Abstract
Background and Aims
To validate prognostic performance of the China liver cancer (CNLC) staging system as well as to compare these parameters with those of the Barcelona Clinic Liver Cancer (BCLC) staging system for Chinese hepatocellular carcinoma (HCC) treated with transarterial chemoembolization (TACE).
Methods
This multicenter retrospective study included 1,124 patients with HCC between January 2012 and December 2020 from six Chinese hospitals. Based on overall survival (OS), the prognostic performance outcomes for the CNLC and BCLC staging systems were compared by model discrimination [C statistic and Akaike information criterion (AIC)], monotonicity of the gradient (linear trend chi-square test), homogeneity (likelihood ratio chi-square test), and calibration (calibration plots). A prospective cohort of 44 patients receiving TACE-based therapy included between January 2021 and December 2022 was used to prospectively validate the outcomes.
Results
Median OS was 19.1 (18.2–20.0) months, with significant differences in OS between stages defined by the CNLC and BCLC observed (p<0.001). The CNLC performed better than the BCLC regarding model discrimination (C-index: 0.661 vs. 0.644; AIC: 10,583.28 vs. 10,583.72), model monotonicity of the gradient (linear trend chi-square test: 66.107 vs. 57.418; p<0.001), model homogeneity (159.2 vs. 158.7; p<0.001). Both staging systems had good model calibration. Similar results were observed in the prospective cohort.
Conclusions
Combining model discrimination, gradient monotonicity, homogeneity, and calibration, the CNLC performed better than the BCLC for Chinese HCC patients receiving TACE.
Keywords: Hepatocellular carcinoma, CNLC, BCLC, TACE
Graphical abstract
Introduction
An ideal staging system for patients with malignancy should provide accurate treatment guidelines and prognostic information and stratify patients to different prognostic groups.1 Establishing a worldwide-adopted staging system in hepatocellular carcinoma (HCC) has been a challenge owing to etiologic and clinical heterogeneity of patients around the world. Staging systems for HCC differ from those of other solid tumors, given that the prognosis is influenced by two diseases, underlying liver disease and liver cancer.2,3 Tumor growth and underlying liver disease worsen liver function and impact prognosis directly. The prognosis of HCC is also influenced by the patient’s personal status.4
Several HCC staging systems have been established in the past 30 years, among which the Barcelona Clinic Liver Cancer (BCLC) staging system is currently the most widely applied around the world.5–7 The BCLC staging system was initially established in 1999 and was the only staging system providing both prognostic classification and treatment recommendations for more than 10 years.8 Notably, the BCLC staging system was based on HCC cohort in Europe, where alcoholic steatohepatitis (ASH) and hepatitis C virus (HCV) are the major causes of HCC.8,9 It is uncertain whether the BCLC staging system is still suitable with high accuracy in China, where hepatitis B virus (HBV) is the major cause of HCC.10,11 The China liver cancer (CNLC) staging system was established with the aim to combine global evidence and patient characteristics in China.12 It is now widely applied for staging and treatment recommendations for Chinese HCC in clinical practice.13 Similar to the BCLC, the CNLC incorporates tumor stage, liver function, and performance status. The predictive ability of the CNLC staging system has yet to be validated externally.
Approximately 80% of patients are initially diagnosed with an intermediate-to-advanced stage because of the insidious onset of HCC.14 For these patients, transarterial chemoembolization (TACE) is the most widely recommended and applied approach in clinical practice.15,16 To date, limited evidence has been reported to validate and compare prognostic performance between the CNLC and BCLC staging systems for HCCs treated with TACE. The purpose of this study was to validate the prognostic performance of the CNLC staging system and to compare it with that of the BCLC staging system for Chinese treatment-naïve HCC receiving TACE.
Methods
With the approval by the institutional review board of the hospital, the multicenter retrospective study was performed in accordance with the Declaration of Helsinki. The requirement for informed consent was waived because of the retrospective nature of the study. Patients with treatment-naïve HCC treated with TACE from January 2012 to December 2020 at six participating hospitals were screened. The diagnosis of HCC was based on the CNLC diagnostic criteria.12 Multidisciplinary discussion was carried out prior to treatment to determine whether TACE should be recommended as the best treatment method for the patient. Advantages and disadvantages of the TACE treatment, including treatment-related morbidities, potential treatment outcomes, and costs, were explained to the patient. The final decision on the treatment choice was made by the patients or their relatives.
