Key Points
Question
When considering extended-release buprenorphine or transmucosal buprenorphine for treatment for opioid use disorder, which therapy provides the best value for money?
Findings
This economic evaluation, which used a state transition model to simulate a population with opioid use disorder and included cohorts of 100 000 simulated individuals in each category of intervention, found that when compared with no medication treatment, treatment with transmucosal buprenorphine yielded an incremental cost-effectiveness ratio of $19 740 quality-adjusted life-years gained. In comparison, treatment with extended-release buprenorphine yielded lower effectiveness by 0.03 quality-adjusted life-years per person at higher cost, suggesting that treatment with extended-release buprenorphine was dominated and therefore not preferred.
Meaning
These results suggest that at current medication cost and retention rates, extended-release buprenorphine was not associated with efficient allocation of limited resources when transmucosal buprenorphine was available; future initiatives should aim to improve retention rates and decrease cost.
This economic evaluation examines the cost-effectiveness of extended-release buprenorphine compared with transmucosal buprenorphine.
Abstract
Importance
In 2017, the US Food and Drug Administration (FDA) approved a monthly injectable form of buprenorphine, extended-release buprenorphine; published data show that extended-release buprenorphine is effective compared with no treatment, but its current cost is higher and current retention is lower than that of transmucosal buprenorphine. Preliminary research suggests that extended-release buprenorphine may be an important addition to treatment options, but the cost-effectiveness of extended-release buprenorphine compared with transmucosal buprenorphine remains unclear.
Objective
To evaluate the cost-effectiveness of extended-release buprenorphine compared with transmucosal buprenorphine.
Design, Setting, and Participants
This economic evaluation used a state transition model starting in 2019 to simulate the lifetime of a closed cohort of individuals with OUD presenting for evaluation for opioid agonist treatment with buprenorphine. The data sources used to estimate model parameters included cohort studies, clinical trials, and administrative data. The model relied on pharmaceutical costs from the Federal Supply Schedule and health care utilization costs from published studies. Data were analyzed from September 2021 to January 2023.
Interventions
No treatment, treatment with transmucosal buprenorphine, or treatment with extended-release buprenorphine.
Main Outcomes and Measures
Mean lifetime costs per person, discounted quality-adjusted life-years (QALYs), and incremental cost-effectiveness ratios (ICERs).
Results
The simulated cohort included 100 000 patients with OUD receiving (61% male; mean [SD] age, 38 [11] years) or not receiving medication treatment (58% male, mean [SD] age, 48 [18] years). Compared with no medication treatment, treatment with transmucosal buprenorphine yielded an ICER of $19 740 per QALY. Compared with treatment with transmucosal buprenorphine, treatment with extended-release buprenorphine yielded lower effectiveness by 0.03 QALYs per person at higher cost, suggesting that treatment with extended-release buprenorphine was dominated and not preferred. In probabilistic sensitivity analyses, treatment with transmucosal buprenorphine was the preferred strategy 60% of the time. Treatment with extended-release buprenorphine was cost-effective compared with treatment with transmucosal buprenorphine at a $100 000 per QALY willingness-to-pay threshold only after substantial changes in key parameters.
Conclusions and Relevance
In this economic evaluation of extended-release buprenorphine compared with transmucosal buprenorphine for the treatment of OUD, extended-release buprenorphine was not associated with efficient allocation of limited resources when transmucosal buprenorphine was available. Future initiatives should aim to improve retention rates or decrease costs associated with extended-release buprenorphine.
Introduction
The US recorded 83 000 opioid-related overdose deaths in 2022.1 Evidence shows that medications for opioid use disorder (MOUD) are a tool that helps reduce opioid-related overdose mortality.2,3,4 Although MOUD are highly effective, many eligible individuals are not receiving them due in part to the limited number of prescribers, financial accessibility, restrictions on methadone care, and stigma.5,6 Although access to and uptake of MOUD has risen, the increase has not been substantial enough to reverse the trajectory of opioid overdose–related death rates.7,8
MOUD currently approved by the Food and Drug Administration (FDA) are buprenorphine, methadone, and naltrexone. Buprenorphine is available as transmucosal buprenorphine, which is most commonly taken daily as a sublingual film and extended-release buprenorphine, which is administered as a monthly injection.9,10 According to Federal Supply Schedule data from 2022, monthly costs are $196 for transmucosal buprenorphine and $1136 for extended-release buprenorphine.11 In addition, a study of commercially insured individuals focusing on persons who had initiated extended-release buprenorphine within 13 months after FDA approval found lower retention on treatment for extended-release buprenorphine compared with other forms of MOUD.12,13 Nevertheless, qualitative research suggests some notable benefits associated with extended-release buprenorphine, including a decrease in stigma, ability to lead a “normal life,” and increased convenience.14,15 These factors all suggest a potential for improved retention on extended-release buprenorphine for later adopters.14,15
Given the urgency of opioid overdose–related deaths, it would be useful for patients, clinicians, and policy makers to know the relative cost-effectiveness of extended-release buprenorphine vs transmucosal buprenorphine. Recent changes to address barriers to prescribing buprenorphine also make the current analysis timely.16 Therefore, we used a computer simulation model to investigate the cost-effectiveness of extended-release buprenorphine when compared with transmucosal buprenorphine.17 We also explored under which conditions extended-release buprenorphine would not provide a good value for money given its known limitations.
Methods
This economic evaluation was deemed exempt from review and informed consent by the institutional review board at the Boston University Medical Campus because the simulation modeling used either previously published data from the literature or publicly available data. We followed the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) reporting guideline.
