Abstract
Background:
Anticoagulants are the mainstay of treatment for venous thromboembolism (VTE). Studies have shown conflicting results regarding statins ability to reduce the incidence of VTE.
Aims:
To perform a network meta-analysis to determine which lipid-lowering agent was more efficacious in and had more evidence regarding reducing the VTE risk.
Study Design:
Network meta-analysis of the randomized controlled trials (RCTs).
Methods:
RCTs that assessed the effectiveness and safety of statins or fibrates and compared them to a placebo or another statin were eligible for the study. The outcomes examined in the study were deep vein thrombosis, pulmonary embolism, and/or VTE. We conducted a comprehensive search of the Medline database from 1966 to February 2017, using specific search terms related to VTE and statins. Additionally, we screened, and cross-checked relevant systematic reviews and meta-analyses. We performed a network meta-analysis to compare the different lipid-lowering agents to each other and the placebo and their effectiveness.
Results:
Twenty-seven RCTs were included in the network meta-analysis (n = 137,940). Pairwise meta-analysis revealed a statistically significant lower incidence of VTE with statins than with placebos (0.79% vs 0.99%, respectively; risk ratios: 0.87, 0.77-0.98; p = 0.022). Rosuvastatin had the most favorable effect in reducing VTE risk than the other statins, fenofibrate, and placebo. Fenofibrate was ranked the worst drug choice, because it increased risk of VTE when compared with the other statins. Rosuvastatin was the best choice for reducing the VTE risk when compared with the placebo (OR: 0.56, 0.42-0.75), atorvastatin (OR: 0.64, 0.44-0.95), pravastatin (OR: 0.50, 0.34-0.74), simvastatin (OR: 0.60, 0.42-0.86) and fenofibrate (OR: 0.37, 0.25-0.56). Compared with a placebo, rosuvastatin reduced the VTE risk by around 45% and fenofibrate increased the risk by 65%.
Conclusion:
Rosuvastatin is significantly reduces the risk of VTE when compared with a placebo, other statin subtypes, and fibrate. Furthermore, fenofibrate increased the VTE risk when compared with a placebo and statins.
INTRODUCTION
Venous thromboembolism (VTE), which encompasses pulmonary embolism and deep vein thrombosis (DVT), continues to pose a significant challenge in the field of healthcare. Although the medical agents used for the treatment of VTE are effective, bleeding issues remain an important concern for clinicians.1 Studies have consistently demonstrated the efficacy of statins for both the primary and secondary prevention of cardiovascular diseases.2,3 Statins have a favorable impact on inflammation and coagulation via the pleiotropic effect. In addition, they do not increase the risk of bleeding.4,5 Venous and arterial thromboses frequently share common etiologic risk factors.6 Therefore, this similarity prompted the hypothesis that statins could reduce the incidence of VTE beyond the favorable effect of reducing the LDL cholesterol level. Recent studies indicate that statin might reduce the incidence of VTE via the pleiotropic mechanism.7,8,9,10,11,12 In one meta-analysis which included eight case-control and three cohort studies, Squizzato et al.13 demonstrated that statins do not reduce the incidence of VTE. In contrast, two other meta-analyses conducted by Rahimi et al.14 and Kunutsor et al.,15 which incorporated multiple randomized controlled trials (RCTs), reached a consensus that statins significantly impacted and reduced the occurrence of VTE.
In this meta-analysis, we included both placebo-controlled and active-comparator RCTs to determine which lipid lowering agent, including statins, and fibrate, was more efficacious, and provided more evidence of reducing the VTE risk.
MATERIALS AND METHODS
The study was conducted in accordance with the principles of the Declaration of Helsinki and followed the guidelines outlined in the PRISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-analyses of Health Care Interventions.16
Eligibility criteria
To be considered eligible, the study had to be an RCT assessing the effectiveness and safety of a statin or fibrate in comparison to a placebo or another statin. RCTs with a follow-up period of < 6 months were excluded. No restrictions were imposed on the medication dosage. Initially, all titles, and abstracts were screened to exclude studies that did not match the inclusion criteria. Subsequently, the full texts of the remaining articles were reviewed to identify eligible studies. RCTs that involved the concurrent use of niacin, ezetimibe, or antioxidant vitamins were excluded from the analysis. Furthermore, RCTs without reports published in the English language were also excluded.
Study outcomes
The study focused on evaluating the outcomes of DVT, PE, and/or VTE.
