Abstract
Introduction
Evidence from cardiovascular outcome trials (CVOTs) for newer antidiabetic drugs is increasingly influencing revised recommendations for second-line therapy in type 2 diabetes (T2D). This systematic review aimed to compare the cost-effectiveness of newer antidiabetic drugs specified as sodium-glucose cotransporter 2 inhibitor (SGLT2i), glucagon-like peptide 1 receptor agonist (GLP-1RA), and dipeptidyl peptidase 4 inhibitor (DPP-4i) for T2D in a second-line setting.
Methods
A systematic review was conducted following the Preferred Reporting Items for Systematic Reviews (PRISMA) guidelines, and all relevant published studies were searched comprehensively in electronic databases, including PubMed, Embase, Web of Science, and International Health Technology Assessment database published from April 2023. The quality of the included studies was evaluated using Consolidated Health Economic Evaluation Reporting Standards (CHEERS) 2022 reporting checklists.
Results
We included 28 studies that met the inclusion criteria. Overall reporting of the identified studies largely met CHEERS 2022 recommendations. The CORE and Cardiff models were the most frequently utilized for pharmacoeconomic evaluation in T2D. Four studies consistently discovered that SGLT2i was more cost-effective than GLP-1RA in T2D who were not adequately controlled by metformin monotherapy. Four studies compared GLP-1RA with DPP-4i, sufonylurea (SU), or insulin. Except for one that demonstrated SU was cost-effective, all were GLP-1RA. Five studies revealed that SGLT2i was more cost-effective than DPP-4i or SU. Eleven studies indicated that DPP-4i was more cost-effective than traditional antidiabetic drugs. Four additional studies explored the cost-effectiveness of various antidiabetic drugs as second-line options, indicating that SU, SGLT2i, or meglitinides were more economically advantageous. The most common driven factors were the cost of new antidiabetic drugs.
Conclusion
Newer antidiabetic drugs as second line are the cost-effective option for T2D from the cost-effectiveness perspective, especially SGLT2i.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-023-02612-z.
Keywords: Cost-effectiveness, Dipeptidyl peptidase 4 inhibitor, Glucagon-like peptide 1 receptor agonist, Second-line treatment, Sodium-glucose transporter 2 inhibitor, Type 2 diabetes
Key Summary Points
| Why carry out this study? |
| The surge in fees of high-cost new antidiabetic drugs will likely pose a challenge for the healthcare system. There are weak supporting evidence and extensive debate regarding the best second-line agent considering long-term efficacy, quality of life, and cost. Therefore, whether new antidiabetic drugs are superior to classic ones remains unclear |
| This systematic review summarizes the pharmacoeconomic studies thus far performed in newer antidiabetic drugs as second-line treatment for type 2 diabetes and provides further evidence to guide treatment strategies |
| What was learned from the study? |
| Newer antidiabetic drugs appear to be generally cost-effective therapy in second-line options with T2D compared to classic antidiabetic drugs |
| SGLT2i was superior to GLP-1RA and DPP-4i; DPP-4i has a good safety profile and weight neutrality, making it more cost-effective than other classical antidiabetic drugs, but not as favorable as GLP-1RA and SGLT2 |
| With the continued improvement in the accessibility and affordability of newer antidiabetic drugs, SGLT2i can be a preferred option for second-line treatment with a great future |
Introduction
Global diabetes-related health expenditures were approximately US$ 966 billion in 2021 and are projected to reach 1054 billion USD by 2045 [1]. Cardiovascular disease (CVD), a major public health challenge, is one of the primary cost-drivers. Diabetes medication affordability and the combination of cardiovascular protective drugs are independent protective factors against cardiovascular death [2].
Increasing evidence suggests newer hypoglycemic agents, such as sodium-glucose cotransporter 2 inhibitor (SGLT2i), glucagon-like peptide 1 receptor agonist (GLP-1 RA), and dipeptidyl peptidase 4 inhibitor (DPP4i), carry lower risks of hypoglycemia than conventional hypoglycemic agents, such as sulfonylurea (SU) and insulin, promote weight loss, and are weight neutral. Crucially, these agents are beneficial in reducing CVD events and mortality in type 2 diabetes (T2D) patients at increased cardiovascular risk [3, 4]. Therefore, new classes of hypoglycemic agents have been progressively replacing SU as the most common treatment for metformin monotherapy failure [5]. Nevertheless, shifting to newer hypoglycemic agents increases the diabetes treatment cost, which could outweigh savings from cardiovascular benefits [6].
The second-line choice in clinical practice has become more complex and uncertain with the constant updating of evidence and guidelines and the rapid expansion of newer antidiabetic drugs. Given the many therapeutic options with a wide range of costs, a challenge for the health system is the suitable selection as second line in new antidiabetics to ensure maximum benefit and acceptable cost. Although several studies have systematically evaluated that newer antidiabetics, including GLP-1 RA, DPP-4i, and SGLT2i, are more cost-effective than classical antidiabetics, such as insulin, thiazolidinedione (TZD) and SU [7, 8], these studies are not explicitly designed for second-line strategies in T2D.
The latest American Diabetes Association guidelines state that GLP-1 RA and SGLT2i can be used for patients at high risk of CVD, heart failure, or chronic kidney disease, irrespective of glycemic control and baseline metformin [9]. Thus, this cardiorenal protective effect independent of glycemic control has shifted the paradigm of conventional second-line T2D strategies, resulting in a continued increase in the overall use of these high-cost new antidiabetics. This systematic review aims to evaluate the cost-effectiveness of the newer antidiabetics, including SGLT2i, GLP-1RA, and DPP-4i, as second-line therapy for T2D failed metformin monotherapy to provide a reference for future clinical decision‐making.
Methods
Search Strategy
We conducted a systematic search according to the PRISMA 2020 statement [10]. PubMed, Embase, Web of Science, and International Health Technology Assessment (HTA) databases were searched for eligible articles until April 26, 2023. Search strategies are provided in Supplementary Table S1.
Study Selection
Economic evaluations were selected using the following search technique based on the PICOS (Participants, Intervention, Comparator, Outcome, Study design) criteria in Supplementary Table S2. Two independent investigators (JZ, YZ) extracted eligible studies and relevant data. A third investigator (QL) resolved the discrepancies between the two investigators and verified for data accuracy. This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Participants: Adults with T2D.
Intervention: second-line therapy.
Outcome: cost, life gain year (LYG), quality-adjusted life year (QALY), incremental cost-effective ratio (ICER), and incremental net monetary benefit (INMB).
Study design: cost-effectiveness analysis (CEA) and cost-utility analysis (CUA).
Data Extraction and Synthesis Strategy
Data items extracted included study characteristics (e.g. author, year of publication, region, perspective, interventions, modelling approach, time horizon, sensitivity analysis, funding source, etc.) and primary outcomes (ICER and INMB). INMB = ΔQALY * willingness-to-pay thresholds—ΔCost. All costs were converted into 2022 US dollars using the CCEMG-EPPI-Center Cost Converter Version.1.6 via purchasing power parities to make ICER and INMB comparable across studies [11].
