Abstract
Background
Damage to the healthy intestinal epithelial layer and regulation of the intestinal immune system, closely interrelated, are considered pivotal parts of the curative treatment for inflammatory bowel disease (IBD). Plant-based diets and phytochemicals can support the immune microenvironment in the intestinal epithelial barrier for a balanced immune system by improving the intestinal microecological balance and may have therapeutic potential in colitis. However, there have been only a few reports on the therapeutic potential of plant-derived exosome-like nanoparticles (PENs) and the underlying mechanism in colitis. This study aimed to assess the therapeutic effect of PENs from Panax ginseng, ginseng-derived exosome-like nanoparticles (GENs), in a mouse model of IBD, with a focus on the intestinal immune microenvironment.
Method
To evaluate the anti-inflammatory effect of GENs on acute colitis, we treated GENs in Caco2 and lipopolysaccharide (LPS) -induced RAW 264.7 macrophages and analyzed the gene expression of pro-inflammatory cytokines and anti-inflammatory cytokines such as TNF-α, IL-6, and IL-10 by real-time PCR (RT-PCR). Furthermore, we further examined bacterial DNA from feces and determined the alteration of gut microbiota composition in DSS-induced colitis mice after administration of GENs through 16S rRNA gene sequencing analysis.
Result
GENs with low toxicity showed a long-lasting intestinal retention effect for 48 h, which could lead to effective suppression of pro-inflammatory cytokines such as TNF-α and IL-6 production through inhibition of NF-κB in DSS-induced colitis. As a result, it showed longer colon length and suppressed thickening of the colon wall in the mice treated with GENs. Due to the amelioration of the progression of DSS-induced colitis with GENs treatment, the prolonged survival rate was observed for 17 days compared to 9 days in the PBS-treated group. In the gut microbiota analysis, the ratio of Firmicutes/Bacteroidota was decreased, which means GENs have therapeutic effectiveness against IBD. Ingesting GENs would be expected to slow colitis progression, strengthen the gut microbiota, and maintain gut homeostasis by preventing bacterial dysbiosis.
Conclusion
GENs have a therapeutic effect on colitis through modulation of the intestinal microbiota and immune microenvironment. GENs not only ameliorate the inflammation in the damaged intestine by downregulating pro-inflammatory cytokines but also help balance the microbiota on the intestinal barrier and thereby improve the digestive system.
Keywords: Exosomes, Inflammatory bowel disease (IBD), Panax Ginseng, Plant-derived exosome-like nanoparticles, Ginseng-derived exosomes-like nanoparticles
Graphical abstract
1. Introduction
Inflammatory bowel disease (IBD), including Crohn's disease and ulcerative colitis, is a group of severe diseases that accompany chronic immune response colitis. According to the data from the Centers for Disease Control and Prevention (CDC) in 2015, approximately three million patients reported being diagnosed with IBD in the United States. Although in recent years people have developed more knowledge about health care and try to take care of themselves, bad eating habits and the burden of stress and irregular sleep patterns in modern society cause immune system derangement, which might affect the intestinal environment [1,2]. There are conventional drugs that have been approved to ameliorate IBD such as 5-aminosalicylate (5-ASA), steroids, immunosuppressants, and anti-tumor necrosis factor alpha (TNF-α) drugs [3,4]. However, severe adverse effects of these drugs such as toxicity and drug resistance have been reported; therefore, there is a limit to long-term usage in clinical application [[5], [6], [7], [8]]. Thus, there is an urgent need to identify new therapeutic strategies with new mechanisms to treat IBD.
Edible plants and dietary phytochemicals have been reported to alleviate inflammatory gut conditions and alter the gut microbiota by enriching the gut microenvironment with antioxidant compounds and bioactive components, such as phenolics [9]. Consumption of edible plants rich in antioxidants can suppress pathogeneses of various diseases. However, the bioactive compounds in ingested plants are usually metabolized after these biomolecules are absorbed in the gastrointestinal tract into the body. The resulting plasma metabolites generally have short retention times in the body and lower bioactivity than the precursor biomolecules. Thus, metabolites of ingested bioactive compounds can lower their anti-oxidant and anti-inflammatory activities.
Recently, the therapeutic application potential of plant-derived exosome-like nanoparticles (PENs) has received much attention due to their biocompatibility, low toxicity at a wide range of dosages, and significant bioactivity. Furthermore, the high stability of PENs enables their oral administration to be effective. Deng et al have reported that orally administered broccoli-derived exosome-like nanoparticles are effective against acute and chronic IBDs and show preventive and therapeutic effects by upregulating anti-inflammatory cytokines through activation of AMP-activated protein kinase [10]. Teng et al have demonstrated that ginger-derived exosome-like nanoparticles containing bioactive miRNAs are taken up by the gut microbiota [11]. The lipid constituents of these PENs can sufficiently upregulate IL-22, which modulates the intestinal barrier, composition of the gut microbiota, and host physiology. However, whether PENs containing bioactive molecules can provide health benefits in colitis, directly induce anti-inflammatory activity in immune cells, and influence the intestinal microenvironment in pathological conditions have not been fully understood.
Ginseng (Panax ginseng), one of the most valuable medicinal herbs, has been frequently used to regulate inflammatory diseases. Ginseng contains many bioactive ingredients such as polysaccharides, polyacetylenes, and ginsenosides [12]. Among these components, ginsenosides are considered the main bioactive ingredients of ginseng and are known to be useful in the treatment of inflammatory diseases. Some specific ginsenosides, such as Rg3, Rb1, and Rk, are known to have a strong influence in the anti-inflammatory cascade through downregulation of the activity of inflammatory signaling pathways such as the nuclear factor-κB (NF-κB), activator protein-1, and p38 MAPK signaling pathways [13]. Among the bioactive molecules in ginseng, protopanaxadiol (PPD) ginsenosides such as Rg3, Rb1, and Rh2 have been reported to have the benefit of attenuating colitis and reducing symptoms such as diarrhea and additional inflammation in the surrounding tissues by suppressing increased inflammatory cytokines [[14], [15], [16], [17]]. However, treatment of IBD with ginsenosides may be problematic due to the instability of ginsenosides under physical conditions such as varying temperatures and in liquid forms and the transformation or conversion of ginsenosides after oral administration.
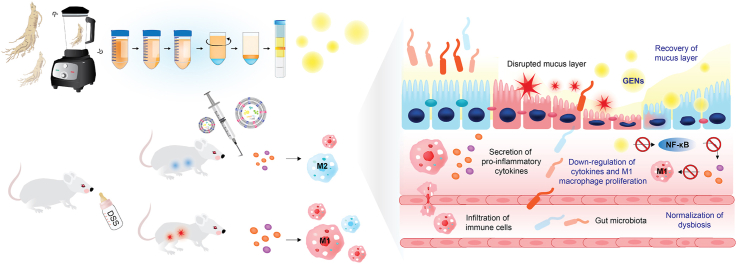
As Panax ginseng is known to have valuable medicinal properties in inflammation, PENs from Panax ginseng may be expected to possess therapeutic potentials. Ginseng-derived exosome-like nanoparticles (GENs) are known to be biocompatible and stable and have a low immunological risk. Due to these characteristics of GENs, the effects of GENs in treating cancer and neural disease have been researched [[18], [19], [20], [21], [22]]. However, there is no evidence of the role of orally administered GENs in inflammatory diseases such as IBD; therefore, we evaluated the influence of GENs on IBD and their ability to regulate pro-inflammatory cytokines and alter gut microbiota components. GENs can help reduce the levels of inflammatory cytokines such as TNF-α and interleukin 6 (IL-6) on inflammatory cells through downregulation of NF-κB expression. Furthermore, we demonstrated that GENs containing various biochemical compounds could support the intestinal microenvironment with an increased level of probiotics such as Lactobacillus. Thus, GENs could be used as a promising therapeutic agent for IBD. The schematic illustration shows the influence of GENs on IBD (Scheme 1) [23].
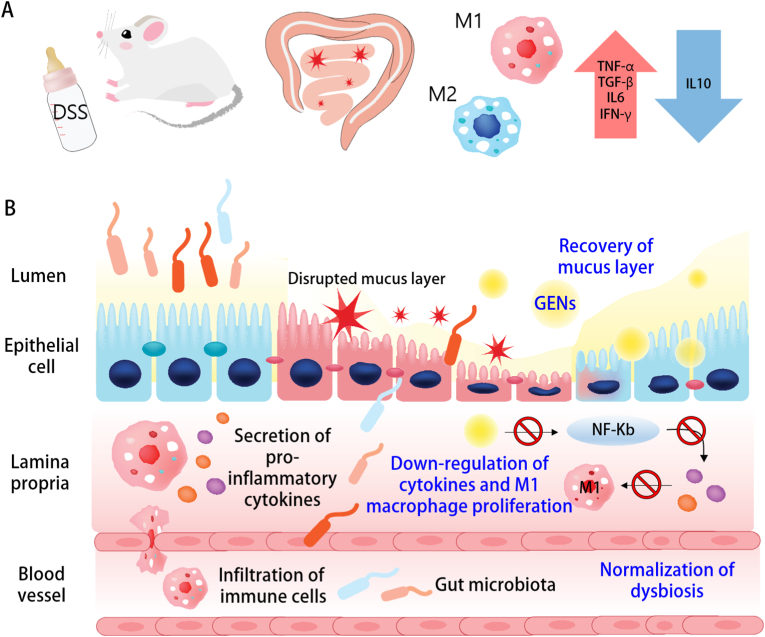
Scheme 1.
