Abstract
Objective:
To describe time trends in cancer incidence in people living with HIV (PLHIV) in Australia between 1982-2012.
Design:
Population-based prospective study using data linkage between the national HIV and cancer registries.
Methods:
Invasive cancers identified in PLHIV were grouped into: AIDS defining cancer (ADC), infection-related non-ADC (NADC), and non-infection related NADC. Crude and age-standardised incidence rates of cancers were calculated and compared over five time periods; 1982-1995, 1996-1999, 2000-2004, 2005-2008, 2009-2012, roughly reflecting advances in HIV antiretroviral therapy. Standardised incidence ratios (SIR) compared with the Australian general population were calculated for each time period. Generalised linear models were developed to assess time trends in crude and age-standardised incidence.
Results:
For ADC, crude and age-standardised incidence of Kaposi sarcoma and non-Hodgkin lymphoma substantially declined over time (p-trend<0.001 for all) but SIR remained significantly elevated. For infection-related NADC, there were significant increases in crude incidence of anal, liver and head and neck cancer. Age-standardised incidence increased for anal (p-trend=0.002) and liver cancer (p-trend<0.001). SIRs were significantly elevated for anal cancer, liver cancer and Hodgkin lymphoma. For non-infection related NADC, the crude incidence of colorectal, lung and prostate cancer increased over time, but age-standardised incidence remained stable.
Conclusions:
Continuous improvements and high coverage of antiretroviral therapy have reduced the incidence of ADC in PLHIV in Australia. Clinical monitoring of anal and liver cancer in people living with HIV should be performed given increasing incidence of these cancers.
Keywords: HIV, Cancer, Incidence, Data linkage, Australia
Introduction
Morbidity and mortality in people living with HIV (PLHIV) have dramatically declined since the advent of combination antiretroviral therapy (ART) in 1996(1). New classes of ART with greater potency and fewer side effects(2, 3) have since been introduced and reductions in AIDS-related complications in HIV populations have continued worldwide(4). The substantially improved survival of PLHIV has increased the size and average age of the population(5, 6) with consequent increases in the incidence of some age-related comorbidities(5).
Cancer is one of the major comorbidities in PLHIV. Improvements in ART potency and coverage and the reversal or prevention of severe immunodeficiency have reduced the incidence of AIDS-defining cancers (ADC) in PLHIV(7, 8). In contrast, the incidence of some infection related non-AIDS defining cancers (NADC) has increased(7, 8). This is likely a result of several factors including prior exposure to oncogenic viruses, the ageing of the HIV population(6), immune dysfunction prior to commencement of ART(9) and ongoing relative immune dysfunction(10). Changes in non-infection related NADC incidence have been less consistently observed, with heterogenous trends for individual cancers in different settings(7, 8, 11).
A previous study examining cancer trends in the Australian HIV-population between 1982 to 2004 showed a dramatic decrease in the incidence of ADC and that anal cancer had become the most common NADC(11). Since then, the Australian HIV population has aged further(12) and the great majority of HIV infected individuals are on treatment with undetectable viral loads(13). The improving immune function and ageing of the Australian HIV-population are likely to impact on cancer risk. Hence trends in cancer incidence require ongoing monitoring to inform health care policy and practice. This study aims to further examine the time trends of cancer incidence in PLHIV in Australia between 1982-2012.
Methods
Australia has routine nationwide data notification systems for both HIV and invasive cancers since 1982. We conducted a nationwide data linkage study to identify cancer diagnoses in PLHIV in Australia between 1982 and 2012. Ethics approvals for the study were granted by the Human Research Ethics Committees of the University of New South Wales, the Australian Institute of Health and Welfare and the health departments of all Australian States and Territories.
Study cohort
The study cohort included people aged 16 and above, diagnosed with HIV between 1982 and 2012 and registered on the Australian National HIV registry. The Australian National HIV registry includes all people notified with HIV in Australia and collects personal identifiers including; name code (first two letters of first and last name), date of birth, sex and postcode of residence at the time of diagnosis. Individuals who had missing information on both the first and last names in the name code were excluded from the study.
Cancer diagnosis
The Australian Cancer Database collects data on all invasive cancers diagnosed in Australian residents from 1982, except for non-melanoma skin cancer. The International Classification of Diseases revision 10 (ICD-10) was used to classify cancers except for Kaposi sarcoma, lymphoid and haemopoietic cancers, where the International Classification of Disease for Oncology revision 3 (ICD-O-3) classification was used. Kaposi sarcoma was defined by the ICD-O-3 code 9140 and the morphology code 8000 (unknown morphology).
HIV, cancer and death data matching
Incident cancer diagnoses were ascertained using probabilistic data linkage between the Australian Cancer Database and the Australian HIV registry using the above-mentioned personal identifiers. Probabilistic data linkages between the Australian National HIV registry and the National Death Index was also conducted to determine dates of death. The linkage algorithm has been described in detail in a previous study(11).
Statistics
Statistical analyses were conducted using Stata version 16 (StataCorp LP, College Station, Texas, USA). Crude cancer incidence (cases per 100,000-person-years) was determined for all cancers by dividing the number of confirmed cancer cases by total person-years accumulated. Age-standardised cancer incidence rates (cases per 100,000-person-years) were calculated using direct standardisation on the basis of sex-specific 5-year age groups. The 2001 Australian Census population was used as the standard population. For each specific cancer, follow up commenced 90 days after the date of HIV diagnosis to minimise the risk of detecting cancers that developed before HIV diagnosis, and ended at the earliest event of either the date of cancer diagnosis, date of death, or 31 December 2012. Therefore, people diagnosed with HIV within 90 days to 31 December 2012 were excluded from the analysis.
Standardised incidence ratio (SIR) and their 95% confidence intervals (CI) were determined for cancers with at least 10 incident cases during the study period. SIRs were calculated by dividing the number of observed cases in each of the five time periods by the expected based on the application of 5-year age group, sex and calendar year specific general Australian population cancer incidence rates, assuming a Poisson distribution. Kaposi sarcoma was the exception, for which the 1982 general population incidence rates were used due to the majority of Kaposi sarcoma cases after 1982 occurring within the HIV population.
Due to the close association between age and cancer risk(14), and the rapid change of the age structure in PLHIV in Australia, the overall and truncated crude and age-standardised cancer incidence and SIR of each cancer were reported. The crude and age standardised incidence and SIR for each cancer were stratified into three age categories; 15-34, 35-64 and >65 years.
Time trends
Crude and age-standardised incidence of cancers were compared over five time periods. These time periods represent different periods of ART treatment advances, including the pre-ART era (1982-1995), the early ART era (1996-1999), the widespread availability of co-formulated protease inhibitors (2000-2004), the advent of fusion inhibitors (2005-2008) and the introduction integrase inhibitors (2009-2012).
Generalised linear regression, assuming Gaussian distribution, was used to determine time trends of crude and age-standardised incidence rates over the five time periods. The cancers were grouped into three categories: 1. ADC, including Kaposi sarcoma and all non-Hodgkin lymphoma; 2. virally mediated infection-related non-AIDS defining cancers, including Hodgkin lymphoma (Epstein Barr virus), anal (human papillomavirus), liver (hepatitis B and C viruses) and head and neck cancer (human papillomavirus)(15); 3. Other NADCs, including lip, prostate, lung, stomach, colorectal cancer and melanoma. Overall time trends of crude anal cancer incidence and its associated SIRs in the Australian HIV-population have previously been reported(16).