Model Overview and Strategies
We conducted an economic evaluation using the Researching Effective Strategies to Prevent Opioid Death (RESPOND) model to evaluate the cost-effectiveness of extended-release buprenorphine. RESPOND is a cohort-based, state transition model of OUD natural history and treatment in Massachusetts (eFigure 1 in Supplement 1).18 Additional details on the model are included in previous work18,19 and in the eAppendix, eFigure 2, eTable 1, and eTable 2 in Supplement 1. We simulated the lifetime of a closed cohort of 100 000 individuals with OUD in Massachusetts starting in 2019. We modeled a base case of 3 strategies: (1) no medication treatment: individuals do not access MOUD or medically managed withdrawal (detoxification); (2) treatment with transmucosal buprenorphine: individuals start receiving transmucosal buprenorphine; if they are not retained, they transition between no treatment, extended-release buprenorphine, methadone, naltrexone, and detoxification; (3) treatment with extended-release buprenorphine: individuals start receiving extended-release buprenorphine; if they are not retained, they transition between the same aforementioned options.
While our goal was to study buprenorphine formulations, individuals in the simulation model transition between receiving and not receiving buprenorphine to reflect that in the real world they can access other treatment. Although data show that detoxification fails to reduce risk of relapse and overdose, we included it to reflect its real-world use.20 As we will describe in more detail later, we do not assume that patients on MOUD are abstinent from drugs.
Model Structure
We utilized RESPOND to simulate the base case over a lifetime. Weekly transitions were used.21,22
Natural History of OUD
The model simulated transitions between 4 health states: active injection opioid use; active noninjection opioid use; nonactive injection opioid use; and nonactive noninjection opioid use. We defined active use as use in the past week. There was bidirectional movement between active and nonactive states. In active use, the rate of overdose was higher among people who inject opioids.
Care Delivery
Each week, a subset of individuals accessed treatment and detoxification. Individuals receiving MOUD experience bidirectional transitions between active and nonactive states, representing the reality that some patients who are taking MOUD use opioids, but the net balance of movement favors nonactive use.23
These individuals also faced a risk of ceasing MOUD. This population entered a 4-week posttreatment state, during which the rates of return to active use and overdose were higher than those for individuals not receiving treatment. In contrast, receiving MOUD also had an independent effect of lowering the rate of overdose among those who were actively using drugs in the model.
Population Dynamics
We ran a closed cohort without adding or removing members from the simulation (except for death) to replicate the experience of an observational cohort study. The total number of individuals in the cohort decreased until everyone had died. Initial cohorts were stratified by age, sex, and OUD status. These data were informed by calibrated proportions of individuals in each category from 2012.24
Mortality
Among individuals in active use states, there was a risk of overdose in every cycle. Overdose incidence was considered as a function of both age and mode of drug consumption (injection or noninjection). A proportion of these overdoses were considered as overdose deaths. We also used a competing risks approach to adjust for death from other causes using standardized mortality ratios (SMRs).
Costs and Utilities
We used MOUD pharmaceutical costs, health care utilization costs, and treatment utilization costs in the model. We stratified the latter 2 (health care utilization costs and treatment utilization costs) by demographic variables. Health care utilization costs accounted for costs associated with prescribing the medication, such as clinical visits. Treatment utilization costs described costs other than the cost of the treatment itself. Costs were reported from the health care system perspective. The model also included demographic-stratified utilities.
Model Data
The epidemiology of OUD and OUD treatment seeking, including the prevalence of drug use, the incidence of overdose, the rate of treatment seeking, and retention in OUD care were specific to the jurisdiction being simulated. The primary data source for these parameters was the Massachusetts Public Health Data Repository (PHD), which is a longitudinal records database that links individual level data across more than 29 sources.24,25 In contrast, we assumed that parameters that simulated the natural history of OUD and the efficacy of pharmaceuticals were generalizable and used data from clinical trials and medical literature. In some cases, data for extended-release buprenorphine were not available and we used estimates based on transmucosal buprenorphine or naltrexone. We summarized our inputs in Table 1.4,11,24,26,27,28,29,30,31,32,33,34,35,36
Table 1. Model Inputs.
| Parameter | Baseline valuea | Range evaluated | Source |
|---|---|---|---|
| Population demographics and epidemiology | |||
| Male, proportion of total people at baseline | Strategy 1: 0.58 | Strategy 1: 0.47-0.70 | Massachusetts Department of Public Health,26 2017 |
| Strategies 2 and 3: 0.61 | Strategies 2 and 3: 0.49-0.73 | ||
| Mean age, y | Strategy 1: 48.21 | Strategy 1: 38.66-58.16 | Massachusetts Department of Public Health,26 2017 |
| Strategies 2 and 3: 38.42 | Strategies 2 and 3: 28.45-48.42 | ||
| Injection drug use, proportion of total people at baseline | 0.25 | 0.20-0.30 | SAMHSA,27 2013 |
| Active drug use, proportion of total people at baseline | Strategy 1: 0.91 | Strategy 1: 0.73-1.00 | Cedarbaum et al,28 2018; Murphy et al,29 2018 |
| Strategy 2: 0.25 | Strategy 2: 0.20-0.28 | ||
| Strategy 3: 0.25 | Strategy 3: 0.20-0.30 | ||
| SMR for injection drug use | 5.07 | 4.06-6.09 | Massachusetts Department of Public Health,26 2017; US Census Bureau,30 2013 |
| SMR for noninjection drug use | 2.05 | 1.64-2.46 | Massachusetts Department of Public Health,26 2017; US Census Bureau,30 2013 |