Quality Assessment Reporting
Reporting quality was assessed using the 28-item checklist of the Consolidated Health Economic Evaluation Reporting Standards (CHEERS 2022) statement [12]. The corresponding scores of every ‘Yes’ (completely fulfill the items), ‘Partly’ (partially fulfill), and ‘NA’ (not applicable) recorded for each item were assigned 1, 0.5, and 0, respectively. The quality of the included studies was ranked as high, moderate, or poor quality depending on the total score: high quality for score ≥ 21 (quality percentage score > 75%); moderate quality for score 14–21 (quality percentage score between 50 and 75%); poor quality for score for score ≤ 14 (quality percentage score < 50%) [13].
Results
Search Results
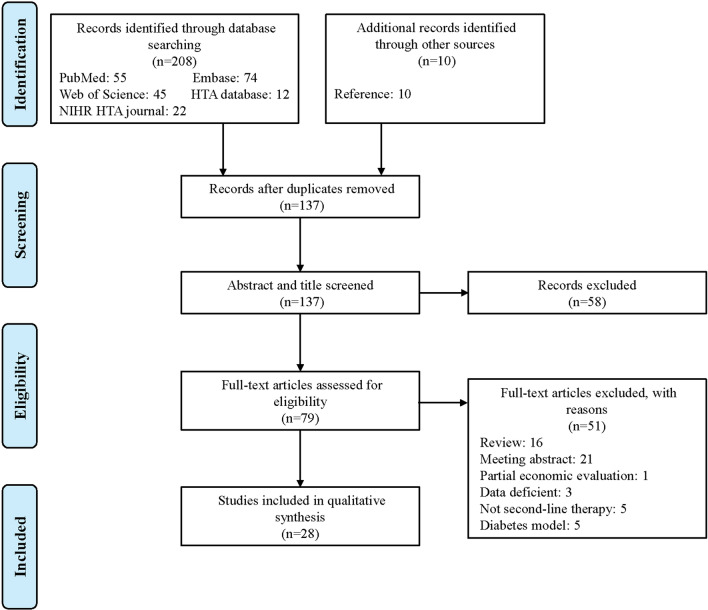
Figure 1 presents the literature search strategy and exclusion criteria. The search yielded 218 publications. Following title and abstract screening, 81 potentially relevant articles were evaluated for full-text eligibility. The systematic review evaluated 28 studies based on the inclusion criteria.
Fig. 1.
PRISMA flowchart of study selection
General Characteristics of the Included Studies
Table 1 presents the general characteristics of the included studies. The most studied population was adults with T2D, and a few focused on specific populations, such as established CVD [14] and patients > 65 years old [15]. Two additional studies analyzed subgroups for heart failure [16] as well as gender and smoking status [17]. Among the included second-line comparison, four studies compared GLP-1RA and SGLT2i [14, 16, 18, 19]. The rest were SGLT2i (n = 5) [20–24], GLP-1RA (n = 4) [17, 25–27], and DPP-4i (n = 11) [15, 28–37] compared to other hypoglycemic agents. Four additional studies compared multiple second-line therapies for T2D [38–41].
Table 1.
Characteristics of the included studies
| Author, year | Region | Perspective | Interventions | Model | Time horizon | Discount rate | Sensitivity analysis | Funding |
|---|---|---|---|---|---|---|---|---|
| GLP-1RA vs. SGLT2i | ||||||||
| Ehlers et al., 2022 [18] | Denmark | Payer | Semaglutide (sc) vs. empagliflozin | CORE | 50Y | 4% | Scenario, DSA and PSA | Industry |
| Ehlers et al., 2021 [14] | Denmark | Healthcare | Liraglutide vs. empagliflozin | CORE | 50Y | 4% | Scenario, DSA and PSA | Industry |
| Ramos et al., 2020 [16] | UK | Payer | Semaglutide (po) vs. empagliflozin | CORE | 50Y | 3.5% | Scenario and PSA | Industry |
| Reifsnider et al., 2022 [19] | USA | Payer | Liraglutide vs. empagliflozin | DICE | Lifetime | 3% | Scenario, DSA and PSA | Industry |
| GLP-1RA vs. othersa | ||||||||
| Steen Carlsson et al., 2014 [17] | Sweden | Societal | Liraglutide vs. Glimepiride or Sitagliptin | IHECM | 40Y | 3% | Scenario and PSA | Industry |
| Davies et al., 2012 [25] | UK | Payer | Liraglutide vs. sitagliptin or glimepiride | CORE | 50Y | 3.5% | DSA and PSA | Industry |
| Sinha et al., 2010 [27] | USA | Healthcare | Exenatide (daily) vs. sitagliptin or glyburide | UKPDS | Lifetime | 3% | DSA | Industry |
| Kiadaliri et al., 2014 [26] | Sweden | Societal | GLP-1RA vs. DPP-4i or NPH insulin | IHECM | 35Y | 3% | DSA and PSA | Public funding |
| SGLT2i vs. othersb | ||||||||
| Reifsnider et al., 2021 [23] | USA | Payer | Empagliflozin vs. sitagliptin | DICE | Lifetime | 3% | Scenario, DSA and PSA | Industry |
| Charokopou et al., 2015 [22] | UK | Healthcare | Dapagliflozin vs. sitagliptin | Cardiff | 40Y | 3.5% | Scenario, DSA and PSA | Industry |
| Bagepally et al., 2021 [20] | India | Payer | Dapagliflozin vs. SU | Markov | Lifetime | 3% | DSA and PSA | Public funding |
| Charokopou et al., 2015 [21] | UK | Healthcare | Dapagliflozin vs. SU | Cardiff | 40Y | 3.5% | Scenario, DSA and PSA | Industry |
| Tzanetakos et al., 2016 [24] | Greece | Payer | Dapagliflozin vs. DPP-4i or SU | Cardiff | 40Y | 3.5% | Scenario, DSA and PSA | Industry |
| DPP-4i vs. othersc | ||||||||
| Gu et al., 2015 [33] | China | Payer | Saxagliptin vs. glimepiride | Cardiff | 40Y | 3% | DSA and PSA | Industry |
| Elgart et al., 2013 [28] | Argentina | Payer | Saxagliptin vs. SU | Cardiff | 20Y | 3.5% | DSA and PSA | Industry |
| Granström et al., 2012 [31] | Sweden | Not specified | Saxagliptin vs. glipizide | Cardiff | 40Y | 3% | DSA and PSA | Not specified |
| Erhardt et al., 2012 [29] | Germany | Payer | Saxagliptin vs. glipizide | Cardiff | 40Y | 3% | DSA and PSA | Industry |
| Grzeszczak et al., 2012 [32] | Poland | Payer | Saxagliptin vs. NPH insulin | Cardiff | 40Y | 3.5% | Scenario, DSA and PSA | Industry |
