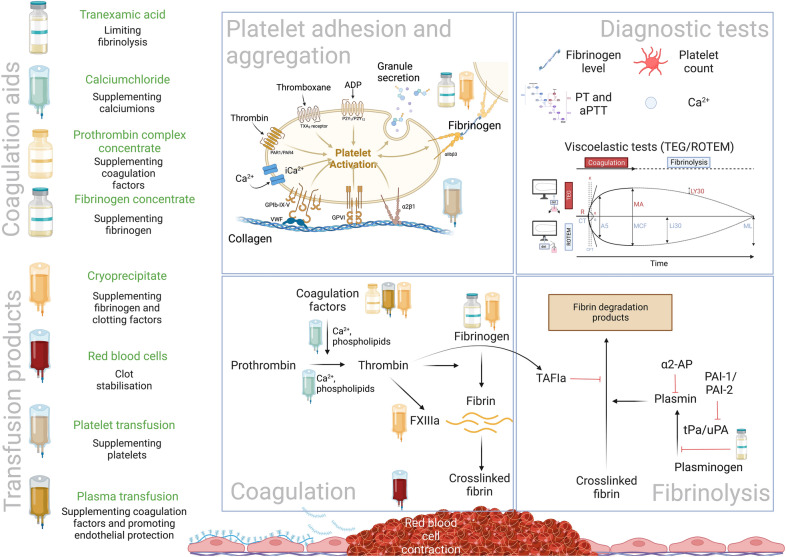
Bleeding is a leading cause of perioperative mortality [1]. In a perioperative setting, damage control surgery with massive transfusion protocols (MTPs) are therapeutic approaches used clinically to manage patients with major bleeding. Massive transfusion is arbitrarily defined as receiving 10 or more red blood cell units in 24 h. MTPs include blood components or whole blood, along with coagulation factor concentrates, including prothrombin complex concentrates (PCCs) and fibrinogen, and are often based on a bleeding management algorithm that may include tranexamic acid, an antifibrinolytic agent [2]. To guide bleeding management, coagulation monitoring includes conventional coagulation tests (e.g., platelet counts, prothrombin time, and fibrinogen level), and/or viscoelastic testing (VET). In addition, haemostatic support is used to optimize haemostasis, while surgeons correct the site-specific bleeding, a strategy that requires collaboration among multiple clinicians, blood services, and in-hospital logistics. In this review, we will examine therapeutic approaches for haemostatic management during perioperative bleeding.
Fibrinogen source, dose, and timing?
Fibrinogen, a critical haemostatic factor for clot formation, is converted into insoluble fibrin by thrombin that undergoes subsequent cross-linking by factor XIII (Fig. 1). Guidelines recommend fibrinogen repletion to a level of 1.5–2 g/L during bleeding using fibrinogen concentrates or cryoprecipitate [3]. The fibrinogen source depends on country-specific availability and local protocols. A recent cardiac surgical randomized clinical trial (RCT) reported no differences using either cryoprecipitate or fibrinogen concentrates [4]. However, no RCT data support the preferred source, dose, minimal targeted fibrinogen level, and timing of fibrinogen supplementation during active bleeding.
Fig. 1.
Coagulation support in perioperative bleeding management. The goal of coagulation support during perioperative bleeding management is to promote clot formation. Components of primary clot formation are fibrinogen which is supplemented by fibrinogen concentrate and/or cryoprecipitate, platelets which can be supplemented by platelet transfusion and activated by calcium supplementation. For secondary coagulation processes, calcium, plasma transfusion, and prothrombin complex concentrate administration promote thrombin and fibrin formation, further stabilizing clot formation. Of note, cryoprecipitate contains more FXIII than most of the fibrinogen concentrates. By thrombin activated FXIII crosslinks fibrin monomers to create a fibrin polymer network. Red blood cells by changing their shape further empower the stabilization of the clot. There is some evidence that plasma transfusion, next to coagulation factor supplementation, also protects endothelial glycocalyx release, limiting excessive clot formation. Finally, tranexamic acid by binding lysine groups of plasminogen limits the transversion of plasminogen into plasmin, reducing the fibrinolysis reaction
Ionized calcium: just counteracting citrated blood products?
Ionized calcium is critical for coagulation (Fig. 1). Rapid infusion of citrated blood products administered during MTPs acutely lower ionized calcium, inhibiting calcium-dependent coagulation factors. Guidelines suggest maintaining normocalcaemia during resuscitation [3]. Of note, recalcification is required for most coagulation assays, a consideration that masks depletion and hypocalcaemia that may occur during resuscitation.
