Abstract
Ferroptosis is a novel form of regulated cellular necrosis that plays a critical role in promoting cancer progression and developing drug resistance. The main characteristic of ferroptosis is iron-dependent lipid peroxidation caused by excess intracellular levels of reactive oxygen species. CUGBP ELAV-like family number 2 (CELF2) is an RNA-binding protein that is downregulated in various types of cancer and is associated with poor patient prognoses. CELF2 can directly bind mRNA to a variety of ferroptosis control factors; however, direct evidence of the regulatory role of CELF2 in ferroptosis is currently limited. The aim of the present review was to summarise the findings of previous studies on CELF2 and its role in regulating cellular redox homeostasis. The present review may provide insight into the possible mechanisms through which CELF2 affects ferroptosis and to provide recommendations for future studies.
Keywords: CUGBP ELAV-like family number 2, ferroptosis, MAPK signalling pathway, PI3K/AKT signalling pathway, autophagy, endoplasmic-reticulum-associated protein degradation, Wnt/β-catenin pathway
1. Introduction
Ferroptosis is a novel form of cell death discovered in recent years, and is characterised by an excessive accumulation of cellular levels of lipid peroxide, caused by elevated levels of reactive oxygen species (ROS) owing to a severe imbalance in the intracellular redox state. This process is closely linked to intracellular iron homeostasis, where an accumulation of the strongly oxidising ferrous ion, which becomes a labile iron pool, can generate ROS via the Fenton or Haber-Weiss reaction, thereby initiating the ferroptosis process. Ferroptosis is classified as a form of regulatory necrosis, a class of genetically regulated cell death. Its similarity to necrosis is the disruption of plasma membrane integrity and the release of cytoplasmic contents, which usually leads to a potent inflammatory response (1,2). However, unlike necrosis, the unregulated decadence process resulting from extreme adverse conditions, different types of regulated necrosis have different downstream execution mechanisms and stimulatory molecular pathways (1,2). Ferroptosis is regulated by a variety of factors, such as the glutathione peroxidase 4 (GPX4) antioxidant system, dihydroorotate dehydrogenase (DHODH), the ferroptosis suppressor protein 1 (FSP1)-mediated ferroptosis protection mechanism and the lipoxygenase trigger mechanism, which are independent of each other and together influence the occurrence of ferroptosis (3). An increasing number of studies have revealed that ferroptosis is a key mechanism involved in the development and progression of cancer, as well as in the development of drug resistance. Therefore, it is crucial to elucidate the regulatory mechanisms that underlie ferroptosis.
CUGBP ELAV-like family (CELF) proteins are a family of RNA-binding proteins (RBPs) that, similar to the majority of RBPs, play broad and diverse roles in RNA regulation. CELF2 is the second member of this family and has been found to play a critical role in cancer development. CELF2 functions as a tumour suppressor in a variety of tumours, and its downregulation is associated with a poor patient prognosis (4,5). The tumour-suppressive effects of CELF2 are dependent on the post-transcriptional regulation of various genes, such as heme oxygenase-1 (HO-1) and cyclooxygenase 1 (COX-1) (6,7), and its inhibition of various cellular signalling pathways.
The present review discusses in detail the possible mechanisms through which CELF2 regulates the mitogen-activated protein kinase (MAPK) signalling pathway, PI3K/AKT signalling pathway, endoplasmic reticulum (ER)-associated protein degradation (ERAD) pathway, autophagy and the Wnt/β-catenin pathway. Subsequently, the role of these pathways in influencing ferroptosis was further summarized. It was hypothsized that the tumour-suppressive effects of CELF2 may be partially dependent on ferroptosis mechanisms.
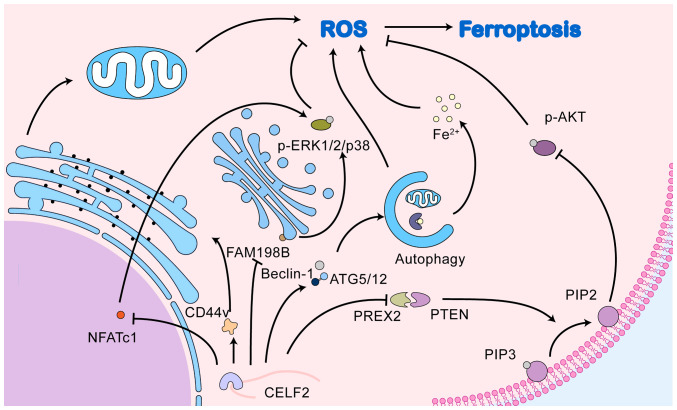
2. CELF2 affects ferroptosis through the MAPK signalling pathway
Association of MAPK signalling pathway with ferroptosis
MAPK is an intracellular signalling pathway that has been extensively studied. This cascade is activated by a sequence of three to five hierarchical layers of protein kinases known as the MAPK kinase kinase kinase (MAPKKKK) class, MAPK kinase kinase (MAPKKK) class, MAPK kinase (MAPKK) class, MAPK and MAPK-activated protein kinase (MAPKAPK) (8). The first three layers are considered the basic core units that recognise and conduct various signals inside and outside the cell via phosphorylation. MAPK activation is a critical step in the MAPK signalling pathway that mainly involves the extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK) and p38 protein families. These proteins can activate a variety of MAPKAPKs, thereby regulating the expression of downstream genes or proteins and allowing cells to respond to intra- and extracellular signals including proliferation, differentiation, apoptosis, senescence and carcinogenesis (9,10). Targeting the MAPK pathway, which is the most commonly mutated signalling pathway in human cancers, has long been considered a promising strategy for cancer therapy. An increasing number of recent studies have demonstrated that influencing the ferroptosis process is a key mechanism by which the MAPK signalling pathway promotes tumour development (11-14). The role of CELF2 in the MAPK signalling pathway and its possible role in ferroptosis is illustrated in Fig. 1.
Figure 1.
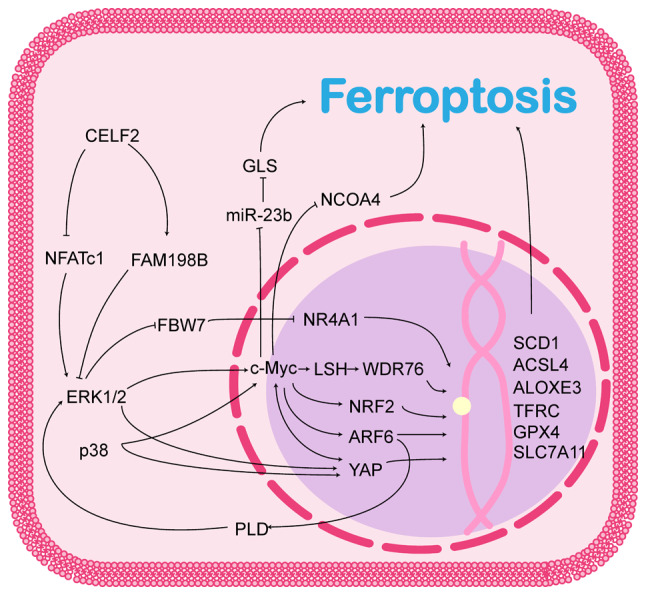
CELF2 affects ferroptosis through the MAPK signalling pathway. CELF2, CUGBP, ELAV-like family number 2; MAPK, mitogen-activated protein kinase; NFATc1, nuclear factor of activated T-cell c1; ERK, extracellular signal-regulated kinase; FAM198B, family with sequence similarity 198, member B; FBW7, F-box and WD repeat domain-containing 7; PLD, phospholipase D; GLS, glutaminase; NR4A1, nuclear receptor subfamily 4 group A member 1; LSH, lymphoid specific helicase; WDR76, WD40-repeat protein 76; NRF2, nuclear factor E2-related factor 2; ARF6, ADP-ribosylation factor 6; YAP, yes-associated protein; SCD1, stearoyl-CoA desaturase 1; ACSL4, acyl-CoA synthetase long-chain family member 4; ALOXE3, arachidonate lipoxygenase 3; TFRC, transferrin receptor protein; GPX4, glutathione peroxidase 4; SLC7A11, solute carrier family 7 member 11.
ERK1/2, two key members of the ERK/MAPK pathway, are normally found in the cytoplasm and, when activated, can enter the nucleus and regulate the activity of various transcription factors and gene expression, playing a crucial role in cell differentiation and proliferation (15). Additionally, ERK1/2 activation can regulate ferroptosis in cancer cells via multiple pathways.
The ERK/MAPK pathway causes resistance to ferroptosis in cancer cells by inhibiting F-box and WD repeat domain-containing 7 (FBW7)
FBW7 is a target of the ERK/MAPK signalling pathway, and ERK1 can directly bind and phosphorylate the Thr205 site of FBW7, which promotes the ubiquitinated degradation of FBW7 in a Pin1-dependent manner, thereby inhibiting FBW7 expression (16). FBW7 is an E3 ubiquitin ligase that ubiquitinates target substrates, usually via K11 or K48 linkages, and consequently degrades target proteins that include a number of crucial human cancer proteins (17,18). Thus, FBW7 functions as a tumour suppressor; its expression is downregulated in several tumours and is associated with patient prognosis (16,18). As previously reported, FBW7 induces cancer cell death by promoting ferroptosis. For example, FBW7 has been shown to reduce the binding of nuclear receptor subfamily 4 group A member 1 to the promoter of stearoyl-CoA desaturase 1 (SCD1), an enzyme that converts saturated fatty acids to monounsaturated fatty acids, and to inhibit its transcription via an unknown mechanism, thereby promoting ferroptosis in pancreatic cancer cells (19). Furthermore, FBW7 has been found to be able to recognize and ubiquitinate c-Myc in a manner dependent on Thr58 and Ser62 phosphorylation, thereby regulating the level of c-Myc in cancer cells and likely influencing the process of ferroptosis in cancer cells (20,21).
c-Myc, a component of the ERK/MAPK pathway, is a key regulator of ferroptosis
c-Myc is located downstream of the ERK/MAPK signalling pathway. In addition to indirectly regulating c-Myc levels through FBW7, ERK1/2 directly phosphorylates Ser62 of c-Myc and activates its transcription under conditions of oxidative stress (22). Moreover, p38a, another MAPK parallel to ERK, promotes c-Myc expression by directly phosphorylating Ser64 and Ser67 and inhibiting its proteasome-dependent degradation (23), or transcriptionally activating c-Myc by directly binding and phosphorylating β-catenin (24). c-Myc is considered an oncogenic transcription factor that binds to the promoters of various oncogenes to promote their transcription. It has been reported that c-Myc is able to transcriptionally activate a variety of ferroptosis suppressor genes, conferring cancer cells the ability to resist ferroptosis. For example, c-Myc is enriched at the solute carrier family 7 member 11 (SLC7A11) and γ-glutamylcysteine synthetase promoters, and transcriptionally activates these genes to help cancer cells resist oxidative stress through a pathway that promotes glutathione synthesis, which also renders cancer cells resistant to chemo- and radiotherapy (22,25-27). c-Myc activates lymphoid specific helicase gene transcription, which promotes WD40-repeat protein 76 enrichment at the SCD1/fatty acid desaturase 2 promoter and inhibits ferroptosis through a pathway that affects lipid metabolism (28). Furthermore, c-Myc can bind to the E-box on the nuclear factor E2-related factor 2 (NRF2) promoter to activate NRF2 transcription, thereby maintaining intracellular redox homeostasis (29). The non-transcription factor activities of c-Myc have also been identified; for example, c-Myc has been found to be able to directly inhibit the expression of miR-23b, which regulates cellular ferroptosis by targeting glutaminase expression at the 3′-untranslated region (3′-UTR) (30,31). c-Myc was also demonstrated to directly bind and inhibit nuclear receptor coactivator 4 (NCOA4) mRNA expression, suppressing ferroptosis by inhibiting ferritinophagy (32).
ADP-ribosylation factor 6 (ARF6), a small GTPase belonging to the Ras superfamily, is mainly localised in the plasma membrane and endosomal compartments, and plays a crucial role in plasma membrane endocytosis, cytokinesis, endosomal recycling, cytokinesis and actin cytoskeletal reorganisation (33). ARF6 is located downstream of the ERK/MAPK signalling pathway and ERK1/2 activates ARF6 transcription via c-Myc (34). Notably, ARF6 continuously activates the ERK/MAPK pathway by interacting with phospholipase D (35). This forms a positive feedback mechanism that maintains high levels of c-Myc in cancer cells (34). Recent studies have revealed that although ARF6 does not directly regulate lipid peroxidation, it can alter the sensitivity of cancer cells to oxidative stress, rendering them less sensitive to ferroptosis induced by RSL3 and erastin, and thereby participating in the development of drug resistance in cancer cells (36,37). ARF6 has been reported to inhibit the expression of acyl-CoA synthetase long-chain family member 4 (ACSL4) (36) and GPX4 (37) at the transcriptional level, allowing pancreatic and gastric cancer cells to develop tolerance to tabine analogues.
Yes-associated protein (YAP) can be activated by the ERK/MAPK pathway and plays a dual role in ferroptosis
YAP is a key effector of the Hippo pathway and is frequently dysregulated in human cancers. Aberrantly activated YAP has emerged as a key driver of tumorigenesis, chemoresistance and tumour metastasis (38). YAP is a co-transcription factor that interacts with DNA-binding transcription factors to regulate diverse cellular behaviours (39). Recent studies have revealed that YAP is located downstream of the MAPK pathway and that ERK1/2/5 (40-42), p38 (41), mechanistic target of rapamycin (mTOR)C2 (43) and c-Myc (44) can activate YAP in a non-Hippo pathway-dependent manner. Notably, the reciprocal upregulation of c-Myc and YAP has been observed in hepatocellular carcinoma. In hepatocellular carcinoma cells, c-Myc activates YAP transcription by interacting with hepatitis B X-interacting protein (44), while YAP promotes c-Myc transcription by binding to c-Abl (45). Taken together, YAP and c-Myc promote the development of hepatocellular carcinoma. However, its role in ferroptosis remains unclear. YAP enhances the binding of transcriptional enhanced associate domain (TEAD)4 to transferrin receptor protein (TFRC), ACSL4, and arachidonate lipoxygenase (ALOX)E3 (46-48), thereby enhancing the sensitivity of cancer cells to ferroptosis, whereas the YAP-TEAD complex inhibits the expression of threonine tyrosine kinase and TFRC via s-phase kinase-associated protein 2 (49), thereby protecting cancer cells from ferroptosis. Moreover, the YAP-activating transcription factor (ATF)4 complex can bind to the SLC7A11 promoter and promote its transcription, thereby promoting the resistance of hepatocellular carcinoma cells to sorafenib (50). These studies suggest that the role of YAP in ferroptosis is complex and may be related to the cancer cell type and genetic background, and that the role of YAP in ferroptosis requires further exploration.
