Abstract
We report the experimental transmission of Ehrlichia equi from naturally infected Ixodes pacificus ticks to horses. Three weeks after exposure to ticks, two of three horses developed clinical signs compatible with E. equi infection, while one horse remained asymptomatic. 16S rRNA gene PCR of blood leukocyte lysates was positive for all horses at various time points; two horses seroconverted. The 16S rRNA gene sequences amplified from tick-exposed horses showed more than 99% homology to corresponding fragments of the 16S rRNA genes of E. equi, Ehrlichia phagocytophila, and the human granulocytic ehrlichiosis agent.
Equine granulocytic ehrlichiosis (EGE) caused by Ehrlichia equi has been a recognized disease of horses since the 1960s (7–10). Clinical manifestations include fever, lethargy, anorexia, limb edema, thrombocytopenia, and petechiae (8). In California, cases of EGE occur in coastal or Sierra foothill ecosystems almost exclusively in certain well-defined geographical areas during the late fall, winter, and spring, corresponding to the season of peak activity of the adult stage of the possible vector, Ixodes pacificus Cooley and Kohls, the Western black-legged tick (2, 3, 10, 15). In New England, Ixodes scapularis Say (= Ixodes dammini Spielman et al.) is considered a likely vector (12), whereas Ixodes ricinus (L.) may be a vector in parts of Europe (9, 17). It has been shown by PCR that I. pacificus ticks are carriers of ehrlichiae of the Ehrlichia phagocytophila genogroup (3). Experimental transmission of E. equi to horses has been accomplished by using laboratory-reared I. pacificus ticks that had previously been fed on ehrlichemic horses (15). However, transmission of E. equi infection from field-derived I. pacificus has not been described. The purpose of the present study was to attempt transmission of infection and/or disease by experimental exposure of horses to I. pacificus ticks collected in areas enzootic for E. equi.
Adult I. pacificus ticks were collected during winter and spring 1996 from the following three locations in the Sierra Nevada and coastal foothills of northern California: Iowa Hill Road near Colfax, Placer County; East Michel Canyon in the University of California Quail Ridge Preserve near Moscovite Corners, Napa County; and Aetna Springs Road near Aetna Springs in Pope Valley, Napa County. The elevation of these three places was approximately 500 m. Ticks were maintained in the laboratory at 98% humidity and at 10 to 12°C until they were used. A confirmed case of E. equi infection had previously occurred in a horse residing near the Pope Valley collection site.
Three clinically normal, ectoparasite-free, E. equi-seronegative horses named Atempo (14-year-old female Thoroughbred), Alice (5-year-old female Standardbred), and Meretricious (14-year-old female Thoroughbred) were used. The horses were maintained at the Center for Equine Health, University of California, Davis, and had no history of residence in the tick collection areas. Atempo received a total of 73 ticks (47 males and 26 females) from Colfax (Placer County), of which 18 were observed to feed. Alice received a total of 67 ticks (39 males and 28 females) from Pope Valley (Napa County), of which 18 were observed to feed; Meretricious received a total of 63 ticks (32 males and 31 females) from Quail Ridge Preserve (Napa County), of which 14 ticks were observed to feed. The ticks were exposed to the horses in tennis hats glued by the brim to the horses’ hides and thus had access to approximately 100 cm2 of nonshaved skin. Approximately 20 to 30 ticks, half of which were male and half of which were female, were contained on each horse at any one time. Sequential batches of ticks were placed at approximately 7- or 8-day intervals, each horse serving as the host for ticks from only one location. The bags were opened and checked each day; fully or partly engorged ticks, dead or alive, were harvested, and their numbers were recorded. The ticks were maintained on each horse for as long as 3 weeks. During this period, the horses were monitored twice daily for clinical signs of illness. Blood samples were obtained for routine hematological, serological, and PCR assays. Hematological parameters included erythrocyte, leukocyte, and platelet counts and microscopic examination for the presence of ehrlichial inclusion bodies in the cytoplasm of neutrophils. Titers of antibody to E. equi in serum were determined by indirect immunofluorescence as described elsewhere (11). A serum titer of 1:10 was considered a positive result.
