Abstract
To diagnose visceral leishmaniasis (kala-azar), we have developed a nested PCR method based on amplification of the mini-exon gene, which is unique and tandomly repeated in the Leishmania genome. Nested PCR was sufficiently sensitive for the detection of DNA in an amount equivalent to a single Leishmania parasite or less. We examined the usefulness of this PCR method using bone marrow aspirates and buffy coat cells collected from kala-azar patients who had or had not received chemotherapy in northwest China. We obtained PCR positivity for all of the parasitologically positive bone marrow samples from the patients. Some ambiguities with the primary PCR results were eliminated by the subsequent nested PCR. The buffy coat samples from 7 of 12 patients with splenomegaly were positive by the nested PCR, although only 2 of them were positive for parasites by culture. However, buffy coat samples from nine children, whose splenomegaly has been reduced and clinically cured by antimony treatment, were all negative. Thus, this nested PCR method represents a new tool for the diagnosis of kala-azar with patient blood samples instead of bone marrow or spleen aspirates obtained by more invasive procedures.
Protozoan parasites of the genus Leishmania cause a wide spectrum of diseases including cutaneous, mucocutaneous, and visceral leishmaniasis (kala-azar) (19). As a diagnostic strategy, a number of different probes derived from nuclear DNA and kinetoplast DNA (kDNA) have been developed for the identification of Leishmania species and determination of the evolutionary relationships of the isolates (1, 3, 6, 14, 15). The PCR method has made these probes especially sensitive for the clinical diagnosis of visceral leishmaniasis in different countries such as India, Kenya, and Brazil as well as in immunocompromised patients (2, 5, 11, 12, 17). In China, new kala-azar cases have been recorded annually, especially in the northwest Xinjiang-Uigur Autonomous Region (4). Examinations of bone marrow aspirates for parasites by microscopy and cultivation remain the methods of diagnosis (4, 18).
In this report, we describe a nested PCR method for the diagnosis of kala-azar and its applications in China. We selected the mini-exon gene as the target DNA for PCR amplification since the gene is unique and is tandemly repeated in the genus Leishmania but is absent from mammalian hosts and sand fly vectors (3). Furthermore, the transcribed region is highly conserved, while the nontranscribed spacer region is different in length and in sequence among different species (3). We used a primer set previously described for the first-round or primary PCR for laboratory studies (3, 14). We developed an additional internal primer set for the subsequent second-round or nested PCR to increase the sensitivity. Here we report that the nested PCR procedure is sufficiently sensitive for the detection of a single Leishmania cell and can detect the parasite DNA from the buffy coat cells of patients with active kala-azar. Thus, the nested PCR may be applied as a new diagnostic technique for the detection of Leishmania in the peripheral blood of patients with active kala-azar and allows us to avoid the use of tissue biopsy specimens which must be obtained by more invasive means.
MATERIALS AND METHODS
Isolation of DNA from liver and bone marrow of mice infected with Leishmania donovani.
Female BALB/c mice (age, 8 weeks) were intravenously inoculated with 108 promastigotes of L. donovani 2S (7). The promastigotes were grown in medium 199 with 25 mM HEPES and 15% heat-inactivated fetal calf serum. One week after infection, a slice of liver (10 to 20 mg) and thigh bone marrow cells (2 × 106 cells) were obtained from each mouse and were stored in absolute ethanol at −20°C until use. The samples were then centrifuged in vacuum to remove ethanol, homogenized, and digested with 0.5 mg of proteinase K per ml and 200 μg of RNase per ml for 16 h. DNA was isolated with a DNA extraction kit (Blood & Cell Culture DNA Kit, QIAGEN Inc., Chatsworth, Calif.) and was stored in TE (10 mM Tris-HCl, 1 mM EDTA [pH 8.0]) at −20°C. Liver and bone marrow cells from uninfected mice were subjected to DNA isolation as described above to serve as negative controls.
The guidelines of the Jikei University School of Medicine for experiments with animals, consistent with national and international guidelines, were followed in this study.
Clinical samples.