Study selection, data extraction, and assessment of the data quality
The Medline, EMBASE, and Cochrane databases were comprehensively and systematically searched from 1966 to February 2017. The following terms related to VTE were searched: “venous thromb*,” “VTE,” “deep vein thrombosis,” and “pulmonary embolism.” These terms were combined with search terms related to statins, including “statin,” “HMG,” “atorvastatin,” “simvastatin,” “statins,” “lovastatin,” “pravastatin,” “fluvastatin,” “fibrate,” and “fenofibrate.” The relevant systematic reviews and meta-analyses were meticulously screened and cross-checked. Two reviewers (I.H.T. and A.K) identified the eligible studies and extracted the key features from the included RCTs. The data quality was evaluated using the Cochrane Collaboration Risk of Bias Tool, which specifically assessed potential selection bias (randomization method and allocation concealment), information bias (blinding of outcome adjudicators), and analysis bias (intention-to-treat analysis and completeness of follow-up). Each study’s overall risk of bias was categorized as low (all analyzed items were appropriate or at least five items were appropriate while the remaining two were unclear), unclear (more than two items were not reported), or high (at least one quality dimension indicated a possible bias).
Statistical Analysis
Two types of meta-analyses were conducted: pairwise and network. All statistical analyses were performed using STATA (version 14.0;).
For the pairwise meta-analysis, the summary risk ratios (RRs) and their corresponding 95% confidence intervals (CIs) were calculated to evaluate the risk of VTE in lipid-lowering drugs and placebo. Both fixed-effects and random-effects models were utilized in the analysis. The random-effects model was employed when there was significant heterogeneity (I2 > 25%) among the outcomes. Conversely, in cases with low heterogeneity, the fixed-effects model was used. The level of heterogeneity was assessed using the I2 statistic. Statistical significance was defined as a p value < 0.05 for two-tailed tests.
We conducted a network meta-analysis to compare the different lipid-lowering agents among themselves as well as with the placebo. Network meta-analysis enables the inclusion of both direct and indirect evidence, even if the treatments have not been directly compared in an RCT,17,18,19 to obtain a more comprehensive and sensitive estimate.20 The network meta-analysis was performed using the “mvmeta” command21,22,23 and self-programmed routines in STATA (version 14.0;).24 To evaluate the presence of small-study effects, a comparison-adjusted funnel plot was employed.
To identify inconsistencies within the network meta-analysis, a loop-specific approach was employed. This approach examines the consistency assumption within each closed loop of the network by comparing the direct and indirect estimates for a specific comparison, referred to as the inconsistency factor. The magnitude of the inconsistency factors and their corresponding 95% CIs were used to determine the presence of inconsistency in each loop. A common heterogeneity estimate within each loop was assumed. The analysis results were presented in a forest plot using the “ifplot” command in STATA (version 14.0;).
To facilitate the interpretation of heterogeneity results, the mean summary effects was presented alongside its predictive intervals (PrIs). The PrI in the interval within which the estimate of a future study is expected to fall.
The ranking probabilities for all the treatments, indicating the likelihood of each intervention being at each possible rank, was calculated using the “mvmeta” command in STATA (version 14.0;).22 Subsequently, a hierarchy of the competing interventions was derived using “rankograms”.25 To establish a treatment hierarchy, the surface under the cumulative ranking curve (SUCRA) and mean ranks was utilized. The relevant plots were generated using the Stata commands described by Chaimani et al.24
RESULTS
Study selection and patient population
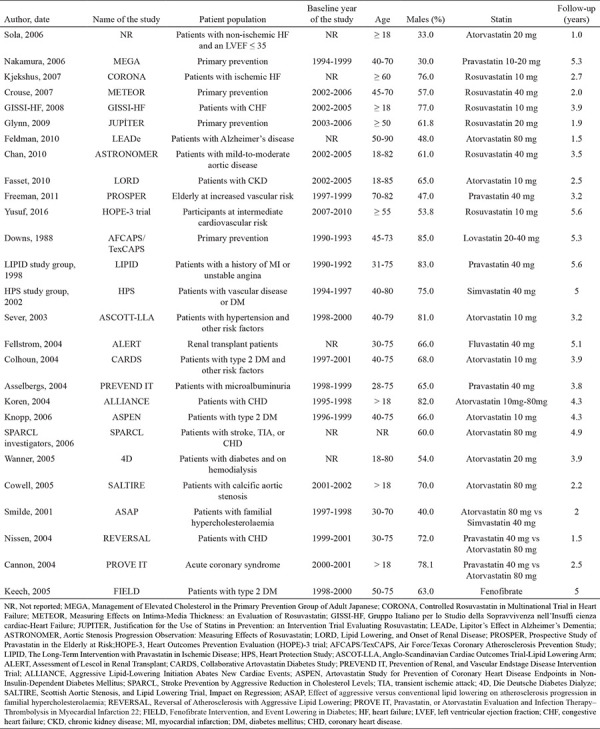
We identified 299 potentially relevant studies through our electronic search. Among these, 55 studies were determined to be eligible. After further analyzing the 55 studies, 28 were excluded as they did not satisfy the inclusion criteria. Finally, a total of 27 RCTs were included in the network meta-analysis. The network structure of the lipid-lowering agents across these 27 RCTs is depicted in Figure 1. Efficacies of the placebo, fluvastatin, lovastatin, simvastatin, pravastatin, atorvastatin, rosuvastatin, and fenofibrate in included studies were evaluated. Overall, 137,940 patients were randomized to either study group. The demographic and clinical characteristics of the enrolled patients are shown in Table 1.
