Abstract
Cell death is associated with a variety of liver diseases, and hepatocyte death is a core factor in the occurrence and progression of liver diseases. In recent years, new cell death modes have been identified, and certain biomarkers have been detected in the circulation during various cell death modes that mediate liver injury. In this review, cell death modes associated with liver diseases are summarized, including some cell death modes that have emerged in recent years. We described the mechanisms associated with liver diseases and summarized recent applications of targeting cell death in liver diseases. It provides new ideas for the diagnosis and treatment of liver diseases. In addition, multiple cell death modes can contribute to the same liver disease. Different cell death modes are not isolated, and they interact with each other in liver diseases. Future studies may focus on exploring the regulation between various cell death response pathways in liver diseases.
Keywords: Cell death, Hepatocytes, Application, Liver diseases
Graphical abstract
Introduction
As a fundamental process of life, cell death plays a crucial role in embryonic development. This process maintains the body’s homeostasis by continuously eliminating damaged cells.1 According to the description listed in the Nomenclature Committee on Cell Death 2018, cell death manifests as macroscopic morphological changes. There are three classic cell death modes: apoptosis, autophagy, and necrosis.2 With the further development of scientific research, a variety of new cell death modes have been discovered. These modes have different signaling pathways and morphological and molecular mechanisms.2,3 Different cell death modes can be regulated by various signaling pathways. When stimulated by internal or external factors, the activation or inhibition of various signaling pathways can cause cell death. There are many different types of liver disease. According to the etiology, liver diseases are divided into: viral hepatitis, alcoholic liver disease, nonalcoholic fatty liver disease, genetic causes, autoimmune causes and liver diseases caused by drugs or other factors, and so on. The final common pathway of any chronic damage to the liver is persistent wound healing, leading to hepatic parenchymal fibrosis.4 During different types of liver disease, such as liver fibrosis, alcoholic liver disease (ALD), steatohepatitis, or liver failure, the most prominent manifestation is hepatocyte death, which results in impaired liver function.5–7 This review mainly summarizes the mechanisms of various cell death modes that mediate liver disease, including some cell death modes that have emerged in recent years. This review provides new ideas for the diagnosis and treatment of liver diseases.
Modes of cell death associated with liver diseases
Apoptosis
Before introducing the mechanism of apoptosis, the B-cell lymphoma 2 (Bcl-2) family needs to be mentioned. Bcl-2 family proteins are divided into three groups according to their roles and domains: antiapoptotic Bcl-2-like proteins (including Bcl-2, Bcl-xL, Bcl-w, Mcl-1, and A1/Bfl-1), proapoptotic Bax-like proteins (including Bax, Bak, and Bok/Mtd) and only proapoptotic BH3 proteins (including Bid, Bim/Bod, Bad, Bmf, Bik/Nbk, Blk, Noxa, Puma/Bbc3, and Hrk/DP5).8 Apoptosis is activated by two pathways: the intrinsic (mitochondrial) pathway and the extrinsic (death receptor) pathway. Studies have shown that the intrinsic pathway is triggered by the Bcl-2 family, which is normally activated by mitochondrial dysfunction, endoplasmic reticulum (ER) stress, lysosomal permeabilization, and nuclear DNA damage.9 Then, toxic proteins such as cytochrome c are released from the intermembrane space through permeabilization of the outer mitochondrial membrane. Ultimately, caspase is activated and promotes apoptosis.5,8 Studies have shown that caspases related to apoptosis include promoters including caspase-8 and -9 and effectors including caspase-3, -6, and -7.10 In addition to being death receptor ligands, tumor necrosis factor (TNF), TNF-related apoptosis-inducing ligand (TRAIL) and Fas ligand (Fas-l) trigger extrinsic apoptosis pathways by binding to death receptors.11 Several studies have shown that apoptosis mediated by death receptors is a key feature of various liver diseases.12 The progression of liver diseases is influenced by the balance between apoptosis and antiapoptotic capacity.13 During liver injury, as hepatocyte apoptosis and its pathophysiology vary among different liver diseases, this review will address them separately (Fig. 1).
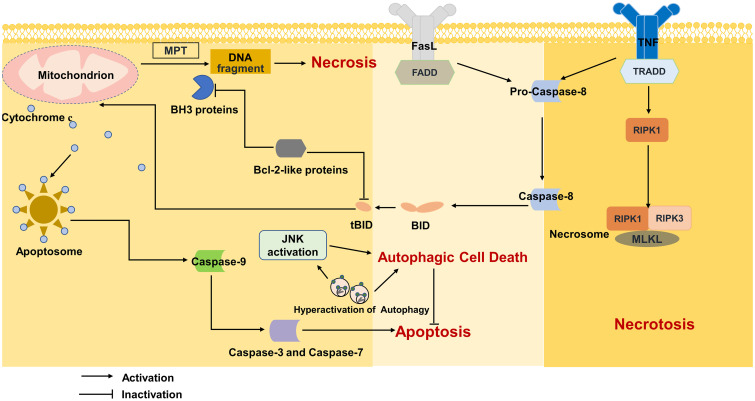
Fig. 1. Mechanism of apoptosis, necrosis, necroptosis and autophagy.
FADD, fas-associated protein; Fas-L, fas ligand; JNK, cJun-N-terminal Kinase; MLKL, mixed lineage kinase domain protein; MPT, mitochondrial permeability transition; RIPK1, receptor interacting protein kinases 1; RIPK3, receptor interacting protein kinases 3; TNF, tumor necrosis factor; TRADD, TNFR1-associated death domain protein.
Viral hepatitis
Hepatitis B virus (HBV) or hepatitis C virus (HCV) infection is the main cause of chronic viral hepatitis. The damage to hepatocytes caused by hepatitis virus is mainly mediated by the immune response. When hepatitis virus is copied in large quantities, cytotoxic T lymphocytes (CTLs) identify and kill hepatocytes that express hepatitis virus antigens and cause hepatocyte damage. Compared with acute viral hepatitis, chronic viral hepatitis is characterized by a large amount of apoptosis during continuous viral infection. Studies have shown that HBV and HCV promote the occurrence of apoptosis via cJun-N-terminal kinase (JNK) and CHOP through the intrinsic pathway.14,15 The extrinsic pathway is typically not sufficient to induce apoptosis, and it needs to be enhanced through the intrinsic pathway. When liver cells are infected by HCV, TNFα/TNF receptor (TNF-R), Fas/Fas-l, and TRAIL/TRAIL receptor-1 (also called death receptor 4, DR4) or TRAIL receptor-2 (also called death receptor 5, DR5) bind to CTLs and undergo apoptosis.16 Death receptors bind with death ligands to form a complex that activates caspase-8,17 which triggers two signaling pathways. In the first pathway, caspase-8 activation directly leads to the activation of effector caspases (caspase-3, -6, -7), which results in massive amplification and promotes apoptosis .18,19 The second pathway is related to the mitochondria, recruited by the intrinsic pathway, and involves the cleavage of Bid. Mitochondria-dependent apoptosis is mediated by Bcl-2 family proteins converged at the mitochondrial permeability transition (MPT) pore, which regulates the release of apoptotic regulatory proteins like cytochrome C and caspase-9 is ultimately activated.20 The hepatocyte is a so-called type II cell in which the activation of caspase-3, caspase -7 is inhibited, so the extrinsic pathway mainly acts through the second pathway.2 HCV also induces apoptosis by generating reactive oxygen species (ROS) and reducing mitochondrial cross-membrane potential.21 During chronic HBV infection, liver expression of TNF α, TRAIL, and Fas is also related to hepatocyte apoptosis.22,23 The expression of Bax in liver cells is positively related to the number of apoptotic cells. HBV X protein can adjust TRAIL receptor expression during apoptosis.23,24
ALD
Various death modes are involved in ALD, and apoptosis plays an important role in ALD.25,26 Caspase-3-positive hepatocytes were significantly increased in liver biopsies taken from patients with alcoholic hepatitis.27 In vivo and in vitro experiments have shown that caspase inhibitors significantly attenuate alcohol-induced hepatocyte apoptosis.28,29 At present, the mechanisms of apoptosis in ALD can be summarized as follows: acetaldehyde metabolism drives the production of ROS, which leads to mitochondrial dysfunction and the release of the proapoptotic factor cytochrome c, which ultimately promotes caspase activation.30 Alcohol and its metabolites trigger ER stress and promote apoptosis via CHOP.14,25,26 Fas-l and TNF play important roles in the pathogenesis of ALD.31,32 Studies have shown that TNF and TNF-R levels were increased in ALD patients, and anti-TNF antibody treatment prevented alcoholic liver injury in animal models.26,33 However, anti-TNF antibodies are currently not clinically recommended for the treatment of ALD owing to increased infection and mortality rates.26,34 In addition, studies have also shown that there is crosstalk between the gut microbiome and the liver. Alcohol increases systemic bacterial production by altering intestinal permeability, which in turn stimulates Kupffer cells (KCs) and promotes TNF production and ultimately leads to apoptosis.35 When cytotoxic T cell sensitivity is increased, Fas receptor expression is significantly increased in ALD through lymphocyte-mediated apoptosis.27,36 Through ER stress, alcohol promotes the phosphorylation of interferon regulatory factor 3, which leads to hepatocyte apoptosis via Bax.26,37
Nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH)
Studies have shown that free fatty acids (FFAs) stimulate TNF-α expression through the lysosomal pathway, which promotes hepatic fat metabolism and apoptosis.38 The release of lysosomal enzymes into the cytosol is dependent on the specific lysosomal enzyme cathepsin B. This enzyme promotes TNF-α-mediated hepatocyte apoptosis by promoting mitochondrial depolarization, cytochrome c release and increased ROS production, thereby exacerbating liver damage.39,40 It has been reported that Fas protein expression is increased in NASH patients.41 In addition, elevated serum TNF-α levels were observed in patients with NASH.42 Studies have shown that Fas/Fas-l,43,44 TNF-α/TNF-R and TRAIL/TRAIL-R2 form a death-inducing signaling complex in NASH.45–47 This complex induces mitochondrial dysfunction through caspase-8-dependent cleavage of Bid. Mitochondrial dysfunction results in the release of cytochrome c, which activates caspase-3 and caspase-7,44,47 and then caspase-6 is activated. Recent studies have shown that activated caspase-6 cleaves Bid, and mitochondrial cytochrome c is released, which in turn activates caspase-3 and caspase-7, resulting in continuous apoptosis in hepatocytes.48 In addition, ER stress plays an important role in the development of NAFLD.49 Hepatocyte ballooning and apoptosis were detected in wild-type mice after the injection of ER stress inducers. Studies have shown that ER stress leads to apoptotic cell death through JNK-mediated Bim activation.26,50 Thus, apoptosis is the predominant mode of cell death in NAFLD. Both the intrinsic and extrinsic pathways play important roles.
Hepatic fibrosis and cirrhosis
Viral hepatitis, ALD, NAFLD, autoimmune hepatitis, and drug-induced liver injury can contribute to liver fibrosis.51 Apoptotic bodies that form during apoptosis can be engulfed by phagocytes in the liver, such as KCs.52 Studies have shown that apoptosis is not only a mode of death but is also associated with inflammatory responses. When the level of hepatocyte apoptosis exceeds the clearance capacity of phagocytes, apoptotic bodies accumulate and release their proinflammatory factors. The phagocytosis of apoptotic bodies by KCs enhances the expression of the death ligands Fas and TNF-α and promotes hepatocyte apoptosis. It also promotes the production of transforming growth factor β (TGF-β), which leads to liver fibrosis.53 Studies have shown that the phagocytosis of apoptotic bodies by hepatic stellate cells (HSCs) directly stimulates fibrogenesis.54 In addition, the destruction of the antiapoptotic factor Bcl-xL in hepatocytes leads to continuous apoptosis in hepatocytes, which increases the production of hepatic TGF-β and leads to liver fibrosis.55 In conclusion, chronic liver diseases cause continuous apoptosis in hepatocytes, induce hepatocyte fibrosis, and gradually cause liver cirrhosis.