Samples were collected from 22 patients from two areas of endemicity, Kashi and Bachu in Kashi Prefecture in the Xinjiang-Uigur Autonomous Region of northwestern China. Bachu is located in the desert area about 200 km east of Kashi. Bone marrow aspirates of about 100 μl were taken from the sphenoid bone with a biopsy needle while the patient was under local anesthesia. This was done for a total of 12 patients in Kashi, including 11 children with splenomegaly and a male adult suspected of having kala-azar, albeit without splenomegaly (Table 1, patients 1 to 11 and 13). Bone marrow aspiration was not done for patient 12. Bone marrow smears were stained with Giemsa and were examined under an oil immersion microscope (magnification, ×1,000). The remaining sample from each subject was diluted with sterile saline. One part was inoculated into at least four culture tubes containing NNN and/or Sloppy Evans medium (20). The cultures were incubated at 25°C and were examined for at least 6 weeks in three different laboratories. The remainder (equivalent to 20 to 30 μl of each original aspirate) was stored in absolute ethanol for subsequent DNA isolation. Peripheral blood of about 1 ml was also taken from each of the 13 patients and was mixed with 10 μl of 100 mM EDTA as an anticoagulant. The samples were either kept at room temperature for at least 30 min or centrifuged at 2,000 × g for 5 min to sediment the erythrocytes. The plasma was saved for immunological tests. Buffy coat cells were collected for inoculation into NNN medium and were stored in absolute ethanol. Buffy coat cells were also similarly collected from a total of nine children in Bachu (Table 1, patients 14 to 22) whose splenomegaly was barely palpable after cure by sodium stibogluconate treatment 1 to 3 years earlier.
TABLE 1.
Comparison of parasitological, serological, and PCR diagnosis of kala-azar in northwest China
| Patient no. (yr) | Age (yr) | Sexa | Splenomegalyb (cm) | Bone marrow
|
Buffy coat
|
Immunoglobulin G titer by IFA | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Smear result | Culture result | PCR result
|
Culture result | PCR result
|
|||||||
| Primary | Nested | Primary | Nested | ||||||||
| Untreated | |||||||||||
| 1 | 4 | F | 5 | − | − | − | − | − | − | − | 320 |
| 2 | 6 | F | 6 | + | + | + | + | − | + | + | 2,560 |
| 3 | 11 | M | 6 | + | + | + | + | + | − | + | 2,560 |
| 4 | 4 | F | 4 | − | − | − | + | − | − | − | 640 |
| 5 | 8 | M | 11.5 | + | + | + | + | − | − | + | 2,560 |
| 6 | 17 | F | 13 | + | + | + | + | NDc | + | + | 5,120 |
| 7 | 11 | M | 6.5 | + | + | + | + | ND | − | − | 1,280 |
| 8 | 35 | M | 0 | + | + | + | + | ND | − | − | 1,280 |
| 9 | 13 | F | 6 | + | + | + | + | − | − | + | 5,120 |
| 10 | 15 | F | 10 | + | + | + | + | − | − | − | 10,240 |
| 11 | 9 | F | 9 | − | − | − | − | − | − | − | 1,280 |
| 12 | 2 | F | 1.5 | ND | ND | ND | ND | + | + | + | 1,280 |
| 13 | 7 | F | 12 | + | + | + | + | − | − | + | 640 |
| Treated | |||||||||||
| 14 (1995) | ? | M | 0 | ND | ND | ND | − | − | − | ND | |
| 15 (1993) | 3 | M | 0 | ND | ND | ND | − | − | − | <20 | |
| 16 (1994) | 5 | F | 0 | ND | ND | ND | − | − | − | <20 | |
| 17 (1994) | 4 | M | 0 | ND | ND | ND | − | − | − | 20 | |
| 18 (1995) | 2 | F | 0 | ND | ND | ND | − | − | − | 20 | |
| 19 (1995) | 2 | M | 0 | ND | ND | ND | − | − | − | <20 | |
| 20 (1994) | 6 | M | 0 | ND | ND | ND | − | − | − | <20 | |
| 21 (1994) | 4 | M | 0 | ND | ND | ND | − | − | − | <20 | |
| 22 (1996) | 2 | M | 0 | ND | ND | ND | − | − | − | <20 | |
F, female; M, male.
Splenomegaly is expressed as the size (in centimeters) of splenic enlargement under the costal diaphragm.
ND, not done.
