Abstract
Introduction
Clinical studies demonstrate an accelerated decline in lung function in patients with moderate chronic obstructive pulmonary disease (COPD) (Global Initiative for Chronic Obstructive Lung Disease [GOLD] grade 2) versus severe and very severe COPD (GOLD grades 3 and 4). This predictive modelling study assessed the impact of initiating pharmacotherapy earlier versus later on long-term disease progression in COPD.
Methods
The modelling approach used data on decline in forced expiratory volume in 1 s (FEV1) extracted from published studies to develop a longitudinal non-parametric superposition model of lung function decline with progressive impact of exacerbations from 0 per year to 3 per year and no ongoing pharmacotherapy. The model simulated decline in FEV1 and annual exacerbation rates from age 40 to 75 years in COPD with initiation of long-acting anti-muscarinic antagonist (LAMA)/long-acting beta2-agonist (LABA) (umeclidinium (UMEC)/vilanterol (VI)) or triple (inhaled corticosteroid (ICS)/LAMA/LABA; fluticasone furoate (FF)/UMEC/VI) therapy at 40, 55 or 65 years of age.
Results
Model-predicted decline in FEV1 showed that, compared with ‘no ongoing’ therapy, initiation of triple or LAMA/LABA therapy at age 40, 55 or 65 years preserved an additional 469.7 mL or 236.0 mL, 327.5 mL or 203.3 mL, or 213.5 mL or 137.5 mL of lung function, respectively, by the age of 75. The corresponding average annual exacerbation rates were reduced from 1.57 to 0.91, 1.06 or 1.23 with triple therapy or to 1.2, 1.26 and 1.4 with LAMA/LABA therapy when initiated at 40, 55 or 65 years of age, respectively.
Conclusions
This modelling study suggests that earlier initiation of LAMA/LABA or triple therapy may have positive benefits in slowing disease progression in patients with COPD. Greater benefits were demonstrated with early initiation therapy with triple versus LAMA/LABA.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-023-02583-1.
Keywords: Chronic obstructive pulmonary disease (COPD), COPD exacerbation, Dual bronchodilator therapy (LAMA/LABA), GOLD grades, Lung function decline, Triple therapy (ICS/LAMA/LABA)
Key Summary Points
| Why carry out this study? |
| There is often a progressive decline in lung function in COPD. |
| While life-long treatment for COPD is usually required, the long-term benefit on lung function decline of initiating a dual bronchodilator (LAMA/LABA) or triple therapy (ICS/LAMA/LABA) early in the natural history of COPD is not known. |
| Using a modelling approach, this study explored the benefits of dual bronchodilator or triple therapies on lung function decline when initiated at 40, 55 and 65 years of age. |
| What was learned from the study? |
| Triple therapy and dual LAMA/LABA therapy when initiated at age 40 years preserved an additional 469.7 mL and 236.0 mL of lung function, respectively, by the age of 75. |
| This modelling study suggests that initiation of pharmacotherapy at an early stage of COPD has the potential to slow disease progression. |
Introduction
Chronic obstructive pulmonary disease (COPD) is a heterogeneous condition characterized by respiratory symptoms and persistent airflow obstruction [1]. There is often a progressive decline in lung function (forced expiratory volume in 1 s [FEV1]) in COPD that is greater than the physiological decline observed with age [1, 2]. The rate of lung function decline varies between individual patients with COPD [3]. In studies of large COPD populations, the average rate of lung function decline is greater in patients with moderate COPD (Global Initiative for Chronic Obstructive Lung Disease [GOLD] grade 2 [1]) compared with severe and very severe COPD (GOLD grades 3 and 4, respectively), suggesting a more accelerated decline in the earlier phases of the disease [4].
One of the aims of COPD management is to reduce the risk of disease progression. While smoking cessation can modify the course of the disease [5], the evidence from randomized controlled trials (RCTs) concerning the effect of pharmacological interventions on lung function decline has been inconsistent [6–11]. These RCTs have often been conducted over relatively short time periods, with 4 years being the longest study. Furthermore, patients with mild COPD (GOLD grade 1) have often been excluded from these studies [12] which have included a high proportion of severe and very severe patients with COPD. Longer-term investigations are warranted in order to understand the impact of pharmacological interventions in patients with COPD of younger age and with milder lung function impairment [13].
An exacerbation of COPD occurs when respiratory symptoms rapidly worsen, often associated with increased local and systemic inflammation, typically (but not always) due to an infection [1]. Exacerbations are a major contributor to impaired health status, lung function decline and mortality [14–17]. The recommended initial pharmacological therapy for patients with COPD is based on individual assessment of symptoms and exacerbation risk [1]. Patients with COPD with low exacerbation risk (GOLD groups A and B) are treated with bronchodilator(s) including long-acting anti-muscarinic antagonists (LAMAs) and long-acting beta2-agonists (LABAs). Patients with higher exacerbation risk (group E) and with higher blood eosinophil counts may also be considered for triple therapy (inhaled corticosteroid (ICS) + LAMA + LABA) as an initial therapy [1]. RCTs in patients with COPD and a history of exacerbations while taking maintenance inhaled therapy have shown considerable benefits of triple therapy over LAMA + LABA treatment for individuals with higher blood eosinophil counts, including prevention of exacerbations and risk of mortality [18–20]. This evidence underpins the GOLD recommendation to consider earlier intervention with triple therapy for patients with COPD with both high exacerbation risk and higher blood eosinophil counts.
The long-term benefit on lung function decline of initiating a dual bronchodilator or triple therapy early in the natural history of COPD is not known. This is practically difficult to assess in a clinical trial setting because of time and cost constraints. However, this can be investigated using a modelling and simulation approach based on existing data. Clinical prediction models are becoming more recognized as a tool for generating evidence to inform public health policy and to support clinical decision-making [21–23]. In the respiratory field, modelling studies have been used to predict factors associated with future exacerbation risk in COPD [24–26] and for comparing different ICS-based treatment regimens in asthma [27, 28].
In COPD, the rate of decline in lung function varies with time, disease severity and in response to exacerbations [1–3, 14, 15, 29, 30]. This modelling analysis investigated the impact of initiating pharmacotherapy earlier versus later on long-term disease progression (lung function decline) in COPD. Data from previously published studies on lung function decline in general population subjects (no known COPD) and in patients with COPD, including data on the impacts of disease severity, exacerbations and pharmacotherapy, were used to develop the model. Clinical trial data for the triple therapy fluticasone furoate (FF)/umeclidinium (UMEC)/vilanterol (VI) and LAMA/LABA therapy UMEC/VI were used for estimates of treatment effects in the model simulations.
