Abstract
A 65-year-old male was admitted for cardiogenic shock (ejection fraction: 15%) with severe aortic stenosis and regurgitation. He underwent emergency angiography and mechanical circulatory support. A multidisciplinary heart team discussed treatment options. Ultimately, he underwent successful emergency surgical aortic valve replacement with recovery on extracorporeal membrane oxygenation. (Level of Difficulty: Intermediate.)
Key Words: cardiogenic shock, “heart team”–based approach, severe critical aortic stenosis, surgical aortic valve replacement
Central Illustration
A 65-year-old male landscaper with a 45-pack/year smoking history was admitted for acute decompensated heart failure exacerbation. Initial cardiac lab results are outlined in Table 1. Intravenous furosemide was administered. The first night of admission, a code blue was called for pulseless electrical activity. After regaining pulse, he was intubated and transferred to the intensive care unit where the cardiology team was consulted for acute decompensated heart failure. Upon initial evaluation, his extremities were cold, there was a 5/6 systolic murmur at the right upper sternal border with absence of second heart sounds, a 2/6 diastolic murmur, decreased carotid upstroke, and elevated jugular venous pressure to approximately 15 mm Hg while on the ventilator. A focused limited echocardiogram showed a severely reduced left ventricular (LV) ejection fraction of 15% with a heavily calcified and stenotic aortic valve (mean gradient 43 mm Hg, peak velocity 4.0 m/s, calculated valve area 0.5 cm2; LV outflow tract velocity time interval 13 cm, aortic valve velocity time interval 90 cm, LV outflow tract diameter 3.3 cm) severe aortic regurgitation (AR), and mild right ventricular dilation.
Learning Objectives
-
•
To be able to identify patients in cardiogenic shock.
-
•
To be able to define hemodynamic changes with different types of mechanical support.
Table 1.
Admission Laboratory Values
| Test | Result |
|---|---|
| B-type natriuretic peptide, pg/mL | >5,000 |
| Troponin I, ng/mL | 0.15 |
The local shock team protocol was initiated, and the patient was taken to the catheterization laboratory for angiography, hemodynamic assessment, and mechanical circulatory support. The shock team comprises a multidisciplinary team of personnel from critical care cardiology, interventional cardiology, and cardiothoracic surgery as well as an advanced heart failure specialist and cardiology fellow. There was no significant angiographic evidence of coronary artery disease. Hemodynamic evaluation with right heart catheterization was performed (Table 2). Notably, the pulmonary artery saturation was 20.9%, cardiac output 1.39 L/min, cardiac index 0.83 L/min per m2, calculated aortic valve area of 0.18 cm2, and aortic valve area index of 0.11 cm2/m2 (using Hakki formula).
Table 2.
Catheterization Laboratory Hemodynamic Assessment at Baseline and With Milrinone Infusion
| Assessment | Baseline | Drug Therapy |
|---|---|---|
| Pressures used in calculation, systolic/diastolic (mean), mm Hg | ||
| Right atrium | 18/14 (14) | |
| Right ventricle | 59/13 (21) | |
| Pulmonary artery | 61/37 (46) | 61/30 (44) |
| Pulmonary capillary wedge | 37/37 (35) | 38/38 (35) |
| Aortic | 88/46 (64) | |
| Left ventricular | 148/17/43 | |
| CO | ||
| Fick CO, L/min | 1.39 | 1.66 |
| Fick cardiac index, L/min/m2 | 0.83 | 0.99 |
| Resistance results, dyne·s–5 (WU)a | ||
| PVR | 602.79 (7.54) | 434.18 (5.43) |
| SVR | 2,868.77 (35.87) | |
| PVR index | 1,007.93 (12.6) | 725.99 (9.08) |
| SVR index | 4,796.88 (59.98) | |
| TPR | 2,520.77 (31.52) | 2,122 65 (26 54) |
| TVR | 3,672.02 (45.91) | |
| TPR index | 4,214.99 (52.7) | 3,549.3 (44.38) |
| TVR index | 6.140.01 (76.77) | |
| PVR/SVR | 0.21 | |
| TPR/TVR | 0.69 | |
| SW results | ||
| Right ventricular SW, g·m | 9.48 | |
| Left ventricular SW, g·m | 8.46 | |
| Right ventricular SW index, g·m/m2 | 5.67 | |
| Left ventricular SW index, g·m/m2 | 5.06 | |
| Valve | ||
| Aortic area, cm2 | 0.18 | |
| Aortic area index, cm2/m2 | 0.11 | |
| Aortic flow, mL/s | 59.27 | |
| Aortic gradient, mm Hg | 52.5 | |
| Blood flow results, % (mL/dL) | ||
| Arterial saturation | 94.4 (19.26) | 94.4 (19.26) |
| Pulmonary artery saturation | 20.9 (4.26) | 32.6 (6.65) |
| VO2, mL/min | 209.01 | 209.01 |
| VO2 source | 125·BSA | 125·BSA |
| Fick CO, L/min | 1.39 | 1.66 |
| Fick cardiac index, L/min/m2 | 0.83 | 0.99 |
| Qp L/min (L/min/m2) | 1.46 (0.87) | 1.66 (0.99) |
| Qs L/min (L/min/m2) | 1.39 (0.83) | 1.66 (0.99) |
| BSA, m2 | 1.67 |
BSA = body surface area; CO = cardiac output; PVR = peripheral vascular resistance; SVR = systemic vascular resistance; SW = stroke work; TPR = total peripheral resistance; TVR = total vascular resistance; VO2 = volume oxygen consumption.