Hepatocellular carcinoma (HCC)
HCC is closely related to chronic inflammation and fibrosis, and is a common end-stage of chronic liver disease. Apoptosis is a prominent defense mechanism against hepatocarcinogenesis. An imbalance of proapoptotic and antiapoptotic members (e.g., activation of the antiapoptotic protein BCL-xL and downregulation of the proapoptotic protein Bax) has been observed in human HCC.56 Most HCCs show insensitivity to TNF-related apoptosis-inducing ligands or Fas-mediated cell death.57 As a strong inducer of apoptosis, sorafenib treatment of different HCC cell lines increased the levels of pro-apoptotic mRNA and proteins and decreased the levels of anti-apoptotic proteins.58
Necrosis
Necrosis is generally considered to be an unprogrammed form of cell death. Necrosis is characterized by cell swelling and plasma membrane rupture.59 Under oxidative stress or toxin stimulation, MPT occurs.60 MPT leads to the opening of mitochondrial pores through interactions with the mitochondrial protein cyclophilin D (CypD).61 The subsequent disturbance in the ion gradient across the inner mitochondrial membrane and the decrease in mitochondrial membrane potential result in the loss of oxidative phosphoric acid and the rapid depletion of ATP. Ultimately, cellular homeostasis and ion pump disruption drive cell necrosis (Fig. 1).59,60
NAFLD and NASH
Studies have shown that in response to a high-fat diet (HFD), CypD-knockout mice exhibited reduced triglyceride levels, steatosis, and mitochondrial stress compared with wild-type mice.62 Similarly, treatment of HFD-fed mice with the pan-cyclophilin inhibitor CRV431 significantly reduced hepatocyte steatosis, ballooning, and liver fibrosis compared with control mice.63 Further studies have shown that CypD induces MPT by disrupting calcium homeostasis and mediates hepatocyte necrosis.62 However, the specific mechanism of necrosis in NAFLD needs further study.
Acetaminophen (APAP)-induced liver injury
APAP is a common cause of drug-induced acute liver failure. Studies have shown that JNK activation plays a role in APAP-induced acute liver failure.64 In a mouse model, the inhibition of JNK protected against APAP-induced liver injury.65 MPT leads to the opening of mitochondrial pores through interactions with the mitochondrial protein CypD.61 Inhibiting CypD prevents APAP-induced acute liver injury.66 Therefore, necrosis is considered to be the main mode of APAP-induced liver injury. Cytochrome P450-mediated metabolism produces the toxic metabolite N-acetyl-p-benzoquinoneimine (NAPQI). Glutathione (GSH) is required for the detoxification of NAPQI, resulting in GSH depletion. GSH depletion increases oxidative stress and alters calcium homeostasis, leading to MPT. Mitochondrial membrane potential is lost, and ATP depletion ultimately leads to hepatocyte necrosis.67
Necroptosis
Unlike necrosis, necroptosis is programmed necrosis mediated by death receptors.68 Necroptosis is also morphologically characterized by cell swelling and plasma membrane rupture. Receptor-interacting protein kinases 1 and 3 (RIPK1, RIPK3) and mixed lineage kinase domain protein (MLKL) play important roles in necroptosis.69 Caspase-8 is a hub that mediates cell death, leading to different cell death modes.70 The binding of TNF and TNF-R1 provides a binding site for receptor-interacting protein 1 (RIP1). After RIP1 is deubiquitinated by cylindromatosis, caspase-8, RIP1, RIP3, and Fas-associated protein form complex IIa, which activates caspase-8 and mediates apoptosis. A necrosome (complex IIb) consisting of RIP1, RIP3 and MLKL induces necroptosis when the activity of caspase-8 is inhibited (Fig. 1).71,72
NAFLD and NASH
Studies have shown that RIPK3 expression is increased in liver biopsy specimens from NASH patients.73 Increased expression of RIPK3 and pMLKL was found in the livers of HFD-fed mice.74 Paradoxically, RIPK3-knockout mice exhibited exacerbated hepatocyte steatosis and liver injury compared with wild-type mice that were fed HFD. A lack of RIP3 exacerbates liver injury in NASH, which may be related to a shift in hepatocytes from necrosis to apoptosis and an increase in the inflammatory response.74 RIP3 is a key mediator of necroptosis. Although its overexpression or deletion may cause liver injury in NASH, the way it mediates cell death is different. In addition, in response to methionine–choline-deficient (MCD) conditions, intrahepatic triglyceride levels in caspase-8-knockout or RIP3-knockout mice were significantly increased. Studies have shown that RIP3 activates the JNK pathway in a caspase-8-dependent manner. This pathway promotes the development of NASH.75 In addition, inhibition of RIPK1 resulted in downregulation of MLKL and reduced liver injury and liver fibrosis in a HFD-fed mouse model. The same study showed that MLKL knockout increases β-oxidation in fatty liver cells.76 Therefore, RIPK1-RIPK3-MLKL-mediated NASH is related to necroptosis, but whether the mechanism involves crosstalk with apoptosis or other modes of cell death needs further study.
ALD
Necroptosis also plays an important role in the occurrence and progression of ALD. Increased expression of RIPK3 and pMLKL was reported in the liver biopsies of ALD patients.77,78 Compared with the control group, RIPK3-knockout mice exhibited reduced liver injury and steatosis in response to ethanol induction.79 This finding suggests that RIPK3 mediates ethanol-induced liver injury. Furthermore, inhibiting RIPK1 reduced alcohol-induced neutrophil recruitment in mice.80 Therefore, RIPK1 mediates inflammatory responses in ALD. Although pMLKL expression was increased in liver biopsies, MLKL knockdown did not alleviate ethanol-induced liver injury compared with that in wild-type mice. This finding suggests that MLKL is not important for ALD.78 It is reported that RIP3 expression was reduced in CYP2E1-deficient mice after chronic ethanol feeding, along with a decrease in plasma aspartate aminotransferase (AST) expression. It suggests that CYP2E1 contributes to ethanol-induced RIP3 expression and liver injury. Furthermore, in RIP3-deficient mouse livers, p-JNK was reduced after chronic ethanol feeding.79,80 CYP2E1 mediates p-JNK via RIPK3, which promotes liver injury and steatosis in response to ethanol induction. Therefore, CYP2E1-RIPK3-JNK mediates necroptosis, leading to ALD.
Autophagy and autophagy-dependent cell death
Autophagy is considered to be a survival mechanism under stressful conditions and is essential for maintaining cellular homeostasis. Misfolded or aggregated proteins, damaged organelles, and intracellular pathogens are sequestered within vesicles that become autophagosomes, which in turn function as lysosomes for degradation.81,82 Theoretically, autophagy mediates cytoprotective effects, and defects in autophagy accelerate cellular stress, leading to cell death.2 However, several studies have shown that cell death does not completely cease when apoptosis is inhibited by caspase inhibitors or apoptosis-related gene knockdown and that autophagy can drive cell death (autophagy-dependent cell death) to compensate.83,84 Autophagy-dependent cell death is also regulated.85 However, its occurrence is dependent on certain environments and needs to be further explored. The dual nature of autophagy means that we have to distinguish autophagy-dependent cell death from autophagy required for cell survival. At present, studies on autophagy in liver diseases such as viral hepatitis, ALD, NAFLD, and HCC have focused on the protective role of autophagy rather than autophagy-dependent cell death.86 Autophagy deficiency is associated with a variety of liver diseases. This review focuses on the mechanisms by which autophagy influences other modes of cell death such as apoptosis and contributes to the development of liver diseases (Fig. 1).
ALD
In general, autophagy activation has a protective effect on ALD. However, the effect of alcohol on autophagy is time- and dose dependent. Acute or low-dose ethanol exposure increases autophagy,87 and chronic or high-dose ethanol exposure decreases autophagy. Chronic and high-dose alcohol exposure inhibits autophagy,88,89 and inhibiting autophagy results in hepatocyte death.88 Crosstalk exists between autophagy and other modes of cell death.90 Alcohol causes apoptosis and the release of ROS, and autophagy is activated to remove cellular debris and lipid droplets, thus protecting hepatocytes.87,91 Apoptosis promotes cell death by inhibiting autophagy-related proteins.92 Studies have shown that inhibiting autophagy enhances cell death and that knocking down autophagy-associated protein 7 (ATG7) leads to liver injury.93 In response to alcohol stimulation, inhibiting autophagy promotes liver injury through apoptosis.87
NAFLD and NASH
Reduced levels of autophagy have been observed in patients with NAFLD and in mouse models.94,95 It has been shown that inhibiting autophagy in HFD-fed mice promotes apoptosis and increases lipid accumulation in hepatocytes.96 Increased JNK signaling and impaired autophagy due to oxidative stress promote NAFLD.97 In NAFLD, autophagy protects hepatocytes from death receptor-mediated liver injury. Macroautophagy inhibits apoptosis mediated by the death receptors TNF and Fas,98,99 and inhibiting hepatocyte autophagy promotes liver injury by activating caspase-8.100 Autophagy is a target of NAFLD therapy and exerts hepatoprotective effects by enhancing mitochondrial autophagy.
HCC
Autophagy has two sides in the occurrence of HCC.101 On the one hand, autophagy plays an inhibitory role in the early stages of tumors by maintaining genome stability, removing damaged mitochondria, and inhibiting malignant transformation of cells.102 On the other hand, autophagy acts as a tumor promoter during malignancy. Once tumors are formed, autophagy promotes tumor proliferation.103
Ferroptosis
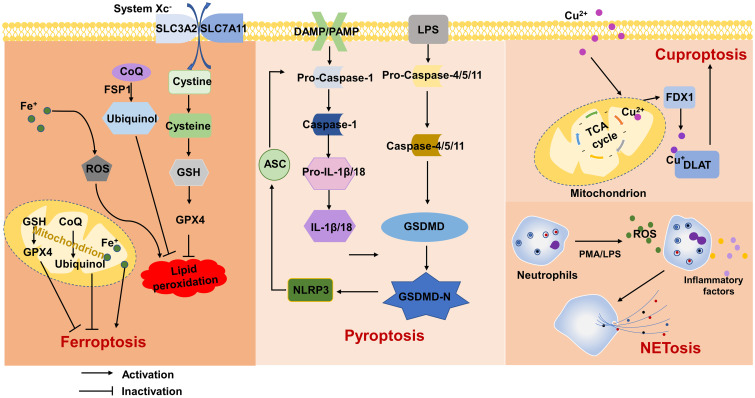
Unlike other types of cell death, ferroptosis is an iron-dependent form of cell death accompanied by extensive lipid peroxidation.104,105 In addition to the role of iron metabolism in ferroptosis, a growing number of metabolic pathways have been implicated in ferroptosis. These pathways include the GSH/GSH peroxidase 4 (GPX4) axis, guanosine triphosphate cyclohydrolase 1 (GCH1)/tetrahydrobiopterin (BH4)/dihydrofolate reductase (DHFR) axis, dihydroorotate dehydrogenase (DHODH)-dihydroubiquione (CoQH2) axis106 and F/coenzyme Q (CoQ) axis.107,108 Ferroptosis is initiated by intracellular iron accumulation, lipid peroxidation, and the oxidation of phospholipids containing polyunsaturated fatty acids (PUFA-PLs).109 According to recent findings, the main mechanism of ferroptosis is the ROS-dependent regulation of cell death. The presence of iron in the cytoplasm leads to the formation of ROS through the Fenton reaction. Through this reaction, H2O2 is converted to hydroxyl radicals. This process leads to lipid peroxidation, which plays an important role in ferroptosis.109 The increase in ROS can be regulated by the quenching effect of GPX4.110 In fact, GPX4 prevents iron-dependent ROS accumulation, and GSH is a cofactor to produce lipidol (R-OH) from lipid hydroperoxides (R-OOH). This metabolic process has a cytoprotective effect against Fe2+-dependent formation and ROS accumulation.109,111 Furthermore, System Xc− supports the efficiency of the antioxidant activity of GPX by inhibiting a series of events that trigger a reduction of GSH levels, lipid peroxidation, and consequent ferroptosis.109 In addition, cytochrome P450 oxidoreductase (POR)-mediated or arachidonic acid lipoxygenase (ALOX)-mediated enzymatic reactions have also been shown to promote lipid peroxidation. Iron not only controls the direct peroxidation of PUFA-PLs through the nonenzymatic Fenton reaction, but also is as an essential cofactor for enzymes involved in lipid peroxidation, such as ALOX and POR, to control ferroptosis.112 A growing number of studies have shown that ferroptosis has an important role in the pathogenesis of liver diseases. Therefore, targeting ferroptosis may provide a promising new therapeutic strategy (Fig. 2).