| Kousoulakou et al., 2017 [35] | Greece | Payer | Vildagliptin vs. glimepiride | UKPDS | Lifetime | 4% | DSA | Industry |
| Viriato et al., 2014 [37] | Portugal | Healthcare | Vildagliptin vs. SU | UKPDS | 40Y | 5% | Scenario, DSA and PSA | Industry |
| Gordon et al., 2016 [30] | UK | Not specified | Alogliptin vs. glipizide | CORE | 50Y | 3.5% | Scenario, DSA and PSA | Industry |
| Schwarz et al., 2008 [36] | Europe | Not specified | Sitagliptin vs. rosiglitazone or SU | JADE | Lifetime | 3%-6% | DSA | Not specified |
| Gu et al., 2016 [34] | China | Payer | Saxagliptin vs. acarbose | Cardiff | 40Y | 3% | DSA and PSA | Industry |
| Gordon et al., 2017 [15] | UK | Payer | DPP-4i vs. SU or TZD | CORE | 50Y | 3.5% | PSA | Industry |
| Multiple antidiabetic drugs | ||||||||
| CADTH, 2017 [40] | Canada | Healthcare | SU, DPP-4i, SGLT2i, GLP-1RA, basal insulin or biphasic insulin | UKPDS | 40Y | NA | Scenario, DSA and PSA | Public funding |
| Klarenbach et al., 2011 [41] | Canada | Payer | Metformin, SU, Meglitinide, AGI, TZD, DPP-4i, basal insulin or biphasic insulin | UKPDS | 40Y | 5% | DSA | Public funding |
| Chien et al., 2020 [39] | Taiwan | Payer | SU, SGLT2i, GLP-1RA, DPP-4i or insulin | Cardiff | 40Y | 3% | Scenario, DSA and PSA | Public funding |
| Gu et al., 2020 [38] | China | Healthcare | SU, TZD, AGI, meglitinide or DPP-4i | Cardiff | 40Y | 3% | Scenario, DSA and PSA | No funding |
AGI α-glucosidase inhibitors, CADTH Canadian Agency for Drugs and Technologies in Health, CORE Centre for Outcomes Research Diabetes Model, DICE discretely integrated condition event, DPP-4i dipeptidyl peptidase-4 inhibitor, DSA deterministic sensitivity analysis, GLP-1RA glucagon-like peptide-1 receptor agonist, IHECM Swedish Institute for Health Economics Cohort Model, JADE Januvia Diabetes Economic Model, NPH neutral protamine Hagedorn, PSA probabilistic sensitivity analysis, SGLT2i sodium-glucose cotransporter-2 inhibitor, SU sulfonylurea, TZD thiazolidinedione, UKPDS UK prospective diabetes study outcomes
aGLP-1RA vs. DPP-4i, SU or insulin
bSGLT2i vs. DPP-4i or SU
cDPP-4i vs. SU, TZD, AGI or insulin
Seventeen studies were conducted in Europe, including six in the UK [8, 16, 21, 22, 25, 30], three in Sweden [17, 26, 31], two in Denmark [14, 18], and two in Greece [24, 35]; the rest were in Poland [32], Germany [29], Portugal [37], and Europe [36]. Six were conducted in North America (two in Canada [40, 41] and three in the USA [19, 23, 27]) and one in South America (Argentina [28]). Five additional studies were from Asia (four in China [33, 34, 38, 39] and one in India [20]). All studies adopted the model-based simulation approach using data predominately derived from clinical trials or literature, except for one based on real-world data.
The most frequently used models in order were Cardiff (n = 11) [21, 22, 24, 28, 29, 31–34, 38, 39], CORE diabetes model (n = 6) [14–16, 18, 25, 30], UK Prospective Diabetes Study Outcomes (UKPDS) (n = 5), discretely integrated condition event (DICE) (n = 2) [19, 23], Swedish Institute for Health economics cohort model (IHECM) (n = 2) [17, 26], Januvia Diabetes Economic (JADE) (n = 1) [36], and Markov (n = 1) [20].
More than half (57.1%, 16/28) of the studies examined the payer perspective [15, 16, 18–20, 23–25, 28, 29, 32–35, 39, 41]. Only two studies adopted societal [17, 26], the remaining seven were healthcare [14, 21, 22, 27, 37, 38, 40], and three were unspecified [30, 31, 36]. The most reported funding was sponsored by pharmaceutical companies (71.4%, 20/28) [14–19, 21–25, 27–30, 32–35, 37], five were public funding [20, 26, 39–41], one was no funding [38], and two were not stated [31, 36].
Quality of the Included Studies
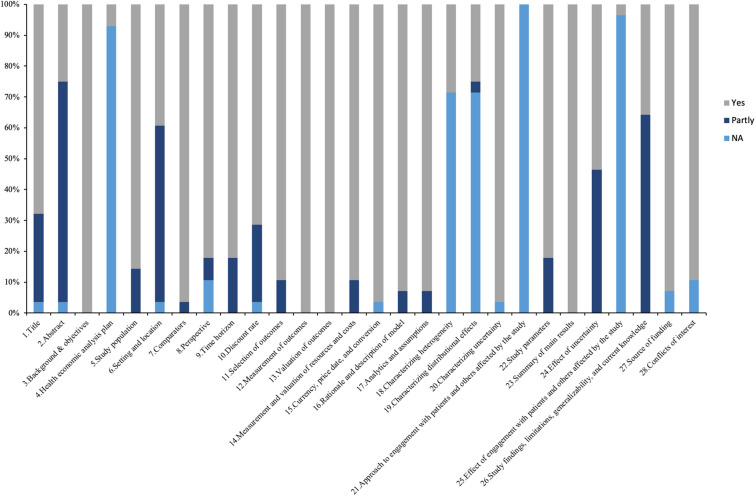
Figure 2 presents the quality assessment results of the design and performance of pharmacoeconomic analysis of second-line therapies for T2D using the CHEERS guideline. According to the quality assessment, approximately 70% (20/28) of studies met the high-quality criteria (Supplementary Table S3). The most frequently unreported were item 21, “Approach to engagement with patients and others affected by the study,” and item 25, “Effect of engagement with patients and others affected by the study.”
Fig. 2.
Quality evaluation result based on CHEERS 2022
Pharmacoeconomics Evaluation Results
Tables 2 and 3 summarize the economic outcomes of the included studies.
Table 2.