DNA obtained from peripheral blood leukocytes (PBL) of the experimental horses was used as the template for a nested PCR as described elsewhere (2). Current cycling parameters were as follows: pre-heat treatment at 94°C for 5 min and then 35 cycles of 94°C for 1 min, 60°C for 2 min, and 72°C for 1.5 min, followed by a final extension at 72°C for 7 min. The expected fragment size for the nested-round PCR was 928 bp. PCR products were visualized in ethidium bromide-stained 1.5% agarose gels. PCR products were purified and sequenced as described elsewhere (14). Sequence from both DNA strands was obtained for all inserts. Sequences were subjected to BLAST analysis (1) of GenBank nucleic acid sequences for similarity rank and alignments. Multiple alignments were made with CLUSTAL W (16) and Geneworks (IntelliGenetics, Mountain View, Calif.).
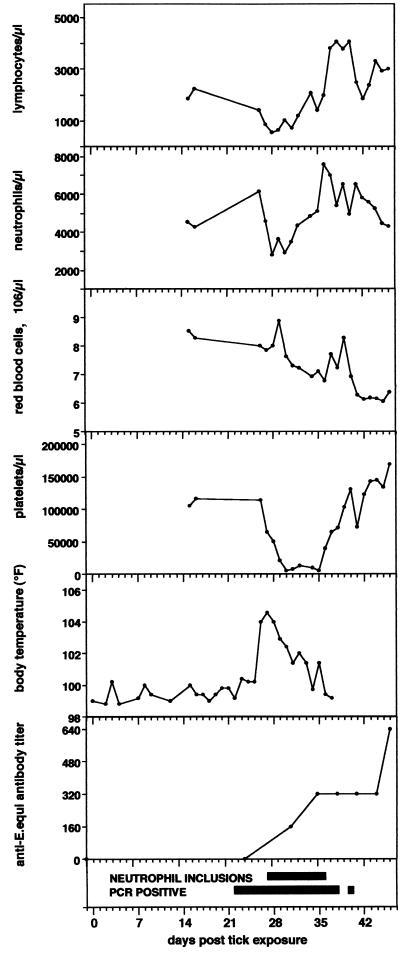
The first horse (Atempo) remained clinically normal for a period of approximately 3 weeks postexposure (p.e.). Clinical signs of disease such as lethargy and fever first appeared on day 25 p.e. and lasted for approximately 6 days (Fig. 1). Slight edema of the rear limbs was observed for several days from day 35 onward. The most dramatic hematological change was a severe decrease in the number of platelets to fewer than 10,000/μl of blood (normal range, 100,000 to 300,000/μl), beginning on approximately day 24 and lasting until day 35 (Fig. 1). The neutrophil and lymphocyte counts decreased considerably at about the time at which the fever occurred (day 25) and then rebounded on approximately day 33 (Fig. 1). Ehrlichial inclusion bodies (morulae) were observed in the neutrophils from days 27 to 36. The erythrocyte count decreased gradually from approximately day 28 until termination of the experiment on day 46 (Fig. 1).
FIG. 1.
Experimental tick-transmitted EGE in a horse. Data show lymphocyte, neutrophil, erythrocyte, platelet count, body temperature, anti-E. equi antibody titer, and microbiological findings in the horse Atempo following experimental exposure to I. pacificus ticks naturally infected with E. equi.
The second horse (Alice) remained clinically normal for 17 days p.e. On day 18, a fever (103.5°F) developed that lasted for 2 days and represented the only overt sign of illness. The most striking hematological result was a decrease in the numbers of platelets on days 18, 19, and 20 to 50% of the baseline counts. Neutrophil inclusion bodies were not observed on these dates.
The third horse (Meretricious) differed from the other two in that it remained normal for the entire observation period (28 days).
All three horses were seronegative for E. equi antibodies prior to tick exposure. Atempo seroconverted on day 32 p.e. (Fig. 1), and Alice seroconverted on day 22 (titer of 1:10, increasing to 1:160 on day 31 p.e.); Meretricious did not seroconvert.