CELF2 is a regulator of the MAPK signalling pathway
Based on the data from available studies, CELF2 may affect the MAPK pathway through both the human family with sequence similarity 198, member B (FAM198B) and nuclear factor of activated T-cell c1 (NFATc1) pathways. FAM198B is an N-linked glycoprotein with unknown functions that is localised to the Golgi membrane (51). CELF2 stabilises FAM198B mRNAs by binding to its AREs (AU/U-rich elements) within the 3′-UTR and then upregulates the expression of FAM198B (52). FAM198B is downregulated in lung and ovarian adenocarcinomas and is associated with a poor patient prognosis (51,52). FAM198B also functions in the tumour microenvironment. For example, Zheng et al (53) observed that the expression level of FAM198B in macrophages of colon cancer tissues correlated with patient prognosis. FAM198B regulates M2 polarisation in macrophages and promotes colon cancer progression by targeting SMAD2 (53). Existing research demonstrates that FAM198B exerts its tumour-suppressive effects mainly by inhibiting the ERK/MAPK pathway (51,52); however, the exact mechanisms involved remain elusive. The only factor that can be determined is that the inhibitory effect of FAM198B on the ERK/MAPK pathway depends on its three major glycosylation sites, namely, Asn98, Asn289 and Asn322, and that defects in the glycosylation sites would result in FAM198B being unable to inhibit the ERK/MAPK signalling pathway (51).
NFATc1 is a major transcription factor involved in osteoblast differentiation. Recent studies have demonstrated that it is upregulated in a variety of cancer types and mediates the malignant behaviour of cancer cells. The cancer-promoting function of NFATc1 is largely dependent on c-Myc expression. In addition to directly binding to the TGFβ inhibitory element of the c-Myc promoter (54), NFATc1 upregulates c-Myc expression by activating the ERK1/2/p38 MAPK signalling pathway (55), thereby promoting the progression of ovarian (55), lung (56) and pancreatic (54) cancers. The knockdown of NFATc1 reduces ERK1/2 phosphorylation, whereas the pharmacological inhibition of ERK1/2 similarly impairs NFATc1 expression (57). NFATc1 and the ERK1/2 MAPK signalling pathway appear to have a mutually reinforcing relationship. The mechanisms through which NFATc1 regulates the MAPK signalling pathway remain unclear. One possible explanation is that NFATc1 maintains the activation of the MAPK signalling pathway by interacting with STAT3 and promoting the transcription of proteins upstream of the MAPK signalling pathway (58). CELF2 exerts tumour-suppressive effects by regulating NFATc1 expression. A previous animal study discovered that tumour size and weight were substantially reduced in mice overexpressing CELF2, and that the overexpression of CELF2 was associated with reduced NFATc1 levels in tumour tissue (59). Furthermore, NFATc1 overexpression significantly reversed the CELF2-mediated reduction in the viability and invasive capacity of MCF-7 cells (59). Taken together, these results suggest that CELF2 affects tumour progression via the NFATc1/MAPK pathway.
2. The PI3K/AKT signalling pathway is critical for the regulation of ferroptosis by CELF2
The PI3K/AKT signalling pathway inhibits the ferroptosis process through multiple mechanisms
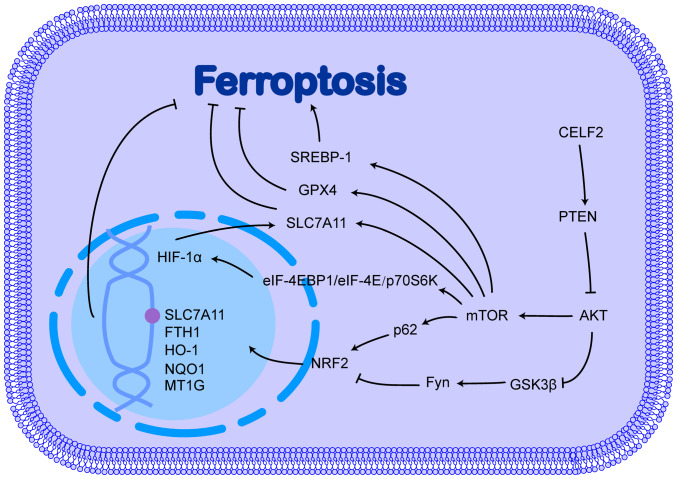
PI3K is a lipid kinase that phosphorylates the 3-OH moiety of phosphatidylinositol in the plasma and cell membranes. There are several PI3K classes, among which, the most extensively studied is class I PI3K, whose activation is involved in a variety of biological behaviours in cancer cells. The main focus of the present review was PI3K signalling pathway. Class I PI3K converts phosphatidylinositol-3,4-bisphosphate (PIP2) on the plasma membrane to phosphatidylinositol 3,4,5-triphosphate (PIP3), which binds AKT and pyruvate dehydrogenase lipoamide kinase isozyme 1 to PH domains and facilitates their interaction to phosphorylate and activate AKT (60,61). Activated AKT phosphorylates several downstream effectors, ultimately leading to cell growth, survival, and proliferation. The PI3K/AKT signalling pathway has been shown to promote tumour progression and drug resistance development by inhibiting ferroptosis (62,63). The role of CELF2 in the PI3K/AKT signalling pathway and its possible role in ferroptosis is illustrated in Fig. 2.
Figure 2.
CELF2 affects ferroptosis through the PI3K/AKT signalling pathway. CELF2, CUGBP, ELAV-like family number 2; HIF-1α, hypoxia inducible factor-1α; SLC7A11, solute carrier family 7 member 11; FTH1, ferritin heavy chain 1; HO-1, heme oxygenase 1; NQO1, NAD(P)H:quinone oxidoreductase 1; MT1G, metallothionein 1G; SREBP-1, sterol regulatory element binding protein-1; GPX4, glutathione peroxidase 4; eIF-4, eukaryotic initiation factor-4E; EBP1, eIF4E-binding protein 1; p70S6K, TRPV1-ribosomal protein 70 S6 kinase; mTOR, mechanistic target of rapamycin; GSK3β, glycogen synthase kinase 3β; PTEN, phosphatase and tensin homolog.
NRF2, a key antioxidant gene, is upregulated by the PI3K/AKT signalling pathway and confers ferroptosis resistance to cancer cells
NRF2 is a critical transcription factor that regulates antioxidant responses and plays a key role in preventing ferroptosis. In response to oxidative stress, the nuclear translocation of NRF2 is caused by the suppressed inhibitory effect of kelch-like ECH-associated protein 1 (Keap1) on NRF2. In the nucleus, NRF2 interacts with antioxidant response elements located in the mRNA promoter region, which encodes a subset of antioxidant genes, such as metallothionein-1G, HO-1, NAD(P)H:quinone oxidoreductase 1, ferritin heavy chain (FTH)1 and SLC7A11, ultimately activating and targeting gene transcription, thereby enhancing resistance to ferroptosis and promoting the development of drug resistance in cancer cells. The activation of the PI3K/AKT pathway induces the development of sorafenib resistance in cancer cells by upregulating NRF2 (64-66). Indeed, the PI3K/AKT pathway can phosphorylate and inhibit glycogen synthase kinase-3β (GSK3β), which attenuates the inhibitory effect of GSK3β on Fyn, which can phosphorylate the Tyr568 site of NRF2, leading to the nuclear export, ubiquitination and degradation of NRF2 (67-69).
mTOR is a major effector of the PI3K/AKT signalling pathway in the inhibition of ferroptosis
mTOR is a protein kinase that regulates cell growth, survival, metabolism and immunity, and is a major effector of the PI3K/AKT signalling pathway. Although mTOR can phosphorylate and activate p62, promoting the binding of p62 to Keap1 and upregulating NRF2 expression (70), its inhibitory effect on ferroptosis is not largely dependent on NRF2 (71). mTOR inhibits ferroptosis by directly upregulating GPX4 and SLC7A11 expression (72,73). In addition, mTOR upregulates sterol regulatory element binding protein-1 (SREBP-1) at both the transcriptional and post-translational levels (74,75), and the overexpression of SREBP-1 promotes resistance to ferroptosis through a pathway that affects lipid metabolism in cancer cells (71).
The PI3K/AKT pathway promotes hypoxia inducible factor-1α (HIF-1α) expression under normoxic conditions and consequently inhibits the onset of ferroptosis
HIF-1α is another gene downstream of the PI3K/AKT/mTOR signalling pathway. The PI3K/AKT/mTOR pathway promotes the translation of HIF-1α by regulating eukaryotic translation initiation factor 4E (eIF4E)-binding protein 1 (eIF-4EBP1), eIF-4E and TRPV1-ribosomal protein 70 S6 kinase (p70S6K) via phosphorylation (76,77). However, the regulation of HIF-1α by the PI3K/AKT signalling pathway is cell-specific and is influenced by environmental oxygenation. The inhibition of the PI3K/AKT pathway significantly inhibits HIF-1α accumulation under normoxic conditions, but not under hypoxic conditions (78,79). Under hypoxic conditions, the PI3K/AKT pathway plays a minimal role in regulating HIF-1α. Additionally, the overexpression of HIF-1α can promote AKT phosphorylation, which is dependent on the activation of autocrine growth factor genes, such as IGF-II and TGF-α by HIF-1α (80). The positive feedback effects of the PI3K/AKT pathway and HIF-1α combine to promote cancer progression (80). HIF-1α has also been found to play an inhibitory role in ferroptosis. For example, under hypoxic conditions, HIF-1α, which is highly expressed in gliomas and gastric cancer, promotes SLC7A11 expression by increasing the stability of SLC7A11 mRNA via the poly (methacrylic acid-niclosamide) polymer/ELAV-like RNA binding protein 1 pathway, thereby promoting resistance to ferroptosis and sulfasalazine (81,82). Moreover, HIF-1α can also promote the production of NADPH from glucose into the pentose phosphate pathway to maintain intracellular redox homeostasis (83).
CELF2 inhibits PI3K/AKT pathway activation via phosphatidylinositol-3,4,5-trisphosphate-dependent Rac exchange factor 2 (PREX2)/phosphatase and tensin homolog (PTEN)
CELF2 influences tumour progression by affecting the PI3K/AKT signalling pathway (84,85). Indeed, the ability of CELF2 to bind to PREX2 and reduce the interaction of PREX2 with PTEN increases the activity of PTEN phosphatase, which reverses the conversion of PIP2 to PIP3, thereby inhibiting activation of the PI3K/AKT signalling pathway (86,87). However, whether CELF2 influences ferroptosis through the PI3K/AKT signalling pathway requires further investigation.
3. CELF2 affects ferroptosis in cancer cells by promoting autophagy
The ferroptosis-promoting effect of autophagy is dependent on a severe imbalance in the intracellular redox state
Ferroptosis is a form of autophagy-dependent cell death (88,89). Autophagy was originally discovered as a cellular self-defence mechanism capable of targeting damaged organelles or various harmful biomolecules, as well as invading pathogens, thereby enabling cells to survive a variety of stimuli in many cases (88,90). Excessive or uncontrolled autophagy can trigger autophagy-dependent cell death (88,90).
NCOA4 mediates ferritinophagy and triggers mitochondrial autophagy
Recent studies have revealed that autophagy plays a crucial role in ferroptosis; for example, ferritinophagy, the degradation of ferritin by autophagy, is a main cause of cellular ferroptosis (91-93). During ferritinophagy, FTH binds specifically to NCOA4 to form a complex and forms cohesions in response to interactions between NCOA4 protein molecules (94,95), which are then degraded by lysosomes via the macroautophagic (92,96) or macroautophagic (97-99) pathways, releasing Fe2+ and causing ferroptosis. During this process, NCOA4 functions as an autophagic cargo receptor and is essential for ferritinophagy. Therefore, NCOA4 is a key target of ferritinophagy. In addition, NCOA4 has been reported to mediate mitochondrial autophagy in the context of iron homeostasis imbalance. For example, deferiprone, an iron chelator, causes cellular iron depletion that increases mitochondrial ferritin (FTMT) expression through the HIF-1α/transcription factor specificity protein 1 axis and localises its precursor form to the outer mitochondrial membrane, whereas NCOA4 interacts with the precursor form of FTMT and triggers mitochondrial autophagy to inhibit hepatocellular carcinoma cell growth (100,101).
The role of mitochondrial autophagy in ferroptosis is dependent on intracellular iron homeostasis and the redox status
The mitochondria play a critical role in ferroptosis. Ferroptosis is accompanied by mitochondrial dysfunction and the accumulation of mitochondrial ROS (mtROS). Mitochondria release mtROS into the cytoplasm to exacerbate their accumulation in the cytoplasm via several mechanisms (102), among which, mitochondrial autophagy may be a key mechanism (103-105). The excessive accumulation of mtROS and its resultant mitochondrial dysfunction, manifested as mitochondrial depolarisation, has been well documented as a cause of mitochondrial autophagy (105-107). Indeed, the excessive accumulation of mtROS and mitochondrial dysfunction can induce the activation of mitochondrial PTEN-induced kinase 1 (PINK1) (108) and its localisation to the outer mitochondrial membrane (109,110), leading to PINK1-mediated ubiquitin-dependent mitochondrial autophagy. Moreover, mitochondrial DNA damage caused by the excessive accumulation of mtROS leads to an increase in intracytoplasmic mitochondrial DNA, which can trigger mitochondrial autophagy via the GCAS (cyclic GMP-AMP synthase)-STING1 (stimulator of interferon response cGAMP interactor 1) signalling pathway (111). Damaged mitochondria are enzymatically cleaved in endolysosomes, which is a cellular defence mechanism that removes dysfunctional mitochondria, thereby preventing excessive ROS from damaging the cell. This also obscures the role of mitochondrial autophagy in tumour development. The analyses of public databases (112,113) have demonstrated that PINK1 expression is decreased in a variety of tumours and plays contradictory roles in various tumours and even in different cells. For example, in hepatocellular carcinoma, some studies have demonstrated a tumour-suppressive effect of PINK1-mediated mitochondrial autophagy (114,115), whereas other studies have revealed opposing results (113,116). These conflicting results indicate that mitochondrial autophagy may be influenced by certain factors that determine the ultimate effect of mitochondrial autophagy on the cell, whether facilitated or inhibited.
Endolysosomes are key sites for removing damaged organelles and abnormal proteins from cells. Endolysosomes contain significant amounts of iron and play a crucial role in maintaining cellular iron homeostasis (117,118). In acidic endolysosomes, iron is mainly present in the ferrous form and excessive iron content renders the endolysosomal membrane more susceptible to oxidative damage by ROS (117,119). It has been reported that in erastin- or RSL3-induced ferroptosis, the ROS content of the endolysosome increases rapidly and leads to endolysosomal membrane permeabilization, resulting in the release of ROS into the cytoplasm, producing a cytoplasmic ROS burst and triggering ferroptosis (120,121). Moreover, excess levels of Fe2+ can also lead to changes in the endolysosomal function and structure. For example, FAC, an iron agent, can significantly increase the number and surface area of endolysosomes and can lead to their dephosphorylation, which drives Fe2+ within endolysosomes into the cytoplasm via divalent metal transporter 1 and the related non-selective two-pore cation channels, thereby exacerbating cytoplasmic iron overload and promoting ferroptosis (122,123). In summary, these findings illustrate that endolysosomes play a role in promoting ferroptosis in the context of iron and ROS overload. During ferroptosis, ferritinophagy and mitochondrial autophagy provide large amounts of Fe2+ and ROS to endolysosomes, which causes endolysosomal dysfunction and exacerbates the ferroptosis-promoting function of the endolysosome, which partially explains the conflicting roles of mitochondrial autophagy.