Figure 1.
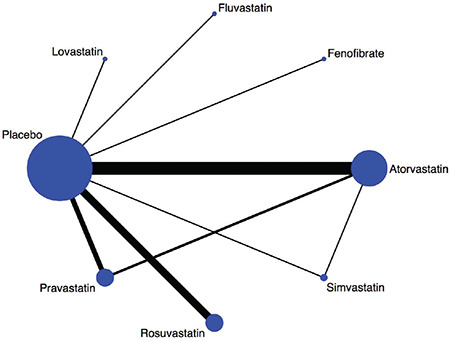
Evidence network of the lipid-lowering agents.
Table 1. Summary of the Trial Characteristics.
The risk of bias is shown in Figure 2. The quality of the RCT’s was usually acceptable. The number of studies with a high risk of bias for random sequence generation and allocation concealment was low. However, there was a high risk of bias for blinding of participants and personnel in most of studies.
Figure 2.
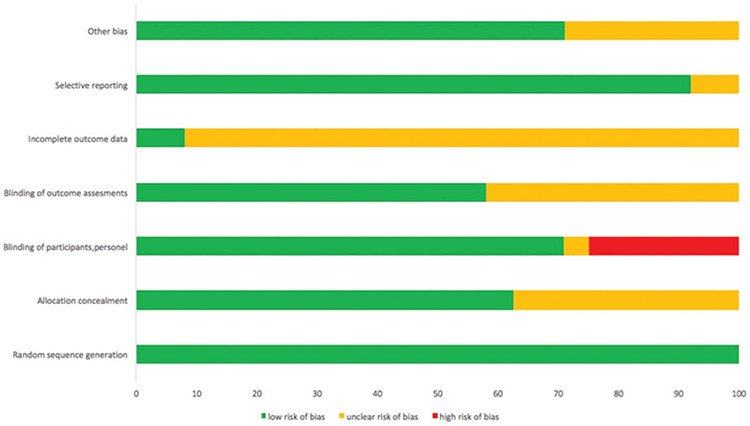
Risk of bias.
Network meta-analysis results
Conventional pairwise meta-analysis of the studies conducted with statins (23 RCT’s) revealed a statistically significant lower incidence of VTE with statins than with placebos (incidence, 0.79% vs 0.99%, RR: 0.87, 0.77-0.98, p = 0.022). No significant heterogeneity was observed among the studies (I2: 12.3%, p = 0.293). However, in the pooled analysis of the 23 RCTs with statins and one RCT with fenofibrate, the risk of VTE was comparable to that of RCTs with placebos. This may be attributable to the increased risk of VTE associated with fenofibrate (incidence, 0.91% vs 0.99%).
Figure 3 illustrates of the contribution of each direct comparison to the estimation of the network summary effects. Among the total number of comparisons, nine were solely informed by direct evidence, nine were informed by a combination of direct and indirect evidence, and 19 were solely informed by indirect evidence. The contribution of the nine comparisons informed by direct evidence was well-balanced and comparable within the network. Furthermore, there was no inconsistency between the direct and indirect point estimates.
Figure 3.
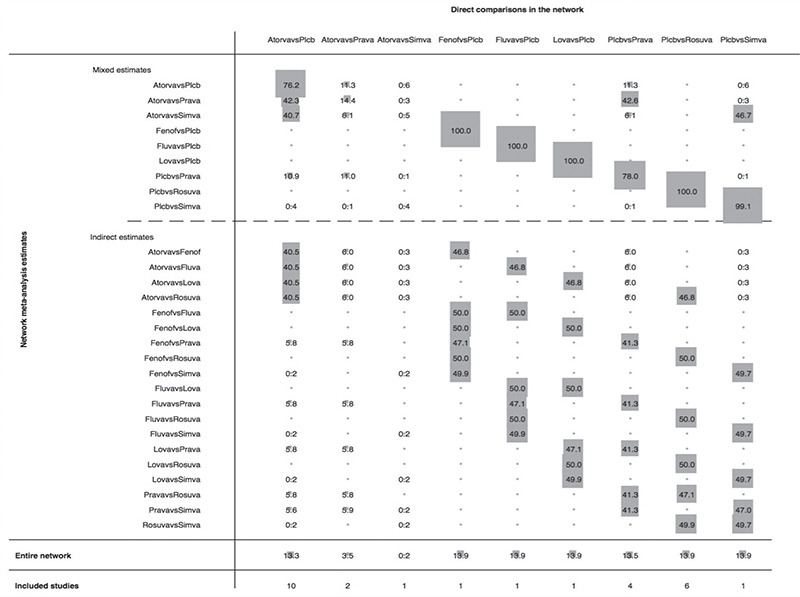
Contribution plot of each direct comparison in the network. The figure depicts the percentage contribution of each direct comparison to the network summary estimates in the entire network.