Methods
Model Overview and Structure
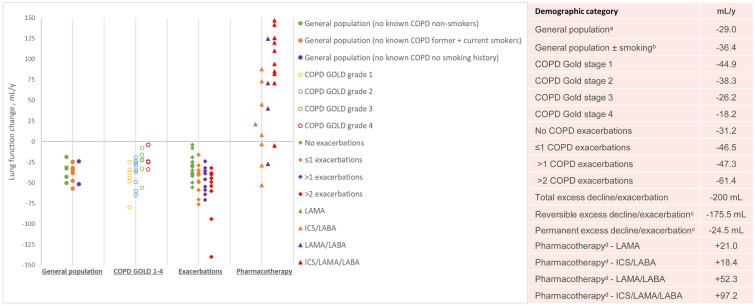
The modelling approach used data extracted from the published literature to develop a longitudinal non-parametric superposition model of lung function decline. In general population subjects without known lung disease, FEV1 declines with age in adult life at a fairly constant rate and can easily be modelled [31], with the average rate of decline among smokers and non-smokers reported as approximately 36 mL/year (Fig. 1). As a result of the variable discontinuous process of lung function decline in COPD, a non-parametric model was considered appropriate. The additive and cumulative effects of exacerbations in accelerating lung function decline and of pharmacotherapy in reducing exacerbations and slowing lung function decline were modelled by superposition of these effects on the underlying loss of lung function with time (FEV1, mL/year) related to the disease process. The FEV1 decline simulation model included the following assumptions:
Lung function decline in general population subjects (no known COPD and never/non-smokers) at a constant rate was used as a benchmark to assess percent decline in COPD, where the lung function decline begins from about the age of 20–25 years [1, 31].
The assumption was that lung function declines in patients with COPD at different rates as disease progresses; initial slow decline in GOLD grade 1, with more rapid decline in GOLD grade 2, and subsequent slowing of the rate of decline in GOLD grades 3 and 4 [4, 32].
COPD lung function decline is accelerated by exacerbations. The impact of exacerbations was simulated where (a) the number of exacerbations per year increased progressively from 0 to 0.5, then 1, then 2 and eventually 3 exacerbations per year [33–35], and (b) each exacerbation caused an initial additional decline (− 200 mL) which was a partially reversible decline in lung function (− 175.5 mL) and which was reduced over 3 months to a smaller permanent decline (− 24.5 mL), based on average data in the literature [3, 14, 36, 37] (Supplementary Materials, Table S1).
COPD lung function decline is modified by pharmacotherapy. The impacts of pharmacotherapy with ICS/LAMA/LABA (FF/UMEC/VI) and LAMA/LABA (UMEC/VI) were assessed as the effects in reducing the annual rate of exacerbations and in increasing lung function (see data in Fig. 1).
Fig. 1.
Summary of published lung function decline data in general population subjects with no known COPD and COPD populations with impact of disease severity, exacerbations and pharmacotherapy. aRefers to general population (no known COPD and never/non-smokers). bRefers to general population (no known COPD ± smoking). cReversible excess decline is defined as decrease in lung function during an exacerbation that is recovered after an exacerbation; permanent excess decline is defined as decrease in lung function during an exacerbation that is not recovered after an exacerbation, based on average data in the literature [3, 14, 36, 37] (Supplementary Materials, Table S1). dData from studies for pharmacotherapy refer to change in FEV1 after 52 weeks of treatment with the exception of two sets of data where change in FEV1 is reported after 26 weeks [38, 41]. These data do not take into account treatment at baseline. Data points on figure represent values from individual published studies [2, 3, 9, 10, 14, 15, 30, 33, 36–55, 57]; values in table represent average values from published data. Data from Kim et al. [47] includes data for FEV1 decline by GOLD grade (1–4) and by GOLD group (A–D). Data from Pauwels 1999 [10] and Tashkin 2012 [50] includes data for FEV1 decline in patients categorized as GOLD grade 1 or 2—on the figure these data points are shown under the grade 2 category. COPD chronic obstructive pulmonary disease, GOLD Global Initiative for Chronic Obstructive Lung Disease, ICS inhaled corticosteroid, LABA long-acting beta2-agonist, LAMA long-acting anti-muscarinic antagonist
This article is not based on any new studies with human participants or animals performed by any of the authors.
Model Input
Targeted literature searches were conducted using PubMed and Google Scholar to identify studies presenting data on FEV1 decline. Additional studies were identified through manual searches of reference lists of relevant articles and through checking papers known to the authors. Only articles published in English were considered and no date limits were set. The four search topics were (1) lung function decline with age in general population (no known COPD) subjects; (2) lung function decline in patients with COPD split by airflow limitation-defined severity (mild or GOLD grade 1, moderate or GOLD grade 2, severe or GOLD grade 3, very severe or GOLD grade 4); (3) impact of COPD exacerbations on lung function decline; (4) impact of pharmacotherapy on lung function decline in COPD. The pharmacotherapy studies considered RCTs that included any triple therapy administered by a single inhaler and included a comparator of dual (LAMA/LABA or ICS/LABA) and/or monotherapy [9, 18, 33, 38–42]. All pharmacotherapy trials were in patients with COPD and a current or recent smoking history, with the exception of one where a smoking history was not specified as an inclusion criterion [38].
Identified studies were screened and selected for inclusion by one reviewer and checked by a second reviewer. A data extraction template was used to extract and capture pre-defined data items, including study design, sample size and duration of follow-up period; baseline/demographic data; follow-up period data (FEV1 decline expressed as mL/year, exacerbations number/rate per year). Data extraction was performed by one person and verified by a second person.
Data from the studies identified in the literature search were used to estimate the model inputs for lung function decline in COPD by age, severity, exacerbation frequency and impact of LAMA/LABA and triple therapy (Table 1). In the model, treatment effects based on data for FF/UMEC/VI and UMEC/VI from the IMPACT study were used for the model simulations [33]. The inputs for increase in FEV1 with triple or dual therapy represent average values across COPD age groups as data split by age groups were not available. These data were regarded as representative of these treatment classes as illustrated by average values across studies for increase in FEV1 (mL) at the end of treatment (Fig. 1).
Table 1.