PCCs and factor concentrates
PCCs generally contain factors (F) II, VII, IX, X, and variable levels of protein C, S, and antithrombin (Fig. 1). PCCs were developed for vitamin K antagonist reversal [5], and are increasingly used for perioperative bleeding management [2, 3]. Although PCC may correct perioperative coagulopathy, there are concerns about safety related to the potential thrombotic risks [6]. Other factor concentrates administered include recombinant FVIIa and factor XIII which are used off-label for refractory bleeding but are not recommended by guidelines.
Tranexamic acid
Multiple trauma trials report the benefit of antifibrinolytic use of tranexamic acid (TXA) with early administration in severely injured patients [2]. However, despite its extensive use, administration for severe (isolated) traumatic brain injury [7] and gastrointestinal bleeding is debated due to the potential of increased thromboembolic risk [8]. In trauma patients, there is concern regarding administering TXA in patients who demonstrate fibrinolytic shutdown, a consideration based on observational data reporting the association of TXA with fibrinolytic shutdown and mortality [9]. However, in European guidelines, TXA is routinely administered [2].
Plasma transfusion: volume or coagulation support?
Plasma transfusion, a component of MTPs, includes fresh-frozen plasma (FFP) obtained from male donors or solvent-detergent plasma (SDP) from collected plasma pools processed to remove enveloped viruses, microparticles, and other contaminants. Large RCTs comparing plasma products in perioperative bleeding are lacking. Plasma does not correct clotting times but may minimize endotheliopathy during massive volume resuscitation, as an important rationale for its use [10]. In a pilot cardiothoracic trial, SDP showed less syndecan-1 release than FFP [11]. The downside of plasma transfusion is its association with transfusion-related acute lung injury (TRALI), circulatory overload, bacterial contamination, and hypersensitivity reactions.
Platelets as primary components
Platelets are critical for haemostasis. Based on MTPs, red blood cells (RBCs) are initially administered, followed by plasma, and platelets. In traumatic bleeding, the Pragmatic, Randomized Optimal Platelet and Plasma Ratios (PROPPR trial) reported platelets-to-red blood cell ratios between 1:1 and 1:2 with no differences in mortality in either of the ratios used. Furthermore, trials evaluating the timing and dose of platelet transfusions in trauma patients are lacking. Currently, cold-stored platelets are being investigated as they have extended storage times and are increasingly studied in perioperative bleeding. Current research is also evaluating synthetic platelet-like particles as potential alternatives for allogeneic platelet transfusions.
Red blood cells: not just for oxygen delivery
RBCs change into a polyhedral shape when incorporated into a forming clot, which has been shown to maximize clot strength [12]. RBCs interact with platelets, fibrinogen, von Willebrand factor, and FXIII to optimize clot formation; however, their role in coagulation is often overlooked in bleeding management.
Moving towards individualized coagulation support
Goal-directed therapy
MTPs provide support for perioperative bleeding management, although additional strategies are needed. Beyond procedural interventions and surgical bleeding repair, goal-directed therapy is increasingly used for major bleeding. As part of this strategy, VET-guided transfusion strategies consistently reduce allogeneic blood administration in cardiothoracic surgical patients. However, studies comparing VET with conventional coagulation testing to guide transfusion support in liver transplant surgery have demonstrated mixed results. In one recent VET-guided liver transplant surgical study, RBCs and FFP transfusions were reduced, and there were no differences in platelet transfusions, but cryoprecipitate administration was increased [13]. After trauma-induced bleeding, transfusion strategies with VET compared to conventional coagulation assays have also been reported to reduce mortality [14]. However, transfusion strategy with VET-augmented coagulation support did not improve survival or reduce the need for MTPs [15]. Most studies of VET compared to conventional coagulation tests use an algorithmic approach; however, the benefits may be due to using an evidence-based, algorithmic approach to bleeding management that avoids any empiric administration of blood products. Patients with acquired coagulopathy and active bleeding may benefit the most from goal-directed coagulation support as part of established algorithms.