Results
Between 1982 and 2012, 33561 individuals were notified to the Australian National HIV Registry. Among them, 4178 (12.4%) individuals were excluded from the analysis due to missing both first and last name codes. Almost all of the missing name codes (97.7%) occurred between 1982-1995, reflecting incomplete identifiers in a period when there were heightened concerns around HIV stigma and breach of confidentiality. A further 313 individuals were excluded after being diagnosed with HIV within 90 days from 31 December 2012, 367 individuals were excluded after dying within 90 days of their HIV diagnosis. A total of 28703 individuals were included in the analysis, with 26187 (91.2%) individuals being male. In over 78% of HIV cases, the likely mode of HIV was determined to be male to male sexual contact in each time period. The mean age at HIV diagnosis was 34.5 years (standard deviation: 12.0 years). The mean age of people living with HIV increased from 37.3 in 1982-1995 to 48.5 in 2009-2012 (p trend<0.001).
AIDS defining Cancer
During the study period, 1369 cases of Kaposi sarcoma and 917 cases of non-Hodgkin lymphoma in PLHIV were diagnosed (Supplementary Table 1).
After age standardisation, there were significant declines over the entire study period in overall incidence for Kaposi sarcoma (from 996.7 to 64.8 per 100 000, p-trend<0.001) and non-Hodgkin lymphoma (from 658.6 to 71.1 per 100 000, p-trend<0.001) (Table 1 Figure 1a). Continued declines in age-standardised incidence were observed since the 2000-2004 period for both Kaposi sarcoma (p-trend=0.050) and non-Hodgkin lymphoma (p-trend=0.034).
Table 1:
Age-standardised incidence of major cancers in people living with HIV in Australia between 1982 – 2012 (per 100,000 person-years) in three age groups, and overall.
| Cancer1 | Age Group | 1982-1995 | 1996-1999 | 2000-2004 | 2005-2008 | 2009-2012 | p-value* |
|---|---|---|---|---|---|---|---|
| AID defining cancers | |||||||
|
| |||||||
| Kaposi sarcoma | Overall | 996.70 | 305.20 | 123.85 | 63.04 | 64.79 | <0.001 |
| 15-34 | 791.77 | 295.36 | 98.84 | 36.59 | 48.73 | <0.001 | |
| 35-64 | 1220.95 | 323.92 | 109.62 | 86.93 | 71.16 | <0.001 | |
| 65- | 144.70 | 0.00 | 35.54 | 0.00 | 19.87 | 0.995 | |
|
| |||||||
| Non-Hodgkin lymphoma | Overall | 658.62 | 343.04 | 121.68 | 112.89 | 71.08 | <0.001 |
| 15-34 | 292.37 | 346.37 | 73.87 | 117.16 | 45.89 | 0.027 | |
| 35-64 | 770.93 | 362.86 | 152.35 | 101.65 | 91.62 | <0.001 | |
| 65- | 901.74 | 133.84 | 52.38 | 101.92 | 82.29 | 0.001 | |
|
| |||||||
| Infection related non-AID defining cancers | |||||||
|
| |||||||
| Anal cancer | Overall | 26.82 | 18.76 | 25.40 | 38.41 | 44.97 | 0.002 |
| 15-34 | 4.41 | 12.40 | 0.00 | 4.55 | 4.34 | 0.621 | |
| 35-64 | 24.49 | 16.99 | 41.13 | 41.69 | 66.44 | <0.001 | |
| 65- | 84.83 | 0.00 | 34.86 | 105.22 | 71.43 | 0.553 | |
|
| |||||||
| Hodgkin lymphoma | Overall | 13.63 | 26.58 | 16.14 | 29.23 | 23.39 | 0.314 |
| 15-34 | 10.48 | 18.20 | 5.90 | 9.11 | 4.35 | 0.222 | |
| 35-64 | 14.19 | 20.40 | 17.70 | 32.04 | 37.25 | <0.001 | |
| 65- | 0.00 | 45.27 | 34.62 | 41.38 | 24.14 | 0.550 | |
|
| |||||||
| Liver cancer | Overall | 2.00 | 4.04 | 9.06 | 8.96 | 14.79 | <0.001 |
| 15-34 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | N/A | |
