Abstract
Many inborn errors of immunity (IEI) manifest with hallmarks of both immunodeficiency and immune dysregulation due to uncontrolled immune responses and impaired immune homeostasis. A subgroup of these disorders frequently presents with autoimmunity and lymphoproliferation (ALPID phenotype). After the initial description of the genetic basis of autoimmune lymphoproliferative syndrome (ALPS) more than 20 years ago, progress in genetics has helped to identify many more genetic conditions underlying this ALPID phenotype. Among these, the majority is caused by a group of autosomal-dominant conditions including CTLA-4 haploinsufficiency, STAT3 gain-of-function disease, activated PI3 kinase syndrome, and NF-κB1 haploinsufficiency. Even within a defined genetic condition, ALPID patients may present with staggering clinical heterogeneity, which makes diagnosis and management a challenge. In this review, we discuss the pathophysiology, clinical presentation, approaches to diagnosis, and conventional as well as targeted therapy of the most common ALPID conditions.
Keywords: Autoimmune lymphoproliferative immunodeficiencies, Inborn errors of immunity, Immune dysregulation, Pathogenesis, Targeted therapy
Introduction
Lymphoproliferation and autoimmunity are relevant manifestations of immuno-hematological diseases. While lymphadenopathy or autoimmune cytopenia in isolation are relatively common and, in most cases, due to secondary causes, the combination of these manifestations raises suspicion for a genetic disease, in particular if associated with other warning signs of an inborn error of immunity (IEI) [1]. These warning signs include clinical manifestations such as increased susceptibility to infection, additional autoimmune manifestations, lymphoma, and indicators raising the likelihood of a genetic disease such as a positive family history, consanguinity or syndromal manifestations, and immunological abnormalities upon laboratory screening. Diagnosis of an underlying IEI is important because of its consequences for prognosis and therapy.
Immune responses are highly dynamic and require tight regulation of proliferation and cell death to maintain homeostasis. Impaired immune homeostasis leads to uncontrolled proliferation of immune cells, manifesting as benign or malignant lymphoproliferation in the form of lymphadenopathy, proliferation of mucosa-associated lymphoid tissue, and hepato- or splenomegaly. Uncontrolled, overactive adaptive immune response may also lead to autoimmunity of almost any organ, and favor immune exhaustion or senescence, paradoxically leading to increased infection susceptibility. It is therefore no surprise that many IEI manifest with hallmarks of both immunodeficiency and autoimmune-lymphoproliferative manifestations [2]. Autoimmune lymphoproliferative syndrome (ALPS) caused by mutations in the genes responsible for the extrinsic apoptotic pathway (FAS, FASLG, CASP10) was the first inherited disease presenting with autoimmunity and lymphoproliferation (autoimmune lymphoproliferative immunodeficiency (ALPID) phenotype) that could be linked to a genetic deficiency [3, 4]. However, ALPS could only explain a part of these cases (around 20%). In recent years, progress in genetics has helped to provide a molecular diagnosis to many more ALPID patients. Next to ALPS, several autosomal-dominant conditions have been identified, which explains another around 20% of cases. They include cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) haploinsufficiency [5, 6], signal transducer and activator of transcription 3 (STAT3) gain-of-function (GOF) disease [7], activated phosphoinositide 3-kinase δ syndrome (APDS) [8–11], and nuclear factor kB1 (NF-κB1) haploinsufficiency [12]. Patients with mutations in more than 50 rare autosomal-recessive genes can also present with an ALPID phenotype (around 10% of cases) [13]. Among these, lipopolysaccharide-responsive vesicle trafficking, beach- and anchor-containing (LRBA) deficiency is particularly notable because of its pathophysiological relationship to CTLA4 haploinsufficiency [14]. Despite all progress, however, half of the patients still cannot be assigned a molecular diagnosis.
In this review, we discuss the pathophysiological basis of the more frequent and mostly autosomal-dominant ALPID conditions and their phenotypic spectrum, as well as laboratory and immunological abnormalities, the approach to diagnosis, and targeted therapies.
Mechanisms of immune tolerance and homeostasis
The development of a functionally competent adaptive immune system involves the generation of a wide repertoire of B (BCR) and T cell receptors (TCR) during the maturation of B and T cells in the bone marrow and the thymus, respectively. A high percentage of TCRs and BCRs generated on maturing cells has been shown to be self-reactive by recognizing self-antigens. In the bone marrow, for instance, up to 75% of the antibodies generated by immature B cells have been reported to be self-reactive [15]. Depending on the lineage, cell clones with high BCR/TCR affinity for self-antigens may undergo clonal deletion via apoptosis, clonal anergy due to a lack of co-stimulation, clonal diversion to regulatory T cells, or secondary gene rearrangement (receptor editing) [16, 17]. A proportion of self-reactive T cells transforms in an interleukin-2 (IL-2)-dependent manner into CD25 + forkhead box protein P3 (FoxP3) + regulatory T (Treg) cells, which in turn are master effectors of peripheral tolerance [18].
To maintain peripheral immunological tolerance and prevent autoimmunity, the processes of anergy and apoptosis play an essential role. The two-step process of B and T cell activation is tightly regulated. Major histocompatibility complex (MHC) molecules on antigen-presenting cells (APCs) are recognized by TCR on naïve T cells (first signal), which in turn may lead to the formation of an immunological synapse favoring engagement of the co-stimulatory or cytokine receptors (second signal) (e.g., in T cells CD28 binds to the CD80/CD86 ligands on APCs) [19]. If this second signal is missing, a state of hyporesponsiveness (anergy) ensues. CTLA-4 expression on T reg cells can downregulate the CD80/CD86 ligands, thus leading to anergy [20, 21]. The CTLA-4-independent functions of Treg in the maintenance of peripheral tolerance include secretion of inhibitory cytokines and metabolic disruption [22]. Deletion of self-reactive or activated lymphocytes via apoptosis is another important mechanism in the maintenance of immune homeostasis and prevention of autoimmunity [23].
Autoimmune lymphoproliferative syndrome (ALPS)
Lymphocyte apoptosis contributes to peripheral immune homeostasis by regulating the pool size of certain lymphocyte populations in lymph nodes and spleen. Receptor-mediated apoptosis via the Fas signaling pathway is essential to control unique populations of mammalian targets of rapamycin (mTOR)-dependent hyperproliferative T and B cells, which contain autoreactive specificities. Fas (also called CD95, Fas antigen, Apo-1) is a trimeric receptor of the tumor necrosis factor-receptor (TNF-R) family found on a variety of cells including mature lymphocytes. Upon interaction with its ligand, FasL, the Fas receptor recruits the adaptor Fas-associated death domain (FADD) [24–26]. FADD in turn forms the so-called death-induced signaling complex (DISC) together with pro-caspase-8. Following activation, caspase-8 and caspase-10 then initiate apoptosis [24, 25]. Since apoptosis is induced in a controlled manner through the interaction of a death factor and its receptor, FasL/Fas-induced apoptosis is classified as activation-induced cell death (AICD) [27].
Mutations in the genes encoding modules of the Fas-FasL apoptotic pathway (FAS, FASLG, FADD, CASP10) lead to ALPS (Fig. 1) [28]. Research into ALPS as a non-malignant, non-infectious cause of uncontrolled lymphocyte proliferation and accompanying autoimmunity was initiated after the first description of dramatic lymphoproliferation in lpr strain mice with lupus-like phenotype [29] and the subsequent discovery of deleterious homozygous mutations in the murine FAS receptor [30]. Heterozygous FAS mutations affect the intracellular death domain of the protein, but still allow Fas expression on the cell surface, acting dominant-negative because they prevent trimerization and successful DISC formation. They can be either germline (ALPS-FAS) [3, 4] or somatic (ALPS-sFAS). Acquisition of a somatic mutation in hematopoietic progenitor cells leads to growth advantage for Fas-controlled T cells. They accumulate as CD3 + CD4-CD8-TCRab + double negative T cells (DNT) which are enriched for the disease-causing mutation [31, 32]. Isolation of DNA from sorted DNT therefore facilitates diagnosis of somatic variants. Dominant-negative somatic FAS mutations underlie around 15% of all ALPS cases [32].
Fig. 1.

Illustration of the Fas-FasL pathway. Fas (CD95) is a trimeric receptor of the tumor TNF-R family and after binding of the Fas ligand (FasL, CD95L), recruits the adaptor FADD, which in turn forms the so-called DISC together with pro-caspase-8 and pro-caspase-10. Following activation, caspase-8 and caspase-10 then initiate the extrinsic apoptotic pathway leading to proteolysis, DNA degradation, and apoptosis. Mutations in the genes responsible for the Fas-FasL signaling cascade (FAS, FASLG, FADD, CASP10) lead to the development of ALPS. Hallmarks of the disease are increased ALPS biomarkers (Vitamin B12 and sFasL), as well as a massive expansion of double-negative T cells (DNT). Increased AKT/mTOR activation leads to DNT hyperproliferation and can be inhibited via mTOR inhibitors such as sirolimus (rapamycin)
In contrast, heterozygous mutations completely abolishing Fas expression, mostly affecting the extracellular or transmembrane domain, do not act dominant-negative, but cause a 50% reduction of total protein expression [33]. These mutations have a very low penetrance unless combined with an additional, somatic “hit” in the second allele. This might be a somatic missense mutation leading to compound-heterozygous mutations in cells affected by the second “hit,” or loss of heterozygosity (LOH) via uniparental disomy (ALPS-FAS-sLOH) [34, 35]. These second genetic events usually occur in hematopoietic progenitors, but are enriched in DNT to which they confer a selective growth advantage. Rarely, biallelic germline mutations in FAS lead to the development of ALPS [33].
Most patients with ALPS due to a FAS mutation present at a young age (median age of onset 2, 7 years) with lymphadenopathy, splenomegaly, and autoimmunity, mainly but not limited to autoimmune cytopenia [36]. Patients also frequently show polyclonal hypergammaglobulinemia (IgG and IgA), but can also develop hypogammaglobulinemia. The carrier status of a deleterious FAS mutation does not necessarily lead to clinical manifestations, even if such individuals exhibit reduced apoptosis, an increased percentage of DNT, and elevated biomarkers such as sFasL and vitamin B12 [36]. A sometimes massive expansion of DNT is observed in most ALPS patients [4]. Fas-controlled DNT are highly proliferative and secrete high amounts of IL-10, sFASL, and the vitamin B12 carrier protein haptocorin. They can be delineated from conventional DNT by the marker combination CD38 and CD45RA, which are not co-expressed on any other known T cell subset [37]. sFASL and vitamin B12 are excellent diagnostic biomarkers for the disease with positive and negative predictive values above 90% [38–40]. FAS-deficient B cells show an increased propensity to develop into auto-reactive switched memory B cells [41]. The aberrant class-switching explains the hypergammaglobulinemia (IgG and/or IgA) and the reduction in IgM. At the same time, marginal zone B cells are reduced, which leads to an impaired anti-polysaccharide response [42]. In contrast to other ALPID, such as CTLA-4 haploinsufficiency or LRBA deficiency, tissue lymphocytosis in ALPS is mostly confined to the secondary lymphoid organs. On histopathological examination, follicular and paracortical hyperplasia of lymphoid organs with expansion of DNT cells is usually observed [43].
