Abstract
Introduction
Despite availability of advanced therapies (ATs) for ulcerative colitis (UC), many patients fail to respond to treatment. This study examined real-world clinical and humanistic outcomes associated with current treatments in patients with UC.
Methods
This cross-sectional study used US data from the Adelphi Real World Disease Specific Programme for inflammatory bowel disease from before (2017–2018) and during the COVID-19 pandemic (2020–2021). Physicians (gastroenterologists) seeing > 5 patients/month reported patients’ disease characteristics, current symptoms and treatments, and reasons for treatment choices for their next seven consecutive patients aged ≥ 18 years with moderately to severely active UC before current treatment. Patients were asked to complete the EQ-5D-5L health-related quality of life (HRQoL) measure. ATs included tumor necrosis factor inhibitors (TNFis), integrin receptor antagonists, interleukin-12/23 antagonists, and Janus kinase inhibitors. Patients were classified as AT-naïve or AT-experienced based on current treatment received for ≥ 8 weeks and further classified as responders or non-responders based on symptoms, disease flare status, and remission. Descriptive analyses are presented.
Results
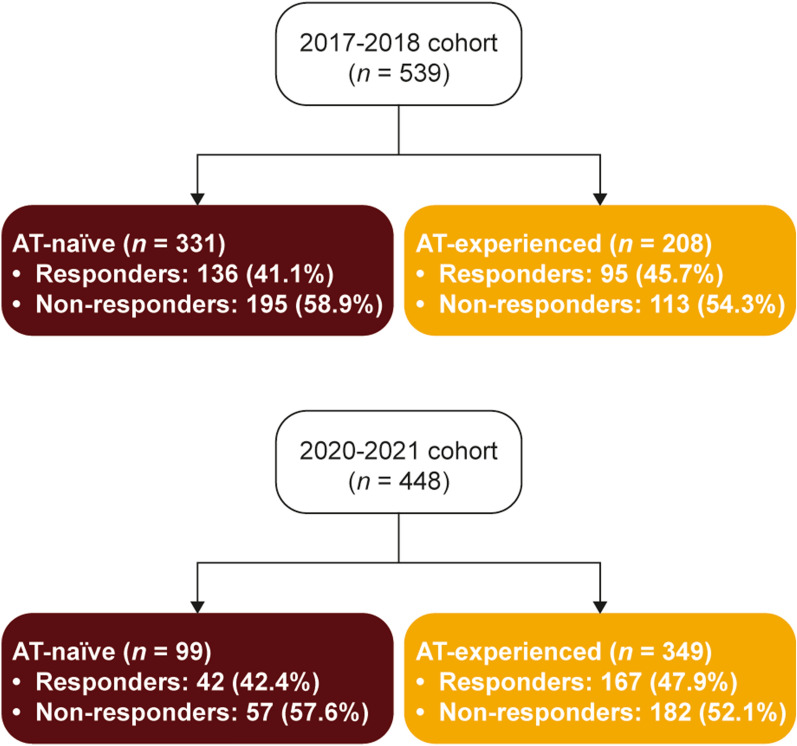
The 2017–2018 cohort included 92 physicians and 539 patients (208 [38.6%] AT-experienced). The 2020–2021 cohort included 73 physicians and 448 patients (349 [77.9%] AT-experienced). TNFis were the most common ATs. In 2017–2018, 195 (58.9%) AT-naïve and 113 (54.3%) AT-experienced patients were non-responders; in 2020–2021 this was 57 (57.6%) and 182 (52.1%). Efficacy and induction of remission were physicians’ most common reasons for AT choice. Dislike of injections/infusions was the most common reason for eligible patients not receiving biologic therapy. Numerically, non-responders (both AT-naïve and AT-experienced) had more symptoms, overall pain and fatigue, and lower HRQoL scores than responders.
Conclusions
Before (2017–2018) and during the pandemic (2020–2021), over half of patients with UC did not respond to AT. Non-responders carried a high burden of disease. Alternative therapies are urgently needed to treat UC.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-023-02605-y.
Keywords: Janus kinase inhibitor, Quality of life, Treatment, Advanced therapy, Tumor necrosis factor inhibitor, Ulcerative colitis, Unmet needs
Key Summary Points
| Why carry out this study? |
| Several advanced therapies (ATs) are available for the treatment of moderate to severe ulcerative colitis (UC); these ATs include biologics and small molecules. |
| Research indicates that a substantial proportion of patients with UC do not respond initially to AT or lose their initial response over time. |
| This study used real-world evidence from the US during the pre-pandemic (2017–2018) and COVID-19 pandemic era (2020–2021) to examine the clinical and humanistic outcomes associated with current treatments in patients with UC who were classified as AT-naïve or AT-experienced. |
| What was learned from this study? |
| The study findings demonstrate that more than half of patients do not respond to current AT for UC, and non-responding patients have a high burden of disease. |
| There is a need for new therapies for UC to increase response rates and provide long-term control of disease and improvement of humanistic outcomes. |
Introduction
Ulcerative colitis (UC) is a chronic inflammatory disease of the colon characterized by intermittent periods of disease flaring and remission [1, 2]. Although the primary clinical symptom of UC is the presence of bloody diarrhea, patients can experience a range of additional symptoms including rectal bleeding, tenesmus (a continual or recurrent need to evacuate the bowels), urgency (feeling of immediate necessity to evacuate the bowels), and abdominal pain [2–4]. Symptoms can be highly disruptive or debilitating, and UC has a substantial negative impact on patients’ quality of life [5–8].
Severity of UC is typically assessed based on symptom severity and the extent of colon involvement [2, 3, 9]. A systematic review of the natural history of adult UC found that most patients have a mild to moderate disease course characterized by periods of remission, while about 14–17% of patients experience an aggressive course [10]. Among 1160 patients diagnosed with UC in Denmark between 1962 and 1987 and followed until 1997 (before the introduction of immunosuppressive drugs), 60% had moderate and 24% had aggressive disease [11].
The goal of treatment is to induce and maintain steroid-free disease remission [3, 9]. Traditional treatments for UC, including 5-aminosalicylic acid (5-ASA), systemic corticosteroids, and immunomodulators (IMs; e.g., methotrexate, thiopurines), may be used alone or in combination to induce remission [3]. Several advanced therapies (ATs) are available for the treatment of moderate to severe UC, including biologics and small molecules [3, 9]. Biologic therapies include tumor necrosis factor inhibitors (TNFis; e.g., infliximab, adalimumab, golimumab), an interleukin (IL)12/23 antagonist (ustekinumab), and an integrin receptor antagonist (vedolizumab) [3, 9]. Advanced small-molecule oral therapies include Janus kinase inhibitors (JAKis; tofacitinib, upadacitinib) as well as a sphingosine 1-phosphate receptor modulator (ozanimod) [3, 9, 12, 13].