Modelling methodology: values used for simulations
| Subject | Age range (years) | FEV1 lung function decline (mL/year)a | Moderate/severe exacerbation rate/yearb | Months between exacerbationsb | Months between exacerbations therapy with ICS/LAMA/LABAc | Months between exacerbations therapy with LAMA/LABAc | FEV1 increase with ICS/LAMA/LABA at week 52 (mL)c | FEV1 increase with LAMA/LABA at week 52 (mL)c |
|---|---|---|---|---|---|---|---|---|
| General population (no known COPD and never/non-smokers) | 25–75 | 29 | – | – | – | – | – | – |
| COPD | 40–49 | 40 | 0.5 | 24 | 44 | 32 | 94 | 40 |
| COPD | 50–57 | 63 | 1 | 12 | 22 | 16 | 94 | 40 |
| COPD | 58–67 | 58 | 2 | 6 | 11 | 8 | 94 | 40 |
| COPD | 68–75 | 35 | 3 | 4 | 7 | 5 | 94 | 40 |
COPD chronic obstructive pulmonary disease, FEV1 forced expiratory volume in 1 s, ICS inhaled corticosteroid, LABA long-acting beta2-agonist, LAMA long-acting anti-muscarinic antagonist
aBased on typical values and pattern of decline over time from the literature [2, 3, 9, 10, 14, 15, 30, 33, 36–55, 57]
bBased on assumption of increasing annual exacerbation frequency from 0.5 to 3 with age; frequency assigned to correspond with typical lifetime exacerbation frequency [33]
cAverage values for magnitude of ICS/LAMA/LABA and LAMA/LABA treatment effects were used, corresponding with those seen in the IMPACT study [33]
No subgroups were evaluated, for example in terms of eosinophil counts or smoking status.
Model Outputs/Outcomes
The model output was decline in FEV1 over the age range 40–75 years. This age range was chosen to reflect the greater prevalence of COPD in those ≥ 40 years of age compared with those < 40, and with the assumption that the disease severity increases as the age advances [1]. Data summaries, graphical output, calculations and simulations were produced in Microsoft Excel (Version 2208).
Base Case, Scenario and Sensitivity Analyses
The assumed base case was model-simulated decline in FEV1 over 40–75 years with progressive impact of exacerbations from 0 per year to 3 per year and no ongoing pharmacotherapy. The scenarios of interest were simulated decline in FEV1 when triple or LAMA/LABA therapy was initiated at either 40, 55 or 65 years of age.
A sensitivity analysis was conducted whereby the assumption on exacerbation rates was progression from 0 to 1 per year, and the same scenarios of interest were investigated.
Results
Literature Search and Summary of Extracted Data
The studies identified by the literature research [2, 3, 9, 10, 14, 15, 18, 29, 30, 33, 36–57] and that were used to inform development of the model are summarized in the Online Data Supplement (Appendix 1). Figure 1 summarizes the published data on lung function decline for different populations. In general population subjects, lung function decline was similar among smokers and non-smokers. Lung function decline was numerically greater in patients with COPD with milder versus more severe disease (assessed by GOLD grade), and with increasing rate of exacerbations. However, these data are heterogeneous with large overlaps in average values. Pharmacotherapy had a positive impact on lung function, with greatest effects observed with triple therapy versus dual or monotherapy.
Model-Simulated Decline in Lung Function
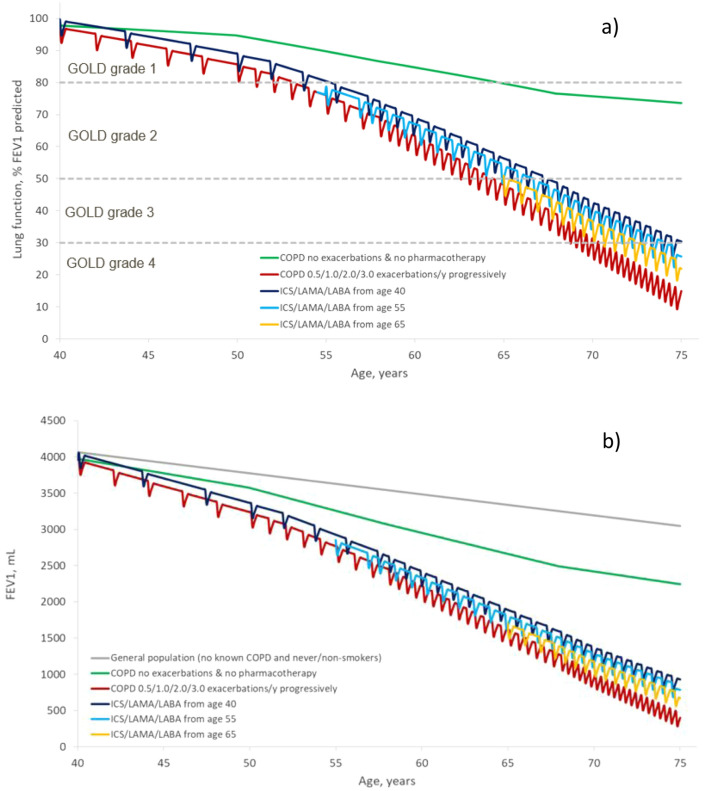
Figure 2a shows that the model-simulated rate of decline in lung function (expressed as FEV1% predicted) from age 40 to 75 years in a scenario of increasing exacerbations with disease progression (red line) was slowed with triple therapy, and larger effects were seen when therapy is initiated earlier versus later. The same pattern was observed when lung function decline was expressed as absolute volume (FEV1 mL) (Fig. 2b). Compared with the baseline assumption of no ongoing pharmacological therapy, initiation of triple therapy at age 40 years preserved an additional 469.7 mL of lung function by the age of 75 and a delay in progress to GOLD grade 4 of 3.66 years (Table 2). If triple therapy was started at either 55 or 65 years, the potential preservation of lung function was 327.5 mL or 213.5 mL, and the delay to GOLD grade 4 was 3.16 years or 1.58 years, respectively. This was largely driven by the reductions in exacerbations rates, as defined in the model, which were reduced on average between 40 and 75 years from 1.57 (no ongoing therapy) to 0.91, 1.06 or 1.23 if triple therapy was initiated at 40, 55 or 65 years, respectively.
Fig. 2.
COPD lung function decline and impact of commencing ICS/LAMA/LABA triple therapy at age 40, 55 or 65 years, measured as a FEV1 percent of predicted and b FEV1 mL. COPD chronic obstructive pulmonary disease, FEV1 forced expiratory volume in 1 s, GOLD Global Initiative for Chronic Obstructive Lung Disease, ICS inhaled corticosteroid, LABA long-acting beta2-agonist, LAMA long-acting anti-muscarinic antagonist
Table 2.
