Summary
Data from animal models suggest a role of early-life gut microbiota in lung immune development, and in establishing susceptibility to respiratory infections and asthma in humans. This systematic review summarises the association between infant (ages 0–12 months) gut microbiota composition measured by genomic sequencing, and childhood (ages 0–18 years) respiratory diseases (ie, respiratory infections, wheezing, or asthma). Overall, there was evidence that low α-diversity and relative abundance of particular gut-commensal bacteria genera (Bifidobacterium, Faecalibacterium, Ruminococcus, and Roseburia) are associated with childhood respiratory diseases. However, results were inconsistent and studies had important limitations, including insufficient characterisation of bacterial taxa to species level, heterogeneous outcome definitions, residual confounding, and small sample sizes. Large longitudinal studies with stool sampling during the first month of life and shotgun metagenomic approaches to improve bacterial and fungal taxa resolution are needed. Standardising follow-up times and respiratory disease definitions and optimising causal statistical approaches might identify targets for primary prevention of childhood respiratory diseases.
Introduction
Childhood respiratory diseases, including respiratory infection, recurrent wheezing, and asthma, are important causes of morbidity and mortality in children and at ages thereafter. Up to 30% of children worldwide will develop at least one viral lower respiratory tract infection (vLRTI) during their first 2 years of life,1 mainly due to respiratory syncytial virus, and a third of these children will have subsequent recurrent wheezing episodes.2 Although asthma-like symptoms might be present before age 2 years, there are no reliable diagnostic tools to ascertain a diagnosis of asthma in children younger than 5 years.3, 4 Asthma prevalence from age 5 years to 14 years is estimated to be around 10%,5 making it the most prevalent chronic disease in childhood globally.6 Despite the huge health burden of vLRTIs, there are currently no widespread licensed primary prevention strategies for them or for asthma in children.3, 7 This absence of strategies is partly due to an incomplete understanding of disease pathogenesis—although host immune response seems to play an important role in susceptibility to both vLRTI and asthma.8, 9 It is now known that the innate and adaptive immune systems of individuals are influenced by their gut microbiota composition during the first year of life,10, 11 which might be a determinant of childhood respiratory disease aetiology.
Animal models have provided evidence that early-life gut microbiota composition might influence respiratory immunity and susceptibility to both asthma and respiratory infections.12, 13 This organ-level interaction is referred to as the gut–lung axis. Mechanistically, bioactive bacterial ligands and metabolites derived from the gut might enter the circulation to affect immune cell migration in the lung.14 Summarising available human observational evidence exploring the association between early-life gut microbiota composition and childhood respiratory diseases might help inform future intervention studies.
The gut microbiota is the largest and most diverse microbiota in the body, harbouring billions of bacteria (the predominant organisms), archaea, eukaryotes, and viruses.15 Gut microbiota colonisation starts at birth and is highly dynamic during the first years of life, stabilising after 1–3 years.16 Clinical, maternal, feeding, and environmental factors shape the early-life gut microbiota composition.17, 18 For example, infants delivered by caesarean section have a higher abundance of opportunistic pathogens during the neonatal period than do infants delivered by a vaginal birth.18 To a lesser extent, the same is true for infants who are not breastfed.18 Gut microbiota is usually measured by stool sample collection and can broadly be described in terms of diversity and abundance. Diversity describes the number of different taxa within a community. α-diversity refers to the number of taxa detected per sample, whereas β-diversity indicates the difference in composition between samples.19 More nuanced comparisons identify specific relative abundance of bacteria or fungi at different taxonomic levels.
In the past decade, genomic sequencing technologies and bioinformatic analytical tools have advanced considerably. Available platforms now allow the simultaneous sequencing of most or all genetic material present in stool samples,20 enabling an untargeted and more in-depth exploration of the gut microbiota composition and functional community dynamics.21 A widely used genomic sequencing technique is the amplicon approach, which sequences the ribosomal 16S rRNA gene and is highly conserved across all bacterial species. This sequencing technique enables resolution to the bacterial genus level.22 Shotgun metagenomic sequencing refers to the sequencing of all DNA present in a sample without targeting.22 This approach can readily resolve bacteria, fungi, viruses, and other microorganisms to strain level and can be used to infer gene functionality.20 However, both 16S rRNA gene sequencing and shotgun metagenomic sequencing involve multiple steps and complex technical challenges that can introduce measurement bias, including sample storage, DNA extraction, sample quality control, and bioinformatic pipelines.19, 20, 23 Moreover, studies combining epidemiological and genomic sequencing data could be subject to a range of limitations that might threaten legitimate inference.20
We systematically reviewed the existing literature to examine the association between gut microbiota composition during infancy (measured by genomic sequencing) and the subsequent development of respiratory disease during childhood.
Method
A systematic literature review was done in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.24 The protocol was prospectively registered in the International Prospective Register of Systematic Reviews (CRD42020184094).
Search strategy and selection criteria
We comprehensively searched five electronic databases (MEDLINE [Ovid interface], Embase, Web of Science, Scopus, and Cochrane) for articles published with the full text in English between Jan 1, 2010, and April 27, 2021. A start year of 2010 was selected to ensure all studies using genomic sequencing for gut microbiota characterisation were captured.25 Four broad search terms were considered: “infancy”, “intestine”, “microbiota”, and “respiratory disease”. An information specialist was consulted and the search strategy was refined with an iterative process on the basis of inclusion of key studies to optimise the selection of MeSH terms and keywords. Additional studies were identified by searching the references of relevant systematic reviews and included studies (appendix pp 2–3).
Screening and data extraction
The first reviewer (CG-MA) screened all titles, abstracts, and full texts of shortlisted articles. A second reviewer (VMP) screened 10% of studies at each stage of the screening process and results were compared between reviewers to check agreement. Any disagreement was resolved with a third reviewer (CO). Data were extracted by the first reviewer. The second reviewer independently extracted information from 80% of included studies to ensure accuracy in reporting and to minimise reviewer bias.
Strategy for data synthesis
Differences in microbiota diversity, relative abundance of bacteria or fungi taxa (at species, genus, or family level), and any measure of association with 95% CIs relative to either diversity or abundance were extracted and reported as the main result. Other gut microbiota parameters analysed and findings related to gut microbiota composition and respiratory disease were also extracted (appendix pp 8–10).
Assessment of methodological quality
Critical appraisal was performed independently by two reviewers (CG-MA and VMP) to assess the quality of included studies and provide context for the interpretation of the findings. Studies were assessed with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) metagenomics framework for reporting of metagenomic studies.20 A checklist to evaluate reporting of the main sources of bias in metagenomic studies was generated from the framework (appendix pp 15–17). Studies were also assessed with the Newcastle-Ottawa Scale (NOS) quality assessment scale for evaluation of bias and study design limitations.26 By use of defined thresholds, the results from the NOS were translated to the Agency for Health Research and Quality standards and studies were rated as good, fair, or poor (table 1; appendix pp 11–12).38 When studies included multiple analyses aimed at answering several research questions within the same study, quality assessments were only applied to the analyses relevant to this systematic review.
Table 1.