Therefore, the following conclusions can be inferred about autophagy and ferroptosis: During ferroptosis, autophagy is activated to eliminate excess Fe2+ and ROS from the cell; however, dysfunctional endolysosomes do not assist the cell to deal with the excess ROS and even leak iron and ROS from the lysosome, causing a burst of intracytoplasmic ROS and allowing ferroptosis to occur in cancer cells.
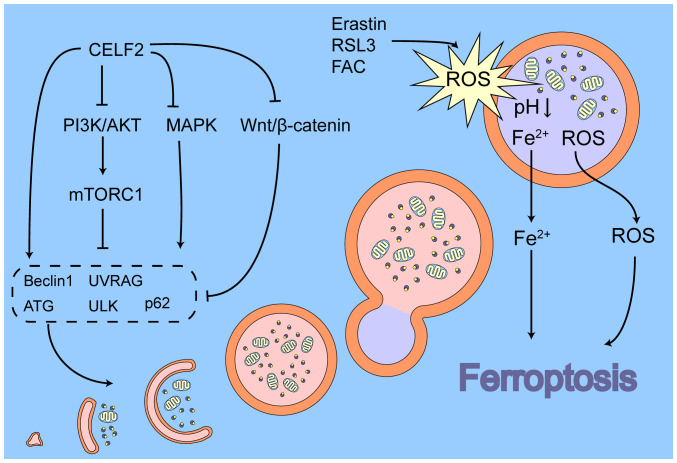
CELF2 can regulate the biological process of autophagy
CELF2, an RNA-binding protein, can directly bind to the mRNA of autophagy-related factors, allowing CELF2 to be directly associated with cellular autophagy. In colorectal cancer, the overexpression of CELF2 induced by radiotherapy is able to bind to Beclin1 and autophagy related gene (ATG)5/12 mRNAs, increasing their half-life and promoting the onset of autophagic cell death (124).
In addition to directly regulating autophagy-related factors, CELF2 may indirectly regulate cellular autophagy via the MAPK and PI3K/AKT pathways. The p38/MAPK signalling pathway plays a dual role in autophagy. p38 can inhibit the activity of unc-51 like autophagy activating kinase 1 (ULK1) (125,126), ATG5 (127), ATG8 (128) and mATG9 (129), thereby reducing autophagic flux; by contrast, p38 can also promote autophagy in some cases. For example, p38 has been found to promote autophagy through the heat shock protein 27/CREB pathway, which activates the transcription of ATG7 (130); the proteasome inhibitor MG231 is also able to induce LC3II production via the p38/GSK3β pathway, thereby activating autophagy (131). p38 also inhibits TP53 ubiquitinated degradation via phosphorylation modifications, the latter activating the downstream DNA damage-regulated autophagy modulator 1 gene, mediating autophagy induced by ROS accumulation (132). In the JNK/MAPK signalling pathway, activated JNK can enter the nucleus and promote the transcription of various autophagy regulators, such as LC3, Beclin1, Sestrin2 and ATG5/7 (133-137). Simultaneously, JNK can also promote autophagy through non-transcriptional mechanisms in some cases, such as through phosphorylation of BCL2. In addition, ERK1/2 and JNK, but not p38, can localise to the mitochondria, increasing the stability of PINK1 and promoting mitochondrial autophagy (138,139). In recent years, researchers have found that the MAPK and PI3K/AKT pathways activate p62 via the NRF2-Keap1 axis, which functions as an autophagic cargo receptor that binds to LC3 and promotes autophagosome formation (140).
mTORC1, located downstream of the MAPK and PI3K/AKT pathways, is a key junction in the regulation of autophagy. mTORC1 is a critical inhibitor of autophagy, and available studies have shown that mTORC1 affects autophagy through several mechanisms. First, ULK1 is the primary downstream target of mTORC1 that can affect ULK1 activity through phosphorylation modifications and post-translational pathways. Activated mTORC1 interacts with ULK1 and joins the ULK1-ATG13-FAK-family interacting protein of 200 kDa complex, whereas mTORC1 directly phosphorylates the Ser757 site in ULK1 (141,142). Ser757 is a key regulatory site of ULK1. The phosphorylation of ULK1 Ser757 not only prevents the activation of ULK1 by AMPK, which inhibits the phosphatase kinase activity of ULK1 (143), but also disrupts the interaction of ULK1 with ATG13, which helps localise ULK1 to the detached membrane, thereby inhibiting the initiation of autophagy (125). Moreover, mTORC1 promotes the ubiquitination of ULK1 by tumour necrosis factor receptor-associated factor 6 by phosphorylating the Ser52 site of the activating molecule in Beclin1-regulated autophagy protein 1, thereby reducing the stability of ULK1 (144). Second, activated mTORC1 phosphorylates the Ser113 and Ser120 (nuclear receptor binding factor 2 (NRBF2) sites. When mTORC1 is inhibited, the dephosphorylated form of NRBF2 binds ATG14-BECN1, facilitating the assembly of the Ptdlns3K complex and stimulating the production of Ptdlns3P on the isolated membrane, which can link to ULK1 and activate autophagy (145). Third, mTORC1 binds and phosphorylates the Ser498 site of the UV radiation resistance-associated gene (UVRAG). Phosphorylated UVRAG is able to inhibit the activity of Vps34 via RUN domain Beclin 1-interacting and cysteine-rich containing protein, whereas its function to activate homotypic fusion and vacuole protein sorting is diminished, resulting in a decrease in ras-like small GTPase superfamily member 7 activity, thereby inhibiting the initiation of autophagy and the maturation of autophagosomes and endosomes (146). Finally, mTORC1 can also reduce autophagic flux by phosphorylating autophagy regulators, such as death-associated protein 1 (147) and p70S6K (148).
The Wnt/β-catenin signalling pathway has beeb found to be a negative regulator of autophagy. This pathway inhibits autophagosome maturation mainly by suppressing p62/SQSTM1 expression (149,150). Fan et al (151) found that miR-363-3p led to the activation of the Wnt/β-catenin signalling pathway by targeting CELF2 and induced epithelial-mesenchymal transition (EMT) in glioma cells. That study demonstrated for the first time that CELF2 may be located upstream of the Wnt/β-catenin signalling pathway; however, it did not explain the specific regulatory mechanisms. The mechanisms through which CELF2 affects autophagy through the Wnt/β-catenin signalling pathway require further investigation. The association between CELF2 and autophagy and its possible role in ferroptosis is illustrated in Fig. 3.
Figure 3.
Possible association between CELF2 and autophagy and the promotion of ferroptosis. CELF2, CUGBP, ELAV-like family number 2; mTOR, mechanistic target of rapamycin; MAPK, mitogen-activated protein kinase; ATG, autophagy related gene; UVRAG, UV radiation resistance-associated gene; ULK, unc-51 like autophagy activating kinase; ROS, reactive oxygen species.
4. CELF2 may influence ferroptosis through the ERAD pathway
ER stress and ferroptosis
The ER is a crucial site for protein synthesis and processing, and its function is vulnerable to external factors. A variety of conditions, such as nutritional deprivation, hypoxia, viral infection, oxidative stress and calcium depletion can cause an imbalance in cellular compartment homeostasis and lead to ER stress, which is characterised by the accumulation of misfolded proteins within the ER lumen.
ER stress is also involved in ferroptosis. Increased acidity and viscosity within the ER have been reported in erastin-induced ferroptosis, and the combination of erastin and dithiothreitol, an ER stress inducer, caused significant increases in acidification and viscosity in the ER over a short period, suggesting the involvement of ER stress in the ferroptosis process (152,153). However, the contribution of ER stress to ferroptosis is so complex that it cannot be explained merely by changes in ER content. ER stress exerts varying, or even contrasting, effects on ferroptosis under different conditions. For example, in renal tubular epithelial cells (154) and hepatocytes (155), ER stress induced by cadmium exposure leads to ferritinophagy, whereas in lung cancer, ER stress caused by Ca2+ bursts can lead to the reprogramming of Ca2+ distribution and mitochondrial dysfunction, facilitating the ferroptosis process through ROS production (156). Moreover, another study reported that the pharmacological inhibition or siRNA knockdown of zrt-like, and Irt-like protein family member 7 induced ER stress by affecting zinc metabolism, which promoted the transcription of homocysteine-responsive endoplasmic reticulum-resident ubiquitin-like domain member 1 and protected MDA-MB-231, RCC1 and HT1080 cells from ferroptosis damage through an unknown mechanism (157). The unfolded protein response (UPR), a signalling mechanism induced by ER stress aimed at resolving misfolded ER proteins and restoring ER homeostasis, is one of the most critical downstream pathways of ER stress and plays an unknown role in ferroptosis. Protein kinase R-like endoplasmic reticulum kinase) and ATF6α, two major UPR effectors, have been found to play opposing roles in ferroptosis (158-161). In summary, these studies collectively suggest that ER stress is involved in ferroptosis and that its function is influenced by a variety of factors.
The ERAD pathway may be involved in the process of ferroptosis
Similar to the UPR, ERAD is a key quality control mechanism in cells capable of degrading natural or misfolded proteins within the ER and maintaining ER homeostasis (162). The ERAD process is broadly divided into three stages. First, ERAD substrates are recognised by chaperone proteins or chaperone-like lectins and are retained within the ER. Depending on their nature or sorting mechanisms within the ER, ERAD substrates are ubiquitinated by various E3 ubiquitin-linked enzymes and transported to the cytoplasm by p97/VCP proteins in an ATP-dependent manner. Finally, the substrate proteins are degraded by the proteasome (163,164). This involves complex molecular mechanisms that will not be described herein, as they exceed the scope of the present review. During ER stress, both the UPR and ERAD are activated and both mechanisms play a crucial role in restoring ER homeostasis. For example, the activation of the UPR promotes protein folding, while also increasing the expression of ERAD-related proteins and promoting the role of ERAD in degrading misfolded proteins; by contrast, defects in ERAD can also lead to the accumulation of misfolded proteins within the ER, resulting in sustained ER stress, subsequently leading to cell death (165). Therefore, these two mechanisms have complementary, synergistic and irreplaceable functions. The ER and mitochondria are highly functionally associated; therefore, the status of the ERAD pathway also appears to be associated with mitochondrial function. Eeyarestatin I, an ERAD inhibitor, reportedly reorganises the overall mitochondrial activity in HepG2 cells, resulting in mitochondrial dysfunction by elevating the intramitochondrial Ca2+ and ROS levels (166). In addition, the inhibition of the ERAD pathway leads to the accumulation of various substrate proteins on the ER membrane, such as sigma non-opioid intracellular receptor 1 (SigmaR1) (167) and diacylglycerol o-acyltransferase 2 (168), and the excessive accumulation of these proteins can affect mitochondrial function through various pathways and may therefore be involved in the ferroptosis process (169).
In addition to resisting ER stress, ERAD maintains a basal state of activation under normal conditions and is involved in the degradation of normal proteins within the ER. A previous study identified SigmaR1 as an ERAD substrate in brown adipocytes (167). That study demonstrated that the knockdown of the Sel1L-Hrd1 complex, the most conserved form of ERAD from yeast to humans, resulted in SigmaR1 accumulation on ER membranes, which allowed the mitochondria to fuse in response to cold stimulation and reduced the mitochondrial utilisation of lipid droplets. This phenomenon was independent of ER stress (167). Of note, SigmaR1 has also been found in various cancer cells (170-172) and has been shown to function as an inhibitor of ferroptosis in hepatocellular carcinoma cells (173,174). Furthermore, cytochrome P450, an upstream regulator of ferroptosis (175), can be degraded as a substrate for ERAD (176). Therefore, the ERAD pathway may function as an upstream regulator of ferroptosis.
CELF2 affects the ERAD pathway by mediating the alternative splicing of CD44
CD44 is a hyaluronan-binding cell surface signal transduction receptor that plays a crucial role in the genesis, invasion and metastasis of a number of tumours, and is widely considered a marker of cancer stem cells. CD44 contains two variable regions encoded by variable exons; therefore, there are multiple isoforms of CD44, including standard CD44 (CD44s) and variant CD44 (CD44v). The dysregulation of alternative splicing frequently occurs in cancer, resulting in a shift from CD44s to CD44v, which can profoundly affect tumour biology (177). Lai et al (178) observed that CELF2, an important factor for mRNA alternative splicing, was involved in the alternative splicing of CD44 and led to a transition from CD44s to CD44v. Lai et al (178) also found that the role of CD44v in promoting pancreatic cancer development was dependent on the ERAD pathway, and that an inhibitor of the ERAD pathway was effective in reversing the effects of CD44 on cancer cells. Although the exact mechanisms of ERAD regulation by CD44 have not yet been elucidated, it is suggested that CELF2 functions as an upstream regulator of the ERAD pathway. The association between CELF2 and ERAD and their possible role in ferroptosis are illustrated in Fig. 4.
Figure 4.
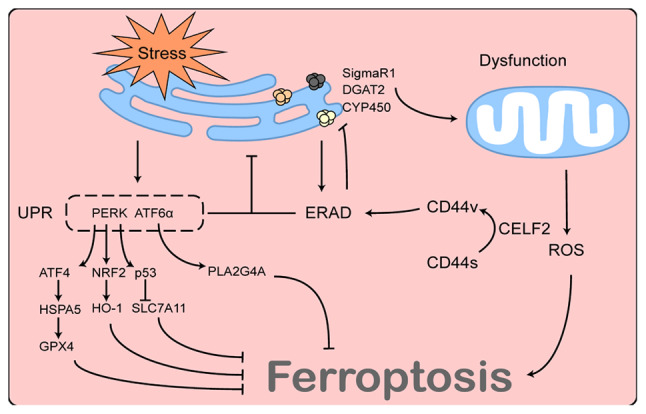
CELF2 affects ferroptosis via ERAD. CELF2, CUGBP, ELAV-like family number 2; ERAD, endoplasmic-reticulum-associated protein degradation; UPR, unfolded protein response; ATF4, activating transcription factor 4; HSPA5, heat shock protein family A (Hsp70) member 5; GPX4, glutathione peroxidase 4; PERK, protein kinase R-like endoplasmic reticulum kinase; ATF6α, activating transcription factor 6α; NRF2, nuclear factor E2-related factor 2; HO-1, heme oxygenase 1; SLC7A11, solute carrier family 7 member 11; DGAT2, diacylglycerol o-acyltransferase 2; SigmaR1, sigma non-opioid intracellular receptor 1; CYP450, cytochrome P450; ROS, reactive oxygen species.