There were two closed loops identified in the network structure. All the CIs for the relative odds ratios (RoRs) were compatible with zero inconsistency, indicating that there was no significant deviation from consistency in the study outcomes (Figure 4). With an RoR value of one, there was no inconsistency between the direct and indirect evidence in the network.
Figure 4.
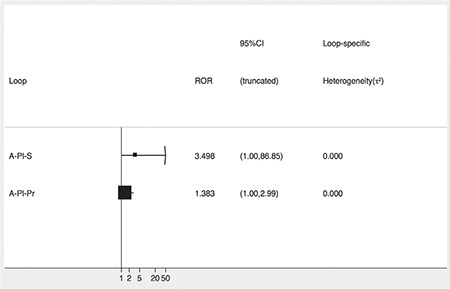
Inconsistency plot for the risk of venous thromboembolism (VTE). The forest plot shows the ratio of the two odds ratios (RoR) from direct and indirect evidence in the loop. The confidence intervals are truncated at zero given that the direction of the inconsistency factor (IF) is unimportant.
Rosuvastatin had the most favorable effect on reducing the VTE risk among all statins, fenofibrate, and placebo. Fenofibrate was ranked the worst in terms of increased risk of VTE when compared with other statins. We ranked and compared the effects of all the drugs in relation to each other and the placebo, which were analyzed and evaluated using SUCRA probabilities (Figure 5). Rosuvastatin was ranked the highest for reducing VTE risk when compared with the placebo (OR: 0.56, 0.42-0.75), atorvastatin (OR: 0.64, 0.44-0.95), pravastatin (OR: 0.50, 0.34-0.74), simvastatin (OR: 0.60, 0.42-0.86), and fenofibrate (OR: 0.37, 0.25-0.56) (Figures 6 and 7). Compared with the placebo, rosuvastatin reduced the risk of VTE by around 45% and fenofibrate increased the risk of VTE by 65%. Figure 8 highlights the ranking of each lipid-lowering drugs for reducing the VTE risk. The probability of being the best drug to reduce VTE risk was > 50% (i.e., pure chance) for rosuvastatin (69.2%). The probability of being the worst drug that increased the VTE risk was > 50% for fenofibrate (80.6%). In our network analysis, the study size did not appear to influence the effect size. Additionally, the funnel plots for all the study outcomes exhibited symmetry around the zero line, indicating a lack of publication bias (Figure 9).
Figure 5.
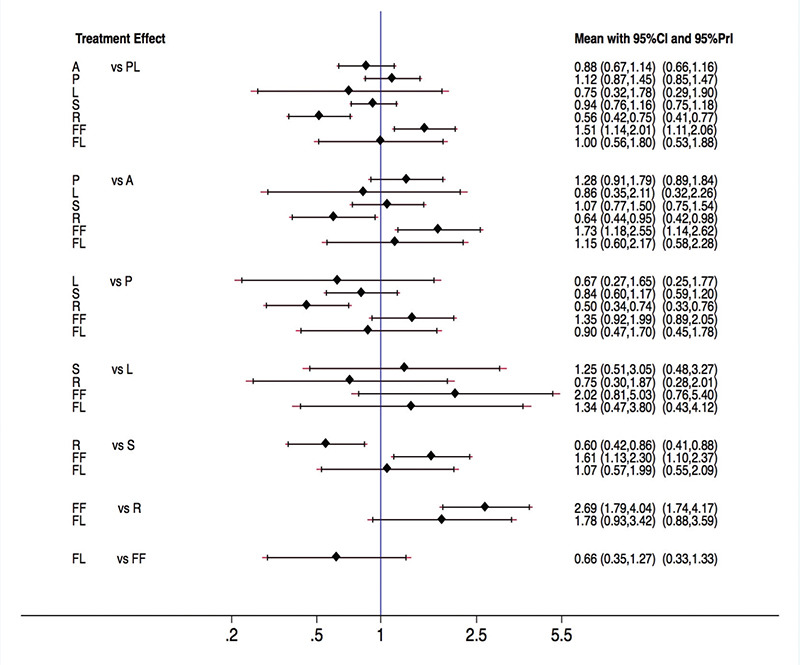
Rankogram of the available lipid-lowering agents for reducing the risk of venous thromboembolism (VTE) based on the surface under the cumulative ranking curve (SUCRA).
Figure 6.
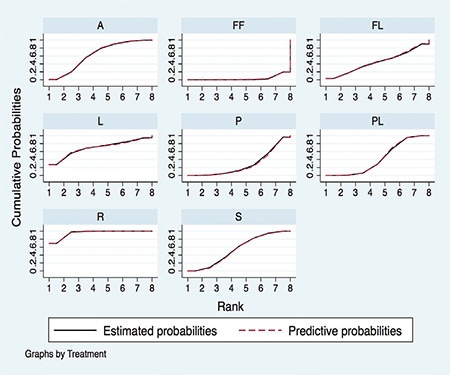
Pooled odds ratio (95% confidence interval [CI]) determined by network meta-analysis for the risk of venous thromboembolism (VTE).