Research indicates that about one-third of patients do not respond initially to AT (primary non-response), and an additional 10% of responders lose their initial response over the course of treatment [14–18], resulting in dosage adjustment or treatment switch. High rates of non-response highlight a need for alternative therapeutic targets and novel mechanisms of action in treatment of UC. Several studies have demonstrated that the coronavirus disease 2019 (COVID-19) pandemic had a notable impact on patients’ treatment choices and access to care and on physicians’ prescribing behavior [19–21]. This highlights the potential health and psychologic impact that infectious diseases pose to patients who require IMs, biologics, and oral small-molecule drugs for immune-mediated inflammatory diseases [19–21]. Prescription of AT for the treatment of autoimmune diseases such as rheumatoid arthritis was shown to decline during this period, which may have been in direct response to patients’ fears that such therapies have the potential to increase infection risk [19]. Emerging data also show that the COVID-19 pandemic led to challenges for the effective and safe management of patients with UC [22].
The broad aim of the present study was to evaluate the unmet disease management needs of AT-naïve and AT-experienced patients with moderately to severely active UC and to further assess these outcomes by responders and non-responders within each group. We examined the clinical and humanistic outcomes associated with current treatments in patients with moderately to severely active UC using both pre-pandemic (2017–2018) and COVID-19 pandemic (2020–2021) real-world data in the US.
Methods
Survey Design
Data were collected in the US in the Adelphi Real World Disease Specific Programme (DSP) [23] for inflammatory bowel disease between September 2017 and January 2018 (2017–2018 cohort) and between January 2020 and March 2021 (2020–2021 cohort). DSPs are large, real-world, cross-sectional, multi-country surveys of physicians and their consulting patients in clinical practice; the DSP methodology has previously been published and validated [23–25].
Physicians were identified by local fieldwork agents from publicly available lists of gastroenterologists. Physician recruitment was monitored to ensure balance across geographic regions in the US and inclusion of both academic and community settings. After completing a short screening questionnaire, physicians were invited to participate in the DSP if they qualified as a gastroenterologist, were actively involved in treatment decisions for patients with UC, and saw ≥ 5 and ≥ 7 patients with UC in a typical month in the 2020–2021 and 2017–2018 cohort, respectively. Physicians received remuneration at fair market rates for their time.
Participating physicians were asked to complete a patient record form for their next seven consecutive consulting patients with UC. An additional oversample of two patients per physician receiving tofacitinib was also collected in 2020–2021. Consulting patients were included in the DSP if they were diagnosed with UC, were ≥ 18 years old, and had no current or previous involvement in clinical trials. Patients included in this analysis had moderate to severe physician-reported disease severity immediately before initiation of current treatment and had been on their current treatment line for ≥ 8 weeks.
The respective consulting patients were invited to complete optional patient self-completed forms independently of their physicians, which included well-established patient-reported outcomes measures. The patient-reported measures presented in this paper are findings from the EQ-5D-5L [26].
Patient and physician identities were not known to the research team, no identifiers were recorded for the patients, and patient record forms and patient self-completed forms for each patient were linked by unique numeric codes preprinted on the forms.
Objective
The objective of the study was to evaluate the clinical and humanistic outcomes between AT-naïve and AT-experienced patients with UC and to further assess these outcomes by responders and non-responders within each group.
Measures
Information recorded in the patient record form included patient demographics, disease characteristics and history, current symptoms, current UC treatment, and UC treatment history. The patient record form included an assessment of the patient’s current status/humanistic outcomes, in which the physician assessed the patient’s pain, fatigue, sleep disturbance, and sexual dysfunction (as well as abdominal pain in 2020–2021) on a scale of 0–10 (0 = none, 10 = extremely severe) in the 2017–2018 cohort and on a scale of 0–5 (0 = none, 5 = extremely severe) in the 2020–2021 cohort. Physicians also reported reasons for choice of current AT (for AT-experienced patients), whether each patient’s current condition indicated suitable candidacy for treatment with biologics or JAKis, reasons why AT-naïve candidates for biologics had not received biologics (2017–2018 and 2020–2021 cohorts), and reasons why AT-naïve candidates for JAKis had not received JAKis (2020–2021 cohort).
The EQ-5D-5L, completed by the patient, assesses health-related quality of life (HRQoL), which was also considered a humanistic outcome in this study [26]. The measure comprises five items: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. Each item is scored from 1–5, and a single health utility index score is generated using a country-specific algorithm, with 1 indicating perfect health; 0, health state equivalent to death; < 0 (negative), health state worse than death.
Statistical Analysis
As the objective of the survey was descriptive (i.e. no a priori hypotheses were specified), the sample size was fixed by the duration of the survey period. Therefore, formal sample size calculations are not applicable and were not performed.
Included patients were classified as AT-naïve or AT-experienced based on treatment history. AT-naïve patients had never received an AT and were currently receiving conventional treatments for ≥ 8 weeks. AT-experienced patients were currently receiving their first AT and had been for ≥ 8 weeks. ATs included TNFis (infliximab, adalimumab, and golimumab), integrin receptor antagonists (vedolizumab), IL12/23 antagonists (ustekinumab), and JAKis (tofacitinib). Upadacitinib and ozanimod were not yet commercially available for treatment of UC at the time the data were collected.
Within the AT-naïve and AT-experienced samples, patients were further classified as responders or non-responders. Responder status was defined in a multipronged approach that considered symptoms, flaring status, and remission. In this approach, a factor analysis was conducted for the 27 common UC symptoms collected through the survey; this analysis simplified the symptoms into groups by correlating how patients naturally experience them and, with clinical input, allowed the symptoms to be further refined into seven general groups (Supplementary Table 1). Although the factor analysis (principal component factors with varimax rotation) was conducted using the 2020–2021 dataset, the same symptom groups were used in the 2017–2018 analyses, based on the expectation that groups of symptoms frequently occurring together will vary little over time.
Next, current flaring status, current remission status (clinical/symptomatic with or without mucosal healing, and histologic [clinical/symptomatic remission and full mucosal healing]), the current seven symptom groups for 2017–2018, and the change in the seven symptom groups (before treatment vs. current) for 2020–2021 were used in a latent class cluster analysis, with Bayesian information criteria used to determine the optimal number of clusters. The model with two clusters provided a reasonable fit. The clusters were profiled and named responders and non-responders accordingly.
Analyses were conducted within the 2017–2018 and 2020–2021 datasets. A subgroup analysis of AT status, responder status, and current treatment according to patient sex and age was also conducted. The Fisher exact test and chi-square test were used to compare binary categorical variables and unordered categorical variables with more than two groups, respectively. Means and standard deviations were calculated for continuous variables and frequency counts and percentages for categorical variables. Missing data were not imputed but remained missing; the base of patients for analysis could therefore vary from variable to variable and is reported for each analysis.
Ethics Compliance
This study was conducted in accordance with European Pharmaceutical Marketing Research Association guidelines [27], International Society for Pharmacoepidemiology Guidelines for Good Pharmacoepidemiology Practices [28], the principles of the Declaration of Helsinki [29], and applicable laws and regulatory requirements [30, 31]. This research was submitted to and obtained a waiver from the Western Institutional Review Board, study protocol numbers 1–1034944-1 (2017–2018 cohort) and 1–1238963-1 (2020–2021 cohort). Physicians provided informed consent for their participation, and patients provided informed consent for the results of their patient self-completed forms to be analyzed by academic researchers and analysts within pharmaceutical companies and used for publication. Patients who did not wish to participate did not return a completed patient self-completed form.