5. CELF2 activates the Wnt/β-catenin pathway and promotes cancer cell resistance to ferroptosis
Activation of the Wnt/β-catenin signalling pathway inhibits ferroptosis
In the classical Wnt/β-catenin signalling pathway, the Wnt protein binds to the Frizzled and low-density lipoprotein receptor-related protein 5/6 (LRP5/6) co-receptors, thereby activating Dishevelled protein (DVL). This prevents adenomatous polyposis coli, axis inhibition protein (AXIN) and GSK3β from forming a destructive complex that prevents the phosphorylation and subsequent degradation of β-catenin. The accumulated β-catenin then translocates to the nucleus and activates downstream genes by binding to different co-transcription factors to form transcriptional complexes. The Wnt/β-catenin signalling pathway plays an inhibitory role in ferroptosis. β-catenin translocated to the nucleus promotes the activation of ferroptosis regulatory genes, such as GPX4 (179), COX2 (182), SCD1 (181), peroxisome proliferator-activated receptor-γ coactivator 1-α (181), matrix metalloproteinases (182) and c-Myc (182,183), and promotes tolerance to platinum-based chemotherapeutic agents through ferroptosis resistance in gastric (184) and ovarian (179) cancers and brain metastases from lung adenocarcinoma (185).
CELF2 affects the activation state of the Wnt/β-catenin signalling pathway through multiple pathways
Fan et al (150) first identified an association between CELF2 and the Wnt/β-catenin signalling pathway; however, its specific regulatory mechanisms require further exploration. Based on previous studies, it is suggested that several potential mechanisms may be involved in the regulation of the Wnt/β-catenin pathway by CELF2.
The MAPK cascade, and PI3K/AKT and Wnt/β-catenin signalling pathways crosstalk in a variety of tumours, and all three pathways play synergistic or alternative roles in promoting tumour progression. The pro-tumorigenic effects of the MAPK and PI3K/AKT pathways are partly dependent on the activation of the Wnt/β-catenin signalling pathway. Thus, the MAPK and PI3K/AKT pathways may play regulatory roles upstream of the Wnt/β-catenin signalling pathway. As previously reported, p38, JNK and ERK1/2 are all able to phosphorylate the Ser1490 and Thr1572 sites of LRP6, which allows LRP6 to provide more AXIN1 and GSK3β binding sites, while isolating these two proteins from the β-catenin destruction complex and thereby reducing the degradation of β-catenin (186,187). Therefore, GSK3β is a critical regulatory target. AKT directly phosphorylates the Ser9 site of GSK3β to negatively regulate GSK3β activity, inhibit β-catenin degradation and promote Wnt/β-catenin pathway activation (188). In addition, β-catenin is a direct target of the MAPK and PI3K/AKT pathways. (p21-Activated kinase 1, located downstream of the PI3K/AKT and ERK/MAPK signalling pathways, activates the Wnt/β-catenin pathway by directly phosphorylating β-catenin and promoting its nuclear localization (189); ERK2 also promotes the nuclear translocation of β-catenin by inhibiting the linkage of α-catenin and β-catenin through the phosphorylation of casein kinase 2α (190).
As previously mentioned (149,150), the Wnt/β-catenin pathway negatively regulates autophagy. In fact, the Wnt/β-catenin pathway has a profound interaction with autophagy. The present review demonstrated that both DVL and β-catenin can function as substrates for autophagy. For example, DVL can be ubiquitinated by Von Hippel-Lindau protein and then degraded by autophagy through a p62-mediated interaction with LC3 (191); β-catenin is degraded by autophagy through interactions with LC3 under both nutrient-dense and starvation conditions (150). Moreover, the inhibition of autophagy can alter the activation of the Wnt/β-catenin signalling pathway (150,191).
Taken together, these results suggest that CELF2 may affect the Wnt/β-catenin signalling pathway via MAPK, PI3K/AKT and autophagy. The association between CELF2 and the Wnt/β-catenin pathway and its possible role in ferroptosis are illustrated in Fig. 5.
Figure 5.
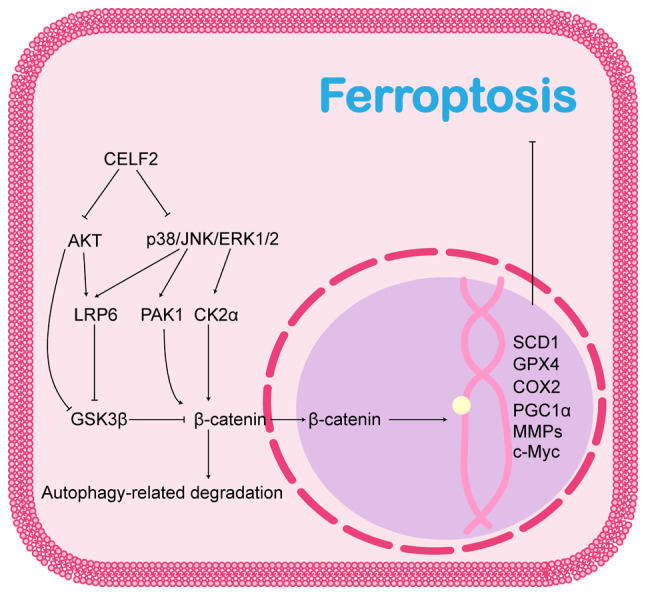
CELF2 affects ferroptosis via the Wnt/β-catenin pathway. CELF2, CUGBP, ELAV-like family number 2; LRP6, low-density lipoprotein receptor-related protein 6; GSK3β, glycogen synthase kinase 3β; PAK1, p21-activated kinase 1; CK2α, casein kinase 2α; SCD1, stearoyl-CoA desaturase 1; GPX4, glutathione peroxidase 4; COX2, cyclooxygenase 2; PGC1α, peroxisome proliferator-activated receptor-γ coactivator 1-α; MMPs, matrix metalloproteinases.
6. Conclusions and future perspectives
Ferroptosis is a novel form of cell death characterised by the excessive accumulation of intracellular lipid peroxide, which is dependent on an increase in intracellular iron-dependent ROS. Ferroptosis involves a variety of factors, such as the GPX4 antioxidant system, the ALOX and Ca2+-independent phospholipase A2β pathways, DHODH and FSP1, and occurs under the combined regulatory effect of these factors. Moreover, the pro-tumour effects of signalling pathways, such as MAPK, PI3K/AKT and Wnt/β-catenin, are partly dependent on the resistance of cancer cells to ferroptosis. Ferroptosis plays a crucial role in the development of cancer cells and may serve as a mechanism for tumour therapy.
CELF2 contains three RNA recognition motifs, two at the N-terminus and one at the C-terminus, and a segment of a divergent structural domain that may mediate interactions with RNA (192). This determines the RNA-binding properties of CELF2 (192). Indeed, CELF2 expression is reduced in a variety of cancers and is significantly associated with tumour stage and a poor prognosis of patient patients with various types of cancer, including in non-small cell lung (87), colorectal (5,193), glioblastoma (151), nasopharyngeal (194), gastric (195), breast (4), ovarian (52) and pancreatic cancers (178), and CELF2 may be a key locus for the action of various dysregulated miRNAs or lncRNAs (84,86,151,193-195). The overexpression of CELF2 in these tumour cell lines has been reported to inhibit their biological behaviours, including proliferation, invasion, migration, EMT, and resistance to radio- and chemotherapy (7,85,124,193-199), although the exact mechanisms involved remain unclear. The activation of MAPK or PI3K/AKT signalling pathways owing to the downregulation of CELF2 has been found to be sufficient for inducing proliferation, invasion and migration of cancer cells in a variety of tumours (84,86,87), but CELF2 may act through multiple mechanisms throughout tumour development. For example, in gliomas, the migration and invasion of cancer cells caused by CELF2 downregulation may be associated with the activation of EMT and Wnt/β-catenin signalling pathways (151). In pancreatic cancer, the CELF2-mediated CD44 alternative splicing affects apoptosis and cell stemness by regulating the ERAD signalling pathway (178). Furthermore, the downregulation of CELF2 confers chemoresistance to cancer cells through HO-1- and COX-2-mediated cytoprotective effects (6,7). Thus, CELF2 appears to function as a key target in tumour development. The anticancer potential of CELF2 was initially demonstrated in several in vitro studies (7,52,200). Curcumin, a natural polyphenolic compound derived from turmeric, enhances the sensitivity of pancreatic and ovarian cancer cell lines to gemcitabine and cisplatin, respectively, by upregulating CELF2 (7,52,200). Although previous studies have identified the effects of CELF2 on the genes that regulate ferroptosis (6,7), the specific role it plays in ferroptosis remains unclear.
The present review summarised the downstream targets of CELF2 in detail and speculated on their role in ferroptosis in a continuous context, which also poses as a limitation of the present review. The present review identified several avenues for further research to improve the understanding of ferroptosis. Fig. 6 broadly illustrates that CELF2 affects ferroptosis through a variety of mechanisms. Overall, CELF2 can exert its oncogenic effects through multiple pathways that may be partly dependent on ferroptosis.
Figure 6.
CELF2 is the upstream regulation factor of MAPK signalling pathway, PI3K/AKT signalling pathway, autophagy and ERAD pathway, which can promote cancer cell ferroptosis through these pathways. CELF2, CUGBP, ELAV-like family number 2; ERAD, endoplasmic-reticulum-associated protein degradation; NFATc1, nuclear factor of activated T-cell c1; FAM198B, family with sequence similarity 198, member B; ATG, autophagy related gene; PTEN, phosphatase and tensin homolog; PIP2, phosphatidylinositol-3,4-bisphosphate; PIP3, phosphatidylinositol 3,4,5-triphosphate.
Acknowledgments
Not applicable.
Funding Statement
The present study was funded by the Jilin Provincial Science and Technology Foundation (grant no. 20200201487JC).
Availability of data and materials
Not applicable.
Authors' contributions
JiahaoL and LX conceived the study. ZZ was involved in the search methods for relevant literature, as well as the structure of the review. JiahaoL provided the software used to prepare the figures (Adobe Illustrator CC 2018), and was also involved in determining the novelty and innovation of the direction of the topic, and in the writing and preparation of the original draft. WZ, RZ, WX and JiahaoL were involved in reading and evaluating the retrieved literature to determine whether it could be included in the review. YW was involved in the evaluation of the retrieved literature for inclusion in the review. LX, WX and JiaruiL were involved in the writing, reviewing and editing of the manuscript. ZZ and WX were involved in visualization. JiaruiL supervised the study. JiaruiL was involved in project administration. JiaruiL was involved in funding acquisition. All authors have read and agreed to the published version of the manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Sun Y, Chen P, Zhai B, Zhang M, Xiang Y, Fang J, Xu S, Gao Y, Chen X, Sui X, Li G. The emerging role of ferroptosis in inflammation. Biomed Pharmacother. 2020;127:110108. doi: 10.1016/j.biopha.2020.110108. [DOI] [PubMed] [Google Scholar]
- 2.Peng JJ, Song WT, Yao F, Zhang X, Peng J, Luo XJ, Xia XB. Involvement of regulated necrosis in blinding diseases: Focus on necroptosis and ferroptosis. Exp Eye Res. 2020;191:107922. doi: 10.1016/j.exer.2020.107922. [DOI] [PubMed] [Google Scholar]
- 3.Ma T, Du J, Zhang Y, Wang Y, Wang B, Zhang T. GPX4-independent ferroptosis-a new strategy in disease's therapy. Cell Death Discov. 2022;8:434. doi: 10.1038/s41420-022-01212-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang L, Liu Z, Liu L, Guo C, Jiao D, Li L, Zhao J, Han X, Sun Y. CELF2 is a candidate prognostic and immunotherapy biomarker in triple-negative breast cancer and lung squamous cell carcinoma: A pan-cancer analysis. J Cell Mol Med. 2021;25:7559–7574. doi: 10.1111/jcmm.16791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramalingam S, Ramamoorthy P, Subramaniam D, Anant S. Reduced expression of RNA binding protein CELF2, a putative tumor suppressor gene in colon cancer. Immunogastroenterology. 2012;1:27–33. doi: 10.7178/ig.1.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sureban SM, Murmu N, Rodriguez P, May R, Maheshwari R, Dieckgraefe BK, Houchen CW, Anant S. Functional antagonism between RNA binding proteins HuR and CUGBP2 determines the fate of COX-2 mRNA translation. Gastroenterology. 2007;132:1055–1065. doi: 10.1053/j.gastro.2006.12.031. [DOI] [PubMed] [Google Scholar]
- 7.Jakstaite A, Maziukiene A, Silkuniene G, Kmieliute K, Dauksa A, Paskauskas S, Gulbinas A, Dambrauskas Z. Upregulation of cugbp2 increases response of pancreatic cancer cells to chemotherapy. Langenbecks Arch Surg. 2016;401:99–111. doi: 10.1007/s00423-015-1364-1. [DOI] [PubMed] [Google Scholar]