Take-home messages
Perioperative bleeding management currently includes a multimodal approach of MTPs, TXA, VET-guided transfusion algorithms, and potentially factor concentrates. With ongoing blood shortages, factor concentrates represent an important alternative therapeutic approach to facilitate haemostasis. Additional modifications of allogeneic blood products, including cold-stored platelets, may provide additional availability of critical haemostatic factors. Ongoing efforts to optimize our current transfusion and coagulation supportive strategies during active bleeding continue to be evaluated. The future of perioperative bleeding management will likely be individualized, and additional ongoing research will guide our therapeutic approaches.
Acknowledgements
Figure was created with biorender.com.
Data availability
Not applicable.
Declarations
Conflicts of interest
None.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Derek J. B. Kleinveld, Email: d.kleinveld@erasmusmc.nl
Nicola Curry, Email: Nicola.Curry@ouh.nhs.uk.
Jerrold H. Levy, Email: jerrold.levy@duke.edu
References
- 1.Roshanov PS, Eikelboom JW, Sessler DI, Kearon C, Guyatt GH, Crowther M, Tandon V, Borges FK, Lamy A, Whitlock R, Biccard BM, Szczeklik W, Panju M, Spence J, Garg AX, McGillion M, VanHelder T, Kavsak PA, de Beer J, Winemaker M, Le Manach Y, Sheth T, Pinthus JH, Siegal D, Thabane L, Simunovic MRI, Mizera R, Ribas S, Devereaux PJ. Bleeding independently associated with mortality after noncardiac surgery (BIMS): an international prospective cohort study establishing diagnostic criteria and prognostic importance. Br J Anaesth. 2021;126:163–171. doi: 10.1016/j.bja.2020.06.051. [DOI] [PubMed] [Google Scholar]
- 2.Rossaint R, Afshari A, Bouillon B, Cerny V, Cimpoesu D, Curry N, Duranteau J, Filipescu D, Grottke O, Grønlykke L, Harrois A, Hunt BJ, Kaserer A, Komadina R, Madsen MH, Maegele M, Mora L, Riddez L, Romero CS, Samama C-M, Vincent J-L, Wiberg S, Spahn DR, (2023) The European guideline on management of major bleeding and coagulopathy following trauma: sixth edition. Critical Care 27: 80. DOI 10.1186/s13054-023-04327-7 [DOI] [PMC free article] [PubMed]
- 3.Kietaibl S, Ahmed A, Afshari A, Albaladejo P, Aldecoa C, Barauskas G, De Robertis E, Faraoni D, Filipescu DC, Fries D, Godier A, Haas T, Jacob M, Lancé MD, Llau JV, Meier J, Molnar Z, Mora L, Rahe-Meyer N, Samama CM, Scarlatescu E, Schlimp C, Wikkelsø AJ, Zacharowski K. Management of severe peri-operative bleeding: guidelines from the european society of anaesthesiology and intensive care: second update 2022. Eur J Anaesthesiol. 2023;40:226–304. doi: 10.1097/eja.0000000000001803. [DOI] [PubMed] [Google Scholar]
- 4.Callum J, Farkouh ME, Scales DC, Heddle NM, Crowther M, Rao V, Hucke H-P, Carroll J, Grewal D, Brar S, Bussières J, Grocott H, Harle C, Pavenski K, Rochon A, Saha T, Shepherd L, Syed S, Tran D, Wong D, Zeller M, Karkouti K, Group ftFR Effect of fibrinogen concentrate vs cryoprecipitate on blood component transfusion after cardiac surgery: the fibres randomized clinical trial. JAMA. 2019;322:1966–1976. doi: 10.1001/jama.2019.17312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldstein JN, Refaai MA, Milling TJ, Jr, Lewis B, Goldberg-Alberts R, Hug BA, Sarode R. Four-factor prothrombin complex concentrate versus plasma for rapid vitamin K antagonist reversal in patients needing urgent surgical or invasive interventions: a phase 3b, open-label, non-inferiority, randomised trial. The Lancet. 