| Transition to MOUD treatment and detoxification, monthly rate per 1000 people | |||
| Transmucosal buprenorphineand extended-release buprenorphine | 8.41 | 6.81-10.00 | Massachusetts Department of Public Health,26 2017 |
| Methadone | 2.40 | 1.92-2.92 | Massachusetts Department of Public Health,26 2017 |
| Naltrexone (injectable) | 1.08 | 0.88-1.32 | Massachusetts Department of Public Health,26 2017 |
| Detoxification | 6.80 | 5.20-8.00 | Massachusetts Department of Public Health,242020 |
| Retained on MOUD treatment, proportion at 6 mo | |||
| Transmucosal buprenorphine | 0.47 | 0.45-0.47 | IBM,31 2021 |
| Extended-release buprenorphine | 0.29 | 0.24-0.33 | IBM,31 2021 |
| Methadone | 0.66 | 0.64-0.69 | IBM,31 2021 |
| Naltrexone (injectable) | 0.32 | 0.30-0.34 | IBM,31 2021 |
| Overdose, monthly rate per 1000 people | |||
| No treatment | 6.76 | 5.40-8.00 | Massachusetts Department of Public Health,26 2017 |
| Transmucosal buprenorphineand extended-release buprenorphine | 2.72 | 2.20-3.28 | Morgan et al,32 2019 |
| Methadone | 5.08 | 4.04-6.08 | Sordo et al,4 2017 |
| Naltrexone (injectable) | 5.80 | 4.68-7.00 | Morgan et al,32 2019 |
| Fatal overdoses, proportion of total overdoses | |||
| All treatment states | 0.14 | 0.11-0.16 | Massachusetts Department of Public Health,26 2017 |
| Pharmaceutical cost | |||
| Transmucosal buprenorphine (16 mg, daily), $ | 49 | 39-58 | US Department of Veterans Affairs,11 2021 |
| Extended-release buprenorphine (100 mg or 300 mg, injection, monthly), $ | 284 | 227-341 | US Department of Veterans Affairs,11 2021 |
| Methadone (80 mg, daily), $ | 4 | 3-5 | US Department of Veterans Affairs,11 2021 |
| Naltrexone (380 mg, injection, monthly), $ | 303 | 242-363 | US Department of Veterans Affairs,11 2021 |
| Treatment utilization costb | |||
| Transmucosal buprenorphine, $ | 65 | 52-72 | Expert opinionc |
| Extended-release buprenorphine and naltrexone (injectable), $ | 24 | 19-29 | Expert opinionc |
| Methadone, $ | 123 | 99-148 | NIDA,33 2021 |
| Detoxification, $ | 2863 | 2290-3436 | McCollister et al,342018; Murphy et al,29, 2019 |
| Overdose cost, weekly | |||
| Fatal overdose cost, $ | 4557 | 3646-5469 | Coffin et al,35 2017; Jiang et al,36 2017 |
| Nonfatal overdose cost, $ | 858 | 686-1030 | Coffin et al,35 2017; Jiang et al,36 2017 |
Abbreviations: MOUD, medications for opioid use disorder; NIDA, the National Institute of Drug Abuse; SAMHSA, Substance Abuse and Mental Health Services Administration; SMR, standardized mortality ratio.
Values for all strategies are the same unless otherwise noted. Where noted, strategy 1 is no medication treatment; strategy 2 is treatment with transmucosal buprenorphine; strategy 3 is treatment with extended-release buprenorphine.
Treatment costs include any costs required in addition to the pharmaceutical cost for administration of the drug.
For parameters that were not available from the literature, addiction medicine specialists listed as coauthors for the current study were consulted for reasonable estimates. Extensive sensitivity analyses were then used to assess the robustness of all parameters in the model.
Demography and Opioid Use
We estimated the demographics of the OUD population in Massachusetts using results from a capture-recapture analysis that utilized data from the Massachusetts PHD.25 We also used data from multiple cross-sectional surveys to estimate the proportion of people in different health states for each cohort.27,28,37
The mean age (SD) in the no medication treatment strategy was 48 (18) years, and in the treatment strategies it was 38 (11) years.25 The proportion of male individuals at baseline was 58% in the no medication treatment strategy, and 61% in the treatment strategies.25
Overdose
The monthly rate of overdose among people not receiving treatment is 6.76 per 1000 people.24,26 The monthly rate of overdose among individuals treated with transmucosal buprenorphine or extended-release buprenorphine is 2.72 per 1000 people.4,32 Among those who experience an overdose, 14% die, which is the same proportion for both injection and noninjection use.24,26 We used data from the Massachusetts PHD to determine overdose rates for individuals not on treatment and proportion of fatal overdoses.24,26 We modeled overdose rates while receiving treatment using data from cohort studies.4,32
OUD Treatment Modalities
We used weekly probabilities of MOUD treatment retention with data from the IBM Watson MarketScan Commercial Claims Database, a nationally representative data set of US commercially insured individuals.31 Retention on transmucosal buprenorphine is 47% at 6 months, whereas retention on extended-release buprenorphine is 29% at 6 months.31
Costs
We used pharmaceutical costs from the Federal Supply Schedule and estimated health care utilization costs and treatment utilization costs from published studies.11,29,33,34 The weekly pharmaceutical cost of extended-release buprenorphine is $284, while the cost of transmucosal buprenorphine is $49. We included the overdose costs from published studies in the model.35,36
Utilities
We assigned utility weights, representing general well-being, based on age, sex, treatment state, and OUD status (eTable 1 in Supplement 1). We used utility weights from published studies.29,38
Statistical Analysis
First, we used the model to simulate the base case clinical progression. Outcomes from the simulation included life expectancy, life-years, quality-adjusted life-years (QALYs) using utility weights, and lifetime costs from the health sector perspective. We reported QALYs as their increase from the base level age. We discounted costs and QALYs by 3% annually and used a minimal approach for estimating utilities, meaning that when multiple conditions affecting utility are experienced simultaneously, we assigned the utility weight associated with the worst condition.39 When necessary, we adjusted costs for inflation to 2019 USD. We compared all strategies using incremental cost-effectiveness ratios (ICERs) using a willingness-to-pay threshold of $100 000 per QALY, which we calculated as the incremental cost divided by the incremental change in QALYs of the next most costly strategy.39 We considered strategies to be dominated if they were more costly and less effective than the next most costly strategy.