Only a few patients with disease-causing mutations in other components of the Fas pathway have been described in the literature. FAS ligand deficiency (ALPS-FASLG) follows an autosomal-recessive inheritance and presents clinically like homozygous ALPS-FAS [44–46]. No convincing disease association of heterozygous FASLG mutations has so far been described [47–49]. FADD deficiency also follows an autosomal-recessive mode of inheritance, but the clinical phenotype of this disease is more complex. While patients show variable lymphoproliferation/splenomegaly, they have also been reported to present with recurrent episodes of encephalopathy and invasive pneumococcal disease, as well as severe viral infections [50–52]. This points to additional Fas-independent effects in FADD deficiency. Similarly, CASP8 mutations cause a more complex combined immunodeficiency. Finally, CASP10 mutations were initially reported to be associated with ALPS, but the evidence is not convincing so far [53, 54].
Malignancies, mostly B and T cell lymphomas, and rarely non-lymphoid malignancies have been described in patients with ALPS-FAS with median onset in late adolescence or early adulthood [36, 55, 56]. Nonetheless, a long-term survival of ALPS-FAS was estimated to be about 85% by age 50 [36]. Interestingly, non-malignant lymphoproliferation and autoimmune manifestations such as cytopenia may spontaneously improve with age [36, 55].
CTLA-4 insufficiency and LRBA deficiency
Inhibitory receptors such as CTLA-4 play an important role in immune regulation and peripheral immunological tolerance by inhibiting immune cell activation [21]. CTLA-4 is upregulated on activated T cells and constitutively expressed on FoxP3 + Tregs [57, 58]. Upon T cell stimulation, endosomal CTLA-4 is transported to the cell surface, where it negatively impacts the immunological synapse by outcompeting CD28 for binding to the costimulatory ligands CD80/CD86. Moreover, CTLA4 downregulates costimulation by ripping out CD80/86 from the membrane via a process called trogocytosis or trans-endocytosis [59, 60]. While FoxP3 + Tregs limit autoimmunity, they are also enriched in tumors and impair anti-tumor immunity [61], which can be dramatically improved via immune checkpoint inhibition [62]. CTLA-4 checkpoint inhibitors are currently a part of the standard therapy regimen in many malignancies.
Heterozygous autosomal-dominant mutations in the CTLA4 gene in humans were first described in 2014 (Fig. 2). They can be associated with an ALPS-like condition with prominent immune dysregulation and lymphoproliferation [5, 6]. Age of onset can be in early childhood but is often in late adolescence; however, clinical penetrance is variable, since 30–40% of mutation carriers remain broadly asymptomatic. No correlation between specific mutations and the disease severity has been observed [6, 63]. In contrast to ALPS, the lymphocyte accumulation is not limited to secondary lymphoid organs: infiltrates can be seen in diverse organs with predominance of the intestine, brain, and lungs [63]. This is associated with various autoimmune complications including autoimmune cytopenias, enteropathy, thyroiditis, diabetes mellitus type 1, or autoimmune skin conditions [5, 6, 63]. This broad phenotype is explained by the important immune tolerance mechanisms mediated via CTLA-4. Patients with CTLA-4 haploinsufficiency patients frequently fulfill diagnostic criteria for common variable immunodeficiency (CVID) due to a marked decrease in switched memory B cells and hypogammaglobulinemia (IgA, IgG, and/or IgM). This is associated with increased infection susceptibility [63]. Interestingly, a subset of exhausted B cells including self-reactive specificities, called CD21low B cells is frequently also elevated in mutation carriers [5, 6, 63, 64], which highlights the importance of CTLA-4-mediated regulation of humoral immunity and prevention of autoantibody formation [65]. The percentage of FoxP3 + Treg cells is increased, even in asymptomatic mutation carriers [63].
Fig. 2.
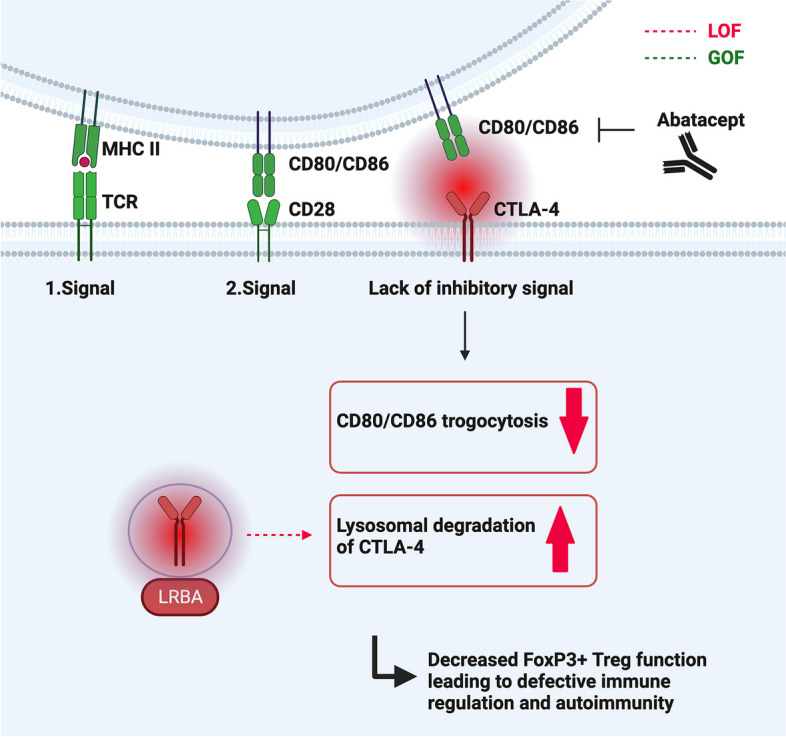
The two-step process of T cell activation. To counteract it, CTLA-4 in endosomes reaches the cell surface, outcompetes CD28, and binds and downregulates CD80/CD86 in a process called trogocytosis. LRBA acts intracellularly to stabilize and protect intracellular CTLA-4 from lysosomal degradation, thus maintaining the pool of available CTLA-4. Both in CTLA-4 haploinsufficiency and LRBA deficiency, lack of CTLA-4 (either due to decreased translation or increased lysosomal degradation) limits the function of regulatory T cells. Abatacept is a soluble CTLA-4 immunoglobulin fusion protein (Fc-region of human IgG1 linked to the extracellular domain of CTLA-4), which mimics CTLA-4 function and can be used successfully as a targeted therapy in both conditions
Functional CTLA-4 protein deficiency leading to impaired Treg function, but without mutations in the CTLA-4 gene, can be observed in LRBA deficiency [66]. The LRBA protein protects intracellular CTLA-4 from lysosomal degradation [66], maintaining the pool of available CTLA-4 protein prior to immune cell activation, thus acting indirectly in concert with CTLA-4 as an immune checkpoint. Hence, biallelic mutations in LRBA are associated with a phenotypically similar syndrome of immune dysregulation, lymphoproliferation, hypogammaglobulinemia, enteropathy, and increased infection susceptibility [14, 66]. The disease is more severe with onset in early childhood (median 2 years) and almost complete penetrance. Autoimmune manifestations are more common when compared to CTLA-4 haploinsufficiency [67–70]. The increased penetrance of LRBA deficiency might be due to the even lower total levels of the CTLA-4 protein than in CTLA-4 haploinsufficiency due to its increased lysosomal degradation [69]. In terms of immune phenotype, FoxP3 + Tregs, switched memory B cells and plasmablasts are usually reduced, while CD21low B cells are increased [67, 71].
There are no reliable biomarkers for the diagnosis of CTLA4 haploinsufficiency or LRBA deficiency, rendering genetic analysis the key diagnostic procedure. If mutations of unknown significance are detected, further diagnostic procedures may include LRBA protein expression via flow cytometry or Western blot, or CTLA-4 expression and CTLA-4-dependent trans-endocytosis of CD80 via Treg cells [68].
In a review of published CTLA-4 haploinsufficiency cases, autoimmunity and hypogammaglobulinemia preceded the development of malignancy, with a cumulative incidence of disease manifestations increasing up to 70% at age 40 [72]. In a cohort of 131 patients, 12.9% developed malignancy with a median onset between 32 and 34 years of age, most commonly lymphoma or gastric cancer, with EBV viremia posing a significant risk factor [73]. LRBA deficiency is a more severe disease, although the occurrence of cancer is less frequent [67, 70]. A current report by Tesch et al. showed a 50–60% probability of survival 15–20 years after disease onset, irrespective of the treatment modality [70].
Germline STAT3 gain-of-function (GOF)
STAT3 is a part of the family of STAT, and as such, a key transcription factor involved in the regulation of multiple immune activation and differentiation pathways [74]. STAT3 is activated by numerous cytokine receptors after binding of their ligand. They include the common gamma chain (IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21), the gp130 (e.g., IL-6, IL-11, IL-27), IL-10 (e.g., IL-10, IL-22), IL-12, IL-23, and interferon (IFNα, IFNβ, and IFNγ) receptor families, as well as receptors for macrophage and granulocyte colony-stimulating factors and hormones such as epidermal growth factor, growth hormone, or insulin-like growth factor. The binding of these cytokines to their specific receptor allows for the activation of an associated Janus Kinase (JAKs) and subsequent phosphorylation of the tyrosine residues on the intracellular domains of the receptor [75]. This in turn leads to the recruitment and phosphorylation of the STAT3 transcription regulator and its homo- or heterodimerization, translocation to the nucleus, and binding to STAT3-responsive DNA sequences which initiates transcription of cytokine-responsive genes and thus a variety of immune responses [75]. Unphosphorylated STAT3 also mediates a variety of non-canonical pathways, such as IL-6-mediated activation of NF-κB [76]. Suppressors of cytokine signaling 3 (SOCS3) and protein inhibitors of activated STAT3 (PIAS3) negatively regulate STAT3 function [77].
The essential function of STAT3 in maintaining immune homeostasis was first shown in hyper-immunoglobulin E syndrome (HIES), a severe immunodeficiency due to dominant-negative mutations in the STAT3 gene [78, 79]. Somatic activating (GOF) mutations in STAT3 were first described in relation to malignant lymphoproliferation and often cluster in the SH2 dimerization and activation domain of STAT3 [80, 81]. In contrast, germline STAT3 GOF mutations (Fig. 3) lead to an early-onset disease of poly-autoimmunity and lymphoproliferation and are found in all functional domains of the protein [7, 82–84].