| 35-64 | 4.13 | 8.35 | 13.32 | 18.53 | 26.65 | <0.001 | |
| 65- | 0.00 | 0.00 | 16.57 | 0.00 | 12.03 | 0.470 | |
|
| |||||||
| Head and neck cancer | Overall | 10.67 | 2.87 | 9.61 | 5.77 | 7.64 | 0.765 |
| 15-34 | 0.00 | 2.99 | 0.00 | 0.00 | 0.00 | N/A | |
| 35-64 | 22.05 | 3.72 | 14.43 | 11.93 | 15.79 | 0.839 | |
| 65- | 0.00 | 0.00 | 16.67 | 0.00 | 0.00 | N/A | |
|
| |||||||
| Non-infection related non-AID defining cancers | |||||||
|
| |||||||
| Lip cancer | Overall | 12.10 | 18.23 | 7.10 | 3.86 | 7.56 | 0.147 |
| 15-34 | 1.88 | 0.00 | 0.00 | 4.55 | 0.00 | 0.917 | |
| 35-64 | 22.07 | 22.86 | 14.68 | 4.60 | 9.58 | 0.006 | |
| 65- | 0.00 | 45.42 | 0.00 | 0.00 | 18.55 | 0.912 | |
|
| |||||||
| Lung cancer | Overall | 13.85 | 26.48 | 27.47 | 16.72 | 31.17 | 0.300 |
| 15-34 | 4.41 | 0.00 | 0.00 | 0.00 | 0.00 | N/A | |
| 35-64 | 9.88 | 39.82 | 29.04 | 27.09 | 39.96 | 0.227 | |
| 65- | 47.42 | 45.71 | 85.02 | 22.89 | 75.01 | 0.716 | |
|
| |||||||
| Colorectal cancer | Overall | 11.51 | 8.98 | 5.99 | 7.73 | 22.63 | 0.238 |
| 15-34 | 0.00 | 0.00 | 0.00 | 0.00 | 7.47 | N/A | |
| 35-64 | 8.27 | 3.72 | 12.38 | 12.22 | 19.97 | 0.003 | |
| 65- | 47.58 | 45.49 | 0.00 | 11.50 | 44.59 | 0525 | |
|
| |||||||
| Stomach cancer | Overall | 7.44 | 0.00 | 7.47 | 8.34 | 5.81 | 0.665 |
| 15-34 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | N/A | |
| 35-64 | 0.00 | 0.00 | 10.03 | 0.00 | 9.91 | 0.382 | |
| 65- | 47.10 | 0.00 | 16.58 | 52.81 | 6.45 | 0.736 | |
|
| |||||||
| Melanoma | Overall | 39.61 | 31.98 | 28.02 | 30.67 | 33.00 | 0.249 |
| 15-34 | 14.59 | 15.40 | 23.43 | 0.00 | 4.35 | 0.291 | |
| 35-64 | 57.25 | 42.09 | 21.48 | 32.09 | 37.33 | 0.142 | |
| 65- | 47.42 | 0.00 | 16.72 | 95.97 | 84.77 | 0.132 | |
|
| |||||||
| Prostate cancer | Overall | 49.72 | 35.47 | 24.28 | 66.39 | 39.44 | 0.853 |
| 15-34 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | N/A | |
| 35-64 | 59.00 | 16.43 | 5.48 | 44.66 | 50.17 | 0.869 | |
| 65- | 134.18 | 174.35 | 137.00 | 283.65 | 96.13 | 0.906 | |
Linear regression model p value for trend
ICD10/O-3 codes: Kaposi sarcoma 9140, 8000 if KS on AIDS Registry, non-Hodgkin lymphoma 9591 9596, 9670-9729, 9820-9837, 9940, 9948 and 9590 if ICD10 C82-C85; Diffuse large B cell lymphoma 9680, 9684, 9678, 9679; Burkitt 9687, 9826; CNS lymphoma, non-Hodgkin lymphoma with C70-C72 topography; Hodgkin lymphoma 9650-9667; anus C21; Head and Neck cancer C01-C10, excluding C07-C08; liver C22; lip C00; melanoma c43; trachea, bronchus and lung C33-C34; colorectal C18-C20; prostate C61
Figure 1:
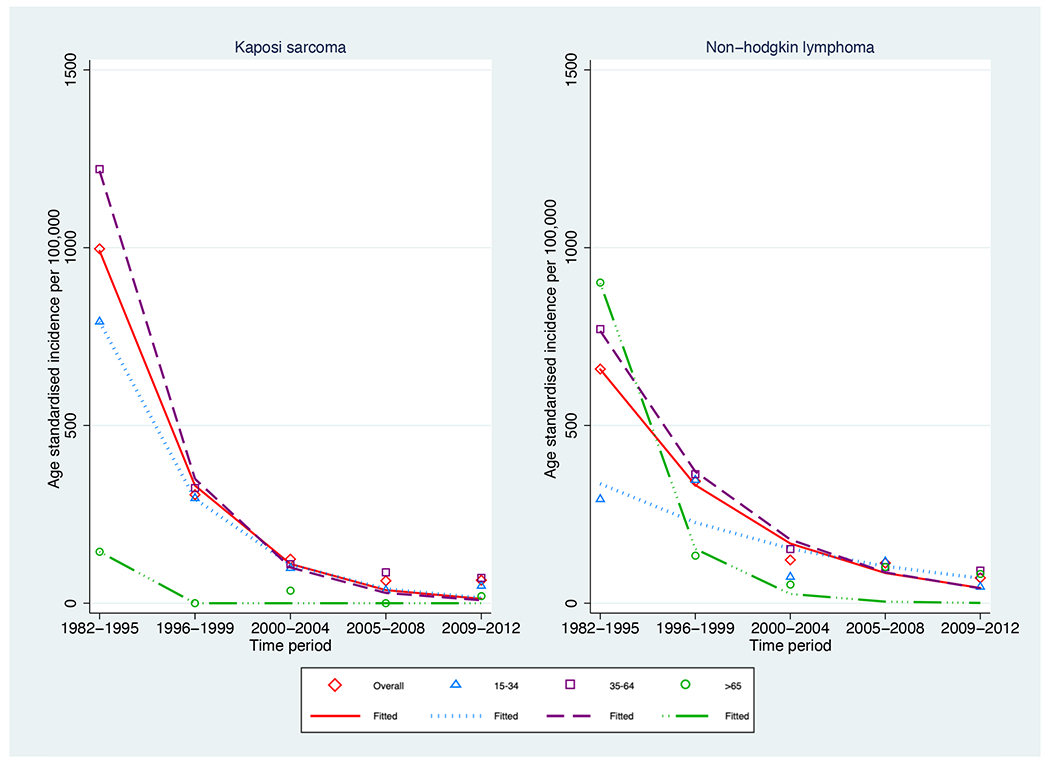
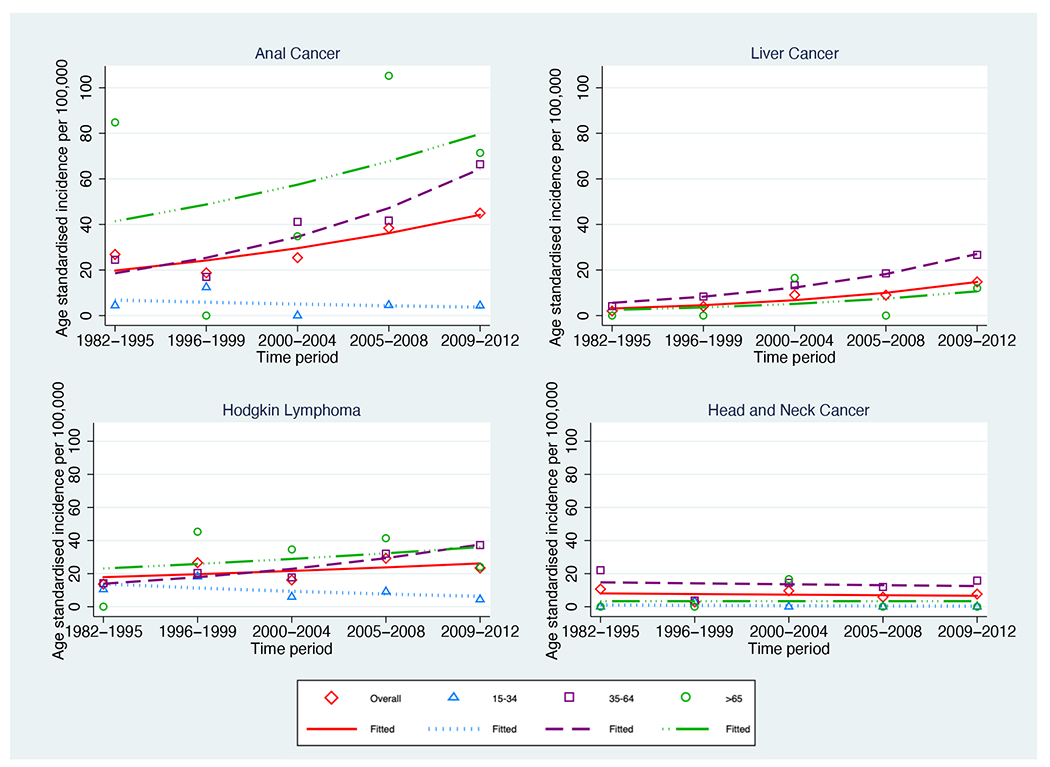
Age-standardised incidence per 100,000 of cancer in the Australian HIV population between 1982-2012 stratified by age group a) AIDS defining cancers b) infection related non-AIDS defining cancers
Similar to age standardised rates, overall crude incidence for both Kaposi sarcoma (p-trend<0.001) and non-Hodgkin lymphoma (p-trend<0.001) declined over time (Supplementary Table 1).
SIRs decreased over time for both Kaposi sarcoma (from 19024.5 to 454.5) and non-Hodgkin lymphoma (from 64.8 to 3.3), but remained significantly greater than one at all time periods (Table 2). For each time period, for both cancers, SIRs were much higher in the younger age groups.