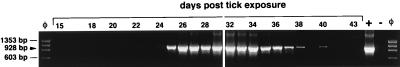
Prior to tick exposure, PBL lysates obtained from the horses were PCR negative for the 16S rRNA gene of E. equi. PBL from Atempo became positive on day 22 p.e., remained positive through day 38, and were positive for one last time on day 40 (Fig. 2). PBL from Alice were positive from days 14 through day 27 (data not shown). PBL from Meretricious were positive for E. equi on days 12, 13, and 14 and then negative on days 15, 16, and 17 (data not shown).
FIG. 2.
E. equi 16S rRNA PCR performed with serial PBL samples from the horse Atempo following experimental exposure to I. pacificus ticks naturally infected with E. equi collected in northern California. Arrowhead, position of the 16S rRNA PCR product (928 bp). Lanes: +, positive E. equi control; −, double-distilled water; and φ, phage fX174 digested with HaeIII.
BLAST searches with the nucleotide sequences from cloned 16S rRNA PCR products from these horses consistently resulted in more than 99% homology with corresponding fragments of the 16S rRNA genes of E. equi, E. phagocytophila, and the human granulocytic ehrlichiosis (HGE) agent. The 16S rRNA gene fragment amplified from Atempo revealed three nucleotide changes compared to E. equi and two nucleotide changes compared to the HGE agent (Table 1). The changes at positions 78 and 439 were unique. The 16S rRNA gene fragment obtained from Alice had one nucleotide change compared to both the California strain of E. equi and the HGE agent. However, the fragment was identical to the corresponding region of the E. equi MRK strain (6) and the llama ehrlichia (4). The 16S rRNA gene fragment from Meretricious had one nucleotide change (position 74) that was not observed in the other 16S rRNA sequences used for multiple alignment; otherwise, the sequence was identical to those of Alice, the E. equi MRK strain, and the llama ehrlichia. None of the three newly obtained strains had a nucleotide deletion at position 886, which is seen in the type (California) strain of E. equi and in E. phagocytophila (Table 1).
TABLE 1.
Nucleotide differences in a 925-bp fragment of the 16S rRNA gene of three equine Ehrlichia strains in comparison with other ehrlichiaea
| DNA source (reference) | Nucleotide at the position following positionsb:
|
||||
|---|---|---|---|---|---|
| 74 | 78 | 84 | 439 | 886 | |
| E. equic | A | A | A | G | —d |
| E. phagocytophilae | A | A | A | G | —d |
| E. equi MRK (6) | A | A | A | G | G |
| HGE agent | A | A | G | G | G |
| Llama ehrlichia (4) | A | A | A | G | G |
| E. equi (Atempo) | A | G | A | C | G |
| E. equi (Alice) | A | A | A | G | G |
| E. equi (Meretricious) | G | A | A | G | G |
Strains were obtained from three horses (Atempo, Alice, and Meretricious) following experimental transmission from naturally infected I. pacificus ticks collected in northern California.
Numbers refer to positions in the 16S rRNA gene of the HGE agent (GenBank accession no. UO2521); the amplified 16S rRNA fragment corresponds to positions 45 through 970 of the HGE agent. Nucleotide differences are underlined.
California strain of E. equi (GenBank accession no. M73223).
—, deletion at this position.
GenBank accession no. M73220.
Here, we report the successful transmission of E. equi, a causative agent of EGE and HGE, to horses through the bites of naturally infected I. pacificus ticks. Some of the ticks originated from an enzootic area where a confirmed case of E. equi infection had previously occurred. In one of the three horses (Atempo), clinical disease that was similar to that observed in experimentally induced E. equi infection resulted. However, the onset of both clinical signs and PCR positivity was considerably delayed and the duration and severity of clinical signs were considerably reduced compared to previous studies in which experimentally infected ticks were the source of E. equi. In addition, we noted mild (Alice) and subclinical (Meretricious) E. equi infections after experimental tick exposure, the latter having only a short period of PCR positivity. These seem to mirror more closely the situation in the field, where the seroprevalence of E. equi infections in horses residing in a hot zone may locally be high but where signs of disease are mild or completely absent (8). I. pacificus has been identified as a likely vector for E. phagocytophila genogroup rickettsiae in California by means of demography (19), tick transmission studies (15), and PCR (2, 4). This situation differs from that in the midwestern and eastern United States, where I. scapularis is the principal vector (5, 13). The results of the present study, together with the seasonal and geographic coincidence of tick feeding activity, reported EGE cases, and the consistent finding of I. pacificus attached to horses with EGE, establish I. pacificus as a vector of E. equi in California.