Study characteristic and main results
| Study participants; age at stool sample collection | Technique of microbiota determination* | Respiratory outcome; age at outcome evaluation; follow-up n/N (%) | Method of evaluation; outcome definition | Participants in each outcome group |
Children with respiratory disease versus no respiratory disease |
Adjustments for diversity; adjustments for relative abundance | Study quality | ||
|---|---|---|---|---|---|---|---|---|---|
| Diversity and relative abundance of taxa | Validation | ||||||||
| Arrieta et al (2015);27 nested case-control study | 319 healthy, more than 35 weeks' gestation, singleton newborn babies; 3 months and 1 year | 16S rRNA V3, Ilumina HiSeq, and Greengenes database (2006); validation with qPCR and 16S rRNA V3 amplicon | Atopic wheeze (as a proxy for later asthma);† 1 year; NA (loss to follow-up does not apply in case-control study designs) | Parental questionnaires at 3 months, 6 months, and 1 year of age; study clinical assessment at age 1 year; four phenotypes: atopy (positive prick test at age 1 year), wheeze (≥1 wheezing episodes in first year of life), atopy and wheeze, and control individuals (no asthma or atopy at age 1 year) | 87 (27%) in atopy group; 136 (43%) in wheeze group; 22 (7%) in atopy and wheeze group; 74 (23%) control individuals | No difference in α-diversity (Shannon index) between the four phenotypes at ages 3 months or 1 year; relative abundance of bacteria taxa (genera): atopy and wheeze (compared with control individuals) showed lower FLRV and Peptostreptococcus (p>0·05) at age 3 months, atopy and wheeze (compared with control individuals) showed lower Oscillospira at age 1 year (p>0·05), and no differences between participants with wheeze and participants without wheeze | Validation with qPCR (for FLRV): individuals with atopy and wheeze have lower relative abundance of FLRV genera at age 3 months than control individuals (p<0.0001) | Unadjusted; unadjusted | Poor |
| Laursen et al (2015);28 cohort study | 114 healthy, singleton children; 9 months and 18 months | 16S rRNA V3, Ion Torrent, OneTouch, and Ion Personal Genome Machine systems; CLC Genomic workbench, Ribosomal database project classifier | Asthmatic bronchitis (cumulative prevalence); 3 years; at 3 years: 104/114 (91·2%) | Parental interviews when child was age 9 months, 18 months, and 3 years; cumulative prevalence (age 0–3 years) of diagnosed asthmatic bronchitis defined as squeaky and wheezing breathing in connection with cold or other viral infections in the respiratory system | 19/104 (18%) had asthmatic bronchitis | No differences in α-diversity (Shannon Index) at ages 9 months or 18 months; relative abundance of bacteria taxa (genera): no differences at ages 9 months or 18 months (p>0·05 after correction for multiple testing) | NA | Unadjusted; unadjusted | Poor |
| Fujimura et al (2016);29 cohort study | 298 children (all births); 130 at 1 month (neonatal) or‡ 168 at 6 months (infants) | 16S rRNA V4, Illumina MiSeq, Greengenes database (2013), and Ribosomal Database Project classifier; fungal internal transcribed spacer region 2, UNITE database V6 | Asthma; 4 years; at 4 years: 111/130 (85%, neonatal samples), 168/168 (100%, infant samples) | Parental interviews at months 1, 6, 12, 24, and 48, and clinical study visit at age 2 years; asthma according to parental-reported doctor diagnosis of asthma | 39/279 (14%) had asthma; 17/111 (15%) were neonatal, 22/168 (13%) were infants | α-diversity not reported relative to asthma risk; bacterial β-diversity (PERMANOVA; R2=0·09, p<0·001) and fungal β-diversity (Bray–Curtis; PERMANOVA, R2=0·037, p=0·068) differed between clusters; relative abundance of bacteria taxa (genera): no differences in infant group (age 6 months), newborn babies with high risk of asthma at age 4 years (OR 1·09–10·3; p<0·05) had lower Bifidobacterium, Lactobacillus Faecalibacterium, and Akkermansia compared with low-risk asthma participants (adjusting for false discovery rate: B-H q<0·05); relative abundance of fungal taxa: newborn babies with high risk of asthma had lower Malassezia and higher Candida and Rhodotorula compared with low-risk asthma participants (adjusting for false discovery rate: B-H q<0·20) | NA | Unadjusted; unadjusted (data reported and individually adjusted for current breastfeeding, detectable cat allergen, detectable dog allergen, ever breastfed, female, first-born, maternal age, maternal education, maternal history allergic disease, birth delivery method, pets, and race) | Poor |
| Stiemsma et al (2016);30 nested case-control study | 76 healthy, term (>35 weeks), singleton newborn babies; 3 months and 1 year | 16S rRNA V3, Hiseq Ilumina, and Greengenes database (2006); validation with qPCR and 16S rRNA V3 amplicon | Asthma; 4 years; NA | Multiple questionnaires and clinical assessments by study physicians at ages 1 year and 3 years; cases defined as physician diagnosis of asthma by age 4 years or prescribed inhaled medications from ages 3–4 years and controls defined as negative for asthma or inhaled medication, wheezing, and atopy (based on standardised allergen skin prick testing at ages 1 year and 3 years) | 39 (51%) with asthma; 37 (49%) matched healthy controls | No differences in α-diversity (Shannon index) or β-diversity at ages 3 months or 1 year; relative abundance of bacteria taxa: individuals with asthma showed lower Clostridiales (class; log2FC 1–2; p=0·035) and Lachnospira (genera; log2FC 1–2; p=0·098), higher Clostridium neonatale (species; log2FC 1–2; p=0·076), Clostridiaceae (family; p=0·005), and Firmicutes (phylum; log2FC 1–3; p=0·035) than did control individuals at age 3 months, individuals with asthma showed higher Lachnospiraceae (family; p=0·032) and Rothia (genera; p=0·003) than did control individuals at age 1 year | Validation with qPCR: individuals with asthma showed lower relative abundance of Lachnospira at age 3 months (p=0·008) and lower Clostridium neonatale at age 1 year (p=0·02) | Matched on gender, birth delivery method, feeding practices, antibiotic exposure; matched on gender, birth delivery method, feeding practices, antibiotic exposure | Good |
| Arrieta et al (2018);31 nested case-control study | 97 healthy newborn babies; 3 months | 16S rRNA V4, Miseq Ilumina, Greengenes database (2006), 18S rRNA V4 (fungi), SILVA database (2013); validation with qPCR | Atopic wheeze; 5 years; NA | Parental interviews; cases defined as maternally reported wheeze in the previous 12 months at age 5 years and positive skin prick test response and controls defined as random sample of children with no previous history of wheeze and no evidence of atopy at age 5 years | 27 (28%) with atopic wheeze, 70 (72%) healthy controls | No differences in α-diversity (Chao1 index) or β-diversity, bacterial or fungal; relative abundance of bacteria taxa (genera): children with atopic wheeze had low Bifidobacterium (log2FC>4; p<0·001) and high Streptoccocus (log2FC 2–4; p=0·044) and Veillonella (log2FC 2–4; p=0·031), adjusted for false discovery rate at age 3 months; relative abundance of fungal taxa (genera): children with atopic wheeze had higher Pichia Kudriavzevii at age 3 months (log2FC 2–6; p<0·01) | Validated increase in Pichia kudriavzevii species using qPCR at 3 months in children with atopic wheeze compared with healthy controls | Unadjusted; adjusted for antibiotic use during pregnancy or during first year of life, antibiotic duration, birth delivery method, household potable water, number of respiratory tract infections during first year of life, eosinophilia at age 7 months, number of diarrhoeal episodes during first year of life | Fair |
| Stockholm et al (2018);32 cohort study | 690 newborn babies (all births); 1 week, 1 month, and 1 year | 16S rRNA V4, Illumina Miseq, and Greengenes database (2013) | Asthma; 5 years; at 5 years: 648/690 (94%) | Clinical visits at age 1 week, ages 1, 3, 6, 12, 18, 24, 30, and 36 months, and yearly thereafter (including during acute respiratory episodes); asthma diagnosis if all of 5 episodes of lung symptoms within 6 months (at least for 3 consecutive days), exercise-induced symptoms, prolonged nocturnal cough, or persistent cough outside of common colds, need for intermittent rescue use β2-agonist, and response to 3 months of inhaled steroids and relapse after stopping | 60/648 (9%) had asthma | No differences in α-diversity (Shannon and Chao1 indices) at any timepoint; no differences in β-diversity at ages 1 week and 1 month; β-diversity at 1 year in individuals with asthma vs individuals without asthma (PERMANOVA; F=3·4, R2=0·6%, p=0·003); relative abundance of bacteria taxa (genera): no differences at ages 1 week or 1 month; for the 20 most abundant bacterial genera, individuals with asthma had lower Roseburia (median relative abundance 0·27% vs 0·66%; p=0·042), Alistipes (median relative abundance 0·04% vs 0·35%; p=0·002), and Flavonifractor (0·05% vs 0·07%; p=0·002) and higher Veillonella (0·94% vs 0·29%; p=0·035) at age 1 year than had controls; diversity and relative abundance differences were influenced by children born to mothers with asthma (effect modifier; p=0·011) | NA | Adjusted for birth delivery method, duration of exclusive breastfeeding, older sibling hospitalisation after birth, and antibiotic use; unadjusted | Good |
