Abstract
Objective
To examine the effects of a 6-month weight loss intervention on physical function, inflammatory biomarkers, and metabolic biomarkers in both those with and without osteoarthritis (OA).
Design
59 individuals ≥60 years old with obesity and a functional impairment were enrolled into this IRB approved clinical trial and randomized into one of two 6-month weight loss arms: a higher protein hypocaloric diet or a standard protein hypocaloric diet. All participants were prescribed individualized 500-kcal daily-deficit diets, with a goal of 10% weight loss. Additionally, participants participated in three, low-intensity, exercise sessions per week. Physical function, serum biomarkers and body composition data were assessed at the baseline and 6-month timepoints. Statistical analyses assessed the relationships between biomarkers, physical function, body composition, and OA status as a result of the intervention.
Results
No group effects of dietary intervention were detected on any outcome measures (multiple p > 0.05). During the 6-month trial, participants lost 6.2 ± 4.0% of their bodyweight (p < 0.0001) and experienced improved physical function on the Short-Performance-Physical-Battery (p < 0.0001), 8-foot-up-and-go (p < 0.0001), and time to complete 10-chair-stands (p < 0.0001). Adiponectin concentrations (p = 0.0480) were elevated, and cartilage oligomeric matrix protein (COMP) concentrations (p < 0.0001) were reduced; further analysis revealed that reductions in serum COMP concentrations were greater in OA-negative individuals.
Conclusions
These results suggest that weight loss in older adults with and without OA may provide a protective effect to cartilage and OA. In particular, OA-negative individuals may be able to mitigate changes associated with OA through weight loss.
Keywords: (3–6): Obesity, Cartilage, Osteoarthritis, Lifestyle intervention, Older adults, Biomarkers
1. Introduction
Obesity is a complex disease that impacts more than 40% of adults aged 60 and older [1]. Furthermore, obesity is a strong risk factor for the development and progression of osteoarthritis (OA) [2]. Since obesity is modifiable, its role in the etiology of OA is increasingly becoming a key focus of OA-related research [[3], [4], [5], [6], [7], [8]]. Specifically, obesity modulates both joint mechanics and inflammatory pathways, resulting in deleterious changes to cartilage that may lead to the development of OA [[5], [6], [7], [8]].
From a mechanical perspective, obesity-related OA development may be due, in part, to aberrant joint loading [3,5,9,10]. Excessive loading may cause tissue damage or lead to upregulation of inflammatory factors that negatively impact cartilage health. Recent in vivo studies have shown altered cartilage mechanics and composition in individuals with obesity, suggesting that functional changes occur prior to clinically evident OA [[11], [12], [13], [14]]. Studies relating mechanical loading to changes in cartilage metabolism have observed that cartilage oligomeric matrix protein (COMP) plays a key regulatory role in the remodeling of the cartilage extracellular matrix (ECM) [15]. COMP is involved in both collagen fibril formation and structural maintenance of the cartilage ECM [[16], [17], [18], [19]]; as such, its concentration in serum and synovial fluid has been hypothesized to be a useful diagnostic and prognostic indicator of OA [[20], [21], [22], [23]].
Moreover, obesity exhibits systemic proinflammatory effects [[5], [6], [7], [8],24] and evidence increasingly supports the notion that inflammatory pathways may be a key factor related to OA development and progression [[5], [6], [7], [8],25]. For example, recent studies suggested that the upregulation of inflammatory biomarkers such as adiponectin, C-reactive protein (CRP), interleukin 6 (IL-6), and tumor necrosis factor alpha (TNF-α) may have adverse effects on cartilage health [[5], [6], [7], [8],[24], [25], [26], [27], [28], [29]].
Physical function is another important factor to consider when examining obesity-related OA development [30]. Individuals with obesity [31] or OA [4,[32], [33], [34]] tend to be more sedentary than those without obesity or OA. Excess adiposity in older adults is associated with impaired physical function, loss of independence, and decreased quality of life [4,31,[34], [35], [36], [37]]. Combined, obesity and OA have the capacity to enact a vicious cycle characterized by increasing weight gain, disability, and concomitant reductions in physical function, independence, and quality of life [4,31].
Given this important etiological role of obesity, weight reduction induced via diet and exercise has been explored as a potential means for ameliorating the onset, progression, and symptoms of OA [4,9,32,33,38,39]. Weight loss has previously been shown to diminish circulating inflammatory biomarkers and improve the symptoms of OA [4,32,33]. However, to date, little data exists examining the effects of weight loss on measures of physical function and OA-related serum biomarkers in individuals with and without OA.
As such, the goal of this study was to examine the effects of a 6-month weight loss intervention on physical function, inflammatory biomarkers, and metabolic biomarkers in both those with and without OA. Specifically, we performed a secondary analysis of data from a 6-month randomized clinical trial (NCT02437643) designed to investigate the effects of a higher protein hypocaloric diet compared to a standard protein hypocaloric diet on weight loss in older adults with obesity [36]. We hypothesized that both physical function and OA-related serum biomarker levels would be improved by weight loss in both those with and without clinical OA.
2. Methods
2.1. Study Design
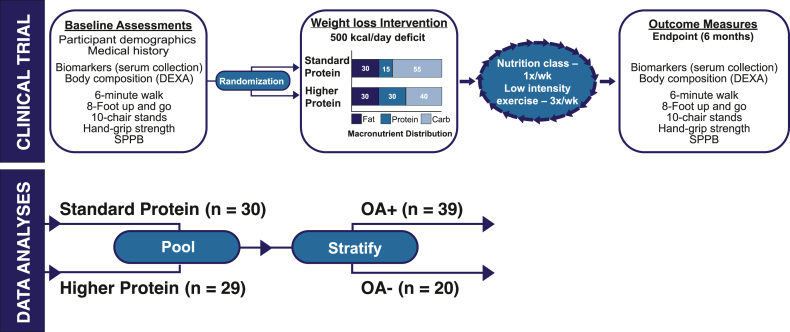
Free-living older adults were recruited in Durham, North Carolina and surrounding areas using flyers, health fair presentations, and other community outreach as part of an institutional review board (IRB) approved randomized clinical trial (NCT02437643). Methods for this parent trial have been previously published [36]. Briefly, older (≥60 years of age) men and women with obesity (body mass index (BMI) ≥30 kg/m2) and functional impairment (score of 4–10; Short Physical Performance Battery (SPPB)) [40] were enrolled and randomized into one of two weight loss intervention arms using block randomization. Both groups received a weight-reduction treatment (diet plus exercise) with a goal of attaining 10% weight loss over 6-months. The two dietary intervention groups were: a standard protein hypocaloric diet or a higher protein hypocaloric diet (Fig. 1). The OA status of participants was ascertained at baseline, based on a medical history of clinical OA diagnosis. All of the participants who were enrolled in the parent trial [36] and had provided blood samples were included in this analysis. Preliminary statistical analyses did not show a group-effect of dietary intervention (standard versus higher protein) on outcome measures in this study (approach detailed in statistical analyses section ‘Impact of Dietary Arm on Outcome Measures’). Thus, for the present analyses, participants were divided into two groups by clinical OA diagnosis (OA-positive or OA-negative). Of the 59 individuals in the study, n = 39 had clinical OA (OA-positive), while n = 20 had no medical history of clinical OA diagnosis (OA-negative). Descriptive characteristics of the participants in these groups can be seen in Table 1.