Results
Disposition and Demographics
The study sample included 92 physicians and 539 patients in the 2017–2018 cohort and 73 physicians and 448 patients (357 patients in the main sample and 91 in the oversample) in the 2020–2021 cohort. For 2017–2018, 222 patient self-completed forms were included in the analysis, and for 2020–2021, 103 patient self-completed forms were included. A numerically smaller proportion of patients with moderately to severely active UC were AT-experienced in the 2017–2018 cohort (n = 208; 38.6%) than in the 2020–2021 cohort (n = 349; 77.9%; Fig. 1). Furthermore, a numerically smaller proportion of male patients were AT-experienced than female patients in the 2017–2018 cohort (35.2% and 41.8%, respectively) and 2020–2021 cohort (75.7% and 81.0%, respectively), although these differences were not statistically significant; Supplementary Table 2). No difference in AT status was observed across age groups (18–34, 35–49, and ≥ 50 years; Supplementary Table 3). Patients were assigned to responder or non-responder groups through cluster analysis (Supplementary Table 1). Across the AT-naïve and AT-experienced groups in the 2017–2018 and 2020–2021 cohorts, 52.1% to 58.9% of patients were not responding to their current therapies, respectively (Fig. 1). The proportion of male and female patients not responding to their current treatment was numerically similar in both the 2017–2018 (55.7% and 58.5%, respectively) and 2020–2021 cohorts (51.4% and 56.1%, respectively; Supplementary Table 2). In the 2017–2018 cohort, responder status was significantly associated with age (p = 0.006; Supplementary Table 3); the highest response rate to current treatment was observed in patients aged ≥ 50 years (51.4%), whereas this balanced out across the age groups in 2020–2021 (43.6–49.4%).
Fig. 1.
Proportions of responders and non-responders among patients with UC by cohort and AT experience. AT advanced therapy, UC ulcerative colitis
In the 2017–2018 cohort, mean age was numerically higher for AT-experienced than AT-naïve patients (43.7 and 42.4 years); most patients in each AT group worked full or part time (64.9% and 71.6%), and the time from diagnosis to first treatment was numerically longer among AT-experienced than AT-naïve patients (Table 1). In the 2020–2021 cohort, demographics were numerically similar between AT-naïve and AT-experienced patients, with a similar mean age (40.5 years), a numerically larger proportion of men among AT-naïve than AT-experienced patients (63.6% and 56.2%), and most patients working full or part time (75.8% and 78.5%). AT-naïve patients had numerically longer mean duration on current therapy than AT-experienced patients in each cohort (32.2 and 15.2 years; Table 1).
Table 1.
Physician-reported characteristics of patients with UC by cohort and AT experience
| 2017–2018 cohort (n = 539) | 2020–2021 cohort (n = 448) | |||
|---|---|---|---|---|
| AT-naïve (n = 331) | AT-experienced (n = 208) | AT-naïve (n = 99) | AT-experienced (n = 349) | |
| Age, mean (SD), years | 42.4 (15.1) | 43.7 (14.8) | 40.5 (16.1) | 40.5 (13.9) |
| Male sex, n (%) | 171 (51.7) | 93 (44.7) | 63 (63.6) | 196 (56.2) |
| Employment, n (%) | ||||
| Working full- or part-time | 237 (71.6) | 135 (64.9) | 75 (75.8) | 274 (78.5) |
| Unemployed/sick leave/othera | 94 (28.4) | 73 (35.1) | 24 (24.2) | 75 (21.5) |
| Time since diagnosis, months | ||||
| Mean (SD) | 50.4 (67.2) | 58.8 (64.8) | 48.0 (70.8) | 48.0 (57.6) |
| Median (IQR) | 33.6 (12.0–66.0) | 39.6 (20.4–72.0) | 21.6 (10.8–57.6) | 27.6 (14.4–55.2) |
| CCI, mean (SD) | 0.1 (0.5) | 0.1 (0.6) | 0.2 (0.7) | 0.2 (0.8) |
| Time from diagnosis to first treatment, months | ||||
| Mean (SD) | 20.5 (38.7) | 31.3 (62.3) | 7.1 (23.8) | 7.6 (21.5) |
| Median (IQR) | 6.9 (1.4–21.1) | 11.3 (3.7–27.2) | 0.4 (0.0–2.4) | 0.7 (0.1–3.3) |
| Duration on current therapy, months | ||||
| Mean (SD) | 15.0 (22.0) | 12.5 (12.5) | 32.2 (57.2) | 15.2 (15.1) |
| Median (IQR) | 11.1 (4.6–18.5) | 11.1 (5.5–12.3) | 16.3 (7.3–29.2) | 15.1 (5.8–20.3) |
AT advanced therapy, CCI Charlson Comorbidity Index, IQR interquartile range, SD standard deviation, UC ulcerative colitis
aIncludes homemaker, student, retired, unknown
Current Treatment
A large proportion of AT-naïve responders (> 60%) and non-responders (≥ 40%) were receiving only 5-ASA in both the 2017–2018 and 2020–2021 cohorts (Table 2). Numerically, a higher proportion of AT-naïve non-responders were receiving corticosteroids (± 5-ASA) than responders in both cohorts (Table 2). A numerically larger proportion of AT-naïve non-responders in the 2017–2018 cohort received an IM (± steroid ± 5-ASA) than responders (43.6% and 22.1%; Table 2).
Table 2.
Current physician-reported combinations of treatments received by patients with UC by cohort, responder status, and AT experience
| n (%) | 2017–2018 cohort (n = 539) | 2020–2021 cohort (n = 448) | ||
|---|---|---|---|---|
| Responders | Non-responders | Responders | Non-responders | |
| AT-naïve, n | 136 | 195 | 42 | 57 |
| Unconventional/non-ATa | 10 (7.4) | 5 (2.6) | 2 (4.8) | 0 |
| 5-ASA | 89 (65.4) | 78 (40.0) | 27 (64.3) | 23 (40.4) |
| Corticosteroid (± 5-ASA) | 7 (5.1) | 27 (13.8) | 3 (7.1) | 19 (33.3) |
| IM (± corticosteroid ± 5-ASA) | 30 (22.1) | 85 (43.6) | 10 (23.8) | 15 (26.3) |
| AT-experienced, n | 95 | 113 | 129 | 129 |
| AT only | 47 (49.5) | 44 (38.9) | 83 (64.3) | 60 (46.5) |
| TNFi | 40 (42.1)b | 42 (37.2)b | 52 (40.3)c | 31 (24.0)c |
| Integrin receptor antagonistd | 6 (6.3) | 2 (1.8) | 21 (16.3) | 12 (9.3) |
| IL12/23 antagoniste | 1 (1.1) | 0 | 5 (3.9) | 11 (8.5) |
| JAKif | 0 | 0 | 5 (3.9) | 6 (4.7) |
| AT + 5-ASA | 35 (36.8) | 38 (33.6) | 28 (21.7) | 45 (34.9) |
| AT + corticosteroid (± 5-ASA) | 1 (1.1) | 12 (10.6) | 5 (3.9) | 12 (9.3) |
|
AT + IM (± corticosteroid ± 5-ASA) |
12 (12.6) | 19 (16.8) | 13 (10.1) | 12 (9.3) |
5-ASA aminosalicylic acid, AT advanced therapy, IL interleukin, IM immunomodulator, JAKi Janus kinase inhibitor, TNFi tumor necrosis factor inhibitor, UC ulcerative colitis
aIncluding pain medication and enteral nutrition
bIncluding infliximab (plus biosimilar), adalimumab (plus biosimilar), golimumab and certolizumab-pegol
cIncluding infliximab (plus biosimilar), adalimumab (plus biosimilar), and golimumab
dIncluding vedolizumab
eIncluding ustekinumab
fIncluding tofacitinib, but excluding tofacitinib oversample in the 2020–2021 cohort
The proportions of AT-experienced responders and non-responders receiving AT only were numerically higher in the 2020–2021 cohort (64.3% and 46.5%) than in the 2017–2018 (49.5% and 38.9%) cohort. Also in each cohort, a numerically larger proportion of responders than non-responders were receiving AT monotherapy. TNFis were the most common class of AT among AT-experienced patients in both cohorts (Table 2).