DNA was extracted from the bone marrow aspirates and buffy coat cells stored in ethanol with a DNA extraction kit (Blood & Cell Culture DNA Kit; QIAGEN Inc.). Bone marrow cells from five uninfected Japanese children were supplied by the Jikei Hospital in Tokyo, and peripheral buffy coat cells from four healthy Japanese adult volunteers were used as negative controls. DNA was similarly extracted from the ethanol-fixed uninfected control tissues as described above.
Informed consent was obtained from all patients or their guardians and all volunteers involved in this study.
PCR amplification and Southern hybridization.
Standard PCR amplification was carried out in a reaction mixture of 10 to 20 μl containing 10 mM Tris-HCl (pH 9.3), 50 mM KCl, 1.5 mM MgCl2, the four deoxynucleoside triphosphates each at a concentration of 0.2 mM, 10% dimethylsulfoxide, each primer at a concentration of 0.25 to 0.5 μM, 1 fg to 100 ng of Leishmania or tissue DNA, and 0.025 U of Taq DNA polymerase (Amersham Pharmacia Biotech Ltd., Uppsala, Sweden) per μl. For the first-round or primary PCR we used a primer set of S-1629 (5′-gggaattCAATAT/AAGTACAGAAACTG) and S-1630 (5′-gggaagcTTCTGTACTT/ATATTGGTA) sequences (lowercase letters indicate noncomplementary bases) (14) (Fig. 1). Each reaction mixture was overlaid with 20 μl of paraffin oil. The PCR was run in a thermocycler (Perkin-Elmer Corp., Norwalk, Conn.) as follows. The samples were first denatured at 95°C for 5 min and were then subjected to 35 cycles of denaturation at 95°C for 1 min, annealing at 50°C for 30 s, and extension at 72°C for 1 min; the final extension was at 72°C for 10 min. For the second-round or subsequent nested PCR, a different primer set, MIN-S1 (5′-GGTATGCGAAACTTCCGGA/GA) and MEX-A1 (5′-ggGTATACTTATATAGCGTTAG), was used (Fig. 1). The standard nested PCR amplification was run in a 20-μl reaction mixture containing 1 μl of the primary PCR products as the template and primers MIN-S1 and MEX-A1 at 0.25 μM each. The PCR was carried out for 25 cycles under the same conditions except that the annealing temperature was raised from 50 to 55°C. The PCR products in 10-μl aliquots were electrophoresed in 2% agarose in 1× TAE (40 mM Tris-acetate and 1 mM EDTA) at 100 V. The gels were stained with 0.5 μg of ethidium bromide per ml and photographed.
FIG. 1.
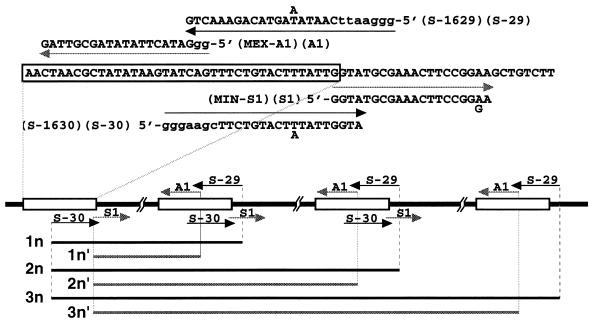
Leishmania mini-exon gene amplification by PCR. Oligonucleotide primers S-1629 (S-29) and S-1630 (S-30) were used for primary PCR. Internal primers MEX-A1 (A1) and MIN-S1 (S1) were used for nested PCR. Lowercase letters indicate noncomplementary bases. The expected DNA fragments of amplified mini-exon gene repeats are indicated by 1n, 2n, and 3n for the primary PCR and by 1n′, 2n′, and 3n′ for the nested PCR. Terminal redundancy of S-1629 and S-1630 may generate PCR products which are 29 nt larger than the genomic repeats (3). Nested PCR may generate PCR products which are 44 nt smaller than those generated by the primary PCR.
In some experiments, the DNAs in the agarose gels were transferred to a nylon membrane (Hybond-N+; Amersham Pharmacia Biotech Ltd.) in 20× SSC (1× SSC is 150 mM NaCl plus 15 mM sodium citrate [pH 7.4]) overnight. The membrane blot was then hybridized with the DNA probe in a hybridization solution (5× SSC, 2% no-fat milk, 0.02% sodium dodecyl sulfate, 0.1% sarcosyl, 50% formamide) at 42°C overnight. The probe was prepared by labeling the 450-bp primary PCR products (see Fig. 3, lane P) amplified from L. donovani 2S with digoxigenin (DIG). The DIG-labeled probe was detected immunologically with a DIG DNA labeling and detection kit (Boehringer GmbH, Mannheim, Germany).