We conducted deterministic sensitivity analyses on all inputs by varying point estimates over a feasible range to evaluate the effects of uncertainty around model parameters (Table 1). We also conducted sensitivity analyses on costs and retention to identify threshold values for cost-effectiveness. Additionally, we performed a sensitivity analysis on utility weights using the multiplicative method, where the utility weights are multiplied.39
We conducted probabilistic sensitivity analyses by defining probability density functions (distributions) for overdose rates, treatment initiation, fatal overdose rate, SMRs, transitions between treatments (including retention and uptake), costs, and utility. We obtained the distribution of values (including shape and mean) from published literature sources and existing data. We repeated each simulation 10 000 times, using a different random value from the feasible range for every input. Statistical analysis was performed using R Studio version 3.5.1 (R Project for Statistical Computing) from September 2021 to January 2023.
Results
Base Case
A cohort of 100 000 simulated individuals was used in each category of intervention (among no medication treatment cohort: 58% were male and mean [SD] age was 48 [18] years; among treatment cohort: 61% were male and mean [SD] age was 38 [11] years) (Table 1). When compared with no medication treatment, treatment with transmucosal buprenorphine was associated with an increased life expectancy (27.44 vs 20.54 life-years) (Table 2). This increase represents an additional 3.22 discounted QALYs, and raised discounted lifetime costs per person ($304 700 vs $241 070). When compared with no medication treatment, treatment with transmucosal buprenorphine had an ICER of $19 740 per QALY, which is below the commonly cited willingness-to-pay threshold of $100 000 per QALY.
Table 2. Results of Base Case Analysis.
| Strategy | Annual fatal overdose rate per 1000 peoplea | Remaining undiscounted LYs per personb | Total discounted cost per person, $b | Change in discounted cost per person, $b | Remaining discounted QALYs per personb | Change in remaining discounted QALYs per personb | ICER ($/LY)b | ICER ($/QALY)b |
|---|---|---|---|---|---|---|---|---|
| No medication treatment strategy | 7.77 | 20.54 | 241 070 | NA | 8.32 | NA | NA | NA |
| Treatment with transmucosal buprenorphine strategy | 6.27 | 27.44 | 304 700 | 63 630 | 11.55 | 3.22 | 18 380 | 19 740 |
| Treatment with extended-release buprenorphine strategy | 6.42 | 27.39 | 308 700 | 4000 | 11.52 | −0.03 | Dominatedc | Dominatedc |
Abbreviations: ICER, incremental cost-effectiveness ratio; LY, life-years; NA, not applicable; QALY, quality-adjusted life-years.
Calculated over the first 10 years of the simulation.
Costs and ICERs were rounded to nearest $10 and QALYs and LYs to nearest 0.01.
A strategy was considered dominated when costing more and achieving a lower QALY than the next least expensive strategy.
When treatment with extended-release buprenorphine was compared with treatment with transmucosal buprenorphine, it had fewer remaining undiscounted life-years per person (27.44 vs 27.39 life-years), a decrease of 0.03 QALYs, and an increase in discounted lifetime costs per person ($308 700 vs $304 700). The treatment with extended-release buprenorphine strategy was therefore dominated.
Sensitivity Analyses
Deterministic Sensitivity Analyses
Pharmaceutical Cost
The cost-effectiveness of treatment with extended-release buprenorphine varied depending on changes in extended-release buprenorphine pharmaceutical cost (Table 3). After applying an 80% decrease in pharmaceutical cost, corresponding to a monthly pharmaceutical cost of $57, the following observations were noted. The total cost for treatment with extended-release buprenorphine was lower than that for treatment with transmucosal buprenorphine ($293 040 vs $293 730). When compared with no medication treatment, treatment with extended-release buprenorphine was associated with an ICER of $16 300 per QALY gained. In addition, when compared with treatment with extended-release buprenorphine, treatment with transmucosal buprenorphine had an ICER of $19 760 per QALY gained.
Table 3. Results of Deterministic Sensitivity Analyses.
| Strategy | Annual overdose rate per 1000 peoplea | Remaining undiscounted LYs per personb | Total discounted cost per person, $b | Change in discounted cost per person, $b | Remaining discounted QALYs per personb | Change in remaining discounted QALYs per personb | ICER ($/LY)b | ICER ($/QALY)b |
|---|---|---|---|---|---|---|---|---|
| Sensitivity analysis: 80% decreased pharmaceutical cost | ||||||||
| No medication treatment strategy | 7.77 | 20.54 | 241 070 | NA | 8.33 | NA | NA | NA |
| Treatment with extended-release buprenorphine strategy | 6.53 | 27.39 | 293 040 | 51 970 | 11.52 | 3.19 | 15 140 | 16 300 |
| Treatment with transmucosal buprenorphine strategy | 6.49 | 27.44 | 293 730 | 680 | 11.55 | 0.03 | 23 410 | 19 760 |
| Sensitivity analysis: 191% increased retention | ||||||||
| No medication treatment strategy | 7.77 | 20.54 | 241 070 | NA | 8.33 | NA | NA | NA |
| Treatment with transmucosal buprenorphine strategy | 5.10 | 28.26 | 338 480 | 97 410 | 12.07 | 3.75 | 25 560 | 25 990 |
| Treatment with extended-release buprenorphine strategy | 4.27 | 28.57 | 358 280 | 19 800 | 12.28 | 0.20 | 118 610 | 97 840 |
Abbreviations: ICER, incremental cost-effectiveness ratio; LY, life-years; QALY, quality-adjusted life-years.
Calculated over the first 10 years of the simulation.
Costs and ICERs were rounded to nearest $10 and QALYs and LYs to nearest 0.01.