Fig. 3.

Illustration of the JAK/STAT pathway. After binding to their receptor, cytokines such as IL-6 activate an associated Janus Kinase (JAK), which upon phosphorylation of its tyrosine residues recruits and phosphorylates the STAT3 transcription regulator. Phosphorylated STAT3 in turn forms homo- or heterodimers which translocate into the nucleus and impact the transcription of cytokine-responsive genes. In STAT3 GOF, the signaling pathway can lead to increased phosphorylation, altered dimer formation, as well as changes in gene expression. Targeting molecules which are part of the STAT3 pathway leads to improved STAT3 GOF disease control, e.g., disruption of the IL-6/IL-6R interaction via the anti-IL-6R monoclonal antibody tocilizumab. Another strategy is the inhibition of JAK by jakinibs such as ruxolitinib
Patients present with variable symptoms, such as lymphoproliferation/splenomegaly, autoimmune cytopenia, type I diabetes in infancy, and enteropathy, as well as short stature and increased susceptibility to viral and bacterial infections [7, 82, 83]. Early severe interstitial lung disease affects some patients, and systemic vasculopathy may also develop [84]. Of note, early-onset diabetes (< 2 years of age) is rarely seen in other IEI from the ALPID spectrum. The age of onset is early (2–3 years of age) with incomplete clinical penetrance and sequential development of disease manifestations [84, 85]. Jägle et al. characterized different STAT3 GOF mutations and clustered them in three groups depending on their molecular activation mechanisms, which correlated to some extent with the observed variable clinical penetrance [86]. Some of the disease manifestations, such as short stature and susceptibility to infection, can be explained by the effect of constitutive STAT3 activation on the activity of other STATs, e.g., decreased growth hormone-STAT5 and Interferon-STAT1 signaling, respectively [82, 83, 87]. The deficient STAT5-response explains the clinical overlap between STAT3 GOF and STAT5B loss-of-function (LOF)-associated disease [88].
The immune phenotype is not sufficiently characteristic for disease diagnosis. Hypogammaglobulinemia with reduced switched memory and increased CD21low B cells is a common feature, whereas reduced naive CD4 T cells, elevated CD57 + CD8, and DNT cells can be observed in some of STAT3 GOF patients [83, 84, 89]. Interestingly, STAT3 GOF mutations lead to reduced Treg populations, in line with the known suppressive effect of STAT3 activity on FoxP3 expression and Treg development [90, 91]. Decreased IL-2-mediated STAT5 signaling in STAT3 GOF may also contribute to the observed defect in Tregs [90, 92]. There is no gold standard for functional analyses of STAT3 mutations, but most frequently, the mutant allele is expressed in a STAT3-deficient cell line followed by a luciferase reporter assay to measure its transcriptional activity [86].
In contrast to other disorders of the ALPID spectrum, malignancy seems to occur less frequently in patients with activating germline STAT3 mutations: in a cohort of 191 patients, 12 developed cancer, most commonly marginal zone B cell and LGL lymphomas [84]. STAT3 GOF patients usually present with very early-onset endocrine and gastrointestinal manifestations, which may be diagnostic clues for the disease, followed by lymphoproliferation, autoimmune cytopenia, and interstitial lung disease [84, 85]. Early diagnosis is essential for survival, since severe cases with enteropathy, autoimmune hepatitis, and oxygen dependence are associated with early death [84].
NF-κB1 haploinsufficiency
NF-κB transcription factors play an essential role as regulators of the innate and adaptive immunity. The NF-κB family consists of NF-κB1 (p50 and its precursor p105), NF-κB2 (p52 and its precursor p100), RelA (p65), c-Rel, and RelB [93–95]. These proteins share a Rel homology domain for DNA binding and dimerization, with a variety of dimers (the two canonical are p50:p65 and p52:RelB) forming in the cytosol [93–95]. At rest, NF-κB dimers are bound to inhibitory IκB proteins in the cytoplasm. Degradation of the IκB proteins occurs through phosphorylation by the IκB kinase (IKK) complex consisting of the catalytically active IKKα and IKKβ, and the regulatory subunit IKKγ (NEMO), and leads to the release of bound NF-κB dimers, which then translocate to the nucleus [93–95]. The canonical NF-κB1 pathway can be activated by a variety of signals from receptors, such as the tumor necrosis factor receptor (TNF-R), antigen, and pattern-recognition receptors (PRR). After IKKβ and NEMO-dependent degradation of IκB, p65-containing heterodimers translocate into the nucleus, where they regulate gene expression [93]. While increased activation of the NF-κB pathways is associated with malignancy [96], loss-of-function mutations affecting NF-κB signaling may lead to a variety of immunodeficiencies [97].
Heterozygous loss-of-function mutations in NFKB1 associated with reduced protein levels of the p105 and/or p50 subunit cause a complex immunodeficiency (Fig. 4), frequently associated with lymphoproliferation and autoimmune manifestations [12, 98–100]. Age of onset and clinical penetrance are highly variable, with symptoms developing between 6 months and 79 years (median 12 years) of age [100]. In a report by Tuijnenburg et al. about 40% of mutation carriers remained asymptomatic, even though p50 expression was reduced in all carriers [98]. Patients with NF-κB1 haploinsufficiency present most commonly with respiratory infections, hypogammaglobulinemia, autoimmune cytopenia, and organ-specific autoimmunity, as well as lymphoproliferation [12, 98, 100–102]. Necrotizing fasciitis is a rare, but severe and characteristic manifestation of the disease. In some rare cases, episodes of severe autoinflammation with increased production of IL-1 and TNF were observed [99].
Fig. 4.

Illustration of the NF-κB pathway. The NF-κB1 transcriptional factor (p50 and its precursor p105) is active upon dimerization (p50:p65) in the cytosol. At rest, NF-κB dimers are bound to inhibitory IκB proteins. After activation (here via PRR signaling), IκB proteins are phosphorylated by the IκB kinase (IKK) complex, which releases the NF-κB dimers. p65-containing heterodimers can then translocate into the nucleus and regulate gene expression. Heterozygous loss-of-function mutations in NFKB1 are associated with reduced protein levels of the p105 and/or p50 subunit and lead to the development of a complex immunodeficiency
In terms of immune phenotype, most mutation carriers showed reduced switched memory B cells [98–100]. Interestingly, increased CD21low B cells may differentiate between symptomatic and asymptomatic carriers [98]. Immunoglobulin class-switching is regulated by NF-κB through various mechanisms [103], e.g., expression of the gene AICD (activation-induced cytidine deaminase) [97, 104], which may account for the decreased switched memory B cells and hypogammaglobulinemia (IgM, IgA, and/or IgG) in NF-κB haploinsufficiency. Even though the B cell defect is more pronounced, some patients present with chronic viral infection due to functional impairment of T cell immunity, including reduced effector memory and Th17 cells, as well as impaired proliferative response [99, 101]. Increased activation of the inflammasome and IL-1 secretion led to severe autoinflammation in some patients [99]. Possible pathophysiological mechanisms include reduced activity of the NF-κB-p62-mitophagy regulatory loop [105], decreased p50:p50 homodimers [106], and increased binding between NF-κB1 and IKKγ [99]. Regarding functional analysis of NFKB1 mutations, there are no simple screening assays. The p105 and/or p50 levels in transfected cells can be measured via Western blot. Moreover, the mutant allele can be expressed in a cell line and transcriptional activity measured via a NF-κB1-responsive reporter assay [100].
NFKB1 is a tumor suppressor gene, and NF-κB1 haploinsufficiency has been shown to promote tumorigenesis in murine models [107]. Malignancy (T and B cell lymphomas as well as solid tumors) also occurs in human NF-κB1 haploinsufficient patients at a median age of diagnosis of 46 years [100]. Age of onset and disease manifestations including cancer are highly variable even within the same family [12, 98], with clinical penetrance increasing in an age-dependent manner [100]. In a cohort of 121 affected patients, death occurred at a median age of 52 years [100].
Activated phosphoinositide 3-kinase δ syndrome (APDS)
Class I phosphoinositide 3-kinases (PI3K) play an essential role in signal transduction through tyrosine kinase- and heterotrimeric G-protein-linked receptors. In the family of class I kinases, PI3Kδ activity is restricted to leukocytes and activated through a variety of receptors, such as cytokine, growth factor, and antigen receptors [108, 109]. PI3Kδ consists of a catalytic subunit (p110δ) and a regulatory subunit (p85α), which are encoded by the PIK3CD and PIK3R1 genes, respectively [109]. PI3Kδ is responsible for the generation of the second messenger molecule phosphatidylinositol 3,4,5-trisphosphate (PIP3) by phosphorylating its precursor phosphatidylinositol 4,5-bisphosphate (PIP2). Downstream PIP3 signaling is mediated by intracellular enzymes, such as the serine/threonine kinase AKT, which phosphorylates the FOXO transcription factors inactivating them, as well as regulators of the mTOR complex 1 (mTORC1), which is in turn activated [109].
Increased activity of the PI3Kδ pathway leads to an autosomal dominant primary immunodeficiency called APDS (Fig. 5). Two entities have been described: APDS1 due to heterozygous GOF mutations in the PIK3CD gene [8, 9] and APDS2 resulting from heterozygous LOF mutations in the PIK3R1 gene [10, 11], which are phenotypically similar due to an overall increase in PI3Kδ activity (“gain-of-PI3Kδ-activity” mutations). Compared to other ALPID conditions, APDS has higher clinical penetrance and lower genetic heterogeneity [110]. Thus, more than 90% of APDS1 patients carry the PIK3CD E1021K mutation. PIK3R1 encodes the subunits p85α, p55α, and p50α as alternative splicing products; thus, loss-of-function mutations may cause different splice variants and consequently affect PI3Kδ signaling and disease phenotype [111].
Fig. 5.