Fig. 1.
Schematic of study design. Baseline assessments were collected following consent and enrollment into the study. Participants were randomized into a dietary arm and received individually tailored dietary plans that aimed to cut 500 kcal/day. Participants received weekly nutrition counseling and participated in low intensity exercise. At the 6-month endpoint, outcome measures were collected. For data analysis, initial tests did not detect effects of the dietary arm on outcome measures. Thus, subjects were pooled by dietary arm and stratified according to OA status.
Table 1.
Descriptive characteristics of participants.
| OA (−) | OA (+) | Combined | |
|---|---|---|---|
| Age (y) | 72.15 ± 5.3 | 69.13 ± 6.1 | 70.15 ± 6.0 |
| Body mass (kg) | 100.51 ± 17.1 | 95.11 ± 14.6 | 96.97 ± 15.6 |
| BMI (kg/m2) | 35.33 ± 4.8 | 34.16 ± 4.6 | 34.56 ± 4.7 |
| Gender | |||
| Female (%) | 14 (70.0%) | 30 (76.9%) | 44 (74.6%) |
| Male (%) | 6 (30.0%) | 9 (23.1%) | 15 (25.4%) |
| Race | |||
| Black (%) | 8 (40.0%) | 19 (48.8%) | 27 (45.8%) |
| White (%) | 12 (60.0%) | 20 (51.3%) | 32 (54.2%) |
| Education | |||
| ≤ High school (%) | 3 (15.0%) | 2 (5.1%) | 5 (8.5%) |
| > High school (%) | 17 (85.0%) | 37 (94.9%) | 54 (91.5%) |
Notes: BMI = Body mass index.
OA (−) n = 20, OA (+) n = 39.
Data reported as mean ± SD or total number (%).
Inclusion criteria stipulated that participants must be willing to participate in weekly group meetings and to consume dairy products. Participants were excluded if they had significant renal impairment (estimated glomerular filtration rates [eGFR] <45 mL/min/1.73 m2) [36], dementia, neurological conditions causing functional limitations, unstable or terminal medical conditions (e.g. active cancer), were taking prescription weight loss medications or monoamine oxidase inhibitors, or participation was contraindicated by their primary care provider. Participants with an eGFR of 45–59 mL/min/1.73 m2 were enrolled but an eGFR was repeated every 2 months to monitor kidney function.
2.2. Diets
Following enrollment, participants were block randomized into either a higher protein hypocaloric diet or a standard protein hypocaloric diet (Fig. 1). Both study groups received a supervised treatment program (hypo-caloric diet plus exercise). The weight loss interventions were delivered by Registered Dietitian Nutritionists (RDNs), who provided individualized kcal prescriptions and meal plans, led weekly group meetings for counseling and peer support, reviewed weekly food logs, and supervised weekly weigh-ins.
A detailed description of the parent study methodology has been published previously [36]. Briefly, participants in both study arms were prescribed a 500-kcal deficit diet with a macronutrient distribution of 30% protein, 30% fat, and 40% carbohydrate for the higher protein hypocaloric diet group and 15% protein, 30% fat, and 55% carbohydrate for the standard protein hypocaloric diet group. Participants in the higher protein group were supplied with >420 g of protein per week from low-fat dairy foods (e.g. cottage cheese, cheese sticks, Quest Whey ProteinTM powder (Quest Nutrition, El Segundo, CA), Fairlife® milk (Fairlife®, Chicago, IL), and Greek yogurt and were counseled to consume other high-quality proteins as well (e.g. lean meats, eggs, and seafood). Both groups were provided a low dose multivitamin (Teen Multivitamin for Boys 12–17, GNC, Pittsburgh, PA) to ensure nutritional adequacy. Additionally, calcium and vitamin D were provided to equalize intake between groups: 400 mg calcium and 500 IU vitamin D (Citracal®, Bayer, Leverkusen, Germany) daily for the standard protein hypocaloric diet group and 200 mg calcium, 250 IU vitamin D daily for the higher protein hypocaloric diet group. All other dietary supplements were prohibited. Adherence to the intervention was monitored through weekly attendance at group meetings and weigh-ins.
2.3. Exercise
All participants completed three, 30-min low-intensity exercise sessions per week during the 6-month intervention, two supervised and one on their own. The in-person sessions utilized resistance bands and balls to conduct movements to improve strength, flexibility, and balance. A certified fitness instructor delivered the two supervised exercise sessions, while written instructions and a DVD recording of guided exercises was provided for the third session.
2.4. Body Composition
At the baseline and endpoint (6-month) (Fig. 1), measures of body composition (e.g. total lean and total fat mass) were measured using Dual Energy X-ray Absorptiometry (DEXA; Hologic 4500, Marlborough, MA).
2.5. Biomarkers
Participants were instructed to refrain from beverages (except for water), food, and exercise for 12 h prior to the morning of data collection. At baseline and 6-month visits (Fig. 1), blood was drawn into 8.5 mL SST tubes and spun at 3000 rpm. Serum was aliquoted and stored at −80 °C until biomarker analyses were performed. The following biomarkers were assessed by ELISA: adiponectin (R&D Systems, Minneapolis, MN), cartilage oligomeric matrix protein (COMP, Abcam, Boston, MA), C-reactive protein (CRP, Abcam), interleukin-6 (IL-6, R&D Systems), and tumor necrosis factor alpha (TNF-α, Abcam). Assays were performed following the manufacturer's instructions.