FIG. 3.
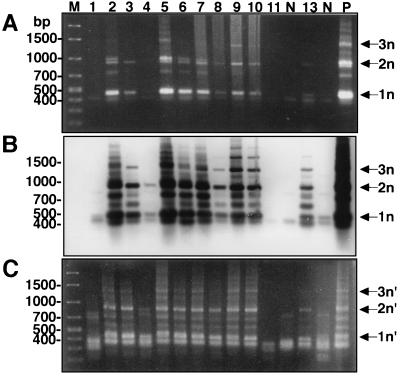
Mini-exon gene amplification for DNA samples from bone marrow aspirates of kala-azar patients. (A) PCR products from first-round primary PCR. (B) Hybridization of the blot in panel A with the L. donovani mini-exon gene probe. (C) PCR products from second-round nested PCR. The lane numbers correspond to the numbers for the patients from Kashi (Table 1). One microliter of each primary PCR product was used as a template DNA for the subsequent nested PCR. Amplified mini-exon gene repeats are indicated by 1n, 2n, and 3n for the primary PCR and by 1n′, 2n′, and 3n′ for the nested PCR. Lane M, DNA molecular marker; lane P, positive control with L. donovani DNA; lane N, uninfected bone marrow DNA.
IFA test.
The sera that had been collected were subjected to an indirect immunofluorescent-antibody (IFA) test for kala-azar by a method modified as described previously (10). Leishmania infantum (MHOM/TN/80/IPT1), kindly provided by D. A. Evans (London School of Hygiene and Tropical Medicine), was used as an antigen. Promastigotes (103) were fixed with 3% formaldehyde for 30 min on a microscope slide. After blocking with 1% bovine serum albumin in phosphate-buffered saline (PBS), the antigen preparations were reacted with the patient sera in various dilutions for 30 min at room temperature. Each slide was washed with PBS three times and was incubated with fluorescein isothiocyanate-conjugated goat anti-human immunoglobulin G (Mo Bio Laboratories Inc., Solana Beach, Calif.), diluted to 1:50 with PBS, for 30 min at room temperature. After a second wash with PBS, the preparations were examined by UV fluorescence microscopy. On the basis of observations made with negative sera including 37 samples from healthy Japanese and Chinese volunteers and a total of 20 samples from patients with malaria, toxoplasmosis, amoebiasis, or American trypanosomiasis, a reaction was considered positive only when the titer was 1:20 or greater.
RESULTS
Sensitivity of PCR for mini-exon gene amplification.
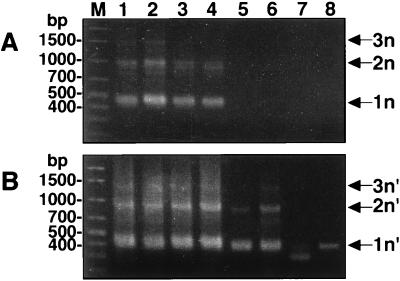
Serial dilutions of L. donovani 2S DNA were subjected to primary PCR followed by a second-round nested PCR for mini-exon gene amplification. The reaction was positive when 10 pg of total parasite DNA was used as the template (Fig. 2A, lanes 1 to 4). The nested PCR increased the sensitivity by at least 100 times. Specific amplification products were detected in a nested reaction mixture containing only 1 μl of the primary PCR products which were estimated to have been derived from 100 fg of original parasite DNA (Fig. 2B, lanes 1 to 6). This nested PCR method is thus able to detect an amount of DNA equivalent to that from not more than a single parasite, i.e., 0.1 pg (8).
FIG. 2.
Sensitivity of mini-exon gene amplification by PCR. DNA samples isolated from L. donovani 2S were serially diluted 10-fold. DNA amounts of approximately 10 ng, 1 ng, 100 pg, 10 pg, 1 pg, 100 fg, 10 fg, and 1 fg (A, lanes 1 to 8, respectively) were subjected to primary PCR. One microliter of each primary PCR product was used as a template DNA for the subsequent nested PCR (B, lanes 1 to 8, respectively). Amplified mini-exon gene repeats are indicated by 1n, 2n, and 3n for the primary PCR and by 1n′, 2n′, and 3n′ for the nested PCR. Lanes M, DNA molecular marker.