A second major result of this study concerns the sequence variability in the strains obtained from the tick-exposed horses. The three EGE 16S rRNA gene sequences differed from each other, and only one was identical to previously isolated ehrlichial strains from California in the homologous fragments available for analysis. The two other E. equi 16S rRNA gene sequences had either one or two unique nucleotide changes compared to E. equi or the HGE agent. Because there were very few base differences among the three new equine strains and E. equi, E. phagocytophila, and the HGE agent, it is not possible to classify them solely by their 16S rRNA gene nucleotide sequences. The sequences of this gene are known to vary in an orderly manner throughout the phylogenetic tree and hence represent desirable targets for PCR and phylogenetic analyses (20). However, whether minor base changes in the 16S rRNA gene might be indicative of changes in host range, the infectivity or pathogenicity of different ehrlichial strains is unknown. In light of the observed genetic polymorphism in the 16S rRNA gene, it is likely that genetic diversity will be more profound in genes coding for antigenic determinants serving as targets for antibody- or cell-mediated immune selection. Future molecular epidemiological studies of the genetic diversity of the ehrlichiae should therefore focus on less-conserved genes such as those coding for surface antigens. One question regarding the observed genetic polymorphism naturally arises: how different (and in which genes) must strains be to be considered different or the same species? In agreement with guidelines on the demarcation of virus species (18), we would respond that beyond its genome sequence relatedness and physicochemical properties, the biological properties of an organism, such as its host range, cell and tissue tropism, pathogenicity, cytopathology, antigenicity, and infectivity, should remain equally important criteria for classification.
Acknowledgments
We thank Elfriede DeRock, University of California, Davis, for technical assistance and the animal caretakers of the Center for Equine Health for their assistance throughout this study.
This work was supported by a grant from the Center for Equine Health, School of Veterinary Medicine, University of California, Davis (J.E.M. and R.B.K.).
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Barlough J E, Madigan J E, DeRock E, Bigornia L. Nested polymerase chain reaction for detection of Ehrlichia equi genomic DNA in horses and ticks (Ixodes pacificus) Vet Parasitol. 1996;63:319–329. doi: 10.1016/0304-4017(95)00904-3. [DOI] [PubMed] [Google Scholar]
- 3.Barlough J E, Madigan J E, Kramer V L, Clover J R, Hui L T, Webb J P, Vredevoe L K. Ehrlichia phagocytophila genogroup rickettsiae in ixodid ticks from California collected in 1995 and 1996. J Clin Microbiol. 1997;35:2018–2021. doi: 10.1128/jcm.35.8.2018-2021.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barlough J E, Madigan J E, Turoff D R, Clover J R, Shelly S M, Dumler J S. An Ehrlichia strain isolated from a llama (Lama glama) and llama-associated ticks (Ixodes pacificus) J Clin Microbiol. 1997;35:1005–1007. doi: 10.1128/jcm.35.4.1005-1007.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DesVignes F, Fish D. Transmission of the agent of human granulocytic ehrlichiosis by host-seeking Ixodus scapularis (Acari: Ixodidae) in southern New York state. J Med Entomol. 1997;34:379–382. doi: 10.1093/jmedent/34.4.379. [DOI] [PubMed] [Google Scholar]