2.6. Physical Function Measurements
Primary assessments of physical function were conducted during the baseline and 6-month visits using the SPPB score [41] (Fig. 1). The SPPB test consists of three component domains (balance, strength, and gait speed) yielding a total score ranging from 0 (poor) to 12 (high). Additional measures of physical function collected during the baseline and 6-month visits included the 6-Minute Walk Test, 8-Foot Up and Go, time to complete 10 chair stands and hand-grip strength (Sammons Preston, Bolingbrook, IL) [36].
2.7. Statistical Analyses
2.7.1. Exploratory Data Analysis
All methods discussed herein were conducted using intent-to-treat analyses. Exploratory analyses revealed that serum biomarker levels were not normally distributed (Shapiro-Wilk tests). Thus, biomarker data were natural log transformed prior to further statistical analysis.
2.7.2. Pre-Post Changes
T-tests were utilized to evaluate group changes in outcome measures as a result of the dietary intervention, without consideration for dietary arm. Changes in body composition and physical function were modeled as normalized difference scores. Changes in serum biomarker concentrations from the baseline to 6-month timepoints were modeled as logged differences, where .
2.7.3. Impact of Dietary Arm on Outcome Measures
The effect of dietary arm (i.e. higher protein hypocaloric diet versus standard protein hypocaloric diet) on outcome measures over the course of the weight loss intervention was evaluated using ordinary least squares regression (OLS). Change scores for all outcome measures were calculated as described above. Dietary arm was coded as a binary, categorical factor. Notably, no effect of dietary arm was detected in any of the aforementioned analyses. Thus, data from participants in the two dietary arms were pooled and subsequently stratified as a function of their clinical OA status prior to statistical analysis.
2.7.4. Impact of OA on Outcome Measures
Accordingly, OLS analyses were then constructed to evaluate the effect of clinical OA status on the change in outcome measures (i.e. body composition, physical function, serum biomarker concentrations). OLS models included clinical OA status, sex, age, and percent change in BMI as covariates. All models were developed in R (version 4.1.2; R Core Team, 2021) using the STATS packages. Statistical significance was set a-priori at .
3. Results
3.1. Pre-Post Changes
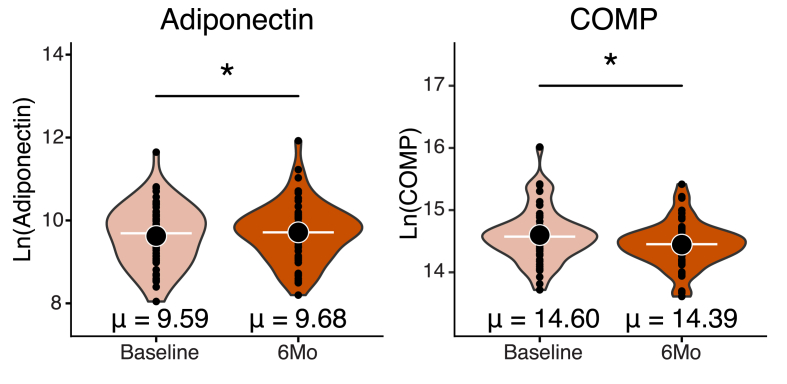
As a whole, participants lost 6 ± 4% of their body weight (p < 0.0001), decreased fat mass (p < 0.0001), fat mass percentage (p < 0.0001), and BMI (p < 0.0001) (Table 2). In addition, participants increased lean body mass percentage from baseline to 6-months (p < 0.0001). Analysis of biomarker concentrations from the baseline to 6-month timepoints revealed that participants had higher levels of serum adiponectin (p = 0.0481) and lower serum COMP concentrations at the 6-month timepoint (p < 0.0001) (Fig. 2). We did not detect differences in CRP, IL-6, or TNF-α concentrations between the baseline and 6-month timepoints (Table 2).
Table 2.
Baseline and 6-month outcome measures stratified by clinical OA status.
| Outcome | Baseline |
6-Mo |
P-value | ||||
| OA (−) | OA (+) | Combined | OA (−) | OA (+) | Combined | ||
| Body Composition | |||||||
| BMI (kg/m2) | 35.3 ± 4.8 | 34.2 ± 4.6 | 34.6 ± 4.7 | 33.0 ± 4.4 | 31.7 ± 4.5 | 32.1 ± 4.4 | P < 0.0001† |
| Body Mass (% change) | – | – | - | −5.8 ± 4.1 | −6.3 ± 4.0 | −6.2 ± 4.0 | P < 0.0001† |
| Lean Body Mass (%) | 56.0 ± 6.0 | 56.3 ± 5.4 | 56.2 ± 5.6 | 58.8 ± 6.0 | 59.7 ± 6.0 | 59.4 ± 6.0 | P < 0.0001† |
| Fat Mass (%) | 44.0 ± 6.0 | 43.6 ± 5.7 | 43.8 ± 5.7 | 41.2 ± 6.0 | 40.4 ± 5.9 | 40.7 ± 5.9† | P < 0.0001† |
| Lean Body Mass (kg) | 54.2 ± 10.4 | 53.6 ± 10.0 | 53.8 ± 10.0 | 54.2 ± 10.6 | 52.9 ± 9.4 | 53.3 ± 9.8 | P = 0.224 |
| Fat Mass (kg) | 43.6 ± 9.2 | 41.2 ± 9.7 | 42.0 ± 8.9 | 37.8 ± 7.6 | 36.0 ± 8.8 | 36.6 ± 8.4 | P < 0.0001† |
| Biomarkers | |||||||