Base case analyses of newer antidiabetic drugs combined with metformin as second-line treatment
| Author | Currency year | Interventions | Comparator | ICERa | INMBb | Cost-effectiveness | ||
|---|---|---|---|---|---|---|---|---|
| Original | 2022 US dollars | Original | 2022 US dollars | |||||
| GLP-1RA vs. SGLT2i | ||||||||
| Ehlers et al. [18] | 2019 | Semaglutide 1 mg/week | Empagliflozin 25 mg/day | DKK 745,561 | USD 108,332 | DKK -50,500 | USD -7338 | SGLT-2i |
| Ehlers et al. [14] | 2019 | Liraglutide 1.8 mg/day | Empagliflozin 25 mg/day | Dominated | DKK -88,420 | USD -12,848 | SGLT-2i | |
| Ramos et al. [16] | NA | Semaglutide 14 mg/day | Empagliflozin 25 mg/day | Dominated | GBP -6648 | USD -9940 | SGLT-2i | |
| Reifsnider et al. [19] | 2019 | Liraglutide 1.8 mg/day | Empagliflozin 25 mg/day | Dominated | USD -27,244 | USD -28,978 | SGLT-2i | |
| GLP-1RA vs. othersc | ||||||||
| Steen Carlsson et al. [17] | 2013 | Liraglutide 1.2 mg/day | Glimepiride 4 mg/day | SEK 226,047 to 255,121f | USD 30,563 to 34,494f |
SEK 77,765 to 99,642f SEK 113,102 to 133,377f |
USD 10,514 to 13,472 f USD 15,292 to 18,034 f |
GLP-1RA |
| Liraglutide 1.2 mg/day | Sitagliptin 100 mg/day | SEK 148,766 to 160,827 f | USD 20,114 to 21,745 f | |||||
| Davies et al. [25] | 2008 | Liraglutide 1.2/1.8 mg/day | Glimepiride 4 mg/day | GBP 9449/16,501 | USD 17,337/30,275 | GBP 3397/1,112 | USD 12,104/7,361 | GLP-1RA |
| Liraglutide 1.2/1.8 mg/day | Sitagliptin 100 mg/day | GBP 9851/10,465 | USD 18,074/19,201 | GBP 1758/2776 | USD 6528/10,598 | |||
| Sinha et al. [27] | 2008 | Exenatide 20 μg/d | Glyburide 7.5 mg/d | USD 278,935 | USD 353,523 | USD -14,253 | USD -18,064 | SU |
| Sitagliptin 100 mg/day | Glyburide 7.5 mg/day | USD 169,572 | USD 214,916 | USD -19,574 | USD -24,808 | |||
| Exenatide 20 μg/d | Sitagliptin 100 mg/d | Dominated | USD -5321 | USD -6744 | ||||
| Kiadaliri et al. [26] | 2013 | GLP-1RA | DPP-4i | SEK 353,172 | USD 47,752 | SEK 15,135 | USD 2046 | GLP-1RA |
| GLP-1RA | NPH insulin 40 IU/day | SEK 160,618 | USD 21,717 | SEK 84,198 | USD 11,384 | |||
| DPP-4i | NPH insulin 40 IU/day | SEK 36,050 | USD 4874 | SEK 69,063 | USD 9338 | |||
| SGLT2i vs. othersd | ||||||||
| Reifsnider et al. [23] | 2018 | Empagliflozin 10–25 mg/day | Sitagliptin 50–100 mg/day | USD 6967 | USD 7542 | USD 8182 | USD 8858 | SGLT-2i |
| Charokopou et al. [22] | 2011 | Dapagliflozin 10 mg/day | Sitagliptin 100 mg/day | GBP 6761 | USD 11,797 | GBP 424 | USD 740 | SGLT-2i |
| Bagepally et al. [20] | 2017 | Dapagliflozin 10 mg/day | SU | INR 106,133 | USD 6596 | USD 87,061 | USD 5410 | SGLT-2i |
| Charokopou et al. [21] | 2011 | Dapagliflozin 10 mg/day | SU | GBP 1246 | GBP 2671 | GBP 7954 | USD 13,878 | SGLT-2i |
| Tzanetakos et al. [24] | 2015 | Dapagliflozin 10 mg/day | DPP-4i | EUR 17,695 | USD 32,665 | EUR 944 | USD 1743 | SGLT-2i |
| SU | EUR 10,623 | USD 19,610 | EUR 11,518 | USD 21,262 | ||||
| DPP-4i vs. otherse | ||||||||
| Gu et al. [33] | 2014 | Saxagliptin 5 mg/day | Glimepiride 2.8 mg/day | Dominate | CNY 88,136 | USD 28,697 | DPP-4i | |
| Elgart et al. [28] | 2009 | Saxagliptin | SU | USD 7374 | USD 11,666 | USD 45 | USD 2 | DPP-4i |
| Granström et al. [31] | 2008 | Saxagliptin 5 mg/day | Glipizide 14.7 mg/day | SEK 91,260 | USD 13,184 | SEK 40,516 | USD 5853 | DPP-4i |
| Erhardt et al. [29] | 2009 | Saxagliptin | Glipizide | EUR 13,931 | USD 22,199 | NA | NA | DPP-4i |
| Grzeszczak et al. [32] | 2009 | Saxagliptin 5 mg/day | NPH insulin 25 IU/day | PLN 27,454 | USD 19,245 | PLN 9336 | USD 6544 | DPP-4i |
| Kousoulakou et al. [35] | 2014 | Vildagliptin | Glimepiride | Dominate | EUR 1561 | USD 2872 | DPP-4i | |
| Viriato et al. [37] | 2013 | Vildagliptin | SU | EUR 9072 | USD 17,291 | EUR 2679 | USD 5106 | DPP-4i |
| Gordon et al. [30] | 2015 | Alogliptin 12.5/25 mg/day | Glipizide 5 mg/day | GBP 10,959/7217 | USD 18,092/11,915 | GBP 1989/3218 | USD 3284/5313 | DPP-4i |
| Schwarz et al. [36] | 2007 | Sitagliptin | Rosiglitazone | Dominate to EUR 4766 | Dominate to USD 6918 | NA | NA | DPP-4i |
| Sitagliptin g | SU g | EUR 5949 to 20,350 | USD 11,923 to 31,880 | NA | NA | |||
| Sitagliptinh | SUh | EUR 6029 to 13,655 | USD 12,084 to 21,392 | NA | NA | |||
| Gu et al. [34] | 2014 | Saxagliptin 5 mg/day | Acarbose 150 mg/day | Dominate | CNY 41,304 | USD 12,927 | DPP-4i | |
| Gordon et al. [28] | 2015 | DPP-4i | SU | GBP 18,680 | USD 30,839 | GBP 531 | USD 877 | DPP-4i |
| TZD | GBP 15,343 | USD 25,480 | GBP 1431 | USD 2362 | ||||
DPP-4i dipeptidyl peptidase-4 inhibitor, GLP-1RA glucagon-like peptide-1 receptor agonist, ICER incremental cost-effectiveness ratio; INMB incremental net monetary benefit, NPH neutral protamine Hagedorn, SGLT2i sodium-glucose cotransporter-2 inhibitor, SU sulfonylurea, TZD thiazolidinedione
aIf ICER > WTP, indicated intervention is not cost-effective; if ICER < WTP, indicated intervention is cost-effective; if ΔCost < 0 and ΔQALY > 0, indicated intervention is dominant (cheaper and more effective); if ΔCost > 0 and ΔQALY < 0, indicated intervention is dominated (costly and less effective)
bIf INMB > 0, indicated intervention is cost-effective; if INMB < 0, indicated intervention is not cost-effective
cGLP-1RA versus DPP-4i, SU, or Insulin
dSGLT2i versus DPP-4i or SU
eDPP-4i versus SU, TZD, AGI, or insulin
fSubgroup analyses stratified according to gender and smoking status
gMetformin combined with basal insulin as third line
hMetformin combined with rosiglitazone as third line
Table 3.