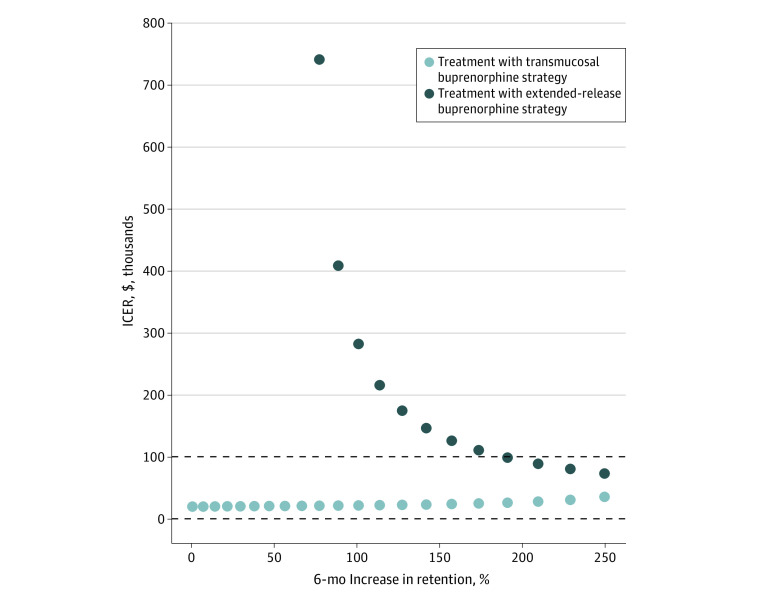
Retention
The cost-effectiveness of treatment with extended-release buprenorphine was also sensitive to changes in retention on extended-release buprenorphine (Table 3; Figure 1). When we increased the 6-month retention rate from 29% to 83%, or an overall increase of 191%, QALYs associated with treatment with extended-release buprenorphine were slightly higher than that of treatment with transmucosal buprenorphine (12.28 QALYs vs 12.07 QALYs) and the ICER was $25 990 per QALY gained for treatment with transmucosal buprenorphine when compared with no medication treatment (eTable 1 in Supplement 1). As for the treatment with extended-release buprenorphine strategy, comparison with treatment with transmucosal buprenorphine had an ICER of $97 840 per QALY gained.
Figure 1. One-Way Sensitivity Analysis of Retention on Medications for Opioid Use Disorder.
The figure shows the cost-effectiveness of treatment with transmucosal buprenorphine and treatment with extended-release buprenorphine strategies as the 6-month rate of retention is increased. The dotted line at the incremental cost-effectiveness ratio (ICER) of $100 000 represents a threshold of $100 000 per quality-adjusted life years (QALY) willingness-to-pay and the dotted line at $0 represents the lowest value at which any strategy is not dominated. Any dots shown between the 2 sets of dotted lines are considered to be cost-effective strategies.
Two-Way Cost and Retention
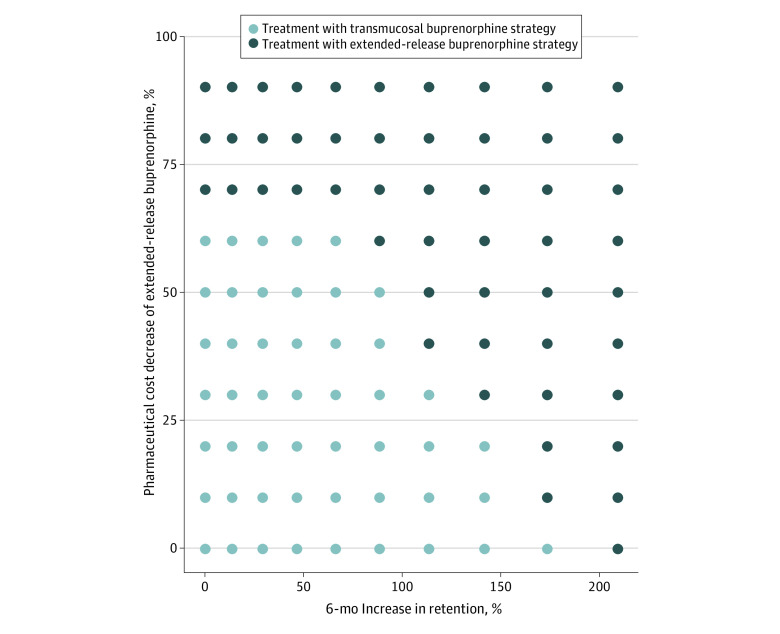
The cost-effectiveness of treatment with extended-release buprenorphine changed in response to simultaneous changes in the pharmaceutical cost and extended-release buprenorphine retention (2-way sensitivity analysis). In Figure 2, we show extended-release buprenorphine retention values and pharmaceutical costs for which treatment with extended-release buprenorphine was cost-effective when compared with treatment with transmucosal buprenorphine. For example, a combination of a 40% decrease in cost and an approximately 2 times improvement in retention led to extended-release buprenorphine as a good value for money. Given that the current study is modeling a cohort of individuals in 2019, Figure 2 may be particularly helpful to understand the cost-effectiveness of interventions considered as cost and retention continue to fluctuate.
Figure 2. Two-Way Deterministic Sensitivity Analysis of Pharmaceutical Cost and Retention for Extended-Release Buprenorphine Cost-Effectiveness Plot.
This figure represents the cost-effective strategy at a $100 000 per quality-adjusted life years (QALY) willingness-to-pay threshold when we vary both retention and cost for extended-release buprenorphine at the same time in increments of 5%. The values in the upper right side of the figure, where the dots turn to darkest, are combinations of retention increases and cost reductions resulting in the treatment with extended-release buprenorphine strategy no longer being dominated.
Other Sensitivity Analyses
Findings were robust to variations in demographic characteristics, epidemiological model inputs, costs, and utilities. Results for key ranges evaluated are included in Table 1. We also included sensitivity analysis results on the type of utility used (multiplicative vs minimal approach) in eTable 2 in Supplement 1. Remaining ranges are included in the eAppendix in Supplement 1.
Probabilistic Sensitivity Analysis
We used probabilistic sensitivity analyses to incorporate uncertainty. Assuming a willingness to pay of $100 000 per QALY gained, treatment with transmucosal buprenorphine was the optimal strategy in 60% of simulations, whereas treatment with extended-release buprenorphine was cost-effective in the remaining 40%.