| Adiponectin Ln(ng/mL) | 9.5 ± 0.7 | 9.7 ± 0.8 | 9.6 ± 0.8 | 9.6 ± 0.7 | 9.7 ± 0.8 | 9.7 ± 0.7 | P = 0.0480† |
| COMP Ln(ng/mL) | 14.7 ± 0.5 | 14.5 ± 0.4 | 14.7 ± 0.4 | 14.4 ± 0.4 | 14.4 ± 0.4 | 14.4 ± 0.4 | P < 0.0001†,‡ |
| CRP Ln(ng/mL) | 10.6 ± 0.6 | 10.4 ± 0.5 | 10.5 ± 0.6 | 10.7 ± 0.7 | 10.3 ± 0.6 | 10.5 ± 0.5 | P = 0.710 |
| IL-6 Ln(pg/mL) | 2.0 ± 0.5 | 1.9 ± 0.7 | 1.9 ± 0.6 | 1.9 ± 0.5 | 1.8 ± 0.9 | 1.9 ± 0.8 | P = 0.414 |
| TNF- Ln(pg/mL) | 1.5 ± 0.7 | 1.2 ± 1.0 | 1.3 ± 0.9 | 1.4 ± 0.7 | 1.2 ± 0.8 | 1.3 ± 0.8 | P = 0.752 |
| Physical Function | |||||||
| SPPB (score) | 9.4 ± 1.2 | 9.1 ± 1.3 | 9.2 ± 1.3 | 11.3 ± 1.1 | 11.1 ± 1.5 | 11.1 ± 1.4 | P < 0.0001† |
| 6-Minute walk (m) | 474.4 ± 124.0 | 499.3 ± 86.7 | 485.4 ± 101.4 | 553.4 ± 77.3 | 524.3 ± 79.7 | 520.6 ± 99.9 | P = 0.091 |
| 8-Foot up and go (sec) | 7.6 ± 1.6 | 7.7 ± 2.0 | 7.6 ± 2.9 | 6.2 ± 0.9 | 7.1 ± 1.6 | 6.8 ± 1.4 | P < 0.0001† |
| 10-Chair Stands (sec) | 24.2 ± 7.7 | 25.5 ± 6.1 | 25.1 ± 6.6 | 19.3 ± 4.0 | 22.2 ± 6.4 | 21.2 ± 5.8 | P < 0.0001† |
| Left Hand Strength (kg) | 24.8 ± 8.3 | 23.7 ± 8.6 | 24.1 ± 8.4 | 25.3 ± 6.8 | 22.8 ± 9.0 | 23.7 ± 8.2 | P = 0.830 |
| Right Hand Strength (kg) | 25.9 ± 7.3 | 25.0 ± 8.9 | 25.4 ± 8.3 | 26.5 ± 7.3 | 24.5 ± 10.1 | 25.3 ± 9.1 | P = 0.910 |
Notes: Since analysis of dietary arm (i.e., standard protein vs higher protein) did not detect any effect of dietary arm on the outcome measures, subjects were stratified only by clinical OA status.
†p < 0.05 for group change from baseline to 6-Mo (See: ‘Pre-Post Changes’).
‡p < 0.05 for between OA group change from baseline to 6-Mo (See: ‘Impact of OA on Outcome Measures’).
OA (−) n = 20, OA (+) n = 39.
Data reported as mean ± SD or total number (%). Bolding highlights the differences in grouped values which were found to be different between the baseline and 6-Month timepoints.
Fig. 2.
Change in biomarker concentration from baseline to 6-month timepoint. Serum concentrations of adiponectin were increased from the baseline to 6-month timepoints, whereas COMP decreased. T-tests were utilized to evaluate changes in biomarker concentrations from the baseline to 6-month timepoints. ∗p < 0.05.
Improvements in physical function were also observed from the baseline to 6-month timepoints. Participants had increased SPPB scores (p < 0.0001) and reduced times to complete the 8-foot up and go (p < 0.0001) and time to complete 10 chair stand tasks (p < 0.0001) (Table 2). No difference in left- or right-hand strength or 6-min walk distances were observed from the baseline to 6-month measurements.
3.2. Impact of OA on Outcome Measures
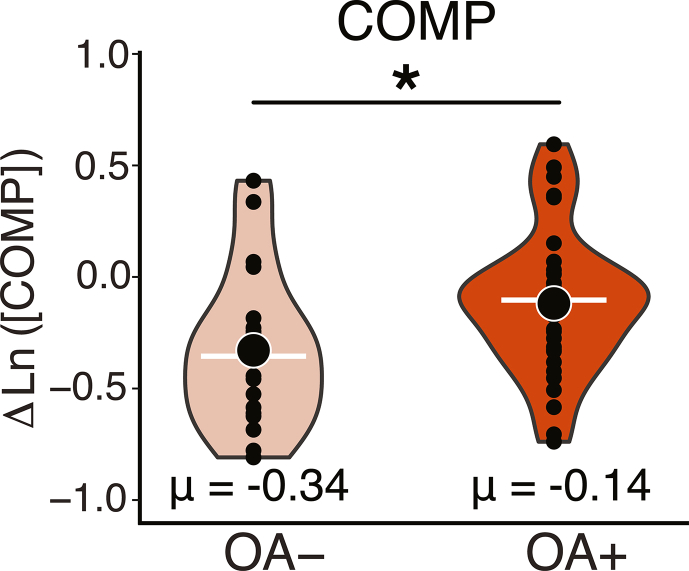
Finally, OLS regression analyses revealed that the OA-negative group had a greater reduction in COMP concentrations, compared to the OA-positive group, when adjusting for sex and percent change in BMI. ( p = 0.026, Fig. 3). No appreciable effect of clinical OA status was detected amongst the other outcome measures tested.
Fig. 3.
Logged change in serum biomarker concentration versus OA status. OLS regression revealed that OA-negative individuals experienced a greater reduction in COMP concentrations than OA-positive individuals. ∗p < 0.05.
4. Discussion
The results of this study reveal that, in older adults with obesity, a weight loss intervention using diet and exercise may improve overall physical function and modulate catabolic pathways associated with cartilage degeneration in both those with and without clinical OA. Specifically, improvements in physical function measures (SPPB, time to complete 10 chair stands, 8 foot up-and-go) and metabolic biomarkers (adiponectin, COMP) were observed in both those with and without clinical OA. Interestingly, reductions in serum COMP concentrations were observed in both those with and without clinical OA, but were more pronounced in individuals who were OA-negative. Thus, the results of this study suggest that weight loss may be able to mitigate degenerative changes associated with OA in both those with and without clinical OA.