The inclusion criteria were: (1) confirmed diagnosis of HCC with no prior HCC-related treatment and were unresectable or unwilling to receive curative treatment; (2) Eastern Cooperative Oncology Group (ECOG) performance score of 0 or 1; (3) adequate liver function [Child-Turcotte-Pugh (CTP) grade A or B]; and (4) adequate renal, hematologic, and clotting function. The exclusion criteria were: (1) a contraindication to TACE; or (2) incomplete or missing follow-up data. To further validate outcomes of the study, the study prospectively included patients treated with TACE-based therapy between January 2021 and December 2022 at the leading participating hospital.
The study included patients with HCC who were unresectable or unwilling to receive curative treatment and initially treated with TACE. In other words, all the stages except for the terminal stage (CNLC stage IV and BCLC stage D) were screened for inclusion in the study. Intermediate-stage HCC referred to CNLC stage IIa/IIb and BCLC stage B, respectively. Intermediate stage in CNLC staging system is defined as HCC with a ECOG performance score of 0 to 2, Child-Pugh A/B liver function, 2 to 3 tumors with a maximum diameter >3 cm (IIa) or ≥4 tumors regardless of tumor diameter (IIb), and absence of vascular invasion or extrahepatic metastasis. Intermediate stage in the BCLC staging system is defined as multifocal HCC (>3 lesions and/or a diameter of the largest one >3 cm), with preserved liver function, no cancer-related symptoms (ECOG performance score of 0) and no vascular invasion or extrahepatic spread.
TACE procedure
All patients received either conventional (c)TACE or TACE with drug-eluting beads (DEBs). Details of the TACE procedure have been described previously.17 In brief, superselective chemoembolization was routinely applied. cTACE was performed with doxorubicin (20–40 mg/m2) and/or oxaliplatin (85 mg/m2) mixed with ethiodized oil (2–20 mL Lipiodol Ultra Fluid) in a 1:3 ratio. Then, injection of bead agent (gelatin foam particles, polyvinyl alcohol particles, or Embosphere microspheres) was performed. In the DEB-TACE procedure, epirubicin was used. The dose mainly depended on the intrahepatic tumor burden and hepatic reserve, with a maximum of 100 mg. All TACE procedures were performed by experienced interventional physicians with more than 10 years of experience in interventional oncology. Repeated TACE was assessed according to “on demand” mode. Patients were followed-up at 8–12 weeks by contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI). Evaluation for repeated TACE was performed if contrast-enhanced CT/MRI presented progression of the treated lesion(s) or new lesions for the patient.18 Choice of subsequent treatment after TACE was mainly based on the intrahepatic tumor burden and extrahepatic spread, and included other local-reginal therapies (hepatic arterial infusion chemotherapy, ablation, or 125I seed implantation), systemic therapies or combination therapy.19,20 Patients did not have diet and lifestyle restrictions, but avoiding smoking, alcohol intake, and increase of the body mass index were recommended.
Data assessment and statistical analysis
Patients were staged using the CNLC staging system and the BCLC staging system. The primary outcome of the study was overall survival (OS), which was calculated from the initial TACE to death from any cause or the last follow-up (May 31, 2022 for the retrospective cohort and April 30, 2023 for the prospective cohort). Model discrimination, monotonicity of the gradient, homogeneity, and calibration were assessed to validate and compare the performance outcomes of the CNLC and BCLC staging systems.21,22 Model discrimination associated with OS was measured with the Harrell C statistic, a rank-order statistic that relates to the area under curve (AUC).23 In addition, 6-, 12-, and 24-month AUC estimations were calculated. The Akaike information criterion (AIC) calculated from the Cox proportional hazards model was used to further confirm model discrimination. The model monotonicity of the gradient was compared using the linear trend chi-square test.21 Model homogeneity was compared using the likelihood ratio chi-square test.21 Model calibration was validated using calibration plots (R2) of the validation cohort versus the original predictions.24 Discrimination represents the spread ability of the model.21 The monotonicity of the gradient represents the consistency of worsening patient survival with worsening stages, while homogeneity represents similarity in patient survival within a given stage.21 Calibration represented agreement between observed outcomes and predictions.24 A high C statistic, linear trend and likelihood chi-square values. and low AIC values were associated with better staging system performance. Secondary outcomes included modified Response Evaluation Criteria In Solid Tumor (mRECIST)-based progression-free survival (PFS) and objective response rate (ORR). PFS was defined as time from initiation of TACE to first tumor progression or death from any cause. ORR was defined as percentage of HCCs with confirmed complete or partial response.