The smallest product produced by the primary PCR was about 450 bp, which is comparable to a mini-exon gene unit in the L. donovani complex (3). The DNA ladders seen were present at multiples of ∼450 bp, corresponding to multiple repeats of the mini-exon unit (indicated by 1n, 2n, and 3n in Fig. 1 and 2A). A terminal redundancy of 13 nucleotides (nt) in primer S-1629 and 16 nt in primer S-1630 generated PCR products which were 29 nt longer than the genomic repeats (3). The mini-exon products from the nested PCR were 44 nt smaller than those generated by the primary PCR, as expected (indicated by 1n′, 2n′, and 3n′ in Fig. 1 and 2B).
Amplification of the mini-exon gene in DNA samples from liver and bone marrow cells of mice infected with L. donovani.
DNA samples isolated from liver and bone marrow of mice infected with L. donovani were serially diluted 10-fold and were subjected to primary PCR followed by nested PCR as described above. A DNA ladder of PCR products was generated from 10 ng of infected liver DNA by the primary PCR. The subsequent nested PCR increased the sensitivity by at least 100 times (data not shown). The sensitivities of the primary and nested PCRs were correlated with the presumed number of parasites in the infected liver. Similar results were obtained with the bone marrow DNAs, but 1,000-fold more DNAs were needed to produce a positive reaction compared to the amount of infected liver DNA required (data not shown). It is possible that the parasites were present in the bone marrow in much smaller quantities than in the liver when the infected mice were killed.
The appearance of at least one and two repeats of the mini-exon gene (1n and 2n for the primary PCR and 1n′ and 2n′ for the nested PCR in Fig. 2 to 4) was considered a positive result since nonspecific bands, mostly smaller than 500 bp, were sometimes observed in DNA samples from uninfected control tissues including liver, bone marrow, and buffy coat cells.
FIG. 4.
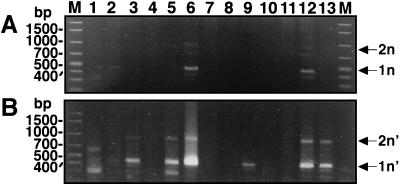
Mini-exon gene amplification for DNA samples from buffy coat cells of kala-azar patients by primary PCR (A) followed by subsequent nested PCR (B). One microliter of each primary PCR product was used as a template DNA for the nested PCR. The lane numbers correspond to the numbers for the patients from Kashi (Table 1). Amplified mini-exon gene repeats are indicated by 1n and 2n for the primary PCR and by 1n′ and 2n′ for the nested PCR. Lanes M, DNA molecular marker.
Comparison of parasitological, serological, and PCR examinations of kala-azar patients in China.
Table 1 summarizes the results of the parasitological, serological, and PCR studies with patients in Kashi and Bachu. Splenomegaly, sometimes combined with a hepatomegaly, is a typical symptom of visceral leishmaniasis. The diagnosis of kala-azar is often based on this criterion alone in the Xinjiang-Uigur region since in that region there are no other diseases such as malaria and schistosomiasis that can cause hepatosplenomegaly. Thus, spleen palpation allowed us to identify suspected kala-azar patients. In Kashi 12 individuals ranging in age from 2 to 17 years had enlarged spleens that extended 1.5 to 13 cm below the costal diaphragm. All of them were serologically positive according to the IFA test, the antibody titers being in the range of 1:320 to 1:10,240. An additional 35-year-old male patient (patient 8) who had no signs of splenomegaly was also seropositive. The bone marrow sample collected from this adult patient was positive by cultivation, suggestive of asymptomatic Leishmania infections perhaps in the adult population in this area of endemicity. Overall, nine of the 12 bone marrow aspirates (75%) collected were microscopically positive for amastigotes in smears and positive by cultivation for promastigotes (Table 1).