Dextran sulfate sodium (DSS)-Induced colitis in vivo. (A) Administration of 2.5% (w/v) DSS in drinking water and the inflammatory responses in macrophages. (B) After administration of 2.5% DSS, the intestinal environment was greatly altered and disturbed with translocation of the bacterial community through the damaged intestinal mucosa structure.
2. Materials and methods
2.1. DLS measurement of GENs
The size and surface charge were measured via dynamic light scattering (DLS) using a Zetasizer Nano ZS (Malvern Instrument, UK). The GENs were diluted 1000 times with DEPC water. The diluted GENs were added in a cuvette (12 mm square polystyrene cuvette, DTS0012, Malvern, UK) and in a disposable cuvette for zeta potential measurement (disposable capillary cell, DTS1070, Malvern, UK). The measurement was conducted at 37 °C, and all experiments were run in triplicate.
2.2. Measurement of of protein concentration of GENs
To quantify GENs, the protein concentration of GENs was determined by Bradford protein assay (Yeasen, China). The fresh GENs were diluted for 10 times with 1X PBS and 5 μL of the diluted GENs was used for measurement of the protein concentration. Bradford assay was performed as described in the previous report [24].
2.3. Analysis of cellular uptake of GENs by flow cytometry
To determine cellular uptake of GENs, Caco2 and RAW 264.7 cells (2 × 105 cells/well) were seeded in 12 well. The cells were incubated for 24 h and subsequently 20 μL of DiD-labeled GENs was injected in the cells. The cells were incubated at 37°C for 6h. Then, the cells were collected by trypsin treatment for detection of the internalized fluorescence of GENs. The result was organized by FlowJo software (BD biosciences, USA).
2.4. Mice and ethical approval of animal experiment
18-20 g of 6-8 week old Balb/C male mice were purchased from Sino-British SIPPR/BK Lab. Animal Co., Ltd. (Shanghai, China). All animal experiments were performed at Fudan university are scientific and ethical in accordance with the Guiding Principles for the Care and Use of Experimental Animals with ethical approval number of 2021-07-YJ-JZS-90 (Shanghai, China). The mice were gavaged with 2.5% (W/V) DSS water under standard conditions. On day 7 post-gavage, the mice were randomly divided into groups (N = 6) and orally administrated with 1 mL of 1 mg/mL of GENs (based on the protein concentration analyzed by Bradford assay) every day for 7 times. On day 15 post-gavage, the mice were anesthetized and humanly sacrificed by cervical dislocation and the large intestine was extracted for subsequent analysis.
3. Results and discussion
3.1. Characterization of GENs
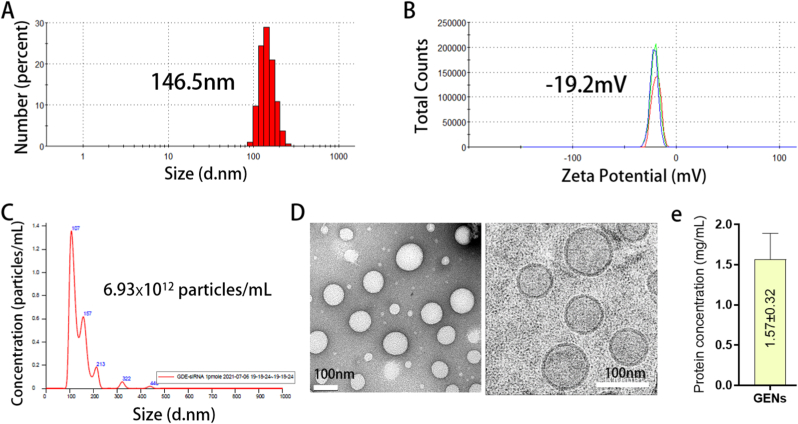
The diameter, size, and zeta potential of GENs were measured using dynamic light scattering (DLS). The average GEN size was 146.5 nm with a low polydispersity index (PDI) range, and the zeta potential of GENs was a negatively charged value of −19.2 mV. (Fig. 1A and B) The average size distribution and concentration of GENs were analyzed by nanoparticle tracking analysis (NTA). (Fig. 1C) The average number of nanoparticles was 6.93 × 1012 particles. Data were collected in triplicate. To determine the morphology of GENs, GENs were visualized by transmission electron microscopy (TEM) and Cryo-TEM. According to TEM and Cryo-TEM images, GENs were homogenous particles within cup-shaped vesicles with diameters of approximately above 100 nm. (Fig. 1D) To quantify GENs, the protein concentration of GENs was measured using a Bradford assay. 1 mL of fresh GENs was obtained per 10 g of ginseng, and a concentration of 1.57 ± 0.32 mg/mL of fresh GENs was measured. (Fig. 1E)
Fig. 1.
Characterization of ginseng derived exosome-like nanoparticles (GENs). (A) (B) The measurement of size and surface charge of GENs by dynamic light scattering (DLS) analysis. (C) Counting of the particles of GENs by nano tracking analysis (NTA). (D) TEM (left) and Cryo-TEM (right) imaging of GENs. (E) Quantification of Protein concentration of GENs by Bradford assay.
3.2. Suppression of M1-like macrophage polarization and increased M2-like macrophage polarization
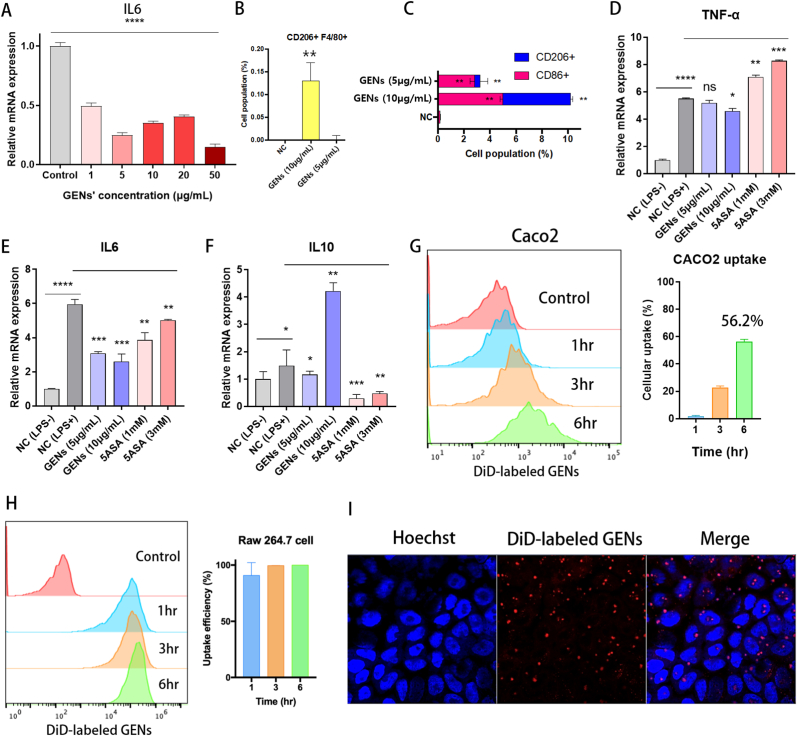
An inappropriate immune response might indicate a poor prognosis in IBD. To assess whether GENs can modulate immune reactions and influence the macrophages in the intestinal microenvironment, lipopolysaccharide (LPS)-induced RAW 264.7 macrophages were used as an in vitro model. M1 macrophages can induce inflammatory responses by secreting pro-inflammatory cytokines into the tissue to be inflamed. Thus, to evaluate whether GENs have anti-inflammatory effects on M1 macrophages, we stimulated the polarization of RAW 264.7 macrophages into these pro-inflammatory macrophages via LPS treatment (0.1 μg/mL) [[25], [26], [27]], followed by GENs treatment (1–50 μg/mL for 24 h). Interleukin (IL)-6 and IL-10 were used as phenotypic markers for M1 and M2 macrophages, respectively. GENs significantly downregulated IL-6 in the M1-polarized RAW 264.7 cells in a concentration-dependent manner (Fig. 2A). In addition, the effect of GENs on the polarization of RAW 264.7 cells was analyzed via flow cytometry (Fig. 2B and C). The results showed that the M2 macrophage marker CD 206 was significantly upregulated after the cells were treated with GENs. Furthermore, we evaluated the expression levels of pro- and anti-inflammatory cytokines in these cells by RT-qPCR (Fig. 2D–F). The results indicated that the levels of the pro-inflammatory cytokines TNF-α and IL-6 were significantly decreased in the cells treated with GENs compared with the levels in the negative-control group, which is cells treated with 1X PBS, while treatment of the cells with 1 mM and 3 mM 5-ASA did not significantly affect the levels of any of these anti-inflammatory cytokines. Conversely, the anti-inflammatory cytokine IL-10 was significantly upregulated by GENs.