Clinical parameters were reported as frequencies and percentages for categorical variables and means with standard deviations or medians and 95% confidence intervals (CIs) for continuous variables. Baseline characteristics of the six hospitals were compared using Fisher’s exact test or the chi-square test for categorical variables and t-test for continuous variables. OS was calculated by the Kaplan–Meier method and survival curves were compared with log-rank tests. Statistical significance was considered if the p-value was ≤0.05. Univariate analysis for OS was performed and variables with p≤0.05 were considered as strong risk factors and were then included in multivariate Cox proportional hazards analysis. Variables with p≤0.05 in the multivariate Cox proportional hazards analysis were considered as independent risk factors associated with OS. The statistical analysis was performed with SPSS version 21.0 for Windows (IBM Corp., Armonk, NY, USA) and R language version 3.4.3 for Windows (R Package for Statistical Computing; www.r-project.org).
Results
Patient characteristics
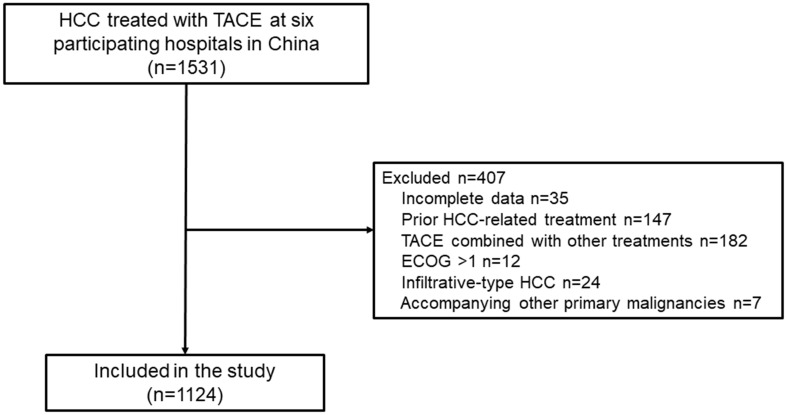
The study screened 1,531 patients with HCC and initially treated with TACE monotherapy, and 1,124 patients were included for final analyses (Fig. 1). The patient baseline characteristics in the participating hospitals are shown and compared in Table 1. In brief, the the patients had a mean age of 60 years old and the majority were men (n=938, 83.5%). The majority (763/1,124, 67.9%) of the included patients had HBV-related HCC, and their tumor burden and liver function were relatively higher than the other included patients. A total of 44 patients were included in the prospective cohort. Details summarized in the Supplementary File 1.
Fig. 1. Flowchart of patient selection.
ECOG, Eastern Cooperative Oncology Group; HCC, hepatocellular carcinoma; TACE, transarterial chemoembolization.
Table 1. Patient characteristics.
| Characteristic | Overall, n=1,124 | HBV cohort, n=763 | Others, n=361 | p-value* |
|---|---|---|---|---|
| Sex | 0.003 | |||
| Male | 938 (83.5%) | 654 (85.7%) | 284 (78.7%) | |
| Female | 186 (16.5%) | 109 (14.3%) | 77 (21.3%) | |
| Age in years | 60 (11) | 58 (11) | 65 (12) | <0.001 |
| ECOG | 0.01 | |||
| 0 | 1,042 (92.7%) | 718 (94.1%) | 324 (89.8%) | |
| 1 | 82 (7.3%) | 45 (5.9%) | 37 (10.2%) | |
| Child-Pugh grade | 0.11 | |||
| A | 1,024 (91.1%) | 688 (90.2%) | 336 (93.1%) | |
| B | 100 (8.9%) | 75 (9.8%) | 25 (6.9%) | |
| Tumor size in cm | 0.01 | |||
| ≤5 | 462 (41.1%) | 330 (43.3%) | 132 (36.5%) | |