A numerically smaller proportion of female patients received 5-ASA only and corticosteroids (± 5-ASA) compared with male patients in both the 2017–2018 (5-ASA only, 27.6% and 34.5%; corticosteroids [± 5-ASA], 4.4% and 8.3%) and 2020–2021 cohorts (5-ASA only, 9.5% and 12.4%; corticosteroids [± 5-ASA], 3.2% and 6.2%; Supplementary Table 2). Furthermore, a numerically smaller proportion of male patients received IMs (± corticosteroid ± 5-ASA) or AT than female patients in both the 2017–2018 (IM [± corticosteroid ± 5-ASA], 20.1% and 22.5%; AT, 35.2% and 41.8%) and 2020–2021 cohorts (IM [± corticosteroid ± 5-ASA], 5.0% and 6.3%; AT, 75.7% and 81.0%; Supplementary Table 2).
In the 2017–2018 cohort, current treatment was significantly associated with age (p < 0.001; Supplementary Table 3). A numerically higher proportion of patients aged 18–34 years received 5-ASA only and corticosteroid (± 5-ASA; 41.3% and 8.7%) than those > 35–49 years (23.9% and 6.3%) and ≥ 50 years (27.4% and 3.9%; Supplementary Table 3). The opposite was observed for IMs (± corticosteroid ± 5-ASA), with a numerically higher proportion of patients aged 35–49 years (31.3%) and ≥ 50 years (21.2%) receiving IMs (± corticosteroid ± 5-ASA) than those aged 18–34 years (12.0%). The proportion of patients receiving AT (biologic/JAKi) was similar across all age groups in both cohorts (Supplementary Table 3). In contrast, there was no observed difference in the proportion of patients receiving 5-ASA only, corticosteroid (± 5-ASA), IMs (± corticosteroid ± 5-ASA), and AT (biologic/JAKi) across the age groups in the 2020–2021 cohort.
Reasons for Treatment Choices
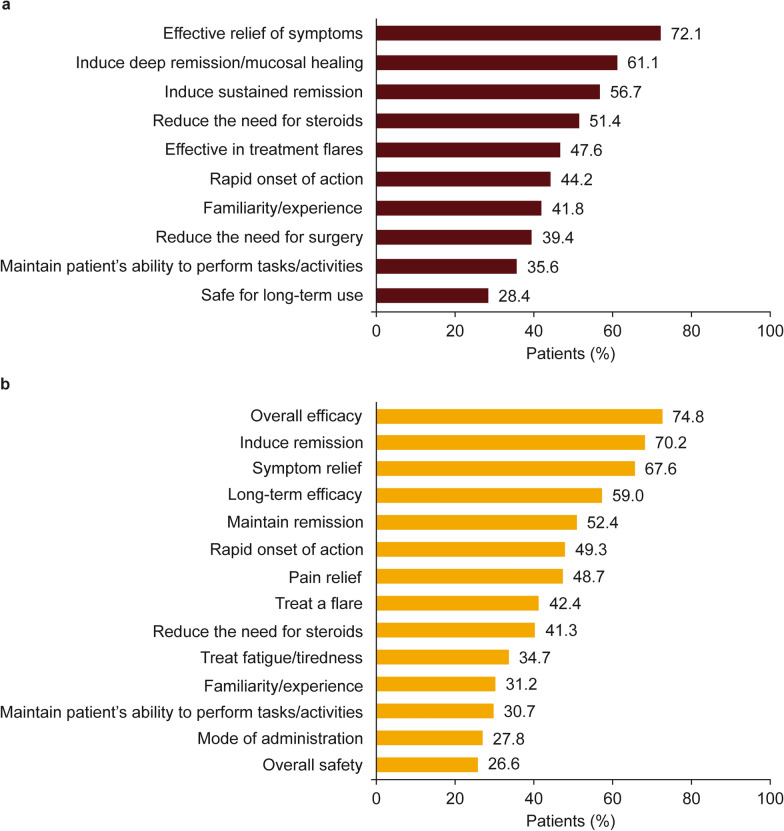
Efficacy (including symptom relief) and induction of remission/mucosal healing were the most common reasons reported by physicians for AT treatment choices in both the 2017–2018 and 2020–2021 AT-experienced cohorts (Fig. 2). Among AT-naïve and AT-experienced patients in the 2017–2018 cohort, failure to induce remission (percentage of total responders and non-responders combined: AT-naïve, 31.0% and AT-experienced, 41.6%), loss of response over time/condition worsened (39.6% and 39.0%), lack of alleviation of pain (46.5% and 17.5%), failure to maintain remission (27.7% and 23.4%), and treatment did not clear the patient’s diarrheal symptoms specifically (38.0% and 16.9%) were cited as the most common reasons for switching treatment (data not shown in table/figure); reasons for switching treatment among responders and non-responders in the 2017–2018 cohort are presented in detail in Supplementary Tables 4 and 5. In the 2020–2021 cohort, the most common reasons cited for switching treatment among AT-naïve and AT-experienced patients were disease progression (percentage of total responders and non-responders combined: 36.1% and 33.5%), loss of response over time (25.0% and 42.9%), failure to induce remission (19.4% and 31.7%), failure to maintain remission (13.9% and 29.0%), lack of flare control (8.3% and 34.4%), and patient requiring an AT with a different mode of action (11.1% and 21.9%; data not shown in table/figure); reasons for switching treatment among responders and non-responders in the 2020–2021 cohort are presented in detail in Supplementary Tables 6 and 7.
Fig. 2.