Index values based on body surface area.
Emergency transcatheter aortic valve replacement (AVR) was believed to be too prohibitive of risk for 2 reasons. First, he would likely require predilation with balloon valvuloplasty due to significant calcium burden, which was contraindicated due to severe AR. Second, it was believed that he would not tolerate rapid ventricular pacing for valve placement in his current state.
Despite an elevated Society of Thoracic Surgery risk score of 20%, without consideration of frailty, it was decided that emergency salvage surgical aortic valve replacement with plans to recover on venoarterial extracorporeal membrane oxygenation (VA ECMO) was the best option for the patient. The use of ECMO as an initial strategy was not sought due to its added afterload requirements on an already severely failing LV with severe AR as it would have caused further ventricular dilation and failure. Importantly, isolated AVR mortality, defined as either death within 30 days or during hospitalization (which may surpass the 30-day timeframe), is monitored on an ongoing 3-year basis. Therefore, a single operative mortality could affect a program’s Medicare “3 Star” rating; thus, emergency salvage AVR is not often performed.
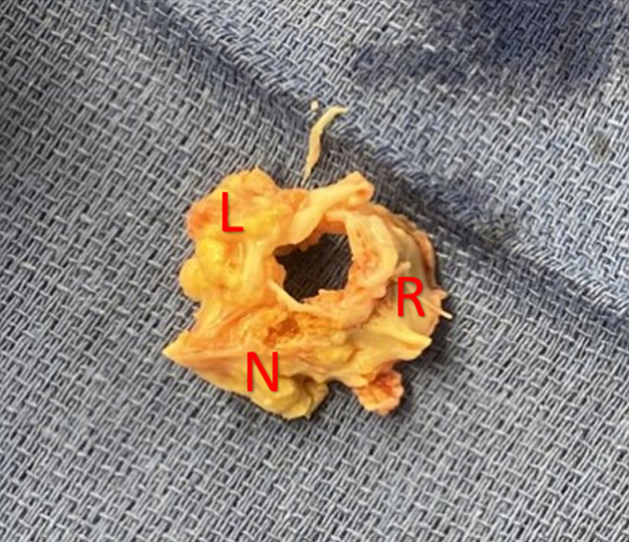
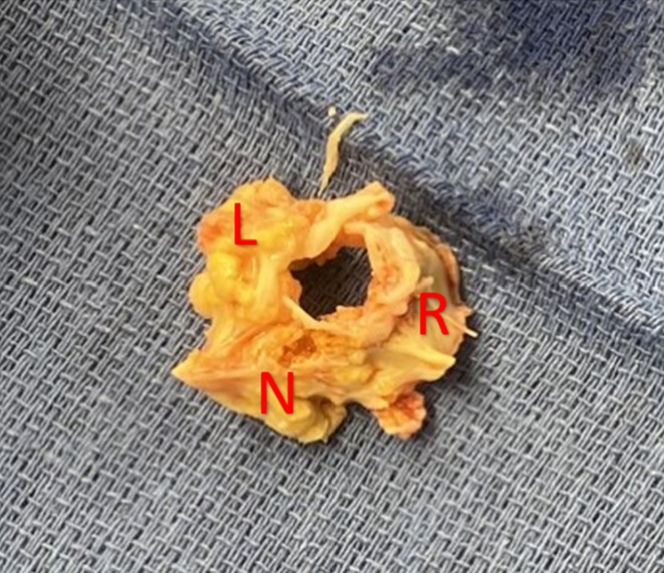
Operatively, the aortic valve was found to be trileaflet but functionally unicuspid due to complete immobility of the right coronary leaflet which was fused to both the left and noncoronary leaflets (Figure 1). A 25-mm bioprosthetic valve was implanted in the aortic valve position. Intraoperatively, he underwent central VA ECMO cannulation and standard local anticoagulation. Peripheral extremity monitoring protocols were followed.
Figure 1.
Ex Vivo View of Heavily Calcified Aortic Valve From Aortic View
R = right cusp; L = left cusp; N = Noncoronary cusp.
The patient successfully recovered and was discharged home on hospital day 24.