Table 2:
Standardised incidence ratio of major cancers in people living with HIV in Australia between 1982 – 2012 in 3 age groups and overall
| Cancer1 | Age group | 1982 – 1995 | 1996 – 1999 | 2000 – 2004 | 2005 – 2008 | 2009 - 2012 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SIR | 95%CI | SIR | 95%CI | SIR | 95%CI | SIR | 95%CI | SIR | 95%CI | ||
| AID defining cancers | |||||||||||
|
| |||||||||||
| Kaposi sarcoma | Overall | 19024.47 | 17860.02-20244.91 | 3826.70 | 3273.07-4447.15 | 1027.30 | 822.83 - 1257.17 | 617.39 | 471.14-794.71 | 454.45 | 345.08 - 587.48 |
| 15-34 | 42000.00 | 38078.81 - 46215.37 | 6100.00 | 4666.02–7835.71 | 2000.00 | 1221.65–3088.83 | 600.00 | 220.19–1305.95 | 900.00 | 411.54 – 1708.48 | |
| 35-64 | 11682.17 | 10743.50 - 12680.89 | 2668.84 | 2191.39-3219.41 | 859.22 | 663.13 - 1095.15 | 641.28 | 481.75 - 836.73 | 447.76 | 329.00 - 595.43 | |
| 65 - | 890.05 | 183.55 - 2601.10 | 0.00 | 0.00 - 1029.58 | 221.29 | 26.80 - 799.38 | 0.00 | 0.00 - 294.27 | 88.25 | 10.69 - 318.81 | |
|
| |||||||||||
| Non-Hodgkin lymphoma | Overall | 64.76 | 29.14 - 70.77 | 23.82 | 20.29 - 27.80 | 8.45 | 6.96 - 10.16 | 4.96 | 3.82 - 7.18 | 3.25 | 2.56 - 4.07 |
| 15-34 | 117.27 | 99.86 - 136.85 | 74.49 | 54.33 - 99.67 | 31.45 | 18.94 - 49.12 | 33.16 | 17.13 - 57.92 | 14.73 | 5.41 - 32.06 | |
| 35-64 | 58.08 | 51.89 - 64.81 | 21.73 | 17.93 - 26.11 | 8.82 | 7.10 - 10.82 | 5.27 | 4.04 - 6.75 | 4.07 | 3.14 - 5.20 | |
| 65 - | 10.72 | 4.31 - 22.09 | 2.20 | 0.27 - 7.95 | 1.23 | 0.25 - 3.58 | 1.31 | 0.43 - 3.07 | 0.83 | 0.30 - 1.80 | |
|
| |||||||||||
| Infection related non-AIDS defining cancers | |||||||||||
|
| |||||||||||
| Anal cancer | Overall | 43.03 | 21.48 - 76.99 | 33.71 | 15.42 - 64.00 | 33.39 | 21.39 - 49.68 | 31.76 | 21.58 - 45.08 | 37.71 | 28.33 - 49.21 |
| 15-34 | 148.73 | 30.67 - 434.66 | 178.18 | 21.58 - 643.66 | 0.00 | 0.00 - 225.47 | 141.45 | 3.58 - 788.12 | 107.19 | 2.71 - 597.23 | |
| 35-64 | 33.34 | 13.40 - 69.69 | 31.93 | 12.84 - 65.80 | 38.05 | 24.12 - 57.09 | 31.91 | 20.84 - 46.75 | 43.01 | 31.82 - 56.86 | |
| 65 - | 39.24 | 0.99 - 218.62 | 0.00 | 0.00 - 100.99 | 10.22 | 0.26 - 56.93 | 25.94 | 7.07 - 66.42 | 14.13 | 3.85 - 36.17 | |
|
| |||||||||||
| Hodgkin lymphoma | Overall | 5.88 | 3.04 - 10.26 | 10.98 | 6.01 - 18.43 | 7.42 | 4.06 - 12.45 | 9.90 | 6.13 - 15.14 | 9.58 | 6.31 - 13.93 |
| 15-34 | 6.12 | 2.25 - 13.33 | 11.31 | 3.67 - 26.40 | 4.96 | 0.60 - 17.93 | 3.17 | 0.08 - 17.65 | 2.54 | 0.06 - 14.13 | |
| 35-64 | 5.88 | 2.16 - 12.80 | 10.15 | 4.38 - 19.99 | 8.02 | 4.00 - 14.35 | 11.81 | 7.11 - 18.44 | 11.87 | 7.60 - 17.66 | |
| 65 - | 0.00 | 0.00 - 87.19 | 22.64 | 0.57 - 126.15 | 8.92 | 0.23 - 49.69 | 5.10 | 0.13 - 28.40 | 4.97 | 0.60 - 17.97 | |
|
| |||||||||||
| Liver cancer | Overall | 0.85 | 0.02 - 4.76 | 2.69 | 0.73 - 6.89 | 2.23 | 1.02 - 4.24 | 1.68 | 0.84 - 3.00 | 1.94 | 1.22 - 2.94 |
| 15-34 | 0.00 | 0.00 - 30.77 | 0.00 | 0.00 - 73.83 | 0.00 | 0.00 - 92.77 | 0.00 | 0.00 - 109.70 | 0.00 | 0.00 - 112.29 | |
| 35-64 | 1.14 | 0.03 - 6.34 | 3.60 | 0.98 - 9.21 | 2.65 | 1.14 - 5.22 | 2.25 | 1.12 - 4.03 | 2.59 | 1.60 - 3.96 | |
| 65 - | 0.00 | 9.00 - 21.61 | 0.00 | 0.00 - 11.34 | 1.03 | 0.03 - 5.73 | 0.00 | 0.00 - 2.24 | 0.31 | 0.01 - 1.74 | |
|
| |||||||||||
| Head and neck cancer | Overall | 0.78 | 0.16 - 2.29 | 0.84 | 0.173 - 2.45 | 1.13 | 0.49 - 2.22 | 0.74 | 0.30 - 1.53 | 0.74 | 0.37 - 1.32 |
| 15-34 | 0.00 | 0.00 - 22.62 | 11.94 | 0.30 - 66.64 | 0.00 | 0.00 - 38.98 | 0.00 | 0.00 - 58.83 | 0.00 | 0.00 - 40.17 | |
| 35-64 | 0.90 | 0.19 - 2.63 | 0.64 | 0.08 - 2.32 | 1.15 | 0.46 - 2.37 | 0.89 | 0.36 - 1.83 | 0.92 | 0.46 - 1.64 | |
| 65 - | 0.00 | 0.00 - 11.23 | 0.00 | 0.00 - 9.53 | 1.07 | 0.03 - 5.94 | 0.00 | 0.00 - 2.49 | 0.00 | 0.00 - 1.30 | |
|
| |||||||||||
| Non-infection related non-AIDS defining cancers | |||||||||||
|
| |||||||||||
| Lip cancer | Overall | 1.81 | 0.83 - 3.44 | 1.75 | 0.70 - 3.60 | 1.32 | 0.57 - 2.61 | 0.73 | 0.20 - 1.86 | 1.04 | 0.45 - 2.05 |
| 15-34 | 1.07 | 0.03 - 5.94 | 0.00 | 0.00 - 8.87 | 0.00 | 0.00 - 9.86 | 5.51 | 0.14 - 30.72 | 0.00 | 0.00 - 24.64 | |
| 35-64 | 2.15 | 0.93 - 4.24 | 1.86 | 0.68 - 4.04 | 1.61 | 0.69 - 3.16 | 0.68 | 0.14 - 1.99 | 1.02 | 0.37 - 2.21 | |
| 65 - | 0.00 | 0.00-12.07 | 2.75 | 0.07 - 15.34 | 0.00 | 0.00 - 5.33 | 0.00 | 0.00 - 3.97 | 1.22 | 0.15 - 4.40 | |
|
| |||||||||||
| Lung cancer | Overall | 0.56 | 0.26 - 1.06 | 0.83 | 0.41 - 1.48 | 0.76 | 0.46 - 1.17 | 0.50 | 0.29 - 0.79 | 0.82 | 0.58 - 1.11 |