Physician-reported reasons for choice of current AT for AT-experienced patients in the 2017–2018 cohort (n = 208) (a) and 2020–2021 cohort (n = 349) (b). Physician-reported reasons applying to > 25% of patients are shown; “select all that apply” survey question. AT advanced therapy
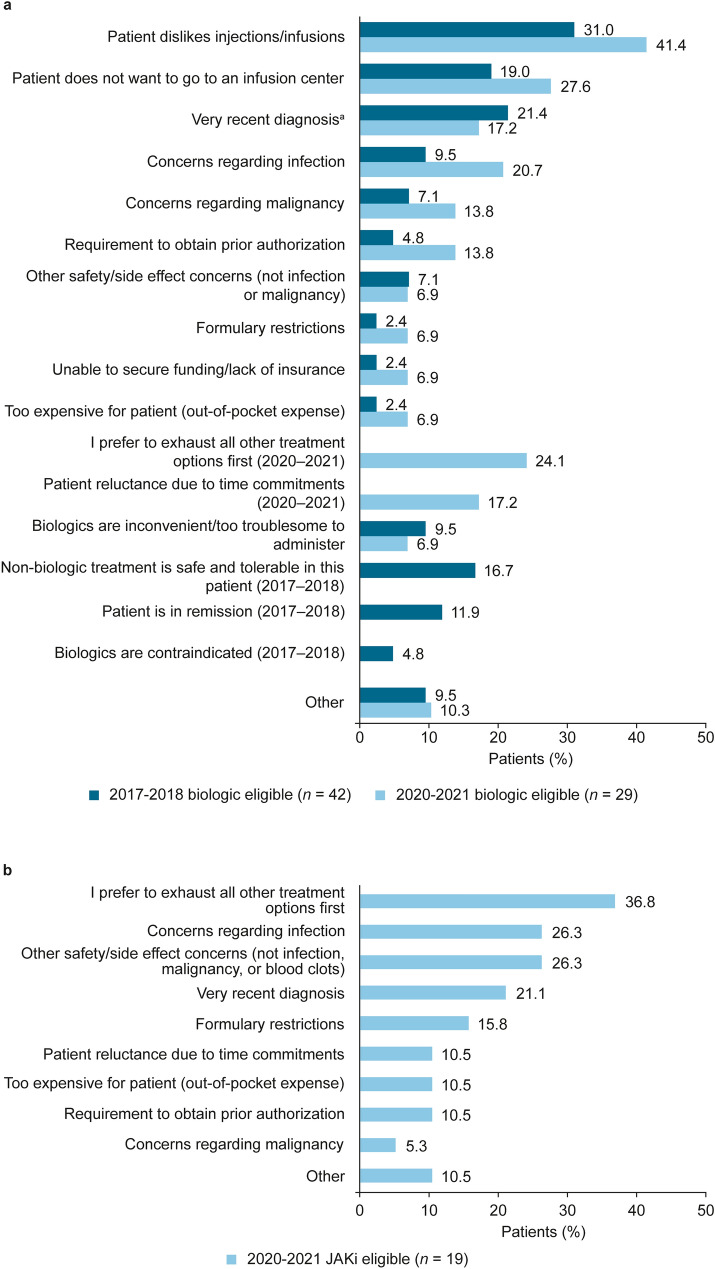
The most common physician-reported reasons for AT-naïve “candidates for biologics” in each cohort not to receive biologics included the patient’s dislike of injections/infusions and not wanting to go to an infusion center; these reasons were reported more frequently in the 2020–2021 cohort than in the 2017–2018 cohort (Fig. 3A). The most common reasons provided for AT-naïve “candidates for JAKis” in the 2020–2021 cohort not to be receiving JAKis were preference to exhaust all other treatment options and concerns regarding infection and other safety/side effects (Fig. 3B).
Fig. 3.
Physician-reported reasons for AT-naïve “candidates for biologics” not receiving biologics in the 2017–2018 and 2020–2021 cohorts (a) and reasons for AT-naïve “candidates for JAKis” not receiving JAKis in the 2020–2021 cohort (b). “Select all that apply” survey question. Response options are shown as they were presented to responding physicians. For panel a, some response options were offered only in 2017–2018 or in 2020–2021, as indicated. The question concerning patients not receiving JAKis (panel b) was not asked in the 2017–2018 survey. aIncluded “Very recent diagnosis/too early to prescribe biologic/still receiving their first treatment” in the 2017–2018 biologic-eligible population. AT advanced therapy, JAKi Janus kinase inhibitor
Patient Disease Characteristics and Patient-Reported Outcomes
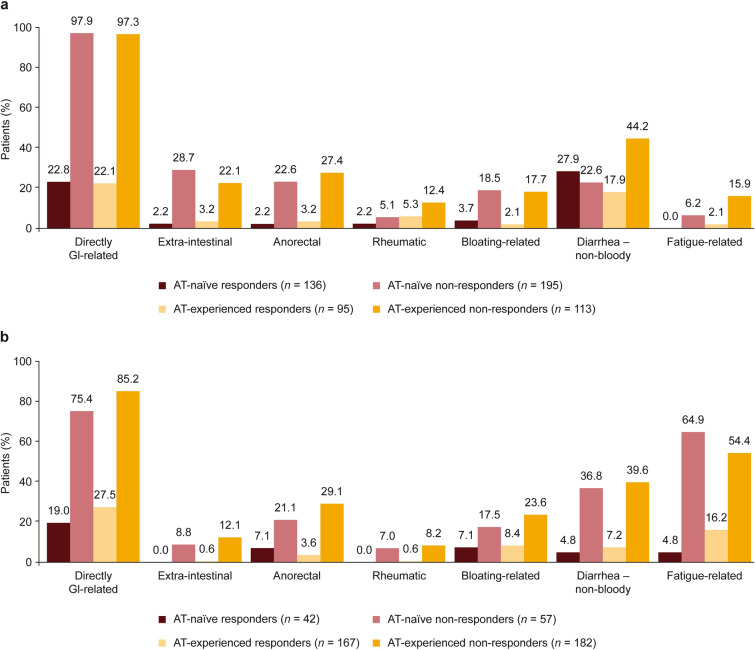
Non-responders were more likely to experience symptoms than responders, regardless of whether they were AT-naïve or AT-experienced (Fig. 4). The most common symptom grouping in both the 2017–2018 and 2020–2021 cohorts was directly gastrointestinal-related symptoms, which were experienced by > 75.0% of non-responders regardless of AT experience. A larger proportion of non-responders experienced fatigue-related symptoms in the 2020–2021 cohort (AT-naïve, 64.9%; AT-experienced, 54.4%) than in the 2017–2018 cohort (AT-naïve, 6.2%; AT-experienced, 15.9%; Fig. 4).
Fig. 4.
Physician-reported symptoms: proportion of patients with UC currently experiencing each symptom group in the 2017–2018 (a) and 2020–2021 (b) cohorts. See Supplementary Table 1 for symptom groups. AT advanced therapy, GI gastrointestinal, UC ulcerative colitis
In the 2017–2018 cohort, 0.0% of AT-naïve and 2.1% of AT-experienced responders and 12.8% of AT-naïve and 29.2% of AT-experienced non-responders were currently experiencing a disease flare (data not shown in table/figure); similarly, in the 2020–2021 cohort, no responders were currently flaring, whereas 8.8% of AT-naïve and 17.0% of AT-experienced non-responders were currently flaring. In the 2017–2018 cohort, 85.6% of AT-naïve and 74.3% of AT-experienced non-responders had not achieved remission; in the 2020–2021 cohort, most responders were in full/partial remission, whereas 61.4% of AT-naïve and 58.8% of AT-experienced non-responders had not achieved remission (data not shown in table/figure).