Fig. 2. Mechanisms of ferroptosis, pyroptosis, NETosis and cuproptosis.
ASC, apoptosis-associated speck-like protein; CoQ, coenzyme Q;DAMP, damage-associated molecular modes; DLAT, dihydrolipoic acid s-acetyltransferase; FDX1, iron oxidation reduction protein 1; FSP1, ferroptosis inhibitory protein 1; GPX4, glutathione peroxidase 4; GSDMD, gasdermin D; GSH, glutathione; IL, interleukin; LPS, lipopolysaccharide; PAMP, pathogen-associated molecular modes; PMA, phorbol 12-myristate 13-acetate; ROS, reactive oxygen species; SLC3A2, solute carrier family 3 member 2; SLC7A11, solute carrier family 7 member 11; TAC, tricarboxylic acid cycle.
ALD
Ferroptosis is associated with the development of ALD, and approximately half of patients with ALD exhibit hepatic iron overload.113 Alcohol metabolism mediates the accumulation of large amounts of acetaldehyde, which causes lipid peroxidation in hepatocytes through mitochondrial GSH depletion and excessive ROS production.5 GPX4 protein expression was observed in the hepatocytes of alcohol-treated mice. In vitro studies have shown that ferrostatin-1 (Fer-1) partially alleviates the cytotoxicity of alcohol, and Fer-1 almost completely reverses alcoholic liver injury.114 Further studies have shown that Fer-1 significantly attenuates ALD lipid peroxidation and reduces liver injury. Other studies have shown that activation of the nuclear factor erythroid 2-related factor 2 (Nrf2) pathway inhibits ferroptosis and exerts a protective effect against ALD.115 Ferroptosis is a promising therapeutic target for ALD treatment.
NAFLD and NASH
Lipid ROS cause hepatic steatosis through the formation of lipid droplets. A study showed that iron accumulation enhanced lipid ROS levels in MCD-fed mice. Treatment of MCD-fed mice with Fer-1 or liproxstatin-1 significantly improved hepatic steatosis and liver injury.116 GPX4 is most highly expressed in the liver, and GPX4 protects the liver from lipid peroxidation.117 It has been shown that inhibiting GPX4 in MCD-fed mice promoted NASH through ferroptosis-related effects. Conversely, GPX4 activation inhibited ferroptosis and improved the severity of NASH.118 Thymidine β4 delays NAFLD development by upregulating GPX4, which inhibits ferroptosis. In addition, Nrf2 inhibits ferroptosis by promoting antioxidant responses and eliminating lipid ROS accumulation in the liver.119 The activation of Nrf2 in HFD-fed mice delays the occurrence of NAFLD.120
APAP-induced liver injury
A study showed that ferroptosis occurred in APAP-induced liver injury, and Fer-1 provided modest protection against APAP-induced primary hepatocyte death in mice.121 Ferroptosis occurred in a mouse model of APAP-induced acute liver failure with elevated lipid peroxide derived from n-6 PUFAs. In addition, the ferroptosis inhibitor UAMC-3203 and the VDAC1 oligomerization inhibitor VBIT-12 reduced ferroptosis in APAP-induced liver injury animal models by preserving mitochondrial function.122 These studies suggest that ferroptosis may serve as a therapeutic target for APAP-induced liver injury.
Hepatic fibrosis and cirrhosis
Ferroptosis is a double-edged sword in liver fibrosis. When ferroptosis targets activated HSCs, ferroptosis suppresses liver fibrosis.123 HBx promotes the development of liver fibrosis by inhibiting ferroptosis. Chrysophanol promotes ferroptosis by downregulating GPX4 and SLC7A11 (a key part of System Xc−) levels. Thus, chrysophanol attenuates the activation of HSCs and improves liver fibrosis.124 Erastin/sorafenib alleviates liver fibrosis in mice by inducing HSC ferroptosis.125 Magnesium isoglycyrrhizinate promotes HSC ferroptosis by regulating heme oxygenase 1, which significantly reduces liver fibrosis and attenuates liver injury.126 P53 plays a crucial role in the induction of ferroptosis.127 Artemether, a derivative of artemisinin, reduced liver fibrosis in a mouse model of liver fibrosis. Knockdown of P53 exacerbated liver fibrosis by blocking artemether-induced HSC ferroptosis, which in turn exacerbated liver fibrosis.128 Hepatocyte ferroptosis leads to liver fibrosis. Studies have shown that increased hepatocyte ferroptosis promotes the occurrence of liver fibrosis. Liver fibrosis was reversed when Fer-1 inhibited hepatocyte ferroptosis.129 It is important to explore the role of ferroptosis in the development of hepatic fibrosis.
HCC
The role of ferroptosis in HCC is complex. On the one hand, ferroptosis associated with iron overload is involved in the progression of different types of liver diseases, which promotes the development of HCC.130 On the other hand, ferroptosis inhibits HCC cell proliferation. PUFAs reduce HCC growth by inhibiting β-catenin and cyclooxygenase 2 (COX-2).131 Studies have shown that ferroptosis regulators GPX4 is overexpressed in HCC.132 Therefore, induction of ferroptosis may be a promising therapeutic strategy for HCC.
Pyroptosis
Pyroptosis is a type of programmed cell death that is usually induced in cells in the innate immune system.133 Pyroptosis is activated by the presence of pathogen-associated molecular patterns (PAMPs) or cell-derived damage-associated molecular patterns (DAMPs). There are two main molecular mechanisms of pyroptosis, the classic caspase-1-dependent pathway and the noncaspase-1-dependent pathway.134,135 In caspase-1-dependent pyroptosis, the activation of inflammasome receptors such as NLRP1, NLRP3, NLRC4, AIM2 by PAMPs or DAMPs triggers the recruitment of the adapter proteins ASC and caspase-1 to form a macromolecular complex in which caspase-1 is activated. Activated caspase-1 directly cleaves gasdermin D (GSDMD), and the precursor cytokines pro-interleukin (IL)1β and pro-IL18, leading to pyroptosis and the maturation of IL1β and IL18. The cleaved gasdermin D N-terminal fragment (GSDMD-NT) forms pores in the cell membrane and mediates the release of cytoplasmic contents.135,136 During noncaspase-1-dependent cell pyroptosis, caspase-4 or caspase-5 in human cells or caspase-11 in murine cells recognize lipopolysaccharide (LPS) in the cytosol.137 These caspases directly cleave GSDMD and induce pyroptosis.135–137 Recent studies have shown that pyroptosis plays a role in various liver diseases, such as viral hepatitis, ALD,26 NAFLD,138 liver fibrosis, and liver failure.139 This review focuses on the mechanism of pyroptosis in liver diseases (Fig. 2).
Viral hepatitis
Pyroptosis is associated with viral hepatitis. Previous studies have suggested that in patients with chronic hepatitis B, HBcAg induces IL18 secretion by inducing caspase-1.140 HBeAg inhibits LPS-induced activation of the NLRP3 inflammasome and IL1β production in two ways: direct inhibition by inhibiting NF-κB phosphorylation and through inhibition of ROS production, which inhibits caspase-1 activation and IL1β maturation.141 Furthermore, in patients with chronic HCV infection, HCV RNA directly induces NLRP3 inflammasome activation in infected hepatocytes.142 In KCs, IL1β production via the NLRP3 inflammasome promotes HCV virus amplification,143 and the activation of natural killer cells inhibits HCV replication.144 Although the mechanisms of pyroptosis in viral hepatitis need to be further explored, inhibiting NLRP3 or IL1β activity is an effective strategy.
ALD
In ALD, IL1β promotes hepatic steatosis and liver injury. The upregulation of caspase-1 and inflammasome activation in KCs increases IL1β. However, recombinant IL1Ra blocks IL1 signaling in vivo.145 In an alcohol-induced mouse model, alcohol promoted TXNIP overexpression and NLRP3 activation, which induced hepatocyte pyroptosis.146 In addition, a noncaspase-1-dependent pathway induced pyroptosis in ALD. The activation of caspase-11 in alcoholic hepatitis mice and caspase-4 in alcoholic hepatitis patients promotes pyroptosis mediated by GSDMD.147
NAFLD and NASH
Compared with other liver diseases, pyroptosis has been most studied in NAFLD/NASH. GSDMD-N expression is significantly upregulated in the liver biopsies of NASH patients.148 Similarly, GSDMD expression was increased in an MCD-fed mouse model. GSDMD-knockout mice have less steatosis than wild-type mice.148 Further studies have shown that pyroptosis driven by GSDMD-N increased the severity of steatohepatitis in mouse models.148 NLRP3 knockdown attenuated liver injury in a mouse model of NAFLD.149 In contrast, NLRP3 activation promoted the development of NASH or NAFLD through hepatocyte pyroptosis.150 The selective NLRP3 inhibitor MCC950 was used in a steatohepatitis model constructed by HFD and MCD feeding and resulted in reduced NFκB activation and reduced liver inflammation.151 MCC950 inhibited inflammation and liver fibrosis in NAFLD mouse models by reducing the expression of caspase-1, IL6, and IL1β.152 Inhibiting NLRP3 and GSDMD significantly reduces inflammation and fibrosis and ultimately alleviates NAFLD by regulating the pyroptosis signaling pathway.153
Liver fibrosis
Pyroptosis is also associated with liver fibrosis.148 Activated HSCs promote collagen deposition through pyroptosis.154 IL1β promotes the proliferation of HSCs in rats, which in turn enhances the expression of collagen and TGFβ and induces liver fibrosis.155 In addition, eosinophils promote liver fibrosis by inducing the secretion of IL1β and IL18, which leads to pyroptosis. However, a caspase-1 inhibitor can reverse liver fibrosis.156 Studies have shown that GSDMD promotes the progression of liver fibrosis in MCD-fed mice.148 Excessive activation of NLRP3 exacerbates liver inflammation and liver fibrosis. NLRP3 knockdown protects the liver from drug-induced liver fibrosis.157
NETosis
It has been shown that activated neutrophils capture and kill pathogens by releasing neutrophil extracellular traps (NETs), which consist of depolymerized chromatin and intracellular granule proteins, to the outside of the cell. This novel form of inflammatory cell death, which is accompanied by neutrophil death during the formation of NETs, is known as NETosis.158 NETosis is a specific form of programmed cell death in which NADPH oxidase is activated when the concentration of Ca2+ in the cytoplasm increases, leading to the massive production of ROS and ultimately inducing NETosis.159 ROS trigger the release of NE into the cytoplasm, where myeloperoxidase (MPO) activates its protein hydrolytic activity, ultimately leading to chromatin decondensation and disruption of the nuclear membrane.160 Although NETs do not cause hepatocyte death, they are involved in the pathogenesis of various chronic liver diseases, such as autoimmune liver disease, NASH, and HCC(Fig. 2).161
Viral hepatitis
Studies have shown that HBV C protein (HBc) and HBV E protein (HBe) caused lower cfDNA levels in patients with chronic hepatitis B infection. It shows that HBc and HBe proteins inhibit NETs release. Further studies revealed that HBc and HBe proteins reduce neutrophil autophagic activity and inhibit ROS production by enhancing mTOR activity. In turn, the inhibition of NETs release ultimately delays the elimination of HBV. It makes HBV to evade the immune system and eventually leads to the establishment of persistent infection in this way.162
NAFLD and NASH
Studies have shown that serum neutrophil levels are significantly elevated in patients with NASH. NETs were detected in MCD/HF diet-fed mouse models. The removal of NETs significantly reduced liver injury in MCD/HF diet-fed mouse models.163 In vivo and in vitro experiments revealed that FFAs stimulated the formation of NETs in a mouse model of NASH. Inhibiting NETs reduces the production of inflammatory cytokines and inflammatory cell infiltration, which in turn slows the progression of NASH.164 Other studies have shown a positive correlation between serum levels of NETs markers (MPO-DNA complexes) and sphingosine kinase 1 (S1P rate-limiting enzyme) levels in damaged livers in a mouse model of NASH. In vitro, S1P receptor 2 (S1PR2) is involved in regulating the transition of neutrophils from apoptosis to NETosis. Knockdown of S1PR2 in MCD/HF diet-fed mice significantly inhibited NETs formation in damaged liver tissue, which in turn reduced liver inflammation and fibrosis.163
Cuproptosis
Copper is an important catalytic cofactor involved in oxygen metabolism and energy conversion.165 Changes in the concentration of copper cause significant damage to cells.166 Cuproptosis is a novel type of cell death, and studies have shown that intracellular copper accumulation leads to the aggregation of mitochondrial lipoproteins and protein destabilization.167 In addition, copper ions regulate cell death by targeting lipidylated mitochondrial tricarboxylic acid cycle (TAC) proteins.168 Further studies revealed that iron oxidation reduction protein 1 (FDX1) encodes a reductase that reduces Cu2+ to Cu+. In contrast, dihydrolipoic acid S-acetyltransferase (DLAT), a protein target of lipid acylation, is involved in the TAC cycle. FDX1 promotes DLAT lipid acylation, Cu+ enhancement of lipid acylated protein aggregation and the reduction of iron-sulfur cluster proteins, thereby promoting cell death.168,169 The liver is an important organ for regulating energy metabolism in the body.170 Impaired energy metabolism is associated with the exacerbation of acute liver failure. Studies have shown that an imbalance in copper metabolism affects normal liver metabolism.171,172 Thus, cuproptosis may be a potential cell death mode in liver diseases, which can be used as a new research direction and therapeutic target (Fig. 2).