Fig. 2.
Suppression of M1 macrophage polarization, pro-inflammatory cytokines and induction of anti-inflammatory cytokine in M1 polarization and in vitro uptake of GENs by Caco2 and RAW 264.7 cells. (A) RAW 264.7 cells were used and proliferated into M1 macrophage with 0.1 μg/mL of LPS. After treatment of GENs from 1 to 50 μg/mL, the gene expression of IL-6 was analyzed. (B) Cell population of F4/80+CD206+ induced by treatment of GENs. (C) M1 and M2 macrophage subtypes induced by GENs. (D,E,F) In M1 macrophage, the gene expression of proinflammatory cytokines including TNF-α and IL-6 and anti-inflammatory cytokine were analyzed. (G) (H) The representative histograms and cellular uptake efficiency of DiD-labeled GENs were analyzed using flow cytometry analysis after 1, 3, 6 hr of incubation. (I) Caco2 cell lines were incubated with DiD-labeled GENs for 6 hr and the image was observed by confocal microscopy. (Blue: Hoechst, Red: DiD-labeled GENs).
3.3. Cellular uptake of GENs in vitro
Efficient cellular delivery of GENs to cells could yield an improved therapeutic effect. Thus, we used DiD-labeled GENs and observed their cellular uptake. As shown in Fig. 2G and H, in Caco2 and RAW 264.7 cells, the cellular uptake of GENs gradually increased for up to 6 h. The internalization efficiency of the DiD-labeled GENs in Caco2 and RAW 264.7 was 56.2% ± 1.91 and 98.95% ± 0.25, respectively. Furthermore, using confocal microscopy, Caco2 cells incubated with DiD-labeled GENs were examined. (Fig. 2I) The results indicated that GENs could be efficiently taken up by Caco2 and RAW 264.7 cell lines at human body temperature (37°C).
3.4. Stability of GENs in vitro digestion
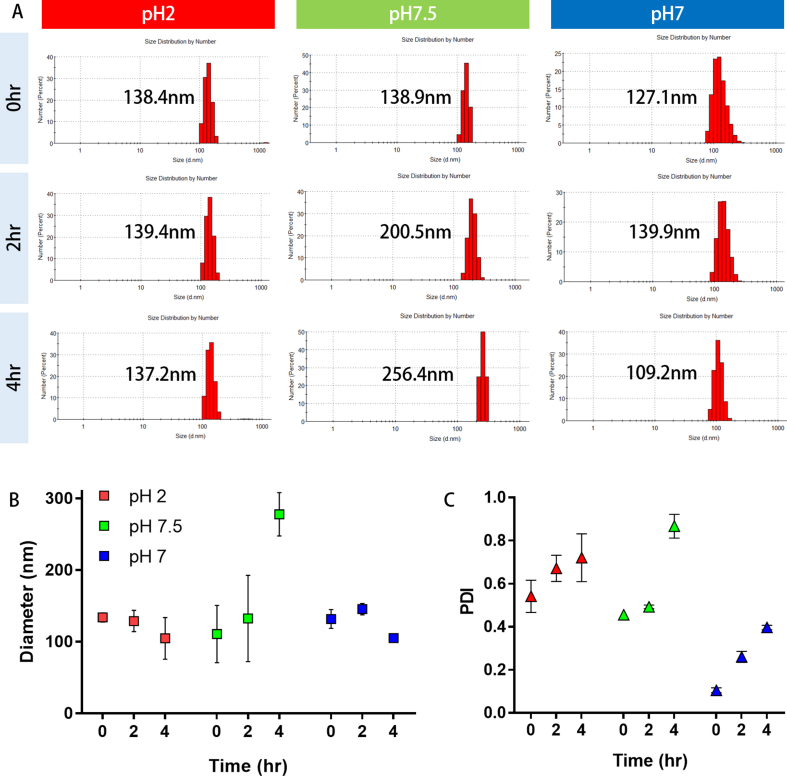
To confirm the stability of GENs under physiological conditions, we incubated GENs in different pH solutions similar to the human digestive system at 37°C and observed at 0, 2, and 4 h. (Fig. S1) Fig. S1 shows the change in the size of GENs in pH 2, pH 7.5, and pH 7 solution. At 0 h, in pH 2 solution, which mimics gastric pepsin solution, the surface size of GENs remained almost unchanged compared with GENs in saline solution (pH 7) at all-time points. In pH 7.5 solution, which mimics bile extract solution, the size of GENs dramatically changed compared with GENs in saline solution (pH 7) over time. The heterogeneity of GENs also gradually increased in all groups at all-time points. (Fig. S1c) As a result, there was no significant difference in GENs in pH 2 solution for 4 h, which indicated that GENs could be very stable under varying pH conditions. However, enlarged particles were observed in pH 7.5 solution at 4 h. It was difficult to tell whether GENs aggregated or individual GENs swelled up in basic solution. Collectively, GENs are highly resistant to the digestive system in both gastric pepsin solution and bile extract solution. The properties of GENs may be pH-dependent specifically at high pH values.
3.5. Suppression of inflammation by inhibition of the NF-κB
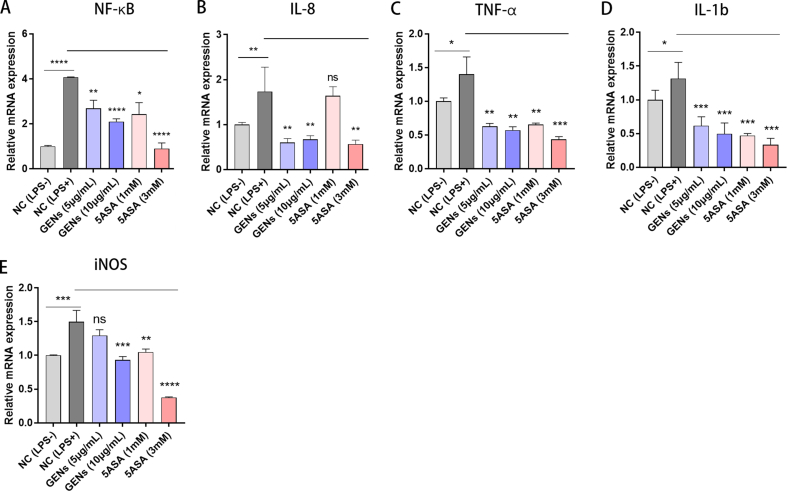
To explore if GENs have an anti-inflammatory effect in inflammatory disease, 5 μg/mL and 10 μg/mL GENs were treated in Caco2 cells exposed to bacterial-derived LPS, and inflammatory genes were analyzed. (Fig. S2) 5-ASA, which is the active compound that is a highly effective treatment for IBD was also used as a control group. The mRNA expression of NF-κB, cytokines, and chemokines including TNF-α, IL-1b, IL-8, and iNOS was evaluated by RT-PCR. As expected, after treatment with GENs of both concentrations, the expression levels of NF-κB, TNF-α, IL-1b, and IL-8 mRNA were statistically decreased in Caco2 cells. Specifically, the mRNA expression levels of IL-8, TNF-α, and IL-1b were significantly decreased as much as those in the 5-ASA-treated groups. With regard to the gene expression of iNOS, the GENs-treated groups exhibited decreased iNOS expression, but this was statistically detectable only at the highest concentration of GENs and both of the 5-ASA-treated groups.
According to previous reports about the anti-inflammatory effects of Panax ginseng [[28], [29], [30], [31]], Panax ginseng contains various bioactive constituents such as ginsenosides. Tung et al [32] evaluated some of the compounds in ginseng that have anti-inflammatory effects and anti-oxidant activity. Furthermore, Ahn et al [33] analyzed NF-κB signaling pathway suppression induced by the ginsenoside Rf in macrophages. Ginseng and its bioactive components regulated cytokines and chemokines, suggesting suppression of the NF-κB signaling pathway. In light of these studies, GENs may contain various bioactive components that originate from Panax ginseng; thus, GENs could have a potential benefit as anti-inflammatories. The RT-qPCR results indicated that GENs attenuate inflammation by downregulating the pro-inflammatory mediator NF-κB.