| 15-34 | 12.30 | 2.54 - 35.96 | 0.00 | 0.00 - 42.01 | 0.00 | 0.00 - 38.68 | 0.00 | 0.00 - 42.64 | 0.00 | 0.00 - 43.82 | |
| 35-64 | 0.41 | 0.13 - 0.96 | 1.10 | 0.53 - 2.03 | 0.96 | 0.55 - 1.56 | 0.76 | 0.42 - 1.25 | 1.24 | 0.84 - 1.76 | |
| 65 - | 0.28 | 0.01 - 1.54 | 0.24 | 0.01 - 1.35 | 0.42 | 0.11 - 1.07 | 0.14 | 0.02 - 0.50 | 0.38 | 0.27 - 0.72 | |
|
| |||||||||||
| Colorectal cancer | Overall | 0.16 | 0.03 - 0.47 | 0.11 | 0.01 - 0.39 | 0.18 | 0.07 - 0.37 | 0.16 | 0.07 - 0.32 | 0.30 | 0.19 - 0.46 |
| 15-34 | 0.00 | 0.00 - 4.52 | 0.00 | 0.00 - 8.76 | 0.00 | 0.00 - 7.48 | 0.00 | 0.00 - 9.70 | 1.90 | 0.05 - 10.58 | |
| 35-64 | 0.14 | 0.02 - 0.49 | 0.07 | 0.00 - 0.40 | 0.26 | 0.11 - 0.54 | 0.22 | 0.09 - 0.45 | 0.34 | 0.18 - 0.57 | |
| 65 - | 0.30 | 0.01 - 1.69 | 0.23 | 0.01 - 1.28 | 0.00 | 0.00 - 0.32 | 0.06 | 0.00 - 0.32 | 0.21 | 0.08 - 0.47 | |
|
| |||||||||||
| Stomach cancer | Overall | 0.23 | 0.01 - 1.28 | 0.00 | 0 - 1.07 | 0.91 | 0.33 - 2.00 | 0.26 | 0.03 - 0.93 | 0.72 | 0.310 - 1.42 |
| 15-34 | 0.00 | 0.00 - 17.16 | 0.00 | 0.00 - 49.06 | 0.00 | 0.00 - 45.09 | 0.00 | 0.00 - 71.54 | 0.00 | 0.00 - 71.77 | |
| 35-64 | 0.00 | 0.00 - 1.12 | 0.00 | 0.00 - 1.48 | 1.11 | 0.36 - 2.60 | 0.00 | 0.00 - 0.74 | 1.07 | 0.43 - 2.20 | |
| 65 - | 1.16 | 0.03 - 6.45 | 0.00 | 0.00 - 4.17 | 0.50 | 0.01 - 2.77 | 0.73 | 0.09 - 2.63 | 0.22 | 0.01 - 1.23 | |
|
| |||||||||||
| Melanoma | Overall | 0.80 | 0.50 - 1.23 | 0.85 | 0.52 - 1.31 | 0.41 | 0.25 - 0.65 | 0.46 | 0.29 - 0.69 | 0.54 | 0.37 - 0.74 |
| 15-34 | 0.80 | 0.26 - 1.86 | 1.07 | 0.22 - 3.12 | 1.86 | 0.60 - 4.33 | 0.00 | 0.00 - 2.22 | 0.65 | 0.02 - 3.61 | |
| 35-64 | 0.80 | 0.45 - 1.33 | 0.91 | 0.53 - 1.46 | 0.34 | 0.18 - 0.60 | 0.49 | 0.30 - 0.77 | 0.61 | 0.41 - 0.88 | |
| 65 - | 0.79 | 0.02 - 4.41 | 0.00 | 0 - 1.76 | 0.17 | 0 - 0.94 | 0.40 | 0.11 - 1.02 | 0.33 | 0.12 - 0.71 | |
|
| |||||||||||
| Prostate cancer | Overall | 0.86 | 0.39 - 1.63 | 0.25 | 0.08 - 0.58 | 0.15 | 0.07 - 0.29 | 0.29 | 0.20 - 0.40 | 0.26 | 0.19 - 0.34 |
| 15-34 | 0.00 | 0.00 - 322.11 | 0.00 | 0.00 - 936.17 | 0.00 | 0.00 - 1292.17 | 0.00 | 0.00 - 1608.34 | 0.00 | 0.00 - 448.15 | |
| 35-64 | 1.32 | 0.53 - 2.73 | 0.16 | 0.02 - 0.59 | 0.05 | 0.01 - 0.20 | 0.31 | 0.19 - 0.46 | 0.32 | 0.22 - 0.44 | |
| 65 - | 0.38 | 0.05 - 1.39 | 0.39 | 0.08 - 1.15 | 0.32 | 0.13 - 0.66 | 0.26 | 0.13 - 0.46 | 0.17 | 0.09 - 0.29 | |
SIR standardised incidence ratio, CI Confidence interval
ICD10/O-3 codes: Kaposi sarcoma 9140, 8000 if KS on AIDS Registry, non-Hodgkin lymphoma 9591 9596, 9670-9729, 9820-9837, 9940, 9948 and 9590 if ICD10 C82-C85; Diffuse large B cell lymphoma 9680, 9684, 9678, 9679; Burkitt 9687, 9826; CNS lymphoma, non-Hodgkin lymphoma with C70-C72 topography; Hodgkin lymphoma 9650-9667; anus C21; Head and Neck cancer C01-C10, excluding C07-C08; liver C22; lip C00; melanoma c43; trachea, bronchus and lung C33-C34; colorectal C18-C20; prostate C61
Non-AIDS Defining Cancers
Between 1982 and 2012, 710 NADC were diagnosed. For infection-related-NADC, 129 cases of anal cancer, 88 cases of Hodgkin lymphoma, 47 cases of liver cancer and 32 cases of head and neck cancer were diagnosed (Supplementary Table 1). For non-infection related NADC, 118 cases of melanoma, 105 cases of prostate cancer, 97 cases of lung cancer, 41 cases of colorectal cancer, 36 cases of lip cancer and 17 cases of stomach cancer were diagnosed (Supplementary Table 1).
Infection-related non-AIDS defining cancers incidence
Age-standardised incidence rates increased significantly for liver cancer (from 2.00 to 14.79 per 100,000 p-trend <0.001) (Table 1 Figure 1b) and anal cancer (from 26.82 to 44.97 per 100,000 p-trend=0.002) (Table 1 Figure 1b). The increase in overall age-standardised incidence for anal cancer continued between the 2000-2004 and 2009-2012 time periods where it increased from 25.40 to 44.97 per 100,000 (p-trend<0.001).
Like age-standardised incidence rates, the overall crude incidence of anal cancer and liver cancer increased (p-trend<0.001 for both) over the study period (Supplementary Table 1). The crude incidence of head and neck caner also increased (p-trend=0.001) (Supplementary Table 1), but the age-standardised incidence rate remained stable (p-trend=0.765).
Compared with the general population, the incidence rates for anal cancer, liver cancer, and Hodgkin lymphoma were significantly higher in PLHIV (Table 2).