Base case analyses of multiple antidiabetic drugs combined with metformin as second-line treatment
| Author | Currency year | Second line | QALYs | Total costs | Rank of cost-effectiveness | |
|---|---|---|---|---|---|---|
| Original | 2022 US dollar | |||||
| CADTH [40] | 2016 | SU | 8.8784 | CAD 39,251 | USD 35,219 | 1 |
| Metformin | 8.8369 | CAD 37,648 | USD 33,780 | 2 | ||
| SGLT2i | 8.9530 | CAD 49,308 | USD 44,242 | 3 | ||
| DPP-4i | 8.8998 | CAD 48,859 | USD 45,674 | 4 | ||
| Basal insulin | 8.8998 | CAD 54,852 | USD 51,276 | 5 | ||
| GLP-1RA | 8.9894 | CAD 55,946 | USD 52,299 | 6 | ||
| Biphasic insulin | 8.9340 | CAD 63,719 | USD 59,565 | 7 | ||
| Klarenbach et al. [41] | 2009 | SU | 8.72 | CAD 40,669 | USD 42,318 | 1 |
| AGI | 8.77 | CAD 42,797 | USD 44,532 | 2 | ||
| Meglitinide | 8.78 | CAD 42,269 | USD 43,983 | 3 | ||
| Metformin | 8.72 | CAD 39,924 | USD 41,543 | 4 | ||
| TZD | 8.78 | CAD 46,202 | USD 48,075 | 5 | ||
| DPP-4i | 8.78 | CAD 47,191 | USD 49,104 | 6 | ||
| Basal insulin | 8.78 | CAD 47,348 | USD 49,268 | 7 | ||
| Biphasic insulin | 8.77 | CAD 52,367 | USD 54,490 | 8 | ||
| Chien et al. [39] | 2019 | SGLT2i (SU as third line) | 12.483 | NTD 283,709 | USD 7593 | 1 |
| SGLT2i (DPP-4i as third line) | 12.548 | NTD 287,891 | USD 7705 | 2 | ||
| SU (SGLT2i as third line) | 11.943 | NTD 249,626 | USD 6681 | 3 | ||
| DPP-4i (SGLT2i as third line) | 12.345 | NTD 282,722 | USD 7566 | 4 | ||
| DPP-4i (SU as third line) | 11.931 | NTD 270,820 | USD 7248 | 5 | ||
| GLP-1RA (SU as third line) | 12.453 | NTD 452,043 | USD 12,098 | 6 | ||
| SU (DPP-4i as third line) | 11.469 | NTD 246,858 | USD 6607 | 7 | ||
| Insulin (SU as third line) | 11.064 | NTD 278,502 | USD 7453 | 8 | ||
| Gu et al. [38] | 2019 | Meglitinide (Insulin as third line) | 14.085 | CNY 55,729 | USD 16,872 | 1 |
| AGI (Insulin as third line) | 14.019 | CNY 60,741 | USD 18,390 | 2 | ||
| DPP-4i (insulin as third line) | 14.051 | CNY 69,467 | USD 21,031 | 3 | ||
| Meglitinide (GLP-1RA as third line) | 14.117 | CNY 85,142 | USD 25,777 | 4 | ||
| SU (insulin as third line) | 13.965 | CNY 52,923 | USD 16,023 | 5 | ||
| TZD (INS as third line) | 13.978 | CNY 56,374 | USD 17,067 | 6 | ||
| AGI (GLP-1RA as third line) | 14.053 | CNY 89,690 | USD 27,154 | 7 | ||
| DPP-4i (GLP-1RA as third line) | 14.084 | CNY 98,597 | USD 29,850 | 8 | ||
| SU (GLP-1RA as third line) | 13.997 | CNY 81,569 | USD 24,695 | 9 | ||
| TZD (GLP-1RA as third line) | 14.011 | CNY 85,095 | USD 25,763 | 10 | ||
AGI α-glucosidase inhibitors, CADTH Canadian Agency for Drugs and Technologies in Health, DPP-4i dipeptidyl peptidase-4 inhibitor, GLP-1RA glucagon-like peptide-1 receptor agonist, ICER incremental cost-effectiveness ratio, NPH neutral protamine Hagedorn, QALYs quality adjusted life years, SGLT2i sodium-glucose cotransporter-2 inhibitor, SU sulfonylurea, TZD thiazolidinedione
GLP-1 RA vs. SGLT2i
Four studies compared the cost-effectiveness of GLP-1 RA and SGLT2i as second-line treatments, with SGLT2i consistently indicating greater cost-effectiveness [14, 16, 18, 19]. Three studies agree that SGLT2i conferred more QALYs at less cost than GLP-1RA, making it an economically dominant treatment strategy [14, 16, 19]. Sensitivity analysis revealed that the main driver of cost-effectiveness was drug price, with the average annual treatment costs for GLP-1RA (oral/subcutaneous semaglutide or liraglutide) being two to three times that of SGLT2i (empagliflozin) (Supplementary Table S4). One of the studies performed a subgroup analysis based on the inclusion or exclusion of the effect of treatment on heart failure [16]. After excluding the effect on heart failure, empagliflozin plus metformin continued to dominate oral semaglutide plus metformin.
GLP-1 RA vs. Others
Two cost-utility studies based on LEAD-2 [42] and 1860-LIRA-DPP-4 [43] trial indicated that liraglutide combined with metformin monotherapy was a highly cost-effective second-line strategy for treating T2D versus sitagliptin or glimepiride, respectively [17, 25]. Similar conclusions were also observed in a cost-utility analysis of GLP-1 RA compared to DPP-4i or neutral protamine Hagedorn insulin in Sweden [26]. However, short-acting exenatide and sitagliptin as second-line therapy for new-onset diabetes in the USA have cost-effectiveness ratios that exceed the threshold, making them expensive [27].
SGLT2i vs. Others
Two studies compared SGLT2i to DPP-4i [22, 23], two studies compared SGLT2i to SU [8, 21], and one study compared SGLT2i to both DPP-4i and SU [24]. The cost-effectiveness analysis consistently revealed that SGLT2i was more cost-effective than DPP-4i or SU over a range of values for the accepted cost-effectiveness threshold. Although SGLT2i has higher therapy costs than DPP4i or SU, the cost may be partially counterbalanced by the lower total cost of diabetes-related complications and managing severe hypoglycemia due to the lower cumulative incidence of these events. One study analyzed the baseline presence or absence of cardiovascular disease and payer perspectives on health insurance type (commercial or Medicare) using scenario analysis. The cost per QALY ranged between USD 3589 and USD 12,577, below the USD 50,000 willingness to pay (WTP) threshold commonly suggested for health intervention cost-effectiveness [23].
DPP-4i vs. Others
Eleven studies compared cost-utility in DPP-4i and conventional oral hypoglycemic agents [α-glucosidase inhibitors (AGI), TZD, SU] or NPH insulin as second-line therapy added to metformin in T2D, more than half of which were saxagliptin [28, 29, 31–34], one of them was DPP-4i [15], and the rest were two vildagliptin [35, 37], one alogliptin [30], and one sitagliptin [36]. These results consistently indicated that DPP-4i was cost-effective. Sensitivity analysis exhibited that DPP-4i had fewer hypoglycemia adverse effects and a weight-neutral profile than traditional antidiabetic drugs. The resulting health and economic benefits offset the overall increased costs associated with managing diabetes-related complications and purchasing medications. This conclusion was validated in economic assessment based on a real‐world observational study in the older population with T2D [15].
Multiple Antidiabetic Drugs
Four studies conducted a cost-utility analysis of multiple second-line T2D treatment strategies (Supplementary Table S5). One study excluded SGLT2i and GLP-1RA from the comparative analysis because they were commercially unavailable at the start of this study. Two Canadian cost-effectiveness studies comparing multiple antidiabetic agents as second-line treatment options indicated that SU was the most cost-effective choice [40, 41]. The other two studies evaluating the cost-utility of different hypoglycemic agent classes combined with second-line escalation therapies [38, 39] reached contradictory results. SGLT2i as the second line and DPP-4i as the third line were the most cost-effective in Chinese Taiwan. However, meglitinide as the second line and insulin as the third line were the most cost-effective in China.