3.6. Protection against DSS-induced colitis by oral gavage with GENs
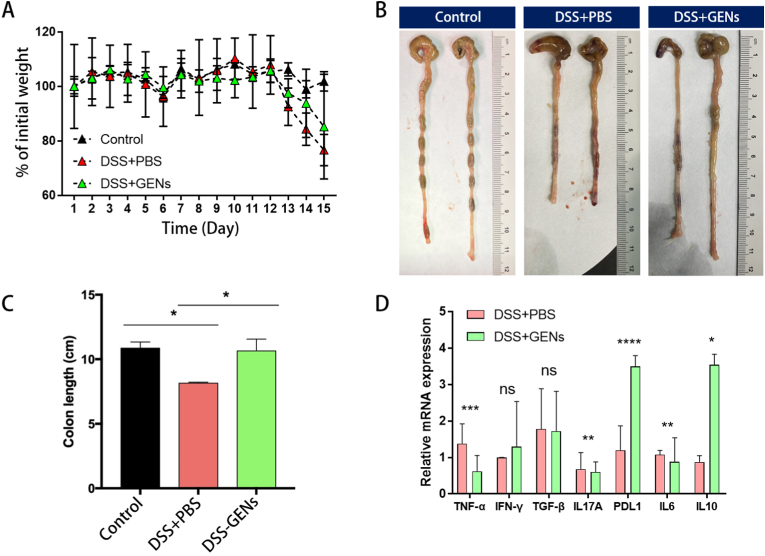
We used a DSS-induced acute colitis model to explore the biological effects of treatment with an oral gavage of GENs on IBD. To confirm whether GENs can exert a protective effect against DSS-induced colitis, 0.02 g of freeze-dried GENs was dissolved in 200 μL of 1X PBS, and GENs were orally administered to Balb/C mice daily for 7 days before gavage with 2.5% DSS. At day 8 of oral administration of GENs, 2.5% DSS gavage was started via drinking water. (Fig. 3) When 2.5% DSS was consistently administered, there was an obviously lower reduction of body weight in the mice treated with GENs compared with the control mice treated with PBS. Furthermore, the body weights of the control mice treated with PBS rapidly decreased at day 13. (Fig. 3A) The results indicated that GENs significantly inhibited DSS-induced colitis progression as evidenced by the fact that the body weight was only slightly reduced in the mice treated with GENs. We compared the lengths of the intestine exerted from the mice treated with 1X PBS and GENs at day 15, and the colon lengths of the mice treated with PBS were noticeably shorter than those of mice in the control group and mice treated with GENs. (Fig. 3B and C)
Fig. 3.
Administration of GENs protects DSS-induced colitis. Balb/C mouse were orally treated with 0.02g of freeze-dried GENs dissolved in 200 μL of 1X PBS and PBS every day for 7 days before administration of 2.5% DSS in water. At day 8 of oral gavage of GENs, 2.5% DSS gavage was started via drinking water. (A) The weight loss of mouse treated with GENs and PBS after administration of 2.5% DSS in water. (B) (C) After administration of GENs every day for 7 days, the mouse were sacrificed and the colon length were measured. (D) mRNA gene expression of inflammatory genes were detected by RT-qPCR. (∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001).
After treatment with GENs, the intestines of the mice were collected, and the total RNA was extracted for further analysis. Then, the relative mRNA expression of genes involved in the inflammatory response was analyzed. According to the results, GENs can selectively inhibit TNF-α, IL-17A, and IL-6, while programmed death-ligand 1 (PD-L1) and IL-10 were upregulated. (Fig. 3D) However, unexpectedly, the mRNA expression of IFN-γ and TGF-β did not show statistically significant differences after treatment with GENs.
3.7. Therapeutic effect of orally administered GENs in vivo
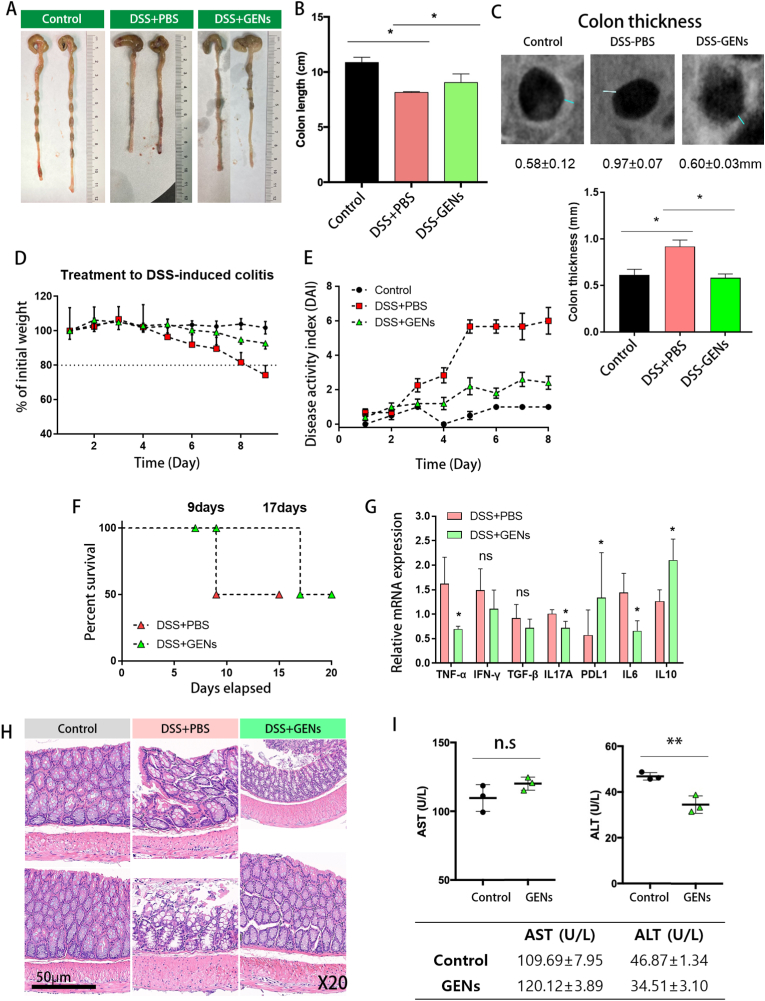
To determine the effect of GENs on the development of DSS-induced colitis, we administered GENs (0.02g of freeze-dried GENs dissolved in 200μL of 1X PBS) via oral gavage to mice DSS-induced colitis daily for 7 days. As shown in Fig. 4, the administration of GENs in mice with DSS-induced colitis resulted in a reduction in colon shortening compared with that of the mice in the PBS group. (Fig. 4A and B) Subsequently, the colon thicknesses of the mice were observed using a CT scan. The colonic wall thicknesses of the mice treated with GENs were significantly reduced at 0.6 ± 0.03 mm compared with that of the DSS-PBS-treated group at 1.1 ± 0.32 mm. (Fig. 4C) With regard to the body weights of the mice, there was no significant change in body weights of mice in the control group, and the mice treated with GENs had significantly reduced weight loss on day 9, whereas the mice gavaged with 2.5% DSS and PBS demonstrated weight loss. (N = 6) (Fig. 4D)
Fig. 4.
Therapeutic effect of GENs in DSS-induced colitis. (A) (B) Measurement of colon length. (C) Colon thickness imaged by CT scan. (D) The weight loss of DSS-induced colitis mice treated with 1X PBS and GENs. (E) The Disease Activity Index (DAI) assessment. DAI index was evaluated with the three parameters (body weight loss, stool consistency, rectal bleeding). (F) Survival rate of Balb/C mice gavaged with 2.5% DSS treated with PBS and GENs. (G) Gene expression of inflammatory genes was analyzed. (H) Representative H&E stained colonic tissue from Balb/C mice with or without 2.5% DSS gavage at day 8 after treatment of GENs. (I) Evaluation of serum AST and ALT levels after administration of GENs (∗P < 0.05).