Summary of modelling simulation outputs
| Start of therapy | Age at which lung function first drops below FEV1 pred 30% | Time delay in progress to COPD Gold stage 4 (< 30% FEV1 pred) | Lung function remaining at age 75 (FEV1 mL) | Additional lung function remaining at age 75 (FEV1 mL) | Exacerbations total between 40 and 75 (average/year) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Triple | LAMA/LABA | Triple | LAMA/LABA | Triple | LAMA/LABA | Triple | LAMA/LABA | Triple | LAMA/LABA | |
| None | 69.0 years | 69.0 years | – | – | 456.5 mL | 456.5 mL | – | – | 55 (1.57) | 55 (1.57) |
| 829 months | 829 months | – | – | |||||||
| From age 40 |
72.5 years 873 months |
70.58 years 847 months |
3.66 years 44 months |
1.5 years 18 months |
926.2 mL | 692.5 mL | 469.7 mL | 236.0 mL | 32 (0.91) | 42 (1.20) |
| From age 55 |
72.25 years 867 months |
70.25 years 843 months |
3.16 years 38 months |
1.16 years 14 months |
784.0 mL | 659.8 mL | 327.5 mL | 203.3 mL | 37 (1.06) | 44 (1.26) |
| From age 65 |
70.66 years 848 months |
69.4 years 833 months |
1.58 years 19 months |
0.33 years 4 months |
670.0 mL | 594.0 mL | 213.5 mL | 137.5 mL | 43 (1.23) | 49 (1.40) |
FEV1 forced expiratory volume in 1 s, LABA long-acting beta2-agonist, LAMA long-acting anti-muscarinic antagonist
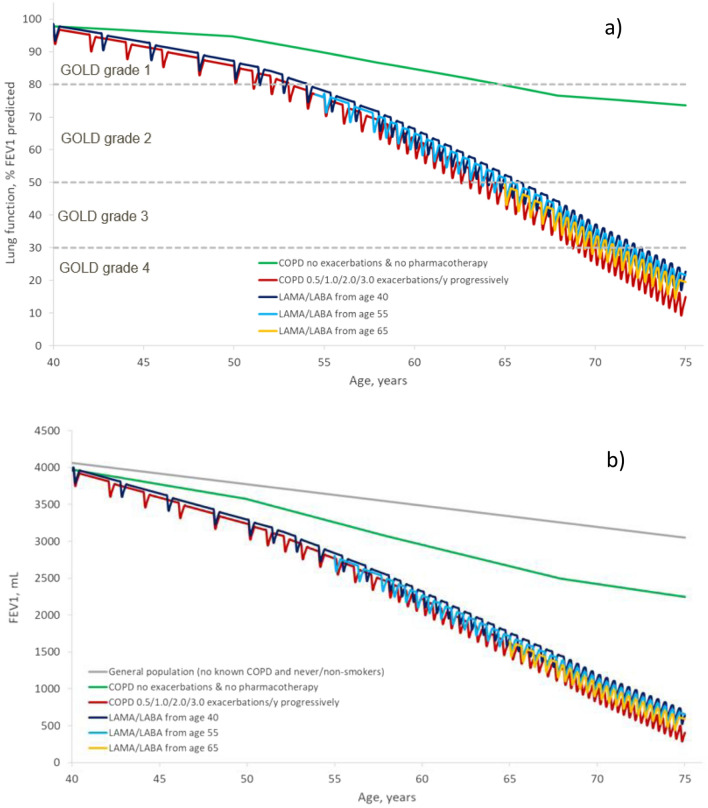
Simulated decline in lung function was also reduced by earlier versus later intervention with LAMA/LABA therapy (Fig. 3a, b). Initiation of LAMA/LABA therapy at ages 40, 55 or 65 years preserved an additional 236.0 mL, 203.3 mL or 137.5 mL of lung function, respectively, by the age of 75; delayed progression to GOLD grade 4 by 1.5, 1.16 and 0.33 years, respectively; and reduced average annual exacerbation rates from 1.57 to 1.20, 1.26 and 1.40, respectively (Table 2).
Fig. 3.
COPD lung function decline and impact of commencing of LABA/LAMA therapy at age 40, 55 or 65 years, measured as a FEV1 percent of predicted and b FEV1 mL. COPD chronic obstructive pulmonary disease, FEV1 forced expiratory volume in 1 s, GOLD Global Initiative for Chronic Obstructive Lung Disease, ICS inhaled corticosteroid, LABA long-acting beta2-agonist, LAMA long-acting anti-muscarinic antagonist
Triple therapy had a greater benefit on lung function decline compared to LAMA/LABA treatment (Table 2). The numerical difference between treatments (triple versus no therapy and LABA/LAMA versus no therapy) in additional lung function remaining at 75 years was 233.7 mL, 124.2 mL and 76.0 mL greater with triple therapy, following initiation of therapy at ages 40, 55 or 65 years, respectively. The simulation of lung function decline when initiating triple or LABA/LAMA treatment starting at age 40 is illustrated in Supplementary Materials, Fig. S1.
The sensitivity analysis, which modelled the exacerbation rate as progressing from 0 to 0.5 to 1 per year, showed a similar pattern of results to the baseline model with FEV1 decline slowed with earlier versus later intervention with triple therapy (Supplementary Materials, Fig. S2), and to a lesser magnitude with LAMA/LABA therapy (Supplementary Materials, Fig. S3).
Discussion
This modelling analysis shows that starting pharmacotherapy at an earlier versus later stage in patients with COPD has the potential for long-term benefits in terms of lung function preservation, delaying progression to GOLD grade 4. Pharmacological treatment reduces the average annual rate of exacerbations, which is a mechanism by which the delay in FEV1 decline can be achieved. These benefits were greater with triple therapy FF/UMEC/VI versus dual bronchodilator treatment with UMEC/VI. The greatest overall benefit in delaying disease progression was observed with initiation of triple therapy at 40 years. This simulation model provides new information on the potential long-term benefits on lung function decline of initiating LAMA/LABA or triple pharmacotherapy, at an earlier versus later stage of disease.