| 5–10 | 399 (35.5%) | 259 (33.9%) | 140 (38.8%) | |
| >10 | 263 (23.4%) | 174 (22.8%) | 89 (24.7%) | |
| No. of nodules | 0.004 | |||
| 1 | 398 (35.4%) | 248 (32.5%) | 150 (41.5%) | |
| 2–3 | 335 (29.8%) | 247 (32.4%) | 88 (24.4%) | |
| ≥4 | 391 (34.8%) | 268 (35.1%) | 123 (34.1%) | |
| Cirrhosis | 620 (55.2%) | 449 (58.8%) | 171 (47.4%) | <0.001 |
| Macroscopic portal vein invasion | 0.41 | |||
| Absent | 917 (81.6%) | 617 (80.9%) | 300 (83.1%) | |
| Present | 207 (18.4%) | 146 (19.1%) | 61 (16.9%) | |
| BCLC | 0.006 | |||
| A | 369 (32.8%) | 228 (29.9%) | 141 (39.1%) | |
| B | 478 (42.5%) | 345 (45.2%) | 108 (36.8%) | |
| C | 277 (24.7%) | 190 (24.9%) | 154 (24.1%) | |
| CNLC | 0.02 | |||
| Ia | 155 (13.8%) | 94 (12.3%) | 61 (16.9%) | |
| Ib | 214 (19.0%) | 134 (17.6%) | 80 (22.2%) | |
| IIa | 204 (18.1%) | 149 (19.5%) | 55 (15.2%) | |
| IIb | 274 (24.4%) | 196 (25.7%) | 78 (21.6%) | |
| IIIa | 168 (15.0%) | 121 (15.9%) | 47 (13.0%) | |
| IIIb | 109 (9.7%) | 69 (9.0%) | 40 (11.1%) | |
| TACE Type | 0.02 | |||
| cTACE | 1,042 (92.7%) | 717 (94.0%) | 325 (90.0%) | |
| DEB-TACE | 82 (7.3%) | 46 (6.0%) | 36 (10.0%) | |
| AFP in ng/dL | 0.001 | |||
| ≤200 | 647 (57.6%) | 413 (54.1%) | 234 (64.8%) | |
| >200 | 477 (42.4%) | 350 (45.9%) | 127 (35.2%) | |
*Chi-square test or one-way analysis of variance. AFP, alpha fetoprotein; BCLC, Barcelona Clinic Liver Cancer; CNLC, China liver cancer; cTACE, conventional transarterial chemoembolization; DEB-TACE, drug-eluting beads transarterial chemoembolization; ECOG, Eastern Cooperative Oncology Group; TACE, transarterial chemoembolization.
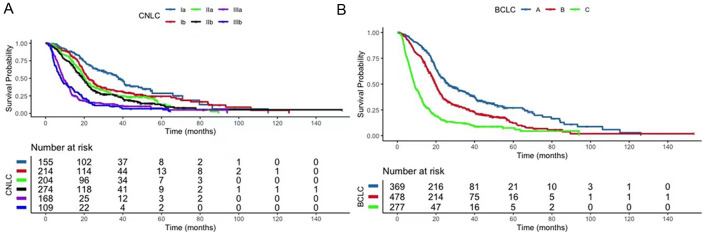
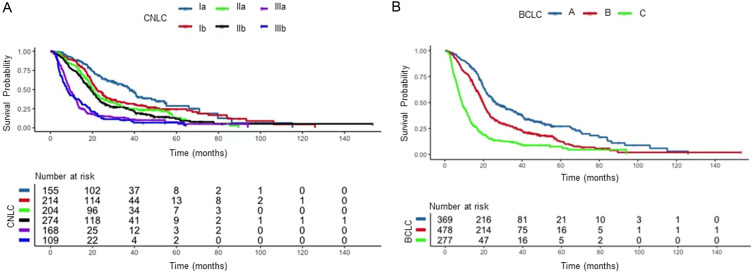
The median OS was 19.1 (18.2–20.0) months. Significant differences in OS between stages defined by the CNLC and BCLC were observed, without obvious overlap between substages in both the CNLC and BCLC staging systems (p<0.001; Fig. 2). For patients whose recommended treatment was curative according to the CNLC and BCLC, the median OS was shorter in the CNLC (n=573) than in the BCLC (n=369) [23.4 (21.3–25.5) months vs. 26.2 (22.4–30.1) months]. Notably, for patients whose recommended treatment was TACE according to the CNLC and systemic therapy according to the BCLC (n=168), the median OS was 9.1 (7.7–10.5) months, which was longer than in the entire BCLC C cohort [8.8 (7.8–9.9) months]. The median PFS was 8.9 (3.5–18.1) months and the ORR was 30.6%.
Fig. 2. Kaplan–Meier survival curves.
(A, B) Patients were stratified by the CNLC (A) and the BCLC (B) staging systems. BCLC, Barcelona Clinic Liver Cancer; CNLC, China liver cancer.