The findings that both those with and without clinical OA experience improvements in inflammatory and metabolic biomarkers is important because recent work has suggested that obesity potentiates the development of degenerative changes associated with OA. For example, murine models of obesity suggest that increased adiposity is associated with alterations in cartilage structure and function, without apparent development of OA by histological OARSI grading [42]. These findings have been echoed in human-subjects research, whereby cartilage mechanical function and composition have been measured in vivo using magnetic resonance imaging. Specifically, individuals with higher BMIs have thinner tibial cartilage [11], which experiences greater diurnal deformations [12,13] and greater deformations in response to walking [11,43] as compared to individuals with a BMI in the normal range. These changes in cartilage function were related to altered cartilage composition, assessed via T1rho relaxation imaging. Namely, T1rho relaxation times in the knee cartilage of individuals with high BMIs were elevated, which may be indicative of degeneration [43,44]. Together, these findings suggest that obesity may exert antagonistic effects on joint metabolism that enact changes associated with the development of OA [5,8].
Serum concentrations of adiponectin increased from the baseline to 6-month timepoints, with no group differences as a function of OA status. Increased adiponectin in the context of obesity reduction has typically been seen as a positive response to weight loss [24]. Adiponectin is an adipokine, whose function has been linked to both metabolic and inflammatory processes [24]. With respect to obesity-related OA, however, adiponectin has been reported to exert both anabolic and catabolic effects on cartilage, leading to uncertainty as to whether serum concentrations of adiponectin reflect cartilage health [8,24,45,46]. For example, adiponectin can down-regulate pro-inflammatory biomarkers [24] and has been inversely associated with obesity [24,39]. Additionally, adiponectin may inhibit extracellular matrix degradation in cartilage [8]. Thus, though adiponectin mediates both metabolic and inflammatory processes, the increased serum concentrations of adiponectin observed in this study may be due to diminished adiposity due to weight loss in these individuals.
IL-6, TNF-, and CRP are inflammatory cytokines that have been linked to the development and progression of OA [6,8]. The serum concentrations of these biomarkers have also been positively linked to obesity [6,8]. Despite the observation that most individuals experienced improved physical function and weight loss, we did not detect changes in serum concentrations of IL-6, TNF-, or CRP following the weight loss intervention. Notably, studies involving comparable weight loss targets and sample demographics (i.e. older individuals with obesity and physical impairment) have observed mixed results regarding the modulation of inflammatory biomarkers through mild weight loss [4,[31], [32], [33],[47], [48], [49]]. On the other hand, studies that achieved more aggressive weight loss benchmarks (e.g. 10% weight loss) have observed more consistent improvement in inflammatory biomarkers profiles, pain, and measures of physical function [32,47,48], with some studies demonstrating a dose-dependent response of weight loss on such improvements [33,48,50]. Thus, it is possible that the average weight loss (6 ± 4%) in the present study was not sufficient to induce appreciable reductions in these select inflammatory biomarkers.
COMP plays an important role in regulating the structural integrity of the cartilage extracellular matrix [19] and its presence in serum is indicative of cartilage breakdown [18]. Indeed, prior research has shown that serum COMP may be useful for diagnostic, prognostic, and therapeutic effect monitoring of OA [16,18,20]. Previous work has revealed that COMP levels are positively associated with radiographic OA severity and obesity [18,51] and thus COMP has gained increasing support as an indicator of OA severity and progression [18]. Furthermore, serum COMP concentrations are increased following acute bouts of cyclic mechanical loading [15,21,52]. Thus, provided that weight loss between OA-negative and OA-positive groups was comparable (p > 0.05), the observation that serum COMP concentrations were reduced to a greater extent in OA-negative individuals may suggest that these individuals were able to reduce cartilage breakdown to a greater extent than OA-positive individuals.
Diminishing physical function in older adults is associated with OA and obesity [4,[31], [32], [33], [34]]. Therefore, periodic assessment of physical function in individuals afflicted with OA and obesity may be a useful prognostic indicator of disease severity, progression, and overall quality of life [30,31,33,34]. In the present study, no differences in physical function measures were observed at baseline between the two OA groups. This may be due, in part, to the enrollment requirement of mild to moderate functional impairment (SPPB), however, it may also be attributable to the reduced physical function generally observed in older adults [4,30,31,53]. Encouragingly, most participants in this clinical trial experienced marked improvements in their physical function despite the fact that, due to their functional status, the prescribed exercise treatment fell below that of the physical activity recommendations for older adults [54]. Nevertheless, in other studies, diet and exercise combined were more effective at improving physical function than diet or exercise alone [33,48,51]. This may suggest that improvements in physical function observed in this study are more likely due to the effects of weight loss than through exercise-specific interventions [47,48]. While hand grip strength is an indicator of functionality in older adults [55] and correlates to weight in healthy individuals [53], we saw no statistically meaningful change among the participants over the course of the intervention. Of note, there are inconsistent results in the literature over the use of hand grip strength in the elderly as a predictor of physical function [56,57]. However, all other physical function measurements for this study were improved throughout the course of this intervention.
This study includes a few limitations. One limitation of the study is the variability in weight loss achieved by participants, with change in percent body weight ranging from a gain of 2% to a loss of 16% body weight. However, the mean weight loss was 6%, which is commonly associated with clinically important improvements in weight-related complications, including hypertension, type II diabetes, and dyslipidemia [58,59]. An additional limitation is that OA status of participants was ascertained based on medical history. Hence, we were not able to relate the outcome measures in the current study to clinical imaging markers. Thus, because participants did not receive imaging in this study, it is possible that individuals considered to be clinically OA-negative may actually have radiographic OA. Nevertheless, the observation that COMP concentrations were reduced to a greater extent in this group of OA-negative individuals underscores the importance of this finding and highlights that individuals are able to improve markers of cartilage health through modest weight loss.
In light of recent findings that obesity alters cartilage mechanical function and composition [14,15], the results of this study suggest that cartilage health may be improved through weight loss. Specifically, the serum COMP reductions observed in both those with and without OA provide encouraging evidence that weight loss may be beneficial for cartilage health in adults with obesity. Future studies should explore how weight loss may relate to cartilage composition and mechanical function.
5. Conclusion
The results of the present study suggest that weight loss may be beneficial to cartilage health in both those with and without clinical OA diagnosis. These findings demonstrate the beneficial effects of weight loss on cartilage health and physical function regardless of clinical OA status, but with the potential for greater benefits to cartilage health in those without diagnosed OA. Future work investigating the relationship between weight loss and cartilage function may help inform how weight loss may improve OA prognosis and cartilage health.
