Abstract
A 77-year-old male presented to the emergency department with dyspnea. A third-degree atrioventricular block was present in the electrocardiogram and an echocardiography showed a moderate mitral regurgitation with a diastolic functional insufficiency. Hemodynamic variations were assessed in the context of heart rhythm disturbances. (Level of Difficulty: Intermediate.)
Key Words: acute heart failure, atrioventricular block, diastolic mitral regurgitation
A 77-year-old former smoker and dyslipidemic man presented to the emergency department with a 10-day history of progressive dyspnea and fatigue. His heart rate was 43 beats/min, his respiratory rate was 22 breaths/min, and his oxygen saturation was 92% on face mask (8L, fraction of inspired oxygen 40%). Physical examination revealed a systolic murmur and some crackles.
Investigations
Initial laboratory investigations revealed an acute respiratory failure (pH 7.4, pco2 23 mm Hg, po2 52 mm Hg, HCO3- 13.9 mmol/L), leukocytosis of 17.9 × 109/L, N-terminal pro–B-type natriuretic peptide of 4,451 pg/mL, C-reactive protein of 80 mg/L, and negative high-sensitivity troponin T, procalcitonin, and D-dimer. An electrocardiogram showed a third-degree atrioventricular block (AVB) with a broad complex escape rhythm with right bundle branch block morphology (Supplemental Figure 1). Chest x-ray showed a patchy infiltrate (Supplemental Figure 2). Severe acute respiratory syndrome coronavirus 2 polymerase chain reaction test was negative.
Because of suspicion for acute heart failure, a transthoracic echocardiogram was performed and showed preserved left ventricle (LV) function, moderate mitral regurgitation (MR), severe pulmonary hypertension (PH), and nondilated inferior vena cava.
Management
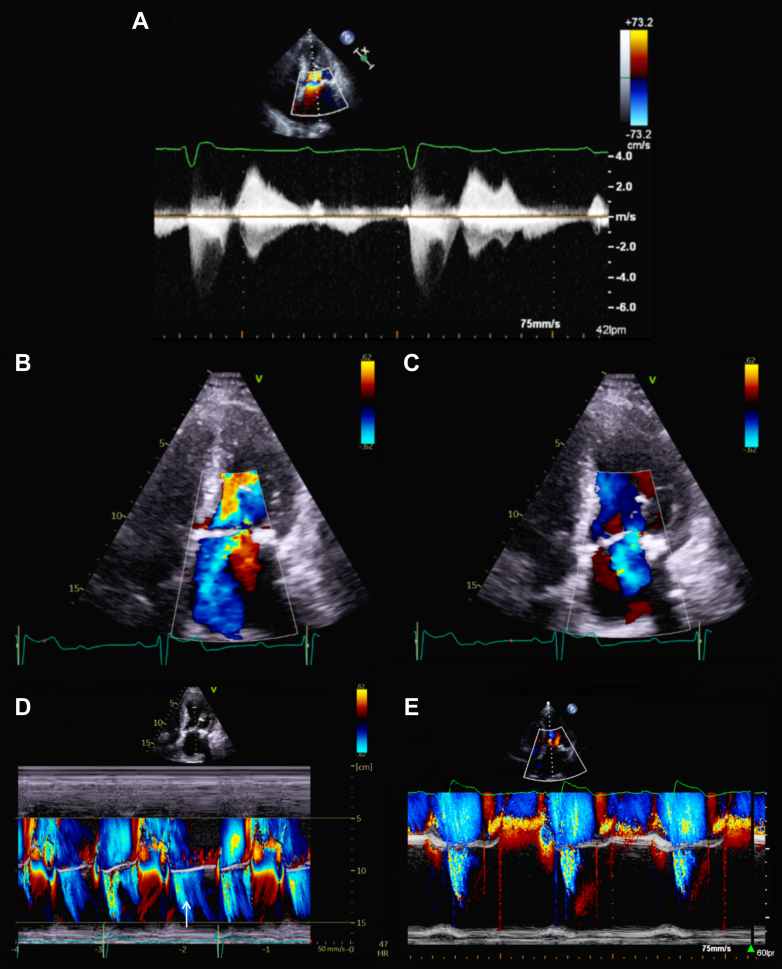
Changes in the hemodynamic situation according to the cardiac rhythm were assessed with a pulmonary artery catheter placement. When dissociated, the pressures of right cavities showed a postcapillary PH (right atrium pressure of 6 mm Hg, pulmonary artery pressure of 55/17/30 mm Hg, and pulmonary capillary wedge pressure of 25 mm Hg with a prominent V-wave). However, when atrioventricular synchronization was artificially achieved with atropine, the right pressures normalized and the postcapillary PH virtually disappeared (pulmonary artery pressure 48/14/28 mm Hg, pulmonary capillary wedge pressure 19 mm Hg with no prominent V-wave). We noticed the same behavior in the MR. When AVB was present, the MR seemed to be severe, with normal systolic component and a diastolic one (Figures 1A to 1C), and when the AVB disappeared, the MR seemed to be lesser, and the diastolic component vanished (Figures 1D and 1E).
Figure 1.
Echocardiography
(A) Continuous Doppler over mitral valve. (B, C) Apical 4 chamber view with (B) systolic and (C) diastolic mitral regurgitation (MR) components. (D, E) M mode view with systolic and diastolic (arrow) MR components during (D) atrioventricular block and only systolic component when atrioventricular synchronization was achieved with atropine (E).
A dual-chamber pacemaker was placed. After 4 days of depletive treatment, the patient was discharged home with mild-degree MR. At 6-month follow-up, the patient remained asymptomatic, and no MR was observed.
Discussion
Diastolic MR is generally described in the setting of high-degree AVB with underlying sinus rhythm. The prolonged diastole with superimposed left atrium, contractions leads to a significant elevation in LV end-diastolic pressure, creating a reverse gradient that favors flow from the LV into the left atrium during diastole.1 This phenomenon may also occur in restrictive cardiomyopathies, acute severe aortic regurgitation, and in patients with long filling periods in atrial tachyarrhythmias.1, 2, 3
Conclusions
Clinical and echocardiographic reassessment takes hold a starring role in acute heart failure management, highlighting the utility of invasive hemodynamic monitoring when diagnosis is uncertain. Understanding the relationship between MR and AVB was the key in this case; specifically, recognizing the presence of diastolic MR as a cause of heart failure.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental figures, please see the online version of this paper.
Appendix
References
- 1.Attar R., El-Tallawi K.C. Diastolic mitral regurgitation. Methodist DeBakey Cardiovasc J. 2021;17(5):89–90. doi: 10.14797/mdcvj.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sisu R.C., Vinereanu D. Different mechanisms for diastolic mitral regurgitation illustrated by three comparative cases. Echocardiography. 2011;28(4):476–479. doi: 10.1111/j.1540-8175.2010.01323.x. [DOI] [PubMed] [Google Scholar]
- 3.Li Q., Liu Y., Zuo W., et al. Mechanisms, features, and significance of diastolic mitral regurgitation: a case series. Eur Heart J Case Rep. 2020;4(5):1–8. doi: 10.1093/ehjcr/ytaa203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.