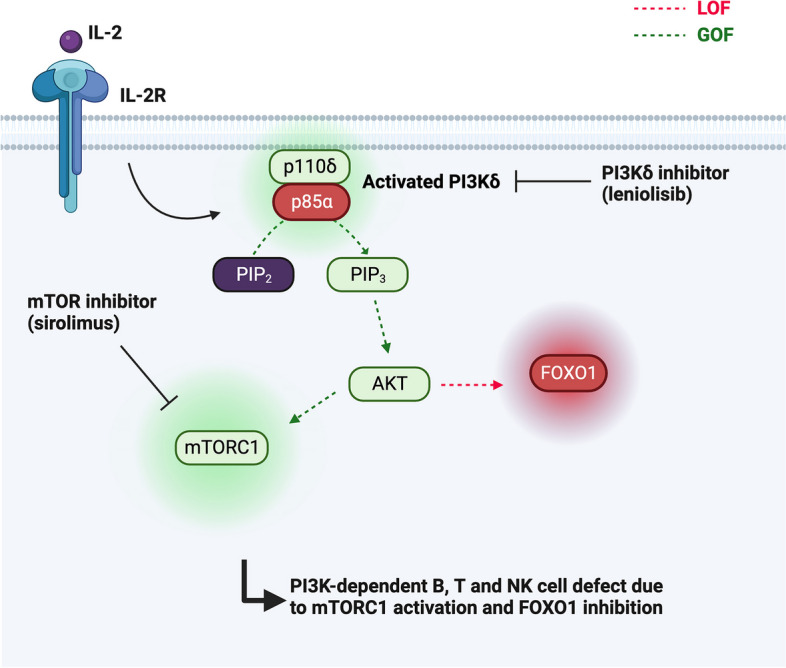
Illustration of the PI3Kδ pathway. PI3Kδ is activated through a variety of receptors (shown here is activation via IL-2 and its associated receptor). PI3Kδ typically consists of a catalytic subunit (p110δ) and a regulatory subunit (p85α) and leads to the generation of phosphatidylinositol 3,4,5-trisphosphate (PIP3) by phosphorylating its precursor phosphatidylinositol 4,5-bisphosphate (PIP2), both located in the cell membrane. Downstream PIP3 signaling is mediated by intracellular enzymes, such as the serine/threonine kinase AKT, which phosphorylates the FOXO transcription factors inactivating them, as well as regulators of the mTOR complex 1 (mTORC1), which is in turn activated. Increased activity of the PI3Kδ pathway leads to APDS. Patients with APDS are responsive to mTOR inhibition. More targeted approaches include selective PI3Kδ inhibitors, such as leniolisib or idealisib
A hallmark of APDS is recurrent respiratory tract infections as early as in the first year of life in almost all patients, as well as early-onset bronchiectasis (especially in APDS1) [110, 112–114]. Lymphadenopathy, hepatosplenomegaly, and recurrent/chronic herpesvirus infections, as well as a variety of autoimmune manifestations such as cytopenia, glomerulonephritis, primary sclerosing cholangitis, or inflammatory bowel disease can be observed [8, 10, 11, 112, 113, 115]. Some non-immunologic complications are developmental delay and growth impairment, particularly in APDS2 patients [112, 113]. The median age of onset is below 2 years of age [110].
Patients often have increased IgM and normal to decreased IgA and total IgG levels, which prior to the first description of APDS often led to the incorrect diagnosis of hyper IgM syndrome (HIGM) [116]. The observed hypogammaglobulinemia might be due to a PI3K-dependent B cell intrinsic defect (e.g., B cell survival [117]), extrinsic defect (e.g., increased AICD of T lymphocytes [8]), or a combination of both [11]. Moreover, increased PI3Kδ signaling has been shown to impair antibody maturation, since it actively suppresses class-switch recombination, e.g., through FOXO inactivation [118]. B cell counts in most patients are progressively decreasing with an expansion of transitional B cells and reduction of class-switched B cells [112, 119]. In terms of T cell phenotype, CD4 T cell counts are reduced, especially CD45RA + T cells [112]. mTORC1 signaling has been shown to differentially regulate the development of T effector and regulatory cells [120], with overactivation of the AKT/mTOR pathway leading to a burst in proliferation and accumulation of terminally differentiated and senescent effector T cells including expansion of CD57 + CD8 + T cells by various mechanisms, such as a metabolic bias towards glycolysis [121, 122]. Susceptibility to herpes infection, especially EBV [112, 123], was shown to develop due to reduced cytotoxicity of the exhausted/senescent CD8 + T and the abnormally differentiated NK cells [124, 125]. For functional testing, mutated p110δ or p85α can be expressed in a cell line and lipid kinase activity measured via a membrane capture assay [126]. A more common functional assay is the measurement of AKT and S6 phosphorylation, reflecting the activated PI3Kδ pathway [127].
Despite high phenotypic overlap, APDS1 patients more commonly present with bronchiectasis, splenomegaly, cytopenia, and skin disease, whereas APDS2 patients develop growth impairment and lymphoma more frequently [110]. Ten to 15% of patients developed malignant disease at a median age of 19 years, most commonly diffuse large B cell lymphoma (DLBCL) and classical Hodgkin lymphoma, with prior EBV infection posing a risk for malignant lymphoproliferation [110]. However, an even more important oncogenic driver is the increased PI3Kδ/AKT/mTOR signaling leading to B and T cell defects [128, 129]. In the largest cohort to date (the ESID APDS registry), death occurred at a median age of 18.5 years [110].
Treating ALPID: basic concepts and targeted therapies
Most patients with ALPID benefit from conventional PID treatments. Since they often present with hypogammaglobulinemia and/or respond poorly to vaccines, many are treated with immunoglobulin replacement therapy to correct for the secondary antibody deficiency [130]. If recurrent bacterial infections are present, this conventional therapy is often supplemented with prophylactic antibiotics with the aim of reducing infection susceptibility and secondary complications such as chronic lung inflammation and bronchiectasis.
Next to infection susceptibility, many inborn errors of immunity present with immune dysregulation [131]. The basic pillars of the treatment of autoimmunity and inflammation are steroids and other non-selective immunosuppressants, such as mycophenolate mofetil (MMF), azathioprine, and cyclosporine. Monoclonal antibodies (e.g., anti-TNF, anti-IL-17) are used for the treatment of inflammatory bowel disease or inflammatory skin disease. The use of these substances usually follows the standard recommendations, but the increased infection susceptibility of ALPID patients has to be carefully considered.
In recent years, targeted therapies have become available for several autoimmune-lymphoproliferative diseases. Rapamycin (sirolimus) is an mTOR inhibitor that can be considered a targeted therapy for ALPS patients [132]. mTOR inhibition targets DNT hyperproliferation and inappropriate survival of autoreactive B cells [133]. After the initial successful treatment of ALPS with rapamycin [134, 135], further studies could show rapid improvement of non-malignant lymphoproliferation and autoimmune cytopenia, which was mirrored in a decrease in DNT cells and ALPS biomarkers [133, 136]. Rapamycin is increasingly used as a first-line treatment for ALPS and shows amazing efficacy in this disease. It is often used as an immunosuppressive agent in CTLA-4 haploinsufficiency and LRBA deficiency where it can improve both lymphoproliferation and autoimmunity, especially enteropathy [70, 137].
Since mTOR signaling is activated downstream of PI3Kδ, patients with APDS also benefited from the use of rapamycin, especially in controlling benign lymphoproliferation. However, non-lymphoproliferative complications (cytopenia, enteropathy) are less responsive to mTOR inhibition [112–114, 138].
The development of selective PI3Kδ inhibitors, such as leniolisib or idealisib, allows for a specific inhibition of the hyperactive PI3Kδ pathway in APDS patients. A clinical trial of leniolisib (NCT02435173) or seletalisib (European Clinical Trials Database 2015–002900-10), oral inhibitors of the p110δ subunit, showed a decrease in lymphoproliferation and some improvement in autoimmune complications (cytopenia) [127, 139, 140]. Treatment was well tolerated with very little side effects. Notably, the treatment also resulted in the normalization of several features of the abnormal immune phenotype, allowing to stop immunoglobulin substitution in some patients.
Targeted treatment is also available for patients with CTLA-4 haploinsufficiency or LRBA deficiency. The soluble CTLA-4 immunoglobulin fusion protein abatacept consisting of the Fc-region of human IgG1 linked to the extracellular domain of CTLA-4 can mimic CTLA-4 function. It has been shown to successfully control lymphoproliferation and many autoimmune manifestations, such as cytopenia and enteropathy [70, 137, 141]. In a cohort of 123 CTLA-4 haploinsufficient patients, abatacept led to an improvement of interstitial lung disease and enteropathy with a response rate above 70% [137]. However, steroids continue to play an essential role in disease management and abatacept often needs to be combined with other immunosuppressive agents, especially if symptomatic lymphoid infiltrates are present. Moreover, abatacept may lead to viral reactivation; therefore, EBV and CMV viremia should be routinely monitored [63]. Data on long-term disease control under abatacept are still lacking.
Although hyperactive STAT3 cannot be targeted directly, other molecules which are part of the STAT3 pathway can be inhibited. Disruption of the IL-6/IL-6R interaction upstream of STAT3 via the anti-IL-6R monoclonal antibody tocilizumab was shown to partially improve autoimmunity [82, 85] by increasing FoxP3 + Treg cells [142]. However, small molecule inhibitors of JAK activation (jakinibs such as ruxolitinib) lead to an even more impressive control of autoimmunity and immune dysregulation, especially if initiated early [85, 143]. Whether Jakinib therapy represents a life-long treatment concept for STAT3 GOF disease remains to be evaluated.
For ALPID patients, hematopoietic stem cell transplantation (HSCT) is usually restricted to patients with early onset of disease, severe disease manifestations of critical organs (lung, brain), and insufficient response to treatment, including targeted therapies. The hyperactive signaling pathways, resulting in autoimmunity and hyperinflammation, frequently combined with persistent viral infections, poses a great challenge for successful stem cell transplantation [144]. Especially when conventional treatments fail, HSCT is the only long-term curative therapy. There have been multiple retrospective studies showing an improvement in disease manifestations, e.g., in APDS [145, 146], CTLA-4 haploinsufficient [137], LRBA deficient [70], and STAT3 GOF [84, 143] patients. In an initial cohort of 23 STAT3 GOF patients, overall survival was 62% [84]. Curative HSCT in patients with LRBA deficiency showed an overall survival rate of 70.8%, and all deaths could be attributed to a short-term transplant-related mortality [70]. Moreover, more than 2/3 (70.6%) of those patients required no further immunosuppressive therapy, which was in stark contrast to non-transplanted LRBA-deficient patients [70]. Similar results were reported regarding CTLA-4 haploinsufficiency: 13 out of 18 transplanted patients (72.2%) remained disease-free with only one requiring IRT [137]. Overall survival in APDS1 and APDS2 was 86% in a cohort of 57 patients, with poor graft performance being the most common complication [146]. Based on these retrospective data, primary HSCT is a potentially curative treatment option in patients presenting with an ALPID phenotype. However, additional data are needed to provide criteria for the selection of suitable patients and the right time point based on risk factor stratification.