| Reyman et al (2019);33 cohort study | 120 healthy, term (>37 weeks) newborn babies; 1 week | 16S rRNA V4, llumina MiSeq, Naive Bayesian Ribosomal Database Project classifier (version 2.2), and SILVA database (2012); validation with qPCR | Respiratory infection (cumulative incidence); 1 year; at 1 year: 118/120 (98%) | Structured interview and questionnaire; respiratory infection defined as fever (>38·0°C) and any of cough, wheezing, dyspnoea, earache, or malaise (events in the first year of life were summed up to a cumulative number and categorised into 0–2 vs 3–7) | 77 (65%) 3–7 respiratory infections; 41 (35%) with 0–2 respiratory infections (considered healthy) | Diversity not reported relative to respiratory infection; relative abundance of bacterial taxa (genera): children with more respiratory infections (those with 3–7 compared with those with 0–2) had lower Bifidobacterium (log2FC 2·1; p=0·049) and higher Klebsiella (log2FC 3·2; p=0·007) and Enterococcus (log2FC 2·8; p=0·009) at age 1 week | Validation with qPCR: children with 3–7 respiratory infections had higher relative abundance of Enterococcus spp (p=0·015) than did children with 0–2 respiratory infections | NA; adjusted for birth delivery method | Good |
| Galazzo et al (2020);34 cohort study | 440 healthy, term newborn babies and parents with atopic disease; 5 weeks, 3·3 months, 5·3 months, and 7·8 months | 16S rRNA V3, Illumina MiSeq, and Greengenes database (2011) | Asthma; 6–11 years; at 6–11 years: 292/440 (66%) | Clinical visit; parent-reported doctor diagnosis of asthma in combination with any indicative symptoms in the past 12 months (wheezing, shortness of breath, nocturnal awakening due to symptoms; included lung function testing) | Not reported | No differences in α-diversity (Shannon index); relative abundance of bacteria taxa (genera): children with asthma showed, at all ages, lower Lachnobacterium, Lachnospira, and Dialister (p<0·001) than children without asthma | NA | Adjusted for breastfeeding, age at introduction to solid food, birth delivery method, treatment (placebo vs probiotic), birthweight, sex, mother or father with atopic dermatitis, >2 older siblings, and pets; adjusted for breastfeeding, age at introduction to solid food, birth delivery method, treatment (placebo vs probiotic), birthweight, sex, mother or father with atopic dermatitis, >2 older siblings, and pets | Good |
| Boutin et al (2020);35 cohort study | 837 healthy, term (>35 weeks), singleton newborn babies; 3 months | 16S rRNA V4, HiSeq Ilumina, and Greengenes database (2013) | Recurrent wheeze and atopic wheeze; 1 year; 659/837 (79%) after 1 year | Questionnaires and clinical assessments by study physicians at ages 1, 3, and 5 years; recurrent wheezing defined as ≥2 episodes of wheezing in the first year of life, healthy control individuals did not have asthma, recurrent wheeze, atopic dermatitis, atopy, or allergic sensitisation at ages 1, 3, and 5 years | 142/659 (21%) had recurrent wheeze; 45/659 (6%) had atopic wheeze; 16/659 (2%) had both (included in recurrent wheeze and atopic wheeze groups) | Children with recurrent wheeze and atopic wheeze had lower α-diversity at age 3 months than individuals without; increased α-diversity at age 3 months was protective of recurrent wheeze (OR 0·75 [0·6–0·95]; p=0·007) and atopic wheeze (OR 0·55 [0·1–0·90]; p=0·016); relative abundance of bacterial taxa (analyses aimed at identifying only decreased bacteria genus): children with recurrent wheeze had lower Faecalibacterium, Lachnospira, Coprococcus, and Oscillospira at age 3 months than did healthy children and children with atopic wheeze showed lower Faecalibacterium, Lachnospira, Coprococcus, Roseburia, Blautia, Parabacteroides, and Ruminococcus at age 3 months than did healthy children | NA | Unadjusted; unadjusted (machine learning predictive methods) | Poor |
| Patrick et al (2020);36 cohort study | 917 healthy, term (>35 weeks), singleton newborn babies; 3 months and 1 year | 16S rRNA V4, Hiseq Ilumina, and Greengenes database (2013) | Asthma; 5 years; outcome evaluated in those with stool sample processed at 1 year: 570/917 (62%) | Multiple questionnaires and clinical assessments by study physicians at ages 1, 3, and 5 years; diagnosis of asthma based on questionnaire data, clinical history, and medical examination | 63/570 (11%) had asthma | At age 1 year, children with asthma showed decreased α-diversity (Chao1 index) compared with children without asthma; having increased α-diversity at age 1 year protected from asthma (OR 0·68 [0·46–0·99]; p=0·046); relative abundance of bacterial taxa (only summarising those with at least 1·5 log2FC): at age 1 year, children with asthma had lower Faecalibacterium prausnitzii (log2FC −1·57 to 1·77), Ruminococcus bromii (log2FC −2·07), and Rikenellaceae (family) (log2FC −2·59) and higher Dialister (genus) (log2FC 2·04; false discovery rate p<0·05) than did children without asthma§ | NA | Adjusted for antibiotic use at age 1 year, race, birth delivery method, older siblings, sex, birthweight, prenatal atopy, breastfeeding at age 6 months, tobacco smoke exposure in first year of life, NO2 exposure during first year of life, season of birth, living area (urban or rural); adjusted for antibiotic use at age 1 year, race, birth delivery method, older siblings, sex, birthweight, prenatal atopy, breastfeeding at age 6 months, tobacco smoke exposure in first year of life, NO2 exposure during first year of life, season of birth, living area (urban or rural), sequencing batch, and study centre | Good |
| Depner et al (2020);37 cohort study | 720 newborn babies; 2 months and 1 year | 16S rRNA V4, Illumina MiSeq, and Greengenes database (2013); fungal internal transcribed spacer region 1, UNITE dynamic database (2010) | Asthma; 6 years; at 6 years: 626/720 (87%) | Parent-reported doctor diagnosis of asthma at least once or recurrent diagnoses of obstructive bronchitis or asthmatic bronchitis; atopic and non-atopic asthma defined as the presence or absence of concomitant sensitisation to inhalant allergens (seasonal or perennial) with specific IgE concentrations higher than 0·7 IU/mL–1 at age 6 years (lung function measured by spirometry at age 6 years) | 53/626 (9%) had asthma | Reported EMA¶ as a proxy for α-diversity (positive correlation [r=0·70] for number of different bacteria genera); children with asthma had a difference in EMA at age 2 months and lower EMA at age 1 year than did children without asthma; relative abundance of bacteria taxa (genera): children with asthma had, at age 2 months, lower Bacteroides and Parabacteroides and higher Enterococcus than did children without asthma; having a high relative abundance of Bacteroides and Parabacteroides and low relative abundance of Enterococcus at age 2 months protected from both atopic and non-atopic asthma (OR 0·68 [0·49–0·95]; p=0·024); children with asthma showed, at age 1 year, lower Roseburia, Ruminococcus, and Faecalibacterium than did children without asthma; having higher relative abundance of Roseburia, Ruminococcus, and Faecalibacterium at age 1 year protected from non-atopic asthma (OR 0·62 [0·39–1·00]; p=0·048; association fully explained by EMA) | NA | Unclear if adjusted (collected clinical variables including birth delivery method, breastfeeding, antibiotic use, gestation age, birthweight, Apgar score, parental history of atopy, farm exposure, pets, smoke exposure, number of siblings, and parental education) | Good |
API= Asthma Predictive Index. B-H=Benjamini-Hochberg. EMA=estimated microbiome age. FLRV=Faecalibacterium, Lachnospira, Rothia, Veillonella. Log2FC=log2 fold change. NA=not available. OR=odds ratio. qPCR=quantitative PCR.
Target, sequencing platform, pipeline, and database.
Children were followed up for 3 years. At age 3 years children had a clinical visit to predict asthma development between ages 6 years and 11 years using the API. The atopy and wheezing group at age 1 year were 5·4 times more likely to have a positive API score at age 3 years compared with the wheeze only group at 1 year.
All other studies used and instead of or (participants were followed up and samples were collected at different timepoints).
Findings extracted from supplementary materials. Findings reported in main paper were that patients with asthma and antibiotic use at age 1 year had relative decreased abundance of Faecalibacterium prausnitzii, Roseburia (genera), and Ruminococcus bromii and increased abundance of Clostridium perfringens (false discovery rate p<0·05).
Random forest analysis (machine learning) was used to estimate the healthy age of gut microbiota sampled at ages 2 months and 1 year in 133 healthy individuals (no diarrhoea, wheezing, or asthma in the first year of life).
