Abstract
A dog was euthanatized because of progressive neurological signs. Histologically, a nonsuppurative meningoencephalitis was found. By immunohistochemistry, in situ hybridization, and nested PCR procedures, Borna disease virus (BDV) antigen and BDV-specific RNA were demonstrated in brain tissues of the dog. The nucleotide sequence of the PCR product showed 94 to 98% homology to published BDV sequences. This is the first description of Borna disease in a dog.
Borna disease (BD) is a fatal disease of the central nervous system that was originally recognized in horses and sheep in certain areas of Germany (6). Subsequently, naturally occurring BD has also been diagnosed in other animal species, such as rabbits, cattle, and certain zoo animals (2, 4, 11, 21). Cases of spontaneous BD have been found only in Germany (6), Switzerland (12), Liechtenstein (unpublished data), and, recently, Austria (24). A condition discovered in cats of Sweden and Austria, known as feline nonsuppurative meningoencephalitis or staggering disease, and a paretic syndrome in ostriches of Israel have also been linked to an infection with Borna disease virus (BDV) (9, 10, 15). Furthermore, BDV antibodies and BDV nucleic acid sequences have been detected in specimens from clinically healthy horses (7, 13) and cats (14) in the United States and Japan, where classical BD has never been seen. BD has become of increasing interest to human medicine since BDV-specific antibodies, antigen, and RNA were found in humans with certain mental disorders (3, 5, 16, 20) but also in individuals without neurological disease (1). The relevance of these findings is controversially discussed (17), and formal proof of the causative role of BDV in psychiatric diseases is still lacking. Another important but still unsolved question is whether BDV can be transmitted from animals to humans; this question would be of particular interest if BDV infections were to be demonstrated in pet animal species. In addition, BDV infection achieved significance in biomedical research by serving as a model for immune-mediated disease of the central nervous system (23).
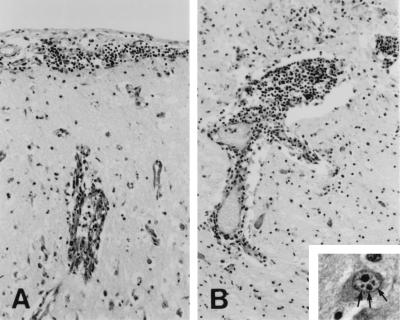
Dogs have never been suspected as being possible host species of BDV infection, and neither natural nor experimental BD has ever been described in the dog to date (19). Also, there is no report of serological surveys of BDV infection in canines. This communication reports the first known case of naturally occurring BD in a dog. During a retrospective study of etiologically unclear cases of nonsuppurative encephalitis in dogs, we came upon a case from September 1994. A 2-year-old female husky from Vorarlberg, the most western federal state of Austria, was presented to a veterinarian with a clinical history of anorexia and lethargy. After 2 days of unsuccessful treatment of symptoms with electrolytes, glucose, vitamins, amino acids, a nonsteroidal antiphlogistic agent, and antibiotics, the dog developed severe central nervous signs and was euthanatized 1 day later because of clinical signs suspicious of rabies or canine distemper. A neuropathological examination revealed a severe nonsuppurative meningoencephalitis that was characterized by the following: lymphocytic meningitis (Fig. 1A); perivascular lymphomonocytic cuffs (Fig. 1B); neuronal necrosis in the neocortex, allocortex, and hippocampus; subpial endothelial cell swelling (Fig. 1A); and focal gliosis. The lesions were most severe in the piriform lobe, rostral neocortex, periventricular gray matter, hippocampus, and mesencephalon, while minor changes were found in the occipital neocortex, medulla oblongata, and cerebellum. Some neurons contained single or several eosinophilic intranuclear Joest-Degen inclusion bodies (Fig. 1B, inset), which are considered characteristic of BD. In Vorarlberg, BD had already been diagnosed in horses, and this federal state is in close proximity to the area in eastern Switzerland in which BD is endemic. However, the histological lesions of the dog, in particular the widespread neuronal necroses and the swelling of endothelial cells, do not fully correspond with the changes associated with BD seen in horses; in the latter, large perivascular cuffs with infiltration of the inflammatory cells into the adjacent neuroparenchyma, without significant neuronal changes, are conspicuous. It may be assumed that the changes seen in the dog were due to ischemia of the cerebral cortex, perhaps as a result of seizures, rather than to primary viral effects.
FIG. 1.