Outlook: variable penetrance and somatic mutations
These examples illustrate the clinical and pathophysiological overlaps and differences of selected ALPID diseases. Even within a defined genetic condition, clinical heterogeneity makes diagnosis and management a challenge (Table 1). Moreover, many patients with similar clinical and immunological presentation remain without a genetic diagnosis, despite significant advances in next-generation sequencing (NGS), particularly whole exome sequencing (WES) [147]. Several recent examples have shown that somatic mutations providing a proliferative advantage to lymphocytes can lead to complex ALPID phenotypes. Genetic analysis must pay particular attention to such non-mendelian constellations, which require deeper sequencing efforts than currently offered by exome or genome analysis.
Table 1.
Comparison of the ALPID syndromes
| Disease | ALPS | CTLA-4 haploinsufficiency and LRBA deficiency | STAT3 GOF | NF-kB1 haploinsufficiency | APDS |
|---|---|---|---|---|---|
| Genes | FAS, FASLG, FADD | CTLA4, LRBA | STAT3 | NFKB1 | PIK3CD, PIK3R1 |
| Pattern of heredity | Variable inheritance, somatic variants (LOF) | CTLA4: autosomal-dominant, LRBA: autosomal-recessive (LOF) | Autosomal-dominant, somatic variants (GOF) | Autosomal-dominant (LOF) | Autosomal dominant, gain-of-PI3Kδ-activity |
| Genetic heterogeneity | High | High | High | High | Low (E1021K hotspot in PIK3CD—90% of cases) |
| Penetrance | Incomplete | CTLA-4: incomplete, LRBA: complete | Incomplete | Incomplete | Almost complete |
| Mechanism | Defective extrinsic apoptotic pathway | Lack of CTLA-4 and decreased Treg function | Increased STAT3-dependent transcription, decreased function of other STATs | Reduced levels of p105/p50 subunit | Increased activity of PI3Kδ with mTORC1 activation and FOXO1 inhibition |
| Age of onset | Variable, majority in childhood | CTLA-4: median 11 years, LRBA: infancy | Median 2,3 years | Highly variable, median 12 years | Median 1 year |
| Most common first symptom(s) | Lymphoproliferation | Immune dysregulation | Immune dysregulation | Infections | Infections |
| Autoimmunity | Cytopenia | Cytopenia, enteropathy, encephalitis | Cytopenia, enteropathy, diabetes, growth failure | Cytopenia, inflammatory disease | Cytopenia, enteropathy |
| Lymphoproliferation | Splenomegaly, lymphadenopathy + + | Splenomegaly, lymphadenopathy | Splenomegaly, lymphadenopathy | Splenomegaly, lymphadenopathy | Splenomegaly, lymphadenopathy + + + , mucosal lymphoid hyperplasia |
| Immunoglobulins | Hyper-IgG, 5–10% hypogammaglobulinemia | Frequent hypogammaglobulinemia | Sometimes hypogammaglobulinemia | Frequent hypogammaglobulinemia | Increased IgM, sometimes hypogammaglobulinemia |
| Recurrent infections | Not prominent | Yes, interstitial lung disease | Yes, interstitial lung disease | Yes | Yes, early-onset bronchiectasis |
| Immunological findings | Expansion of DNT, sometimes low class-switched B cells | Increased Tfh, increased CD21low, decreased class-switched B cells | Increased CD21low B cells, reduced Treg | Increased CD21low B cells, decreased T cell function | Increased transitional, reduced class-switched B cells, increased senescent T cells |
| Disease-specific assay | ALPS biomarkers | Trans-endocytosis assay | STAT3 reporter assay | NF-kB1 reporter assay | S6 phosphorylation |
| Targeted therapy | mTOR inhibitor | CTLA-4 fusion protein | JAK inhibitor, anti-IL-6R monoclonal antibody | - | PI3Kδ inhibitor |
Another fascinating research topic is the variable clinical penetrance of these mostly autosomal dominant conditions. While some cases may be explained by second-hit somatic mutations leading to clinical manifestation of the disease, other factors that need to be considered are other genetic or epigenetic factors or environmental factors such as microbiota or metabolic cues. A better understanding of penetrance factors may result in prophylactic measures and will allow better discussion of prognosis for affected patients.
All figures were created via BioRender.
Acknowledgements
We thank the CCI Advanced Diagnostic Unit, the FREEZE Biobank, the CCI Clinical Research Unit, the working group “Genetic disorders of T cell immunity,” and the team of the outpatient clinic and of the transplantation team for their engaged work for patients with inborn errors of immunity.
Abbreviations
- AICD
Activation-induced cytidine deaminase
- AICD
Activation-induced cell death
- ALPID
Autoimmune lymphoproliferative immunodeficiencies
- ALPS
Autoimmune lymphoproliferative syndrome
- APDS
Activated PI3Kδ syndrome
- APC
Antigen-presenting cells
- BCR
B cell receptor
- CD
Cluster of differentiation
- CTLA-4
Cytotoxic T lymphocyte-associated antigen-4
- CVID
Common variable immunodeficiency
- DISC
Death-induced signaling complex
- DNT
Double-negative T cells
- FADD
Fas-associated death domain
- FoxP3
Forkhead box P3
- GOF
Gain of function
- HIES
Hyper-immunoglobulin E syndrome
- HIGM
Hyper IgM syndrome
- HSCT
Hematopoietic stem cell transplantation
- IFN
Interferon
- Ig
Immunoglobulin
- IL
Interleukin
- IKK
IκB kinase
- JAK
Janus kinase
- LOF
Loss of function
- LOH
Loss of heterozygosity
- LRBA
Lipopolysaccharide-responsive vesicle trafficking, beach- and anchor-containing
- MHC
Major histocompatibility complex
- mTOR
Mammalian target of rapamycin
- mTORC1
MTOR complex 1
- NF-κB
Nuclear factor kB
- PI3K
Phosphoinositide 3-kinases
- PIAS3
Protein inhibitor of activated STAT3
- PIP2
Phosphatidylinositol 4,5-bisphosphate
- PIP3
Phosphatidylinositol 3,4,5-trisphosphate
- PRR
Pattern-recognition receptors
- sFasL
Soluble Fas ligand
- SOCS3
Suppressor of cytokine signaling 3
- STAT
Signal transducer and activator of transcription
- TCR
T cell receptor
- Tfh
T follicular helper cells
- TNF-R
Tumor necrosis factor-receptor
- Treg
Regulatory T cells
- WES
Whole exome sequencing
Authors’ contributions
V.T. drafted the manuscript and created the figures and table. S.E. edited the manuscript. Both authors read and approved the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was supported by the BMBF-funded German Auto-Immunity Network (GAIN), grant code 197 01GM1910A, and the Deutsche Forschungsgemeinschaft under Germany’s Excellence Strategy (CIBSS—EXC-21899—Project ID 390939984).
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Farmand S, Baumann U, von Bernuth H, et al. Interdisziplinäre AWMF-Leitlinie zur Diagnostik von primären Immundefekten (S2k) Klin Pädiatrie. 2011;223:378–385. doi: 10.1055/s-0031-1287835. [DOI] [PubMed] [Google Scholar]
- 2.Walter JE, Ayala IA, Milojevic D. Autoimmunity as a continuum in primary immunodeficiency. Curr Opin Pediatr. 2019;31:851–862. doi: 10.1097/mop.0000000000000833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rieux-Laucat F, Deist FL, Hivroz C, et al. Mutations in Fas associated with human lymphoproliferative syndrome and autoimmunity. Science. 1995;268:1347–1349. doi: 10.1126/science.7539157. [DOI] [PubMed] [Google Scholar]
- 4.Fisher GH, Rosenberg FJ, Straus SE, et al. Dominant interfering fas gene mutations impair apoptosis in a human autoimmune lymphoproliferative syndrome. Cell. 1995;81:935–946. doi: 10.1016/0092-8674(95)90013-6. [DOI] [PubMed] [Google Scholar]
- 5.Kuehn HS, Ouyang W, Lo B, et al. Immune dysregulation in human subjects with heterozygous germline mutations in CTLA4. Science. 2014;345:1623–1627. doi: 10.1126/science.1255904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schubert D, Bode C, Kenefeck R, et al. Autosomal dominant immune dysregulation syndrome in humans with CTLA4 mutations. Nat Med. 2014;20:1410–1416. doi: 10.1038/nm.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flanagan SE, Haapaniemi E, Russell MA, et al. Activating germline mutations in STAT3 cause early-onset multi-organ autoimmune disease. Nat Genet. 2014;46:812–814. doi: 10.1038/ng.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Angulo I, Vadas O, Garçon F, et al. Phosphoinositide 3-kinase δ gene mutation predisposes to respiratory infection and airway damage. Science. 2013;342:866–871. doi: 10.1126/science.1243292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lucas CL, Kuehn HS, Zhao F, et al. Dominant-activating germline mutations in the gene encoding the PI(3)K catalytic subunit p110δ result in T cell senescence and human immunodeficiency. Nat Immunol. 2014;15:88–97. doi: 10.1038/ni.2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lucas CL, Zhang Y, Venida A, et al. Heterozygous splice mutation in PIK3R1 causes human immunodeficiency with lymphoproliferation due to dominant activation of PI3K. J Exp Med. 2014;211:2537–2547. doi: 10.1084/jem.20141759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deau M-C, Heurtier L, Frange P, et al. A human immunodeficiency caused by mutations in the PIK3R1 gene. J Clin Investig. 2014;124:3923–3928. doi: 10.1172/jci75746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fliegauf M, Bryant VL, Frede N, et al. Haploinsufficiency of the NF-κB1 subunit p50 in common variable immunodeficiency. Am J Hum Genet. 2015;97:389–403. doi: 10.1016/j.ajhg.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.López-Nevado M, González-Granado LI, Ruiz-García R, et al. Primary immune regulatory disorders with an autoimmune lymphoproliferative syndrome-like phenotype: immunologic evaluation, early diagnosis and management. Front Immunol. 2021;12:671755. doi: 10.3389/fimmu.2021.671755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopez-Herrera G, Tampella G, Pan-Hammarström Q, et al. Deleterious mutations in LRBA are associated with a syndrome of immune deficiency and autoimmunity. Am J Hum Genetics. 2012;90:986–1001. doi: 10.1016/j.ajhg.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wardemann H, Yurasov S, Schaefer A, et al. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 16.Xing Y, Hogquist KA. T-cell tolerance: central and peripheral. Csh Perspect Biol. 2012;4:a006957. doi: 10.1101/cshperspect.a006957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pelanda R, Torres RM. Central B-cell tolerance: where selection begins. Csh Perspect Biol. 2012;4:a007146. doi: 10.1101/cshperspect.a007146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 19.June CH, Ledbetter JA, Linsley PS, Thompson CB. Role of the CD28 receptor in T-cell activation. Immunol Today. 1990;11:211–216. doi: 10.1016/0167-5699(90)90085-n. [DOI] [PubMed] [Google Scholar]