Results
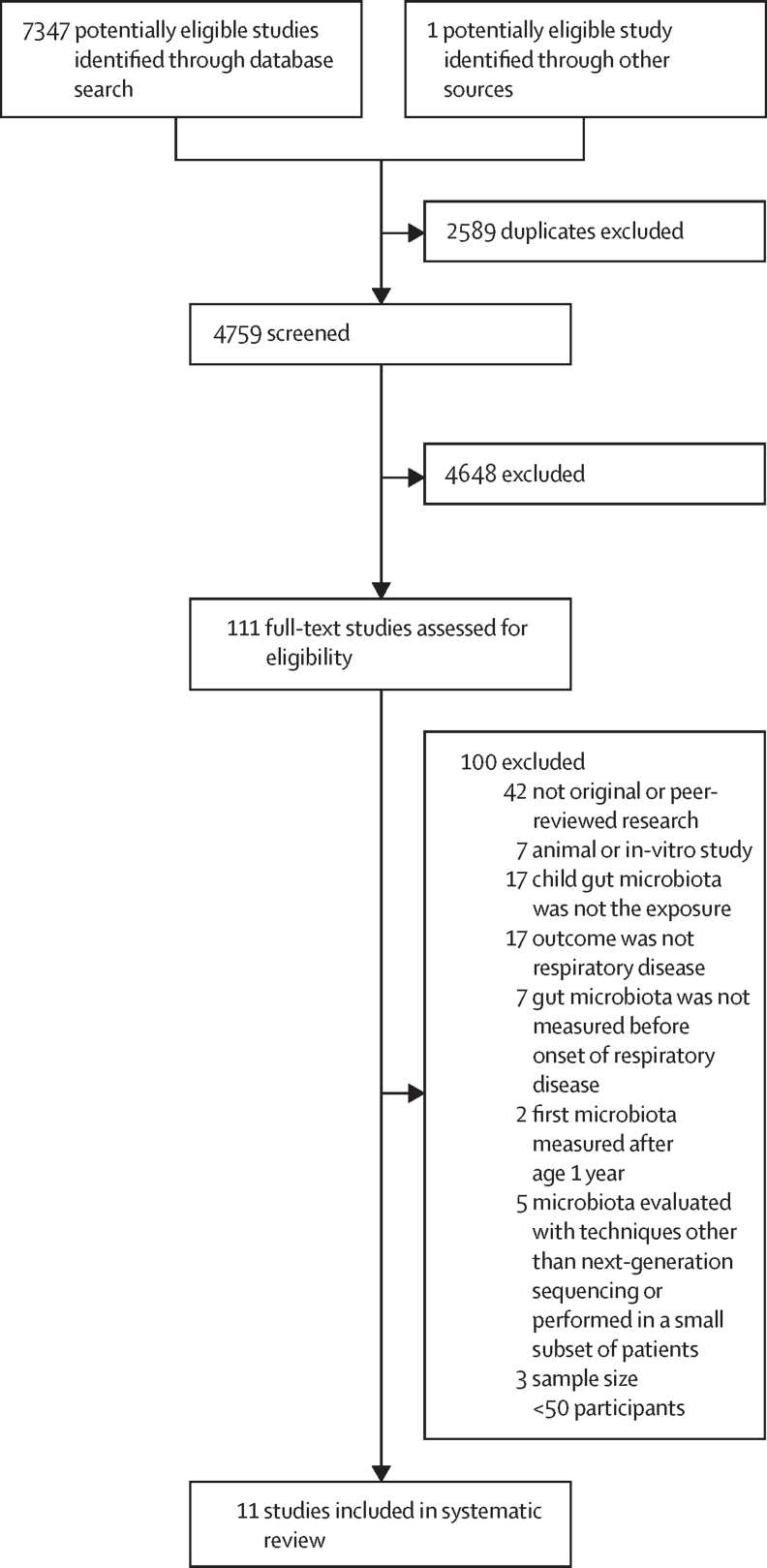
4759 titles and abstracts were screened and 4648 of these studies were excluded. Reasons for exclusion included non-primary research, animal studies, in-vitro studies, or studies in which exposure or outcome were not relevant to the research question. After a full-text review of the remaining 111 studies, 11 were included (figure). Agreement between the first and second reviewer was high (κ=0·98).
Figure.
Study selection
Of the 11 included studies, eight were cohort studies28, 29, 32, 33, 34, 35, 36, 37 and three were nested case-control studies (table 1).27, 30, 31 Nine studies were done in urban areas in Europe or the USA and two studies were done in rural areas of Europe and Ecuador.31, 37 Four studies were from the same research group and based on the same birth cohort, with potential overlap of participants.27, 30, 35, 36 Sample size ranged from 76 participants to 917 participants, with a median of 319. Eight studies27, 28, 29, 30, 34, 36, 37 characterised the gut microbiota composition at more than one timepoint, but only three studies collected stool samples in the neonatal period (first month of life).29, 32, 33 All studies used amplicon sequencing targeting the 16S rRNA (V3 or V4 regions) gene for gut microbiome determination. Three studies included evaluation of the presence of fungi.29, 31, 37 Respiratory disease status was ascertained on the basis of parental reported symptoms or reported doctor diagnosis of respiratory disease in all but one study, in which the outcome was ascertained with clinical visits during acute respiratory episodes.32 Two studies mentioned that lung function tests were done,32, 34 but it was unclear how these tests were integrated into respiratory disease definitions.
Four of the 11 included studies were classified as poor quality,27, 28, 29, 35 one was classified as fair quality,31 and six were classified as good quality (table 1, appendix pp 11–13).30, 32, 33, 34, 36, 37 Study design limitations were mainly due to potential selection bias or unadjusted confounders. Most studies selected participants on the basis of completed follow-up or availability of stool samples, whereas only three studies31, 32, 36 compared participants with and without stool availability or complete follow-up, showing low probability of selection bias. No studies presented hypothesised a-priori causal pathways regarding variables associated with exposures or outcomes (potential confounders),39 and three studies did not mention adjusting for confounding factors.27, 28, 35 The other eight studies adjusted for some potential confounders, such as breastfeeding or delivery method. However, other important potential confounders, such as socioeconomic status or smoking exposure,40 were not accounted for in most analyses of these eight studies.
Regarding the STROBE metagenomics checklist (appendix pp 14–17), all included studies provided at least partial methods for specimen collection, storage, and DNA extraction, although six studies27, 30, 31, 34, 35, 36 did not clearly report time between sample collection and freezing. Six studies27, 29, 30, 31, 32, 33 explicitly reported use of negative control samples to exclude contamination. Sequencing methods and bioinformatic pipelines were at least partly reported by all included studies. Seven studies amplified the V4 hypervariable region of the 16S rRNA gene, and four studies amplified the V3 region of the 16S rRNA gene.27, 28, 30, 34 All studies except one28 used Illumina HiSeq or MiSeq sequencing platforms. Bioinformatic pipelines for data cleaning varied between studies. Four used Mothur27, 30, 31, 32 and six used QIIME or QIIME2.29, 31, 33, 35, 36, 37 All but one study33 used a version of the Greengenes database (2006 or 2013) as a reference for defining bacterial operational taxonomic units.
The statistical analyses used to establish the association between diversity and relative abundance of taxa and respiratory disease included univariate statistical tests,27 correlation matrices adjusted for false discovery rates,28 DESeq2,30, 31, 36 multivariate regression models adapted to microbiome data (eg, MaAsLin),41 and predictive machine learning approaches.33, 35 Most included studies compared microbiota diversity or raw relative abundance of taxa between participants with and without respiratory disease, whereas others used clustering approaches based on microbiota composition.29, 32, 34, 37 Four studies used quantitative PCR as a confirmatory assay27, 30, 31, 33 and one performed next-generation sequencing in a subset of 20 samples.33 Two studies used experimental models (animal or in-vitro experiments) to validate observational findings.27, 29 Four studies27, 29, 31, 37 evaluated gut microbiota functionality by measuring concentrations of short chain fatty acids and bacterial metabolites42 in stool samples (appendix pp 8–10). Only two studies considered a-priori power calculations; however, no specific estimations of power were reported.30, 33 Although cross-sectional studies were excluded, the potential for reverse causality was identified in two of the included studies, in which respiratory disease was assessed before, at the same time as, or after one of the timepoints for stool collection.27, 28
Study results have been summarised and stratified by type of respiratory disease and age at stool sample collection17 (Table 1, Table 2, Table 3). Two groups of studies were identified on the basis of respiratory outcome definition, including whether atopy was considered and age at outcome determination.3 The first group included nine studies that explored atopic wheeze or asthma27, 29, 30, 31, 32, 34, 35, 36, 37 (table 2), of which four studies followed up participants to age 5 years,31, 32, 35, 36 one study followed up participants to age 6 years,37 and one study followed up participants between age 6 years and age 11 years.34 The second group included studies exploring respiratory infections or studies exploring wheezing in the context of respiratory infections (first year of life), referred to as “respiratory infection or wheezing” (table 3).27, 28, 33, 35 Two studies explored wheeze both with and without atopy (defined as positive prick test) at age 1 year and were included in both groups.27, 35
Table 2.