NAFLD and NASH
Copper and lipid metabolism have a complex relationship, and increased cellular copper may downregulate lipid and lipogenesis genes.173 Study carried out transcriptome analysis on the liver tissues of HFD-induced NAFLD mice and normal mice. Gene set variation analysis results showed that the cuproptosis was aberrantly activated in NAFLD. Among them, DLD and PDHB may be potential hub genes for the diagnosis and treatment of NAFLD.174
Targeting cell death in liver diseases’ application
Currently, some preclinical or clinical studies has provided the basis to transfer cell death–based therapies for liver diseases to the clinic. In this section, we summarize the involvement of cell death in liver diseases.
Targeting apoptosis in liver diseases
Clinical trials have shown that inhibition of hepatocyte apoptosis delays the progression of liver disease. Pan-caspase inhibitors PF-03491390 improved liver function in patients with chronic HCV infection.175 The selective caspase inhibitor GS-9450 significantly reduced alanine transaminase (ALT) levels in NASH patients by reducing hepatocyte apoptosis.176 Besides, as small noncoding RNAs, miRNAs are highly stable and easily detected in the circulation compared with traditional biomarkers. Studies have shown that many miRNAs are specifically expressed in hepatocytes. MiR-34a/SIRT1/p53 leads to NASLD by promoting apoptosis of hepatocytes. It is reported that miR-296-5p levels are inversely correlated with PUMA (pro-apoptotic protein) mRNA levels in human liver samples. In NASH patients, hepatic miR-296 expression was decreased, PUMA was increased, and hepatic steatosis was observed.177 MiR-34a and miR-296 play a role in NAFLD by regulating hepatocyte apoptosis. Sorafenib is approved for the first-line treatment of advanced liver cancer, and its main antitumor activity is related to caspase activation, Bax and Bak activation.57
Targeting necrosis in liver diseases
Clinical studies have shown that glycyrrhizin, as an HMGB1 inhibitor, reduces serum ALT levels in patients with HCV infection and improves hepatic necroinflammation and fibrosis. Glycyrrhizin can be used as an adjuvant drug in viral hepatitis.178
Targeting ferroptosis in liver diseases
Targeting ferroptosis therapy plays important role in nonviral liver diseases.179 Preclinical studies have found that Fer-1 improves ALD and NAFLD by inhibiting ferroptosis in hepatocytes.108,114 As a ferroptosis inducer, erastin improves liver fibrosis by promoting ferroptosis by inhibiting the system Xc−.125 In addition to apoptosis inducers, sorafenib can also induce a new type of programmed cell death, ferroptosis. However, it shows that inhibition of ferroptosis mediates solafenib resistance in HCC. Studies have shown that solute carrier family 27 member 5 (SLC27A5) is downregulated in sorafenib-resistant HCC. Loss of SLC27A5 enhances GSH reductase (GSR) expression in a Nrf2-dependent manner, thereby maintaining GSH homeostasis and it promotes HCC by inhibiting ferroptosis.180 It provides a potential therapeutic strategy for overcoming sorafenib resistance.
Targeting pyroptosis in liver diseases
Inhibition of NLRP3 or its downstream signaling pathways (e.g., caspases and IL1) is the main strategy for the treatment of pyroptosis. Animal experiments have shown that taurine or the ER stress inhibitor Tudaka relieves nonalcoholic fatty liver/NASH by inhibiting the activity of NLRP3 inflammasome.150,181 A pan-caspase inhibitor, IDN 6556, reduced liver injury and fibrosis in a mouse model of NASH. Clinical trials have shown that pan-caspase inhibitors IDN 6556 or PF-034 reduce serum AST and ALT levels in patients with chronic hepatitis C.175,182
Future directions and therapeutic implications
Cell death is central to the study of the development and progression of liver diseases. In recent years, cell death in liver diseases has been intensively studied and new cell death modes that enhance our understanding of the pathophysiology of liver diseases have been discovered. In this review, it was found that different cell death modes occur simultaneously in certain liver diseases. Therefore, the various cell death modes are not independent or compartmentalized, and different cell death modes crosstalk with each other. The activation or inhibition of one cell death mode may activate other cell death modes. Future studies should focus on exploring the regulatory relationship between various cell death response pathways in the liver. Moreover, the application of cell death in liver diseases is mostly in the preclinical stage. We should aim to translate cell death into clinical applications and predict the onset and regression of liver diseases using hepatocyte death-related biomarkers as early as possible. That will provide a basis for the diagnosis, monitoring and treatment of liver diseases in the future and could help to block liver diseases in the initial stage or delay the progression of liver disease and improve the cure rate.
Abbreviations
- ALD
alcoholic liver disease
- ALT
alanine transaminase
- APAP
acetaminophen
- AST
aspartate aminotransferase
- CypD
cyclophilin D
- DAMP
damage-associated molecular modes
- ER
endoplasmic reticulum
- Fas-l
Fas ligand
- Fer-1
Ferrostatin-1
- GPX4
glutathione peroxidase 4
- GSDMD
gasdermin D
- GSDMD-NT
gasdermin D N-terminal fragment
- GSH
glutathione
- HBV
hepatitis B virus
- HBc
HBV C protein
- HBe
HBV E protein
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HSCs
hepatic stellate cells
- HFD
high-fat diet
- IL
interleukin
- JNK
cJun-N-terminal Kinase
- KC
kupffer cells
- LPS
lipopolysaccharide
- MCD
methionine–choline-deficient
- MLKL
mixed lineage kinase domain protein
- MPT
mitochondrial permeability transition
- NASH
nonalcoholic steatohepatitis
- NAFLD
nonalcoholic fatty liver disease
- NETs
neutrophil extracellular traps
- Nrf2
nuclear factor erythroid 2-related factor 2
- PAMP
pathogen-associated molecular modes
- RIPK
receptor interacting rotein kinases
- RIP1
receptor-interacting protein
- ROS
reactive oxygen species
- SLC27A5
solute carrier family 27 member 5
- TAC
tricarboxylic acid cycle
- TNF
tumor necrosis factor
- TNF-R
TNF receptor
- TRAIL
TNF-related apoptosis-inducing ligand
References
- 1.Yan G, Elbadawi M, Efferth T. Multiple cell death modalities and their key features (Review) World Acad Sci J. 2020;2(2):39–48. doi: 10.3892/wasj.2020.40. [DOI] [Google Scholar]
- 2.Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25(3):486–541. doi: 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu X, Yang W, Guan Z, Yu W, Fan B, Xu N, et al. There are only four basic modes of cell death, although there are many ad-hoc variants adapted to different situations. Cell Biosci. 2018;8:6. doi: 10.1186/s13578-018-0206-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heidelbaugh JJ, Bruderly M. Cirrhosis and chronic liver failure: part I. Diagnosis and evaluation. Am Fam Physician. 2006;74(5):756–762. [PubMed] [Google Scholar]
- 5.Luedde T, Kaplowitz N, Schwabe RF. Cell death and cell death responses in liver disease: mechanisms and clinical relevance. Gastroenterology. 2014;147(4):765–783.e4. doi: 10.1053/j.gastro.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang L, Liu J, Bie C, Liu H, Ji Y, Chen D, et al. Advances in cell death - related signaling pathways in acute-on-chronic liver failure. Clin Res Hepatol Gastroenterol. 2022;46(2):101783. doi: 10.1016/j.clinre.2021.101783. [DOI] [PubMed] [Google Scholar]
- 7.Eguchi A, Wree A, Feldstein AE. Biomarkers of liver cell death. J Hepatol. 2014;60(5):1063–1074. doi: 10.1016/j.jhep.2013.12.026. [DOI] [PubMed] [Google Scholar]
- 8.Martinou JC, Youle RJ. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev Cell. 2011;21(1):92–101. doi: 10.1016/j.devcel.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35(4):495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McIlwain DR, Berger T, Mak TW. Caspase functions in cell death and disease. Cold Spring Harb Perspect Biol. 2013;5(4):a008656. doi: 10.1101/cshperspect. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muppidi JR, Tschopp J, Siegel RM. Life and death decisions: secondary complexes and lipid rafts in TNF receptor family signal transduction. Immunity. 2004;21(4):461–465. doi: 10.1016/j.immuni.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Akazawa Y, Gores GJ. Death receptor-mediated liver injury. Semin Liver Dis. 2007;27(4):327–338. doi: 10.1055/s-2007-991510. [DOI] [PubMed] [Google Scholar]
- 13.Wang K. Molecular mechanisms of hepatic apoptosis. Cell Death Dis. 2014;5(1):e996. doi: 10.1038/cddis.2013.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dara L, Ji C, Kaplowitz N. The contribution of endoplasmic reticulum stress to liver diseases. Hepatology. 2011;53(5):1752–1763. doi: 10.1002/hep.24279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asselah T, Bièche I, Mansouri A, Laurendeau I, Cazals-Hatem D, Feldmann G, et al. In vivo hepatic endoplasmic reticulum stress in patients with chronic hepatitis C. J Pathol. 2010;221(3):264–274. doi: 10.1002/path.2703. [DOI] [PubMed] [Google Scholar]
- 16.Fischer R, Baumert T, Blum HE. Hepatitis C virus infection and apoptosis. World J Gastroenterol. 2007;13(36):4865–4872. doi: 10.3748/wjg.v13.i36.4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar S. Caspase function in programmed cell death. Cell Death Differ. 2007;14(1):32–43. doi: 10.1038/sj.cdd.4402060. [DOI] [PubMed] [Google Scholar]
- 18.Faouzi S, Burckhardt BE, Hanson JC, Campe CB, Schrum LW, Rippe RA, et al. Anti-Fas induces hepatic chemokines and promotes inflammation by an NF-kappa B-independent, caspase-3-dependent pathway. J Biol Chem. 2001;276(52):49077–49082. doi: 10.1074/jbc.M109791200. [DOI] [PubMed] [Google Scholar]
- 19.Jones RA, Johnson VL, Buck NR, Dobrota M, Hinton RH, Chow SC, et al. Fas-mediated apoptosis in mouse hepatocytes involves the processing and activation of caspases. Hepatology. 1998;27(6):1632–1642. doi: 10.1002/hep.510270624. [DOI] [PubMed] [Google Scholar]
- 20.Schaefer U, Voloshanenko O, Willen D, Walczak H. TRAIL: a multifunctional cytokine. Front Biosci. 2007;12:3813–3824. doi: 10.2741/2354. [DOI] [PubMed] [Google Scholar]