Author contributions
JAC: Conceptualization, Methodology, Software, Validation, Formal analysis, Statistical expertise, Data curation, Writing – Original draft preparation, Writing – Reviewing and Editing; ALM: Project administration, Investigation, Methodology, Writing – Original draft preparation, Writing – Reviewing and Editing; AGH: Methodology, Validation, Formal analysis, Data curation; MSB: Methodology, Validation, Formal analysis, Data curation; ASK: Methodology, Statistical expertise, Formal analysis, Writing – Original draft preparation, Writing – Reviewing and Editing; ATC: Formal analysis, Data curation, Writing – Original draft preparation, Writing – Reviewing and Editing; CWB: Conceptualization, Methodology, Resources, Supervision, Project administration, Funding acquisition, Methodology, Investigation, Writing - Review & Editing; KNPS: Conceptualization, Methodology, Resources, Supervision, Project administration, Funding acquisition, Investigation, Writing - Review & Editing; LED: Conceptualization, Methodology, Resources, Supervision, Project administration, Funding acquisition, Investigation, Writing - Review & Editing.
Role of the funding source
This work was supported by the National Dairy Council, National Institutes of Health (5T32 AG00029), Claude Pepper Independence Center (5P30AG028716-13), U.S. Department of Veterans Affairs Rehabilitation Research and Development Service Program (IK2 RX002348; I01 RX002843) and the NIH (AR074800, AR065527, AR075399, and AR079184).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Handling Editor: Professor H Madry
Contributor Information
James A. Coppock, Email: james.coppock@duke.edu.
Amy L. McNulty, Email: alr@duke.edu.
Kathryn N. Porter Starr, Email: kathryn.starr@duke.edu.
Abigail G. Holt, Email: abigail.gh573@gmail.com.
Michael S. Borack, Email: michael.borack@gmail.com.
Amber T. Collins, Email: amber.collins@duke.edu.
Connie W. Bales, Email: connie.bales@duke.edu.
Louis E. DeFrate, Email: lou.defrate@duke.edu.
References
- 1.Porter Starr K.N., Bales C.W. Excessive body weight in older adults. Clin. Geriatr. Med. 2015;31:311–326. doi: 10.1016/j.cger.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reyes C., Leyland K.M., Peat G., Cooper C., Arden N.K., Prieto-Alhambra D. Association between overweight and obesity and risk of clinically diagnosed knee, hip, and hand osteoarthritis: a population-based cohort study. Arthritis Rheumatol. 2016;68:1869–1875. doi: 10.1002/art.39707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Griffin T.M., Guilak F. The role of mechanical loading in the onset and progression of osteoarthritis. Exerc. Sport Sci. Rev. 2005;33:195–200. doi: 10.1097/00003677-200510000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Miller G.D., Nicklas B.J., Loeser R.F. Inflammatory biomarkers and physical function in older, obese adults with knee pain and self-reported osteoarthritis after intensive weight-loss therapy. J. Am. Geriatr. Soc. 2008;56:644–651. doi: 10.1111/j.1532-5415.2007.01636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Issa R.I., Griffin T.M. Pathobiology of obesity and osteoarthritis: integrating biomechanics and inflammation. Pathobiol. Aging Age Relat. Dis. 2012;2 doi: 10.3402/pba.v2i0.17470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berenbaum F., Eymard F., Houard X. Osteoarthritis, inflammation and obesity. Curr. Opin. Rheumatol. 2013;25:114–118. doi: 10.1097/BOR.0b013e32835a9414. [DOI] [PubMed] [Google Scholar]
- 7.Cicuttini F.M., Wluka A.E. Not just loading and age: the dynamics of osteoarthritis, obesity and inflammation. Med. J. Aust. 2016;204:47. doi: 10.5694/mja15.01069. [DOI] [PubMed] [Google Scholar]
- 8.Wang T.T., He C.Q. Pro-inflammatory cytokines: the link between obesity and osteoarthritis. Cytokine Growth Factor Rev. 2018;44:38–50. doi: 10.1016/j.cytogfr.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Felson D.T., Goggins J., Niu J., Zhang Y., Hunter D.J. The effect of body weight on progression of knee osteoarthritis is dependent on alignment. Arthritis Rheum. 2004;50:3904–3909. doi: 10.1002/art.20726. [DOI] [PubMed] [Google Scholar]
- 10.Andriacchi T.P., Mundermann A. The role of ambulatory mechanics in the initiation and progression of knee osteoarthritis. Curr. Opin. Rheumatol. 2006;18:514–518. doi: 10.1097/01.bor.0000240365.16842.4e. [DOI] [PubMed] [Google Scholar]
- 11.Collins A.T., Kulvaranon M., Spritzer C.E., McNulty A.L., DeFrate L.E. The influence of obesity and meniscal coverage on in vivo tibial cartilage thickness and strain. Orthopaed. J. Sports Med. 2020;8 doi: 10.1177/2325967120964468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coleman J.L., Widmyer M.R., Leddy H.A., Utturkar G.M., Spritzer C.E., Moorman C.T., 3rd, et al. Diurnal variations in articular cartilage thickness and strain in the human knee. J. Biomech. 2013;46:541–547. doi: 10.1016/j.jbiomech.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Widmyer M.R., Utturkar G.M., Leddy H.A., Coleman J.L., Spritzer C.E., Moorman C.T., et al. High body mass index is associated with increased diurnal strains in the articular cartilage of the knee. Arthritis Rheum. 2013;65:2615–2622. doi: 10.1002/art.38062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collins A.T., Kulvaranon M.L., Cutcliffe H.C., Utturkar G.M., Smith W.A.R., Spritzer C.E., et al. Obesity alters the in vivo mechanical response and biochemical properties of cartilage as measured by MRI. Arthritis Res. Ther. 2018;20 doi: 10.1186/s13075-018-1727-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piscoya J.L., Fermor B., Kraus V.B., Stabler T.V., Guilak F. The influence of mechanical compression on the induction of osteoarthritis-related biomarkers in articular cartilage explants. Osteoarthritis Cartilage. 2005;13:1092–1099. doi: 10.1016/j.joca.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 16.Saxne T., Heinegard D. Cartilage oligomeric matrix protein - a novel marker of cartilage turnover detectable in synovial-fluid and blood. Br. J. Rheumatol. 1992;31:583–591. doi: 10.1093/rheumatology/31.9.583. [DOI] [PubMed] [Google Scholar]