Prognostic validation and comparison
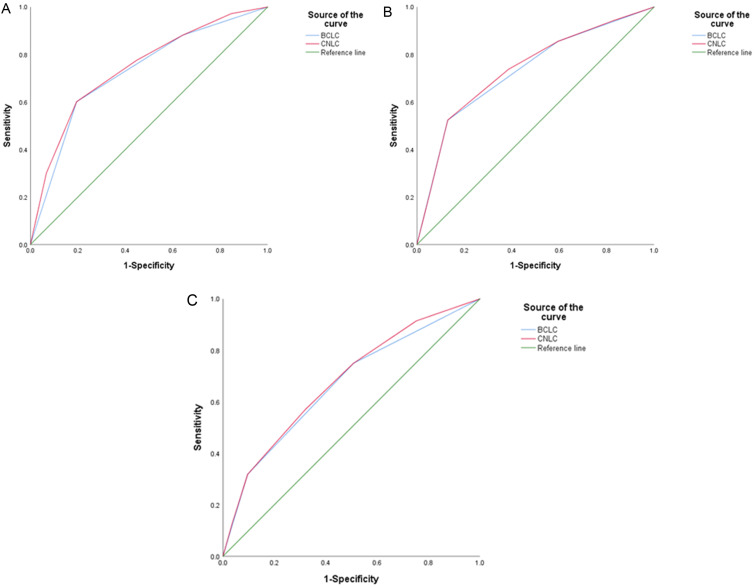
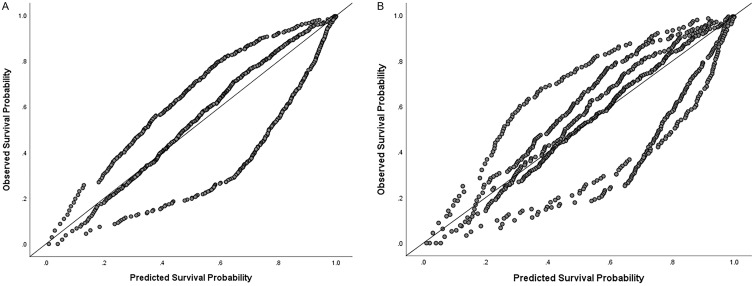
The prognostic performance outcomes of the CNLC and BCLC staging systems are shown in Table 2. The CNLC had better prognostic performance outcomes than the BCLC did. For model discrimination, the CNLC had a higher C-index (0.661 vs. 0.644) and a lower AIC (10,583.28 vs. 10,583.72) compared with the BCLC. In addition, the CNLC had a greater AUC estimate at 6, 12, and 24 months (Fig. 3). The AUCs at 6 months were 0.744 (0.699–0.788) for the CNLC and 0.727 (0.682–0.772) for the BCLC (p<0.001). The corresponding values were 0.738 (0.705–0.771) and 0.731 (0.697–0.764) at 12 months (p<0.001) and 0.679 (0.647–0.712) and 0.666 (0.633–0.699) at 24 months (p<0.001). For model monotonicity of the gradient, the CNLC had higher linear trends of chi-square tests than the BCLC (66.107 vs. 57.418; p<0.001). For model homogeneity, the CNLC had higher likelihood ratios of chi-square tests than the BCLC (159.2 vs. 158.7; p<0.001). For model calibration, both staging systems achieved matching of OS in the validation and original cohorts (Fig. 4). R2 was slightly better in the CNLC than in the BCLC (0.916 vs. 0.912). Similar outcomes were observed for the performance comparison between the CNLC and BCLC in the prospective cohort. Details are shown in the Supplementary File 1. After univariate analysis, six variables, the CNLC staging system, the BCLC staging system, Child-Pugh grade, serum alpha fetoprotein level, intrahepatic tumor number, and maximum intrahepatic tumor diameter, were identified as significant risk factors. Considering the overlapped influence between the CNLC and BCLC staging systems, multivariate analysis was performed separately for these two risk factors. For the CNLC-based multivariate analysis, the CNLC staging system, Child-Pugh grade, serum alpha fetoprotein level, and intrahepatic tumor number were identified as independent risk factors associated with OS (Supplementary Table 1). Similarly, for the BCLC-based multivariate analysis, the BCLC staging system, Child-Pugh grade, serum alpha fetoprotein level, and intrahepatic tumor number were identified as independent risk factors associated with OS (Supplementary Table 2).
Table 2. Prognostic performance of the CNLC and BCLC staging systems.
| Staging system | C-index (SD) | AIC | Linear trend | Likelihood ratio | R2 |
|---|---|---|---|---|---|
| CNLC | 0.661 (0.019) | 10,583.28 | 66.107 | 159.2 | 0.916 |
| BCLC | 0.644 (0.019) | 10,583.72 | 57.418 | 158.7 | 0.912 |
CNLC, China liver cancer; BCLC, Barcelona Clinic Liver Cancer; SD, standard deviation; AIC, Akaike information criterion.