(A) Cresyl echt violet staining of neocortex tissue, showing lymphocytic meningitis and endothelial cell swelling of a subpial blood vessel. Magnification, ×110. (B) Cresyl echt violet staining of thalamus tissue, showing lymphomonocytic perivascular cuffing. Magnification, ×110. The inset shows hematoxylin- and eosin-stained thalamus tissue, with the arrows indicating intranuclear Joest-Degen inclusion bodies in a neuron (magnification, ×510).
The histological diagnosis was confirmed by immunohistochemistry (IHC), in situ hybridization (ISH), and nested PCR procedures and by sequencing of the PCR product. Serum or cerebrospinal fluid (CSF) was not available for this retrospectively diagnosed case; therefore, it was not possible to determine BDV antibodies.
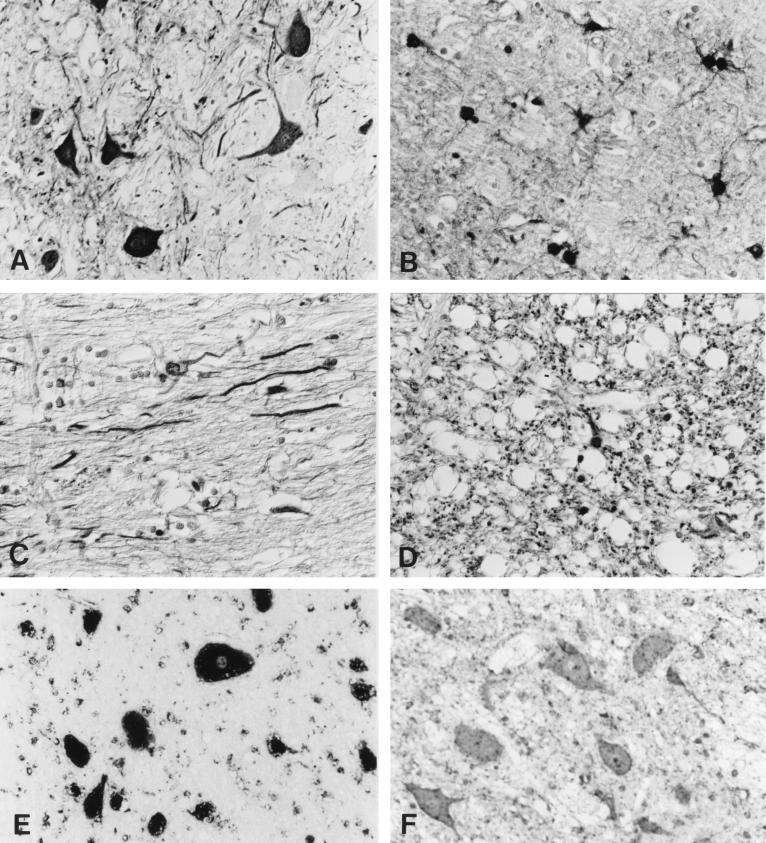
For IHC, we used an avidin-biotin complex technique and monoclonal antibody BO 18, directed against the 38/40-kDa protein (p38/40) of BDV, as the primary antibody (18). Brain tissue samples from two different horses with BD were taken as positive controls, while brain tissue samples from a normal dog and from various dogs with different nonsuppurative encephalitides (i.e., rabies, canine distemper, tick-borne encephalitis, and etiologically unresolved cases) served as negative controls. Another negative control was carried out by replacing the first antibody with a monoclonal antibody directed against bovine viral diarrhea virus. In brain tissue of the reported dog, strong immunoreactivity was confined to areas with severe encephalitis: the piriform lobe, the medio- and laterobasal areas of the neocortex, the basal ganglia, the hippocampus, the basal area of the thalamus, the hypothalamus, and the mesencephalon. In these regions, there was diffuse immunostaining of the neuropil and white matter but also of the nuclei, perikarya, and neurites of many neurons, astrocytes, and oligodendrocytes (Fig. 2A to D). The immunoreactivity in the remaining brain regions (the upper portions of the neocortex and thalamus, as well as pons, medulla oblongata, and cerebellum), which also displayed minor inflammatory changes, was less diffuse and restricted to groups of neurons and glial cells. Diffuse labelling of the neuropil was rarely found, and it was only focally present in these areas. This pattern of immunostaining was largely consistent with that of tissues from horses with BD examined with the same antibody (24).
FIG. 2.