Discussion
Aortic stenosis (AS) is a chronic disease process which usually develops over decades. Severe AS has been well studied and defined. It can be due to congenital processes (such as bicuspid aortic valve, occurring in 1% to 2% of the population), rheumatic disease, or calcific/senile disease processes.1 Criteria for severe AS include: maximal aortic jet velocity >4.0 m/s, mean gradient >40 mm Hg, aortic valve area <1.0 cm2, indexed aortic valve area <0.6 cm2/m2, and dimensionless index velocity ratio <0.25.1,2 Different criteria and testing modalities exist for other low-flow states when criteria are incongruent. Evidence from the PARTNER (Placement of Aortic Transcatheter Valve Trial) trial showed worse outcomes for patients with severe AS without valve replacement, up to 50% 1-year mortality, and even poorer prognosis in those with evidence of LV dysfunction.3 The American College of Cardiology/American Heart Association 2020 valvular guidelines define severe AS and classify a staging system, which is dependent upon symptoms, valve area, peak and mean gradients, and LV function. It also outlines indications and level of evidence regarding valve replacement.2 Mixed valvular lesions pose a complicated challenge due to differing remodeling properties of AS and AR. Pressure overload is the predominant phenotype of AS and results in concentric hypertrophy, whereas AR is driven by volume overload and results in concentric hypertrophy with LV dilation.
The heart team approach to patient care is paramount in treating patients, especially due to the increasing complexity and treatment options. The heart team is a Class 1 indication in various American College of Cardiology clinical guidelines.4, 5, 6, 7
In this case report, we highlight the use of our “shock heart team” approach for management of patients with mixed valvular lesions and cardiogenic shock. One noteworthy aspect of this case was the decision to go to the operating room with such a complex and high-risk patient. Our case emphasizes the importance of rapid evaluation of patients in cardiogenic shock as well as the extreme importance of a collaborative heart team discussion/approach to managing such complex patients.
Conclusions
Using a heart team approach, patients with critical AS and cardiogenic shock can have a favorable outcome.
Question 1: based on the physical exam and echocardiographic findings, what would be the next step in management of this patient?
The patient was in cardiogenic shock, so we elected to take him to the cardiac catheterization laboratory to assess hemodynamics, provide any necessary mechanical circulatory support, and to evaluate for significant coronary artery disease. Early identification and prompt treatment of cardiogenic shock is imperative to give patients their best odds of survival.
Question 2: based on the hemodynamic profile in the catheterization lab, what potential treatment options would you consider?
Options for this patient depend on resource availability at the local institution. This patient had a significantly reduced cardiac output in the setting of a cardiogenic shock. Complicating this was a mixed valvular lesion of both a critically stenosed aortic valve and severe regurgitation. Because emergent transcatheter replacement and left atrial to aortic bypass were not available options, surgical replacement was selected.
Question 3: what are the pros/cons of various support devices in the setting of severe AS and severe AR?
The hemodynamic profile of various forms of mechanical circulatory support devices must be considered. In this case, initial use of VA ECMO would have significantly increased afterload. Continuous flow devices would increase cardiac output while decreasing afterload and LV end diastolic pressure must be able to cross the aortic valve, which was not possible in this case. Last, counter-pulsation with an intra-aortic balloon pump can be used to decrease afterload, increase cardiac output, and increase coronary perfusion, but should be avoided in patients with significant aortic regurgitation.
Question 4: define the hemodynamic changes associated with VA-ECMO
Important hemodynamic parameters should be carefully assessed when evaluating patients for the use of VA ECMO. Namely, although it provides some improvement in cardiac flow, it significantly increases afterload. It also reduces LV preload and has minimal effect on LV end diastolic pressure, myocardial oxygen demand, and coronary perfusion.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Mann D.L., Zipes D.P., Libby P., Bonow R.O., Braunwald E. Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine. Tenth Edition; 2015. Chapter 68: Aortic Valve Disease; pp. 1389–1412. [Google Scholar]
- 2.Otto C.M., Nishimura R.A., Bonow R.O., et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. J Am Coll Cardiol. 2020;77(4):450–500. doi: 10.1016/j.jacc.2020.11.035. [DOI] [PubMed] [Google Scholar]
- 3.Mack M.J., Leon M.B., Smith C.R., et al. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. 2015;385(9986):2477–2484. doi: 10.1016/S0140-6736(15)60308-7. [DOI] [PubMed] [Google Scholar]
- 4.Lawton J., Tamis-Holland J., et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization. J Am Coll Cardiol. 2022;79(2):e21–e129. doi: 10.1016/j.jacc.2021.09.006. [DOI] [PubMed] [Google Scholar]
- 5.Isselbacher E., Preventza O., et al. 2022 ACC/AHA guideline for the diagnosis and management of aortic disease. J Am Coll Cardiol. 2022;80(24):e223–e393. doi: 10.1016/j.jacc.2022.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heidenreich P., Bozkurt B., Aguilar D., et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure. J Am Coll Cardiol. 2022;79(17):e263–e421. doi: 10.1016/j.jacc.2021.12.012. [DOI] [PubMed] [Google Scholar]
- 7.Otto C., Nishimura R., Bonow R.O., et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease. J Am Coll Cardiol. 2021;77(4):e25–e197. doi: 10.1016/j.jacc.2020.11.018. [DOI] [PubMed] [Google Scholar]