Overall, these results demonstrated that SU was still highly cost-effective in second-line antidiabetic drugs when antidiabetic drug availability was poor or the WTP cost threshold was low. Otherwise, SGLT2i was more cost-effective because of weight loss, resulting in lower follow-up treatment costs and greater utility.
Discussion
The present study systematically reviewed the cost-effectiveness of second‐line antidiabetic therapy in T2D, including newer (GLP-1RA, SGLT2i and DPP-4i) and traditional hypoglycemic agents (SU, TZD, AGI, and insulin). The Cardiff and CORE models are the most commonly suggested health economic model of T2D. Based on the CHEERS checklist, most reviewed studies have good quality.
The base case analysis indicates that SGLT2i was more cost-effective than GLP-1RA and DPP-4i. GLP-1RA had better cost-effectiveness than DPP-4i and insulin in three evaluations. DPP-4i was cost-effective compared to traditional hypoglycemic agents, consistent with previously reported cost-effectiveness analyses. The remaining four comparative evaluations analyzed multiple strategies that SU or SGLT2i was the preferred second-line option for treatment failure with metformin monotherapy. New hypoglycemic agents have better cost-effectiveness as second-line therapy in treating T2D with the continuous improvement of the accessibility of new hypoglycemic agents, and the efficacy and safety data accumulation, especially the conclusions of cost–benefit advantages of SGLT2i, are mostly consistent.
A systematic review evaluated the cost-effectiveness of SGLT2i for T2D revealed that SGLT2i was more cost-effective than GLP-1RA and classic antidiabetic treatment options among patients who were not meeting HbA1c goals on metformin, especially for elevated CVD risk [7]. A similar study indicated that SGLT-2i and GLP-1RA were more cost-effective than DPP-4i and conventional hypoglycemic agents as second-line T2D treatment options [44]. Our comprehensive pharmacoeconomic evaluation of second-line treatment options for T2D has yielded some inconsistent results. This may be related to heterogeneity in economic simulation modeling in T2D, simulated treatment pathways and switching thresholds for treatment escalation in the study design, and susceptibility of different ethnicities to T2D.
The Canadian Agency for Drugs and Technologies in Health suggested that replacing SU as the most cost-effective option would result in price reductions of 60% and 70% on SGLT2i and GLP-1RA, respectively. Since the HTA report used the UKPDS model, the independent assessment of SGLT-2 and GLP-1 cardiovascular benefits may have been limited. Furthermore, it was hypothesized that metformin, in combination with a hypoglycemic agent as a long-term treatment for T2D, might not entirely reflect standard clinical practice.
Other cost-utility analyses using the Cardiff model revealed that SGLT2i as second line and DPP-4i as third line were the most cost-effective choices in Chinese Taiwan, but in China, meglitinide sequential add-on insulin was the best in the absence of SGLT2i. There are several potential reasons for this difference. Second-line escalation strategies were set inconsistently. Unlike the second-line setting of GLP-1RA in Chinese Taiwan, GLP-1RA was assumed as the third line in China. However, the efficacy data input models are heterogeneous because they are obtained from different included studies meta-analyzed. According to Gu et al., meglitinide as second line has better weight control, HbA1c reduction, and lower risk of hypoglycemia compared to SU, TZD, AGI, and DPP-4i, contradicting other cost-effective analyses. This may be related to the effect of ethnicity on glucose metabolism and insulin regulation. Compared with Caucasian people, the onset of T2D in the Asian population is characterized by limited β cell reserve, and inability to compensate for the slight decrease in insulin sensitivity, which can lead to β cell dysfunction prior to the decrease in insulin sensitivity and an increased risk for developing T2D [45]. In addition, since the Asian population has a higher carbohydrate content in its diet than the Western population, the glycemic response to the same glycemic load is also greater. Thus, the hypoglycemic regimen is more suitable for insulin secretagogue, which mainly reduces postprandial glycemia.
The current studies have several limitations. First, classical economic evaluation models of diabetes, such as UKPDS, may not meet all requirements because of the unique cardiovascular protective effects of GLP-1RA and SGLT-2i that are independent of their hypoglycemic effects. Therefore, there are growing appeals for encouraging the incorporation of new CVOT data on drug-mediated cardioprotection in the T2D economic model [46, 47]. Second, most clinical trial data of hypoglycemic drugs are currently based on the Caucasian population, and there is a lack of data support for the Asian population. Additional prospective studies are therefore needed to understand better the effects of different ethnic groups on the efficacy and safety of novel hypoglycemic agents. This can provide more personalized and targeted management strategies, especially for Asian populations. Third, in terms of study design, only a few studies have included costs from a societal perspective. An economic and health burden analysis of cardiovascular disease in the T2D population suggested that total healthcare costs were comparable to total lost productivity costs. The productivity loss due to premature mortality accounted for 42.65% of the indirect costs [48]. Hence, omitting the indirect costs is likely to underestimate the cost-effectiveness of SGLT2i in reducing all-cause mortality. In addition, the pharmaceutical industry funded approximately 70% of the included studies, which may cause bias in favor of newer antidiabetic agents' cost-effectiveness.
Despite these limitations, the current studies’ findings have important implications for future research and clinical practice, suggesting that the pattern of glucose-lowering drug use has changed substantially, with novel antidiabetic drugs increasingly being used as second-line therapies. The trade-off among efficacy, safety, and cost of novel hypoglycemic drugs underscores the importance of cost-effectiveness analyses in practical clinical practice with accumulating CVOT evidence for hypoglycemic agents. Although only one second-line study in our systematic review used real-world data, this method of using real-world evidence in pharmacoeconomics will become more mainstream, potentially identifying subgroups that benefit the most from particular interventions to facilitate clinical care and health policy decisions and optimize health care resource allocation.
Conclusion
Our systematic review suggests that newer antidiabetic drugs are cost-effective therapy in second-line options following metformin monotherapy with T2D than classic glucose-lowering agents. Cost-effectiveness analysis suggested that SGLT2i was a dominant strategy compared with GLP-1RA as an add-on treatment to metformin. DPP-4i has good safety profiles and weight neutrality, showing predominant cost-effectiveness in relation to other classical antidiabetic drugs but is less favorable than GLP-1RA and SGLT2i. When comparing various classes of antidiabetic drugs, the simulated treatment pathways by different studies and the accessibility and affordability of new antidiabetic drugs in different regions had significant heterogeneity. Therefore, it is difficult to draw definitive conclusions regarding which hypoglycemic agents should be recommended as the preferred second-line treatment from a cost-effectiveness perspective. However, as newer antidiabetic drugs reach patent expiry, SGLT2i may have potential as the preferred second-line treatment after metformin failure in T2D as the potential impact of generics could decrease the economic burden.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
This study was supported by the Medical and Health Research Project of Zhejiang Province (grant no. 2022KY255), which also funded the Rapid Service and Open Access fees.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Jiejin Zhu, Ying Zhou, and Qingyu Li. The first draft of the manuscript was written by Jiejin Zhu, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Disclosures
Jiejin Zhu, Ying Zhou, Qingyu Li, and Gang Wang declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
This article is based upon previously conducted studies, and all data are publicly available in the referenced publications.