- 8.Guo YJ, Pan WW, Liu SB, Shen ZF, Xu Y, Hu LL. ERK/MAPK signalling pathway and tumorigenesis. Exp Ther Med. 2020;19:1997–2007. doi: 10.3892/etm.2020.8454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee S, Rauch J, Kolch W. Targeting MAPK Signaling in Cancer: Mechanisms of Drug Resistance and Sensitivity. Int J Mol Sci. 2020;21:1102. doi: 10.3390/ijms21031102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sui X, Kong N, Ye L, Han W, Zhou J, Zhang Q, He C, Pan H. p38 and JNK MAPK pathways control the balance of apoptosis and autophagy in response to chemotherapeutic agents. Cancer Lett. 2014;344:174–179. doi: 10.1016/j.canlet.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 11.Chang WT, Bow YD, Fu PJ, Li CY, Wu CY, Chang YH, Teng YN, Li RN, Lu MC, Liu YC, Chiu CC. A Marine terpenoid, heteronemin, induces both the apoptosis and ferroptosis of hepatocellular carcinoma cells and involves the ROS and MAPK pathways. Oxid Med Cell Longev. 2021;2021:7689045. doi: 10.1155/2021/7689045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou D, Wu Q, Qiu H, Li M, Ji Y. Simvastatin inhibits endometrial cancer malignant behaviors by suppressing R AS/ M itogen-Activated protei n k i nase ( M A PK) Pathway-Mediated reactive oxygen species (ROS) and ferroptosis. Evid Based Complement Alternat Med. 2022;2022:6177477. doi: 10.1155/2022/6177477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He T, Lin X, Yang C, Chen Z, Wang L, Li Q, Ma J, Zhan F, Wang Y, Yan J, Quan Z. Theaflavin-3,3′-Digallate Plays a ROS-Mediated dual role in ferroptosis and apoptosis via the MAPK pathway in human osteosarcoma cell lines and xenografts. Oxid Med Cell Longev. 2022;2022:8966368. doi: 10.1155/2022/8966368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhatt V, Lan T, Wang W, Kong J, Lopes EC, Wang J, Khayati K, Raju A, Rangel M, Lopez E, et al. Inhibition of autophagy and MEK promotes ferroptosis in Lkb1-deficient Kras-driven lung tumors. Cell Death Dis. 2023;14:61. doi: 10.1038/s41419-023-05592-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Santarpia L, Lippman SM, El-Naggar AK. Targeting the MAPK-RAS-RAF signaling pathway in cancer therapy. Expert Opin Ther Targets. 2012;16:103–119. doi: 10.1517/14728222.2011.645805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ji S, Qin Y, Shi S, Liu X, Hu H, Zhou H, Gao J, Zhang B, Xu W, Liu J, et al. ERK kinase phosphorylates and destabilizes the tumor suppressor FBW7 in pancreatic cancer. Cell Res. 2015;25:561–573. doi: 10.1038/cr.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis RJ, Welcker M, Clurman BE. Tumor suppression by the Fbw7 ubiquitin ligase: mechanisms and opportunities. Cancer Cell. 2014;26:455–464. doi: 10.1016/j.ccell.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y, Hu K, Xiao X, Wu W, Yan H, Chen H, Chen Z, Yin D. FBW7 suppresses cell proliferation and G2/M cell cycle transition via promoting γ-catenin K63-linked ubiquitylation. Biochem Biophys Res Commun. 2018;497:473–479. doi: 10.1016/j.bbrc.2018.01.192. [DOI] [PubMed] [Google Scholar]
- 19.Ye Z, Zhuo Q, Hu Q, Xu X, Mengqi Liu, Zhang Z, Xu W, Liu W, Fan G, Qin Y, et al. FBW7-NRA41-SCD1 axis synchronously regulates apoptosis and ferroptosis in pancreatic cancer cells. Redox Biol. 2021;38:101807. doi: 10.1016/j.redox.2020.101807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yada M, Hatakeyama S, Kamura T, Nishiyama M, Tsunematsu R, Imaki H, Ishida N, Okumura F, Nakayama K, Nakayama KI. Phosphorylation-dependent degradation of c-Myc is mediated by the F-box protein Fbw7. EMBO J. 2004;23:2116–2125. doi: 10.1038/sj.emboj.7600217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen J, Ding C, Chen Y, Hu W, Lu Y, Wu W, Zhang Y, Yang B, Wu H, Peng C, et al. ACSL4 promotes hepatocellular carcinoma progression via c-Myc stability mediated by ERK/FBW7/c-Myc axis. Oncogenesis. 2020;9:42. doi: 10.1038/s41389-020-0226-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benassi B, Fanciulli M, Fiorentino F, Porrello A, Chiorino G, Loda M, Zupi G, Biroccio A. c-Myc phosphorylation is required for cellular response to oxidative stress. Mol Cell. 2006;21:509–519. doi: 10.1016/j.molcel.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 23.Lepore Signorile M, Grossi V, Fasano C, Forte G, Disciglio V, Sanese P, De Marco K, La Rocca F, Armentano R, Valentini AM, et al. c-MYC protein stability is sustained by MAPKs in colorectal cancer. Cancers (Basel) 2022;14:4840. doi: 10.3390/cancers14194840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lepore Signorile M, Grossi V, Di Franco S, Forte G, Disciglio V, Fasano C, Sanese P, De Marco K, Susca FC, Mangiapane LR, et al. Pharmacological targeting of the novel β-catenin chromatin-associated kinase p38α in colorectal cancer stem cell tumorspheres and organoids. Cell Death Dis. 2021;12:316. doi: 10.1038/s41419-021-03572-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang X, Guo S, Xu M, Ma B, Liu R, Xu Y, Zhang Y. TFAP2C-Mediated lncRNA PCAT1 inhibits ferroptosis in docetaxel-resistant prostate cancer through c-Myc/miR-25-3p/SLC7A11 signaling. Front Oncol. 2022;12:862015. doi: 10.3389/fonc.2022.862015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benassi B, Zupi G, Biroccio A. Gamma-glutamylcysteine synthetase mediates the c-Myc-dependent response to antineoplastic agents in melanoma cells. Mol Pharmacol. 2007;72:1015–1023. doi: 10.1124/mol.107.038687. [DOI] [PubMed] [Google Scholar]
- 27.Kim BY, Kwak SY, Yang JS, Han YH. Phosphorylation and stabilization of c-Myc by NEMO renders cells resistant to ionizing radiation through up-regulation of γ-GCS. Oncol Rep. 2011;26:1587–1593. doi: 10.3892/or.2011.1432. [DOI] [PubMed] [Google Scholar]
- 28.Jiang Y, Mao C, Yang R, Yan B, Shi Y, Liu X, Lai W, Liu Y, Wang X, Xiao D, et al. EGLN1/c-Myc induced lymphoid-specific helicase inhibits ferroptosis through lipid metabolic gene expression changes. Theranostics. 2017;7:3293–3305. doi: 10.7150/thno.19988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liang C, Shi S, Liu M, Qin Y, Meng Q, Hua J, Ji S, Zhang Y, Yang J, Xu J, et al. PIN1 maintains redox balance via the c-Myc/NRF2 axis to counteract kras-induced mitochondrial respiratory injury in pancreatic cancer cells. Cancer Res. 2019;79:133–145. doi: 10.1158/0008-5472.CAN-18-1968. [DOI] [PubMed] [Google Scholar]
- 30.Lu H, Yin H, Qu L, Ma X, Fu R, Fan D. Ginsenoside Rk1 regulates glutamine metabolism in hepatocellular carcinoma through inhibition of the ERK/c-Myc pathway. Food Funct. 2022;13:3793–3811. doi: 10.1039/D1FO03728E. [DOI] [PubMed] [Google Scholar]
- 31.Gao P, Tchernyshyov I, Chang TC, Lee YS, Kita K, Ochi T, Zeller KI, De Marzo AM, Van Eyk JE, Mendell JT, Dang CV. c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature. 2009;458:762–765. doi: 10.1038/nature07823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jin Y, Qiu J, Lu X, Li G. C-MYC inhibited ferroptosis and promoted immune evasion in ovarian cancer cells through NCOA4 mediated ferritin autophagy. Cells. 2022;11:4127. doi: 10.3390/cells11244127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hongu T, Kanaho Y. Activation machinery of the small GTPase Arf6. Adv Biol Regul. 2014;54:59–66. doi: 10.1016/j.jbior.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 34.Liang C, Qin Y, Zhang B, Ji S, Shi S, Xu W, Liu J, Xiang J, Liang D, Hu Q, et al. ARF6, induced by mutant Kras, promotes proliferation and Warburg effect in pancreatic cancer. Cancer Lett. 2017;388:303–311. doi: 10.1016/j.canlet.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 35.Knizhnik AV, Kovaleva OV, Komelkov AV, Trukhanova LS, Rybko VA, Zborovskaya IB, Tchevkina EM. Arf6 promotes cell proliferation via the PLD-mTORC1 and p38MAPK pathways. J Cell Biochem. 2012;113:360–371. doi: 10.1002/jcb.23362. [DOI] [PubMed] [Google Scholar]
- 36.Ye Z, Hu Q, Zhuo Q, Zhu Y, Fan G, Liu M, Sun Q, Zhang Z, Liu W, Xu W, et al. Abrogation of ARF6 promotes RSL3-induced ferroptosis and mitigates gemcitabine resistance in pancreatic cancer cells. Am J Cancer Res. 2020;10:1182–1193. [PMC free article] [PubMed] [Google Scholar]
- 37.Geng D, Wu H. Abrogation of ARF6 in promoting erastin-induced ferroptosis and mitigating capecitabine resistance in gastric cancer cells. J Gastrointest Oncol. 2022;13:958–967. doi: 10.21037/jgo-22-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yan F, Qian M, He Q, Zhu H, Yang B. The posttranslational modifications of Hippo-YAP pathway in cancer. Biochim Biophys Acta Gen Subj. 2020;1864:129397. doi: 10.1016/j.bbagen.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 39.Jang JW, Kim MK, Bae SC. Reciprocal regulation of YAP/TAZ by the Hippo pathway and the Small GTPase pathway. Small GTPases. 2020;11:280–288. doi: 10.1080/21541248.2018.1435986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meng XY, Zhang HZ, Ren YY, Wang KJ, Chen JF, Su R, Jiang JH, Wang P, Ma Q. Pinin promotes tumor progression via activating CREB through PI3K/AKT and ERK/MAPK pathway in prostate cancer. Am J Cancer Res. 2021;11:1286–1303. [PMC free article] [PubMed] [Google Scholar]
- 41.Lee CW, Nam JS, Park YK, Choi HK, Lee JH, Kim NH, Cho J, Song DK, Suh HW, Lee J, et al. Lysophosphatidic acid stimulates CREB through mitogen- and stress-activated protein kinase-1. Biochem Biophys Res Commun. 2003;305:455–461. doi: 10.1016/S0006-291X(03)00790-3. [DOI] [PubMed] [Google Scholar]
- 42.Ippolito F, Consalvi V, Noce V, Battistelli C, Cicchini C, Tripodi M, Amicone L, Marchetti A. Extracellular signal-Regulated Kinase 5 (ERK5) is required for the Yes-associated protein (YAP) co-transcriptional activity. Cell Death Dis. 2023;14:32. doi: 10.1038/s41419-023-05569-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holmes B, Benavides-Serrato A, Saunders JT, Kumar S, Nishimura RN, Gera J. mTORC2-mediated direct phosphorylation regulates YAP activity promoting glioblastoma growth and invasive characteristics. Neoplasia. 2021;23:951–965. doi: 10.1016/j.neo.2021.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Y, Fang R, Cui M, Zhang W, Bai X, Wang H, Liu B, Zhang X, Ye L. The oncoprotein HBXIP up-regulates YAP through activation of transcription factor c-Myb to promote growth of liver cancer. Cancer Lett. 2017;385:234–242. doi: 10.1016/j.canlet.2016.10.018. [DOI] [PubMed] [Google Scholar]
- 45.Xiao W, Wang J, Ou C, Zhang Y, Ma L, Weng W, Pan Q, Sun F. Mutual interaction between YAP and c-Myc is critical for carcinogenesis in liver cancer. Biochem Biophys Res Commun. 2013;439:167–172. doi: 10.1016/j.bbrc.2013.08.071. [DOI] [PubMed] [Google Scholar]
- 46.Qin Y, Pei Z, Feng Z, Lin P, Wang S, Li Y, Huo F, Wang Q, Wang Z, Chen ZN, et al. Oncogenic activation of YAP signaling sensitizes ferroptosis of hepatocellular carcinoma via ALOXE3-mediated lipid peroxidation accumulation. Front Cell Dev Biol. 2021;9:751593. doi: 10.3389/fcell.2021.751593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu J, Minikes AM, Gao M, Bian H, Li Y, Stockwell BR, Chen ZN, Jiang X. Intercellular interaction dictates cancer cell ferroptosis via NF2-YAP signalling. Nature. 2019;572:402–406. doi: 10.1038/s41586-019-1426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fang K, Du S, Shen D, Xiong Z, Jiang K, Liang D, Wang J, Xu H, Hu L, Zhai X, et al. SUFU suppresses ferroptosis sensitivity in breast cancer cells via Hippo/YAP pathway. iScience. 2022;25:104618. doi: 10.1016/j.isci.2022.104618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang WH, Lin CC, Wu J, Chao PY, Chen K, Chen PH, Chi JT. The Hippo pathway effector YAP promotes ferroptosis via the E3 ligase SKP2. Mol Cancer Res. 2021;19:1005–1014. doi: 10.1158/1541-7786.MCR-20-0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gao R, Kalathur RKR, Coto-Llerena M, Ercan C, Buechel D, Shuang S, Piscuoglio S, Dill MT, Camargo FD, Christofori G, Tang F. YAP/TAZ and ATF4 drive resistance to Sorafenib in hepatocellular carcinoma by preventing ferroptosis. EMBO Mol Med. 2021;13:e14351. doi: 10.15252/emmm.202114351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hsu CY, Chang GC, Chen YJ, Hsu YC, Hsiao YJ, Su KY, Chen HY, Lin CY, Chen JS, Chen YJ, et al. FAM198B is associated with prolonged survival and inhibits metastasis in lung adenocarcinoma via blockage of ERK-mediated MMP-1 expression. Clin Cancer Res. 2018;24:916–926. doi: 10.1158/1078-0432.CCR-17-1347. [DOI] [PubMed] [Google Scholar]
- 52.Guo Q, Wu Y, Guo X, Cao L, Xu F, Zhao H, Zhu J, Wen H, Ju X, Wu X. The RNA-binding protein CELF2 inhibits ovarian cancer progression by stabilizing FAM198B. Mol Ther Nucleic Acids. 2021;23:169–184. doi: 10.1016/j.omtn.2020.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zheng X, Chen J, Nan T, Zheng L, Lan J, Jin X, Cai Y, Liu H, Chen W. FAM198B promotes colorectal cancer progression by regulating the polarization of tumor-associated macrophages via the SMAD2 signaling pathway. Bioengineered. 2022;13:12435–12445. doi: 10.1080/21655979.2022.2075300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Buchholz M, Schatz A, Wagner M, Michl P, Linhart T, Adler G, Gress TM, Ellenrieder V. Overexpression of c-myc in pancreatic cancer caused by ectopic activation of NFATc1 and the Ca2+/calcineurin signaling pathway. EMBO J. 2006;25:3714–3724. doi: 10.1038/sj.emboj.7601246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu W, Gu J, Ren Q, Shi Y, Xia Q, Wang J, Wang S, Wang Y, Wang J. NFATC1 promotes cell growth and tumorigenesis in ovarian cancer up-regulating c-Myc through ERK1/2/p38 MAPK signal pathway. Tumour Biol. 2016;37:4493–4500. doi: 10.1007/s13277-015-4245-x. [DOI] [PubMed] [Google Scholar]
- 56.Ren F, Zhu K, Wang Y, Zhou F, Pang S, Chen L. Proliferation, apoptosis and invasion of human lung cancer cells are associated with NFATc1. Exp Ther Med. 2023;25:49. doi: 10.3892/etm.2022.11748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Russo R, Mallia S, Zito F, Lampiasi N. Long-lasting activity of ERK kinase depends on NFATc1 induction and is involved in cell migration-fusion in murine macrophages RAW264.7. Int J Mol Sci. 2020;21:8965. doi: 10.3390/ijms21238965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baumgart S, Chen NM, Siveke JT, König A, Zhang JS, Singh SK, Wolf E, Bartkuhn M, Esposito I, Heßmann E, et al. Inflammation-induced NFATc1-STAT3 transcription complex promotes pancreatic cancer initiation by KrasG12D. Cancer Discov. 2014;4:688–701. doi: 10.1158/2159-8290.CD-13-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou L, Xie X. RNA-binding protein CELF2 inhibits breast cancer cell invasion and angiogenesis by downregulating NFATc1. Exp Ther Med. 2021;22:898. doi: 10.3892/etm.2021.10330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Faes S, Dormond O. PI3K and AKT: Unfaithful partners in cancer. Int J Mol Sci. 2015;16:21138–21152. doi: 10.3390/ijms160921138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hashemi M, Taheriazam A, Daneii P, Hassanpour A, Kakavand A, Rezaei S, Hejazi ES, Aboutalebi M, Gholamrezaie H, Saebfar H, et al. Targeting PI3K/Akt signaling in prostate cancer therapy. J Cell Commun Signal. 2022 Nov 11; doi: 10.1007/s12079-022-00702-1. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ma RH, Ni ZJ, Thakur K, Cespedes-Acuña CL, Zhang JG, Wei ZJ. Transcriptome and proteomics conjoint analysis reveal metastasis inhibitory effect of 6-shogaol as ferroptosis activator through the PI3K/AKT pathway in human endometrial carcinoma in vitro and in vivo. Food Chem Toxicol. 2022;170:113499. doi: 10.1016/j.fct.2022.113499. [DOI] [PubMed] [Google Scholar]
