K, Onizawa K, Yanagawa T, Hasegawa Y, Kojima H, Nagasawa T, et al. A prospective study to evaluate a new dental management protocol before hematopoietic stem cell transplantation. Bone Marrow Transplant. 2006;38(3):237–242. doi: 10.1038/sj.bmt.1705429. [DOI] [PubMed] [Google Scholar]