Studies exploring asthma or atopic wheeze
| Age at gut microbiota determination | α-diversity (respiratory disease vs no respiratory disease) | Relative abundance of bacteria taxa (or fungal taxa) in respiratory disease versus no respiratory disease | Age of participants at respiratory disease determination (outcome) | AHRQ rating | |
|---|---|---|---|---|---|
| Fujimura et al (2016)29 | ≤1 month | Not reported | Lower Bifidobacterium, Lactobacillus, Faecalibacterium, and Akkermansia; lower Malassezia; higher Candida and Rhodotorula | 4 years (high risk of asthma) | Poor |
| Stockholm et al (2018)32 | ≤1 month | No difference | No difference | 5 years (asthma) | Good |
| Arrieta et al (2015)27* | 3 months | No difference | Lower Faecalibacterium, Lachnospira, Rothia, Veillonella, and Peptostreptococcus | 1 year (atopic wheeze) | Poor |
| Boutin et al (2020)35* | 3 months | α-diversity decreased | Lower Faecalibacterium, Lachnospira, Coprococcus, Roseburia, Blautia, Parabacteroides, and Ruminococcus | 1 year (atopic wheeze) | Poor |
| Stiemmsa et al (2016)30* | 3 months | No difference | Lower Clostridiales and Lachnospira; higher Clostridium neonatale (species), Clostridiaceae (family), and Firmicutes (phylum) | 4 years (asthma) | Good |
| Arrieta et al (2018)31 | 3 months | No difference | Lower Bifidobacterium; higher Streptococcus, Veillonella, and Pichia kudriavzevii | 5 years (atopic wheeze) | Fair |
| Arrieta et al (2015)27* | 1 year | No difference | Lower Oscillospira | 1 year (atopic wheeze) | Poor |
| Stiemmsa et al (2016)30* | 1 year | No difference | Lower Clostridium neonatale; higher Lachnospiraceae and Rothia | 4 years (asthma) | Good |
| Stockholm et al (2018)32 | 1 year | No difference | Lower Roseburia, Alistipes, and Flavonifractor; higher Veillonella | 5 years (asthma) | Good |
| Patrick et al (2020)36* | 1 year | α-diversity decreased | Lower Faecalibacterium prausnitzii, Ruminococcus bromii, and Rikenellaceae (family); higher Dialister | 5 years (asthma) | Good |
| Depner et al (2020)37 | 1 year | α-diversity decreased | Lower Faecalibacterium, Roseburia, and Ruminococcus | 6 years (non-atopic asthma) | Good |
Studies from the same cohort. AHRQ=Newcastle-Ottawa Quality assessment for cohort and case-control studies converted to the Agency for Healthcare Research and Quality scale. One paper29 did not report results independently by time of stool sample collection, but the authors reported consistent decreases in relative abundance of certain bacteria genera in gut microbiota (Lachnobacterium, Lachnospira, and Dialister) at all timepoints examined (5 weeks, 3·3 months, 5·3 months, and 7·8 months) in children who developed asthma (parent-reported doctor diagnosis of asthma at age 6–11 years) compared with children who did not develop asthma.
Table 3.
Studies exploring respiratory infections or studies exploring wheezing in the context of respiratory infections
| Age at gut microbiota determination | Diversity (respiratory disease vs non-respiratory disease) | Relative abundance of bacteria taxa (fungal taxa) in respiratory disease versus non-respiratory disease | Age of participants at respiratory disease determination (outcome) | AHRQ rating | |
|---|---|---|---|---|---|
| Reyman et al (2019)33 | ≤1 month | Not reported | Lower Bifidobacterium; higher Klebsiella and Enterococcus | 1 year (cumulative incidence of respiratory infections) | Good |
| Arrieta et al (2015)27 | 3 months | No difference | No difference | 1 year (wheezing) | Poor |
| Boutin et al (2020)35 | 3 months | α-diversity decreased | Lower Faecalibacterium, Lachnospira, Coprococcus, and Oscillospira | 1 year (recurrent wheezing) | Poor |
| Laursen et al (2015)28 | 9 months | No difference | No difference | 3 years (wheezing in respiratory infection context) | Poor |
| Arrieta et al (2015)27 | 1 year | No difference | No difference | 1 year (wheezing) | Poor |
AHRQ=Newcastle-Ottawa Quality assessment for cohort and case-control studies converted to the Agency for Healthcare Research and Quality scale.
Asthma and atopic wheezing
Of the seven studies27, 30, 31, 32, 35, 36, 37 that explored the direct association between α-diversity and asthma or atopic wheeze, two reported that higher gut microbiota α-diversity in the first year of life, compared with lower α-diversity, was significantly associated with not having atopic wheeze at age 1 year, and not having asthma at ages 5 and 6 years.35, 36, 37 The other five studies observed no association. Three studies used an alternative measure for describing overall gut microbiota composition. These studies explored gut microbial maturity on the basis of bacterial taxa compositional changes over time in a subset of healthy participants and compared this microbiota maturity with that of participants who developed childhood respiratory disease (appendix pp 7–10). One study used this method as a proxy for diversity, as they were positively correlated.37 One study reported that increased gut microbiota maturity at age 5 weeks, compared with decreased gut microbiota maturity, was associated with high risk of asthma in participants aged 6–11 years,34 and two studies reported an association between an immature gut microbiota at age 12 months and increased risk of asthma at ages 5 years and 6 years.32, 37
Although results varied between studies, overall there was evidence of a low relative abundance of the genera Bifidobacterium29, 31 in stools collected at ages 1 month and 3 months and low abundance of the genera Faecalibacterium,27, 35, 36, 37 Roseburia,32, 35, 37 and Ruminococcus35, 36, 37 in stools collected at ages 3 months and 1 year being associated with asthma and atopic wheeze at ages 1–6 years. Low relative abundance of Lachnospira27, 30, 35 at age 3 months but increased abundance of Lachnospira at age 1 year30, 32 was also associated with asthma and atopic wheeze at ages 1–6 years. One study showed low relative abundance of Veillonella27 at age 3 months was associated with atopic wheeze at age 1 year, whereas two studies reported that high relative abundance of Veillonella at ages 3 months and 1 year was associated with asthma and atopic wheeze at age 5 years.
Three studies29, 31, 37 explored the association between fungi and asthma. One study sequenced the conserved fungal marker genes, including the 18S rRNA gene,31 and two studies sequenced the nuclear ribosomal Internal Transcribed Spacer (ITS1 and ITS2).29, 37 High relative abundance of Candida and Rhodotorula and low abundance of Malassezia taxa measured at age 1 month in one study,29 and an increase of Pichia kudriavzevii at age 3 months measured in another,34 were associated with asthma and atopic wheeze at ages 4–5 years. The third study found no associations between fungal maturity and asthma at age 6 years.37
Respiratory infections and wheezing
Four studies were included in the respiratory infections and wheezing group, in which respiratory disease definitions were highly heterogeneous. One study evaluated respiratory infections,33 another study evaluated wheezing in the context of respiratory infection,28 and two studies evaluated non-atopic wheeze27, 35 at age 1 year. Three studies27, 28, 35 explored the association between α-diversity and respiratory infections or wheezing and one study reported that high α-diversity in the first year of life was associated with reduced recurrent wheezing at age 1 year.35 The other two studies did not find an association between α-diversity and respiratory infections or wheezing. Two studies showed no association between relative abundance of species measured at ages 3, 9, and 12 months and wheezing at ages 1–3 years.28, 31 One study showed a lower relative abundance of Bifidobacterium and higher abundance of Klebsiella and Enterococcus at age 1 week in children with higher cumulative incidence of respiratory infections at age 1 year than for children with lower cumulative incidence of respiratory infections.33 No study specifically explored vLRTI as an outcome.
Discussion
To our knowledge, this systematic review is the first to consider the association between early-life gut microbiota and childhood respiratory diseases, including respiratory infections, with a focus on genomic sequencing to measure the gut microbiota. Large studies (>700 participants) reported high α-diversity as being protective of asthma and wheezing.35, 36, 37 Overall, there was evidence of low relative abundance of Bifidobacterium29, 31, 33 in stools collected before age 3 months being associated with respiratory infections at age 1 year and asthma at ages 4–5 years. Generally, low abundance of the genera Faecalibacterium,28, 35, 36, 37 Roseburia,32, 35, 37 and Ruminococcus,35, 36, 37 in stool samples collected at ages 3–12 months were associated with asthma and atopic wheeze at ages 1–6 years. However, there were important study limitations, including heterogeneous outcome definitions and follow-up times, residual confounding, small sample sizes, and heterogeneous bioinformatic and statistical approaches, with most studies not reporting effect estimates.
A previous systematic review by Zimmerman and colleagues25 in 2019 assessed the association between gut microbiota and atopy, including asthma. Study inclusion was not restricted by method of microbiota determination, and 11 studies that independently reported wheezing or asthma as their outcome were included. Four of those studies were also included in this systematic review.27, 29, 30, 31 The other seven studies were excluded, as gut microbiota determination was not done with genomic sequencing. Instead, these seven studies used culture,43, 44 PCR testing targeting five bacteria,45, 46 or denaturing gradient gel electrophoresis.47, 48, 49 Zimmerman and colleagues summarised study results at a bacterial family taxa resolution. They concluded that high relative abundance of Bacteroidaceae, Clostridiaceae, and Enterobacteriaceae and low relative abundance of Bifidobacteriaceae and Lactobacillaceae were associated with the development of allergic sensitisation, eczema, or asthma.
All studies included in this systematic review used 16S rRNA gene amplicon sequencing, allowing a relatively untargeted starting point for exploring the gut microbiome and permitting us to summarise and compare study results at a bacterial genera level. Some studies reported that high relative abundance of non-commensal gut bacteria such as Klebsiella and Enterococcus at age 1 week33 was associated with respiratory infections at age 1 year, that high relative abundance of Streptococcus at age 3 months was associated with atopic wheeze at age 5 years, and that high relative abundance of Rothia30 or Dialister36 at age 1 year was associated with asthma at ages 4–5 years. However, study results seem to show more consistency with regard to low relative abundance of particular gut-commensal bacteria genera, such as Bifidobacterium,29, 31 Faecalibacterium,27, 29, 35, 36 Ruminococcus,35, 36, 37 or Roseburia,35, 37 in the first 1–12 months of life being associated with respiratory disease.