This FEV1 decline model made several assumptions with respect to the occurrence and patterns of exacerbation rates. The model assumed that exacerbations of COPD accelerate the decline of FEV1 and this was supported by the data from studies identified in the literature search that show an increasing impact on loss of lung function with increased exacerbation frequency [3, 14, 15, 36, 37, 51, 53–55]. Exacerbation rates (number per patient per year) were modelled to progressively worsen over time from 0 to 3 exacerbations per year. Real-life patterns of exacerbation frequency vary among individuals [58] and the results of the sensitivity analysis which modelled exacerbation rates progressing from 0.5 to 1 per year provide reassurance about the model robustness, showing the potential for triple therapy benefits on disease progression even in patients with only 1 exacerbation per year. The model also assumed that treatment with triple and LABA/LAMA therapy modifies the decline in FEV1, based on data from large, 1-year RCTs as shown in Fig. 1 [9, 33, 38–40, 42], and that this treatment effect would be similar over a long-term period. The model outputs in this analysis provide average values and do not consider separately the contribution of other factors which may impact lung function decline such as smoking history [59], or variation in ICS response which is associated with eosinophil counts [60]. These may be seen as limitations of the model; nevertheless, this analysis provides information on potential long-term treatment-related benefits that are difficult to study in the real world. Further analysis that accounts for different clinical characteristics may be informative.
In clinical practice, a variety of pathways to triple therapy are observed within and between countries [61]. However, the impact of pharmacological interventions at an earlier stage in disease progression has been unclear [13]. The benefits of early versus late initiation of triple therapy after an exacerbation have been shown in retrospective studies in terms of significantly reduced exacerbation rates in the following year [62, 63]. Our analysis adds to these findings by showing that reductions in exacerbations can lead to long-term benefits in slowing lung function decline and disease progression.
Although starting triple therapy in all patients at an early stage of COPD may not be practical, use of a treatable traits approach to determine patients at high risk of exacerbation and rapid FEV1 decline could be one way of identifying patients who would benefit from early triple therapy [60]. In these subgroups, optimization to triple therapy at an early stage of COPD could preserve valuable lung function and delay disease progression. In the Subpopulations and Intermediate Outcome Measures in COPD Study (SPIROMICS), Woodruff et al. reported that current/former smokers with respiratory symptoms but preserved airway function had a higher risk of exacerbations than asymptomatic smokers [64]. In an analysis of 30 months treatment with ICS in 50 steroid-naïve patients with moderate to severe COPD, long-term predictors of better ICS effects on FEV1 decline were a lower smoking burden, less hyperinflation and more preserved diffusion capacity, i.e. in patients with less advanced disease [65]. Previous modelling prediction studies have identified factors associated with the risk of future exacerbations in COPD [24–26]. For example, prior exacerbations, FEV1 percent predicted, eosinophil count, sex, region, COPD Assessment Test score, prior treatment and reliever medication use were highly significant factors associated with future exacerbation risk, and showed a greater benefit of ICS-containing treatments in patients with higher eosinophil counts, greater number of prior exacerbations and those who had received more prior maintenance therapies [24].
A limitation of this analysis is that exacerbations were modelled as occurring at regular intervals with the assumption that there would be recovery from each exacerbation before the next occurred, whereas in real-life, patterns of exacerbations are likely to be more random [58]. However, this approach was not thought to have influenced the overall outcome since we modelled the average outcomes over a long period of time. In addition, the sensitivity analysis, which assumed a different exacerbation rate pattern, also demonstrated the benefit of triple over LAMA/LABA treatment. Another limitation is that the inputs for increase in FEV1 with triple or dual therapy represent average values across COPD age groups as data split by age groups were not available. In addition, the scenarios we focused on were therapy (LAMA/LABA or triple) or ‘no therapy’ from age 40, 55 or 65, whereas in clinical practice patients follow several different treatment pathways to triple therapy, and patients may change treatments between classes [61]. This has not been factored into the current model. Nonetheless, we can still infer from our findings the likely outcomes for these alternative scenarios. A further limitation is that we did not consider patient mortality in this model and the model outputs show lung function declining beyond a point where many patients may not survive. However, if the model had omitted data once lung function fell below a “survivability threshold”, it would not have changed the observed conclusions. There may be many other causes of death in this population making such events difficult to predict and model. It should also be noted that we did not consider the risk/benefit aspect or the cost implications of starting triple therapy earlier versus later. Finally, in this analysis, reduction in exacerbations by pharmacotherapy was the model input for simulation of lung function decline. This may have introduced an unintentional bias in favour of ICS-containing treatments including triple therapy and, as previously highlighted, starting triple therapy early may require a treatable traits approach to identify patients most eligible for early treatment. On the other hand, as pharmacotherapy can improve lung function through other mechanisms, this model may underestimate the benefits of early pharmacotherapy on long-term disease progression. In this study we developed a simple, hypothesis-generating model and future, more refined models could consider other factors that were not included in our model.
Conclusion
This simulation modelling analysis has demonstrated the potential benefits of starting pharmacotherapy earlier in the course of COPD in terms of slowing disease progression. Greater benefits were demonstrated with early initiation of triple versus LAMA/LABA therapy.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
This study was funded by GSK. The Rapid Service and Open Access Fees for publication were funded by GSK. Dave Singh is supported by the National Institute for Health Research (NIHR) Manchester Biomedical Research Centre (BRC).
Medical Writing/Editorial Assistance
Writing assistance and editorial support was provided by Kate Hollingworth of Continuous Improvement Ltd and funded by GSK.
Author Contributions
The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors. Peter Daley-Yates, Sudeep Acharya and Bhumika Aggarwal were involved in the study concept and design. Peter Daley-Yates was responsible for the data analysis. All authors discussed and interpreted the results, contributed to the writing and reviewing of the manuscript, and gave approval for the final version to be published.
Disclosures
Dave Singh has received consulting fees from Aerogen, AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, CSL Behring, Epiendo, Genentech, GSK, Glenmark, Gossamerbio, Kinaset, Menarini, Novartis, Orion, Pulmatrix, Sanofi, Synairgen, Teva, Theravance and Verona. Diego Litewka has received honoraria for lectures, presentations, speakers’ bureaus or educational events and participation in an advisory board from GSK; and support for travel/attending meetings from Boehringer Ingelheim and Tuteur. Rafael Páramo has received consulting fees from Chiesi and GSK; payment for lectures and presentations at medical meetings from AstraZeneca, Boehringer Ingelheim, GSK and Novartis; and support for travel/attending meetings from AstraZeneca, Boehringer Ingelheim, Chiesi, GSK and Novartis. Adrian Rendon has received consulting fees, honoraria for lectures, presentations, speakers’ bureaus or educational events, and participation in advisory boards from AstraZeneca, Boehringer Ingelheim, Chiesi, GSK and Sanofi; and has received support for travel/attending meetings from Chiesi. Abdullah Sayiner has received honoraria for lectures, presentations, speakers’ bureaus or educational events from Abdi Ibrahim, Bilim, Deva and GSK; and fees for participation in advisory boards from Abdi Ibrahim and GSK. Suzana Erico Tanni has received honoraria for educational events from Gilead. Sudeep Acharya, Bhumika Aggarwal, Afisi S Ismaila and Raj Sharma are employees of GSK and hold GSK shares. Afisi S Ismaila is also an unpaid part-time faculty member at the McMaster University in Canada. Peter Daley-Yates is a former employee of GSK and holds GSK shares. Peter Daley-Yates is currently an independent clinical pharmacology consultant.