Discussion
This economic evaluation found that when using currently available cost and retention data, extended-release buprenorphine was not a cost-effective strategy when transmucosal buprenorphine was available. This finding was robust over a reasonable range of variations in key parameters, and we also found that substantial changes in pharmaceutical cost and retention rates would be necessary for extended-release buprenorphine to become a good value for money.
Our findings do not imply that extended-release buprenorphine has no role in the market. Previous studies show that extended-release buprenorphine has the potential to be a helpful addition to existing forms of MOUD. Injectables include a consistent dose of medication and reduced burden of daily medication. Extended-release buprenorphine may decrease stigma that is associated with daily dosing of medication.14 In addition, extended-release buprenorphine may be particularly useful for populations at high risk for overdose fatalities, including survivors of opioid overdose.40 Furthermore, a study showed that almost a quarter of respondents preferred extended-release buprenorphine when given a choice between transmucosal buprenorphine, extended-release buprenorphine, or a buprenorphine implant.41 Although we found that at current cost and retention rates, extended-release buprenorphine was not preferred when compared with transmucosal buprenorphine, not all patients are willing to initiate transmucosal buprenorphine. Our study specifically contributes to the literature by estimating changes in key parameters such as cost and retention that would lead to extended-release buprenorphine becoming a cost-effective option when other buprenorphine formulations are available. In addition, the current analysis is especially timely given that another buprenorphine extended-release subcutaneous injection drug was recently approved by the FDA.42 The availability of new drugs might lead to more competition and a decreased price.43 This situation might be akin to what was observed with another health condition, hepatitis C. In this particular case, our team and others found that some drugs that were not a good value for money at higher prices, later became acceptable options when prices were lowered.44,45 As opioid overdoses continue to surge nationwide, unabated and likely worsened by the COVID-19 pandemic, it is crucial to develop a broad menu of effective MOUD for a population with diverse needs.
Although our study found that extended-release buprenorphine may not currently be a cost-effective option when transmucosal buprenorphine is available, we also found that if this medication’s cost were decreased by 80%, or retention over 6 months were to increase from 29% to 83%, this conclusion may change. Given that implementing these changes might require substantial adjustments, our 2-way sensitivity analysis shows combinations of cost and retention values for which extended-release buprenorphine may become a cost-effective option when transmucosal buprenorphine is available.
We found that our results were robust with variations over a wide range of deterministic sensitivity analyses; however, by assigning a distribution around key input parameters in probabilistic sensitivity analyses, we found that extended-release buprenorphine was cost-effective in 40% of the simulations. This result suggests that our findings might be substantially influenced by uncertainty around some baseline parameters such as retention in care. Therefore, additional information on some parameters would improve estimates and might modify our conclusions. Addressing the overdose crisis will require a variety of options in the OUD treatment toolbox and extended-release buprenorphine has the potential to improve outcomes.
Limitations
Our study has limitations. First, available data on extended-release buprenorphine are for patients who were prescribed extended-release buprenorphine within 13 months of its approval. Some parameter estimates, particularly related to retention, may change as later adopters constitute a larger proportion of prescribers. Also, our results may not be applicable outside of the US even though our 2-way analysis of cost and retention may help a diverse range of stakeholders with decisions related to the use of extended-release buprenorphine. We used Massachusetts data because this state has some of the best data on OUD and its complications.46 Our results are, therefore, likely applicable to other places in the country with a similar mix of urban and rural communities. Furthermore, our data on utilities for MOUD may not reflect patients’ preferences for one medication over another. Additionally, our use of QALYs may not fully reflect quality of life as experienced by people who use drugs. Nonetheless, they provide a basis for comparing our results with those reported by others in the literature, and we also report life-year results. Next, given that data on nonfatal overdoses are incomplete, the proportion of fatal overdoses might need to be refined. Nevertheless, our estimated overdoses from the calibrated simulation match the overdoses reported in Massachusetts in 2012 from the PHD data set. Also, there are no clear data of counseling interventions effective in combination with buprenorphine to inform detailed simulation of the bidirectional effect of counseling and MOUD.47 Additionally, our retention values are from a commercial claims database and therefore they may not be representative of overall retention rates on MOUD.
Conclusions
In this economic evaluation of extended-release buprenorphine compared with transmucosal buprenorphine for patients with OUD, we found that extended-release buprenorphine was not a cost-effective treatment option when transmucosal buprenorphine was available, and we identified thresholds in cost and MOUD retention that would change the main conclusion of our analysis. The ongoing nationwide surge in opioid overdose deaths underscores the need for a broad menu of effective treatments to address the diverse needs of all patients. Our findings on important thresholds in 2 key parameters, namely cost and retention, might be helpful to policy makers. Future research should evaluate the experience of later extended-release buprenorphine adopters as well as the role of interventions aimed to enhance retention or decrease extended-release buprenorphine cost.