These nine parasitologically confirmed bone marrow aspirates (from patients 2, 3, 5 to 10, and 13) were all (100%) positive by primary PCR (Fig. 3A). Primary PCR amplified the mini-exon gene repeats as a ladder of three bands (indicated by 1n, 2n, and 3n in Fig. 3A), comparable to the result for the positive control, i.e., L. donovani 2S strain (Fig. 3A, lane P). Subsequent Southern hybridization of this blot with the mini-exon gene probe prepared from L. donovani 2S confirmed the authenticity of the PCR products from the patients (Fig. 3B). These nine bone marrow samples were also positive by the second-round nested PCR. Nested PCR generated three major bands of mini-exon gene repeats (indicated by 1n′, 2n′, and 3n′ in Fig. 3C). However, the sizes of the bands were smaller than those obtained by the primary PCR, as expected. Significantly, faint bands produced by the primary PCR with two samples (from patients 8 and 13) became clearly positive after the hybridization and further amplification by the nested PCR (Fig. 3, lanes 8 and 13, respectively).
Interestingly, the bone marrow sample from patient 4, which was negative by cultivation, microscopy, and primary PCR, was positive by Southern hybridization and the nested PCR, revealing two clear bands of the mini-exon gene corresponding to one and two repeats (Fig. 3B and C, lane 4). The result revealed that the sensitivity of this nested PCR was comparable to that of Southern hybridization for primary PCR products.
Promastigotes were detected by culture in only two of the 10 buffy coat samples (from patients 3 and 12). Three buffy coat DNA samples (from patients 2, 6, and 12) were found to be positive by the primary PCR, showing amplification of the mini-exon gene as one and two repeats (indicated by 1n and 2n in Fig. 4A). Notably, four more buffy coat DNA samples (from patients 3, 5, 9, and 13) were positive after the nested PCR and had a double banding pattern (indicated by 1n′ and 2n′ in Fig. 4B). The PCR products from patients 2 and 9 were further confirmed to be positive in different experiments (data not shown). Overall, 7 of the 12 buffy coat samples (58%) collected from these patients with splenomegaly (patients 1 to 7 and 9 to 13) were positive by the nested PCR (Table 1).
A total of nine children in Bachu (patients 14 to 22), who had been treated with antimony 1 to 3 years earlier because of splenomegaly, had very low (1:20) or negative (<1:20) titers by the IFA test. Their buffy coat samples were also negative by culture and PCR (Table 1).
DISCUSSION
In the present investigation we have developed a nested PCR method based on amplification of the Leishmania mini-exon gene repeats. We used the primers described previously (3, 14) for the first-round primary PCR. Internal primers were designed from conserved spliced leader and intron sequences of the mini-exon gene for the subsequent second-round nested PCR. Our internal primers amplify multiple units of the mini-exon gene repeats known to exist in different Leishmania species (unpublished data). The nested PCR step was sufficiently sensitive for the detection of DNA in an amount equivalent to or less than that from a single Leishmania cell. The sensitivity of the nested PCR was comparable to that of Southern hybridization for primary PCR products. Our PCR procedure proved to be effective for the diagnosis of kala-azar in the Xinjiang-Uigur Autonomous Region of China. Leishmania mini-exon genes were efficiently detected in DNA extracted not only from bone marrow aspirates but also from buffy coat cells of patients with splenomegaly. Additional work is under way to extend this finding with a larger sample size.
For the diagnosis of kala-azar, the use of peripheral blood is advantageous because the collection procedure is less invasive and less painful than the splenic or bone marrow biopsy specimen collection procedure. Leishmania kDNA or mini-exon DNA from blood samples has been amplified for the diagnosis of kala-azar in India, but the success rate was lower with blood samples than with splenic biopsy samples (5, 17). Recently, there have been reports of PCR amplification of patient blood samples for the detection of kDNA or small-subunit rRNA genes, but these methods require Southern hybridization with enzyme-linked immunosorbent assay (2, 11). The nested PCR method reported here is so simple and sensitive that this method may be used to monitor circulating parasites in the peripheral blood of patients with kala-azar. Isolation of infected phagocytes from buffy coat cells may further increase the sensitivity of the PCR.