- 21.Machida K, Cheng KT, Lai CK, Jeng KS, Sung VM, Lai MM. Hepatitis C virus triggers mitochondrial permeability transition with production of reactive oxygen species, leading to DNA damage and STAT3 activation. J Virol. 2006;80(14):7199–7207. doi: 10.1128/jvi.00321-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feitelson MA, Reis HM, Tufan NL, Sun B, Pan J, Lian Z. Putative roles of hepatitis B x antigen in the pathogenesis of chronic liver disease. Cancer Lett. 2009;286(1):69–79. doi: 10.1016/j.canlet.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Du J, Liang X, Liu Y, Qu Z, Gao L, Han L, et al. Hepatitis B virus core protein inhibits TRAIL-induced apoptosis of hepatocytes by blocking DR5 expression. Cell Death Differ. 2009;16(2):219–229. doi: 10.1038/cdd.2008.144. [DOI] [PubMed] [Google Scholar]
- 24.Liang X, Liu Y, Zhang Q, Gao L, Han L, Ma C, et al. Hepatitis B virus sensitizes hepatocytes to TRAIL-induced apoptosis through Bax. J Immunol. 2007;178(1):503–510. doi: 10.4049/jimmunol.178.1.503. [DOI] [PubMed] [Google Scholar]
- 25.Wang S, Pacher P, De Lisle RC, Huang H, Ding WX. A Mechanistic Review of Cell Death in Alcohol-Induced Liver Injury. Alcohol Clin Exp Res. 2016;40(6):1215–1223. doi: 10.1111/acer.13078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shojaie L, Iorga A, Dara L. Cell Death in Liver Diseases: A Review. Int J Mol Sci. 2020;21(24):9682. doi: 10.3390/ijms21249682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Natori S, Rust C, Stadheim LM, Srinivasan A, Burgart LJ, Gores GJ. Hepatocyte apoptosis is a pathologic feature of human alcoholic hepatitis. J Hepatol. 2001;34(2):248–253. doi: 10.1016/s0168-8278(00)00089-1. [DOI] [PubMed] [Google Scholar]
- 28.Hao F, Cubero FJ, Ramadori P, Liao L, Haas U, Lambertz D, et al. Inhibition of Caspase-8 does not protect from alcohol-induced liver apoptosis but alleviates alcoholic hepatic steatosis in mice. Cell Death Dis. 2017;8(10):e3152. doi: 10.1038/cddis.2017.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilson CH, Kumar S. Caspases in metabolic disease and their therapeutic potential. Cell Death Differ. 2018;25(6):1010–1024. doi: 10.1038/s41418-018-0111-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cahill A, Cunningham CC, Adachi M, Ishii H, Bailey SM, Fromenty B, et al. Effects of alcohol and oxidative stress on liver pathology: the role of the mitochondrion. Alcohol Clin Exp Res. 2002;26(6):907–915. [PMC free article] [PubMed] [Google Scholar]
- 31.Lambert JC, Zhou Z, Kang YJ. Suppression of Fas-mediated signaling pathway is involved in zinc inhibition of ethanol-induced liver apoptosis. Exp Biol Med (Maywood) 2003;228(4):406–412. doi: 10.1177/153537020322800411. [DOI] [PubMed] [Google Scholar]
- 32.Nagy LE, Ding WX, Cresci G, Saikia P, Shah VH. Linking Pathogenic Mechanisms of Alcoholic Liver Disease With Clinical Phenotypes. Gastroenterology. 2016;150(8):1756–1768. doi: 10.1053/j.gastro.2016.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naveau S, Emilie D, Balian A, Grangeot-Keros L, Borotto E, Portier A, et al. Plasma levels of soluble tumor necrosis factor receptors p55 and p75 in patients with alcoholic liver disease of increasing severity. J Hepatol. 1998;28(5):778–784. doi: 10.1016/s0168-8278(98)80227-4. [DOI] [PubMed] [Google Scholar]
- 34.Naveau S, Chollet-Martin S, Dharancy S, Mathurin P, Jouet P, Piquet MA, et al. A double-blind randomized controlled trial of infliximab associated with prednisolone in acute alcoholic hepatitis. Hepatology. 2004;39(5):1390–1397. doi: 10.1002/HEP.20206. [DOI] [PubMed] [Google Scholar]
- 35.Hartmann P, Seebauer CT, Schnabl B. Alcoholic liver disease: the gut microbiome and liver cross talk. Alcohol Clin Exp Res. 2015;39(5):763–775. doi: 10.1111/acer.12704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ziol M, Tepper M, Lohez M, Arcangeli G, Ganne N, Christidis C, et al. Clinical and biological relevance of hepatocyte apoptosis in alcoholic hepatitis. J Hepatol. 2001;34(2):254–260. doi: 10.1016/s0168-8278(00)00047-7. [DOI] [PubMed] [Google Scholar]
- 37.Petrasek J, Iracheta-Vellve A, Csak T, Satishchandran A, Kodys K, Kurt-Jones EA, et al. STING-IRF3 pathway links endoplasmic reticulum stress with hepatocyte apoptosis in early alcoholic liver disease. Proc Natl Acad Sci U S A. 2013;110(41):16544–16549. doi: 10.1073/pnas.1308331110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feldstein AE, Werneburg NW, Canbay A, Guicciardi ME, Bronk SF, Rydzewski R, et al. Free fatty acids promote hepatic lipotoxicity by stimulating TNF-alpha expression via a lysosomal pathway. Hepatology. 2004;40(1):185–194. doi: 10.1002/hep.20283. [DOI] [PubMed] [Google Scholar]
- 39.Guicciardi ME, Deussing J, Miyoshi H, Bronk SF, Svingen PA, Peters C, et al. Cathepsin B contributes to TNF-alpha-mediated hepatocyte apoptosis by promoting mitochondrial release of cytochrome c. J Clin Invest. 2000;106(9):1127–1137. doi: 10.1172/jci9914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Z, Berk M, McIntyre TM, Gores GJ, Feldstein AE. The lysosomal-mitochondrial axis in free fatty acid-induced hepatic lipotoxicity. Hepatology. 2008;47(5):1495–1503. doi: 10.1002/hep.22183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feldstein AE, Canbay A, Angulo P, Taniai M, Burgart LJ, Lindor KD, et al. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology. 2003;125(2):437–443. doi: 10.1016/s0016-5085(03)00907-7. [DOI] [PubMed] [Google Scholar]
- 42.Hui JM, Hodge A, Farrell GC, Kench JG, Kriketos A, George J. Beyond insulin resistance in NASH: TNF-alpha or adiponectin? Hepatology. 2004;40(1):46–54. doi: 10.1002/hep.20280. [DOI] [PubMed] [Google Scholar]
- 43.Peter ME, Krammer PH. The CD95(APO-1/Fas) DISC and beyond. Cell Death Differ. 2003;10(1):26–35. doi: 10.1038/sj.cdd.4401186. [DOI] [PubMed] [Google Scholar]
- 44.Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli KJ, et al. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17(6):1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marí M, Caballero F, Colell A, Morales A, Caballeria J, Fernandez A, et al. Mitochondrial free cholesterol loading sensitizes to TNF- and Fas-mediated steatohepatitis. Cell Metab. 2006;4(3):185–198. doi: 10.1016/j.cmet.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 46.MacFarlane M. TRAIL-induced signalling and apoptosis. Toxicol Lett. 2003;139(2-3):89–97. doi: 10.1016/s0378-4274(02)00422-8. [DOI] [PubMed] [Google Scholar]
- 47.Guicciardi ME, Gores GJ. Apoptosis: a mechanism of acute and chronic liver injury. Gut. 2005;54(7):1024–1033. doi: 10.1136/gut.2004.053850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao P, Sun X, Chaggan C, Liao Z, In Wong K, He F, et al. An AMPK-caspase-6 axis controls liver damage in nonalcoholic steatohepatitis. Science. 2020;367(6478):652–660. doi: 10.1126/science.aay0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maiers JL, Malhi H. Endoplasmic Reticulum Stress in Metabolic Liver Diseases and Hepatic Fibrosis. Semin Liver Dis. 2019;39(2):235–248. doi: 10.1055/s-0039-1681032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nakagawa H, Umemura A, Taniguchi K, Font-Burgada J, Dhar D, Ogata H, et al. ER stress cooperates with hypernutrition to trigger TNF-dependent spontaneous HCC development. Cancer Cell. 2014;26(3):331–343. doi: 10.1016/j.ccr.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khanam A, Saleeb PG, Kottilil S. Pathophysiology and Treatment Options for Hepatic Fibrosis: Can It Be Completely Cured? Cells. 2021;10(5):1097. doi: 10.3390/cells10051097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gregory CD, Devitt A. The macrophage and the apoptotic cell: an innate immune interaction viewed simplistically? Immunology. 2004;113(1):1–14. doi: 10.1111/j.1365-2567.2004.01959.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Canbay A, Feldstein AE, Higuchi H, Werneburg N, Grambihler A, Bronk SF, et al. Kupffer cell engulfment of apoptotic bodies stimulates death ligand and cytokine expression. Hepatology. 2003;38(5):1188–1198. doi: 10.1053/jhep.2003.50472. [DOI] [PubMed] [Google Scholar]
- 54.Canbay A, Taimr P, Torok N, Higuchi H, Friedman S, Gores GJ. Apoptotic body engulfment by a human stellate cell line is profibrogenic. Lab Invest. 2003;83(5):655–663. doi: 10.1097/01.lab.0000069036.63405.5c. [DOI] [PubMed] [Google Scholar]
- 55.Takehara T, Tatsumi T, Suzuki T, Rucker EB, 3rd, Hennighausen L, Jinushi M, et al. Hepatocyte-specific disruption of Bcl-xL leads to continuous hepatocyte apoptosis and liver fibrotic responses. Gastroenterology. 2004;127(4):1189–1197. doi: 10.1053/j.gastro.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 56.Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer. 2006;6(9):674–687. doi: 10.1038/nrc1934. [DOI] [PubMed] [Google Scholar]
- 57.Moreno-Càceres J, Fabregat I. Apoptosis in liver carcinogenesis and chemotherapy. Hepat Oncol. 2015;2(4):381–397. doi: 10.2217/hep.15.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fernando J, Sancho P, Fernández-Rodriguez CM, Lledó JL, Caja L, Campbell JS, et al. Sorafenib sensitizes hepatocellular carcinoma cells to physiological apoptotic stimuli. J Cell Physiol. 2012;227(4):1319–1325. doi: 10.1002/jcp.22843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guicciardi ME, Malhi H, Mott JL, Gores GJ. Apoptosis and necrosis in the liver. Compr Physiol. 2013;3(2):977–1010. doi: 10.1002/cphy.c120020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Malhi H, Gores GJ, Lemasters JJ. Apoptosis and necrosis in the liver: a tale of two deaths? Hepatology. 2006;43(Suppl 1):S31–S44. doi: 10.1002/hep.21062. [DOI] [PubMed] [Google Scholar]
- 61.Giorgio V, von Stockum S, Antoniel M, Fabbro A, Fogolari F, Forte M, et al. Dimers of mitochondrial ATP synthase form the permeability transition pore. Proc Natl Acad Sci U S A. 2013;110(15):5887–5892. doi: 10.1073/pnas.1217823110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang X, Du H, Shao S, Bo T, Yu C, Chen W, et al. Cyclophilin D deficiency attenuates mitochondrial perturbation and ameliorates hepatic steatosis. Hepatology. 2018;68(1):62–77. doi: 10.1002/hep.29788. [DOI] [PubMed] [Google Scholar]