The genus Bifidobacterium constitutes one of the most abundant bacteria in the gut of children during the first 4 months of life50, 51 and has been shown to modulate the systemic immune response of individuals through surface-associated molecules and microbiota-derived metabolites both in vitro and in vivo.52 Specific Bifidobacterium spp have been shown to affect respiratory disease susceptibility in mouse models of asthma and respiratory infection.12, 13, 53 A 2020 study showed that gut colonisation with Bifidobacterium infantis regulates the equilibrium between Th1 and Th2 responses, reducing symptoms of atopic asthma in an induced mouse model.12 Another study reported that, when challenged with influenza virus, mice with higher gut abundance of Bifidobacterium and Bacteroides showed increased influenza survival through an enhanced CD8 T-cell and well regulated macrophage response than mice with lower gut abundance, preventing excessive airway neutrophil influx.13
The bacteria genera Faecalibacterium, Ruminococcus, Lachnospira, Roseburia, and Veillonella correspond to the clostridia class, which has been described in the guts of children from around 4–6 months of age, coinciding with weaning off breastmilk.16 Ruminococcus (specifically Ruminococcus gnavus) and Roseburia (specifically Roseburia inulinivorans) have been described as signature taxa in infants aged 12 months.16 Potential immune-modulation mechanisms have been described for Roseburia and Faecalibacterium, which produce butyrate, a bacterial metabolite with anti-inflammatory properties in animal and in-vitro models.42 Although one study included in this systematic review showed that inoculating germ-free mice with Lachnospira, Veillonella, Faecalibacterium, and Rothia improved airway inflammation in the adult progeny of these mice,27 little is known about the mechanistic role of these bacteria in respiratory disease.
Comparison of studies exploring respiratory infections and wheezing episodes in children younger than 5 years is challenging because of the inconsistent definitions for upper and lower viral respiratory infections and recurrent wheezing in the literature and clinical guidelines, and the difficulty in diagnosing asthma at ages 0–6 years.54 Correctly classifying wheezing phenotypes could be crucial as they seem to have divergent underlying pathophysiology, shown by the variability in response to different treatments (eg, steroids or β2 agonists).55 In turn, these phenotypes might be linked to different early gut microbiota compositions. We stratified results by respiratory disease type but still found that outcome definitions were inconsistent within groups, were mostly ascertained by parental interviews (with potential recall bias), and follow-up of children only went beyond age 5 years in two asthma studies. Wheezing in children younger than 5 years was used in most studies as a proxy for asthma, with some studies considering atopy and other studies not considering atopy. However, wheezing is a symptom and not a disease,55 so the results of these studies should be compared with caution and the measurements of outcomes are at risk of misclassification bias.
Only two studies included in this systematic review contained respiratory infections per se in their outcome definition.28, 33 The largest and most robust study showed that low relative abundance of Bifidobacterium and high relative abundance of Klebsiella and Enterococcus in stool samples collected at age 1 week were associated with a higher number of respiratory infections evaluated at age 1 year compared with those with low numbers of respiratory infections.33 As such, a considerable knowledge gap exists with respect to respiratory infections.
Neonatal gut microbiota composition is known to influence subsequent colonisation patterns, which might also affect subsequent microbiota and immune crosstalk and development in the long term.13 However, only three studies included in this systematic review collected stool samples in the neonatal period (first month of life),28, 35, 37 even though this age has been highlighted as an important time period for potential microbiota-altering interventions.56, 57 Although Zimmerman and colleagues25 reported consistency regarding study results in stool samples collected in the neonatal period in six wheezing or asthma studies, as our review only included three studies with neonatal stool samples, we found more consistency in results from studies with stool samples collected at ages 3–12 months. Gut microbiota composition beyond the first year of life might also be important, although careful assessment of timing of respiratory disease ascertainment will be needed to avoid reverse causality.
Despite relatively good reporting of laboratory methods and bioinformatic pipelines, these methods and pipelines were heterogeneous, important information was sometimes missing, and potential procedure-specific bias was barely discussed. For example, six studies did not clearly report time between sample collection and freezing and no studies mentioned freeze –thaw cycles, although both can affect microbial profiles.23, 58 Similarly, time between sample collection and processing, which introduces artifact to measures of relative abundance (eg, Bacteroides spp are selectively depleted at –80°C dependent on time in storage),59 was only explicitly mentioned by one study.37 Although microbiota characterisation through 16S rRNA gene amplicon sequencing allows bacterial determination robustly down to the genus level and is cost-effective, it is subject to bias associated with PCR primer-binding and bacterial taxonomic classification.60 Furthermore, the choice of reference database for bacterial taxonomic classification is crucial, and could lead to different results.61 In this systematic review, most studies used the Greengenes database; however, of note, this database was last updated in 2013.62 Use of an up-to-date reference database is important for accurate and high-resolution taxonomic assignment to enable comparisons of the sequencing output with a rapidly expanding and improving microbial genome taxonomy.63
A more informative genomic sequencing approach than the targeted amplicon sequencing method is shotgun metagenomic sequencing, which enables completely untargeted sequencing of all genetic material present in a stool sample; robust bacterial resolution down to a species or strain level; provides information about functionality; and detects viruses, archaea, fungi, and other microeukaryotes that do not possess the 16S rRNA gene.19, 23 Reporting microorganisms down to a strain or species taxa is crucial, as different species within the same genus can have different immunomodulatory effects.64
This systematic review has additional limitations. The search was restricted to studies published after 2010, and it is possible that some smaller studies were missed. One included study had important issues with reverse causality, and one study was a post-hoc analysis of a randomised controlled trial (RCT).28, 34 These studies did not meet the predetermined exclusion criteria but have important limitations and should be interpreted cautiously. Findings were summarised in two groups on the basis of outcome definitions, but alternative ways of grouping study findings could be used. As most studies did not report effect estimates, publication bias cannot be assessed with a funnel plot. However, the fact that some of the studies included in this systematic review reported null findings is somewhat reassuring.
Several frameworks have been developed to aid decisions in establishing causation in microbiome studies.65, 66 An important point in these frameworks is that inoculating a host and generating or preventing disease is a key step in providing evidence for causation.20, 67, 68 However, although some Bifidobacterium spp have been shown to influence respiratory disease pathogenesis in animal studies,13, 53 meta-analyses published between 2015 and 2020, including RCTs testing the efficacy of probiotics and prebiotics to prevent respiratory disease, have not shown a reduction in childhood risk of asthma, wheezing, or respiratory infections.69, 70, 71 Improving RCT study design and optimising observational studies to identify key bacterial species (most RCTs have focused on particular Bifidobacterium spp and Lactobacillus spp) for subsequently informing intervention studies,70 alongside optimal timing of intervention,72 is important.
Another important consideration is that the entire process of microbiota ecosystem development could be the cause of health or disease,66 rather than the absence or presence of specific microorganisms. The role of gut fungi, only explored in three studies,29, 31, 37 and gut viruses should be considered.56, 72 This concept has implications for future prevention strategies, potentially shifting the focus from introduction of single species towards designing probiotics with a rational mixture of species, or towards holistic interventions that might include changing perinatal clinical practice (eg, antibiotic use guidelines or diet).18, 40 However, such interventions might prove more complex to design, implement, and evaluate.
Another outstanding question when evaluating causality between gut microbiota and lung disease is the role of the respiratory microbiota.57 Given evidence of bidirectional influences between gut and respiratory microbiota,73 the potential effect of respiratory microbiota on the immune system, and the observed association between respiratory microbiota and childhood respiratory disease,57 respiratory microbiota might act as a confounder, mediator, or effect modifier in the association between gut microbiota and childhood respiratory disease.57 Longitudinal studies collecting both stool and respiratory samples might help to understand these complex interactions and elucidate the role of the gut–lung axis to identify targets for primary prevention interventions for childhood respiratory diseases.
Overall, there is observational evidence that low α-diversity and relative abundance of particular gut-commensal bacterial genera in the first year of life are associated with subsequent respiratory disease, especially asthma. There is less evidence for the association between low α-diversity and relative abundance of particular bacterial taxa in the first year of life and respiratory infections. However, the available evidence showed important limitations, and gut microbiota composition might not have a causal role in subsequent respiratory disease (despite the observed associations). Large longitudinal studies with stool and respiratory sampling during the neonatal period that use shotgun metagenomic sequencing to improve measurement resolution of the microbiome to a species or strain level are needed. Optimising statistical approaches for causal inference, standardising outcome definitions, and validating findings with experimental models will help move knowledge forward in the area of early-life microbiota and in the development of potential preventive and therapeutic interventions for childhood respiratory diseases.