We obtained PCR positivity for all of the parasitologically positive bone marrow and buffy coat samples from Chinese kala-azar patients. Some uncertain results by the primary PCR were solved by the subsequent second-round nested PCR. We could not detect positive reactions by nested PCR for two bone marrow samples from patients with splenomegaly. They were also parasitologically negative by bone marrow culture and microscopy but serologically positive by the IFA test, suggesting that the number of parasites in these samples was below the level of detection. Interestingly, the children examined in Bachu, whose splenomegaly had already been significantly reduced after antimony treatment, were negative by nested PCR and buffy coat culture. They also had no anti-Leishmania antibody titers by the IFA test. Although we did not have the opportunity to examine their antibody titers before chemotherapy, reduction of antibody titers may also be a good indicator of the clinical cure of kala-azar. In this sense, a drastic reduction of the anti-rK39 antibody titers has been reported with Indian kala-azar patients who became parasitologically negative after treatment with sodium stibogluconate (16). Serodiagnosis with the rk39 antigen has recently been introduced for the epidemiological study of Chinese kala-azar (13).
Finally, Leishmania isolates from kala-azar patients in China appear to be different from the reference strains of L. donovani in India, an important neighboring country of endemicity. This was determined on the basis of the isoenzyme profiles (21) and restriction fragment length polymorphisms of kDNA (9). By nucleotide sequence analysis of the PCR products amplified from Chinese patients with kala-azar in this study, we confirmed that the products were derived from the Leishmania mini-exon gene. However, the nucleotide sequences were not identical to those of the reference strains of L. donovani in India or L. infantum in Tunisia (unpublished data). Further analysis of the mini-exon gene of Leishmania isolates in China is required to obtain an understanding of the relationship among strains persisting in China and in the surrounding countries.
ACKNOWLEDGMENTS
This work was supported by International Scientific Research Program, the Ministry of Education, Science and Culture, Japan (research grant 10041190).
We thank Z. Feng and the staff of the Institute of Parasitic Diseases, the staff of National Hydatic Disease Center of China, and the staff of Kashgar Regional Health and Epidemic Prevention Station, Xinjiang Uigur Autonomous Region, for valuable suggestions and information. We also thank K. Morinaga and T. Okita for technical assistance.
REFERENCES
- 1.Barker D C. DNA diagnosis of human leishmaniasis. Parasitol Today. 1987;3:177–184. doi: 10.1016/0169-4758(87)90174-8. [DOI] [PubMed] [Google Scholar]
- 2.Costa J-M, Durand R, Deniau M, Rivollet D, Izri M, Houin R, Vidaud M, Bretagne S. PCR enzyme-linked immunosorbent assay for diagnosis of leishmaniasis in human immunodeficiency virus-infected patients. J Clin Microbiol. 1996;34:1831–1833. doi: 10.1128/jcm.34.7.1831-1833.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fernandes O, Murthy V K, Kurath U, Degrave W M, Campbell D A. Mini-exon gene variation in human pathogenic Leishmania species. Mol Biochem Parasitol. 1994;66:261–271. doi: 10.1016/0166-6851(94)90153-8. [DOI] [PubMed] [Google Scholar]
- 4.Guan L-R. Current status of kala-azar and vector control in China. Bull W H O. 1991;69:595–601. [PMC free article] [PubMed] [Google Scholar]
- 5.Hassan M D, Ghosh A, Ghosh S S, Gupta M, Basu D, Mallik K K, Adhya S. Enzymatic amplification of mini-exon-derived RNA gene spacers of Leishmania donovani: primers and probes for DNA diagnosis. Parasitology. 1993;107:509–517. doi: 10.1017/s0031182000068086. [DOI] [PubMed] [Google Scholar]