To assess the symptoms of colitis, the signs of DSS-induced colitis were evaluated using the disease activity index (DAI). The DAI score is used to determine the severity of DSS-induced colitis, and can be calculated with three parameters, which are body weight loss, stool consistency, and rectal bleeding [34]. We evaluated the severity of the DSS-induced colitis and disease progression in mice using the DAI assessment. Over time, we observed significantly increased DAI scores of up to 6 for the PBS group after the first day of the experiment, whereas the DAI scores for the GENs treatment group were 2 points. Although the scores in the GEN treatment group tended to increase from 0 to 2 points, the symptoms were significantly relieved. (Fig. 4E) As expected, due to improvement in the progression of DSS-induced colitis with GENs treatment, the long-term survival rate was 17 days in the GENs treatment group compared with 9 days in the PBS-treated group. (Fig. 4F)
Accompanied by the injured colonic tissue and improved colonic tissue, a quantitative analysis of gene expression was performed. GENs treatment resulted in the downregulation of pro-inflammatory related cytokines including TNF-α, interleukin 17A (IL-17A), and IL-6, while the gene expression levels of PDL-1 and IL-10 statistically increased. (Fig. 4G) The mRNA expression levels of IFN-γ and TGF-β slightly decreased, but this change was not statistically significant. Assuming that inflammatory progression was ameliorated and that the damaged tissues were normalized by administration of GENs, GENs could aid in the downregulation of NF-κB responses and activation of pro-inflammatory macrophages in the affected colon. Thus, mice treated with DSS and PBS were especially vulnerable to damage to the intestinal mucosa and showed severe intestinal inflammatory progression during all experimental days, while those in the GENs treatment group exhibited slightly increased DAIs, and the inflammatory response was ameliorated.
3.8. Histopathological findings of DSS-induced colitis
To determine the severity of colitis, hematoxylin and eosin (H&E)-stained colonic tissues were analyzed. (Fig. 4H) The results of H&E-stained colonic tissues from mice treated with 2.5% DSS showed histological and pathological changes. The colonic tissues of mice with 2.5% DSS-induced colitis were consistent with gross lesions such as crypt loss or damage, destroyed mucosa, and inflammatory cell infiltration. In the presence of GENs in colitis, the colonic tissue showed comparatively little crypt damage and only mild degeneration of the crypts and mucosa. There were fewer histological changes in the colonic tissue of mice treated with GENs than in the colonic tissue of mice in the DSS and PBS treatment groups.
3.9. Liver toxicity of GENs in vivo
To evaluate the liver toxicity of GENs in mice treated with GENs, the serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels were measured after 7 days of oral administration of GENs. (Fig. 4I) In mice administered 1X PBS (control group) and GENs, there was no significant difference in the serum AST and ALT levels between the control and GEN-treated groups. Our data showed minimal hepatic toxicity of regularly orally administered GENs.
3.10. Accumulation of orally administered GENs
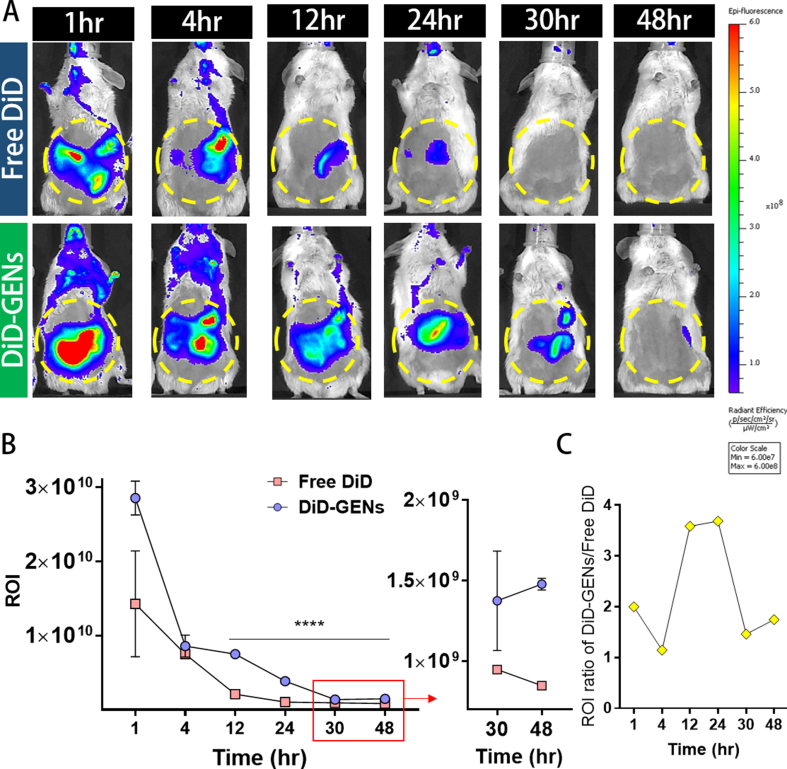
To our knowledge, no study has reported whether orally ingested GENs can reach the intestine without degradation or how long they can accumulate there. Thus, we GENs with a lipophilic carbocyanine dye, DiD (DiIC18(5); 1,1′-dioctadecyl-3,3,3′,3′- tetramethylindodicarbocyanine, 4-chlorobenzenesulfonate salt), and administered a single dose of oral DiD-labeled GENs to the mice. The results indicated that high amounts of DiD-labeled GENs were distributed in the intestines at 30 h and lasted for 48 h with slight intensity of DiD-labeled GENs, whereas the intensity of free DiD was completely excreted at 30 h after administration. (Fig. S3A, B)
The mean ratio of the ROI of DiD-labeled GENs and free DiD in the intestines was significantly increased by approximately 3.6 times at 24 h after administration. (Fig. S3C) This finding indicates that DiD-labeled GENs are physically and chemically tolerable in gastric transit, and may facilitate sustained delivery of GENs at the site of the intestine in GI tract compared with free DiD, suggesting that GENs can be delivered throughout the large intestine and that the medicinal effects of GENs might last for a long time in vivo. GENs typically consist of lipids; thus, highly stable GENs could provide a strategy for long-term accumulation in the intestines without early elimination even under various pH conditions. To avoid the limitation of orally administered drug for colon-targeted delivery, GENs can replace edible plants, wild phytochemicals, or unstable chemical drugs, while including the advantages and inducing maximum therapeutic outcomes.
3.11. Alteration of the gut microbiota induced by GENs
According to previous articles, ginseng affects the structure of gut microbiota [12]. Among various biochemical metabolites in ginseng, ginsenosides such as Rg3 and Rb1 exhibit particularly potent pharmacological effects on the gut microenvironment and can enhance beneficial microbiota under pathological conditions. Based on these findings, it is expected that GENs containing various metabolites could promote the growth of probiotics. Thus, we investigated whether administration of GENs could alter the abundance of the microbiome and gut microbiota to improve intestinal immune homeostasis in mice.
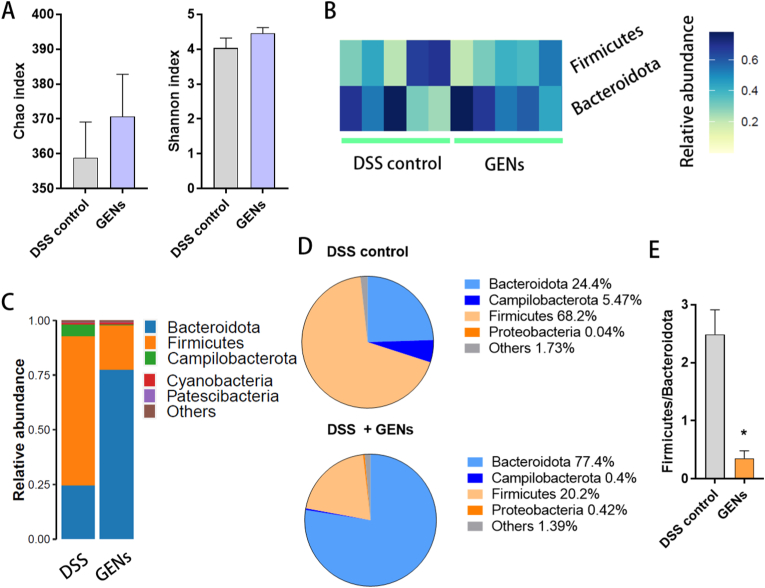
As displayed in Fig. 5, the phylum profiles of the microbiome showed that the relative abundance of the gut microbiome in the mice gavaged with 2.5% DSS changed after administration of GENs. The number of species in the intestinal microbial community was determined by the Chao and Shannon index. The diversity and community abundance in the mice treated with GENs seemed to improve slightly compared with mice in the DSS control group. (Fig. 5A) The species composition of the microbiome appeared different between the relative abundance in the the DSS control group and GEN treatment group. (Fig. 5B and C) The phylum profiles in the GEN treatment group exhibited significant induction of Bacteroidota and reduction in Firmicutes. (Fig. 5D) A microbial imbalance was observed in the DSS control group at the phylum level by the major bacterial phyla including Firmicutes and Bacteroidota. An increased level of Firmicutes is found in most IBD cases. There is considerable focus on the Firmicutes/Bacteroidota ratio because it is affected by increased dysbiosis. The decreased ratio of Firmicutes/Bacteroidota in the GEN treatment group indicates the therapeutic effectiveness against IBD. (Fig. 5E)
Fig. 5.