References
- 1.Sun H, Saeedi P, Karuranga S, et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. doi: 10.1016/j.diabres.2021.109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Einarson TR, Acs A, Ludwig C, Panton UH. Economic burden of cardiovascular disease in type 2 diabetes: a systematic review. Value Health. 2018;21:881–890. doi: 10.1016/j.jval.2017.12.019. [DOI] [PubMed] [Google Scholar]
- 3.Marsico F, Paolillo S, Gargiulo P, et al. Effects of glucagon-like peptide-1 receptor agonists on major cardiovascular events in patients with Type 2 diabetes mellitus with or without established cardiovascular disease: a meta-analysis of randomized controlled trials. Eur Heart J. 2020;41:3346–3358. doi: 10.1093/eurheartj/ehaa082. [DOI] [PubMed] [Google Scholar]
- 4.Zelniker TA, Wiviott SD, Raz I, et al. Comparison of the effects of glucagon-like peptide receptor agonists and sodium-glucose cotransporter 2 inhibitors for prevention of major adverse cardiovascular and renal outcomes in type 2 diabetes mellitus. Circulation. 2019;139:2022–2031. doi: 10.1161/CIRCULATIONAHA.118.038868. [DOI] [PubMed] [Google Scholar]
- 5.Greiver M, Havard A, Bowles JK, et al. Trends in diabetes medication use in Australia, Canada, England, and Scotland: a repeated cross-sectional analysis in primary care. Br J Gen Pract. 2021;71(704):e209–e218. doi: 10.3399/bjgp20X714089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou X, Shrestha SS, Shao H, Zhang P. Factors contributing to the rising national cost of glucose-lowering medicines for diabetes during 2005–2007 and 2015–2017. Diabetes Care. 2020;43(10):2396–2402. doi: 10.2337/dc19-2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoshida Y, Cheng X, Shao H, Fonseca VA, Shi L. A systematic review of cost-effectiveness of sodium-glucose cotransporter inhibitors for type 2 diabetes. Curr Diab Rep. 2020;20:12. doi: 10.1007/s11892-020-1292-5. [DOI] [PubMed] [Google Scholar]
- 8.Hong D, Si L, Jiang M, et al. Cost Effectiveness of sodium-glucose cotransporter-2 (SGLT2) inhibitors, glucagon-like peptide-1 (GLP-1) receptor agonists, and dipeptidyl peptidase-4 (DPP-4) inhibitors: a systematic review. Pharmacoeconomics. 2019;37:777–818. doi: 10.1007/s40273-019-00833-1. [DOI] [PubMed] [Google Scholar]
- 9.American Diabetes Association Professional Practice Committee.9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes-2022. Diabetes Care. 2022;45:S125–43. [DOI] [PubMed]
- 10.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campbell and Cochrane Economics Methods Group (CCEMG), Evidence for Policy and Practice Information and Coordinating Centre (EPPI-Centre). CCEMG - EPPI-Centre Cost Converter v.1.4. 2019. http://eppi.ioe.ac.uk/costconversion/default.aspx (last accessed Jun 2, 2023)
- 12.Husereau D, Drummond M, Augustovski F, et al. Consolidated health economic evaluation reporting standards (CHEERS) 2022 explanation and elaboration: a report of the ISPOR CHEERS II good practices task force. Value Health. 2022;25:10–31. doi: 10.1016/j.jval.2021.10.008. [DOI] [PubMed] [Google Scholar]
- 13.Geng J, Yu H, Mao Y, Zhang P, Chen Y. Cost effectiveness of dipeptidyl peptidase-4 inhibitors for type 2 diabetes. Pharmacoeconomics. 2015;33:581–597. doi: 10.1007/s40273-015-0266-y. [DOI] [PubMed] [Google Scholar]
- 14.Ehlers LH, Lamotte M, Monteiro S, et al. The cost-effectiveness of empagliflozin versus liraglutide treatment in people with type 2 diabetes and established cardiovascular disease. Diabetes Ther. 2021;12(5):1523–1534. doi: 10.1007/s13300-021-01040-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gordon J, McEwan P, Evans M, Puelles J, Sinclair A. Managing glycaemia in older people with type 2 diabetes: a retrospective, primary care-based cohort study, with economic assessment of patient outcomes. Diabetes Obes Metab. 2017;19(5):644–653. doi: 10.1111/dom.12867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramos M, Cummings MH, Ustyugova A, Raza SI, de Silva SU, Lamotte M. Long-Term cost-effectiveness analyses of empagliflozin versus oral semaglutide, in addition to metformin, for the treatment of type 2 diabetes in the UK. Diabetes Ther. 2020;11(9):2041–2055. doi: 10.1007/s13300-020-00883-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steen Carlsson K, Persson U. Cost-effectiveness of add-on treatments to metformin in a Swedish setting: liraglutide vs sulphonylurea or sitagplitin. J Med Econ. 2014;17:658–669. doi: 10.3111/13696998.2014.933110. [DOI] [PubMed] [Google Scholar]
- 18.Ehlers LH, Lamotte M, Ramos MC, et al. The Cost-effectiveness of subcutaneous semaglutide versus empagliflozin in type 2 diabetes uncontrolled on metformin alone in Denmark. Diabetes Ther. 2022;13:489–503. doi: 10.1007/s13300-022-01221-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reifsnider OS, Pimple P, Brand S, Bergrath Washington E, Shetty S, Desai NR. Cost-effectiveness of second-line empagliflozin versus liraglutide for type 2 diabetes in the United States. Diabetes Obes Metab. 2022;24(4):652–661. doi: 10.1111/dom.14625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bagepally BS, Chaikledkaew U, Youngkong S, et al. Cost-utility analysis of dapagliflozin compared to sulfonylureas for type 2 diabetes as second-line treatment in Indian healthcare payer’s perspective. Clinicoecon Outcomes Res. 2021;13:897–907. doi: 10.2147/CEOR.S328433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Charokopou M, McEwan P, Lister S, et al. The cost-effectiveness of dapagliflozin versus sulfonylurea as an add-on to metformin in the treatment of Type 2 diabetes mellitus. Diabet Med. 2015;32(7):890–898. doi: 10.1111/dme.12772. [DOI] [PubMed] [Google Scholar]
- 22.Charokopou M, McEwan P, Lister S, et al. Cost-effectiveness of dapagliflozin versus DPP-4 inhibitors as an add-on to metformin in the treatment of type 2 diabetes mellitus from a UK healthcare system perspective. BMC Health Serv Res. 2015;15:496. doi: 10.1186/s12913-015-1139-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reifsnider O, Kansal A, Pimple P, Aponte-Ribero V, Brand S, Shetty S. Cost-effectiveness analysis of empagliflozin versus sitagliptin as second-line therapy for treatment in patients with type 2 diabetes in the United States. Diabetes Obes Metab. 2021;23:791–799. doi: 10.1111/dom.14268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tzanetakos C, Tentolouris N, Kourlaba G, Maniadakis N. Cost-effectiveness of dapagliflozin as add-on to metformin for the treatment of type 2 diabetes mellitus in Greece. Clin Drug Investig. 2016;36:649–659. doi: 10.1007/s40261-016-0410-2. [DOI] [PubMed] [Google Scholar]