Figure 7.
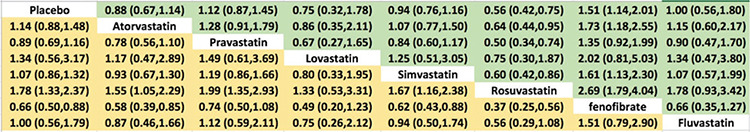
League table for the risk of venous thromboembolism (VTE).
Figure 8.
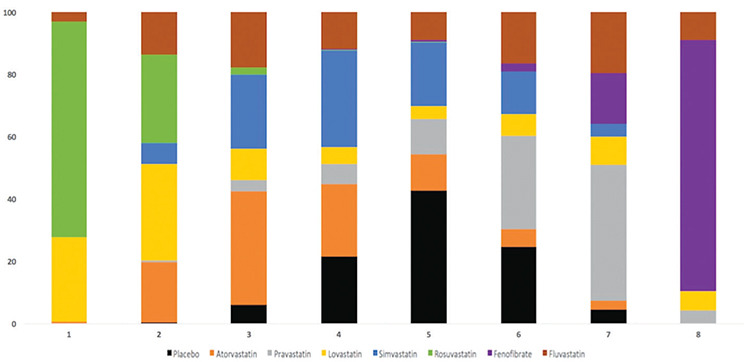
Ranking of the treatment strategies regarding the risk of venous thromboembolism (VTE). Bar plots have been utilized for ranking the probabilities of each treatment strategy. The possible rank of each strategy is represented on the x-axis (from best to worst) and the probability of each strategy to be at a specific rank is on the y-axis.
Figure 9.
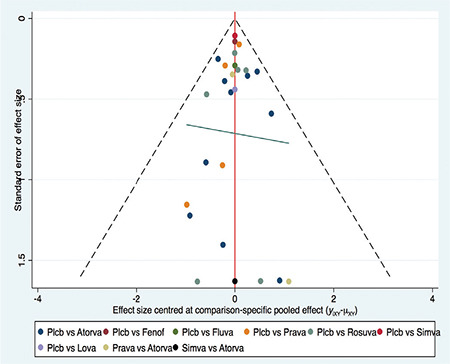
Comparison-adjusted funnel plot for the risk of venous thromboembolism (VTE). The red line represents a null hypothesis, indicating that the study-specific effect sizes do not differ from the respective comparison-specific pooled effect estimates. The two black dashed lines represent a 95% CI for the difference between study-specific sizes and comparison-specific summary estimates. YiXY is the noted effect size in study I that compares x with y. μXY is the comparison-specific summary estimate for x versus y.
DISCUSSION
Despite the comprehensive evidence of long-term efficacy and safety of lipid-lowering agents, including pairwise, and network meta-analyses, data related to their impact on VTE are sparse. Our report is the first meta-analysis conducted on the impact of lipid-lowering agent on VTE risk that included 28 RCTs and a total of 137,000 patients. Our analysis revealed that rosuvastatin is significantly associated with a reduced risk of VTE compared with placebo, other statin subtypes, and fibrate. Fenofibrate showed an increased risk of VTE when compared with both the placebo and statins. These findings highlight the differential effects of various lipid-lowering agents on the risk of VTE. The other statin subgroups, aside from rosuvastatin, demonstrated similar effects on the risk of VTE when compared with the placebo. Thus, the overall impact of statins, excluding rosuvastatin, on the VTE risk did not significantly differ from that of placebos.
Our pairwise meta-analysis demonstrated that compared with the placebo, statins reduced the risk of VTE when the analysis was confined to only studies involving statins (23 RCT’s) (incidences, 0.79% vs 0.99%; RR: 0.87, 0.77-0.98, p = 0.022). However, in the pooled analysis of 23 statin-related RCTs and one fenofibrate-related RCT, the risk of VTE was similar to that with placebo, predominantly due to the increased risk with fenofibrate use (incidences, 0.91% vs 0.99%). These findings are comparable to those of previous meta-analyses, further strengthening the consistency of the evidence across studies.13,15,26 Our network meta-analysis demonstrated that rosuvastatin had the lowest VTE risk than the other statin substypes and fenofibrate did. Fenofibrate ranked the worst drug choice because it increased the risk of VTE. Rosuvastatin was ranked the best drug choice for reducing VTE risk when compared with the placebo (OR: 0.56, 0.42-0.75), atorvastatin (OR: 0.64, 0.44-0.95), pravastatin (OR: 0.50, 0.34-0.74), simvastatin (OR: 0.60, 0.42-0.86) and fenofibrate (OR: 0.37, 0.25-0.56). Furthermore, the probability of being the best drug to reduce VTE was > 50% for rosuvastatin (69.2%), and the probability of being the worst drug was > 50% for fenofibrate (80.6%). These findings indicate that the reduced risk of VTE with statins is mainly associated with rosuvastatin.