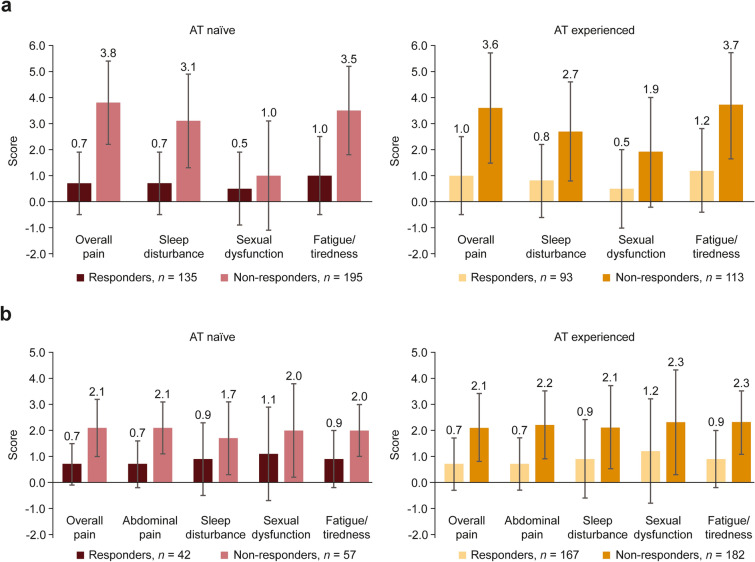
All patient status scores, including overall pain and fatigue scores, appeared to be lower among responders than non-responders regardless of AT experience in both the 2017–2018 and 2020–2021 cohorts (Fig. 5). Similarly, HRQoL scores appeared to be lower among non-responders than responders regardless of AT experience in both cohorts (Table 3).
Fig. 5.
Physician-reported patient status scores for patients with UC by AT experience, responder status, and cohort (response scale differs between cohorts) in the (a) 2017–2018a and (b) 2020–2021 cohorts. bAT advanced therapy, UC ulcerative colitis. a0–10 scale; 0 = none, 10 = extremely severe. b0–5 scale; 0 = none, 5 = extremely severe. Data represent the mean ± standard deviation
Table 3.
Patient-reported health-related quality-of-life (EQ-5D-5L) scores among patients with UC by AT experience, responder status, and cohort
| AT-naïve | AT-experienced | |||
|---|---|---|---|---|
| Responders | Non-responders | Responders | Non-responders | |
| 2017–2018 cohort, n | 42 | 102 | 25 | 53 |
| Mean (SD) scorea | 0.91 (0.15) | 0.83 (0.15) | 0.91 (0.12) | 0.86 (0.16) |
| 2020–21 cohort, n | 7 | 17 | 38 | 41 |
| Mean (SD) scorea | 0.90 (0.20) | 0.86 (0.16) | 0.94 (0.08) | 0.83 (0.12) |
AT advanced therapy, SD standard deviation, UC ulcerative colitis
a0–1 scale: 1 = perfect health; 0 = health state equivalent to death; health states worse than death (negative) are possible
Discussion
Results from this study provide real-world insights into current unmet needs of AT-naïve and AT-experienced patients with moderately to severely active UC in the US and provide a perspective at pre-pandemic (2017–2018) and COVID-19 pandemic (2020–2021) eras, with treatment trends and patient-reported outcomes. Fatigue-related symptoms were much more prevalent among non-responding patients in 2020–2021 than in 2017–2018, likely demonstrating the additional challenge of managing patients with UC in the pandemic era [32].
Most patients in the 2017–2018 cohort were naïve to treatment with ATs, whereas most patients in the 2020–2021 cohort were AT-experienced. In particular, there was a strong uptake in use of integrin receptor antagonists between cohorts. However, despite increased use of ATs in the later (2020–2021) cohort, there were more non-responders than responders during both time periods and among both AT-naïve and AT-experienced patients. In both cohorts, AT-naïve and AT-experienced non-responders reported more current symptoms, lower HRQoL scores, and higher pain and fatigue scores, demonstrating a greater clinical and humanistic burden of disease among non-responding patients and highlighting the importance of effective treatment options for patients with UC.
The most common reasons for choosing the current AT for AT-experienced patients in both cohorts were efficacy related, likely reflecting physicians’ goals of achieving better treatment outcomes for the many non-responding patients. For those AT-naïve candidates who did not want to receive biologics, physicians reported patients’ aversion to injections/infusions and not wanting to visit infusion centers; these reasons were more common in the 2020–2021 cohort than in the 2017–2018 cohort, perhaps in part because of the COVID-19 pandemic [19, 33]. In contrast, physicians cited safety concerns in choosing not to prescribe JAKis to patients otherwise eligible for these agents. However, it is important to note that data collection was initiated before and continued after the initial US Food and Drug Administration warning and subsequent European Medicines Agency investigation of increased risk of serious heart-related events, cancer, blood clots, and death related to JAK inhibitor use, which have since been addressed in product labeling [34, 35].
Several studies have reported sex-based differences in the pathogenesis and progression of UC, which have been shown to impact patient management and therapeutic response [36, 37]. A single-center, retrospective study demonstrated that the likelihood of achieving long-term remission was 3.5-fold higher for female versus male patients with UC when treated with infliximab [36, 37]. In the present study, fewer male patients were AT-experienced than female patients in both the 2017–2018 and 2020–2021 cohort, suggesting that females were more likely to switch from conventional therapy to AT during these periods. Furthermore, a smaller proportion of female patients received 5-ASA and corticosteroids (± 5-ASA), whereas fewer male patients received IMs (± corticosteroid ± 5-ASA) or AT. Despite these trends, no significant association was observed between patient sex and AT status, responder status, or current treatment status. Further investigation on the impact of patient sex on AT status, responder status, and current treatment is warranted.
Epidemiologic observations show that older patients represent a considerable proportion of those with UC, and the prevalence is expected to rise considerably in the future [38–40]. Older patients tend to present with complex disease and a range of comorbidities, representing a substantial financial burden to health care systems and society [39, 40]. The present study found a significant association between patient age and responder status and current treatment. These findings are in line with the American Gastroenterological Association (AGA), American College of Gastroenterology (ACG), European Crohn’s and Colitis Organization (ECCO), and Italian Group-IBD (IG-IBD) clinical guidelines, which recommend that treatment choice be driven by a patient’s age, comorbidities, prior treatments, extent of disease, and the need to induce and maintain disease remission [3, 9, 41, 42]. Availability of health care resources is also an important factor in determining whether clinical guidelines are implemented in many countries [42].
Our findings add to a body of evidence from clinical trials and real-world clinical practice identifying substantial unmet needs in the treatment and management of patients with UC [14]. Non-response to treatment is not clearly defined, with existing literature highlighting the need for new prognostic tools that would predict response to a specific therapy or assess response at an earlier point in treatment [15]. Such tools remain lacking despite considerable research. With no single marker or factor(s) identified, it is likely that multiparametric models will be needed to predict response to therapy [15]. The importance of disease control and response to treatment is demonstrated in studies in which patients with inactive UC have a negligible disease burden, comparable with that of the general population [43].
Limitations
Limitations of this study include recall bias, response bias, and the cross-sectional study design, which does not allow for assessment of cause and effect. The Adelphi DSP data collection approach also has limitations as the sample collected is not fully randomized and did not include data on patient gender. Patients are collected consecutively to avoid selection bias; however, this does mean that patients who consult their physician frequently and physicians who are more willing to take part in the study are more likely to be included in the sample, potentially meaning that the sample is not fully representative of the general UC population.
In addition, diagnosis of UC was based primarily on the judgment and diagnostic skills of the physician, and a formalized diagnostic checklist was not mandated as part of the DSP methodology. However, this situation is consistent with decisions made by physicians in routine clinical practice and is, therefore, reflective of the real world.