- 63.Kuo J, Bobardt M, Chatterji U, Mayo PR, Trepanier DJ, Foster RT, et al. A Pan-Cyclophilin Inhibitor, CRV431, Decreases Fibrosis and Tumor Development in Chronic Liver Disease Models. J Pharmacol Exp Ther. 2019;371(2):231–241. doi: 10.1124/jpet.119.261099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bourdi M, Korrapati MC, Chakraborty M, Yee SB, Pohl LR. Protective role of c-Jun N-terminal kinase 2 in acetaminophen-induced liver injury. Biochem Biophys Res Commun. 2008;374(1):6–10. doi: 10.1016/j.bbrc.2008.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gunawan BK, Liu ZX, Han D, Hanawa N, Gaarde WA, Kaplowitz N. c-Jun N-terminal kinase plays a major role in murine acetaminophen hepatotoxicity. Gastroenterology. 2006;131(1):165–178. doi: 10.1053/j.gastro.2006.03.045. [DOI] [PubMed] [Google Scholar]
- 66.Ramachandran A, Lebofsky M, Baines CP, Lemasters JJ, Jaeschke H. Cyclophilin D deficiency protects against acetaminophen-induced oxidant stress and liver injury. Free Radic Res. 2011;45(2):156–164. doi: 10.3109/10715762.2010.520319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hinson JA, Roberts DW, James LP. Mechanisms of acetaminophen-induced liver necrosis. Handb Exp Pharmacol. 2010;196:369–405. doi: 10.1007/978-3-642-00663-0_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vanden Berghe T, Linkermann A, Jouan-Lanhouet S, Walczak H, Vandenabeele P. Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nat Rev Mol Cell Biol. 2014;15(2):135–147. doi: 10.1038/nrm3737. [DOI] [PubMed] [Google Scholar]
- 69.Molnár T, Mázló A, Tslaf V, Szöllősi AG, Emri G, Koncz G. Current translational potential and underlying molecular mechanisms of necroptosis. Cell Death Dis. 2019;10(11):860. doi: 10.1038/s41419-019-2094-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yuan J, Najafov A, Py BF. Roles of Caspases in Necrotic Cell Death. Cell. 2016;167(7):1693–1704. doi: 10.1016/j.cell.2016.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wegner KW, Saleh D, Degterev A. Complex Pathologic Roles of RIPK1 and RIPK3: Moving Beyond Necroptosis. Trends Pharmacol Sci. 2017;38(3):202–225. doi: 10.1016/j.tips.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li X, Dong G, Xiong H, Diao H. A narrative review of the role of necroptosis in liver disease: a double-edged sword. Ann Transl Med. 2021;9(5):422. doi: 10.21037/atm-20-5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dara L, Liu ZX, Kaplowitz N. Questions and controversies: the role of necroptosis in liver disease. Cell Death Discov. 2016;2:16089. doi: 10.1038/cddiscovery.2016.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Roychowdhury S, McCullough RL, Sanz-Garcia C, Saikia P, Alkhouri N, Matloob A, et al. Receptor interacting protein 3 protects mice from high-fat diet-induced liver injury. Hepatology. 2016;64(5):1518–1533. doi: 10.1002/hep.28676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gautheron J, Vucur M, Reisinger F, Cardenas DV, Roderburg C, Koppe C, et al. A positive feedback loop between RIP3 and JNK controls non-alcoholic steatohepatitis. EMBO Mol Med. 2014;6(8):1062–1074. doi: 10.15252/emmm.201403856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Majdi A, Aoudjehane L, Ratziu V, Islam T, Afonso MB, Conti F, et al. Inhibition of receptor-interacting protein kinase 1 improves experimental non-alcoholic fatty liver disease. J Hepatol. 2020;72(4):627–635. doi: 10.1016/j.jhep.2019.11.008. [DOI] [PubMed] [Google Scholar]
- 77.Zhang Z, Xie G, Liang L, Liu H, Pan J, Cheng H, et al. RIPK3-Mediated Necroptosis and Neutrophil Infiltration Are Associated with Poor Prognosis in Patients with Alcoholic Cirrhosis. J Immunol Res. 2018;2018:1509851. doi: 10.1155/2018/1509851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Miyata T, Wu X, Fan X, Huang E, Sanz-Garcia C, Ross CKC, et al. Differential role of MLKL in alcohol-associated and non-alcohol-associated fatty liver diseases in mice and humans. JCI Insight. 2021;6(4):140180. doi: 10.1172/jci.insight.140180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Roychowdhury S, McMullen MR, Pisano SG, Liu X, Nagy LE. Absence of receptor interacting protein kinase 3 prevents ethanol-induced liver injury. Hepatology. 2013;57(5):1773–1783. doi: 10.1002/hep.26200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang S, Ni HM, Dorko K, Kumer SC, Schmitt TM, Nawabi A, et al. Increased hepatic receptor interacting protein kinase 3 expression due to impaired proteasomal functions contributes to alcohol-induced steatosis and liver injury. Oncotarget. 2016;7(14):17681–17698. doi: 10.18632/oncotarget.6893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. J Pathol. 2010;221(1):3–12. doi: 10.1002/path.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nazio F, Bordi M, Cianfanelli V, Locatelli F, Cecconi F. Autophagy and cancer stem cells: molecular mechanisms and therapeutic applications. Cell Death Differ. 2019;26(4):690–702. doi: 10.1038/s41418-019-0292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shimizu S, Kanaseki T, Mizushima N, Mizuta T, Arakawa-Kobayashi S, Thompson CB, et al. Role of Bcl-2 family proteins in a non-apoptotic programmed cell death dependent on autophagy genes. Nat Cell Biol. 2004;6(12):1221–1228. doi: 10.1038/ncb1192. [DOI] [PubMed] [Google Scholar]
- 84.Arakawa S, Tsujioka M, Yoshida T, Tajima-Sakurai H, Nishida Y, Matsuoka Y, et al. Role of Atg5-dependent cell death in the embryonic development of Bax/Bak double-knockout mice. Cell Death Differ. 2017;24(9):1598–1608. doi: 10.1038/cdd.2017.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Denton D, Nicolson S, Kumar S. Cell death by autophagy: facts and apparent artefacts. Cell Death Differ. 2012;19(1):87–95. doi: 10.1038/cdd.2011.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ueno T, Komatsu M. Autophagy in the liver: functions in health and disease. Nat Rev Gastroenterol Hepatol. 2017;14(3):170–184. doi: 10.1038/nrgastro.2016.185. [DOI] [PubMed] [Google Scholar]
- 87.Ding WX, Li M, Chen X, Ni HM, Lin CW, Gao W, et al. Autophagy reduces acute ethanol-induced hepatotoxicity and steatosis in mice. Gastroenterology. 2010;139(5):1740–1752. doi: 10.1053/j.gastro.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lin CW, Zhang H, Li M, Xiong X, Chen X, Chen X, et al. Pharmacological promotion of autophagy alleviates steatosis and injury in alcoholic and non-alcoholic fatty liver conditions in mice. J Hepatol. 2013;58(5):993–999. doi: 10.1016/j.jhep.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Khambu B, Wang L, Zhang H, Yin XM. The Activation and Function of Autophagy in Alcoholic Liver Disease. Curr Mol Pharmacol. 2017;10(3):165–171. doi: 10.2174/1874467208666150817112654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dolganiuc A, Thomes PG, Ding WX, Lemasters JJ, Donohue TM., Jr Autophagy in alcohol-induced liver diseases. Alcohol Clin Exp Res. 2012;36(8):1301–1308. doi: 10.1111/j.1530-0277.2012.01742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Eid N, Ito Y, Maemura K, Otsuki Y. Elevated autophagic sequestration of mitochondria and lipid droplets in steatotic hepatocytes of chronic ethanol-treated rats: an immunohistochemical and electron microscopic study. J Mol Histol. 2013;44(3):311–326. doi: 10.1007/s10735-013-9483-x. [DOI] [PubMed] [Google Scholar]
- 92.Li H, Wang P, Sun Q, Ding WX, Yin XM, Sobol RW, et al. Following cytochrome c release, autophagy is inhibited during chemotherapy-induced apoptosis by caspase 8-mediated cleavage of Beclin 1. Cancer Res. 2011;71(10):3625–3634. doi: 10.1158/0008-5472.Can-10-4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Komatsu M, Waguri S, Ueno T, Iwata J, Murata S, Tanida I, et al. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J Cell Biol. 2005;169(3):425–434. doi: 10.1083/jcb.200412022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Noureddin M, Yates KP, Vaughn IA, Neuschwander-Tetri BA, Sanyal AJ, McCullough A, et al. Clinical and histological determinants of nonalcoholic steatohepatitis and advanced fibrosis in elderly patients. Hepatology. 2013;58(5):1644–1654. doi: 10.1002/hep.26465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fontana L, Zhao E, Amir M, Dong H, Tanaka K, Czaja MJ. Aging promotes the development of diet-induced murine steatohepatitis but not steatosis. Hepatology. 2013;57(3):995–1004. doi: 10.1002/hep.26099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tanaka S, Hikita H, Tatsumi T, Sakamori R, Nozaki Y, Sakane S, et al. Rubicon inhibits autophagy and accelerates hepatocyte apoptosis and lipid accumulation in nonalcoholic fatty liver disease in mice. Hepatology. 2016;64(6):1994–2014. doi: 10.1002/hep.28820. [DOI] [PubMed] [Google Scholar]
- 97.Singh R, Wang Y, Xiang Y, Tanaka KE, Gaarde WA, Czaja MJ. Differential effects of JNK1 and JNK2 inhibition on murine steatohepatitis and insulin resistance. Hepatology. 2009;49(1):87–96. doi: 10.1002/hep.22578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lin HZ, Yang SQ, Chuckaree C, Kuhajda F, Ronnet G, Diehl AM. Metformin reverses fatty liver disease in obese, leptin-deficient mice. Nat Med. 2000;6(9):998–1003. doi: 10.1038/79697. [DOI] [PubMed] [Google Scholar]
- 99.Feldstein AE, Canbay A, Guicciardi ME, Higuchi H, Bronk SF, Gores GJ. Diet associated hepatic steatosis sensitizes to Fas mediated liver injury in mice. J Hepatol. 2003;39(6):978–983. doi: 10.1016/s0168-8278(03)00460-4. [DOI] [PubMed] [Google Scholar]