- 63.Lu Y, Mao J, Xu Y, Pan H, Wang Y, Li W. Ropivacaine represses the ovarian cancer cell stemness and facilitates cell ferroptosis through inactivating the PI3K/AKT signaling pathway. Hum Exp Toxicol. 2022;41:9603271221120652. doi: 10.1177/09603271221120652. [DOI] [PubMed] [Google Scholar]
- 64.Wang L, Wang J, Chen L. TIMP1 represses sorafenib-triggered ferroptosis in colorectal cancer cells by activating the PI3K/Akt signaling pathway. Immunopharmacol Immunotoxicol. 2022;45:419–425. doi: 10.1080/08923973.2022.2160731. [DOI] [PubMed] [Google Scholar]
- 65.Liu H, Zhao L, Wang M, Yang K, Jin Z, Zhao C, Shi G. FNDC5 causes resistance to sorafenib by activating the PI3K/Akt/Nrf2 pathway in hepatocellular carcinoma cells. Front Oncol. 2022;12:852095. doi: 10.3389/fonc.2022.852095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huang W, Chen K, Lu Y, Zhang D, Cheng Y, Li L, Huang W, He G, Liao H, Cai L, et al. ABCC5 facilitates the acquired resistance of sorafenib through the inhibition of SLC7A11-induced ferroptosis in hepatocellular carcinoma. Neoplasia. 2021;23:1227–1239. doi: 10.1016/j.neo.2021.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jain AK, Jaiswal AK. GSK-3beta acts upstream of Fyn kinase in regulation of nuclear export and degradation of NF-E2 related factor 2. J Biol Chem. 2007;282:16502–16510. doi: 10.1074/jbc.M611336200. [DOI] [PubMed] [Google Scholar]
- 68.Rizvi F, Shukla S, Kakkar P. Essential role of PH domain and leucine-rich repeat protein phosphatase 2 in Nrf2 suppression via modulation of Akt/GSK3 beta/Fyn kinase axis during oxidative hepatocellular toxicity. Cell Death Dis. 2014;5:e1153. doi: 10.1038/cddis.2014.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liao S, Wu JN, Liu RM, Wang SX, Luo J, Yang Y, Qin Y, Li T, Zheng X, Song J, et al. A novel compound DBZ ameliorates neuroinflammation in LPS-stimulated microglia and ischemic stroke rats: Role of Akt(Ser473)/GSK3β(Ser9)-mediated Nrf2 activation. Redox Biol. 2020;36:101644. doi: 10.1016/j.redox.2020.101644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ichimura Y, Waguri S, Sou YS, Kageyama S, Hasegawa J, Ishimura R, Saito T, Yang Y, Kouno T, Fukutomi T, et al. Phosphorylation of p62 activates the Keap1-Nrf2 pathway during selective autophagy. Mol Cell. 2013;51:618–631. doi: 10.1016/j.molcel.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 71.Yi J, Zhu J, Wu J, Thompson CB, Jiang X. Oncogenic activation of PI3K-AKT-mTOR signaling suppresses ferroptosis via SREBP-mediated lipogenesis. Proc Natl Acad Sci USA. 2020;117:31189–31897. doi: 10.1073/pnas.2017152117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu Y, Wang Y, Liu J, Kang R, Tang D. Interplay between MTOR and GPX4 signaling modulates autophagy-dependent ferroptotic cancer cell death. Cancer Gene Ther. 2021;28:55–63. doi: 10.1038/s41417-020-0182-y. [DOI] [PubMed] [Google Scholar]
- 73.Zhang L, Liu W, Liu F, Wang Q, Song M, Yu Q, Tang K, Teng T, Wu D, Wang X, et al. IMCA induces ferroptosis mediated by SLC7A11 through the AMPK/mTOR pathway in colorectal cancer. Oxid Med Cell Longev. 2020;2020:1675613. doi: 10.1155/2020/6901472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li S, Oh YT, Yue P, Khuri FR, Sun SY. Inhibition of mTOR complex 2 induces GSK3/FBXW7-dependent degradation of sterol regulatory element-binding protein 1 (SREBP1) and suppresses lipogenesis in cancer cells. Oncogene. 2016;35:642–650. doi: 10.1038/onc.2015.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang Q, Mao Y, Wang J, Yu H, Zhang X, Pei X, Duan Z, Xiao C, Ma M. Gestational bisphenol A exposure impairs hepatic lipid metabolism by altering mTOR/CRTC2/SREBP1 in male rat offspring. Hum Exp Toxicol. 2022;41:9603271221129852. doi: 10.1177/09603271221129852. [DOI] [PubMed] [Google Scholar]
- 76.Masoud GN, Li W. HIF-1α pathway: Role, regulation and intervention for cancer therapy. Acta Pharm Sin B. 2015;5:378–389. doi: 10.1016/j.apsb.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.van den Beucken T, Koritzinsky M, Wouters BG. Translational control of gene expression during hypoxia. Cancer Biol Ther. 2006;5:749–755. doi: 10.4161/cbt.5.7.2972. [DOI] [PubMed] [Google Scholar]
- 78.Alvarez-Tejado M, Alfranca A, Aragonés J, Vara A, Landázuri MO, del Peso L. Lack of evidence for the involvement of the phosphoinositide 3-kinase/Akt pathway in the activation of hypoxia-inducible factors by low oxygen tension. J Biol Chem. 2002;277:13508–13517. doi: 10.1074/jbc.M200017200. [DOI] [PubMed] [Google Scholar]
- 79.Arsham AM, Plas DR, Thompson CB, Simon MC. Phosphatidylinositol 3-kinase/Akt signaling is neither required for hypoxic stabilization of HIF-1 alpha nor sufficient for HIF-1-dependent target gene transcription. J Biol Chem. 2002;277:15162–15170. doi: 10.1074/jbc.M111162200. [DOI] [PubMed] [Google Scholar]
- 80.Tanaka H, Yamamoto M, Hashimoto N, Miyakoshi M, Tamakawa S, Yoshie M, Tokusashi Y, Yokoyama K, Yaginuma Y, Ogawa K. Hypoxia-independent overexpression of hypoxia-inducible factor 1alpha as an early change in mouse hepatocarcinogenesis. Cancer Res. 2006;66:11263–11270. doi: 10.1158/0008-5472.CAN-06-1699. [DOI] [PubMed] [Google Scholar]
- 81.Sun S, Guo C, Gao T, Ma D, Su X, Pang Q, Zhang R. Hypoxia enhances glioma resistance to sulfasalazine-induced ferroptosis by upregulating SLC7A11 via PI3K/AKT/HIF-1α axis. Oxid Med Cell Longev. 2022;2022:7862430. doi: 10.1155/2022/7862430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lin Z, Song J, Gao Y, Huang S, Dou R, Zhong P, Huang G, Han L, Zheng J, Zhang X, et al. Hypoxia-induced HIF-1α/lncRNA-PMAN inhibits ferroptosis by promoting the cytoplasmic translocation of ELAVL1 in peritoneal dissemination from gastric cancer. Redox Biol. 2022;52:102312. doi: 10.1016/j.redox.2022.102312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Guo S, Miyake M, Liu KJ, Shi H. Specific inhibition of hypoxia inducible factor 1 exaggerates cell injury induced by in vitro ischemia through deteriorating cellular redox environment. J Neurochem. 2009;108:1309–1321. doi: 10.1111/j.1471-4159.2009.05877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang Q, Wang Y. MiR-210-3p targets CELF2 to facilitate progression of lung squamous carcinoma through PI3K/AKT pathway. Med Oncol. 2022;39:161. doi: 10.1007/s12032-022-01752-6. [DOI] [PubMed] [Google Scholar]
- 85.Wu JZ, Jiang N, Lin JM, Liu X. STYXL1 promotes malignant progression of hepatocellular carcinoma via downregulating CELF2 through the PI3K/Akt pathway. Eur Rev Med Pharmacol Sci. 2020;24:2977–2985. doi: 10.26355/eurrev_202003_20662. [DOI] [PubMed] [Google Scholar]
- 86.Shi M, Yang R, Lin J, Wei QI, Chen L, Gong W, Li Y, Guo X. LncRNA-SNHG16 promotes proliferation and migration of acute myeloid leukemia cells via PTEN/PI3K/AKT axis through suppressing CELF2 protein. J Biosci. 2021;46:4. doi: 10.1007/s12038-020-00127-1. [DOI] [PubMed] [Google Scholar]
- 87.Yeung YT, Fan S, Lu B, Yin S, Yang S, Nie W, Wang M, Zhou L, Li T, Li X, et al. CELF2 suppresses non-small cell lung carcinoma growth by inhibiting the PREX2-PTEN interaction. Carcinogenesis. 2020;41:377–389. doi: 10.1093/carcin/bgz113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhou B, Liu J, Kang R, Klionsky DJ, Kroemer G, Tang D. Ferroptosis is a type of autophagy-dependent cell death. Semin Cancer Biol. 2020;66:89–100. doi: 10.1016/j.semcancer.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 89.Kang R, Tang D. Autophagy and Ferroptosis-What's the connection? Curr Pathobiol Rep. 2017;5:153–159. doi: 10.1007/s40139-017-0139-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Denton D, Kumar S. Autophagy-dependent cell death. Cell Death Differ. 2019;26:605–616. doi: 10.1038/s41418-018-0252-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gao M, Monian P, Pan Q, Zhang W, Xiang J, Jiang X. Ferroptosis is an autophagic cell death process. Cell Res. 2016;26:1021–1032. doi: 10.1038/cr.2016.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hou W, Xie Y, Song X, Sun X, Lotze MT, Zeh HJ, III, Kang R, Tang D. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy. 2016;12:1425–1428. doi: 10.1080/15548627.2016.1187366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Park E, Chung SW. ROS-mediated autophagy increases intracellular iron levels and ferroptosis by ferritin and transferrin receptor regulation. Cell Death Dis. 2019;10:822. doi: 10.1038/s41419-019-2064-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gryzik M, Srivastava A, Longhi G, Bertuzzi M, Gianoncelli A, Carmona F, Poli M, Arosio P. Expression and characterization of the ferritin binding domain of Nuclear Receptor Coactivator-4 (NCOA4) Biochim Biophys Acta Gen Subj. 2017;1861:2710–2716. doi: 10.1016/j.bbagen.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 95.Ohshima T, Yamamoto H, Sakamaki Y, Saito C, Mizushima N. NCOA4 drives ferritin phase separation to facilitate macroferritinophagy and microferritinophagy. J Cell Biol. 2022;221:e202203102. doi: 10.1083/jcb.202203102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dowdle WE, Nyfeler B, Nagel J, Elling RA, Liu S, Triantafellow E, Menon S, Wang Z, Honda A, Pardee G, et al. Selective VPS34 inhibitor blocks autophagy and uncovers a role for NCOA4 in ferritin degradation and iron homeostasis in vivo. Nat Cell Biol. 2014;16:1069–1079. doi: 10.1038/ncb3053. [DOI] [PubMed] [Google Scholar]
- 97.Ohnstad AE, Delgado JM, North BJ, Nasa I, Kettenbach AN, Schultz SW, Shoemaker CJ. Receptor-mediated clustering of FIP200 bypasses the role of LC3 lipidation in autophagy. EMBO J. 2020;39:e104948. doi: 10.15252/embj.2020104948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kuno S, Fujita H, Tanaka YK, Ogra Y, Iwai K. Iron-induced NCOA4 condensation regulates ferritin fate and iron homeostasis. EMBO Rep. 2022;23:e54278. doi: 10.15252/embr.202154278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Goodwin JM, Dowdle WE, DeJesus R, Wang Z, Bergman P, Kobylarz M, Lindeman A, Xavier RJ, McAllister G, Nyfeler B, et al. Autophagy-independent lysosomal targeting regulated by ULK1/2-FIP200 and ATG9. Cell Rep. 2017;20:2341–2356. doi: 10.1016/j.celrep.2017.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fuhrmann DC, Mondorf A, Beifuß J, Jung M, Brüne B. Hypoxia inhibits ferritinophagy, increases mitochondrial ferritin, and protects from ferroptosis. Redox Biol. 2020;36:101670. doi: 10.1016/j.redox.2020.101670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hara Y, Yanatori I, Tanaka A, Kishi F, Lemasters JJ, Nishina S, Sasaki K, Hino K. Iron loss triggers mitophagy through induction of mitochondrial ferritin. EMBO Rep. 2020;21:e50202. doi: 10.15252/embr.202050202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev. 2014;94:909–950. doi: 10.1152/physrev.00026.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rademaker G, Boumahd Y, Peiffer R, Anania S, Wissocq T, Liégeois M, Luis G, Sounni NE, Agirman F, Maloujahmoum N, et al. Myoferlin targeting triggers mitophagy and primes ferroptosis in pancreatic cancer cells. Redox Biol. 2022;53:102324. doi: 10.1016/j.redox.2022.102324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Basit F, van Oppen LM, Schöckel L, Bossenbroek HM, van Emst-de Vries SE, Hermeling JC, Grefte S, Kopitz C, Heroult M, Hgm Willems P, Koopman WJ. Mitochondrial complex I inhibition triggers a mitophagy-dependent ROS increase leading to necroptosis and ferroptosis in melanoma cells. Cell Death Dis. 2017;8:e2716. doi: 10.1038/cddis.2017.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wei S, Qiu T, Yao X, Wang N, Jiang L, Jia X, Tao Y, Wang Z, Pei P, Zhang J, et al. Arsenic induces pancreatic dysfunction and ferroptosis via mitochondrial ROS-autophagy-lysosomal pathway. J Hazard Mater. 2020;384:121390. doi: 10.1016/j.jhazmat.2019.121390. [DOI] [PubMed] [Google Scholar]