Fig. 3. ROC curves of the CNLC and BCLC staging systems.
(A) AUC at 6 months (p<0.001). (B) AUC at 12 months (p<0.001). (C) AUC at 24 months (p<0.001). AUC, area under curve; BCLC, Barcelona Clinic Liver Cancer; CNLC, China liver cancer; ROC, receiver operating characteristic.
Fig. 4. Calibration plots.
(A, B) CNLC (A) and the BCLC (B) staging systems. BCLC, Barcelona Clinic Liver Cancer; CNLC, China liver cancer.
Discussion
The study evaluated the prognostic performance of the CNLC and BCLC staging systems in Chinese patients with HCC with TACE as the initial treatment. The CNLC performed better than the BCLC in terms of model discrimination, monotonicity of the gradient, homogeneity, and calibration. In addition, the CNLC staging system was able to select patients classified as BCLC stage C to receive aggressive treatment and achieve a better prognosis. Considering the promising future of TACE combined with immunotherapy and molecular targeted therapies for unresectable HCC, the CNLC system may have a significant impact on patients with HCC, especially on those with HBV-related HCC, for clinical decision and research design.
Considering that staging systems often perform better in the development cohort, external validation and comparison have an important role in assessing the prognostic performance of staging systems.25 Chinese and Western HCC cohorts have major differences in etiology and socioeconomics. Hence, the treatments recommended by the staging systems used in those cohorts are also different.8,12 The major differences between the CNLC and BCLC staging systems are as follows. First, the CNLC staging system was established with targeting specific to Chinese HCC, whereas the BCLC staging system was established mainly for use in Western HCC cohorts. The major etiology for Chinese HCC is chronic HBV infection, whereas it is HCV infection and alcohol intake for Western HCC.9 In addition, the socioeconomic difference between these two cohorts also warrants different HCC management strategies. Second, the CNLC staging system recommends radical therapies and TACE for more advanced HCC. Specifically, the CNLC staging system recommends TACE not only for intermediate HCC but also a first-line choice for patients with portal vein tumor thrombosis (PVTT), but the BCLC staging system recommends TACE just for intermediate HCC. PVTT has an important role in the staging and prognosis of HCC, because the occurrence of PVTT is closely associated with portal hypertension and its complications.26 Clear differences exist in the treatment of HCC with PVTT in the east and west.26 Physicians in west mainly follow the BCLC staging system, which recommends systemic therapies for HCC with PVTT. By contrast, physicians in east, including China prefer more aggressive and multidisciplinary therapies such as surgical resection, TACE, and systemic therapy for PVTT.27 The major advantages and differences of the CNLC compared with the BCLC for Chinese HCCs are as follows. First, compared with the BCLC, the CNLC caters more to the epidemiological characteristics of Chinese HCCs, as the major cause for Chinese HCC is HBV infection. Second, the CNLC recommends more aggressive treatment of patients with vascular invasion, which occurs more frequently in Chinese HCC. For patients with vascular invasion, the CNLC recommends TACE as a first-line choice, which more precisely targets the treated lesions and vascular invasion. The CNLC staging system broadens the application of TACE as the first-line choice for patients with vascular invasion and without extrahepatic metastasis for whom systemic therapies is the standard approach recommended by the BCLC staging system. Previous studies found that more than two-thirds of patients with HCC died because of intrahepatic tumor progression or liver failure rather than extrahepatic progression.28 Hence, TACE targeting intrahepatic lesion(s) should be recommended as feasible for those patients. The median OS of the CNLC stage IIIa patients in this study was longer than that of the entire BCLC C cohort. The superior benefit of the CNLC staging system was apparent for locally advanced HCC according to the BCLC staging system.
In this study, palliative treatment was recommended for 551 patients (49.0%) by the CNLC staging system and for 755 (67.2%) by the BCLC staging system. The median OS was shorter for the former cohort [13.6 (12.3–14.9) months] than for the latter cohort [15.8 (14.5–17.0) months], which indicates that the BCLC recommendation was conservative for unresectable HCC compared with the CNLC. Several previous studies reported that efficacy and the safety profile of DEB-TACE were superior to cTACE for patients with higher tumor burdens (i.e. maximum diameter ≥5 cm). Notably, no high-quality evidence has demonstrated that DEB-TACE was significantly superior to cTACE in those patients, and neither the BCLC nor the CNLC staging system recommends DEB-TACE for certain condition. Therefore, both cTACE and DEB-TACE are widely recommended and used including including for patients with high tumor burdens.