Compliance with Ethics Guidelines
This article is not based on any new studies with human participants or animals performed by any of the authors.
Data Availability
Information on GSK’s data sharing commitments and requesting access can be found at: https://www.gsk-studyregister.com/en/
Footnotes
The original online version of this article was revised due to update in article text.
Change history
7/28/2023
A Correction to this paper has been published: 10.1007/s12325-023-02613-y
References
- 1.Global strategy for the diagnosis, management and prevention of COPD. Global Initiative for Chronic Obstructive Lung Disease (GOLD). 2023. http://goldcopd.org. Accessed 12 Jan 2023.
- 2.Lange P, Celli B, Agustí A, et al. Lung-function trajectories leading to chronic obstructive pulmonary disease. N Engl J Med. 2015;373:111–122. doi: 10.1056/NEJMoa1411532. [DOI] [PubMed] [Google Scholar]
- 3.Vestbo J, Edwards LD, Scanlon PD, et al. Changes in forced expiratory volume in 1 second over time in COPD. N Engl J Med. 2011;365:1184–1192. doi: 10.1056/NEJMoa1105482. [DOI] [PubMed] [Google Scholar]
- 4.Tantucci C, Modina D. Lung function decline in COPD. Int J Chron Obstruct Pulmon Dis. 2012;7:95–99. doi: 10.2147/COPD.S27480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maltais F, Dennis N, Chan CK. Rationale for earlier treatment in COPD: a systematic review of published literature in mild-to-moderate COPD. COPD. 2013;10:79–103. doi: 10.3109/15412555.2012.719048. [DOI] [PubMed] [Google Scholar]
- 6.Burge PS, Calverley PM, Jones PW, Spencer S, Anderson JA, Maslen TK. Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial. BMJ. 2000;320(7245):1297–1303. doi: 10.1136/bmj.320.7245.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356:775–789. doi: 10.1056/NEJMoa063070. [DOI] [PubMed] [Google Scholar]
- 8.Tashkin DP, Celli B, Senn S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359(15):1543–1554. doi: 10.1056/NEJMoa0805800. [DOI] [PubMed] [Google Scholar]
- 9.Rabe KF, Martinez FJ, Singh D, et al. Improvements in lung function with budesonide/glycopyrrolate/formoterol fumarate metered dose inhaler versus dual therapies in patients with COPD: a sub-study of the ETHOS trial. Ther Adv Respir Dis. 2021;15:17534666211034329. doi: 10.1177/17534666211034329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pauwels RA, Löfdahl CG, Laitinen LA, et al. Long-term treatment with inhaled budesonide in persons with mild chronic obstructive pulmonary disease who continue smoking. European Respiratory Society Study on Chronic Obstructive Pulmonary Disease. N Engl J Med. 1999;340:1948–1953. doi: 10.1056/NEJM199906243402503. [DOI] [PubMed] [Google Scholar]
- 11.van Grunsven P, Schermer T, Akkermans R, et al. Short- and long-term efficacy of fluticasone propionate in subjects with early signs and symptoms of chronic obstructive pulmonary disease. Results of the DIMCA study. Respir Med. 2003;97:1303–1312. doi: 10.1016/j.rmed.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Singh D, D'Urzo AD, Donohue JF, Kerwin EM. Weighing the evidence for pharmacological treatment interventions in mild COPD; a narrative perspective. Respir Res. 2019;20:141. doi: 10.1186/s12931-019-1108-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinez FJ, Agusti A, Celli BR, et al. Treatment trials in young patients with chronic obstructive pulmonary disease and pre-chronic obstructive pulmonary disease patients: time to move forward. Am J Respir Crit Care Med. 2022;205:275–287. doi: 10.1164/rccm.202107-1663SO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dransfield MT, Kunisaki KM, Strand MJ, et al. Acute exacerbations and lung function loss in smokers with and without chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;195:324–330. doi: 10.1164/rccm.201605-1014OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57:847–852. doi: 10.1136/thorax.57.10.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wedzicha JA, Mackay AJ, Singh R. COPD exacerbations: impact and prevention. Breathe. 2013;9:434–440. doi: 10.1183/20734735.002913. [DOI] [Google Scholar]
- 17.Hurst JR, Skolnik N, Hansen GJ, et al. Understanding the impact of chronic obstructive pulmonary disease exacerbations on patient health and quality of life. Eur J Intern Med. 2020;73:1–6. doi: 10.1016/j.ejim.2019.12.014. [DOI] [PubMed] [Google Scholar]
- 18.Rabe KF, Martinez FJ, Ferguson GT, et al. Triple inhaled therapy at two glucocorticoid doses in moderate-to-very-severe COPD. N Engl J Med. 2020;383:35–48. doi: 10.1056/NEJMoa1916046. [DOI] [PubMed] [Google Scholar]
- 19.Pascoe S, Barnes N, Brusselle G, et al. Blood eosinophils and treatment response with triple and dual combination therapy in chronic obstructive pulmonary disease: analysis of the IMPACT trial. Lancet Respir Med. 2019;7:745–756. doi: 10.1016/S2213-2600(19)30190-0. [DOI] [PubMed] [Google Scholar]
- 20.Martinez FJ, Rabe KF, Ferguson GT, et al. Reduced all-cause mortality in the ETHOS trial of budesonide/glycopyrrolate/formoterol for chronic obstructive pulmonary disease. A randomized, double-blind, multicenter, parallel-group study. Am J Respir Crit Care Med. 2021;203:553–564. doi: 10.1164/rccm.202006-2618OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Porgo TV, Norris SL, Salanti G, et al. The use of mathematical modeling studies for evidence synthesis and guideline development: a glossary. Res Synth Methods. 2019;10:125–133. doi: 10.1002/jrsm.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Egger M, Johnson L, Althaus C, et al. Developing WHO guidelines: time to formally include evidence from mathematical modelling studies. F1000Res. 2017;6:1584. doi: 10.12688/f1000research.12367.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Younis T, Thana M, Skedgel C. Evidence in medicine: math versus biology! Curr Oncol. 2017;24:349–351. doi: 10.3747/co.24.3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh D, Hurst JR, Martinez FJ, et al. Predictive modeling of COPD exacerbation rates using baseline risk factors. Ther Adv Respir Dis. 2022;16:17534666221107314. doi: 10.1177/17534666221107314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoogendoorn M, Feenstra TL, Boland M, et al. Prediction models for exacerbations in different COPD patient populations: comparing results of five large data sources. Int J Chron Obstruct Pulmon Dis. 2017;12:3183–3194. doi: 10.2147/COPD.S142378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adibi A, Sin DD, Safari A, et al. The acute COPD exacerbation prediction tool (ACCEPT): a modelling study. Lancet Respir Med. 2020;8:1013–1021. doi: 10.1016/S2213-2600(19)30397-2. [DOI] [PubMed] [Google Scholar]