Declaration of interests
We declare no competing interests.
Acknowledgments
Acknowledgments
The work that led to this systematic review was supported by the Wellcome Trust (WT101169MA). CG-MA was supported by an Institute for Global Health University College London teaching fellowship. We thank Heather Chesters, who was consulted as Deputy Librarian at the University College London Great Ormond Street Institute of Child Health Library (London, UK) on the search strategy and selection criteria and homogeneity between databases.
Acknowledgments
Contributors
CG-MA, AM, AR, and NF conceptualised and designed the study. CG-MA designed the search strategy and selection criteria. CG-MA, VMP, and CO contributed to the screening, data extraction, and quality assessment. CG-MA synthesised the study data with help from YS, CO, TDL, PB, AR, and NF. All authors contributed to the interpretation of data. CG-MA wrote the first draft of the paper, and all authors read, commented on, and approved the final manuscript.
Supplementary Material
References
- 1.Meissner HC. Viral bronchiolitis in children. N Engl J Med. 2016;374:62–72. doi: 10.1056/NEJMra1413456. [DOI] [PubMed] [Google Scholar]
- 2.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. N Engl J Med. 1995;332:133–138. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 3.Global Initiative for Asthma GINA Report, global strategy for asthma management and prevention. 2022. https://ginasthma.org/gina-reports/
- 4.National Institute for Health and Care Excellence Asthma: diagnosis, monitoring and chronic asthma management. 2017. https://www.nice.org.uk/guidance/ng80/chapter/recommendations#diagnostic-summary [PubMed]
- 5.Asher MI, Rutter CE, Bissell K, et al. Worldwide trends in the burden of asthma symptoms in school-aged children: Global Asthma Network Phase I cross-sectional study. Lancet. 2021;398:1569–1580. doi: 10.1016/S0140-6736(21)01450-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asher I, Pearce N. Global burden of asthma among children. Int J Tuberc Lung Dis. 2014;18:1269–1278. doi: 10.5588/ijtld.14.0170. [DOI] [PubMed] [Google Scholar]
- 7.Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134:e1474–e1502. doi: 10.1542/peds.2014-2742. [DOI] [PubMed] [Google Scholar]
- 8.Heinonen S, Rodriguez-Fernandez R, Diaz A, Oliva Rodriguez-Pastor S, Ramilo O, Mejias A. Infant immune response to respiratory viral infections. Immunol Allergy Clin North Am. 2019;39:361–376. doi: 10.1016/j.iac.2019.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raedler D, Schaub B. Immune mechanisms and development of childhood asthma. Lancet Respir Med. 2014;2:647–656. doi: 10.1016/S2213-2600(14)70129-8. [DOI] [PubMed] [Google Scholar]
- 10.Zheng D, Liwinski T, Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020;30:492–506. doi: 10.1038/s41422-020-0332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gensollen T, Iyer SS, Kasper DL, Blumberg RS. How colonization by microbiota in early life shapes the immune system. Science. 2016;352:539–544. doi: 10.1126/science.aad9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang W, Luo X, Zhang Q, He X, Zhang Z, Wang X. Bifidobacterium infantis relieves allergic asthma in mice by regulating Th1/Th2. Med Sci Monit. 2020;26 doi: 10.12659/MSM.920583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Renz H, Adkins BD, Bartfeld S, et al. The neonatal window of opportunity—early priming for life. J Allergy Clin Immunol. 2018;141:1212–1214. doi: 10.1016/j.jaci.2017.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Enaud R, Prevel R, Ciarlo E, et al. The gut-lung axis in health and respiratory diseases: a place for inter-organ and inter-kingdom crosstalks. Front Cell Infect Microbiol. 2020;10:9. doi: 10.3389/fcimb.2020.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sender R, Fuchs S, Milo R. Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans. Cell. 2016;164:337–340. doi: 10.1016/j.cell.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 16.Bäckhed F, Roswall J, Peng Y, et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe. 2015;17:690–703. doi: 10.1016/j.chom.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 17.Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shao Y, Forster SC, Tsaliki E, et al. Stunted microbiota and opportunistic pathogen colonization in caesarean-section birth. Nature. 2019;574:117–121. doi: 10.1038/s41586-019-1560-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharpton TJ. An introduction to the analysis of shotgun metagenomic data. Front Plant Sci. 2014;5:209. doi: 10.3389/fpls.2014.00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bharucha T, Oeser C, Balloux F, et al. STROBE-metagenomics: a STROBE extension statement to guide the reporting of metagenomics studies. Lancet Infect Dis. 2020;20:e251–e260. doi: 10.1016/S1473-3099(20)30199-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Browne HP, Forster SC, Anonye BO, et al. Culturing of ‘unculturable’ human microbiota reveals novel taxa and extensive sporulation. Nature. 2016;533:543–546. doi: 10.1038/nature17645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao B, Chi L, Zhu Y, et al. An introduction to next generation sequencing bioinformatic analysis in gut microbiome studies. Biomolecules. 2021;11:530. doi: 10.3390/biom11040530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quince C, Walker AW, Simpson JT, Loman NJ, Segata N. Shotgun metagenomics, from sampling to analysis. Nat Biotechnol. 2017;35:833–844. doi: 10.1038/nbt.3935. [DOI] [PubMed] [Google Scholar]
- 24.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6 [PMC free article] [PubMed] [Google Scholar]
- 25.Zimmermann P, Messina N, Mohn WW, Finlay BB, Curtis N. Association between the intestinal microbiota and allergic sensitization, eczema, and asthma: a systematic review. J Allergy Clin Immunol. 2019;143:467–485. doi: 10.1016/j.jaci.2018.09.025. [DOI] [PubMed] [Google Scholar]
- 26.Wells GA, Shea B, O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2021. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 27.Arrieta MC, Stiemsma LT, Dimitriu PA, et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci Transl Med. 2015;7 doi: 10.1126/scitranslmed.aab2271. [DOI] [PubMed] [Google Scholar]
- 28.Laursen MFZ, Zachariassen G, Bahl MI, et al. Having older siblings is associated with gut microbiota development during early childhood. BMC Microbiol. 2015;15:154. doi: 10.1186/s12866-015-0477-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fujimura KE, Sitarik AR, Havstad S, et al. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat Med. 2016;22:1187–1191. doi: 10.1038/nm.4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stiemsma LT, Arrieta MC, Dimitriu PA, et al. Shifts in Lachnospira and Clostridium sp in the 3-month stool microbiome are associated with preschool age asthma. Clin Sci (Lond) 2016;130:2199–2207. doi: 10.1042/CS20160349. [DOI] [PubMed] [Google Scholar]
- 31.Arrieta MC, Arévalo A, Stiemsma L, et al. Associations between infant fungal and bacterial dysbiosis and childhood atopic wheeze in a nonindustrialized setting. J Allergy Clin Immunol. 2018;142:424–434. doi: 10.1016/j.jaci.2017.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stokholm J, Blaser MJ, Thorsen J, et al. Maturation of the gut microbiome and risk of asthma in childhood. Nat Commun. 2018;9:141. doi: 10.1038/s41467-017-02573-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reyman M, van Houten MA, van Baarle D, et al. Impact of delivery mode-associated gut microbiota dynamics on health in the first year of life. Nat Commun. 2019;10 doi: 10.1038/s41467-019-13014-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galazzo G, van Best N, Bervoets L, et al. Development of the microbiota and associations with birth mode, diet, and atopic disorders in a longitudinal analysis of stool samples, collected from infancy through early childhood. Gastroenterology. 2020;158:1584–1596. doi: 10.1053/j.gastro.2020.01.024. [DOI] [PubMed] [Google Scholar]
- 35.Boutin RCT, Sbihi H, Dsouza M, et al. Mining the infant gut microbiota for therapeutic targets against atopic disease. Allergy. 2020;75:2065–2068. doi: 10.1111/all.14244. [DOI] [PubMed] [Google Scholar]
- 36.Patrick DM, Sbihi H, Dai DLY, et al. Decreasing antibiotic use, the gut microbiota, and asthma incidence in children: evidence from population-based and prospective cohort studies. Lancet Respir Med. 2020;8:1094–1105. doi: 10.1016/S2213-2600(20)30052-7. [DOI] [PubMed] [Google Scholar]
- 37.Depner M, Taft DH, Kirjavainen PV, et al. Maturation of the gut microbiome during the first year of life contributes to the protective farm effect on childhood asthma. Nat Med. 2020;26:1766–1775. doi: 10.1038/s41591-020-1095-x. [DOI] [PubMed] [Google Scholar]