- 25.Davies MJ, Chubb BD, Smith IC, Valentine WJ. Cost–utility analysis of liraglutide compared with sulphonylurea or sitagliptin, all as add-on to metformin monotherapy in Type 2 diabetes mellitus. Diabet Med. 2012;29(3):313–320. doi: 10.1111/j.1464-5491.2011.03429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kiadaliri AA, Gerdtham UG, Eliasson B, Carlsson KS. Cost-utility analysis of glucagon-like Peptide-1 agonists compared with dipeptidyl peptidase-4 inhibitors or neutral protamine hagedorn Basal insulin as add-on to metformin in type 2 diabetes in sweden. Diabetes Ther. 2014;5:591–607. doi: 10.1007/s13300-014-0080-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sinha A, Rajan M, Hoerger T, Pogach L. Costs and consequences associated with newer medications for glycemic control in type 2 diabetes. Diabetes Care. 2010;33:695–700. doi: 10.2337/dc09-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elgart JF, Caporale JE, Gonzalez L, Aiello E, Waschbusch M, Gagliardino JJ. Treatment of type 2 diabetes with saxagliptin: a pharmacoeconomic evaluation in Argentina. Health Econ Rev. 2013;3(1):11. doi: 10.1186/2191-1991-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Erhardt W, Bergenheim K, Duprat-Lomon I, McEwan P. Cost effectiveness of saxagliptin and metformin versus sulfonylurea and metformin in the treatment of type 2 diabetes mellitus in Germany: a Cardiff diabetes model analysis. Clin Drug Investig. 2012;32:189–202. doi: 10.2165/11597060-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 30.Gordon J, McEwan P, Hurst M, Puelles J. The cost-effectiveness of alogliptin versus sulfonylurea as add-on therapy to metformin in patients with uncontrolled type 2 diabetes mellitus. Diabetes Ther. 2016;7:825–845. doi: 10.1007/s13300-016-0206-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Granström O, Bergenheim K, McEwan P, Sennfält K, Henriksson M. Cost-effectiveness of saxagliptin (Onglyza®) in type 2 diabetes in Sweden. Prim Care Diabetes. 2012;6:127–136. doi: 10.1016/j.pcd.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 32.Grzeszczak W, Czupryniak L, Kolasa K, Sciborski C, Lomon ID, McEwan P. The cost-effectiveness of saxagliptin versus NPH insulin when used in combination with other oral antidiabetes agents in the treatment of type 2 diabetes mellitus in Poland. Diabetes Technol Ther. 2012;14:65–73. doi: 10.1089/dia.2011.0092. [DOI] [PubMed] [Google Scholar]
- 33.Gu S, Deng J, Shi L, Mu Y, Dong H. Cost-effectiveness of saxagliptin vs glimepiride as a second-line therapy added to metformin in Type 2 diabetes in China. J Med Econ. 2015;18:808–820. doi: 10.3111/13696998.2015.1049542. [DOI] [PubMed] [Google Scholar]
- 34.Gu S, Zeng Y, Yu D, Hu X, Dong H. Cost-effectiveness of saxagliptin versus acarbose as second-line therapy in type 2 diabetes in China. PLoS ONE. 2016;11:e0167190. doi: 10.1371/journal.pone.0167190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kousoulakou H, Hatzikou M, Baroutsou V, Yfantopoulos J. Cost effectiveness of vildagliptin versus glimepiride as add-on treatment to metformin for the treatment of diabetes mellitus type 2 patients in Greece. Cost Eff Resour Alloc. 2017;15:19. doi: 10.1186/s12962-017-0082-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwarz B, Gouveia M, Chen J, et al. Cost-effectiveness of sitagliptin-based treatment regimens in European patients with type 2 diabetes and haemoglobin A1c above target on metformin monotherapy. Diabetes Obes Metab. 2008;10(Suppl 1):43–55. doi: 10.1111/j.1463-1326.2008.00886.x. [DOI] [PubMed] [Google Scholar]
- 37.Viriato D, Calado F, Gruenberger J-B, et al. Cost-effectiveness of metformin plus vildagliptin compared with metformin plus sulphonylurea for the treatment of patients with type 2 diabetes mellitus: a Portuguese healthcare system perspective. J Med Econ. 2014;17:499–507. doi: 10.3111/13696998.2014.912986. [DOI] [PubMed] [Google Scholar]
- 38.Gu S, Shi L, Shao H, et al. Choice across 10 pharmacologic combination strategies for type 2 diabetes: a cost-effectiveness analysis. BMC Med. 2020;18(1):378. doi: 10.1186/s12916-020-01837-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chien CL, Chen YC, Malone DC, Peng YL, Ko Y. Cost-utility analysis of second-line anti-diabetic therapy in patients with type 2 diabetes mellitus inadequately controlled on metformin. Curr Med Res Opin. 2020;36:1619–1626. doi: 10.1080/03007995.2020.1815686. [DOI] [PubMed] [Google Scholar]
- 40.Canadian Agency for Drugs and Technologies in Health (CADTH). New Drugs for Type 2 Diabetes: Second-Line Therapy - Science Report. 2017. http://www.ncbi.nlm.nih.gov/books/NBK531904/ (last accessed Apr 29, 2023) [PubMed]
- 41.Klarenbach S, Cameron C, Singh S, Ur E. Cost-effectiveness of second-line antihyperglycemic therapy in patients with type 2 diabetes mellitus inadequately controlled on metformin. CMAJ. 2011;183:E1213–1220. doi: 10.1503/cmaj.110178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nauck M, Frid A, Hermansen K, et al. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care. 2009;32:84–90. doi: 10.2337/dc08-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pratley RE, Nauck M, Bailey T, et al. Liraglutide versus sitagliptin for patients with type 2 diabetes who did not have adequate glycaemic control with metformin: a 26-week, randomised, parallel-group, open-label trial. Lancet. 2010;375:1447–1456. doi: 10.1016/S0140-6736(10)60307-8. [DOI] [PubMed] [Google Scholar]
- 44.Ruan Z, Zou H, Lei Q, Ung COL, Shi H, Hu H. Pharmacoeconomic evaluation of dipeptidyl peptidase-4 inhibitors for the treatment of type 2 diabetes mellitus: a systematic literature review. Expert Rev Pharmacoecon Outcomes Res. 2022;22:555–574. doi: 10.1080/14737167.2022.2042255. [DOI] [PubMed] [Google Scholar]
- 45.Vale Moreira NC, Ceriello A, Basit A, et al. Race/ethnicity and challenges for optimal insulin therapy. Diabetes Res Clin Pract. 2021;175:108823. doi: 10.1016/j.diabres.2021.108823. [DOI] [PubMed] [Google Scholar]
- 46.Evans M, Berry S, Nazeri A, et al. The challenges and pitfalls of incorporating evidence from cardiovascular outcomes trials in health economic modelling of type 2 diabetes. Diabetes Obes Metab. 2023;25(3):639–648. doi: 10.1111/dom.14917. [DOI] [PubMed] [Google Scholar]
- 47.Willis M, Asseburg C, Nilsson A, Neslusan C. Challenges and opportunities associated with incorporating new evidence of drug-mediated cardioprotection in the economic modeling of type 2 diabetes: a literature review. Diabetes Ther. 2019;10(5):1753–1769. doi: 10.1007/s13300-019-00681-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abushanab D, Marquina C, Morton JI, et al. Projecting the health and economic burden of cardiovascular disease among people with type 2 diabetes, 2022–2031. Pharmacoeconomics. 2023;41(6):719–732. doi: 10.1007/s40273-023-01258-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article is based upon previously conducted studies, and all data are publicly available in the referenced publications.