- 27.Daley-Yates P, Aggarwal B, Lulic Z, Fulmali S, Cruz AA, Singh D. Pharmacology versus convenience: a benefit/risk analysis of regular maintenance versus infrequent or as-needed inhaled corticosteroid use in mild asthma. Adv Ther. 2022;39:706–726. doi: 10.1007/s12325-021-01976-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh D, Garcia G, Maneechotesuwan K, et al. New versus old: the impact of changing patterns of inhaled corticosteroid prescribing and dosing regimens in asthma management. Adv Ther. 2022;39:1895–1914. doi: 10.1007/s12325-022-02092-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Makris D, Moschandreas J, Damianaki A, et al. Exacerbations and lung function decline in COPD: new insights in current and ex-smokers. Respir Med. 2007;101:1305–1312. doi: 10.1016/j.rmed.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 30.Bridevaux PO, Gerbase MW, Probst-Hensch NM, Schindler C, Gaspoz JM, Rochat T. Long-term decline in lung function, utilisation of care and quality of life in modified GOLD stage 1 COPD. Thorax. 2008;63:768–774. doi: 10.1136/thx.2007.093724. [DOI] [PubMed] [Google Scholar]
- 31.Kohansal R, Martinez-Camblor P, Agustí A, Buist AS, Mannino DM, Soriano JB. The natural history of chronic airflow obstruction revisited: an analysis of the Framingham offspring cohort. Am J Respir Crit Care Med. 2009;180:3–10. doi: 10.1164/rccm.200901-0047OC. [DOI] [PubMed] [Google Scholar]
- 32.Welte T, Vogelmeier C, Papi A. COPD: early diagnosis and treatment to slow disease progression. Int J Clin Pract. 2015;69:336–349. doi: 10.1111/ijcp.12522. [DOI] [PubMed] [Google Scholar]
- 33.Lipson DA, Barnhart F, Brealey N, et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378:1671–1680. doi: 10.1056/NEJMoa1713901. [DOI] [PubMed] [Google Scholar]
- 34.Hoogendoorn M, Feenstra TL, Hoogenveen RT, Al M, Mölken MR. Association between lung function and exacerbation frequency in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2010;5:435–444. doi: 10.2147/COPD.S13826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Donaldson GC, Seemungal TA, Patel IS, Lloyd-Owen SJ, Wilkinson TM, Wedzicha JA. Longitudinal changes in the nature, severity and frequency of COPD exacerbations. Eur Respir J. 2003;22:931–936. doi: 10.1183/09031936.03.00038303. [DOI] [PubMed] [Google Scholar]
- 36.Cote CG, Dordelly LJ, Celli BR. Impact of COPD exacerbations on patient-centered outcomes. Chest. 2007;131:696–704. doi: 10.1378/chest.06-1610. [DOI] [PubMed] [Google Scholar]
- 37.Halpin DMG, Decramer M, Celli BR, Mueller A, Metzdorf N, Tashkin DP. Effect of a single exacerbation on decline in lung function in COPD. Respir Med. 2017;128:85–91. doi: 10.1016/j.rmed.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 38.Lipson DA, Barnacle H, Birk R, et al. FULFIL trial: once-daily triple therapy for patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;196:438–446. doi: 10.1164/rccm.201703-0449OC. [DOI] [PubMed] [Google Scholar]
- 39.Singh D, Papi A, Corradi M, et al. Single inhaler triple therapy versus inhaled corticosteroid plus long-acting β2-agonist therapy for chronic obstructive pulmonary disease (TRILOGY): a double-blind, parallel group, randomised controlled trial. Lancet. 2016;388:963–973. doi: 10.1016/S0140-6736(16)31354-X. [DOI] [PubMed] [Google Scholar]
- 40.Vestbo J, Papi A, Corradi M, et al. Single inhaler extrafine triple therapy versus long-acting muscarinic antagonist therapy for chronic obstructive pulmonary disease (TRINITY): a double-blind, parallel group, randomised controlled trial. Lancet. 2017;389:1919–1929. doi: 10.1016/S0140-6736(17)30188-5. [DOI] [PubMed] [Google Scholar]
- 41.Ferguson GT, Rabe KF, Martinez FJ, et al. Triple therapy with budesonide/glycopyrrolate/formoterol fumarate with co-suspension delivery technology versus dual therapies in chronic obstructive pulmonary disease (KRONOS): a double-blind, parallel-group, multicentre, phase 3 randomised controlled trial. Lancet Respir Med. 2018;6:747–758. doi: 10.1016/S2213-2600(18)30327-8. [DOI] [PubMed] [Google Scholar]
- 42.Papi A, Vestbo J, Fabbri L, et al. Extrafine inhaled triple therapy versus dual bronchodilator therapy in chronic obstructive pulmonary disease (TRIBUTE): a double-blind, parallel group, randomised controlled trial. Lancet. 2018;391:1076–1084. doi: 10.1016/S0140-6736(18)30206-X. [DOI] [PubMed] [Google Scholar]
- 43.Luoto J, Pihlsgård M, Wollmer P, Elmståhl S. Relative and absolute lung function change in a general population aged 60–102 years. Eur Respir J. 2019;53:1701812. doi: 10.1183/13993003.01812-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oelsner EC, Balte PP, Bhatt SP, et al. Lung function decline in former smokers and low-intensity current smokers: a secondary data analysis of the NHLBI Pooled Cohorts Study. Lancet Respir Med. 2020;8:34–44. doi: 10.1016/S2213-2600(19)30276-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wijnant SRA, De Roos E, Kavousi M, et al. Trajectory and mortality of preserved ratio impaired spirometry: the Rotterdam Study. Eur Respir J. 2020;55:1901217. doi: 10.1183/13993003.01217-2019. [DOI] [PubMed] [Google Scholar]