Use of the avidin-biotin complex technique to show specific labelling of neurons (A), astrocytes (B), neurites (C), and white matter (D) with monoclonal antibody BO 18, which specifically recognizes BDV p38/40. Magnification, ×350. (E) In situ hybridization with an RNA probe specific for BDV ORF I reveals that positive signals are predominantly in the cytoplasm of neurons. Magnification, ×570. (F) In situ hybridization with an RNA probe specific for bovine herpesvirus 1 shows the absence of specific signal. Magnification, ×570.
ISH was carried out on a paraffin-embedded sample of mesencephalon, using a digoxigenin-labelled RNA probe specific for open reading frame (ORF) I of BDV. The probe was obtained by runoff synthesis after subcloning of the BDV ORF I cDNA into a transcription vector (pGEM 3z; Promega, Wallisellen, Switzerland) containing the viral RNA polymerase promoters T7 and Sp6. Briefly, the conditions for ISH were as follows: pretreatment with 0.1 N HCl for 10 min at room temperature, digestion with 0.1% (wt/vol) pepsin in 0.1% HCl at 37°C for 20 min, hybridization with partially hydrolyzed probe at 50°C overnight, and washing with 1× SSC (0.15 M NaCl, 0.015 M sodium citrate) at room temperature (twice, for 15 min each time) and with 0.1× SSC at 50°C (twice, for 20 min each time). Specific hybrids were detected by standard IHC using alkaline phosphatase-conjugated antidigoxigenin Fab fragments (Boehringer, Rotkreuz, Switzerland) and nitroblue tetrazolium–5-bromo-4-chloro-3-indolyl phosphate as the chromogen.
ISH revealed intense dark-blue cytoplasmic staining of numerous neurons (Fig. 2E) and astrocytes. Such a reaction was absent from the same tissue sample when it was hybridized with an irrelevant probe (Fig. 2F), and it was also not evident in tissue samples from uninfected animals when the specific probe was used.
To detect BDV-specific RNA, we applied a nested PCR assay to formalin-fixed, paraffin-embedded brain tissue specimens from the dog, since there was no frozen material available. RNA extraction was performed according to the method described by Sorg and Metzler (22); in order to extract as much nucleic acid as possible, proteinase K digestion was extended to 5 days at 37°C. For cDNA synthesis and the first PCR, the Titan One Tube reverse transcription-PCR system (Boehringer, Mannheim, Germany) was used. The primers for PCR and nested PCR as well as the conditions for the nested PCR were the same as those described by Sorg and Metzler (22), yielding a 212-bp PCR product within the ORF encoding p38/40. Formalin-fixed, paraffin-embedded brain tissue specimens from a horse from Vorarlberg and a horse from Bavaria, both with confirmed cases of BD, served as positive controls, and formalin-fixed, paraffin-embedded brain tissue specimens from dogs and horses without neurological diseases served as negative controls. Subsequently, the PCR products were sequenced in both directions by using an ABI Prism 310 genetic analyzer (Perkin-Elmer, Norwalk, Conn.), and the sequences were compared with already published sequences. For the suspected canine case, as well as the horses with BD from Vorarlberg and Bavaria, nested PCR resulted in PCR products of the expected size of 212 bp. In contrast, PCR of the brain tissue specimens from the dogs and horses which died due to other etiologies proved to be negative. Automated DNA sequencing of the amplicons showed a high degree of homology between dog and horse sequences from the same area (only two single-base changes; 99% homology at the nucleotide level and 100% identity at the amino acid level), although the canine case was from 1994 and the equine case was from 1997; the sequence of the Bavarian horse showed 97% nucleotide homology and 100% amino acid identity to the BDV sequence from the canine host. Comparison with sequences deposited in the GenBank database revealed nucleotide homologies of between 94 and 98%; the corresponding amino acid identities were between 97 and 100%. Although we have sequenced only a small fragment, it seems likely that the same virus strain is responsible for the BD cases in the dog and in horses in this area.
The reports of a tentative BDV infection in another carnivore—staggering disease in cats—differ significantly from the observations in this canine case. The cats did not originate from areas in which BD is endemic, and did not show the typical signs of classic BD, and only single positive cells were detected by IHC, which is not consistent with the huge amount of antigen found in horses and the present canine case (8). Taken together, the results of neuropathology, IHC, ISH, PCR, and sequencing studies provide evidence that the reported case is the first confirmed case of BD in a dog, caused by a BDV strain that is endemic to western Austria.