- 38.Agency for Healthcare Research and Quality Newcastle-Ottawa Quality assessment form for cohort studies. https://www.ncbi.nlm.nih.gov/books/NBK115843/bin/appe-fm3.pdf
- 39.Ferguson KD, McCann M, Katikireddi SV, et al. Evidence synthesis for constructing directed acyclic graphs (ESC-DAGs): a novel and systematic method for building directed acyclic graphs. Int J Epidemiol. 2020;49:322–329. doi: 10.1093/ije/dyz150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zimmermann P, Curtis N. Factors influencing the intestinal microbiome during the first year of life. Pediatr Infect Dis J. 2018;37:e315–e335. doi: 10.1097/INF.0000000000002103. [DOI] [PubMed] [Google Scholar]
- 41.Mallick H, Rahnavard A, McIver LJ, et al. Multivariable association discovery in population-scale meta-omics studies. PLoS Comput Biol. 2021;17 doi: 10.1371/journal.pcbi.1009442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Corrêa-Oliveira R, Fachi JL, Vieira A, Sato FT, Vinolo MAR. Regulation of immune cell function by short-chain fatty acids. Clin Transl Immunology. 2016;5:e73. doi: 10.1038/cti.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Verhulst SL, Vael C, Beunckens C, Nelen V, Goossens H, Desager K. A longitudinal analysis on the association between antibiotic use, intestinal microflora, and wheezing during the first year of life. J Asthma. 2008;45:828–832. doi: 10.1080/02770900802339734. [DOI] [PubMed] [Google Scholar]
- 44.Vael C, Nelen V, Verhulst SL, Goossens H, Desager KN. Early intestinal Bacteroides fragilis colonisation and development of asthma. BMC Pulm Med. 2008;8:19. doi: 10.1186/1471-2466-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Penders J, Stobberingh EE, van den Brandt PA, Thijs C. The role of the intestinal microbiota in the development of atopic disorders. Allergy. 2007;62:1223–1236. doi: 10.1111/j.1398-9995.2007.01462.x. [DOI] [PubMed] [Google Scholar]
- 46.van Nimwegen FA, Penders J, Stobberingh EE, et al. Mode and place of delivery, gastrointestinal microbiota, and their influence on asthma and atopy. J Allergy Clin Immunol. 2011;128:948–955. doi: 10.1016/j.jaci.2011.07.027. [DOI] [PubMed] [Google Scholar]
- 47.Vael C, Vanheirstraeten L, Desager KN, Goossens H. Denaturing gradient gel electrophoresis of neonatal intestinal microbiota in relation to the development of asthma. BMC Microbiol. 2011;11:68. doi: 10.1186/1471-2180-11-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bisgaard H, Li N, Bonnelykke K, et al. Reduced diversity of the intestinal microbiota during infancy is associated with increased risk of allergic disease at school age. J Allergy Clin Immunol. 2011;128:646–652. doi: 10.1016/j.jaci.2011.04.060. [DOI] [PubMed] [Google Scholar]
- 49.Murray CS, Tannock GW, Simon MA, et al. Fecal microbiota in sensitized wheezy and non-sensitized non-wheezy children: a nested case-control study. Clin Exp Allergy. 2005;35:741–745. doi: 10.1111/j.1365-2222.2005.02259.x. [DOI] [PubMed] [Google Scholar]
- 50.Avershina E, Storrø O, Øien T, et al. Bifidobacterial succession and correlation networks in a large unselected cohort of mothers and their children. Appl Environ Microbiol. 2013;79:497–507. doi: 10.1128/AEM.02359-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hidalgo-Cantabrana C, Delgado S, Ruiz L, Ruas-Madiedo P, Sánchez B, Margolles A. Bifidobacteria and their health-promoting effects. Microbiol Spectr. 2017;5 doi: 10.1128/microbiolspec.bad-0010-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alessandri G, Ossiprandi MC, MacSharry J, van Sinderen D, Ventura M. Bifidobacterial dialogue with its human host and consequent modulation of the immune system. Front Immunol. 2019;10 doi: 10.3389/fimmu.2019.02348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Raftis EJ, Delday MI, Cowie P, et al. Bifidobacterium breve MRx0004 protects against airway inflammation in a severe asthma model by suppressing both neutrophil and eosinophil lung infiltration. Sci Rep. 2018;8 doi: 10.1038/s41598-018-30448-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Douros K, Everard ML. Time to say goodbye to bronchiolitis, viral wheeze, reactive airways disease, wheeze bronchitis and all that. Front Pediatr. 2020;8:218. doi: 10.3389/fped.2020.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Padem N, Glick Robison R. The infant and toddler with wheezing. Allergy Asthma Proc. 2019;40:393–395. doi: 10.2500/aap.2019.40.4255. [DOI] [PubMed] [Google Scholar]
- 56.Nash AK, Auchtung TA, Wong MC, et al. The gut mycobiome of the Human Microbiome Project healthy cohort. Microbiome. 2017;5:153. doi: 10.1186/s40168-017-0373-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Steenhuijsen Piters WAA, Binkowska J, Bogaert D. Early life microbiota and respiratory tract infections. Cell Host Microbe. 2020;28:223–232. doi: 10.1016/j.chom.2020.07.004. [DOI] [PubMed] [Google Scholar]
- 58.Gorzelak MA, Gill SK, Tasnim N, Ahmadi-Vand Z, Jay M, Gibson DL. Methods for improving human gut microbiome data by reducing variability through sample processing and storage of stool. PLoS One. 2015;10 doi: 10.1371/journal.pone.0134802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dorsaz S, Charretier Y, Girard M, et al. Changes in microbiota profiles after prolonged frozen storage of stool suspensions. Front Cell Infect Microbiol. 2020;10:77. doi: 10.3389/fcimb.2020.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Johnson JS, Spakowicz DJ, Hong B-Y, et al. Evaluation of 16S rRNA gene sequencing for species and strain-level microbiome analysis. Nat Commun. 2019;10 doi: 10.1038/s41467-019-13036-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Almeida A, Mitchell AL, Tarkowska A, Finn RD. Benchmarking taxonomic assignments based on 16S rRNA gene profiling of the microbiota from commonly sampled environments. Gigascience. 2018;7 doi: 10.1093/gigascience/giy054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McDonald D, Price MN, Goodrich J, et al. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J. 2012;6:610–618. doi: 10.1038/ismej.2011.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Parks DH, Chuvochina M, Waite DW, et al. A standardized bacterial taxonomy based on genome phylogeny substantially revises the tree of life. Nat Biotechnol. 2018;36:996–1004. doi: 10.1038/nbt.4229. [DOI] [PubMed] [Google Scholar]
- 64.Ruiz L, Delgado S, Ruas-Madiedo P, Sánchez B, Margolles A. Bifidobacteria and their molecular communication with the immune system. Front Microbiol. 2017;8 doi: 10.3389/fmicb.2017.02345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Neville BA, Forster SC, Lawley TD. Commensal Koch's postulates: establishing causation in human microbiota research. Curr Opin Microbiol. 2018;42:47–52. doi: 10.1016/j.mib.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 66.Vonaesch P, Anderson M, Sansonetti PJ. Pathogens, microbiome and the host: emergence of the ecological Koch's postulates. FEMS Microbiol Rev. 2018;42:273–292. doi: 10.1093/femsre/fuy003. [DOI] [PubMed] [Google Scholar]
- 67.Lipkin WI. The changing face of pathogen discovery and surveillance. Nat Rev Microbiol. 2013;11:133–141. doi: 10.1038/nrmicro2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brooks JP, Edwards DJ, Harwich MD, Jr, et al. The truth about metagenomics: quantifying and counteracting bias in 16S rRNA studies. BMC Microbiol. 2015;15:66. doi: 10.1186/s12866-015-0351-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wei X, Jiang P, Liu J, Sun R, Zhu L. Association between probiotic supplementation and asthma incidence in infants: a meta-analysis of randomized controlled trials. J Asthma. 2020;57:167–178. doi: 10.1080/02770903.2018.1561893. [DOI] [PubMed] [Google Scholar]
- 70.Hao Q, Dong BR, Wu T. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database Syst Rev. 2015;2 doi: 10.1002/14651858.CD006895.pub3. [DOI] [PubMed] [Google Scholar]
- 71.Chan CKY, Tao J, Chan OS, Li HB, Pang H. Preventing respiratory tract infections by synbiotic interventions: a systematic review and meta-analysis of randomized controlled trials. Adv Nutr. 2020;11:979–988. doi: 10.1093/advances/nmaa003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mukhopadhya I, Segal JP, Carding SR, Hart AL, Hold GL. The gut virome: the ‘missing link’ between gut bacteria and host immunity? Therap Adv Gastroenterol. 2019;12 doi: 10.1177/1756284819836620. 1756284819836620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Grier A, McDavid A, Wang B, et al. Neonatal gut and respiratory microbiota: coordinated development through time and space. Microbiome. 2018;6:193. doi: 10.1186/s40168-018-0566-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.