Missing data were not imputed; therefore, the base of patients for analyses varies from measure to measure and is reported separately for each analysis. Finally, survey questionnaires in the DSPs change over time depending on market changes, needs, and prescribing environments. As a result, questions and data collected may have slightly differed between cohorts.
Conclusion
The results of this study demonstrate the large number of patients with moderately to severely active UC who do not respond to currently available ATs and highlights a need for new therapies that can increase response rates, provide long-term disease control, and offer alternative options to parenteral routes of administration.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the study participants for their involvement in this study.
Funding
This study and the journal’s Rapid Service and Open Access fees were funded by Bristol Myers Squibb (Princeton, NJ, USA). Bristol Myers Squibb did not influence the original survey through either contribution to the design of questionnaires or data collection. The analysis described here used data from the Adelphi Real World Disease Specific Programme for inflammatory bowel disease. The Disease Specific Programme is a wholly owned Adelphi Real World product. Bristol Myers Squibb is one of multiple subscribers to the Disease Specific Programme. Publication of survey results was not contingent on the subscriber's approval or censorship of this manuscript.
Medical Writing and/or Editorial Assistance
Medical writing support was provided by Russell Craddock, PhD, of Parexel International and was funded by Bristol Myers Squibb.
Authorship
All authors met the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Conceptualization: Rina Lukanova, Fritha Hennessy, Sumie Kakehi, Komal Gupte-Singh; formal analysis: Fritha Hennessy, Sumie Kakehi, Gary Milligan; investigation: Fritha Hennessy, Hannah Knight; methodology: all listed authors; project administration: Fritha Hennessy, Sumie Kakehi, Hannah Knight; resources: Fritha Hennessy, Hannah Knight; supervision: Rina Lukanova, Fritha Hennessy; validation and visualization: Fritha Hennessy, Hannah Knight; writing – review & editing: all listed authors.
Prior Publication
Portions of this research have been presented at the 16th Congress of the European Crohn’s and Colitis Organisation (July 2–3 and July 8–10, 2021, virtual) and at the 2021 American College of Gastroenterology Annual Meeting (October 22–27, 2021, in Las Vegas, NV, USA).
Disclosures
Anita Afzali served as consultant for AbbVie, Takeda, Janssen, Bristol Myers Squibb/Celgene, Lilly, and Gilead; and received speaker fees from AbbVie, Takeda, Janssen, and Pfizer. Sumie Kakehi and Komal Gupte-Singh are employees and shareholders of Bristol Myers Squibb. Rina Lukanova, Fritha Hennessy, Gary Milligan, and Hannah Knight are employees of Adelphi Real World.
Compliance with Ethics Guidelines
The study was conducted in accordance with European Pharmaceutical Marketing Research Association guidelines [27], International Society for Pharmacoepidemiology Guidelines for Good Epidemiology Practices [28], the principles of the Declaration of Helsinki [29], and applicable laws and regulatory requirements [30, 31]. This research was submitted to and obtained a waiver from the Western Institutional Review Board, study protocol numbers 1–1034944-1 (2017–2018 cohort) and 1–1238963-1 (2020–2021 cohort). Physicians provided informed consent for their participation, and for the results of their patient self-completed forms to be analyzed by academic researchers and analysts within pharmaceutical companies and used for publication. Patients who did not wish to participate did not return a completed patient self-completed form.
Data Access
All data (methodology, materials, data, and data analysis) that support the findings of this survey are the intellectual property of Adelphi Real World. All relevant data are presented in full, and descriptions of the methods, the tools used, questions asked and how answers were derived are included within the methodology and results sections of the manuscript, where appropriate. All requests for data access should be addressed directly to Rina Lukanova at rina.lukanova@adelphigroup.com. Rina Lukanova is an employee of Adelphi Real World.
References
- 1.Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140(6):1785–1794. doi: 10.1053/j.gastro.2011.01.055. [DOI] [PubMed] [Google Scholar]
- 2.Ordas I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ. Ulcerative colitis. Lancet. 2012;380(9853):1606–1619. doi: 10.1016/S0140-6736(12)60150-0. [DOI] [PubMed] [Google Scholar]
- 3.Rubin DT, Ananthakrishnan AN, Siegel CA, Sauer BG, Long MD. ACG clinical guideline: ulcerative colitis in adults. Am J Gastroenterol. 2019;114(3):384–413. doi: 10.14309/ajg.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 4.Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis. Lancet. 2017;389(10080):1756–1770. doi: 10.1016/S0140-6736(16)32126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Irvine EJ. Quality of life of patients with ulcerative colitis: past, present, and future. Inflamm Bowel Dis. 2008;14(4):554–565. doi: 10.1002/ibd.20301. [DOI] [PubMed] [Google Scholar]
- 6.Ghosh S, Mitchell R. Impact of inflammatory bowel disease on quality of life: results of the European Federation of Crohn's and Ulcerative Colitis Associations (EFCCA) patient survey. J Crohns Colitis. 2007;1(1):10–20. doi: 10.1016/j.crohns.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Han SW, McColl E, Barton JR, James P, Steen IN, Welfare MR. Predictors of quality of life in ulcerative colitis: the importance of symptoms and illness representations. Inflamm Bowel Dis. 2005;11(1):24–34. doi: 10.1097/00054725-200501000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Armuzzi A, Liguori G. Quality of life in patients with moderate to severe ulcerative colitis and the impact of treatment: a narrative review. Dig Liver Dis. 2021;53(7):803–808. doi: 10.1016/j.dld.2021.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Feuerstein JD, Isaacs KL, Schneider Y, Siddique SM, Falck-Ytter Y, Singh S. AGA clinical practice guidelines on the management of moderate to severe ulcerative colitis. Gastroenterology. 2020;158(5):1450–1461. doi: 10.1053/j.gastro.2020.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fumery M, Singh S, Dulai PS, Gower-Rousseau C, Peyrin-Biroulet L, Sandborn WJ. Natural history of adult ulcerative colitis in population-based cohorts: a systematic review. Clin Gastroenterol Hepatol. 2018;16(3):343–56e3. doi: 10.1016/j.cgh.2017.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jess T, Riis L, Vind I, et al. Changes in clinical characteristics, course, and prognosis of inflammatory bowel disease during the last 5 decades: a population-based study from Copenhagen, Denmark. Inflamm Bowel Dis. 2007;13(4):481–489. doi: 10.1002/ibd.20036. [DOI] [PubMed] [Google Scholar]
- 12.Rinvoq (upadacitinib). [package insert]. AbbVie Inc: North Chicago, IL, USA; 2022.
- 13.Zeposia (ozanimod). [package insert]. Celgene Corporation: Summit, NJ, USA; 2022.