It is presently unknown whether this was just an exceptional single case or whether BD is more widespread in dogs. Paraffin blocks of specimens from 14 other dogs with nonsuppurative encephalitis of unresolved etiology were also examined immunohistologically for BDV antigen, and all proved to be negative. No serum or CSF was available for any of these animals. To gain additional insight into the prevalence of BDV infection in dogs, an epidemiological survey is being carried out in the federal state of Vorarlberg. Also, as a consequence of this case, BD has to be taken into account as a potential differential diagnosis in dogs with symptoms of neurological disease, and dogs with nonsuppurative encephalitis of unknown etiology should be checked for the presence of BDV antigen or nucleic acid, at least in endemic areas.
Nucleotide sequence accession numbers.
The nucleotide sequence data reported in this article have been deposited in the GenBank database under accession no. AF054275 (BDV, dog, Vorarlberg), AF054276 (BDV, horse, Vorarlberg), and AF054277 (BDV, horse, Bavaria).
Acknowledgments
We thank R. Waller for submission of the case, S. Herzog for providing monoclonal antibody BO 18, P. Staeheli for providing plasmids containing BDV cDNA inserts, and K. Bitterman, I. Friedl, and H. Lussy for excellent technical assistance.
REFERENCES
- 1.Bode L. Human infections with Borna disease virus and potential pathogenic implications. Curr Top Microbiol Immunol. 1995;190:103–130. doi: 10.1007/978-3-642-78618-1_7. [DOI] [PubMed] [Google Scholar]
- 2.Bode L, Dürrwald R, Ludwig H. Borna virus infections in cattle associated with fatal neurological disease. Vet Rec. 1994;135:283–284. doi: 10.1136/vr.135.12.283. [DOI] [PubMed] [Google Scholar]
- 3.Bode L, Zimmermann W, Ferszt R, Steinbach F, Ludwig H. Borna disease virus genome transcribed and expressed in psychiatric patients. Nat Med. 1995;1:232–236. doi: 10.1038/nm0395-232. [DOI] [PubMed] [Google Scholar]
- 4.Caplazi P, Waldvogel A, Stitz L, Braun U, Ehrensperger F. Borna disease in naturally infected cattle. J Comp Pathol. 1994;111:65–72. doi: 10.1016/s0021-9975(05)80112-4. [DOI] [PubMed] [Google Scholar]
- 5.de la Torre J C, Gonzalez-Dunia D, Cubitt B, Mallory M, Mueller-Lantzsch N, Grässer F A, Hansen L A, Masliah E. Detection of Borna disease virus antigen and RNA in human autopsy brain samples from neuropsychiatric patients. Virology. 1996;223:272–282. doi: 10.1006/viro.1996.0479. [DOI] [PubMed] [Google Scholar]
- 6.Dürrwald R, Ludwig H. Borna disease virus (BDV), a (zoonotic?) worldwide pathogen. A review of the history of the disease and the virus infection with comprehensive bibliography. J Vet Med Ser B. 1997;44:147–184. doi: 10.1111/j.1439-0450.1997.tb00962.x. [DOI] [PubMed] [Google Scholar]
- 7.Kao M, Hamir A N, Rupprecht C E, Fu Z F, Shankar V, Koprowski H, Dietzschold B. Detection of antibodies against Borna disease virus in sera and cerebrospinal fluid of horses in the USA. Vet Rec. 1993;132:241–244. doi: 10.1136/vr.132.10.241. [DOI] [PubMed] [Google Scholar]
- 8.Lundgren A-L, Lindberg R, Ludwig H, Gosztonyi G. Immunoreactivity of the central nervous system in cats with a Borna disease-like meningoencephalomyelitis (staggering disease) Acta Neuropathol. 1995;90:184–193. doi: 10.1007/BF00294319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lundgren A-L, Zimmermann W, Bode L, Czech G, Gosztonyi G, Lindberg R, Ludwig H. Staggering disease in cats: isolation and characterization of the feline Borna disease virus. J Gen Virol. 1995;76:2215–2222. doi: 10.1099/0022-1317-76-9-2215. [DOI] [PubMed] [Google Scholar]