- 106.Liu M, Fan Y, Li D, Han B, Meng Y, Chen F, Liu T, Song Z, Han Y, Huang L, et al. Ferroptosis inducer erastin sensitizes NSCLC cells to celastrol through activation of the ROS-mitochondrial fission-mitophagy axis. Mol Oncol. 2021;15:2084–2105. doi: 10.1002/1878-0261.12936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Xiao B, Deng X, Lim GGY, Xie S, Zhou ZD, Lim KL, Tan EK. Superoxide drives progression of Parkin/PINK1-dependent mitophagy following translocation of Parkin to mitochondria. Cell Death Dis. 2017;8:e3097. doi: 10.1038/cddis.2017.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gan ZY, Callegari S, Cobbold SA, Cotton TR, Mlodzianoski MJ, Schubert AF, Geoghegan ND, Rogers KL, Leis A, Dewson G, et al. Activation mechanism of PINK1. Nature. 2022;602:328–335. doi: 10.1038/s41586-021-04340-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang C, Liu K, Cao J, Wang L, Zhao Q, Li Z, Zhang H, Chen Q, Zhao T. PINK1-mediated mitophagy maintains pluripotency through optineurin. Cell Prolif. 2021;54:e13034. doi: 10.1111/cpr.13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Matsuda N, Sato S, Shiba K, Okatsu K, Saisho K, Gautier CA, Sou YS, Saiki S, Kawajiri S, Sato F, et al. PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J Cell Biol. 2010;189:211–221. doi: 10.1083/jcb.200910140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li C, Zhang Y, Liu J, Kang R, Klionsky DJ, Tang D. Mitochondrial DNA stress triggers autophagy-dependent ferroptotic death. Autophagy. 2021;17:948–960. doi: 10.1080/15548627.2020.1739447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhu L, Wu W, Jiang S, Yu S, Yan Y, Wang K, He J, Ren Y, Wang B. Pan-cancer analysis of the Mitophagy-Related protein PINK1 as a biomarker for the immunological and prognostic role. Front Oncol. 2020;10:569887. doi: 10.3389/fonc.2020.569887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zheng Y, Huang C, Lu L, Yu K, Zhao J, Chen M, Liu L, Sun Q, Lin Z, Zheng J, et al. STOML2 potentiates metastasis of hepatocellular carcinoma by promoting PINK1-mediated mitophagy and regulates sensitivity to lenvatinib. J Hematol Oncol. 2021;14:16. doi: 10.1186/s13045-020-01029-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chen Y, Chen HN, Wang K, Zhang L, Huang Z, Liu J, Zhang Z, Luo M, Lei Y, Peng Y, et al. Ketoconazole exacerbates mitophagy to induce apoptosis by downregulating cyclooxygenase-2 in hepatocellular carcinoma. J Hepatol. 2019;70:66–77. doi: 10.1016/j.jhep.2018.09.022. [DOI] [PubMed] [Google Scholar]
- 115.Kung-Chun Chiu D, Pui-Wah Tse A, Law CT, Ming-Jing Xu I, Lee D, Chen M, Kit-Ho Lai R, Wai-Hin Yuen V, Wing-Sum Cheu J, Wai-Hung Ho D, et al. Hypoxia regulates the mitochondrial activity of hepatocellular carcinoma cells through HIF/HEY1/PINK1 pathway. Cell Death Dis. 2019;10:934. doi: 10.1038/s41419-019-2155-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wu H, Wang T, Liu Y, Li X, Xu S, Wu C, Zou H, Cao M, Jin G, Lang J, et al. Mitophagy promotes sorafenib resistance through hypoxia-inducible ATAD3A dependent Axis. J Exp Clin Cancer Res. 2020;39:274. doi: 10.1186/s13046-020-01768-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lv H, Shang P. The significance, trafficking and determination of labile iron in cytosol, mitochondria and lysosomes. Metallomics. 2018;10:899–916. doi: 10.1039/C8MT00048D. [DOI] [PubMed] [Google Scholar]
- 118.Rizzollo F, More S, Vangheluwe P, Agostinis P. The lysosome as a master regulator of iron metabolism. Trends Biochem Sci. 2021;46:960–975. doi: 10.1016/j.tibs.2021.07.003. [DOI] [PubMed] [Google Scholar]
- 119.Kurz T, Gustafsson B, Brunk UT. Cell sensitivity to oxidative stress is influenced by ferritin autophagy. Free Radic Biol Med. 2011;50:1647–1658. doi: 10.1016/j.freeradbiomed.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 120.Torii S, Shintoku R, Kubota C, Yaegashi M, Torii R, Sasaki M, Suzuki T, Mori M, Yoshimoto Y, Takeuchi T, et al. An essential role for functional lysosomes in ferroptosis of cancer cells. Biochem J. 2016;473:769–777. doi: 10.1042/BJ20150658. [DOI] [PubMed] [Google Scholar]
- 121.Chen Y, Yang Z, Wang S, Ma Q, Li L, Wu X, Guo Q, Tao L, Shen X. Boosting ROS-Mediated lysosomal membrane permeabilization for cancer ferroptosis therapy. Adv Healthc Mater. 2023;12:e2202150. doi: 10.1002/adhm.202202150. [DOI] [PubMed] [Google Scholar]
- 122.Fernández B, Fdez E, Gómez-Suaga P, Gil F, Molina-Villalba I, Ferrer I, Patel S, Churchill GC, Hilfiker S. Iron overload causes endolysosomal deficits modulated by NAADP-regulated 2-pore channels and RAB7A. Autophagy. 2016;12:1487–1506. doi: 10.1080/15548627.2016.1190072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Halcrow PW, Lakpa KL, Khan N, Afghah Z, Miller N, Datta G, Chen X, Geiger JD. HIV-1 gp120-Induced endolysosome de-Acidification leads to efflux of endolysosome iron, and increases in mitochondrial iron and reactive oxygen species. J Neuroimmune Pharmacol. 2022;17:181–194. doi: 10.1007/s11481-021-09995-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.New J, Subramaniam D, Ramalingam S, Enders J, Sayed AAA, Ponnurangam S, Standing D, Ramamoorthy P, O'Neil M, Dixon DA, et al. Pleotropic role of RNA binding protein CELF2 in autophagy induction. Mol Carcinog. 2019;58:1400–1409. doi: 10.1002/mc.23023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.He Y, She H, Zhang T, Xu H, Cheng L, Yepes M, Zhao Y, Mao Z. p38 MAPK inhibits autophagy and promotes microglial inflammatory responses by phosphorylating ULK1. J Cell Biol. 2018;217:315–328. doi: 10.1083/jcb.201701049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Trelford CB, Di Guglielmo GM. Canonical and Non-canonical TGFβ signaling activate autophagy in an ULK1-Dependent manner. Front Cell Dev Biol. 2021;9:712124. doi: 10.3389/fcell.2021.712124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Keil E, Höcker R, Schuster M, Essmann F, Ueffing N, Hoffman B, Liebermann DA, Pfeffer K, Schulze-Osthoff K, Schmitz I. Phosphorylation of Atg5 by the Gadd45β-MEKK4-p38 pathway inhibits autophagy. Cell Death Differ. 2013;20:321–332. doi: 10.1038/cdd.2012.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Comes F, Matrone A, Lastella P, Nico B, Susca FC, Bagnulo R, Ingravallo G, Modica S, Lo Sasso G, Moschetta A, et al. A novel cell type-specific role of p38alpha in the control of autophagy and cell death in colorectal cancer cells. Cell Death Differ. 2007;14:693–702. doi: 10.1038/sj.cdd.4402076. [DOI] [PubMed] [Google Scholar]
- 129.Webber JL, Tooze SA. Coordinated regulation of autophagy by p38alpha MAPK through mAtg9 and p38IP. EMBO J. 2010;29:27–40. doi: 10.1038/emboj.2009.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhao Y, Wu H, Xing X, Ma Y, Ji S, Xu X, Zhao X, Wang S, Jiang W, Fang C, et al. CD13 induces autophagy to promote hepatocellular carcinoma cell chemoresistance through the P38/Hsp27/CREB/ATG7 pathway. J Pharmacol Exp Ther. 2020;374:512–520. doi: 10.1124/jpet.120.265637. [DOI] [PubMed] [Google Scholar]
- 131.Choi CH, Lee BH, Ahn SG, Oh SH. Proteasome inhibition-induced p38 MAPK/ERK signaling regulates autophagy and apoptosis through the dual phosphorylation of glycogen synthase kinase 3β. Biochem Biophys Res Commun. 2012;418:759–764. doi: 10.1016/j.bbrc.2012.01.095. [DOI] [PubMed] [Google Scholar]
- 132.Xie X, Le L, Fan Y, Lv L, Zhang J. Autophagy is induced through the ROS-TP53-DRAM1 pathway in response to mitochondrial protein synthesis inhibition. Autophagy. 2012;8:1071–1084. doi: 10.4161/auto.20250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sun T, Li D, Wang L, Xia L, Ma J, Guan Z, Feng G, Zhu X. c-Jun NH2-terminal kinase activation is essential for up-regulation of LC3 during ceramide-induced autophagy in human nasopharyngeal carcinoma cells. J Transl Med. 2011;9:161. doi: 10.1186/1479-5876-9-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhang XY, Wu XQ, Deng R, Sun T, Feng GK, Zhu XF. Upregulation of sestrin 2 expression via JNK pathway activation contributes to autophagy induction in cancer cells. Cell Signal. 2013;25:150–158. doi: 10.1016/j.cellsig.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 135.Li DD, Wang LL, Deng R, Tang J, Shen Y, Guo JF, Wang Y, Xia LP, Feng GK, Liu QQ, et al. The pivotal role of c-Jun NH2-terminal kinase-mediated Beclin 1 expression during anticancer agents-induced autophagy in cancer cells. Oncogene. 2009;28:886–898. doi: 10.1038/onc.2008.441. [DOI] [PubMed] [Google Scholar]
- 136.Wong CH, Iskandar KB, Yadav SK, Hirpara JL, Loh T, Pervaiz S. Simultaneous induction of non-canonical autophagy and apoptosis in cancer cells by ROS-dependent ERK and JNK activation. PLoS One. 2010;5:e9996. doi: 10.1371/journal.pone.0009996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Byun JY, Yoon CH, An S, Park IC, Kang CM, Kim MJ, Lee SJ. The Rac1/MKK7/JNK pathway signals upregulation of Atg5 and subsequent autophagic cell death in response to oncogenic Ras. Carcinogenesis. 2009;30:1880–1888. doi: 10.1093/carcin/bgp235. [DOI] [PubMed] [Google Scholar]
- 138.Park JH, Ko J, Park YS, Park J, Hwang J, Koh HC. Clearance of damaged mitochondria through PINK1 stabilization by JNK and ERK MAPK signaling in Chlorpyrifos-Treated neuroblastoma cells. Mol Neurobiol. 2017;54:1844–1857. doi: 10.1007/s12035-016-9753-1. [DOI] [PubMed] [Google Scholar]
- 139.Dagda RK, Zhu J, Kulich SM, Chu CT. Mitochondrially localized ERK2 regulates mitophagy and autophagic cell stress: Implications for Parkinson's disease. Autophagy. 2008;4:770–782. doi: 10.4161/auto.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Meng Y, Yang Z, Huo T, Jiang H. Realgar facilitates the Nrf2-Keap1-p62 positive feedback signaling axis via MAPKs and AKT to interfere with autophagy-induced apoptosis and oxidative stress in the hippocampus. Biomed Pharmacother. 2022;150:112964. doi: 10.1016/j.biopha.2022.112964. [DOI] [PubMed] [Google Scholar]
- 141.Hosokawa N, Hara T, Kaizuka T, Kishi C, Takamura A, Miura Y, Iemura S, Natsume T, Takehana K, Yamada N, et al. Nutrient-dependent mTORC1 association with the ULK1-Atg13-FIP200 complex required for autophagy. Mol Biol Cell. 2009;20:1981–1991. doi: 10.1091/mbc.e08-12-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jung CH, Jun CB, Ro SH, Kim YM, Otto NM, Cao J, Kundu M, Kim DH. ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol Biol Cell. 2009;20:1992–2003. doi: 10.1091/mbc.e08-12-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13:132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nazio F, Strappazzon F, Antonioli M, Bielli P, Cianfanelli V, Bordi M, Gretzmeier C, Dengjel J, Piacentini M, Fimia GM, Cecconi F. mTOR inhibits autophagy by controlling ULK1 ubiquitylation, self-association and function through AMBRA1 and TRAF6. Nat Cell Biol. 2013;15:406–416. doi: 10.1038/ncb2708. [DOI] [PubMed] [Google Scholar]
- 145.Ma X, Zhang S, He L, Rong Y, Brier LW, Sun Q, Liu R, Fan W, Chen S, Yue Z, et al. MTORC1-mediated NRBF2 phosphorylation functions as a switch for the class III PtdIns3K and autophagy. Autophagy. 2017;13:592–607. doi: 10.1080/15548627.2016.1269988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kim YM, Jung CH, Seo M, Kim EK, Park JM, Bae SS, Kim DH. mTORC1 phosphorylates UVRAG to negatively regulate autophagosome and endosome maturation. Mol Cell. 2015;57:207–218. doi: 10.1016/j.molcel.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Koren I, Reem E, Kimchi A. DAP1, a novel substrate of mTOR, negatively regulates autophagy. Curr Biol. 2010;20:1093–1098. doi: 10.1016/j.cub.2010.04.041. [DOI] [PubMed] [Google Scholar]
- 148.Yang C, Li Y, Hu W, Wang X, Hu J, Yuan C, Zhou C, Wang H, Du J, Wang Y, Tong X. TEOA promotes autophagic cell death via ROS-Mediated inhibition of mTOR/p70S6k signaling pathway in pancreatic cancer cells. Front Cell Dev Biol. 2021;9:734818. doi: 10.3389/fcell.2021.734818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Nàger M, Sallán MC, Visa A, Pushparaj C, Santacana M, Macià A, Yeramian A, Cantí C, Herreros J. Inhibition of WNT-CTNNB1 signaling upregulates SQSTM1 and sensitizes glioblastoma cells to autophagy blockers. Autophagy. 2018;14:619–636. doi: 10.1080/15548627.2017.1423439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Petherick KJ, Williams AC, Lane JD, Ordóñez-Morán P, Huelsken J, Collard TJ, Smartt HJ, Batson J, Malik K, Paraskeva C, Greenhough A. Autolysosomal β-catenin degradation regulates Wnt-autophagy-p62 crosstalk. EMBO J. 2013;32:1903–1916. doi: 10.1038/emboj.2013.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Fan B, Su B, Song G, Liu X, Yan Z, Wang S, Hu F, Yang J. miR-363-3p induces EMT via the Wnt/β-catenin pathway in glioma cells by targeting CELF2. J Cell Mol Med. 25:10418–10429. doi: 10.1111/jcmm.16970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wei H, Tang X, Chen Q, Yue T, Dong B. An endoplasmic reticulum-targeting fluorescent probe for the visualization of the viscosity fluctuations during ferroptosis in live cells. Anal Chim Acta. 2022;1232:340454. doi: 10.1016/j.aca.2022.340454. [DOI] [PubMed] [Google Scholar]
- 153.Song W, Zhang W, Yue L, Lin W. Revealing the effects of endoplasmic reticulum stress on ferroptosis by Two-Channel Real-Time Imaging of pH and viscosity. Anal Chem. 2022;94:6557–6565. doi: 10.1021/acs.analchem.2c00387. [DOI] [PubMed] [Google Scholar]
- 154.Zhao C, Yu D, He Z, Bao L, Feng L, Chen L, Liu Z, Hu X, Zhang N, Wang T, Fu Y. Endoplasmic reticulum stress-mediated autophagy activation is involved in cadmium-induced ferroptosis of renal tubular epithelial cells. Free Radic Biol Med. 2021;175:236–248. doi: 10.1016/j.freeradbiomed.2021.09.008. [DOI] [PubMed] [Google Scholar]
- 155.He Z, Shen P, Feng L, Hao H, He Y, Fan G, Liu Z, Zhu K, Wang Y, Zhang N, et al. Cadmium induces liver dysfunction and ferroptosis through the endoplasmic stress-ferritinophagy axis. Ecotoxicol Environ Saf. 2022;245:114123. doi: 10.1016/j.ecoenv.2022.114123. [DOI] [PubMed] [Google Scholar]