- 17.Recklies A.D., Baillargeon L., White C. Regulation of cartilage oligomeric matrix protein synthesis in human synovial cells and articular chondrocytes. Arthritis Rheum. 1998;41:997–1006. doi: 10.1002/1529-0131(199806)41:6<997::AID-ART6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 18.Tseng S., Reddi A.H., Di Cesare P.E. Cartilage oligomeric matrix protein (COMP): a biomarker of arthritis. Biomark. Insights. 2009;4 doi: 10.4137/bmi.s645. BMI.S645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Acharya C., Yik J.H., Kishore A., Van Dinh V., Di Cesare P.E., Haudenschild D.R. Cartilage oligomeric matrix protein and its binding partners in the cartilage extracellular matrix: interaction, regulation and role in chondrogenesis. Matrix Biol. 2014;37:102–111. doi: 10.1016/j.matbio.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 20.Vilim V., Olejarova M., Machacek S., Gatterova J., Kraus V.B., Pavelka K. Serum levels of cartilage oligomeric matrix protein (COMP) correlate with radiographic progression of knee osteoarthritis. Osteoarthritis Cartilage. 2002;10:707–713. doi: 10.1053/joca.2002.0819. [DOI] [PubMed] [Google Scholar]
- 21.Mundermann A., Dyrby C.O., Andriacchi T.P., King K.B. Serum concentration of cartilage oligomeric matrix protein (COMP) is sensitive to physiological cyclic loading in healthy adults. Osteoarthritis Cartilage. 2005;13:34–38. doi: 10.1016/j.joca.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 22.Andersson M.L., Thorstensson C.A., Roos E.M., Petersson I.F., Heinegard D., Saxne T. Serum levels of cartilage oligomeric matrix protein (COMP) increase temporarily after physical exercise in patients with knee osteoarthritis. BMC Muscoskel. Disord. 2006;7:98. doi: 10.1186/1471-2474-7-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verma P., Dalal K. Serum cartilage oligomeric matrix protein (COMP) in knee osteoarthritis: a novel diagnostic and prognostic biomarker. J. Orthop. Res. 2013;31:999–1006. doi: 10.1002/jor.22324. [DOI] [PubMed] [Google Scholar]
- 24.Fantuzzi G. Adiponectin and inflammation: consensus and controversy. J. Allergy Clin. Immunol. 2008;121:326–330. doi: 10.1016/j.jaci.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 25.Felson D.T., Lawrence R.C., Dieppe P.A., Hirsch R., Helmick C.G., Jordan J.M., et al. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann. Intern. Med. 2000;133:635–646. doi: 10.7326/0003-4819-133-8-200010170-00016. [DOI] [PubMed] [Google Scholar]
- 26.Marques-Vidal P., Bochud M., Bastardot F., Lüscher T., Ferrero F., Gaspoz J.-M., et al. Levels and determinants of inflammatory biomarkers in a Swiss population-based sample (CoLaus study) PLoS One. 2011;6 doi: 10.1371/journal.pone.0021002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi J., Joseph L., Pilote L. Obesity and C-reactive protein in various populations: a systematic review and meta-analysis. Obes. Rev. 2013;14:232–244. doi: 10.1111/obr.12003. [DOI] [PubMed] [Google Scholar]
- 28.Jin X.Z., Beguerie J.R., Zhang W.Y., Blizzard L., Otahal P., Jones G., et al. Circulating C reactive protein in osteoarthritis: a systematic review and meta-analysis. Ann. Rheum. Dis. 2015;74:703–710. doi: 10.1136/annrheumdis-2013-204494. [DOI] [PubMed] [Google Scholar]
- 29.Runhaar J., Beavers D.P., Miller G.D., Nicklas B.J., Loeser R.F., Bierma-Zeinstra S., et al. Inflammatory cytokines mediate the effects of diet and exercise on pain and function in knee osteoarthritis independent of BMI. Osteoarthritis Cartilage. 2019;27:1118–1123. doi: 10.1016/j.joca.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 30.Corti M.C., Rigon C. Epidemiology of osteoarthritis: prevalence, risk factors and functional impact. Aging Clin. Exp. Res. 2003;15:359–363. doi: 10.1007/BF03327356. [DOI] [PubMed] [Google Scholar]
- 31.Webb E.J., Osmotherly P.G., Baines S.K. Physical function after dietary weight loss in overweight and obese adults with osteoarthritis: a systematic review and meta-analysis. Publ. Health Nutr. 2021;24:338–353. doi: 10.1017/S1368980020002529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richette P., Poitou C., Garnero P., Vicaut E., Bouillot J.L., Lacorte J.M., et al. Benefits of massive weight loss on symptoms, systemic inflammation and cartilage turnover in obese patients with knee osteoarthritis. Ann. Rheum. Dis. 2011;70:139–144. doi: 10.1136/ard.2010.134015. [DOI] [PubMed] [Google Scholar]
- 33.Messier S.P., Resnik A.E., Beavers D.P., Mihalko S.L., Miller G.D., Nicklas B.J., et al. Intentional weight loss in overweight and obese patients with knee osteoarthritis: Is more better? Arthritis Care Res. 2018;70:1569–1575. doi: 10.1002/acr.23608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lo J., Chan L., Flynn S. A systematic review of the incidence, prevalence, costs, and activity and work limitations of amputation, osteoarthritis, rheumatoid arthritis, back pain, multiple sclerosis, spinal cord injury, stroke, and traumatic brain injury in the United States: a 2019 update. Arch. Phys. Med. Rehabil. 2021;102:115–131. doi: 10.1016/j.apmr.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buckwalter J.A., Mankin H.J., Grodzinsky A.J. Articular cartilage and osteoarthritis. Instr. Course Lect. 2005;54:465–480. [PubMed] [Google Scholar]