- 20.Wing K, Onishi Y, Prieto-Martin P, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 21.Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182:459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vignali DAA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rathmell JC, Thompson CB. Pathways of apoptosis in lymphocyte development, homeostasis, and disease. Cell. 2002;109:S97–S107. doi: 10.1016/s0092-8674(02)00704-3. [DOI] [PubMed] [Google Scholar]
- 24.Danial NN, Korsmeyer SJ. Cell death critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 25.Strasser A, Pellegrini M. T-lymphocyte death during shutdown of an immune response. Trends Immunol. 2004;25:610–615. doi: 10.1016/j.it.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 26.Chinnaiyan AM, O’Rourke K, Tewari M, Dixit VM. FADD, a novel death domain-containing protein, interacts with the death domain of fas and initiates apoptosis. Cell. 1995;81:505–512. doi: 10.1016/0092-8674(95)90071-3. [DOI] [PubMed] [Google Scholar]
- 27.Alderson MR, Tough TW, Davis-Smith T, et al. Fas ligand mediates activation-induced cell death in human T lymphocytes. J Exp Med. 1995;181:71–77. doi: 10.1084/jem.181.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Magerus A, Bercher-Brayer C, Rieux-Laucat F. The genetic landscape of the FAS pathway deficiencies. Biomed J. 2021;44:388–399. doi: 10.1016/j.bj.2021.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andrews BS, Eisenberg RA, Theofilopoulos AN, et al. Spontaneous murine lupus-like syndromes. Clinical and immunopathological manifestations in several strains. J Exp Medicine. 1978;148:1198–1215. doi: 10.1084/jem.148.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watanabe-Fukunaga R, Brannan CI, Copeland NG, et al. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992;356:314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- 31.Holzelova E, Vonarbourg C, Stolzenberg M-C, et al. Autoimmune lymphoproliferative syndrome with somatic fas mutations. New Engl J Med. 2004;351:1409–1418. doi: 10.1056/nejmoa040036. [DOI] [PubMed] [Google Scholar]
- 32.Dowdell KC, Niemela JE, Price S, et al. Somatic FAS mutations are common in patients with genetically undefined autoimmune lymphoproliferative syndrome. Blood. 2010;115:5164–5169. doi: 10.1182/blood-2010-01-263145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deist FL, Emile J-F, Rieux-Laucat F, et al. Clinical, immunological, and pathological consequences of Fas-deficient conditions. Lancet. 1996;348:719–723. doi: 10.1016/s0140-6736(96)02293-3. [DOI] [PubMed] [Google Scholar]
- 34.Magerus-Chatinet A, Neven B, Stolzenberg M-C, et al. Onset of autoimmune lymphoproliferative syndrome (ALPS) in humans as a consequence of genetic defect accumulation. J Clin Invest. 2011;121:106–112. doi: 10.1172/jci43752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roesler J, Izquierdo J-M, Ryser M, et al. Haploinsufficiency, rather than the effect of an excessive production of soluble CD95 (CD95ΔTM), is the basis for ALPS Ia in a family with duplicated 3′ splice site AG in CD95 intron 5 on one allele. Blood. 2005;106:1652–1659. doi: 10.1182/blood-2004-08-3104. [DOI] [PubMed] [Google Scholar]
- 36.Price S, Shaw PA, Seitz A, et al. Natural history of autoimmune lymphoproliferative syndrome associated with FAS gene mutations. Blood. 2014;123:1989–1999. doi: 10.1182/blood-2013-10-535393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maccari ME, Fuchs S, Kury P, et al. A distinct CD38+CD45RA+ population of CD4+, CD8+, and double-negative T cells is controlled by FAS. J Exp Med. 2020;218:e20192191. doi: 10.1084/jem.20192191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bowen RAR, Dowdell KC, Dale JK, et al. Elevated vitamin B12 levels in autoimmune lymphoproliferative syndrome attributable to elevated haptocorrin in lymphocytes. Clin Biochem. 2012;45:490–492. doi: 10.1016/j.clinbiochem.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Magerus-Chatinet A, Stolzenberg M-C, Loffredo MS, et al. FAS-L, IL-10, and double-negative CD4−CD8− TCR α/β+ T cells are reliable markers of autoimmune lymphoproliferative syndrome (ALPS) associated with FAS loss of function. Blood. 2009;113:3027–3030. doi: 10.1182/blood-2008-09-179630. [DOI] [PubMed] [Google Scholar]
- 40.Caminha I, Fleisher TA, Hornung RL, et al. Using biomarkers to predict the presence of FAS mutations in patients with features of the autoimmune lymphoproliferative syndrome. J Allergy Clin Immun. 2010;125:946–949.e6. doi: 10.1016/j.jaci.2009.12.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Janda A, Schwarz K, van der Burg M, et al. Disturbed B-lymphocyte selection in autoimmune lymphoproliferative syndrome. Blood. 2016;127:2193–2202. doi: 10.1182/blood-2015-04-642488. [DOI] [PubMed] [Google Scholar]
- 42.Neven B, Bruneau J, Stolzenberg M-C, et al. Defective anti-polysaccharide response and splenic marginal zone disorganization in ALPS patients. Blood. 2014;124:1597–1609. doi: 10.1182/blood-2014-02-553834. [DOI] [PubMed] [Google Scholar]
- 43.Lim MS, Straus SE, Dale JK, et al. Pathological findings in human autoimmune lymphoproliferative syndrome. Am J Pathology. 1998;153:1541–1550. doi: 10.1016/s0002-9440(10)65742-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruiz-García R, Mora S, Lozano-Sánchez G, et al. Decreased activation-induced cell death by EBV-transformed B-cells from a patient with autoimmune lymphoproliferative syndrome caused by a novel FASLG mutation. Pediatr Res. 2015;78:603–608. doi: 10.1038/pr.2015.170. [DOI] [PubMed] [Google Scholar]
- 45.Del-Rey M, Ruiz-Contreras J, Bosque A, et al. A homozygous Fas ligand gene mutation in a patient causes a new type of autoimmune lymphoproliferative syndrome. Blood. 2006;108:1306–1312. doi: 10.1182/blood-2006-04-015776. [DOI] [PubMed] [Google Scholar]
- 46.Magerus-Chatinet A, Stolzenberg M-C, Lanzarotti N, et al. Autoimmune lymphoproliferative syndrome caused by a homozygous null FAS ligand (FASLG) mutation. J Allergy Clin Immun. 2013;131:486–490. doi: 10.1016/j.jaci.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu J, Wilson J, He J, et al. Fas ligand mutation in a patient with systemic lupus erythematosus and lymphoproliferative disease. J Clin Invest. 1996;98:1107–1113. doi: 10.1172/jci118892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bi LL, Pan G, Atkinson TP, et al. Dominant inhibition of Fas ligand-mediated apoptosis due to a heterozygous mutation associated with autoimmune lymphoproliferative syndrome (ALPS) Type Ib. Bmc Med Genet. 2007;8:41. doi: 10.1186/1471-2350-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maccari ME, Schneider P, Smulski CR, et al. Revisiting autoimmune lymphoproliferative syndrome caused by Fas ligand mutations. J Allergy Clin Immun. 2023 doi: 10.1016/j.jaci.2022.11.028. [DOI] [PubMed] [Google Scholar]
- 50.Bolze A, Byun M, McDonald D, et al. Whole-exome-sequencing-based discovery of human FADD deficiency. Am J Hum Genet. 2010;87:873–881. doi: 10.1016/j.ajhg.2010.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Savic S, Parry D, Carter C, et al. A new case of Fas-associated death domain protein deficiency and update on treatment outcomes. J Allergy Clin Immun. 2015;136:502–505.e4. doi: 10.1016/j.jaci.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 52.Kohn LA, Long JD, Trope EC, Kuo CY. Novel compound heterozygote variations in FADD identified to cause FAS-associated protein with death domain deficiency. J Clin Immunol. 2020;40:658–661. doi: 10.1007/s10875-020-00779-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Puck JM, Zhu S. Immune disorders caused by defects in the caspase cascade. Curr Allergy Asthm R. 2003;3:378–384. doi: 10.1007/s11882-003-0070-1. [DOI] [PubMed] [Google Scholar]
- 54.Zhu S, Hsu AP, Vacek MM, et al. Genetic alterations in caspase-10 may be causative or protective in autoimmune lymphoproliferative syndrome. Hum Genet. 2006;119:284–294. doi: 10.1007/s00439-006-0138-9. [DOI] [PubMed] [Google Scholar]
- 55.Neven B, Magerus-Chatinet A, Florkin B, et al. A survey of 90 patients with autoimmune lymphoproliferative syndrome related to TNFRSF6 mutation. Blood. 2011;118:4798–4807. doi: 10.1182/blood-2011-04-347641. [DOI] [PubMed] [Google Scholar]
- 56.Straus SE, Jaffe ES, Puck JM, et al. The development of lymphomas in families with autoimmune lymphoproliferative syndrome with germline Fas mutations and defective lymphocyte apoptosis. Blood. 2001;98:194–200. doi: 10.1182/blood.v98.1.194. [DOI] [PubMed] [Google Scholar]
- 57.Takahashi T, Tagami T, Yamazaki S, et al. Immunologic self-tolerance maintained by Cd25+Cd4+regulatory T cells constitutively expressing cytotoxic T lymphocyte–associated antigen 4. J Exp Med. 2000;192:303–310. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Waterhouse P, Penninger JM, Timms E, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 59.Linsley PS, Bradshaw J, Greene J, et al. Intracellular trafficking of CTLA-4 and focal localization towards sites of TCR engagement. Immunity. 1996;4:535–543. doi: 10.1016/s1074-7613(00)80480-x. [DOI] [PubMed] [Google Scholar]
- 60.Qureshi OS, Zheng Y, Nakamura K, et al. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332:600–603. doi: 10.1126/science.1202947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shimizu J, Yamazaki S, Sakaguchi S. Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J Immunol. 1999;163:5211–5218. doi: 10.4049/jimmunol.163.10.5211. [DOI] [PubMed] [Google Scholar]
- 62.Kwon ED, Hurwitz AA, Foster BA, et al. Manipulation of T cell costimulatory and inhibitory signals for immunotherapy of prostate cancer. Proc National Acad Sci. 1997;94:8099–8103. doi: 10.1073/pnas.94.15.8099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schwab C, Gabrysch A, Olbrich P, et al. Phenotype, penetrance, and treatment of 133 cytotoxic T-lymphocyte antigen 4–insufficient subjects. J Allergy Clin Immun. 2018;142:1932–1946. doi: 10.1016/j.jaci.2018.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Warnatz K, Denz A, Dräger R, et al. Severe deficiency of switched memory B cells (CD27+IgM−IgD−) in subgroups of patients with common variable immunodeficiency: a new approach to classify a heterogeneous disease. Blood. 2002;99:1544–1551. doi: 10.1182/blood.v99.5.1544. [DOI] [PubMed] [Google Scholar]