In a meta-analysis that included an RCT and nine observational studies, Agarwal et al.26 demonstrated that statins were associated with a reduced risk of VTE. This result was similar to that of the study by Kunutsor et al.15 Rosuvastatin appears to have a beneficial effect on VTE events when compared with other statins. Our network meta-analysis that demonstrated that statins reduced the risk of VTE, included the aforementioned studies. However, we also found a significant difference between the effects of different statin groups on VTE risk. Only rosuvastatin was significantly associated with a reduced risk of VTE when compared with placebo; the other statin subgroups had a similar effect to that of placebos. The risk of VTE was significantly higher with fenofibrate administration that with statins and placebos.
Statins have a strong vasculo-protective effect in addition to being lipid-lowering agents. The anti-inflammatory and anti-thrombotic properties of statins are considered to be responsible for the vasculo-protective effect; this leads to the alteration of endothelial dysfunction and blood flow, which opposes the hypercoagulable states. The pairwise meta-analyses results showing that statins reduced the VTE risk can be partly explained by these mechanisms. However, in our network meta-analysis, rosuvastatin alone was associated with a reduced risk of VTE. This can be partly attributed to the inherent properties of rosuvastatin, including its more potent lipid-lowering and anti-inflammatory effects, which produces a more pronounced decrease in CRP level and prominent vascular protection (anti-atherogenic).27,28,29,30
Fibrates are peroxisome proliferator-activated receptor activators that reduce the procoagulant activity and enhance fibrinolysis.31,32,33 Although, fibrates are generally thought to have anti-thrombotic activities, in our network meta-analysis, they were associated with an increased risk of VTE when compared with both statins and the placebo. This can be attributed to the increased homocysteine levels associated with fibrates; however, this remains debatable.34
Ongoing studies and reviews almost always show a relationship between anti-coagulation and statin therapies. However, the mechanism by which statins cause anti-coagulant or protective effects against VTE remains a debate. Although there are theories regarding the mechanism of these effects, there is no hard evidence.35,36
RCT results indicate a potential beneficial effect of rosuvastatin in the prevention of VTE, while suggesting a harmful effect of fibrate use in relation to VTE. However, further studies are necessary to validate these hypotheses and draw definitive conclusions. While our analysis provides valuable insights, additional studies, and robust evidence are required to confirm the observed associations and establish conclusive findings.
VTE is a frequently encountered in clinical practice and has substantial implications in terms of morbidity and mortality. The potential utilization of rosuvastatin for the prevention of VTE could present an additional indication for this medication. This could expand the therapeutic applications of rosuvastatin and potentially improve patient outcomes by reducing the risk of VTE. However, further research and clinical trials are warranted to establish the efficacy and safety of rosuvastatin for VTE prevention, before it can be used worldwide or any definitive recommendations can be made.
The present meta-analysis has several limitations. An important limitation is the variability in the study population characteristics, which is inherent to any meta-analysis. This heterogeneity in participant characteristics may have introduced a potential bias and limited the generalizability of the study findings.
The COVID-19 pandemic caused a new wave of VTE cases.37 Most of the studies included were conducted before the pandemic. Inclusion of studies conducted after the pandemic may change the outcomes of our analyses.
Another limitation of the study is the variation in statin dosages used. The different dosages may have influenced the effectiveness and safety outcomes and could potentially impact the overall results of the network meta-analysis.
Most of the trial evidence used in this study was based on previously unpublished data, which were only recently made available through two reviews. This reliance on unpublished data may have introduced a publication bias and limited the comprehensiveness of the analysis.
Due to the limited number of studies available for the outcomes of DVT and PE, further analysis of these data was not possible. This limitation highlights the need for more studies in these specific areas.
Finally, the trial evidence mainly relied on previously unpublished data, which were collected as adverse events and contributed by investigators. This may introduce have introduced potential biases in the estimates of the analyses.
Considering these limitations, our network meta-analyses findings should be interpreted with caution. Furthermore, there is a need for additional high-quality studies with larger sample sizes and standardized statin dosages to further investigate the effectiveness and safety of statins in relation to VTE.
The present network meta-analysis revealed that rosuvastatin was significantly associated with a reduced risk of VTE, while fenofibrate was associated with an increased VTE risk. Except for rosuvastatin, all other statin subgroups had a neutral effect on the risk of VTE.
Footnotes
Ethics Committee Approval: Since our article was a meta-analysis, we did not receive ethics committee approval.
Data Sharing Statement: The data that support the findings of this study are available from the corresponding author upon reasonable request.
Author Contributions: Concept- O.B.; Design- O.B., A.K., İ.H.T.; Data Collection or Processing- M.S., R.D.; Analysis or Interpretation- A.K.; Literature Search- R.D.; Writing- O.B., O.T.
Conflict of Interest: No conflict of interest was declared by the authors.