- 6.Howard M K, Kelly J M, Lane R P, Miles M A. A sensitive repetitive DNA probe that is specific to the Leishmania donovani complex and its use as an epidemiological and diagnostic reagent. Mol Biochem Parasitol. 1991;44:63–72. doi: 10.1016/0166-6851(91)90221-q. [DOI] [PubMed] [Google Scholar]
- 7.Katakura K, Kobayashi A. Acid phosphatase activity of virulent and avirulent clones of Leishmania donovani promastigotes. Infect Immun. 1988;56:2856–2860. doi: 10.1128/iai.56.11.2856-2860.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leon W, Fouts D L, Manning J. Sequence arrangement of the 16S and 26S rRNA gene in the pathogenic haemoflagellate Leishmania donovani. Nucleic Acids Res. 1978;5:491–503. doi: 10.1093/nar/5.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu H-G, Zhong L, Guan L-R, Qu J-Q, Hu X-S, Chai J-J, Xu Z-B, Wang C-T, Chang K-P. Separation of Chinese Leishmania isolates into five genotypes by kinetoplast and chromosomal DNA heterogeneity. Am J Trop Med Hyg. 1994;50:763–770. doi: 10.4269/ajtmh.1994.50.763. [DOI] [PubMed] [Google Scholar]
- 10.Nagakura K, Tachibana H, Tanaka T, Kaneda Y, Tokunaga M, Sasao M, Takeuchi T. An outbreak of amebiasis in an institution for the mentally retarded in Japan. Jpn J Med Sci Biol. 1989;42:63–76. doi: 10.7883/yoken1952.42.63. [DOI] [PubMed] [Google Scholar]
- 11.Nuzum E, White III F, Thankur C, Dietze R, Wages J, Grogl M, Berman J. Diagnosis of symptomatic visceral leishmaniasis by use of the polymerase chain reaction on patient blood. J Infect Dis. 1995;171:751–754. doi: 10.1093/infdis/171.3.751. [DOI] [PubMed] [Google Scholar]
- 12.Piarroux R, Gambarelli F, Dumon H, Fontes M, Dunan S, Mary C, Toga B, Quilici M. Comparison of PCR with direct examination of bone marrow aspiration, myeloculture, and serology for diagnosis of visceral leishmaniasis in immunocompromised patients. J Clin Microbiol. 1994;32:746–749. doi: 10.1128/jcm.32.3.746-749.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qu J-Q, Zhong L, Masoom-Yasinzai M, Rab M, Aksu H S Z, Reed S G, Chang K-P, Gilman-Sachs A. Serodiagnosis of Asian leishmaniasis with recombinant antigen from repetitive domain of a Leishmania kinesin. Trans R Soc Trop Med Hyg. 1994;88:543–545. doi: 10.1016/0035-9203(94)90154-6. [DOI] [PubMed] [Google Scholar]
- 14.Ramos A, Maslov D A, Fernandes O, Campbell D A, Simpson L. Detection and identification of human pathogenic Leishmania and Trypanosoma species by hybridization of PCR-amplified mini-exon repeats. Exp Parasitol. 1996;82:242–250. doi: 10.1006/expr.1996.0031. [DOI] [PubMed] [Google Scholar]
- 15.Rogers M R, Popper S J, Wirth D F. Amplification of kinetoplast DNA as a tool in the detection and diagnosis of Leishmania. Exp Parasitol. 1990;71:267–275. doi: 10.1016/0014-4894(90)90031-7. [DOI] [PubMed] [Google Scholar]
- 16.Singh S, Gilman-Sachs A, Chang K-P, Reed S G. Diagnostic and prognostic value of K39 recombinant antigen in Indian leishmaniasis. J Parasitol. 1995;81:1000–1003. [PubMed] [Google Scholar]
- 17.Smyth A J, Ghosh A, Hassan M Q, Basu D, De Bruijn M H L, Adhya S, Mallik K K, Barker D C. Rapid and sensitive detection of Leishmania kinetoplast DNA from spleen and blood samples of kala-azar patients. Parasitology. 1992;105:183–192. doi: 10.1017/s0031182000074096. [DOI] [PubMed] [Google Scholar]
- 18.Wang C-T. Leishmaniasis in China: epidemiology and control program. In: Chang K-P, Bray R S, editors. Leishmaniasis. Amsterdam, The Netherlands: Elsevier Biomedical Press; 1985. pp. 469–478. [Google Scholar]
- 19.World Health Organization Expert Committee. Control of the leishmaniases. World Health Organization; 1990. pp. 66–95. Technical Report Series 793. [PubMed] [Google Scholar]
- 20.World Health Organization Expert Committee. Control of the leishmaniases. World Health Organization; 1990. pp. 147–148. Technical Report Series 793. [PubMed] [Google Scholar]
- 21.Xu Z-B, Le Blancq S, Evans D A, Peters W. The characterization by isoenzyme electrophoresis of Leishmania isolated in the People’s Republic of China. Trans R Soc Trop Med Hyg. 1984;78:689–693. doi: 10.1016/0035-9203(84)90243-8. [DOI] [PubMed] [Google Scholar]