- 46.Masuko H, Sakamoto T, Kaneko Y, et al. Lower FEV1 in non-COPD, nonasthmatic subjects: association with smoking, annual decline in FEV1, total IgE levels, and TSLP genotypes. Int J Chron Obstruct Pulmon Dis. 2011;6:181–189. doi: 10.2147/COPD.S16383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim J, Yoon HI, Oh YM, et al. Lung function decline rates according to GOLD group in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2015;10:1819–1827. doi: 10.2147/COPD.S87766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Decramer M, Celli B, Kesten S, et al. Effect of tiotropium on outcomes in patients with moderate chronic obstructive pulmonary disease (UPLIFT): a prespecified subgroup analysis of a randomised controlled trial. Lancet. 2009;374:1171–1178. doi: 10.1016/S0140-6736(09)61298-8. [DOI] [PubMed] [Google Scholar]
- 49.Jenkins CR, Jones PW, Calverley PM, et al. Efficacy of salmeterol/fluticasone propionate by GOLD stage of chronic obstructive pulmonary disease: analysis from the randomised, placebo-controlled TORCH study. Respir Res. 2009;10:59. doi: 10.1186/1465-9921-10-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tashkin DP, Celli BR, Decramer M, Lystig T, Liu D, Kesten S. Efficacy of tiotropium in COPD patients with FEV1 ≥ 60% participating in the UPLIFT® trial. COPD. 2012;9:289–296. doi: 10.3109/15412555.2012.656211. [DOI] [PubMed] [Google Scholar]
- 51.Calverley PMA, Anderson JA, Brook RD, et al. Fluticasone furoate, vilanterol, and lung function decline in patients with moderate chronic obstructive pulmonary disease and heightened cardiovascular risk. Am J Respir Crit Care Med. 2018;197:47–55. doi: 10.1164/rccm.201610-2086OC. [DOI] [PubMed] [Google Scholar]
- 52.Celli BR, Thomas NE, Anderson JA, et al. Effect of pharmacotherapy on rate of decline of lung function in chronic obstructive pulmonary disease: results from the TORCH study. Am J Respir Crit Care Med. 2008;178:332–338. doi: 10.1164/rccm.200712-1869OC. [DOI] [PubMed] [Google Scholar]
- 53.Larsson K, Janson C, Lisspers K, et al. The impact of exacerbation frequency on clinical and economic outcomes in Swedish COPD patients: the ARCTIC study. Int J Chron Obstruct Pulmon Dis. 2021;16:701–713. doi: 10.2147/COPD.S297943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang H, Xiang P, Zhang E, et al. Predictors of exacerbation frequency in chronic obstructive pulmonary disease. Eur J Med Res. 2014;19:18. doi: 10.1186/2047-783X-19-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Spencer S, Calverley PM, Burge PS, Jones PW. Impact of preventing exacerbations on deterioration of health status in COPD. Eur Respir J. 2004;23:698–702. doi: 10.1183/09031936.04.00121404. [DOI] [PubMed] [Google Scholar]
- 56.Kanner RE, Anthonisen NR, Connett JE, Lung Health Study Research Group Lower respiratory illnesses promote FEV1 decline in current smokers but not ex-smokers with mild chronic obstructive pulmonary disease: results from the lung health study. Am J Respir Crit Care Med. 2001;164:358–364. doi: 10.1164/ajrccm.164.3.2010017. [DOI] [PubMed] [Google Scholar]
- 57.Kerkhof M, Voorham J, Dorinsky P, et al. The long-term burden of COPD exacerbations during maintenance therapy and lung function decline. Int J Chron Obstruct Pulmon Dis. 2020;15:1909–1918. doi: 10.2147/COPD.S253812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Han MK, Quibrera PM, Carretta EE, et al. Frequency of exacerbations in patients with chronic obstructive pulmonary disease: an analysis of the SPIROMICS cohort. Lancet Respir Med. 2017;5:619–626. doi: 10.1016/S2213-2600(17)30207-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lange P, Groth S, Nyboe GJ, et al. Effects of smoking and changes in smoking habits on the decline of FEV1. Eur Respir J. 1989;2:811–816. doi: 10.1183/09031936.93.02090811. [DOI] [PubMed] [Google Scholar]
- 60.Singh D, Agusti A, Martinez FJ, et al. Blood eosinophils and chronic obstructive pulmonary disease: a global initiative for chronic obstructive lung disease science committee 2022 review. Am J Respir Crit Care Med. 2022;206:17–24. doi: 10.1164/rccm.202201-0209PP. [DOI] [PubMed] [Google Scholar]
- 61.Quint JK, O'Leary C, Venerus A, et al. Prescribing pathways to triple therapy: a multi-country, retrospective observational study of adult patients with chronic obstructive pulmonary disease. Pulm Ther. 2020;6:333–350. doi: 10.1007/s41030-020-00132-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sicras Mainar A, Huerta A, Navarro Artieda R, Monsó E, Landis SH, Ismaila AS. Economic impact of delaying initiation with multiple-inhaler maintenance triple therapy in Spanish patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2019;14:2121–2129. doi: 10.2147/COPD.S211854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mannino D, Bogart M, Germain G, et al. Benefit of prompt versus delayed use of single-inhaler fluticasone furoate/umeclidinium/vilanterol (FF/UMEC/VI) following a COPD exacerbation. Int J Chron Obstruct Pulmon Dis. 2022;17:491–504. doi: 10.2147/COPD.S337668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Woodruff PG, Couper D, Han MK. Symptoms in smokers with preserved pulmonary function. N Engl J Med. 2016;375:896–897. doi: 10.1056/NEJMc1608235. [DOI] [PubMed] [Google Scholar]
- 65.Snoeck-Stroband JB, Lapperre TS, Sterk PJ, et al. Prediction of long-term benefits of inhaled steroids by phenotypic markers in moderate-to-severe COPD: a randomized controlled trial. PLoS ONE. 2015;10:e0143793. doi: 10.1371/journal.pone.0143793. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Information on GSK’s data sharing commitments and requesting access can be found at: https://www.gsk-studyregister.com/en/