- 100.Amir M, Zhao E, Fontana L, Rosenberg H, Tanaka K, Gao G, et al. Inhibition of hepatocyte autophagy increases tumor necrosis factor-dependent liver injury by promoting caspase-8 activation. Cell Death Differ. 2013;20(7):878–887. doi: 10.1038/cdd.2013.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu L, Liao JZ, He XX, Li PY. The role of autophagy in hepatocellular carcinoma: friend or foe. Oncotarget. 2017;8(34):57707–57722. doi: 10.18632/oncotarget.17202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu K, Lee J, Ou JJ. Autophagy and mitophagy in hepatocarcinogenesis. Mol Cell Oncol. 2018;5(2):e1405142. doi: 10.1080/23723556.2017.1405142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sun K, Guo XL, Zhao QD, Jing YY, Kou XR, Xie XQ, et al. Paradoxical role of autophagy in the dysplastic and tumor-forming stages of hepatocarcinoma development in rats. Cell Death Dis. 2013;4(2):e501. doi: 10.1038/cddis.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ, et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell. 2017;171(2):273–285. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Xie Y, Hou W, Song X, Yu Y, Huang J, Sun X, et al. Ferroptosis: process and function. Cell Death Differ. 2016;23(3):369–379. doi: 10.1038/cdd.2015.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mao C, Liu X, Zhang Y, Lei G, Yan Y, Lee H, et al. DHODH-mediated ferroptosis defence is a targetable vulnerability in cancer. Nature. 2021;593(7860):586–590. doi: 10.1038/s41586-021-03539-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zheng J, Conrad M. The Metabolic Underpinnings of Ferroptosis. Cell Metab. 2020;32(6):920–937. doi: 10.1016/j.cmet.2020.10.011. [DOI] [PubMed] [Google Scholar]
- 108.Shi Z, Naowarojna N, Pan Z, Zou Y. Multifaceted mechanisms mediating cystine starvation-induced ferroptosis. Nat Commun. 2021;12(1):4792. doi: 10.1038/s41467-021-25159-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Forcina GC, Dixon SJ. GPX4 at the Crossroads of Lipid Homeostasis and Ferroptosis. Proteomics. 2019;19(18):e1800311. doi: 10.1002/pmic.201800311. [DOI] [PubMed] [Google Scholar]
- 111.Conrad M, Friedmann Angeli JP. Glutathione peroxidase 4 (Gpx4) and ferroptosis: what’s so special about it? Mol Cell Oncol. 2015;2(3):e995047. doi: 10.4161/23723556.2014.995047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liu J, Kang R, Tang D. Signaling pathways and defense mechanisms of ferroptosis. FEBS J. 2022;289(22):7038–7050. doi: 10.1111/febs.16059. [DOI] [PubMed] [Google Scholar]
- 113.Mueller S, Rausch V. The role of iron in alcohol-mediated hepatocarcinogenesis. Adv Exp Med Biol. 2015;815:89–112. doi: 10.1007/978-3-319-09614-8_6. [DOI] [PubMed] [Google Scholar]
- 114.Liu CY, Wang M, Yu HM, Han FX, Wu QS, Cai XJ, et al. Ferroptosis is involved in alcohol-induced cell death in vivo and in vitro. Biosci Biotechnol Biochem. 2020;84(8):1621–1628. doi: 10.1080/09168451.2020.1763155. [DOI] [PubMed] [Google Scholar]
- 115.Zhang Y, Zhao S, Fu Y, Yan L, Feng Y, Chen Y, et al. Computational repositioning of dimethyl fumarate for treating alcoholic liver disease. Cell Death Dis. 2020;11(8):641. doi: 10.1038/s41419-020-02890-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li X, Wang TX, Huang X, Li Y, Sun T, Zang S, et al. Targeting ferroptosis alleviates methionine-choline deficient (MCD)-diet induced NASH by suppressing liver lipotoxicity. Liver Int. 2020;40(6):1378–1394. doi: 10.1111/liv.14428. [DOI] [PubMed] [Google Scholar]
- 117.Carlson BA, Tobe R, Yefremova E, Tsuji PA, Hoffmann VJ, Schweizer U, et al. Glutathione peroxidase 4 and vitamin E cooperatively prevent hepatocellular degeneration. Redox Biol. 2016;9:22–31. doi: 10.1016/j.redox.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Qi J, Kim JW, Zhou Z, Lim CW, Kim B. Ferroptosis Affects the Progression of Nonalcoholic Steatohepatitis via the Modulation of Lipid Peroxidation-Mediated Cell Death in Mice. Am J Pathol. 2020;190(1):68–81. doi: 10.1016/j.ajpath.2019.09.011. [DOI] [PubMed] [Google Scholar]
- 119.Gao G, Xie Z, Li EW, Yuan Y, Fu Y, Wang P, et al. Dehydroabietic acid improves nonalcoholic fatty liver disease through activating the Keap1/Nrf2-ARE signaling pathway to reduce ferroptosis. J Nat Med. 2021;75(3):540–552. doi: 10.1007/s11418-021-01491-4. [DOI] [PubMed] [Google Scholar]
- 120.Yang Y, Chen J, Gao Q, Shan X, Wang J, Lv Z. Study on the attenuated effect of Ginkgolide B on ferroptosis in high fat diet induced nonalcoholic fatty liver disease. Toxicology. 2020;445:152599. doi: 10.1016/j.tox.2020.152599. [DOI] [PubMed] [Google Scholar]
- 121.Yamada N, Karasawa T, Kimura H, Watanabe S, Komada T, Kamata R, et al. Ferroptosis driven by radical oxidation of n-6 polyunsaturated fatty acids mediates acetaminophen-induced acute liver failure. Cell Death Dis. 2020;11(2):144. doi: 10.1038/s41419-020-2334-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Niu B, Lei X, Xu Q, Ju Y, Xu D, Mao L, et al. Protecting mitochondria via inhibiting VDAC1 oligomerization alleviates ferroptosis in acetaminophen-induced acute liver injury. Cell Biol Toxicol. 2022;38(3):505–530. doi: 10.1007/s10565-021-09624-x. [DOI] [PubMed] [Google Scholar]
- 123.Pan Q, Luo Y, Xia Q, He K. Ferroptosis and Liver Fibrosis. Int J Med Sci. 2021;18(15):3361–3366. doi: 10.7150/ijms.62903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kuo CY, Chiu V, Hsieh PC, Huang CY, Huang SJ, Tzeng IS, et al. Chrysophanol attenuates hepatitis B virus X protein-induced hepatic stellate cell fibrosis by regulating endoplasmic reticulum stress and ferroptosis. J Pharmacol Sci. 2020;144(3):172–182. doi: 10.1016/j.jphs.2020.07.014. [DOI] [PubMed] [Google Scholar]
- 125.Zhang Z, Guo M, Li Y, Shen M, Kong D, Shao J, et al. RNA-binding protein ZFP36/TTP protects against ferroptosis by regulating autophagy signaling pathway in hepatic stellate cells. Autophagy. 2020;16(8):1482–1505. doi: 10.1080/15548627.2019.1687985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sui M, Jiang X, Chen J, Yang H, Zhu Y. Magnesium isoglycyrrhizinate ameliorates liver fibrosis and hepatic stellate cell activation by regulating ferroptosis signaling pathway. Biomed Pharmacother. 2018;106:125–133. doi: 10.1016/j.biopha.2018.06.060. [DOI] [PubMed] [Google Scholar]
- 127.Dixon SJ, Stockwell BR. The Hallmarks of Ferroptosis. Annu Rev Cancer Biol. 2019;3(1):35–54. doi: 10.1146/annurev-cancerbio-030518-055844. [DOI] [Google Scholar]
- 128.Wang L, Zhang Z, Li M, Wang F, Jia Y, Zhang F, et al. P53-dependent induction of ferroptosis is required for artemether to alleviate carbon tetrachloride-induced liver fibrosis and hepatic stellate cell activation. IUBMB Life. 2019;71(1):45–56. doi: 10.1002/iub.1895. [DOI] [PubMed] [Google Scholar]
- 129.Yu Y, Jiang L, Wang H, Shen Z, Cheng Q, Zhang P, et al. Hepatic transferrin plays a role in systemic iron homeostasis and liver ferroptosis. Blood. 2020;136(6):726–739. doi: 10.1182/blood.2019002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Liao H, Shi J, Wen K, Lin J, Liu Q, Shi B, et al. Molecular Targets of Ferroptosis in Hepatocellular Carcinoma. J Hepatocell Carcinoma. 2021;8:985–996. doi: 10.2147/jhc.S325593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lim K, Han C, Dai Y, Shen M, Wu T. Omega-3 polyunsaturated fatty acids inhibit hepatocellular carcinoma cell growth through blocking beta-catenin and cyclooxygenase-2. Mol Cancer Ther. 2009;8(11):3046–3055. doi: 10.1158/1535-7163.Mct-09-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Guerriero E, Capone F, Accardo M, Sorice A, Costantini M, Colonna G, et al. GPX4 and GPX7 over-expression in human hepatocellular carcinoma tissues. Eur J Histochem. 2015;59(4):2540. doi: 10.4081/ejh.2015.2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Orning P, Weng D, Starheim K, Ratner D, Best Z, Lee B, et al. Pathogen blockade of TAK1 triggers caspase-8-dependent cleavage of gasdermin D and cell death. Science. 2018;362(6418):1064–1069. doi: 10.1126/science.aau2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wu J, Lin S, Wan B, Velani B, Zhu Y. Pyroptosis in Liver Disease: New Insights into Disease Mechanisms. Aging Dis. 2019;10(5):1094–1108. doi: 10.14336/ad.2019.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lu L, Zhang Y, Tan X, Merkher Y, Leonov S, Zhu L, et al. Emerging mechanisms of pyroptosis and its therapeutic strategy in cancer. Cell Death Discov. 2022;8(1):338. doi: 10.1038/s41420-022-01101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fink SL, Cookson BT. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell Microbiol. 2006;8(11):1812–1825. doi: 10.1111/j.1462-5822.2006.00751.x. [DOI] [PubMed] [Google Scholar]
- 137.Hagar JA, Powell DA, Aachoui Y, Ernst RK, Miao EA. Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science. 2013;341(6151):1250–1253. doi: 10.1126/science.1240988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Beier JI, Banales JM. Pyroptosis: An inflammatory link between NAFLD and NASH with potential therapeutic implications. J Hepatol. 2018;68(4):643–645. doi: 10.1016/j.jhep.2018.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wree A, McGeough MD, Inzaugarat ME, Eguchi A, Schuster S, Johnson CD, et al. NLRP3 inflammasome driven liver injury and fibrosis: Roles of IL-17 and TNF in mice. Hepatology. 2018;67(2):736–749. doi: 10.1002/hep.29523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Manigold T, Böcker U, Chen J, Gundt J, Traber P, Singer MV, et al. Hepatitis B core antigen is a potent inductor of interleukin-18 in peripheral blood mononuclear cells of healthy controls and patients with hepatitis B infection. J Med Virol. 2003;71(1):31–40. doi: 10.1002/jmv.10445. [DOI] [PubMed] [Google Scholar]