The recovery effect on intestinal microbiome of treatment of GENs. (A) Chao and Shannon index of DSS control group and GENs treatment group. (B) Heatmap representative of the relative abundance of microbiome at the phylum level in each group. (C) The phylum profiles in gut microbiome (D) The pie chart of microbiome at the phylum level. (E) The ratio of Firmicutes/Bacteroidota in each group. (∗P < 0.05).
Maintaining normal intestinal function, homeostasis, and therapeutic strategies are intended to achieve an appropriate balance between Firmicutes and Bacteroidota [35]. The results indicate that the intestinal microenvironment was normalized and stabilized with the treatment of GENs against IBD.
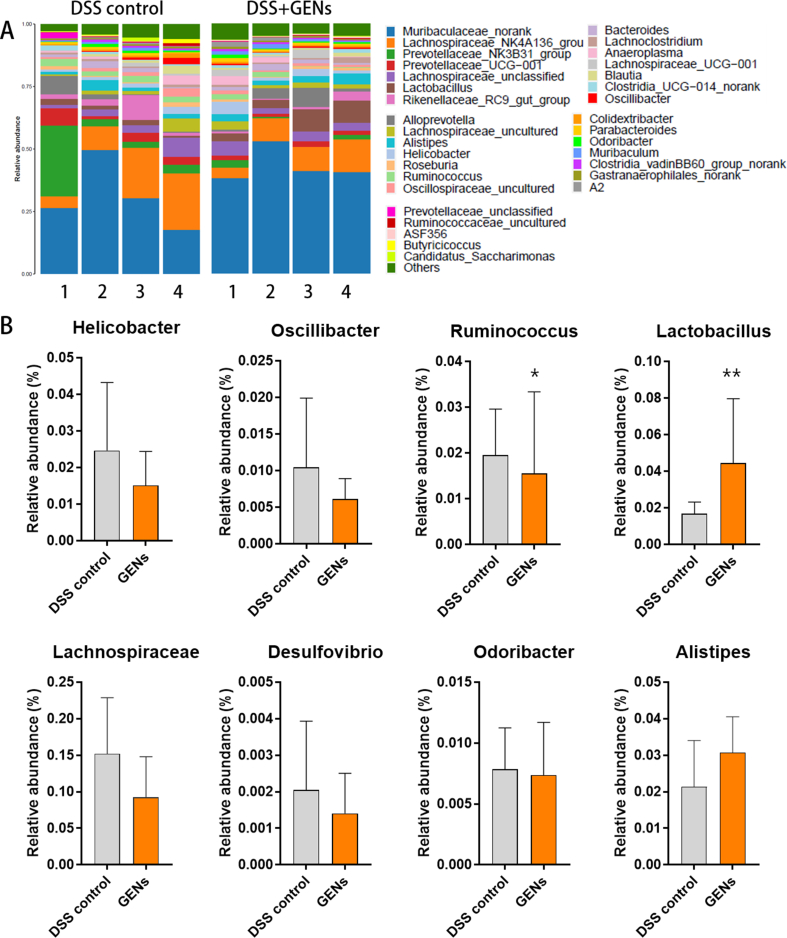
The gut microbiota community members were evaluated. (Fig. S4) An increased population of Helicobacter is known to affect pathogenesis in IBD by triggering an autoimmune reaction, and an increased abundance of Oscillibacter indicates increased severity of enhanced colitis. GENs administration clearly decreased the harmful bacteria in the intestines [36]. Recently, an increased level of Ruminococcus, a prevalent gut microbe, has been linked to aggravated symptoms of IBD by inducing pro-inflammatory complex polysaccharides [37], and the population of Ruminococcus was found to be increased in the DSS control group, while the relative abundance of Ruminococcus was statistically decreased in the GEN treatment group.
According to previous reports, the probiotic Lactobacillus is known to have a preventive effect against colitis [38]. Some probiotics may have a particular relevance to the cellular immune response to intestinal microorganisms because they can help to balance relapsing intestinal conditions by shifting from a T helper cell 1 (Th1)-mediated immune response toward a Th2/Th3-promoting profile. As expected, GENs treatment significantly increased the relative abundance of Lactobacillus, suggesting that GENs notably affected Lactobacillus generation compared with the DSS control group.
Lachnospiraceae, one of the core families of the gut microbiota, is generally detected in the human intestine. Lachnospiraceae are related to producing short-chain fatty acids (SCFAs) that are crucial for maintaining intestinal homeostasis through the activation of regulatory T cells. Unexpectedly, the abundance of Lachnospiraceae was slightly decreased in the GENs treatment group compared with the DSS control group.
Desulfovibrio and Odoribacter are probiotics that protect against colitis by enhancing host immunity and maintaining intestinal integrity. However, the abundances of Desulfovibrio and Odoribacter were slightly increased in the DSS control group, but the difference was not statistically significant.
The increased abundance of Alistipes plays a vital role in the protection of gut barrier function and attenuation of DSS-induced colitis [39,40]. In the GENs treatment group, the abundance of Alistipes was slightly higher than that in the control group.
Thus, the amount of beneficial bacteria including Lactobacillus and Alistipes was significantly increased in the GENs treatment group, while the abundance of harmful bacteria such as Helicobacter and Odoribacter was decreased.
4. Discussion
PENs have received increasing attention in regenerative medicine due to their many clinical advantages, such as relatively few safety concerns, good biocompatibility, high stability, and significant therapeutic activity. PENs can be produced with high yield and high purity, and thus they have been widely studied in various pathological conditions [10,11,41,42]. It is well known that PENs participate in cell-to-cell communications, thereby inducing multiple therapeutic benefits in inflammatory diseases [[43], [44], [45]]. Reports show that PENs can target specific cells and modulate the mammalian innate immune system without any significant toxicity in vivo [41,42].
GENs contain various bioactive biomolecules with pharmacological properties, such as anti-oxidant and anti-inflammation effects, and thus may be applied to the treatment of inflammatory diseases [45]. However, the studies on GENs are still limited, and mechanisms underlying various diseases and the regulatory effects of GENs on pro-inflammatory macrophages in DSS-induced colitis have not been elucidated yet. For the morphological characterization of GENs, we used DLS, TEM, and Cryo-TEM. Our purified sample of GENs consisted of homogenously distributed, cup-shaped and spherical GENs. The pH stability of GENs was determined based on particle size and distribution in a simulated gastrointestinal solution. We expect that orally administered GENs can be stably transmitted through the gastrointestinal tract and properly absorbed by the body. In addition, we observed that orally administered DiD-labeled GENs rapidly reached the intestine, and the fluorescence intensity of GENs was approximately ∼3.6-fold of the control (free-DiD). This observation indicates that GENs can tolerate the intestinal environment and can accumulate in the colonic mucosal barrier.
Although Panax ginseng or its bioactive metabolites have been shown to be effective against many inflammatory diseases, whether GENs can have any effect on colitis and modulate the gut microbiome has not been assessed. Aberrant NF-κB activation has been found in inflamed intestine, thus there are several pharmacological approaches to inhibit the activation of NF-κB signaling pathway [46,47]. From a therapeutic application aspect, we uncovered that GENs suppress the activation of NF-κB under inflammatory conditions and thereby exert anti-inflammatory activity.(Fig. S2) [48,49] Thus it can infer the sequential signal interactions in M2 macrophage proliferation [50]. We also observed that GENs downregulate pro-inflammatory cytokines and promote M2 polarization of macrophages. Additionally, we observed that GENs upregulate the anti-inflammatory cytokine IL-10 in mice. Therefore, we concluded that GENs could elicit anti-inflammatory activities in the immune microenvironment. Furthermore, GENs-mediated suppression of NF-κB needs to be investigated. However, according to previous reports, the role of IL-10 in intestinal inflammation is controversial. The secretion of IL-10 represses the expression of pro-inflammatory cytokines and thereby contributes to restoring the immune balance [51,52]. However, as IL-10 and IL-6 are involved in various diseases, it has been proposed that modulation of the cytokine levels within the microenvironment can improve the immune imbalance. Assuming that, GENs and the bioactive molecules can enhance the polarization of M2 macrophages and may also promote the production of IL-10.
Disruption of the gut microbiota composition promotes inflammatory processes associated with upregulation of pro-inflammatory cytokines [[53], [54], [55]]. GENs reinforce a balanced immune response in colitis, and our gut microbial-profile studies demonstrated that GENs can alter gut bacterial composition of gut phyla and affect gut homeostasis. Thus, this study implied that GENs could elicit improvement of intestinal environment by an increase in beneficial bacteria such as Bacteroidota. The stably remained probiotics in the intestine contribute to the balance of immune response in gut microbiota associated diseases. Increase of probiotics could provide the health effect to the host within the gut [56]. Imbalance in gut bacteria composition in DSS-induced colitis is accompanied with intestinal symptoms. Taken together, we speculate that the oral delivery of GENs to the intestine with longer retention time can coordinate the cross-talk between host cells and the gut microbiota, help slow the progression of DSS-induced colitis, and ameliorate symptoms with strengthening the colonic mucosal barrier. This strategy could provide an alternate approach for edible plants or wild phytochemicals in DSS-induced colitis. However, the exact mechanisms underlying these effects are elusive.