- 36.Miller M.G., Porter Starr K.N., Rincker J., Orenduff M.C., McDonald S.R., Pieper C.F., et al. Rationale and design for a higher (dairy) protein weight loss intervention that promotes muscle quality and bone health in older adults with obesity: a randomized, controlled pilot study. J. Nutr. Gerontol. Geriatr. 2021;40:150–170. doi: 10.1080/21551197.2021.1896615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Porter Starr K.N., Mcdonald S.R., Bales C.W. Obesity and physical frailty in older adults: a scoping review of lifestyle intervention trials. J. Am. Med. Dir. Assoc. 2014;15:240–250. doi: 10.1016/j.jamda.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stannus O., Jones G., Cicuttini F., Parameswaran V., Quinn S., Burgess J., et al. Circulating levels of IL-6 and TNF-alpha are associated with knee radiographic osteoarthritis and knee cartilage loss in older adults. Osteoarthritis Cartilage. 2010;18:1441–1447. doi: 10.1016/j.joca.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 39.Miller G.D., Jenks M.Z., Vendela M., Norris J.L., Muday G.K. Influence of weight loss, body composition, and lifestyle behaviors on plasma adipokines: a randomized weight loss trial in older men and women with symptomatic knee osteoarthritis. J. Obes. 2012;2012:1–14. doi: 10.1155/2012/708505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Messier S.P., Devita P., Cowan R.E., Seay J., Young H.C., Marsh A.P. Do older adults with knee osteoarthritis place greater loads on the knee during gait? A preliminary study. Arch. Phys. Med. Rehabil. 2005;86:703–709. doi: 10.1016/j.apmr.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 41.Guralnik J.M., Simonsick E.M., Ferrucci L., Glynn R.J., Berkman L.F., Blazer D.G., et al. A Short physical performance Battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J. Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 42.Collins A.T., Hu G.L., Newman H., Reinsvold M.H., Goldsmith M.R., Twomey-Kozak J.N., et al. Obesity alters the collagen organization and mechanical properties of murine cartilage. Sci. Rep. 2021;11 doi: 10.1038/s41598-020-80599-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tamayo K.S., Heckelman L.N., Spritzer C.E., DeFrate L.E., Collins A.T. Obesity impacts the mechanical response and biochemical composition of patellofemoral cartilage: an in vivo, MRI-based investigation. J. Biomech. 2022;134 doi: 10.1016/j.jbiomech.2022.110991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Collins A.T., Kulvaranon M.L., Cutcliffe H.C., Utturkar G.M., Smith W.A.R., Spritzer C.E., et al. Obesity alters the in vivo mechanical response and biochemical properties of cartilage as measured by MRI. Arthritis Res. Ther. 2018;20 doi: 10.1186/s13075-018-1727-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Francin P.J., Abot A., Guillaume C., Moulin D., Bianchi A., Gegout-Pottie P., et al. Association between adiponectin and cartilage degradation in human osteoarthritis. Osteoarthritis Cartilage. 2014;22:519–526. doi: 10.1016/j.joca.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 46.Kershaw E.E., Flier J.S. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metabol. 2004;89:2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 47.Messier S.P. Diet and exercise for obese adults with knee osteoarthritis. Clin. Geriatr. Med. 2010;26:461–477. doi: 10.1016/j.cger.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Messier S.P., Beavers D.P., Mihalko S.L., Miller G.D., Lyles M.F., Hunter D.J., et al. The effects of intensive dietary weight loss and exercise on gait in overweight and obese adults with knee osteoarthritis. The Intensive Diet and Exercise for Arthritis (IDEA) trial. J. Biomech. 2020;98 doi: 10.1016/j.jbiomech.2019.109477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bliddal H., Leeds A.R., Christensen R. Osteoarthritis, obesity and weight loss: evidence, hypotheses and horizons – a scoping review. Obes. Rev. 2014;15:578–586. doi: 10.1111/obr.12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Atukorala I., Makovey J., Lawler L., Messier S.P., Bennell K., Hunter D.J. Is there a dose-response relationship between weight loss and symptom improvement in persons with knee osteoarthritis? Arthritis Care Res. 2016;68:1106–1114. doi: 10.1002/acr.22805. [DOI] [PubMed] [Google Scholar]
- 51.Chua S.D., Messier S.P., Legault C., Lenz M.E., Thonar E.J.-M.A., Loeser R.F. Effect of an exercise and dietary intervention on serum biomarkers in overweight and obese adults with osteoarthritis of the knee. Osteoarthritis Cartilage. 2008;16:1047–1053. doi: 10.1016/j.joca.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Palmoski M.J., Brandt K.D. Effects of static and cyclic compressive loading on articular cartilage plugs in vitro. Arthritis Rheum. 1984;27:675–681. doi: 10.1002/art.1780270611. [DOI] [PubMed] [Google Scholar]
- 53.Chandrasekaran B., Ghosh A., Prasad C., Krishnan K., Chandrasharma B. Age and anthropometric traits predict handgrip strength in healthy normals. J Hand Microsurg. 2010;2:58–61. doi: 10.1007/s12593-010-0015-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sparling P.B., Howard B.J., Dunstan D.W., Owen N. Recommendations for physical activity in older adults. BMJ. 2015;350:h100. doi: 10.1136/bmj.h100. [DOI] [PubMed] [Google Scholar]
- 55.Bohannon R.W. Hand-grip dynamometry predicts future outcomes in aging adults. J. Geriatr. Phys. Ther. 2008;31:3–10. doi: 10.1519/00139143-200831010-00002. [DOI] [PubMed] [Google Scholar]
- 56.Burton W.N., Chen C.Y., Conti D.J., Pransky G., Edington D.W. Caregiving for ill dependents and its association with employee health risks and productivity. J. Occup. Environ. Med. 2004;46:1048–1056. doi: 10.1097/01.jom.0000141830.72507.32. [DOI] [PubMed] [Google Scholar]
- 57.Norman K., Smoliner C., Kilbert A., Valentini L., Lochs H., Pirlich M. Disease-related malnutrition but not underweight by BMI is reflected by disturbed electric tissue properties in the bioelectrical impedance vector analysis. Br. J. Nutr. 2008;100:590–595. doi: 10.1017/S0007114508911545. [DOI] [PubMed] [Google Scholar]
- 58.Yanovski S.Z., Yanovski J.A. Obesity. N Engl J Med. 2002;346:591–602. doi: 10.1056/NEJMra012586. [DOI] [PubMed] [Google Scholar]
- 59.Goodpaster B.H., Delany J.P., Otto A.D., Kuller L., Vockley J., South-Paul J.E., et al. Effects of diet and physical activity interventions on weight loss and cardiometabolic risk factors in severely obese adults: a randomized trial. JAMA. 2010;304:1795–1802. doi: 10.1001/jama.2010.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]