eAppendix. Supplemental Materials
eTable 1. Utility Values (For Active Treatment)
eTable 2. Multiplicative Utility Sensitivity Analysis on Base Case
eFigure 1. Model Structure of Researching Effective Strategies to Prevent Opioid Death (RESPOND)
eFigure 2. Sensitivity of Cost and Quality-Adjusted Life Years for the Extended-Release Buprenorphine Strategy
eReferences
Data Sharing Statement
References
- 1.CDC . Provisional drug overdose death counts. 2022. Accessed October 4, 2022. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm
- 2.Bart G. Maintenance medication for opiate addiction: the foundation of recovery. J Addict Dis. 2012;31(3):207-225. doi: 10.1080/10550887.2012.694598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Degenhardt L, Bucello C, Mathers B, et al. Mortality among regular or dependent users of heroin and other opioids: a systematic review and meta-analysis of cohort studies. Addiction. 2011;106(1):32-51. doi: 10.1111/j.1360-0443.2010.03140.x [DOI] [PubMed] [Google Scholar]
- 4.Sordo L, Barrio G, Bravo MJ, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ. 2017;357:j1550. doi: 10.1136/bmj.j1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams AR, Nunes EV, Bisaga A, Levin FR, Olfson M. Development of a Cascade of Care for responding to the opioid epidemic. Am J Drug Alcohol Abuse. 2019;45(1):1-10. doi: 10.1080/00952990.2018.1546862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Volkow ND. Medications for opioid use disorder: bridging the gap in care. Lancet. 2018;391(10118):285-287. doi: 10.1016/S0140-6736(17)32893-3 [DOI] [PubMed] [Google Scholar]
- 7.Morgan JR, Schackman BR, Leff JA, Linas BP, Walley AY. Injectable naltrexone, oral naltrexone, and buprenorphine utilization and discontinuation among individuals treated for opioid use disorder in a United States commercially insured population. J Subst Abuse Treat. 2018;85:90-96. doi: 10.1016/j.jsat.2017.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alderks CE. Trends in the use of methadone, buprenorphine, and extended-release naltrexone at substance abuse treatment facilities: 2003-2015 (update). the CBHSQ report. Substance Abuse and Mental Health Services Administration. Published 2017. Accessed July 18, 2023. https://www.samhsa.gov/data/report/trends-use-methadone-buprenorphine-and-extended-release-naltrexone-substance-abuse-treatment [PubMed] [Google Scholar]
- 9.Indivior UK Limited . SUBLOCADE (buprenorphine extended-release) injection, for subcutaneous use, CIII. Published 2022. Accessed Oct 4, 2022. https://www.sublocade.com/Content/pdf/prescribing-information.pdf
- 10.Hikma Pharmaceutical USA Inc . Buprenorphine (bue” pre nor’ feen) Sublingual Tablets CIII. Accessed October 4, 2022. https://dailymed.nlm.nih.gov/dailymed/medguide.cfm?setid=1bf8b35a-b769-465c-a2f8-099868dfcd2f
- 11.US Department of Veterans Affairs . Office of Procurement, Acquisition and Logistics (OPAL): pharmaceutical prices. Accessed July 25, 2023. https://www.va.gov/opal/nac/fss/pharmprices.asp
- 12.Morgan JR, Walley AY, Murphy SM, et al. Characterizing initiation, use, and discontinuation of extended-release buprenorphine in a nationally representative United States commercially insured cohort. Drug Alcohol Depend. 2021;225:108764. doi: 10.1016/j.drugalcdep.2021.108764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.US Food & Drug Administration . FDA approves first once-monthly buprenorphine injection, a medication-assisted treatment option for opioid use disorder. Published November 30, 2017. Accessed October 4, 2022. https://www.fda.gov/news-events/press-announcements/fda-approves-first-once-monthly-buprenorphine-injection-medication-assisted-treatment-option-opioid
- 14.Treloar C, Lancaster K, Gendera S, et al. Can a new formulation of opiate agonist treatment alter stigma?: place, time and things in the experience of extended-release buprenorphine depot. Int J Drug Policy. 2022;107:103788. doi: 10.1016/j.drugpo.2022.103788 [DOI] [PubMed] [Google Scholar]
- 15.Allen E, Samadian S, Altobelli G, Johnson J, Holmwood C. Exploring patient experience and satisfaction with depot buprenorphine formulations: a mixed-methods study. Drug Alcohol Rev. 2023;42(4):791-802. doi: 10.1111/dar.13616 [DOI] [PubMed] [Google Scholar]
- 16.Knopf A. Spending bill includes X-waiver elimination, more. Alcohol Drug Abuse Wkly. 2022;34(33):4-5. doi: 10.1002/adaw.33531 [DOI] [Google Scholar]
- 17.Schackman BR, Leff JA, Polsky D, Moore BA, Fiellin DA. Cost-effectiveness of long-term outpatient buprenorphine-naloxone treatment for opioid dependence in primary care. J Gen Intern Med. 2012;27(6):669-676. doi: 10.1007/s11606-011-1962-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Linas BP, Savinkina A, Madushani RWMA, et al. Projected estimates of opioid mortality after community-level interventions. JAMA Netw Open. 2021;4(2):e2037259. doi: 10.1001/jamanetworkopen.2020.37259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Savinkina A, Madushani RWMA, Eftekhari Yazdi G, et al. Population-level impact of initiating pharmacotherapy and linking to care people with opioid use disorder at inpatient medically managed withdrawal programs: an effectiveness and cost-effectiveness analysis. Addiction. 2022;117(9):2450-2461. doi: 10.1111/add.15879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wakeman SE, Larochelle MR, Ameli O, et al. Comparative effectiveness of different treatment pathways for opioid use disorder. JAMA Netw Open. 2020;3(2):e1920622. doi: 10.1001/jamanetworkopen.2019.20622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siebert U, Alagoz O, Bayoumi AM, et al. State-transition modeling: a report of the ISPOR-SMDM modeling good research practices task force–3. Med Decis Making. 2012;32(5):690-700. doi: 10.1177/0272989X12455463 [DOI] [PubMed] [Google Scholar]
- 22.Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Med Decis Making. 1993;13(4):322-338. doi: 10.1177/0272989X9301300409 [DOI] [PubMed] [Google Scholar]
- 23.Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318. doi: 10.1016/S0140-6736(17)32812-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mass.gov. Massachusetts Public Health Data Warehouse (PHD). Accessed October 4, 2022. https://www.mass.gov/public-health-data-warehouse-phd