Oral administration of GENs for a therapeutic application is challenging. The relationship between gut microbiota and host immunity is complex, and the mechanisms whereby GENs support gut immune homeostasis and modulate gut dysbiosis in IBDs should be clarified. Overall, this study describes the benefits of GENs on the innate immune system and gut microbiota and suggests that GENs can serve as therapeutic agents in DSS-induced colitis.
5. Conclusion
Although there have been some reports of GENs being involved in modulation of macrophages in the tumor microenvironment and suppression of tumor progression, the function of GENs in inhibiting inflammation is poorly understood. In this study, we found that GENs are very stable in mimicking enzyme solutions and efficiently taken up by immune cells and then induce therapeutic activity in DSS-induced colitis. GENs could prevent and inhibit the inflammatory reaction by inducing downregulation of pro-inflammatory cytokines in pro-inflammatory macrophages, while inducing the anti-inflammatory macrophage expression. Through inhibition of NF-κB. Furthermore, administration of GENs led to modulation of the composition of the gut microbiome and gut microbiota in the inflamed intestinal environment, suggesting that GENs could aid in treatment by altering the regular course of these pathological intestinal conditions. Ingesting GENs would be expected to slow colitis progression, strengthen the gut microbiota, and maintain gut homeostasis by preventing bacterial dysbiosis.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 82074277 and 81773911), and the Development Project of Shanghai Peak Disciplines-Integrated Medicine (No. 20180101).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jgr.2023.01.004.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1
figs2.
figs3.
figs4.
References
- 1.Inflammatory bowel disease (IBD) in the United States. Centers for Disease Control and Prevention.
- 2.Dahlhamer Epz James M., Ward Brian W., Wheaton Anne G., Croft Janet B. Prevalence of inflammatory bowel disease among adults aged ≥18 years — United States, 2015. MMWR Morb Mortal Wkly Rep. 2016;65:1166–1169. doi: 10.15585/mmwr.mm6542a3. [DOI] [PubMed] [Google Scholar]
- 3.Renna S., Cottone M., Orlando A. Optimization of the treatment with immunosuppressants and biologics in inflammatory bowel disease. World J Gastroenterol. 2014;20:9675–9690. doi: 10.3748/wjg.v20.i29.9675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peyrin-Biroulet L. Anti-TNF therapy in inflammatory bowel diseases: a huge review. Minerva Gastroenterol Dietol. 2010;56:233–243. [PubMed] [Google Scholar]
- 5.Sicilia B., Arias L., Hontoria G., Garcia N., Badia E., Gomollon F. Are steroids still useful in immunosuppressed patients with inflammatory bowel disease? A retrospective, population-based study. Front Med (Lausanne) 2021;8 doi: 10.3389/fmed.2021.651685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D'Haens G.R., van Deventer S. 25 years of anti-TNF treatment for inflammatory bowel disease: lessons from the past and a look to the future. Gut. 2021;70:1396–1405. doi: 10.1136/gutjnl-2019-320022. [DOI] [PubMed] [Google Scholar]
- 7.Mishra R., Dhawan P., Srivastava A.S., Singh A.B. Inflammatory bowel disease: therapeutic limitations and prospective of the stem cell therapy. World J Stem Cells. 2020;12:1050–1066. doi: 10.4252/wjsc.v12.i10.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Bawardy B., Shivashankar R., Proctor D.D. Novel and emerging therapies for inflammatory bowel disease. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.651415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sandra Gonçalves E.M., Andrade Paula B., Valentão Patrícia, Romano Anabela. Effect of in vitro gastrointestinal digestion on the total phenolic contents and antioxidant activity of wild Mediterranean edible plant extracts. European Food Research and Technology. 2018;245:753–762. [Google Scholar]
- 10.Deng Z., Rong Y., Teng Y., Mu J., Zhuang X., Tseng M., et al. Broccoli-derived nanoparticle inhibits mouse colitis by activating dendritic cell AMP-activated protein kinase. Mol Ther. 2017;25:1641–1654. doi: 10.1016/j.ymthe.2017.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teng Y., Ren Y., Sayed M., Hu X., Lei C., Kumar A., et al. Plant-derived exosomal MicroRNAs shape the gut microbiota. Cell Host Microbe. 2018;24:637–652 e8. doi: 10.1016/j.chom.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim D.H. Gut microbiota-mediated pharmacokinetics of ginseng saponins. J Ginseng Res. 2018;42:255–263. doi: 10.1016/j.jgr.2017.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim J.H., Yi Y.S., Kim M.Y., Cho J.Y. Role of ginsenosides, the main active components of Panax ginseng, in inflammatory responses and diseases. J Ginseng Res. 2017;41:435–443. doi: 10.1016/j.jgr.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li L., Wang Y., Guo R., Li S., Ni J., Gao S., et al. Ginsenoside Rg3-loaded, reactive oxygen species-responsive polymeric nanoparticles for alleviating myocardial ischemia-reperfusion injury. J Control Release. 2020;317:259–272. doi: 10.1016/j.jconrel.2019.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ni J., Liu Z., Jiang M., Li L., Deng J., Wang X., et al. Ginsenoside Rg3 ameliorates myocardial glucose metabolism and insulin resistance via activating the AMPK signaling pathway. J Ginseng Res. 2022;46:235–247. doi: 10.1016/j.jgr.2021.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dong J.Y., Xia K.J., Liang W., Liu L.L., Yang F., Fang X.S., et al. Ginsenoside Rb1 alleviates colitis in mice via activation of endoplasmic reticulum-resident E3 ubiquitin ligase Hrd1 signaling pathway. Acta Pharmacol Sin. 2021;42:1461–1471. doi: 10.1038/s41401-020-00561-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang C.Z., Yu C., Wen X.D., Chen L., Zhang C.F., Calway T., et al. American ginseng attenuates colitis-associated colon carcinogenesis in mice: impact on gut microbiota and metabolomics. Cancer Prev Res (Phila) 2016;9:803–811. doi: 10.1158/1940-6207.CAPR-15-0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hong J., Gwon D., Jang C.Y. Ginsenoside Rg1 suppresses cancer cell proliferation through perturbing mitotic progression. J Ginseng Res. 2022;46:481–488. doi: 10.1016/j.jgr.2021.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahuja A., Kim J.H., Kim J.H., Yi Y.S., Cho J.Y. Functional role of ginseng-derived compounds in cancer. J Ginseng Res. 2018;42:248–254. doi: 10.1016/j.jgr.2017.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dai D., Zhang C.F., Williams S., Yuan C.S., Wang C.Z. Ginseng on cancer: potential role in modulating inflammation-mediated angiogenesis. Am J Chin Med. 2017;45:13–22. doi: 10.1142/S0192415X17500021. [DOI] [PubMed] [Google Scholar]
- 21.Hou W., Wang Y., Zheng P., Cui R. Effects of ginseng on neurological disorders. Front Cell Neurosci. 2020;14:55. doi: 10.3389/fncel.2020.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ong W.Y., Farooqui T., Koh H.L., Farooqui A.A., Ling E.A. Protective effects of ginseng on neurological disorders. Front Aging Neurosci. 2015;7:129. doi: 10.3389/fnagi.2015.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fuhrer A., Sprenger N., Kurakevich E., Borsig L., Chassard C., Hennet T. Milk sialyllactose influences colitis in mice through selective intestinal bacterial colonization. J Exp Med. 2010;207:2843–2854. doi: 10.1084/jem.20101098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kruger N.J. The Bradford method for protein quantitation. Methods Mol Biol. 1994;32:9–15. doi: 10.1385/0-89603-268-X:9. [DOI] [PubMed] [Google Scholar]
- 25.Cao Y., Chen J., Ren G., Zhang Y., Tan X., Yang L. Punicalagin prevents inflammation in LPS-induced RAW264.7 macrophages by inhibiting FoxO3a/autophagy signaling pathway. Nutrients. 2019;11 doi: 10.3390/nu11112794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim J.R., Oh D.R., Cha M.H., Pyo B.S., Rhee J.H., Choy H.E., et al. Protective effect of polygoni cuspidati radix and emodin on Vibrio vulnificus cytotoxicity and infection. J Microbiol. 2008;46:737–743. doi: 10.1007/s12275-008-0232-x. [DOI] [PubMed] [Google Scholar]