Other non-AIDS defining cancers incidence
There were no significant trends in age-standardised rates of lung, colorectal and prostate cancer (Table 1). In contrast to the stability of age standardised incidence rates, the crude incidence increased over time for colorectal (p-trend<0.001), lung (p-trend=0.002) and prostate cancer (p-trend<0.001) (Supplementary Table 1).
SIRs were significantly less than 1 for prostate cancer, colorectal cancer and melanoma (Table 2). The overall SIR of all non-infection related NADC did not change over time except for lip and colorectal cancer (Table 2). A decrease in overall SIR was observed in lip cancer (from 1.80 to 1.04). The overall SIR of colorectal cancer increased from 0.16 in 1982-1995 to 0.30 in 2009-2012. There was also a decrease in overall SIR for melanoma from 0.80 to 0.54.
Discussion
The patterns of infection and non-infection related cancers incidence continue to evolve in PLHIV three decades into the HIV epidemic. Since the last publication on cancer trends in the Australian HIV-population(11), the age-standardised incidence of ADC continued to decline for both non-Hodgkin lymphoma and Kaposi sarcoma. The SIRs for both Kaposi sarcoma and non-Hodgkin lymphoma, however, remained substantially higher than the general population. For non-infection related NADC, an increasing size and ageing of the HIV population accounted for increases in crude incidence of several cancers that were no longer observed after age standardisation. Finally, PLHIV had an elevated risk of most infection related cancers compared with the general population, and the increases in anal and liver cancer incidence continued. In contrast, the risk of non-infection related NADC in PLHIV was similar or lower than the general population.
The introduction of combination ART has resulted in a dramatic reduction in the occurrence of both Kaposi sarcoma and non-Hodgkin lymphoma. The reductions in the age standardised incidence and SIR of ADC since 2004 is a continuation of declines between 1982 and 2004(11). Elsewhere, the declines in Kaposi sarcoma and non-Hodgkin lymphoma incidence in the ART era have been well described in other similar studies in U.S, European, and Taiwanese HIV-population cohorts(6, 7, 17, 18) and have been attributed to the restoration of immune function and subsequent control of oncogenic viruses as a result of HIV treatment. In Australia, the continual reductions in ADC in recent years likely reflect progress made in early HIV diagnosis and expanding ART uptake in HIV-positive individuals. In 2012, 88% of people in the Australian HIV Observational Database were on ART, and 88% of those on ART were virally suppressed(19). In comparison, in 2004 approximately 70% of people in the Australian HIV Observational database were on ART, and 50% of those on treatment had undetectable viral loads(20).
The decreasing trend in ADC over time was contrasted by the increasing age-standardised incidence of some infection-related NADCs in the Australian HIV population. Anal cancer was the most common NADC in PLHIV and incidence increased further since 2004. Other population-based studies also have reported increasing anal cancer incidence in PLHIV following the introduction of ART(21, 22). French(23), Swiss(7) and U.S data linkage studies(24), however, have seen stabilising incidence after decades of ART use, while a Dutch study(25) reported declining incidence since 2006. Differences in incidence trends may be due to difference in the reported time period, length of follow up, level of ART coverage and differences in the proportion of the HIV-population in whom male to male sexual contact was the method of HIV transmission between HIV cohorts. HIV-positive gay and bisexual men have a higher risk of developing anal cancer compared to HIV-positive heterosexual men and women(26) thus differences in the proportion of HIV transmission occurring through male to male sexual contact may partially explain difference in anal cancer incidence between cohorts.
The lack of decline in anal cancer incidence in the Australian HIV-population, despite a high ART coverage(27), potentially conflicts with expected trends given reported associations between anal cancer risk and immunosuppression(10). In 2009-2012, the risk of anal cancer was 38 times higher in HIV positive individuals than the general population. ART may assist in preventing anal cancer in the early stages of anal carcinogenesis by assisting the clearance of high-risk HPV infection(28). This is supported by CD4 nadir of up to 8.5 years prior to anal cancer diagnosis being a key predictor of anal cancer risk(9). The lack of decline in anal cancer incidence may result from previously treatments guidelines recommendations to commence ART during moderate levels of immunosuppression. The delay may have resulted in these individuals not receiving the protection of ART in preventing the early stages of anal carcinogenesis. Recent changes to commence ART early at HIV diagnosis(29) may result in eventual declines in anal cancer incidence, although, longer follow up is require to observe this trend.
In the 2009-2012 time period the risk of some other infection-related cancers was also substantially higher in PLHIV compared with the general population. They had a 10 and 2-times higher risk of Hodgkin lymphoma and liver cancer respectively. Previous U.S(30), European(7, 17) and Taiwanese(18) studies also reported an elevated risk of these malignancies in HIV-positive cohorts. Trends in infection related NADC risk, however, vary between cohorts. In the U.S HIV/AIDS Cancer Match study, reductions in anal and liver cancer SIR but no change in Hodgkin lymphoma were reported between 1996 and 2012(30). In contrast, the Swiss HIV cohort study reported increases in anal, liver and Hodgkin’s lymphoma SIR between 1985-1996 and 1997-2001 time periods, but no change these cancer SIR between 1997-2001 and 2002-2006(7).
For non-infection related NADC, there were increases in crude incidence rates which were no longer present after age-standardisation. The impact of the increasing age of the HIV population on crude cancer incidence rates has also been reported elsewhere(6, 8, 31). In an Italian HIV/Cancer data linkage study increases in the crude incidence rates of NADC also disappeared after age standardisation with the exception of lung cancer whose incidence increased after age standardisation(31). The ageing of the HIV-population was found to be the most significant factor influencing changes in breast, liver, colorectal, lung and prostate incidence in a U.S HIV/AIDS cancer data linkage study(8).
Lip cancer SIR in PLHIV were not increased overall but declined from 1.8 to 1.0 between 1982 to 2012. A decline in lip cancer SIR has, to our knowledge, not been previously been described in the HIV population. Lip cancer has been associated with immunosuppression, with an increased risk reported in both HIV and transplant populations(30, 32) and a decline in risk observed in renal transplant patients who return to dialysis and cease immunosuppressive drugs(33). Additional research is needed to confirm the decline in Lip cancer SIR in other HIV-populations.
A lower risk of prostate and colorectal cancer and melanoma was observed in the Australian HIV population. The lower risk of prostate cancer is consistent with findings in European and U.S HIV cohorts(34, 35). The lower risk of colorectal cancer and melanoma, however, has not been found in meta-analyses examining colorectal cancer(36) and melanoma(37) risk in PLHV. The lower colorectal cancer SIR observed in this study may be partially explained by the high level of health care engagement in the Australian HIV-positive population(13) which may have facilitated higher than average participation in colorectal cancer screening offered to the Australian general public older than 50 years. Similarly, lower melanoma SIR may result from the medical surveillance of Australian HIV-positive individuals which may result in increased biopsy of suspicious skin lesions and the subsequent diagnosis of in situ melanoma, preventing progression to invasive melanoma.
The changes in cancer incidence in PLHIV have implications for cancer prevention and screening in HIV-positive individuals. Current cancer prevention and screening measures recommended in the general population, such as smoking deterrence and cessation(38) and bowel cancer screening, should be encouraged in PLHIV given the rising crude incidence of lung, head and neck and colorectal cancers. Efforts to increase HIV testing in high-risk individuals and early initiation of ART should also be promoted to minimise the risk of immune dysfunction and reduce the risk of infection-related cancers(39).