- 141.Yu X, Lan P, Hou X, Han Q, Lu N, Li T, et al. HBV inhibits LPS-induced NLRP3 inflammasome activation and IL-1β production via suppressing the NF-κB pathway and ROS production. J Hepatol. 2017;66(4):693–702. doi: 10.1016/j.jhep.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 142.Chattergoon MA, Latanich R, Quinn J, Winter ME, Buckheit RW, 3rd, Blankson JN, et al. HIV and HCV activate the inflammasome in monocytes and macrophages via endosomal Toll-like receptors without induction of type 1 interferon. PLoS Pathog. 2014;10(5):e1004082. doi: 10.1371/journal.ppat.1004082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Negash AA, Ramos HJ, Crochet N, Lau DT, Doehle B, Papic N, et al. IL-1β production through the NLRP3 inflammasome by hepatic macrophages links hepatitis C virus infection with liver inflammation and disease. PLoS Pathog. 2013;9(4):e1003330. doi: 10.1371/journal.ppat.1003330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Serti E, Werner JM, Chattergoon M, Cox AL, Lohmann V, Rehermann B. Monocytes activate natural killer cells via inflammasome-induced interleukin 18 in response to hepatitis C virus replication. Gastroenterology. 2014;147(1):209–220.e3. doi: 10.1053/j.gastro.2014.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Petrasek J, Bala S, Csak T, Lippai D, Kodys K, Menashy V, et al. IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J Clin Invest. 2012;122(10):3476–3489. doi: 10.1172/JCI60777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Heo MJ, Kim TH, You JS, Blaya D, Sancho-Bru P, Kim SG. Alcohol dysregulates miR-148a in hepatocytes through FoxO1, facilitating pyroptosis via TXNIP overexpression. Gut. 2019;68(4):708–720. doi: 10.1136/gutjnl-2017-315123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Khanova E, Wu R, Wang W, Yan R, Chen Y, French SW, et al. Pyroptosis by caspase11/4-gasdermin-D pathway in alcoholic hepatitis in mice and patients. Hepatology. 2018;67(5):1737–1753. doi: 10.1002/hep.29645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Xu B, Jiang M, Chu Y, Wang W, Chen D, Li X, et al. Gasdermin D plays a key role as a pyroptosis executor of non-alcoholic steatohepatitis in humans and mice. J Hepatol. 2018;68(4):773–782. doi: 10.1016/j.jhep.2017.11.040. [DOI] [PubMed] [Google Scholar]
- 149.Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T, et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482(7384):179–185. doi: 10.1038/nature10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Qiu T, Pei P, Yao X, Jiang L, Wei S, Wang Z, et al. Taurine attenuates arsenic-induced pyroptosis and nonalcoholic steatohepatitis by inhibiting the autophagic-inflammasomal pathway. Cell Death Dis. 2018;9(10):946. doi: 10.1038/s41419-018-1004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mridha AR, Wree A, Robertson AAB, Yeh MM, Johnson CD, Van Rooyen DM, et al. NLRP3 inflammasome blockade reduces liver inflammation and fibrosis in experimental NASH in mice. J Hepatol. 2017;66(5):1037–1046. doi: 10.1016/j.jhep.2017.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Qu J, Yuan Z, Wang G, Wang X, Li K. The selective NLRP3 inflammasome inhibitor MCC950 alleviates cholestatic liver injury and fibrosis in mice. Int Immunopharmacol. 2019;70:147–155. doi: 10.1016/j.intimp.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 153.He K, Zhu X, Liu Y, Miao C, Wang T, Li P, et al. Inhibition of NLRP3 inflammasome by thioredoxin-interacting protein in mouse Kupffer cells as a regulatory mechanism for non-alcoholic fatty liver disease development. Oncotarget. 2017;8(23):37657–37672. doi: 10.18632/oncotarget.17489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wree A, Mehal WZ, Feldstein AE. Targeting Cell Death and Sterile Inflammation Loop for the Treatment of Nonalcoholic Steatohepatitis. Semin Liver Dis. 2016;36(1):27–36. doi: 10.1055/s-0035-1571272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yaping Z, Ying W, Luqin D, Ning T, Xuemei A, Xixian Y. Mechanism of interleukin-1β-induced proliferation in rat hepatic stellate cells from different levels of signal transduction. APMIS. 2014;122(5):392–398. doi: 10.1111/apm.12155. [DOI] [PubMed] [Google Scholar]
- 156.Palacios-Macapagal D, Connor J, Mustelin T, Ramalingam TR, Wynn TA, Davidson TS. Cutting Edge: Eosinophils Undergo Caspase-1-Mediated Pyroptosis in Response to Necrotic Liver Cells. J Immunol. 2017;199(3):847–853. doi: 10.4049/jimmunol.1601162. [DOI] [PubMed] [Google Scholar]
- 157.Wree A, Eguchi A, McGeough MD, Pena CA, Johnson CD, Canbay A, et al. NLRP3 inflammasome activation results in hepatocyte pyroptosis, liver inflammation, and fibrosis in mice. Hepatology. 2014;59(3):898–910. doi: 10.1002/hep.26592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Thiam HR, Wong SL, Wagner DD, Waterman CM. Cellular Mechanisms of NETosis. Annu Rev Cell Dev Biol. 2020;36:191–218. doi: 10.1146/annurev-cellbio-020520-111016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Vorobjeva N, Galkin I, Pletjushkina O, Golyshev S, Zinovkin R, Prikhodko A, et al. Mitochondrial permeability transition pore is involved in oxidative burst and NETosis of human neutrophils. Biochim Biophys Acta Mol Basis Dis. 2020;1866(5):165664. doi: 10.1016/j.bbadis.2020.165664. [DOI] [PubMed] [Google Scholar]
- 160.Metzler KD, Goosmann C, Lubojemska A, Zychlinsky A, Papayannopoulos V. A myeloperoxidase-containing complex regulates neutrophil elastase release and actin dynamics during NETosis. Cell Rep. 2014;8(3):883–896. doi: 10.1016/j.celrep.2014.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hilscher MB, Shah VH. Neutrophil Extracellular Traps and Liver Disease. Semin Liver Dis. 2020;40(2):171–179. doi: 10.1055/s-0039-3399562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hu S, Liu X, Gao Y, Zhou R, Wei M, Dong J, et al. Hepatitis B Virus Inhibits Neutrophil Extracellular Trap Release by Modulating Reactive Oxygen Species Production and Autophagy. J Immunol. 2019;202(3):805–815. doi: 10.4049/jimmunol.1800871. [DOI] [PubMed] [Google Scholar]
- 163.Zhao X, Yang L, Chang N, Hou L, Zhou X, Yang L, et al. Neutrophils undergo switch of apoptosis to NETosis during murine fatty liver injury via S1P receptor 2 signaling. Cell Death Dis. 2020;11(5):379. doi: 10.1038/s41419-020-2582-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.van der Windt DJ, Sud V, Zhang H, Varley PR, Goswami J, Yazdani HO, et al. Neutrophil extracellular traps promote inflammation and development of hepatocellular carcinoma in nonalcoholic steatohepatitis. Hepatology. 2018;68(4):1347–1360. doi: 10.1002/hep.29914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kim BE, Nevitt T, Thiele DJ. Mechanisms for copper acquisition, distribution and regulation. Nat Chem Biol. 2008;4(3):176–185. doi: 10.1038/nchembio.72. [DOI] [PubMed] [Google Scholar]
- 166.Rae TD, Schmidt PJ, Pufahl RA, Culotta VC, O’Halloran TV. Undetectable intracellular free copper: the requirement of a copper chaperone for superoxide dismutase. Science. 1999;284(5415):805–808. doi: 10.1126/science.284.5415.805. [DOI] [PubMed] [Google Scholar]
- 167.Tang D, Chen X, Kroemer G. Cuproptosis: a copper-triggered modality of mitochondrial cell death. Cell Res. 2022;32(5):417–418. doi: 10.1038/s41422-022-00653-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Tsvetkov P, Coy S, Petrova B, Dreishpoon M, Verma A, Abdusamad M, et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science. 2022;375(6586):1254–1261. doi: 10.1126/science.abf0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Li SR, Bu LL, Cai L. Cuproptosis: lipoylated TCA cycle proteins-mediated novel cell death pathway. Signal Transduct Target Ther. 2022;7(1):158. doi: 10.1038/s41392-022-01014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Jones JG. Hepatic glucose and lipid metabolism. Diabetologia. 2016;59(6):1098–1103. doi: 10.1007/s00125-016-3940-5. [DOI] [PubMed] [Google Scholar]
- 171.Lutsenko S. Human copper homeostasis: a network of interconnected pathways. Curr Opin Chem Biol. 2010;14(2):211–217. doi: 10.1016/j.cbpa.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kim H, Son HY, Bailey SM, Lee J. Deletion of hepatic Ctr1 reveals its function in copper acquisition and compensatory mechanisms for copper homeostasis. Am J Physiol Gastrointest Liver Physiol. 2009;296(2):G356–G364. doi: 10.1152/ajpgi.90632.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Blades B, Ayton S, Hung YH, Bush AI, La Fontaine S. Copper and lipid metabolism: A reciprocal relationship. Biochim Biophys Acta Gen Subj. 2021;1865(11):129979. doi: 10.1016/j.bbagen.2021.129979. [DOI] [PubMed] [Google Scholar]
- 174.Wu C, Liu X, Zhong L, Zhou Y, Long L, Yi T, et al. Identification of Cuproptosis-Related Genes in Nonalcoholic Fatty Liver Disease. Oxid Med Cell Longev. 2023;2023:9245667. doi: 10.1155/2023/9245667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Shiffman ML, Pockros P, McHutchison JG, Schiff ER, Morris M, Burgess G. Clinical trial: the efficacy and safety of oral PF-03491390, a pancaspase inhibitor - a randomized placebo-controlled study in patients with chronic hepatitis C. Aliment Pharmacol Ther. 2010;31(9):969–978. doi: 10.1111/j.1365-2036.2010.04264.x. [DOI] [PubMed] [Google Scholar]
- 176.Ratziu V, Sheikh MY, Sanyal AJ, Lim JK, Conjeevaram H, Chalasani N, et al. A phase 2, randomized, double-blind, placebo-controlled study of GS-9450 in subjects with nonalcoholic steatohepatitis. Hepatology. 2012;55(2):419–428. doi: 10.1002/hep.24747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Cazanave SC, Mott JL, Elmi NA, Bronk SF, Masuoka HC, Charlton MR, et al. A role for miR-296 in the regulation of lipoapoptosis by targeting PUMA. J Lipid Res. 2011;52(8):1517–1525. doi: 10.1194/jlr.M014654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Manns MP, Wedemeyer H, Singer A, Khomutjanskaja N, Dienes HP, Roskams T, et al. Glycyrrhizin in patients who failed previous interferon alpha-based therapies: biochemical and histological effects after 52 weeks. J Viral Hepat. 2012;19(8):537–546. doi: 10.1111/j.1365-2893.2011.01579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Jia M, Zhang H, Qin Q, Hou Y, Zhang X, Chen D, et al. Ferroptosis as a new therapeutic opportunity for nonviral liver disease. Eur J Pharmacol. 2021;908:174319. doi: 10.1016/j.ejphar.2021.174319. [DOI] [PubMed] [Google Scholar]
- 180.Xu FL, Wu XH, Chen C, Wang K, Huang LY, Xia J, et al. SLC27A5 promotes sorafenib-induced ferroptosis in hepatocellular carcinoma by downregulating glutathione reductase. Cell Death Dis. 2023;14(1):22. doi: 10.1038/s41419-023-05558-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lebeaupin C, Proics E, de Bieville CH, Rousseau D, Bonnafous S, Patouraux S, et al. ER stress induces NLRP3 inflammasome activation and hepatocyte death. Cell Death Dis. 2015;6(9):e1879. doi: 10.1038/cddis.2015.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Pockros PJ, Schiff ER, Shiffman ML, McHutchison JG, Gish RG, Afdhal NH, et al. Oral IDN-6556, an antiapoptotic caspase inhibitor, may lower aminotransferase activity in patients with chronic hepatitis C. Hepatology. 2007;46(2):324–329. doi: 10.1002/hep.21664. [DOI] [PubMed] [Google Scholar]