- 25.Barocas JA, White LF, Wang J, et al. Estimated prevalence of opioid use disorder in Massachusetts, 2011-2015: a capture-recapture analysis. Am J Public Health. 2018;108(12):1675-1681. doi: 10.2105/AJPH.2018.304673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Massachusetts Department of Public Health . An assessment of fatal and nonfatal opioid overdoses in Massachusetts (2011 – 2015). Published August 2017. Accessed October 4, 2022. https://www.mass.gov/files/documents/2017/08/31/legislative-report-chapter-55-aug-2017.pdf
- 27.Substance Abuse and Mental Health Services Administration. Results from the 2013 National Survey on Drug Use and Health: detailed tables. Published September 4, 2014. Accessed July 18, 2023. https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs2013.pdf
- 28.Cedarbaum ER, Banta-Green CJ. Health behaviors of young adult heroin injectors in the Seattle area. Drug Alcohol Depend. 2016;158:102-109. doi: 10.1016/j.drugalcdep.2015.11.011 [DOI] [PubMed] [Google Scholar]
- 29.Murphy SM, McCollister KE, Leff JA, et al. Cost-effectiveness of buprenorphine-naloxone versus extended-release naltrexone to prevent opioid relapse. Ann Intern Med. 2019;170(2):90-98. doi: 10.7326/M18-0227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.United States Census Bureau . Decennial census of population and housing datasets. 2010. Accessed October 4, 2022. https://www.census.gov/programs-surveys/decennial-census/data/datasets.2010.List_327707051.html#list-tab-List_327707051
- 31.IBM . IBM MarketScan Research Databases. Published 2021. Accessed October 4, 2022. https://www.ibm.com/uk-en/products/marketscan-research-databases/databases
- 32.Morgan JR, Schackman BR, Weinstein ZM, Walley AY, Linas BP. Overdose following initiation of naltrexone and buprenorphine medication treatment for opioid use disorder in a United States commercially insured cohort. Drug Alcohol Depend. 2019;200:34-39. doi: 10.1016/j.drugalcdep.2019.02.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.National Institute on Drug Abuse . Medications to Treat Opioid Use Disorder Research Report. Published 2021. Accessed July 25, 2023. https://nida.nih.gov/publications/research-reports/medications-to-treat-opioid-addiction/overview
- 34.McCollister KE, Leff JA, Yang X, et al. Cost of pharmacotherapy for opioid use disorders following inpatient detoxification. Am J Manag Care. 2018;24(11):526-531. [PMC free article] [PubMed] [Google Scholar]
- 35.Coffin PO, Santos GM, Matheson T, et al. Behavioral intervention to reduce opioid overdose among high-risk persons with opioid use disorder: a pilot randomized controlled trial. PLoS One. 2017;12(10):e0183354. doi: 10.1371/journal.pone.0183354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang Y, McDonald JV, Koziol J, McCormick M, Viner-Brown S, Alexander-Scott N. Can emergency department, hospital discharge, and death data be used to monitor burden of drug overdose in Rhode Island? J Public Health Manag Pract. 2017;23(5):499-506. doi: 10.1097/PHH.0000000000000514 [DOI] [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention . HIV Infection, risk, prevention, and testing behaviors among persons who inject drugs: National HIV Behavioral Surveillance: injection drug use, 23 US cities, 2018. Published February 2020. Accessed July 18, 2023. https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-special-report-number-24.pdf
- 38.Wittenberg E, Bray JW, Aden B, Gebremariam A, Nosyk B, Schackman BR. Measuring benefits of opioid misuse treatment for economic evaluation: health-related quality of life of opioid-dependent individuals and their spouses as assessed by a sample of the US population. Addiction. 2016;111(4):675-684. doi: 10.1111/add.13219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neumann PJ. SGD, Russell L.B., Siegel JE, Ganiats T.G. Cost-Effectiveness in Health and Medicine. 2nd ed. Oxford University Press; 2016. doi: 10.1093/acprof:oso/9780190492939.001.0001 [DOI] [Google Scholar]
- 40.Socias ME, Nolan S. Can extended-release injectable medications help curb United States and Canada's opioid overdose epidemic?. J Addict Med. 2021;15(1):15-17. doi: 10.1097/ADM.0000000000000697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kenney SR, Anderson BJ, Bailey GL, Stein MD. Buprenorphine treatment formulations: preferences among persons in opioid withdrawal management. J Subst Abuse Treat. 2018;94:55-59. doi: 10.1016/j.jsat.2018.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.US Food and Drug Administration . FDA approves new buprenorphine treatment option for opioid use disorder. Published May 23, 2023. Accessed July 22, 2023. https://www.fda.gov/news-events/press-announcements/fda-approves-new-buprenorphine-treatment-option-opioid-use-disorder
- 43.Santos C. Costa E, Machado SR. Uncovering competitive forces in prescription drug markets. Published online March 8, 2023. Accessed July 18, 2023. https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4374203
- 44.Linas BP, Barter DM, Morgan JR, et al. The cost-effectiveness of sofosbuvir-based regimens for treatment of hepatitis C virus genotype 2 or 3 infection. Ann Intern Med. 2015;162(9):619-629. doi: 10.7326/M14-1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Linas BP, Morgan JR, Pho MT, et al. Cost effectiveness and cost containment in the era of interferon-free therapies to treat hepatitis C virus genotype 1. Open Forum Infect Dis. 2016;4(1):ofw266. doi: 10.1093/ofid/ofw266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Larochelle MR, Bernson D, Land T, et al. Medication for opioid use disorder after nonfatal opioid overdose and association with mortality: a cohort study. Ann Intern Med. 2018;169(3):137-145. doi: 10.7326/M17-3107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rice D, Corace K, Wolfe D, et al. Evaluating comparative effectiveness of psychosocial interventions adjunctive to opioid agonist therapy for opioid use disorder: a systematic review with network meta-analyses. PLoS One. 2020;15(12):e0244401. doi: 10.1371/journal.pone.0244401 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Supplemental Materials
eTable 1. Utility Values (For Active Treatment)
eTable 2. Multiplicative Utility Sensitivity Analysis on Base Case
eFigure 1. Model Structure of Researching Effective Strategies to Prevent Opioid Death (RESPOND)
eFigure 2. Sensitivity of Cost and Quality-Adjusted Life Years for the Extended-Release Buprenorphine Strategy
eReferences
Data Sharing Statement