Anal and liver cancer prevention in HIV-positive individuals should also be prioritised given the elevated risk and rising incidence. Optimising hepatitis B vaccination(40) and screening and treatment of hepatitis B and hepatitis C infection(41, 42) in PLHIV may decrease the risk liver cancer. Monitoring for development of anal cancer precursor lesions should also be considered in HIV-positive individuals, particularly gay and bisexual men given their substantially higher risk of anal cancer(26).
The strength of this study is that both national HIV and cancer registries were established in 1982, allowing analysis of 30 years of cancer trends throughout the HIV epidemic in Australia. The cancer registries meet international standards for both classification and completeness to ensure the high accuracy of data linkage(43). The HIV registry also captures the majority of people living with HIV in Australia. At the end of 2012, it was estimated that between 28000 and 34000 people were living with HIV in Australia of whom 25700 were diagnosed(19). The study highlights the increasing burden of infection-related NADC, particular anal and liver cancer, in a HIV population with high levels of ART uptake and viral load suppression. It points to the need for additional cancer prevention strategies focussing on these infection-related NADC cancers. The study also shows the lower risk of colorectal cancer and melanoma in the HIV-population compared to the general Australian population which has not been widely observed in other HIV populations(36, 37).
The study is limited by the size of the cohort, which may impair the ability to detect time trends for some rare cancers. Approximately 10% of notifications to the National HIV Registry had missing information for both the first and last name codes which may affect the ability to match data with the cancer registries. This may result in underestimation of cancer cases in the HIV population during the pre-ART era and contribute to the limited ability to detect temporal trends in the over 65 years age group. The 2x2 name code has been found to have a 76.8% sensitivity but a higher specificity of 99.9%(44). The data linkage thus, minimise the chances of ‘false’ cancer diagnoses but also risks missing cancer cases in the HIV population. The male preponderance in PLHIV in Australia also prevented an analysis of female sex-specific cancers for both ADC (cervical) and NADC (breast).
Changes in cancer incidence in PLHIV in Australia continued in the era of effective ART, three decades since the start of the HIV/AIDS epidemic. Declining AIDS defining cancer incidence and increasing non-AIDS defining cancer crude incidence reflect improving ART coverage and rapid ageing of the HIV population. Reducing infection-related non-AIDS defining cancer incidence, in particular, anal and liver cancers, by early detection, is a health priority in PLHIV.
Supplementary Material
Acknowledgement
The authors are indebted to people living with HIV in Australia and thank the Data Linkage Unit of the Australian Institute of Health and Welfare for their assistance in the project.
Sources of Funding
The HIV Cancer Data Linkage Study was funded by the International Epidemiology Databases to Evaluate AIDS (IeDEA; U01AI069907). The IeDEA Asia-Pacific Research Collaboration: Cancer Studies is an initiative of TREAT Asia, a program of amfAR, the Foundation for AIDS Research, with support from the US National Institutes of Health’s National Institute of Allergy and Infectious Disease, the Eunice Kennedy Shriver National Institute of Child Health, and the National Institute on Drug Abuse, as part of the International Epidemiology Databases to Evaluate AIDS (IeDEA; U01AI069907)
The Kirby Institute is affiliated with the Faculty of Medicine, UNSW and funded by the Australian Government of Health and Ageing. The views expressed in this publication do not necessarily represent the position of the Australian Government.
Conflict of Interest Statement
IMP has received travel funding from Seqiris Australia in 2018. MTVL is a Postdoctoral fellow of the National Health and Medical Research Council (1012141). ML has received research grants from Gilead Sciences, Janssen-Cilag and ViiV Healthcare.
References
- 1.Palella FJ Jr., Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338(13):853–60. [DOI] [PubMed] [Google Scholar]
- 2.Hughes A, Barber T, Nelson M. New treatment options for HIV salvage patients: an overview of second generation PIs, NNRTIs, integrase inhibitors and CCR5 antagonists. J Infect. 2008;57(1):1–10. [DOI] [PubMed] [Google Scholar]
- 3.McManus H, Hoy JF, Woolley I, Boyd MA, Kelly MD, Mulhall B, et al. Recent trends in early stage response to combination antiretroviral therapy in Australia. Antivir Ther. 2015;20(2):131–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mocroft A, Ledergerber B, Katlama C, Kirk O, Reiss P, d’Arminio Monforte A, et al. Decline in the AIDS and death rates in the EuroSIDA study: an observational study. Lancet. 2003;362(9377):22–9. [DOI] [PubMed] [Google Scholar]
- 5.Hasse B, Ledergerber B, Furrer H, Battegay M, Hirschel B, Cavassini M, et al. Morbidity and aging in HIV-infected persons: the Swiss HIV cohort study. Clin Infect Dis. 2011;53(11):1130–9. [DOI] [PubMed] [Google Scholar]
- 6.Shiels MS, Pfeiffer RM, Gail MH, Hall HI, Li J, Chaturvedi AK, et al. Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst. 2011;103(9):753–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franceschi S, Lise M, Clifford GM, Rickenbach M, Levi F, Maspoli M, et al. Changing patterns of cancer incidence in the early- and late-HAART periods: the Swiss HIV Cohort Study. Br J Cancer. 2010;103(3):416–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robbins HA, Shiels MS, Pfeiffer RM, Engels EA. Epidemiologic contributions to recent cancer trends among HIV-infected people in the United States. Aids. 2014;28(6):881–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hernández-Ramírez RU, Qin L, Lin H, Leyden W, Neugebauer RS, Althoff KN, et al. Association of Immunosuppression and Human Immunodeficiency Virus (HIV) Viremia With Anal Cancer Risk in Persons Living With HIV in the United States and Canada. Clin Infect Dis. 2020;70(6):1176–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guiguet M, Boue F, Cadranel J, Lang JM, Rosenthal E, Costagliola D. Effect of immunodeficiency, HIV viral load, and antiretroviral therapy on the risk of individual malignancies (FHDH-ANRS CO4): a prospective cohort study. Lancet Oncol. 2009;10(12):1152–9. [DOI] [PubMed] [Google Scholar]
- 11.van Leeuwen MT, Vajdic CM, Middleton MG, McDonald AM, Law M, Kaldor JM, et al. Continuing declines in some but not all HIV-associated cancers in Australia after widespread use of antiretroviral therapy. Aids. 2009;23(16):2183–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cysique LA, Bain MP, Brew BJ, Murray JM. The burden of HIV-associated neurocognitive impairment in Australia and its estimates for the future. Sex Health. 2011;8(4):541–50. [DOI] [PubMed] [Google Scholar]
- 13.Keen P, Gray RT, Telfer B, Guy R, Schmidt HM, Whittaker B, et al. The 2016 HIV diagnosis and care cascade in New South Wales, Australia: meeting the UNAIDS 90–90-90 targets. Journal of the International AIDS Society. 2018;21(4):e25109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yancik R, Ries LAG. AGING AND CANCER IN AMERICA: Demographic and Epidemiologic Perspectives. Hematology/Oncology Clinics of North America. 2000;14(1):17–23. [DOI] [PubMed] [Google Scholar]