- 27.Endale M., Park S.C., Kim S., Kim S.H., Yang Y., Cho J.Y., et al. Quercetin disrupts tyrosine-phosphorylated phosphatidylinositol 3-kinase and myeloid differentiation factor-88 association, and inhibits MAPK/AP-1 and IKK/NF-kappaB-induced inflammatory mediators production in RAW 264.7 cells. Immunobiology. 2013;218:1452–1467. doi: 10.1016/j.imbio.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 28.Lee Y.Y., Kim S.D., Park S.C., Rhee M.H. Panax ginseng: inflammation, platelet aggregation, thrombus formation, and atherosclerosis crosstalk. J Ginseng Res. 2022;46:54–61. doi: 10.1016/j.jgr.2021.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang G., Li J., Peng Y., Shen B., Li Y., Liu L., et al. Ginsenoside Rb1 attenuates methamphetamine (METH)-induced neurotoxicity through the NR2B/ERK/CREB/BDNF signalings in vitro and in vivo models. J Ginseng Res. 2022;46:426–434. doi: 10.1016/j.jgr.2021.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim K.H., Lee D., Lee H.L., Kim C.E., Jung K., Kang K.S. Beneficial effects of Panax ginseng for the treatment and prevention of neurodegenerative diseases: past findings and future directions. J Ginseng Res. 2018;42:239–247. doi: 10.1016/j.jgr.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wan Y., Wang J., Xu J.F., Tang F., Chen L., Tan Y.Z., et al. Panax ginseng and its ginsenosides: potential candidates for the prevention and treatment of chemotherapy-induced side effects. J Ginseng Res. 2021;45:617–630. doi: 10.1016/j.jgr.2021.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tung N.H., Song G.Y., Nhiem N.X., Ding Y., Tai B.H., Jin L.G., et al. Dammarane-type saponins from the flower buds of Panax ginseng and their intracellular radical scavenging capacity. J Agric Food Chem. 2010;58:868–874. doi: 10.1021/jf903334g. [DOI] [PubMed] [Google Scholar]
- 33.Ahn S., Siddiqi M.H., Aceituno V.C., Simu S.Y., Yang D.C. Suppression of MAPKs/NF-kappaB activation induces intestinal anti-inflammatory action of ginsenoside Rf in HT-29 and RAW264.7 cells. Immunol Invest. 2016;45:439–449. doi: 10.3109/08820139.2016.1168830. [DOI] [PubMed] [Google Scholar]
- 34.Cooper H.S., Murthy S.N., Shah R.S., Sedergran D.J. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238–249. [PubMed] [Google Scholar]
- 35.Stojanov S., Berlec A., Strukelj B. The influence of probiotics on the firmicutes/bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease. Microorganisms. 2020;8 doi: 10.3390/microorganisms8111715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Papamichael K., Konstantopoulos P., Mantzaris G.J. Helicobacter pylori infection and inflammatory bowel disease: is there a link? World J Gastroenterol. 2014;20:6374–6385. doi: 10.3748/wjg.v20.i21.6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Henke M.T., Kenny D.J., Cassilly C.D., Vlamakis H., Xavier R.J., Clardy J. Ruminococcus gnavus, a member of the human gut microbiome associated with Crohn's disease, produces an inflammatory polysaccharide. Proc Natl Acad Sci U S A. 2019;116:12672–12677. doi: 10.1073/pnas.1904099116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peran L., Camuesco D., Comalada M., Nieto A., Concha A., Diaz-Ropero M.P., et al. Preventative effects of a probiotic, Lactobacillus salivarius ssp. salivarius, in the TNBS model of rat colitis. World J Gastroenterol. 2005;11:5185–5192. doi: 10.3748/wjg.v11.i33.5185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dziarski R., Park S.Y., Kashyap D.R., Dowd S.E., Gupta D. Pglyrp-regulated gut microflora prevotella falsenii, parabacteroides distasonis and Bacteroides eggerthii enhance and Alistipes finegoldii attenuates colitis in mice. PLoS One. 2016;11 doi: 10.1371/journal.pone.0146162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zu M., Xie D., Canup B.S.B., Chen N., Wang Y., Sun R., et al. 'Green' nanotherapeutics from tea leaves for orally targeted prevention and alleviation of colon diseases. Biomaterials. 2021;279 doi: 10.1016/j.biomaterials.2021.121178. [DOI] [PubMed] [Google Scholar]
- 41.Zhuang X., Deng Z.B., Mu J., Zhang L., Yan J., Miller D., et al. Ginger-derived nanoparticles protect against alcohol-induced liver damage. J Extracell Vesicles. 2015;4 doi: 10.3402/jev.v4.28713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li Z., Wang H., Yin H., Bennett C., Zhang H.G., Guo P. Arrowtail RNA for ligand display on ginger exosome-like nanovesicles to systemic deliver siRNA for cancer suppression. Sci Rep. 2018;8 doi: 10.1038/s41598-018-32953-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mu J., Zhuang X., Wang Q., Jiang H., Deng Z.B., Wang B., et al. Interspecies communication between plant and mouse gut host cells through edible plant derived exosome-like nanoparticles. Mol Nutr Food Res. 2014;58:1561–1573. doi: 10.1002/mnfr.201300729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Q., Zhuang X., Mu J., Deng Z.B., Jiang H., Zhang L., et al. Delivery of therapeutic agents by nanoparticles made of grapefruit-derived lipids. Nat Commun. 2013;4:1867. doi: 10.1038/ncomms2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu X.H., Yuan T.J., Dad H.A., Shi M.Y., Huang Y.Y., Jiang Z.H., et al. Plant exosomes as novel nanoplatforms for MicroRNA transfer stimulate neural differentiation of stem cells in vitro and in vivo. Nano Lett. 2021;21:8151–8159. doi: 10.1021/acs.nanolett.1c02530. [DOI] [PubMed] [Google Scholar]
- 46.Liu T., Zhang L., Joo D., Sun S.C. NF-kappaB signaling in inflammation. Signal Transduct Target Ther. 2017;2 doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karunaweera N., Raju R., Gyengesi E., Munch G. Plant polyphenols as inhibitors of NF-kappaB induced cytokine production-a potential anti-inflammatory treatment for Alzheimer's disease? Front Mol Neurosci. 2015;8:24. doi: 10.3389/fnmol.2015.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Driessler F., Venstrom K., Sabat R., Asadullah K., Schottelius A.J. Molecular mechanisms of interleukin-10-mediated inhibition of NF-kappaB activity: a role for p50. Clin Exp Immunol. 2004;135:64–73. doi: 10.1111/j.1365-2249.2004.02342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hovsepian E., Penas F., Siffo S., Mirkin G.A., Goren N.B. IL-10 inhibits the NF-kappaB and ERK/MAPK-mediated production of pro-inflammatory mediators by up-regulation of SOCS-3 in Trypanosoma cruzi-infected cardiomyocytes. PLoS One. 2013;8 doi: 10.1371/journal.pone.0079445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dorrington M.G., Fraser I.D.C. NF-kappaB signaling in macrophages: dynamics, crosstalk, and signal integration. Front Immunol. 2019;10:705. doi: 10.3389/fimmu.2019.00705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Szczepanik A.M., Funes S., Petko W., Ringheim G.E. IL-4, IL-10 and IL-13 modulate A beta(1--42)-induced cytokine and chemokine production in primary murine microglia and a human monocyte cell line. J Neuroimmunol. 2001;113:49–62. doi: 10.1016/s0165-5728(00)00404-5. [DOI] [PubMed] [Google Scholar]
- 52.Guillot-Sestier M.V., Doty K.R., Gate D., Rodriguez J., Jr., Leung B.P., Rezai-Zadeh K., et al. Il10 deficiency rebalances innate immunity to mitigate Alzheimer-like pathology. Neuron. 2015;85:534–548. doi: 10.1016/j.neuron.2014.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zheng D., Liwinski T., Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020;30:492–506. doi: 10.1038/s41422-020-0332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Souza D.G., Vieira A.T., Soares A.C., Pinho V., Nicoli J.R., Vieira L.Q., et al. The essential role of the intestinal microbiota in facilitating acute inflammatory responses. J Immunol. 2004;173:4137–4146. doi: 10.4049/jimmunol.173.6.4137. [DOI] [PubMed] [Google Scholar]
- 55.Sarubbo F., Cavallucci V., Pani G. The influence of gut microbiota on neurogenesis: evidence and hopes. Cells. 2022:11. doi: 10.3390/cells11030382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang Y.J., Li S., Gan R.Y., Zhou T., Xu D.P., Li H.B. Impacts of gut bacteria on human health and diseases. Int J Mol Sci. 2015;16:7493–7519. doi: 10.3390/ijms16047493. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.