2015;385:2077–2087. doi: 10.1016/S0140-6736(14)61685-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouzat P, Charbit J, Abback PS, Huet-Garrigue D, Delhaye N, Leone M, Marcotte G, David JS, Levrat A, Asehnoune K, Pottecher J, Duranteau J, Courvalin E, Adolle A, Sourd D, Bosson JL, Riou B, Gauss T, Payen JF, Group PS Efficacy and safety of early administration of 4-factor prothrombin complex concentrate in patients with trauma at risk of massive transfusion: The PROCOAG randomized clinical trial. JAMA. 2023 doi: 10.1001/jama.2023.4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roberts I, Shakur-Still H, Aeron-Thomas A, Beaumont D, Belli A, Brenner A, Cargill M, Chaudhri R, Douglas N, Frimley L, Gilliam C, Geer A, Jamal Z, Jooma R, Mansukhani R, Miners A, Pott J, Prowse D, Shokunbi T, Williams J. Tranexamic acid to reduce head injury death in people with traumatic brain injury: the CRASH-3 international RCT. Health Technol Assess. 2021;25:1–76. doi: 10.3310/hta25260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collaborators H-IT. Effects of a high-dose 24-h infusion of tranexamic acid on death and thromboembolic events in patients with acute gastrointestinal bleeding (HALT-IT): an international randomised, double-blind, placebo-controlled trial. Lancet. 2020;395:1927–1936. doi: 10.1016/S0140-6736(20)30848-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore HB, Moore EE, Neal MD, Sheppard FR, Kornblith LZ, Draxler DF, Walsh M, Medcalf RL, Cohen MJ, Cotton BA, Thomas SG, Leeper CM, Gaines BA, Sauaia A. Fibrinolysis shutdown in trauma: historical review and clinical implications. Anesth Analg. 2019;129:762–773. doi: 10.1213/ane.0000000000004234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sperry JL, Guyette FX, Brown JB, Yazer MH, Triulzi DJ, Early-Young BJ, Adams PW, Daley BJ, Miller RS, Harbrecht BG, Claridge JA, Phelan HA, Witham WR, Putnam AT, Duane TM, Alarcon LH, Callaway CW, Zuckerbraun BS, Neal MD, Rosengart MR, Forsythe RM, Billiar TR, Yealy DM, Peitzman AB, Zenati MS, Group PAS Prehospital plasma during air medical transport in trauma patients at risk for Hemorrhagic shock. N Engl J Med. 2018;379:315–326. doi: 10.1056/NEJMoa1802345. [DOI] [PubMed] [Google Scholar]
- 11.Stensballe J, Ulrich AG, Nilsson JC, Henriksen HH, Olsen PS, Ostrowski SR, Johansson PI. Resuscitation of endotheliopathy and bleeding in thoracic aortic dissections: the VIPER-OCTA randomized clinical pilot trial. Anesth Analg. 2018;127:920–927. doi: 10.1213/ane.0000000000003545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tutwiler V, Mukhitov AR, Peshkova AD, Le Minh G, Khismatullin RR, Vicksman J, Nagaswami C, Litvinov RI, Weisel JW. Shape changes of erythrocytes during blood clot contraction and the structure of polyhedrocytes. Sci Rep. 2018;8:17907. doi: 10.1038/s41598-018-35849-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aceto P, Punzo G, Di Franco V, Teofili L, Gaspari R, Avolio AW, Del Tedesco F, Posa D, Lai C, Sollazzi L. Viscoelastic versus conventional coagulation tests to reduce blood product transfusion in patients undergoing liver transplantation: a systematic review and meta-analysis. Eur J Anaesthesiol. 2023;40:39–53. doi: 10.1097/EJA.0000000000001780. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez E, Moore EE, Moore HB, Chapman MP, Chin TL, Ghasabyan A, Wohlauer MV, Barnett CC, Bensard DD, Biffl WL, Burlew CC, Johnson JL, Pieracci FM, Jurkovich GJ, Banerjee A, Silliman CC, Sauaia A. Goal-directed Hemostatic resuscitation of trauma-induced coagulopathy: a pragmatic randomized clinical trial comparing a viscoelastic assay to conventional coagulation assays. Ann Surg. 2016;263:1051–1059. doi: 10.1097/sla.0000000000001608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baksaas-Aasen K, Gall LS, Stensballe J, Juffermans NP, Curry N, Maegele M, Brooks A, Rourke C, Gillespie S, Murphy J, Maroni R, Vulliamy P, Henriksen HH, Pedersen KH, Kolstadbraaten KM, Wirtz MR, Kleinveld DJB, Schafer N, Chinna S, Davenport RA, Naess PA, Goslings JC, Eaglestone S, Stanworth S, Johansson PI, Gaarder C, Brohi K. Viscoelastic haemostatic assay augmented protocols for major trauma haemorrhage (ITACTIC): a randomized, controlled trial. Intensive Care Med. 2021;47:49–59. doi: 10.1007/s00134-020-06266-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.