- 14.Danese S, Allez M, van Bodegraven AA, et al. Unmet medical needs in ulcerative colitis: an expert group consensus. Dig Dis. 2019;37(4):266–283. doi: 10.1159/000496739. [DOI] [PubMed] [Google Scholar]
- 15.Privitera G, Pugliese D, Rapaccini GL, Gasbarrini A, Armuzzi A, Guidi L. Predictors and early markers of response to biological therapies in inflammatory bowel diseases. J Clin Med. 2021;10(4):853. doi: 10.3390/jcm10040853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sands BE, Sandborn WJ, Panaccione R, et al. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2019;381(13):1201–1214. doi: 10.1056/NEJMoa1900750. [DOI] [PubMed] [Google Scholar]
- 17.Tuskey A, Behm BW. Profile of ustekinumab and its potential in patients with moderate-to-severe Crohn's disease. Clin Exp Gastroenterol. 2014;7:173–179. doi: 10.2147/CEG.S39518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varyani F, Argyriou K, Phillips F, Tsakiridou E, Moran GW. Profile of tofacitinib in the treatment of ulcerative colitis: an evidence-based review of recent data. Drug Des Devel Ther. 2019;13:4091–4105. doi: 10.2147/DDDT.S182891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Machado PM, Verschueren P, Grainger R, et al. Impact of COVID-19 pandemic on the management of patients with RA: a survey of rheumatologists in six European countries. Rheumatol Adv Pract. 2023;7(1):rkac108. doi: 10.1093/rap/rkac108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hider S, Muller S, Gray L, et al. Exploring the longer-term impact of the COVID-19 pandemic on physical and mental health of people with inflammatory rheumatic diseases: a cross-sectional survey. Clin Rheumatol. 2023;42:1903–1909. doi: 10.1007/s10067-023-06565-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin S, Lau LH, Chanchlani N, Kennedy NA, Ng SC. Recent advances in clinical practice: management of inflammatory bowel disease during the COVID-19 pandemic. Gut. 2022;71(7):1426–1439. doi: 10.1136/gutjnl-2021-326784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brenner EJ, Ungaro RC, Gearry RB, et al. Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: results from an international registry. Gastroenterology. 2020;159(2):481–91e3. doi: 10.1053/j.gastro.2020.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson P, Benford M, Harris N, Karavali M, Piercy J. Real-world physician and patient behaviour across countries: disease-specific programmes - a means to understand. Curr Med Res Opin. 2008;24(11):3063–3072. doi: 10.1185/03007990802457040. [DOI] [PubMed] [Google Scholar]
- 24.Higgins V, Piercy J, Roughley A, et al. Trends in medication use in patients with type 2 diabetes mellitus: a long-term view of real-world treatment between 2000 and 2015. Diabetes Metab Syndr Obes. 2016;9:371–380. doi: 10.2147/DMSO.S120101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Babineaux SM, Curtis B, Holbrook T, Milligan G, Piercy J. Evidence for validity of a national physician and patient-reported, cross-sectional survey in China and UK: the Disease Specific Programme. BMJ Open. 2016;6(8):e010352. doi: 10.1136/bmjopen-2015-010352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.EuroQol—a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208. [DOI] [PubMed]
- 27.European Pharmaceutical Market Research Association. Code of conduct 2021. https://www.ephmra.org/sites/default/files/2022-03/2021%20EPHMRA%20Code%20of%20Conduct%202.11.21.pdf. Accessed 24 Mar 2023.
- 28.Public Policy Committee, International Society of Pharmacoepidemiology Guidelines for good pharmacoepidemiology practice (GPP) Pharmacoepidemiol Drug Saf. 2016;25(1):2–10. doi: 10.1002/pds.3891. [DOI] [PubMed] [Google Scholar]
- 29.World Medical Association. WMA Declaration of Helsinki—ethical principles for medical research involving human subjects. https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/. Accessed 23 Mar 2023.
- 30.US Department of Health and Human Services. Summary of the HIPAA privacy rule. https://www.hhs.gov/sites/default/files/privacysummary.pdf. Accessed 23 Mar 2023.
- 31.Office of the National Coordinator for Health Information Technology. Health Information Technology (HITECH) Act. https://www.healthit.gov/sites/default/files/hitech_act_excerpt_from_arra_with_index.pdf. Accessed 24 Mar 2023.
- 32.Salvatori S, Baldassarre F, Mossa M, Monteleone G. Long COVID in inflammatory bowel diseases. J Clin Med. 2021;10(23):5575. doi: 10.3390/jcm10235575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hisamatsu T. Management of inflammatory bowel disease during the COVID-19 pandemic. Immunol Med. 2022;45(3):128–135. doi: 10.1080/25785826.2021.1978205. [DOI] [PubMed] [Google Scholar]
- 34.US Food and Drug Administration. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. https://www.fda.gov/media/151936/download. Accessed 1 Feb 2023.
- 35.European Medicines Agency. EMA confirms measures to minimise risk of serious side effects with Janus kinase inhibitors for chronic inflammatory disorders. https://www.ema.europa.eu/en/news/ema-confirms-measures-minimise-risk-serious-side-effects-janus-kinase-inhibitors-chronic#:~:text=Janus%20kinase%20(JAK)%20inhibitors%20used%20to%20treat%20chronic%20inflammatory%20disorders,compared%20with%20TNF%20alpha%20inhibitors. Accessed 1 Feb 2023.
- 36.Nasuno M, Miyakawa M, Tanaka H, Motoya S. Short- and long-term outcomes of infliximab treatment for steroid-refractory ulcerative colitis and related prognostic factors: a single-center retrospective study. Digestion. 2017;95(1):67–71. doi: 10.1159/000452459. [DOI] [PubMed] [Google Scholar]
- 37.Rustgi SD, Kayal M, Shah SC. Sex-based differences in inflammatory bowel diseases: a review. Ther Adv Gastroenterol. 2020;13:1756284820915043. doi: 10.1177/1756284820915043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van der Have M, Mangen MJ, van der Valk ME, et al. Effect of aging on healthcare costs of inflammatory bowel disease: a glimpse into the future. Inflamm Bowel Dis. 2014;20(4):637–645. doi: 10.1097/01.MIB.0000442677.55051.03. [DOI] [PubMed] [Google Scholar]
- 39.Windsor JW, Kaplan GG. Evolving epidemiology of IBD. Curr Gastroenterol Rep. 2019;21(8):40. doi: 10.1007/s11894-019-0705-6. [DOI] [PubMed] [Google Scholar]
- 40.Gisbert JP, Chaparro M. Systematic review with meta-analysis: inflammatory bowel disease in the elderly. Aliment Pharmacol Ther. 2014;39(5):459–477. doi: 10.1111/apt.12616. [DOI] [PubMed] [Google Scholar]
- 41.Macaluso FS, Orlando A, Papi C, et al. Use of biologics and small molecule drugs for the management of moderate to severe ulcerative colitis: IG-IBD clinical guidelines based on the GRADE methodology. Dig Liver Dis. 2022;54(4):440–451. doi: 10.1016/j.dld.2022.01.127. [DOI] [PubMed] [Google Scholar]
- 42.Raine T, Bonovas S, Burisch J, et al. ECCO guidelines on therapeutics in ulcerative colitis: medical treatment. J Crohns Colitis. 2022;16(1):2–17. doi: 10.1093/ecco-jcc/jjab178. [DOI] [PubMed] [Google Scholar]
- 43.Yarlas A, Rubin DT, Panés J, et al. Burden of ulcerative colitis on functioning and well-being: a systematic literature review of the SF-36® Health Survey. J Crohns Colitis. 2018;12(5):600–609. doi: 10.1093/ecco-jcc/jjy024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.