Several study limitations should be acknowledged. First, the retrospective nature of the study could lead to several biases. Further study in a prospective setting would be beneficial. Second, the prognostic performance of the CNLC and BCLC staging systems were validated and compared only for patients treated with TACE, which limited the applicability of the study conclusion. Prognostic performance of the staging systems for other treatments remains unknown. For example, for patients with PVTT and without extrahepatic spread, comparison of TACE following the CNLC system and systemic therapies following the BCLC system is needed to explore which staging system is more suitable for such patients. Further studies that include cohorts with different treatments are to be encouraged. Last but not least, the study consecutively included patients with HCC treated with TACE, without grouping CNLC-based and BCLC-based patients. The patients included in this study were staged by either the CNLC and the BCLC system and comparison of their prognostic performance found that the CNLC was a better choice. Further work on this topic should be carried out using this approach. In conclusion, combining model discrimination, gradient monotonicity, homogeneity, and calibration, found that the CNLC staging system outperformed the BCLC staging system regarding prognostic performance for Chinese treatment-naïve HCC patients receiving TACE.
Supporting information
Abbreviations
- AASLD
American Association for the Study of the Liver Diseases
- AIC
Akaike information criterion
- ASH
alcoholic steatohepatitis
- AUC
area under curve
- BCLC
Barcelona Clinic Liver Cancer
- CI
confidence interval
- CNLC
China liver cancer
- CT
computed tomography
- CTP
Child-Turcotte-Pugh
- DEB
drug-eluting bead
- EASL
European Association for the Study of the Liver
- ECOG
Eastern Cooperative Oncology Group
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- mRECIST
modified Response Evaluation Criteria In Solid Tumors
- MRI
magnetic resonance imaging
- ORR
objective response rate
- OS
overall survival
- PFS
progression-free survival
- PVTT
portal vein tumor thrombosis
- TACE
transarterial chemoembolization
Ethical statement
The study was approved by the institutional review board of the hospital and complied with applicable local laws and regulatory requirements. The requirement for informed consent was waived owing to the retrospective nature of the study.
Data sharing statement
No additional data are available.
References
- 1.Greene FL, Sobin LH. The staging of cancer: a retrospective and prospective appraisal. CA Cancer J Clin. 2008;58(3):180–190. doi: 10.3322/CA.2008.0001. [DOI] [PubMed] [Google Scholar]
- 2.Yang JD, Kim WR, Park KW, Chaiteerakij R, Kim B, Sanderson SO, et al. Model to estimate survival in ambulatory patients with hepatocellular carcinoma. Hepatology. 2012;56(2):614–621. doi: 10.1002/hep.25680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7(1):6. doi: 10.1038/s41572-020-00240-3. [DOI] [PubMed] [Google Scholar]
- 4.Liu PH, Hsu CY, Hsia CY, Lee YH, Huang YH, Su CW, et al. Proposal and validation of a new model to estimate survival for hepatocellular carcinoma patients. Eur J Cancer. 2016;63:25–33. doi: 10.1016/j.ejca.2016.04.023. [DOI] [PubMed] [Google Scholar]
- 5.Vogel A, Meyer T, Sapisochin G, Salem R, Saborowski A. Hepatocellular carcinoma. Lancet. 2022;400(10360):1345–1362. doi: 10.1016/S0140-6736(22)01200-4. [DOI] [PubMed] [Google Scholar]
- 6.Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723–750. doi: 10.1002/hep.29913. [DOI] [PubMed] [Google Scholar]
- 7.European Association for the Study of the Liver EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 8.Reig M, Forner A, Rimola J, Ferrer-Fabrega J, Burrel M, Garcia-Criado A, et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022;76(3):681–693. doi: 10.1016/j.jhep.2021.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rumgay H, Arnold M, Ferlay J, Lesi O, Cabasag CJ, Vignat J, et al. Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol. 2022;77(6):1598–1606. doi: 10.1016/j.jhep.2022.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 11.Zhou M, Wang H, Zeng X, Yin P, Zhu J, Chen W, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2019;394(10204):1145–1158. doi: 10.1016/S0140-6736(19)30427-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou J, Sun H, Wang Z, Cong W, Wang J, Zeng M, et al. Guidelines for the Diagnosis and Treatment of Hepatocellular Carcinoma (2019 Edition) Liver Cancer. 2020;9(6):682–720. doi: 10.1159/000509424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xie DY, Ren ZG, Zhou J, Fan J, Gao Q. 2019 Chinese clinical guidelines for the management of hepatocellular carcinoma: updates and insights. Hepatobiliary Surg Nutr. 2020;9(4):452–463. doi: 10.21037/hbsn-20-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Villanueva A. Hepatocellular Carcinoma. N Engl J Med. 2019;380(15):1450–1462. doi: 10.1056/NEJMra1713263. [DOI] [PubMed] [Google Scholar]