- 65.Lo B, Abdel-Motal UM. Lessons from CTLA-4 deficiency and checkpoint inhibition. Curr Opin Immunol. 2017;49:14–19. doi: 10.1016/j.coi.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 66.Lo B, Zhang K, Lu W, et al. Patients with LRBA deficiency show CTLA4 loss and immune dysregulation responsive to abatacept therapy. Science. 2015;349:436–440. doi: 10.1126/science.aaa1663. [DOI] [PubMed] [Google Scholar]
- 67.Gámez-Díaz L, August D, Stepensky P, et al. The extended phenotype of LPS-responsive beige-like anchor protein (LRBA) deficiency. J Allergy Clin Immun. 2016;137:223–230. doi: 10.1016/j.jaci.2015.09.025. [DOI] [PubMed] [Google Scholar]
- 68.Hou TZ, Verma N, Wanders J, et al. Identifying functional defects in patients with immune dysregulation due to LRBA and CTLA-4 mutations. Blood. 2017;129:1458–1468. doi: 10.1182/blood-2016-10-745174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lo B, Fritz JM, Su HC, et al. CHAI and LATAIE: new genetic diseases of CTLA-4 checkpoint insufficiency. Blood. 2016;128:1037–1042. doi: 10.1182/blood-2016-04-712612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tesch VK, Abolhassani H, Shadur B, et al. Long-term outcome of LRBA deficiency in 76 patients after various treatment modalities as evaluated by the immune deficiency and dysregulation activity (IDDA) score. J Allergy Clin Immunol. 2020;145:1452–1463. doi: 10.1016/j.jaci.2019.12.896. [DOI] [PubMed] [Google Scholar]
- 71.Charbonnier L-M, Janssen E, Chou J, et al. Regulatory T-cell deficiency and immune dysregulation, polyendocrinopathy, enteropathy, X-linked–like disorder caused by loss-of-function mutations in LRBA. J Allergy Clin Immun. 2015;135:217–227.e9. doi: 10.1016/j.jaci.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hoshino A, Toyofuku E, Mitsuiki N, et al. Clinical courses of IKAROS and CTLA4 deficiencies: a systematic literature review and retrospective longitudinal study. Front Immunol. 2022;12:784901. doi: 10.3389/fimmu.2021.784901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Egg D, Schwab C, Gabrysch A, et al. Increased risk for malignancies in 131 affected CTLA4 mutation carriers. Front Immunol. 2018;9:2012. doi: 10.3389/fimmu.2018.02012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Morris R, Kershaw NJ, Babon JJ. The molecular details of cytokine signaling via the JAK/STAT pathway. Protein Sci. 2018;27:1984–2009. doi: 10.1002/pro.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hillmer EJ, Zhang H, Li HS, Watowich SS. STAT3 signaling in immunity. Cytokine Growth F R. 2016;31:1–15. doi: 10.1016/j.cytogfr.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang J, Liao X, Agarwal MK, et al. Unphosphorylated STAT3 accumulates in response to IL-6 and activates transcription by binding to NFκB. Gene Dev. 2007;21:1396–1408. doi: 10.1101/gad.1553707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Carow B, Rottenberg ME. SOCS3, a major regulator of infection and inflammation. Front Immunol. 2014;5:58. doi: 10.3389/fimmu.2014.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Holland MS, Frank D, Houda E, et al. STAT3 mutations in the hyper-IgE syndrome. New Engl J Med. 2007;357:1608–1619. doi: 10.1056/nejmoa073687. [DOI] [PubMed] [Google Scholar]
- 79.Minegishi Y, Saito M, Tsuchiya S, et al. Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome. Nature. 2007;448:1058–1062. doi: 10.1038/nature06096. [DOI] [PubMed] [Google Scholar]
- 80.Jerez A, Clemente MJ, Makishima H, et al. STAT3 mutations unify the pathogenesis of chronic lymphoproliferative disorders of NK cells and T-cell large granular lymphocyte leukemia. Blood. 2012;120:3048–3057. doi: 10.1182/blood-2012-06-435297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kh LM, Samuli E, Pekka E, et al. Somatic STAT3 mutations in large granular lymphocytic leukemia. New Engl J Med. 2012;366:1905–1913. doi: 10.1056/nejmoa1114885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Milner JD, Vogel TP, Forbes L, et al. Early-onset lymphoproliferation and autoimmunity caused by germline STAT3 gain-of-function mutations. Blood. 2015;125:591–599. doi: 10.1182/blood-2014-09-602763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Haapaniemi EM, Kaustio M, Rajala HLM, et al. Autoimmunity, hypogammaglobulinemia, lymphoproliferation, and mycobacterial disease in patients with activating mutations in STAT3. Blood. 2015;125:639–648. doi: 10.1182/blood-2014-04-570101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Leiding JW, Vogel TP, Santarlas VGJ, et al. Monogenic early-onset lymphoproliferation and autoimmunity: natural history of STAT3 gain-of-function syndrome. J Allergy Clin Immun. 2023;151:1081–1095. doi: 10.1016/j.jaci.2022.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fabre A, Marchal S, Barlogis V, et al. Clinical aspects of STAT3 gain-of-function germline mutations: a systematic review. J Allergy Clin Immunol Pract. 2019;7:1958–1969.e9. doi: 10.1016/j.jaip.2019.02.018. [DOI] [PubMed] [Google Scholar]
- 86.Jägle S, Heeg M, Grün S, et al. Distinct molecular response patterns of activating STAT3 mutations associate with penetrance of lymphoproliferation and autoimmunity. Clin Immunol. 2020;210:108316. doi: 10.1016/j.clim.2019.108316. [DOI] [PubMed] [Google Scholar]
- 87.Gutiérrez M, Scaglia P, Keselman A, et al. Partial growth hormone insensitivity and dysregulatory immune disease associated with de novo germline activating STAT3 mutations. Mol Cell Endocrinol. 2018;473:166–177. doi: 10.1016/j.mce.2018.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kanai T, Jenks J, Nadeau KC. The STAT5b pathway defect and autoimmunity. Front Immunol. 2012;3:234. doi: 10.3389/fimmu.2012.00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vogel TP, Milner JD, Cooper MA. The Ying and Yang of STAT3 in human disease. J Clin Immunol. 2015;35:615–623. doi: 10.1007/s10875-015-0187-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yao Z, Kanno Y, Kerenyi M, et al. Nonredundant roles for Stat5a/b in directly regulating Foxp3. Blood. 2007;109:4368–4375. doi: 10.1182/blood-2006-11-055756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xu L, Kitani A, Stuelten C, et al. Positive and negative transcriptional regulation of the Foxp3 gene is mediated by access and binding of the Smad3 protein to enhancer I. Immunity. 2010;33:313–325. doi: 10.1016/j.immuni.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Burchill MA, Yang J, Vogtenhuber C, et al. IL-2 Receptor β-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J Immunol. 2007;178:280–290. doi: 10.4049/jimmunol.178.1.280. [DOI] [PubMed] [Google Scholar]
- 93.Hayden MS, Ghosh S. Signaling to NF-κB. Genes Dev. 2004;18:2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 94.Oeckinghaus A, Hayden MS, Ghosh S. Crosstalk in NF-κB signaling pathways. Nat Immunol. 2011;12:695–708. doi: 10.1038/ni.2065. [DOI] [PubMed] [Google Scholar]
- 95.Vallabhapurapu S, Karin M. Regulation and function of NF-κB transcription factors in the immune system. Immunology. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- 96.Gasparini C, Celeghini C, Monasta L, Zauli G. NF-κB pathways in hematological malignancies. Cell Mol Life Sci. 2014;71:2083–2102. doi: 10.1007/s00018-013-1545-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang Q, Lenardo MJ, Baltimore D. 30 years of NF-κB: a blossoming of relevance to human pathobiology. Cell. 2017;168:37–57. doi: 10.1016/j.cell.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tuijnenburg P, Allen HL, Burns SO, et al. Loss-of-function nuclear factor κB subunit 1 (NFKB1) variants are the most common monogenic cause of common variable immunodeficiency in Europeans. J Allergy Clin Immun. 2018;142:1285–1296. doi: 10.1016/j.jaci.2018.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kaustio M, Haapaniemi E, Göös H, et al. Damaging heterozygous mutations in NFKB1 lead to diverse immunologic phenotypes. J Allergy Clin Immunol. 2017;140:782–796. doi: 10.1016/j.jaci.2016.10.054. [DOI] [PubMed] [Google Scholar]
- 100.Lorenzini T, Fliegauf M, Klammer N, et al. Characterization of the clinical and immunologic phenotype and management of 157 individuals with 56 distinct heterozygous NFKB1 mutations. J Allergy Clin Immunol. 2020;146:901–911. doi: 10.1016/j.jaci.2019.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Boztug H, Hirschmugl T, Holter W, et al. NF-κB1 haploinsufficiency causing immunodeficiency and EBV-driven lymphoproliferation. J Clin Immunol. 2016;36:533–540. doi: 10.1007/s10875-016-0306-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Maffucci P, Filion CA, Boisson B, et al. Genetic diagnosis using whole exome sequencing in common variable immunodeficiency. Front Immunol. 2016;7:220. doi: 10.3389/fimmu.2016.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Snapper CM, Zelazowski P, Rosas FR, et al. (1996) B cells from p50/NF-kappa B knockout mice have selective defects in proliferation, differentiation, germ-line CH transcription, and Ig class switching. J Immunol Baltim Md. 1950;156:183–191. [PubMed] [Google Scholar]
- 104.Revy P, Muto T, Levy Y, et al. Activation-induced cytidine deaminase (AID) deficiency causes the autosomal recessive form of the hyper-IgM syndrome (HIGM2) Cell. 2000;102:565–575. doi: 10.1016/s0092-8674(00)00079-9. [DOI] [PubMed] [Google Scholar]
- 105.Zhong Z, Umemura A, Sanchez-Lopez E, et al. NF-κB restricts inflammasome activation via elimination of damaged mitochondria. Cell. 2016;164:896–910. doi: 10.1016/j.cell.2015.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cao S, Zhang X, Edwards JP, Mosser DM. NF-κB1 (p50) Homodimers differentially regulate pro- and anti-inflammatory cytokines in macrophages*. J Biol Chem. 2006;281:26041–26050. doi: 10.1074/jbc.m602222200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Voce DJ, Schmitt AM, Uppal A, et al. Nfkb1 is a haploinsufficient DNA damage-specific tumor suppressor. Oncogene. 2015;34:2807–2813. doi: 10.1038/onc.2014.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chantry D, Vojtek A, Kashishian A, et al. p110δ, a novel phosphatidylinositol 3-kinase catalytic subunit that associates with p85 and is expressed predominantly in leukocytes*. J Biol Chem. 1997;272:19236–19241. doi: 10.1074/jbc.272.31.19236. [DOI] [PubMed] [Google Scholar]