- 156.Fu F, Wang W, Wu L, Wang W, Huang Z, Huang Y, Wu C, Pan X. Inhalable biomineralized liposomes for cyclic Ca2+-Burst-Centered endoplasmic reticulum stress enhanced lung cancer ferroptosis therapy. ACS Nano. 2023;17:5486–5502. doi: 10.1021/acsnano.2c10830. [DOI] [PubMed] [Google Scholar]
- 157.Chen PH, Wu J, Xu Y, Ding CC, Mestre AA, Lin CC, Yang WH, Chi JT. Zinc transporter ZIP7 is a novel determinant of ferroptosis. Cell Death Dis. 2021;12:198. doi: 10.1038/s41419-021-03482-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chen Y, Mi Y, Zhang X, Ma Q, Song Y, Zhang L, Wang D, Xing J, Hou B, Li H, et al. Dihydroartemisinin-induced unfolded protein response feedback attenuates ferroptosis via PERK/ATF4/HSPA5 pathway in glioma cells. J Exp Clin Cancer Res. 2019;38:402. doi: 10.1186/s13046-019-1413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wei R, Zhao Y, Wang J, Yang X, Li S, Wang Y, Yang X, Fei J, Hao X, Zhao Y, et al. Tagitinin C induces ferroptosis through PERK-Nrf2-HO-1 signaling pathway in colorectal cancer cells. Int J Biol Sci. 2021;17:2703–1277. doi: 10.7150/ijbs.59404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zheng X, Liu B, Liu X, Li P, Zhang P, Ye F, Zhao T, Kuang Y, Chen W, Jin X, Li Q. PERK regulates the sensitivity of hepatocellular carcinoma cells to High-LET carbon ions via either apoptosis or ferroptosis. J Cancer. 2022;13:669–680. doi: 10.7150/jca.61622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhao R, Lv Y, Feng T, Zhang R, Ge L, Pan J, Han B, Song G, Wang L. ATF6α promotes prostate cancer progression by enhancing PLA2G4A-mediated arachidonic acid metabolism and protecting tumor cells against ferroptosis. Prostate. 2022;82:617–629. doi: 10.1002/pros.24308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hwang J, Qi L. Quality control in the endoplasmic reticulum: Crosstalk between ERAD and UPR pathways. Trends Biochem Sci. 2018;43:593–605. doi: 10.1016/j.tibs.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Krshnan L, van de Weijer ML, Carvalho P. Endoplasmic reticulum-associated protein degradation. Cold Spring Harb Perspect Biol. 2022;14:a041247. doi: 10.1101/cshperspect.a041247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lopata A, Kniss A, Löhr F, Rogov VV, Dötsch V. Ubiquitination in the ERAD process. Int J Mol Sci. 2020;21:5369. doi: 10.3390/ijms21155369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Haynes CM, Titus EA, Cooper AA. Degradation of misfolded proteins prevents ER-derived oxidative stress and cell death. Mol Cell. 2004;15:767–776. doi: 10.1016/j.molcel.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 166.Liu Q, Yang X, Long G, Hu Y, Gu Z, Boisclair YR, Long Q. ERAD deficiency promotes mitochondrial dysfunction and transcriptional rewiring in human hepatic cells. J Biol Chem. 2020;295:16743–16753. doi: 10.1074/jbc.RA120.013987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhou Z, Torres M, Sha H, Halbrook CJ, Van den Bergh F, Reinert RB, Yamada T, Wang S, Luo Y, Hunter AH, et al. Endoplasmic reticulum-associated degradation regulates mitochondrial dynamics in brown adipocytes. Science. 2020;368:54–60. doi: 10.1126/science.aay2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wang X, Wang QC, Sun Z, Li T, Yang K, An C, Guo C, Tang TS. ER stress mediated degradation of diacylglycerol acyltransferase impairs mitochondrial functions in TMCO1 deficient cells. Biochem Biophys Res Commun. 2019;512:914–920. doi: 10.1016/j.bbrc.2019.03.115. [DOI] [PubMed] [Google Scholar]
- 169.Takashi Y, Tomita K, Kuwahara Y, Roudkenar MH, Roushandeh AM, Igarashi K, Nagasawa T, Nishitani Y, Sato T. Mitochondrial dysfunction promotes aquaporin expression that controls hydrogen peroxide permeability and ferroptosis. Free Radic Biol Med. 2020;161:60–70. doi: 10.1016/j.freeradbiomed.2020.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sereti E, Tsimplouli C, Kalaitsidou E, Sakellaridis N, Dimas K. Study of the Relationship between sigma receptor expression levels and some common sigma ligand activity in cancer using human cancer cell lines of the NCI-60 cell line panel. Biomedicines. 2021;9:38. doi: 10.3390/biomedicines9010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Oyer HM, Sanders CM, Kim FJ. Small-molecule modulators of sigma1 and Sigma2/TMEM97 in the context of cancer: Foundational concepts and emerging themes. Front Pharmacol. 2019;10:1141. doi: 10.3389/fphar.2019.01141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Gueguinou M, Crottès D, Chantôme A, Rapetti-Mauss R, Potier-Cartereau M, Clarysse L, Girault A, Fourbon Y, Jézéquel P, Guérin-Charbonnel C, et al. The SigmaR1 chaperone drives breast and colorectal cancer cell migration by tuning SK3-dependent Ca2+ homeostasis. Oncogene. 2017;36:3640–3647. doi: 10.1038/onc.2016.501. [DOI] [PubMed] [Google Scholar]
- 173.Bai T, Lei P, Zhou H, Liang R, Zhu R, Wang W, Zhou L, Sun Y. Sigma-1 receptor protects against ferroptosis in hepatocellular carcinoma cells. J Cell Mol Med. 2019;23:7349–7359. doi: 10.1111/jcmm.14594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bai T, Wang S, Zhao Y, Zhu R, Wang W, Sun Y. Haloperidol, a sigma receptor 1 antagonist, promotes ferroptosis in hepatocellular carcinoma cells. Biochem Biophys Res Commun. 2017;491:919–925. doi: 10.1016/j.bbrc.2017.07.136. [DOI] [PubMed] [Google Scholar]
- 175.Zou Y, Li H, Graham ET, Deik AA, Eaton JK, Wang W, Sandoval-Gomez G, Clish CB, Doench JG, Schreiber SL. Cytochrome P450 oxidoreductase contributes to phospholipid peroxidation in ferroptosis. Nat Chem Biol. 2020;16:302–309. doi: 10.1038/s41589-020-0472-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Acharya P, Liao M, Engel JC, Correia MA. Liver cytochrome P450 3A endoplasmic reticulum-associated degradation: A major role for the p97 AAA ATPase in cytochrome P450 3A extraction into the cytosol. J Biol Chem. 2011;286:3815–3828. doi: 10.1074/jbc.M110.186981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Prochazka L, Tesarik R, Turanek J. Regulation of alternative splicing of CD44 in cancer. Cell Signal. 2014;26:2234–2239. doi: 10.1016/j.cellsig.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 178.Lai S, Wang Y, Li T, Dong Y, Lin Y, Wang L, Weng S, Zhang X, Lin C. N6-methyladenosine-mediated CELF2 regulates CD44 alternative splicing affecting tumorigenesis via ERAD pathway in pancreatic cancer. Cell Biosci. 2022;12:125. doi: 10.1186/s13578-022-00844-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wang Y, Zhao G, Condello S, Huang H, Cardenas H, Tanner EJ, Wei J, Ji Y, Li J, Tan Y, et al. Frizzled-7 identifies platinum-tolerant ovarian cancer cells susceptible to ferroptosis. Cancer Res. 2021;81:384–399. doi: 10.1158/0008-5472.CAN-20-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Nuñez F, Bravo S, Cruzat F, Montecino M, De Ferrari GV. Wnt/β-catenin signaling enhances cyclooxygenase-2 (COX2) transcriptional activity in gastric cancer cells. PLoS One. 2011;6:e18562. doi: 10.1371/journal.pone.0018562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wang H, Zhang H, Chen Y, Wang H, Tian Y, Yi X, Shi Q, Zhao T, Zhang B, Gao T, et al. Targeting Wnt/β-Catenin signaling exacerbates ferroptosis and increases the efficacy of melanoma immunotherapy via the regulation of MITF. Cells. 2022;11:3580. doi: 10.3390/cells11223580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chen QF, Shi F, Huang T, Huang C, Shen L, Wu P, Li W. ASTN1 is associated with immune infiltrates in hepatocellular carcinoma, and inhibits the migratory and invasive capacity of liver cancer via the Wnt/β-catenin signaling pathway. Oncol Rep. 2020;44:1425–1440. doi: 10.3892/or.2020.7704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Tu B, Ma TT, Peng XQ, Wang Q, Yang H, Huang XL. Targeting of COX-2 expression by recombinant adenovirus shRNA attenuates the malignant biological behavior of breast cancer cells. Asian Pac J Cancer Prev. 2014;15:8829–8836. doi: 10.7314/APJCP.2014.15.20.8829. [DOI] [PubMed] [Google Scholar]
- 184.Wang Y, Zheng L, Shang W, Yang Z, Li T, Liu F, Shao W, Lv L, Chai L, Qu L, et al. Wnt/beta-catenin signaling confers ferroptosis resistance by targeting GPX4 in gastric cancer. Cell Death Differ. 2022;29:2190–2202. doi: 10.1038/s41418-022-01008-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Liu W, Zhou Y, Duan W, Song J, Wei S, Xia S, Wang Y, Du X, Li E, Ren C, et al. Glutathione peroxidase 4-dependent glutathione high-consumption drives acquired platinum chemoresistance in lung cancer-derived brain metastasis. Clin Transl Med. 2021;11:e517. doi: 10.1002/ctm2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Krejci P, Aklian A, Kaucka M, Sevcikova E, Prochazkova J, Masek JK, Mikolka P, Pospisilova T, Spoustova T, Weis M, et al. Receptor tyrosine kinases activate canonical WNT/β-catenin signaling via MAP kinase/LRP6 pathway and direct β-catenin phosphorylation. PLoS One. 2012;7:e35826. doi: 10.1371/journal.pone.0035826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Červenka I, Wolf J, Mašek J, Krejci P, Wilcox WR, Kozubík A, Schulte G, Gutkind JS, Bryja V. Mitogen-activated protein kinases promote WNT/beta-catenin signaling via phosphorylation of LRP6. Mol Cell Biol. 2011;31:179–189. doi: 10.1128/MCB.00550-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Siddharth S, Mohapatra P, Preet R, Das D, Satapathy SR, Choudhuri T, Kundu CN. Induction of apoptosis by 4-(3-(tert-butylamino)imidazo[1,2-α]pyridine-2-yl) benzoic acid in breast cancer cells via upregulation of PTEN. Oncol Res. 2013;21:1–13. doi: 10.3727/096504013X13786659070190. [DOI] [PubMed] [Google Scholar]
- 189.Khare V, Dammann K, Asboth M, Krnjic A, Jambrich M, Gasche C. Overexpression of PAK1 promotes cell survival in inflammatory bowel diseases and colitis-associated cancer. Inflamm Bowel Dis. 2015;21:287–296. doi: 10.1097/MIB.0000000000000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ji H, Wang J, Nika H, Hawke D, Keezer S, Ge Q, Fang B, Fang X, Fang D, Litchfield DW, et al. EGF-induced ERK activation promotes CK2-mediated disassociation of alpha-Catenin from beta-Catenin and transactivation of beta-Catenin. Mol Cell. 2009;36:547–559. doi: 10.1016/j.molcel.2009.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Gao C, Cao W, Bao L, Zuo W, Xie G, Cai T, Fu W, Zhang J, Wu W, Zhang X, Chen YG. Autophagy negatively regulates Wnt signalling by promoting Dishevelled degradation. Nat Cell Biol. 2010;12:781–790. doi: 10.1038/ncb2082. [DOI] [PubMed] [Google Scholar]
- 192.Nasiri-Aghdam M, Garcia-Garduño TC, Jave-Suárez LF. CELF family proteins in cancer: Highlights on the RNA-binding protein/noncoding RNA regulatory axis. Int J Mol Sci. 2021;22:11056. doi: 10.3390/ijms222011056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zhang Y, Yin C, Wei C, Xia S, Qiao Z, Zhang XW, Yu B, Zhou J, Wang R. Exosomal miR-625-3p secreted by cancer-associated fibroblasts in colorectal cancer promotes EMT and chemotherapeutic resistance by blocking the CELF2/WWOX pathway. Pharmacol Res. 2022;186:106534. doi: 10.1016/j.phrs.2022.106534. [DOI] [PubMed] [Google Scholar]
- 194.Zhao Y, Zhou H, Dong W. LncRNA RHPN1-AS1 promotes the progression of nasopharyngeal carcinoma by targeting CELF2 expression. Exp Mol Pathol. 2021;122:104671. doi: 10.1016/j.yexmp.2021.104671. [DOI] [PubMed] [Google Scholar]
- 195.Wang J, Liu L, Sun Y, Xue Y, Qu J, Pan S, Li H, Qu H, Wang J, Zhang J. miR-615-3p promotes proliferation and migration and inhibits apoptosis through its potential target CELF2 in gastric cancer. Biomed Pharmacother. 2018;101:406–413. doi: 10.1016/j.biopha.2018.02.104. [DOI] [PubMed] [Google Scholar]
- 196.Xu H, Wang F, Wang L. Suppression of miR-106a-5p expression inhibits tumorigenesis via increasing CELF-2 expression in spinal cord glioma. Oncol Lett. 2021;22:627. doi: 10.3892/ol.2021.12888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ge L, Zhou F, Nie J, Wang X, Zhao Q. Hypoxic colorectal cancer-secreted exosomes deliver miR-210-3p to normoxic tumor cells to elicit a protumoral effect. Exp Biol Med (Maywood) 2021;246:1895–1906. doi: 10.1177/15353702211011576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Fan HN, Zhao XY, Liang R, Chen XY, Zhang J, Chen NW, Zhu JS. CircPTK2 inhibits the tumorigenesis and metastasis of gastric cancer by sponging miR-134-5p and activating CELF2/PTEN signaling. Pathol Res Pract. 2021;227:153615. doi: 10.1016/j.prp.2021.153615. [DOI] [PubMed] [Google Scholar]
- 199.Xie SC, Zhang JQ, Jiang XL, Hua YY, Xie SW, Qin YA, Yang YJ. LncRNA CRNDE facilitates epigenetic suppression of CELF2 and LATS2 to promote proliferation, migration and chemoresistance in hepatocellular carcinoma. Cell Death Dis. 2020;11:676. doi: 10.1038/s41419-020-02853-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Subramaniam D, Ramalingam S, Linehan DC, Dieckgraefe BK, Postier RG, Houchen CW, Jensen RA, Anant S. RNA binding protein CUGBP2/CELF2 mediates curcumin-induced mitotic catastrophe of pancreatic cancer cells. PLoS One. 2011;6:e16958. doi: 10.1371/journal.pone.0016958. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.