- 15.Lu J, Zhao M, Arai Y, Zhong BY, Zhu HD, Qi XL, et al. Clinical practice of transarterial chemoembolization for hepatocellular carcinoma: consensus statement from an international expert panel of International Society of Multidisciplinary Interventional Oncology (ISMIO) Hepatobiliary Surg Nutr. 2021;10(5):661–671. doi: 10.21037/hbsn-21-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park JW, Chen M, Colombo M, Roberts LR, Schwartz M, Chen PJ, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int. 2015;35(9):2155–2166. doi: 10.1111/liv.12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhong BY, Yan ZP, Sun JH, Zhang L, Hou ZH, Yang MJ, et al. Prognostic Performance of Albumin-Bilirubin Grade With Artificial Intelligence for Hepatocellular Carcinoma Treated With Transarterial Chemoembolization Combined With Sorafenib. Front Oncol. 2020;10:525461. doi: 10.3389/fonc.2020.525461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Terzi E, Golfieri R, Piscaglia F, Galassi M, Dazzi A, Leoni S, et al. Response rate and clinical outcome of HCC after first and repeated cTACE performed “on demand”. J Hepatol. 2012;57(6):1258–1267. doi: 10.1016/j.jhep.2012.07.025. [DOI] [PubMed] [Google Scholar]
- 19.Zhang S, Wang WS, Zhong BY, Ni CF. Subsequent Treatment after Transarterial Chemoembolization Failure/Refractoriness: A Review Based on Published Evidence. J Clin Transl Hepatol. 2022;10(4):740–747. doi: 10.14218/JCTH.2021.00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhong BY, Jin ZC, Chen JJ, Zhu HD, Zhu XL. Role of Transarterial Chemoembolization in the Treatment of Hepatocellular Carcinoma. J Clin Transl Hepatol. 2023;11(2):480–489. doi: 10.14218/JCTH.2022.00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ueno S, Tanabe G, Sako K, Hiwaki T, Hokotate H, Fukukura Y, et al. Discrimination value of the new western prognostic system (CLIP score) for hepatocellular carcinoma in 662 Japanese patients. Cancer of the Liver Italian Program. Hepatology. 2001;34(3):529–534. doi: 10.1053/jhep.2001.27219. [DOI] [PubMed] [Google Scholar]
- 22.Memon K, Kulik LM, Lewandowski RJ, Wang E, Wang J, Ryu RK, et al. Comparative study of staging systems for hepatocellular carcinoma in 428 patients treated with radioembolization. J Vasc Interv Radiol. 2014;25(7):1056–1066. doi: 10.1016/j.jvir.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15(4):361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 24.Steyerberg EW, Vickers AJ, Cook NR, Gerds T, Gonen M, Obuchowski N, et al. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology. 2010;21(1):128–138. doi: 10.1097/EDE.0b013e3181c30fb2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lemeshow S, le Gall J. Modeling the severity of illness of ICU patients. A systems update. JAMA. 1994;272(13):1049–1055. [PubMed] [Google Scholar]
- 26.Lu J, Zhang XP, Zhong BY, Lau WY, Madoff DC, Davidson JC, et al. Management of patients with hepatocellular carcinoma and portal vein tumour thrombosis: comparing east and west. Lancet Gastroenterol Hepatol. 2019;4(9):721–730. doi: 10.1016/S2468-1253(19)30178-5. [DOI] [PubMed] [Google Scholar]
- 27.Sun J, Guo R, Bi X, Wu M, Tang Z, Lau WY, et al. Guidelines for Diagnosis and Treatment of Hepatocellular Carcinoma with Portal Vein Tumor Thrombus in China (2021 Edition) Liver Cancer. 2022;11(4):315–328. doi: 10.1159/000523997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoo DJ, Kim KM, Jin YJ, Shim JH, Ko GY, Yoon HK, et al. Clinical outcome of 251 patients with extrahepatic metastasis at initial diagnosis of hepatocellular carcinoma: does transarterial chemoembolization improve survival in these patients? J Gastroenterol Hepatol. 2011;26(1):145–154. doi: 10.1111/j.1440-1746.2010.06341.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.