- 6.Goodman J L, Nelson C, Vitale B, Madigan J E, Dumler J S, Kurtti T J, Munderloh U G. Direct cultivation of the causative agent of human granulocytic ehrlichiosis. N Engl J Med. 1996;334:209–215. doi: 10.1056/NEJM199601253340401. . (See comments.) [DOI] [PubMed] [Google Scholar]
- 7.Gribble D H. Equine ehrlichiosis. J Am Vet Med Assoc. 1969;155:462–469. [PubMed] [Google Scholar]
- 8.Madigan J E. Equine ehrlichiosis. Vet Clin N Am Equine Pract. 1993;9:423–428. doi: 10.1016/s0749-0739(17)30408-x. [DOI] [PubMed] [Google Scholar]
- 9.Madigan J E, Barlough J E, Dumler J S, Schankman N S, DeRock E. Equine granulocytic ehrlichiosis in Connecticut caused by an agent resembling the human granulocytotropic ehrlichia. J Clin Microbiol. 1996;34:434–435. doi: 10.1128/jcm.34.2.434-435.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madigan J E, Gribble D. Equine ehrlichiosis in Northern California: 49 cases (1968–1981) J Am Vet Med Assoc. 1987;190:445–448. [PubMed] [Google Scholar]
- 11.Madigan J E, Hietala S, Chalmers S, Derock E. Seroepidemiologic survey of antibodies to Ehrlichia equi in horses of northern California. J Am Vet Med Assoc. 1990;196:1962–1964. [PubMed] [Google Scholar]
- 12.Oliver J H, Jr, Owsley M R, Hutcheson H J, James A M, Chen C, Irby W S, Dotson E M, McLain D K. Conspecificity of the ticks Ixodes scapularis and I. dammini (Acari: Ixodidae) J Med Entomol. 1993;30:54–63. doi: 10.1093/jmedent/30.1.54. [DOI] [PubMed] [Google Scholar]
- 13.Pancholi P, Kolbert C P, Mitchell P D, Reed K D, Jr, Dumler J S, Bakken J S, Telford S R R, Persing D H. Ixodes dammini as a potential vector of human granulocytic ehrlichiosis. J Infect Dis. 1995;172:1007–1012. doi: 10.1093/infdis/172.4.1007. [DOI] [PubMed] [Google Scholar]
- 14.Reubel G H, Barlough J E, Madigan J E. Production and characterization of Ehrlichia risticii, the agent of Potomac horse fever, from snails (Pleuroceridae: Juga spp.) in aquarium culture and genetic comparison to equine strains. J Clin Microbiol. 1998;36:1501–1511. doi: 10.1128/jcm.36.6.1501-1511.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richter P J, Jr, Kimsey R B, Madigan J E, Barlough J E, Dumler J S, Brooks D L. Ixodes pacificus (Acari: Ixodidae) as a vector of Ehrlichia equi (Rickettsiales: Ehrlichieae) J Med Entomol. 1996;33:1–5. doi: 10.1093/jmedent/33.1.1. [DOI] [PubMed] [Google Scholar]
- 16.Thompson J D, Higgins D G, Gibson T J. Improved sensitivity of profile searches through the use of sequence weights and gap excision. Comp Appl Biosci. 1994;10:19–29. doi: 10.1093/bioinformatics/10.1.19. [DOI] [PubMed] [Google Scholar]
- 17.Timoney J F, Gillespie J H, Scott F W, Barlough J E. Hagan and Brunner’s microbiology and infectious diseases of domestic animals. 8th ed. Ithaca, N.Y: Cornell University Press; 1988. The Rickettsiaceae; pp. 326–331. [Google Scholar]
- 18.Van Regenmortel M H, Bishop D H, Fauquet C M, Mayo M A, Maniloff J, Calisher C H. Guidelines to the demarcation of virus species. Arch Virol. 1997;142:1505–1518. [PubMed] [Google Scholar]
- 19.Vredevoe L K, Richter P J, Madigan J E, Kimsey R B. Proceedings of the 44th Annual Meeting of the American Society for Tropical Medicine and Hygiene. 1995. Ecological association between Ixodes pacificus (Acari: Ixodidae) and the spatial and temporal distribution of equine ehrlichiosis in Northern California; p. 166. [Google Scholar]
- 20.Wilson K H, Blitchington R B, Greene R C. Amplification of bacterial 16S ribosomal DNA with polymerase chain reaction. J Clin Microbiol. 1990;28:1942–1946. doi: 10.1128/jcm.28.9.1942-1946.1990. . (Erratum, 29:666, 1991.) [DOI] [PMC free article] [PubMed] [Google Scholar]