- 109.Okkenhaug K. Signaling by the phosphoinositide 3-kinase family in immune cells. Annu Rev Immunol. 2013;31:675–704. doi: 10.1146/annurev-immunol-032712-095946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Maccari ME, Wolkewitz M, Schwab C, et al. Activated phosphoinositide 3-kinase δ syndrome: update from the ESID Registry and comparison with other autoimmune-lymphoproliferative inborn errors of immunity. J Allergy Clin Immunol. 2023 doi: 10.1016/j.jaci.2023.06.015. [DOI] [PubMed] [Google Scholar]
- 111.García-Morato MB, García-Miñaúr S, Garicano JM, et al. Mutations in PIK3R1 can lead to APDS2, SHORT syndrome or a combination of the two. Clin Immunol. 2017;179:77–80. doi: 10.1016/j.clim.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 112.Coulter TI, Chandra A, Bacon CM, et al. Clinical spectrum and features of activated phosphoinositide 3-kinase δ syndrome: a large patient cohort study. J Allergy Clin Immun. 2017;139:597–606.e4. doi: 10.1016/j.jaci.2016.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Elkaim E, Neven B, Bruneau J, et al. Clinical and immunologic phenotype associated with activated phosphoinositide 3-kinase δ syndrome 2: a cohort study. J Allergy Clin Immunol. 2016;138:210–218.e9. doi: 10.1016/j.jaci.2016.03.022. [DOI] [PubMed] [Google Scholar]
- 114.Maccari ME, Abolhassani H, Aghamohammadi A, et al. Disease evolution and response to rapamycin in activated phosphoinositide 3-kinase δ syndrome: the European Society for Immunodeficiencies-activated phosphoinositide 3-kinase δ syndrome registry. Front Immunol. 2018;9:543. doi: 10.3389/fimmu.2018.00543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hartman HN, Niemela J, Hintermeyer MK, et al. Gain of function mutations of PIK3CD as a cause of primary sclerosing cholangitis. J Clin Immunol. 2015;35:11–14. doi: 10.1007/s10875-014-0109-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Crank MC, Grossman JK, Moir S, et al. Mutations in PIK3CD can cause hyper IgM syndrome (HIGM) associated with increased cancer susceptibility. J Clin Immunol. 2014;34:272–276. doi: 10.1007/s10875-014-0012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Srinivasan L, Sasaki Y, Calado DP, et al. PI3 kinase signals BCR-dependent mature B cell survival. Cell. 2009;139:573–586. doi: 10.1016/j.cell.2009.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Omori SA, Cato MH, Anzelon-Mills A, et al. Regulation of class-switch recombination and plasma cell differentiation by phosphatidylinositol 3-kinase signaling. Immunity. 2006;25:545–557. doi: 10.1016/j.immuni.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 119.Jamee M, Moniri S, Zaki-Dizaji M, et al. Clinical, immunological, and genetic features in patients with activated PI3Kδ syndrome (APDS): a systematic review. Clin Rev Allergy Immunol. 2020;59:323–333. doi: 10.1007/s12016-019-08738-9. [DOI] [PubMed] [Google Scholar]
- 120.Delgoffe GM, Kole TP, Zheng Y, et al. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30:832–844. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Araki K, Turner AP, Shaffer VO, et al. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Xu X, Ye L, Araki K, Ahmed R. mTOR, linking metabolism and immunity. Semin Immunol. 2012;24:429–435. doi: 10.1016/j.smim.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Carpier J-M, Lucas CL. Epstein-Barr virus susceptibility in activated PI3Kδ syndrome (APDS) immunodeficiency. Front Immunol. 2018;8:2005. doi: 10.3389/fimmu.2017.02005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Edwards ESJ, Bier J, Cole TS, et al. Activating PIK3CD mutations impair human cytotoxic lymphocyte differentiation and function and EBV immunity. J Allergy Clin Immunol. 2019;143:276–291.e6. doi: 10.1016/j.jaci.2018.04.030. [DOI] [PubMed] [Google Scholar]
- 125.Ruiz-García R, Vargas-Hernández A, Chinn IK, et al. Mutations in PI3K110δ cause impaired natural killer cell function partially rescued by rapamycin treatment. J Allergy Clin Immunol. 2018;142:605–617.e7. doi: 10.1016/j.jaci.2017.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Knight ZA, Feldman ME, Balla A, et al. A membrane capture assay for lipid kinase activity. Nat Protoc. 2007;2:2459–2466. doi: 10.1038/nprot.2007.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rao VK, Webster S, Dalm VASH, et al. Effective “activated PI3Kδ syndrome”–targeted therapy with the PI3Kδ inhibitor leniolisib. Blood. 2017;130:2307–2316. doi: 10.1182/blood-2017-08-801191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhao L, Vogt PK. Class I PI3K in oncogenic cellular transformation. Oncogene. 2008;27:5486–5496. doi: 10.1038/onc.2008.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Xu Z-Z, Xia Z-G, Wang A-H, et al. Activation of the PI3K/AKT/mTOR pathway in diffuse large B cell lymphoma: clinical significance and inhibitory effect of rituximab. Ann Hematol. 2013;92:1351–1358. doi: 10.1007/s00277-013-1770-9. [DOI] [PubMed] [Google Scholar]
- 130.Bonilla FA, Barlan I, Chapel H, et al. International Consensus Document (ICON): common variable immunodeficiency disorders. J Allergy Clin Immunol In Pract. 2016;4:38–59. doi: 10.1016/j.jaip.2015.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fischer A, Provot J, Jais J-P, et al. Autoimmune and inflammatory manifestations occur frequently in patients with primary immunodeficiencies. J Allergy Clin Immun. 2017;140:1388–1393.e8. doi: 10.1016/j.jaci.2016.12.978. [DOI] [PubMed] [Google Scholar]
- 132.Majewski M, Korecka M, Kossev P, et al. The immunosuppressive macrolide RAD inhibits growth of human Epstein-Barr virus-transformed B lymphocytes in vitro and in vivo: A potential approach to prevention and treatment of posttransplant lymphoproliferative disorders. Proc National Acad Sci. 2000;97:4285–4290. doi: 10.1073/pnas.080068597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Völkl S, Rensing-Ehl A, Allgäuer A, et al. Hyperactive mTOR pathway promotes lymphoproliferation and abnormal differentiation in autoimmune lymphoproliferative syndrome. Blood. 2016;128:227–238. doi: 10.1182/blood-2015-11-685024. [DOI] [PubMed] [Google Scholar]
- 134.Teachey DT, Obzut DA, Axsom K, et al. Rapamycin improves lymphoproliferative disease in murine autoimmune lymphoproliferative syndrome (ALPS) Blood. 2006;108:1965–1971. doi: 10.1182/blood-2006-01-010124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Teachey DT, Greiner R, Seif A, et al. Treatment with sirolimus results in complete responses in patients with autoimmune lymphoproliferative syndrome. Br J Haematol. 2009;145:101–106. doi: 10.1111/j.1365-2141.2009.07595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Klemann C, Esquivel M, Magerus-Chatinet A, et al. Evolution of disease activity and biomarkers on and off rapamycin in 28 patients with autoimmune lymphoproliferative syndrome. Haematologica. 2017;102:e52–e56. doi: 10.3324/haematol.2016.153411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Egg D, Rump IC, Mitsuiki N, et al. Therapeutic options for CTLA-4 insufficiency. J Allergy Clin Immunol. 2022;149:736–746. doi: 10.1016/j.jaci.2021.04.039. [DOI] [PubMed] [Google Scholar]
- 138.Olbrich P, Lorenz M, Daball PC, et al. Activated PI3Kδ syndrome type 2: Two patients, a novel mutation, and review of the literature. Pediatr Allergy Immu. 2016;27:640–644. doi: 10.1111/pai.12585. [DOI] [PubMed] [Google Scholar]
- 139.Rao VK, Webster S, Šedivá A, et al. Randomized, placebo-controlled, phase 3 trial of PI3Kδ inhibitor leniolisib for activated PI3Kδ syndrome. Blood. 2022;141:971–983. doi: 10.1182/blood.2022018546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Diaz N, Juarez M, Cancrini C, et al. Seletalisib for activated PI3Kδ syndromes: open-label phase 1b and extension studies. J Immunol. 2020;205:2979–2987. doi: 10.4049/jimmunol.2000326. [DOI] [PubMed] [Google Scholar]
- 141.Kiykim A, Ogulur I, Dursun E, et al. Abatacept as a long-term targeted therapy for LRBA deficiency. The J Allergy Clin Immunol In Pract. 2019;7:2790–2800.e15. doi: 10.1016/j.jaip.2019.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Khoury T, Molho-Pessach V, Ramot Y, et al. Tocilizumab promotes regulatory T-cell alleviation in STAT3 gain-of-function−associated multi-organ autoimmune syndrome. Clin Ther. 2017;39:444–449. doi: 10.1016/j.clinthera.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 143.Forbes LR, Vogel TP, Cooper MA, et al. Jakinibs for the treatment of immune dysregulation in patients with gain-of-function signal transducer and activator of transcription 1 (STAT1) or STAT3 mutations. J Allergy Clin Immunol. 2018;142:1665–1669. doi: 10.1016/j.jaci.2018.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lankester AC, Albert MH, Booth C, et al. EBMT/ESID inborn errors working party guidelines for hematopoietic stem cell transplantation for inborn errors of immunity. Bone Marrow Transplant. 2021;56:2052–2062. doi: 10.1038/s41409-021-01378-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Okano T, Imai K, Tsujita Y, et al. Hematopoietic stem cell transplantation for progressive combined immunodeficiency and lymphoproliferation in patients with activated phosphatidylinositol-3-OH kinase δ syndrome type 1. J Allergy Clin Immunol. 2019;143:266–275. doi: 10.1016/j.jaci.2018.04.032. [DOI] [PubMed] [Google Scholar]
- 146.Dimitrova D, Nademi Z, Maccari ME, et al. International retrospective study of allogeneic hematopoietic cell transplantation for activated PI3K-delta syndrome. J Allergy Clin Immunol. 2022;149:410–421.e7. doi: 10.1016/j.jaci.2021.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Conley ME, Casanova J-L. Discovery of single-gene inborn errors of immunity by next generation sequencing. Curr Opin Immunol. 2014;30:17–23. doi: 10.1016/j.coi.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