References
- 1.Linkins LA, Choi PT, Douketis JD. Clinical impact of bleeding in patients taking oral anticoagulant therapy for venous thromboembolism: a meta-analysis. Ann Intern Med. 2003;139:893–900. doi: 10.7326/0003-4819-139-11-200312020-00007. [DOI] [PubMed] [Google Scholar]
- 2.Cholesterol Treatment Trialists’ (CTT) Collaboration; Baigent C, Blackwell L, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu Y, Cheng Z, Zhao Y, et al. Efficacy and safety of long-term treatment with statins for coronary heart disease: A Bayesian network meta-analysis. Atherosclerosis. 2016;254:215–227. doi: 10.1016/j.atherosclerosis.2016.10.025. [DOI] [PubMed] [Google Scholar]
- 4.Undas A, Brummel-Ziedins KE, Mann KG. Statins and blood coagulation. Arterioscler Thromb Vasc Biol. 2005;25:287–294. doi: 10.1161/01.ATV.0000151647.14923.ec. [DOI] [PubMed] [Google Scholar]
- 5.Krysiak R, Okopień B, Herman Z. Effects of HMG-CoA reductase inhibitors on coagulation and fibrinolysis processes. Drugs. 2003;63:1821–1854. doi: 10.2165/00003495-200363170-00005. [DOI] [PubMed] [Google Scholar]
- 6.Ageno W, Becattini C, Brighton T, Selby R, Kamphuisen PW. Cardiovascular risk factors and venous thromboembolism: a meta-analysis. Circulation. 2008;117:93–102. doi: 10.1161/CIRCULATIONAHA.107.709204. [DOI] [PubMed] [Google Scholar]
- 7.Herrington DM, Vittinghoff E, Lin F, et al. Statin therapy, cardiovascular events, and total mortality in the Heart and Estrogen/Progestin Replacement Study (HERS) Circulation. 2002;105:2962–2967. doi: 10.1161/01.cir.0000019406.74017.b2. [DOI] [PubMed] [Google Scholar]
- 8.Ray JG, Mamdani M, Tsuyuki RT, Anderson DR, Yeo EL, Laupacis A. Use of statins and the subsequent development of deep vein thrombosis. Arch Intern Med. 2001;161:1405–1410. doi: 10.1001/archinte.161.11.1405. [DOI] [PubMed] [Google Scholar]
- 9.Yang CC, Jick SS, Jick H. Statins and the risk of idiopathic venous thromboembolism. Br J Clin Pharmacol. 2002;53:101–105. doi: 10.1046/j.0306-5251.2001.01523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huerta C, Johansson S, Wallander MA, García Rodríguez LA. Risk factors and shortterm mortality of venous thromboembolism diagnosed in the primary care setting in the United Kingdom. Arch Intern Med. 2007;167:935–943. doi: 10.1001/archinte.167.9.935. [DOI] [PubMed] [Google Scholar]
- 11.Smeeth L, Douglas I, Hall AJ, Hubbard R, Evans S. Effect of statins on a wide range of health outcomes: a cohort study validated by comparison with randomized trials. Br J Clin Pharmacol. 2009;67:99–109. doi: 10.1111/j.1365-2125.2008.03308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glynn RJ, Danielson E, Fonseca FA, et al. A randomized trial of rosuvastatin in the prevention of venous thromboembolism. N Engl J Med. 2009;360:1851–1861. doi: 10.1056/NEJMoa0900241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Squizzato A, Galli M, Romualdi E, et al. Statins, fibrates, and venous thromboembolism: a meta-analysis. Eur Heart J. 2010;31:1248–1256. doi: 10.1093/eurheartj/ehp556. [DOI] [PubMed] [Google Scholar]
- 14.Rahimi K, Bhala N, Kamphuisen P, et al. Effect of statins on venous thromboembolic events: a meta-analysis of published and unpublished evidence from randomised controlled trials. PLoS Med. 2012;9:e1001310. doi: 10.1371/journal.pmed.1001310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kunutsor SK, Seidu S, Khunti K. Statins and primary prevention of venous thromboembolism: a systematic review and meta-analysis. Lancet Haematol. 2017;4:e83–e93. doi: 10.1016/S2352-3026(16)30184-3. [DOI] [PubMed] [Google Scholar]
- 16.Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 17.Cipriani A, Higgins JP, Geddes JR, Salanti G. Conceptual and technical challenges in network meta-analysis. Ann Intern Med. 2013;159:130–137. doi: 10.7326/0003-4819-159-2-201307160-00008. [DOI] [PubMed] [Google Scholar]
- 18.Salanti G, Higgins JP, Ades AE, Ioannidis JP. Evaluation of networks of randomized trials. Stat Methods Med Res. 2008;17:279–301. doi: 10.1177/0962280207080643. [DOI] [PubMed] [Google Scholar]
- 19.Caldwell DM, Ades AE, Higgins JP. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ. 2005;331:897–900. doi: 10.1136/bmj.331.7521.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. 1997;50:683–691. doi: 10.1016/s0895-4356(97)00049-8. [DOI] [PubMed] [Google Scholar]