- 15.Humans IWGotEoCRt. Biological agents. Volume 100 B. A review of human carcinogens. IARC monographs on the evaluation of carcinogenic risks to humans / World Health Organization, International Agency for Research on Cancer. 2012;100 (Pt B):1–441. [PMC free article] [PubMed] [Google Scholar]
- 16.Jin F, Vajdic CM, Law M, Amin J, van Leeuwen M, McGregor S, et al. Incidence and time trends of anal cancer among people living with HIV in Australia. Aids. 2019;33(8):1361–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dal Maso L, Polesel J, Serraino D, Lise M, Piselli P, Falcini F, et al. Pattern of cancer risk in persons with AIDS in Italy in the HAART era. Br J Cancer. 2009;100(5):840–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen M, Jen I, Chen YH, Lin MW, Bhatia K, Sharp GB, et al. Cancer incidence in a Nationwide HIV/AIDS patient cohort in Taiwan in 1998–2009. J Acquir Immune Defic Syndr. 2014;65(4):463–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The Kirby Institute. HIV, viral hepatitis and sexually transmissible infections in Australia Annual Surveillance Report 2013. The University of New South Wales; 2013. [Google Scholar]
- 20.The Kirby Institute. HIV, viral hepatitis and sexually transmissible infections in Australia Annual Surveillance Report 2005. The University of New South Wales; 2005. [Google Scholar]
- 21.D’Souza G, Wiley DJ, Li X, Chmiel JS, Margolick JB, Cranston RD, et al. Incidence and epidemiology of anal cancer in the multicenter AIDS cohort study. J Acquir Immune Defic Syndr. 2008;48(4):491–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanaka LF, Latorre M, Gutierrez EB, Heumann C, Herbinger KH, Froeschl G. Trends in the incidence of AIDS-defining and non-AIDS-defining cancers in people living with AIDS: a population-based study from São Paulo, Brazil. International journal of STD & AIDS. 2017;28(12):1190–8. [DOI] [PubMed] [Google Scholar]
- 23.Piketty C, Selinger-Leneman H, Bouvier AM, Belot A, Mary-Krause M, Duvivier C, et al. Incidence of HIV-related anal cancer remains increased despite long-term combined antiretroviral treatment: results from the french hospital database on HIV. J Clin Oncol. 2012;30(35):4360–6. [DOI] [PubMed] [Google Scholar]
- 24.Colon-Lopez V, Shiels MS, Machin M, Ortiz AP, Strickler H, Castle PE, et al. Anal Cancer Risk Among People With HIV Infection in the United States. J Clin Oncol. 2018;36(1):68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richel O, Van Der Zee RP, Smit C, De Vries HJ, Prins JM. Brief Report: Anal Cancer in the HIV-Positive Population: Slowly Declining Incidence After a Decade of cART. J Acquir Immune Defic Syndr. 2015;69(5):602–5. [DOI] [PubMed] [Google Scholar]
- 26.Clifford GM, Georges D, Shiels MS, Engels EA, Albuquerque A, Poynten IM, et al. A meta-analysis of anal cancer incidence by risk group: towards a unified anal cancer risk scale. Int J Cancer. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelly H, Chikandiwa A, Alemany Vilches L, Palefsky JM, de Sanjose S, Mayaud P. Association of antiretroviral therapy with anal high-risk human papillomavirus, anal intraepithelial neoplasia, and anal cancer in people living with HIV: a systematic review and meta-analysis. The Lancet HIV. 2020;7(4):e262–e78. [DOI] [PubMed] [Google Scholar]
- 28.Hidalgo-Tenorio C, Gil-Anguita C, Lopez Ruz MA, Omar M, Lopez-Hidalgo J, Pasquau J. ART is key to clearing oncogenic HPV genotypes (HR-HPV) in anal mucosa of HIV-positive MSM. PloS one. 2019;14(10):e0224183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lundgren JD, Babiker AG, Gordin F, Emery S, Grund B, Sharma S, et al. Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. N Engl J Med. 2015;373(9):795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hernandez-Ramirez RU, Shiels MS, Dubrow R, Engels EA. Cancer risk in HIV-infected people in the USA from 1996 to 2012: a population-based, registry-linkage study. Lancet HIV. 2017;4(11):e495–e504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Polesel J, Franceschi S, Suligoi B, Crocetti E, Falcini F, Guzzinati S, et al. Cancer incidence in people with AIDS in Italy. International journal of cancer. 2010;127(6):1437–45. [DOI] [PubMed] [Google Scholar]
- 32.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370(9581):59–67. [DOI] [PubMed] [Google Scholar]
- 33.van Leeuwen MT, Grulich AE, McDonald SP, McCredie MR, Amin J, Stewart JH, et al. Immunosuppression and other risk factors for lip cancer after kidney transplantation. Cancer Epidemiol Biomarkers Prev. 2009;18(2):561–9. [DOI] [PubMed] [Google Scholar]
- 34.Sun D, Cao M, Li H, Ren J, Shi J, Li N, et al. Risk of prostate cancer in men with HIV/AIDS: a systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2020. [DOI] [PubMed] [Google Scholar]
- 35.Coghill AE, Engels EA, Schymura MJ, Mahale P, Shiels MS. Risk of Breast, Prostate, and Colorectal Cancer Diagnoses Among HIV-Infected Individuals in the United States. J Natl Cancer Inst. 2018;110(9):959–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.OʼNeill TJ, Nguemo JD, Tynan AM, Burchell AN, Antoniou T. Risk of Colorectal Cancer and Associated Mortality in HIV: A Systematic Review and Meta-Analysis. J Acquir Immune Defic Syndr. 2017;75(4):439–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olsen CM, Knight LL, Green AC. Risk of melanoma in people with HIV/AIDS in the pre- and post-HAART eras: a systematic review and meta-analysis of cohort studies. PloS one. 2014;9(4):e95096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shepherd L, Ryom L, Law M, Petoumenos K, Hatleberg CI, d’Arminio Monforte A, et al. Cessation of Cigarette Smoking and the Impact on Cancer Incidence in Human Immunodeficiency Virus-infected Persons: The Data Collection on Adverse Events of Anti-HIV Drugs Study. Clin Infect Dis. 2019;68(4):650–7. [DOI] [PubMed] [Google Scholar]
- 39.Borges AH, Neuhaus J, Babiker AG, Henry K, Jain MK, Palfreeman A, et al. Immediate Antiretroviral Therapy Reduces Risk of Infection-Related Cancer During Early HIV Infection. Clin Infect Dis. 2016;63(12):1668–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chang MH, You SL, Chen CJ, Liu CJ, Lee CM, Lin SM, et al. Decreased incidence of hepatocellular carcinoma in hepatitis B vaccinees: a 20-year follow-up study. J Natl Cancer Inst. 2009;101(19):1348–55. [DOI] [PubMed] [Google Scholar]
- 41.Lui FH, Moosvi Z, Patel A, Hussain S, Duong A, Duong J, et al. Decreased risk of hepatocellular carcinoma recurrence with direct-acting antivirals compared with no treatment for hepatitis C: a meta-analysis. Ann Gastroenterol. 2020;33(3):293–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wandeler G, Mauron E, Atkinson A, Dufour JF, Kraus D, Reiss P, et al. Incidence of hepatocellular carcinoma in HIV/HBV-coinfected patients on tenofovir therapy: Relevance for screening strategies. J Hepatol. 2019;71(2):274–80. [DOI] [PubMed] [Google Scholar]
- 43.Cancer incidence in five continents [Internet]. International Agency for Research on Cancer. 2017. [Google Scholar]
- 44.Swart A, Meagher NS, van Leeuwen MT, Zhao K, Grulich A, Mao L, et al. Examining the quality of name code record linkage: what is the impact on death and cancer risk estimates? A validation study. Aust N Z J Public Health. 2015;39(2):141–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