- 10.Malkinson M, Weisman Y, Perl S, Ashash E. A Borna-like disease of ostriches in Israel. Curr Top Microbiol Immunol. 1995;190:31–38. doi: 10.1007/978-3-642-78618-1_3. [DOI] [PubMed] [Google Scholar]
- 11.Metzler A, Ehrensperger F, Wyler R. Natürliche Bornavirus-Infektion bei Kaninchen. Zentlbl Vetmed Reihe B. 1978;25:161–164. [PubMed] [Google Scholar]
- 12.Metzler A, Minder H-P, Wegmann C, Zindel W. Die Borna’sche Krankheit, ein veterinärmedizinisches Problem von regionaler Bedeutung. Schweiz Arch Tierheilkd. 1979;121:207–213. [PubMed] [Google Scholar]
- 13.Nakamura Y, Kishi M, Nakaya T, Asahi S, Tanaka H, Sentsui H, Ikeda K, Ikuta K. Demonstration of Borna disease virus RNA in peripheral blood mononuclear cells from healthy horses in Japan. Vaccine. 1995;13:1076–1079. doi: 10.1016/0264-410x(95)00050-b. [DOI] [PubMed] [Google Scholar]
- 14.Nakamura Y, Asahi S, Nakaya T, Bahmani M K, Saitoh S, Yasui K, Mayama H, Hagiwara K, Ishihara C, Ikuta K. Demonstration of Borna disease virus RNA in peripheral blood mononuclear cells derived from domestic cats in Japan. J Clin Microbiol. 1996;34:188–191. doi: 10.1128/jcm.34.1.188-191.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nowotny N, Weissenböck H. Description of feline nonsuppurative meningoencephalomyelitis (“staggering disease”) and studies of its etiology. J Clin Microbiol. 1995;33:1668–1669. doi: 10.1128/jcm.33.6.1668-1669.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nowotny N, Windhaber J. Borna disease virus and neuropsychiatric disorders. Lancet. 1997;350:593. doi: 10.1016/s0140-6736(05)63184-4. [DOI] [PubMed] [Google Scholar]
- 17.Richt J A, Alexander R C, Herzog S, Hooper D C, Kean R, Spitsin S, Bechter K, Schüttler R, Feldmann H, Heiske A, Fu Z F, Dietzschold B, Rott R, Koprowski H. Failure to detect Borna disease virus infection in peripheral blood leukocytes from humans with psychiatric disorders. J Neurovirol. 1997;3:174–178. doi: 10.3109/13550289709015807. [DOI] [PubMed] [Google Scholar]
- 18.Richt J A, Pfeuffer I, Christ M, Frese K, Bechter K, Herzog S. Borna disease virus infection in animals and humans. Emerg Infect Dis. 1997;3:343–352. doi: 10.3201/eid0303.970311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rott R, Becht H. Natural and experimental Borna disease in animals. Curr Top Microbiol Immunol. 1995;190:17–30. doi: 10.1007/978-3-642-78618-1_2. [DOI] [PubMed] [Google Scholar]
- 20.Rott R, Herzog S, Fleischer B, Winokur A, Amsterdam J, Dyson W, Koprowski H. Detection of serum antibodies to Borna disease virus in patients with psychiatric disorders. Science. 1985;228:755–756. doi: 10.1126/science.3922055. [DOI] [PubMed] [Google Scholar]
- 21.Schüppel K-F, Kinne J, Reinacher M. Bornavirus-Antigennachweis bei Alpakas (Lama pakos) sowie einem Faultier (Choloepus didactylus) und einem Zwergflußpferd (Choeropsis liberiensis) In: Hofmann R R, Ippen R, editors. Verhandlungsbericht XXXVI. Internationales Symposium über Erkrankungen der Zootiere. Berlin: Akademie Verlag; 1994. pp. 189–194. [Google Scholar]
- 22.Sorg I, Metzler A. Detection of borna disease virus RNA in formalin-fixed, paraffin-embedded brain tissues by nested PCR. J Clin Microbiol. 1995;33:821–823. doi: 10.1128/jcm.33.4.821-823.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stitz L, Dietzschold B, Carbone K M. Immunopathogenesis of Borna disease. Curr Top Microbiol Immunol. 1995;190:75–92. doi: 10.1007/978-3-642-78618-1_5. [DOI] [PubMed] [Google Scholar]
- 24.Suchy A, Weissenböck H, Waller R, Schmidt P, Nowotny N. Nachweis der Bornaschen Krankheit bei einem Pferd in Österreich. Wien Tieraerztl Monschr. 1997;84:317–321. [Google Scholar]