- 21.White IR. Multivariate random effects meta- regression: Updates to mvmeta. Stata J. 2011;11:255–270. [Google Scholar]
- 22.White IR. Multivariate random effects meta- analysis. Stata J. 2009;9:40–56. [Google Scholar]
- 23.White IR, Barrett JK, Jackson D, Higgins JP. Consistency and inconsistency in network meta-analysis: model estimation using multivariate meta-regression. Res Synth Methods. 2012;3:111–125. doi: 10.1002/jrsm.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in STATA. PLoS One. 2013;8:e76654. doi: 10.1371/journal.pone.0076654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011;64:163–171. doi: 10.1016/j.jclinepi.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 26.Agarwal V, Phung OJ, Tongbram V, Bhardwaj A, Coleman CI. Statin use and the prevention of venous thromboembolism: a meta-analysis. Int J Clin Pract. 2010;64:1375–1383. doi: 10.1111/j.1742-1241.2010.02439.x. [DOI] [PubMed] [Google Scholar]
- 27.Betteridge DJ, Gibson JM, Sager PT. Comparison of effectiveness of rosuvastatin versus atorvastatin on the achievement of combined C-reactive protein (<2 mg/L) and low-density lipoprotein cholesterol (< 70 mg/dl) targets in patients with type 2 diabetes mellitus (from the ANDROMEDA study) Am J Cardiol. 2007;100:1245–1248. doi: 10.1016/j.amjcard.2007.05.044. [DOI] [PubMed] [Google Scholar]
- 28.Pitt B, Loscalzo J, Monyak J, Miller E, Raichlen J. Comparison of lipid-modifying efficacy of rosuvastatin versus atorvastatin in patients with acute coronary syndrome (from the LUNAR study) Am J Cardiol. 2012;109:1239–1246. doi: 10.1016/j.amjcard.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 29.Lee CW, Kang SJ, Ahn JM, et al. Comparison of effects of atorvastatin (20 mg) versus rosuvastatin (10 mg) therapy on mild coronary atherosclerotic plaques (from the ARTMAP trial) Am J Cardiol. 2012;109:1700–1704. doi: 10.1016/j.amjcard.2012.01.399. [DOI] [PubMed] [Google Scholar]
- 30.Jones PH, Hunninghake DB, Ferdinand KC, et al. Effects of rosuvastatin versus atorvastatin, simvastatin, and pravastatin on non-high-density lipoprotein cholesterol, apolipoproteins, and lipid ratios in patients with hypercholesterolemia: additional results from the STELLAR trial. Clin Ther. 2004;26:1388–1399. Erratum in: Clin Ther. 2005;27:142. doi: 10.1016/j.clinthera.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 31.Kilicarslan A, Yavuz B, Guven GS, et al. Fenofibrate improves endothelial function and decreases thrombin-activatable fibrinolysis inhibitor concentration in metabolic syndrome. Blood Coagul Fibrinolysis. 2008;19:310–314. doi: 10.1097/MBC.0b013e3283009c69. [DOI] [PubMed] [Google Scholar]
- 32.Undas A, Celinska-Löwenhoff M, Domagala TB, et al. Early antithrombotic and anti-inflammatory effects of simvastatin versus fenofibrate in patients with hypercholesterolemia. Thromb Haemost. 2005;94:193–199. doi: 10.1160/TH05-01-0067. [DOI] [PubMed] [Google Scholar]
- 33.Ali FY, Armstrong PC, Dhanji AR, et al. Antiplatelet actions of statins and fibrates are mediated by PPARs. Arterioscler Thromb Vasc Biol. 2009;29:706–711. doi: 10.1161/ATVBAHA.108.183160. [DOI] [PubMed] [Google Scholar]
- 34.Sahebkar A, Pirro M, Reiner Ž, et al. A Systematic Review and Meta-Analysis of Controlled Trials on the Effects of Statin and Fibrate Therapies on Plasma Homocysteine Levels. Curr Med Chem. 2016;23:4490–4503. doi: 10.2174/0929867323666161007155310. [DOI] [PubMed] [Google Scholar]
- 35.Undas A. Statins in prevention of thromboembolic events: from seminal studies to recent advances. Pol Arch Intern Med. 2022;132:16208. doi: 10.20452/pamw.16208. [DOI] [PubMed] [Google Scholar]
- 36.Zhu H, Zheng H, Xu T, et al. Effects of statins in primary and secondary prevention for venous thromboembolism events: A meta analysis. Vascul Pharmacol. 2022;142:106931. doi: 10.1016/j.vph.2021.106931. [DOI] [PubMed] [Google Scholar]
- 37.Di Minno A, Ambrosino P, Calcaterra I, Di Minno MND. COVID-19 and Venous Thromboembolism: A Meta-analysis of Literature Studies. Semin Thromb Hemost. 2020;46:763–771. doi: 10.1055/s-0040-1715456. [DOI] [PMC free article] [PubMed] [Google Scholar]


