This randomized clinical trial investigates if evinacumab provides a sustained reduction in low-density lipoprotein cholesterol level in patients with hypercholesterolemia refractory to maximum-tolerated combinations of currently available lipid-lowering therapies.
Key Points
Question
In patients with refractory hypercholesterolemia who have been treated with maximally tolerated combinations of currently available lipid-lowering therapies (LLTs), does evinacumab provide a sustained reduction in low-density lipoprotein cholesterol (LDL-C) level?
Findings
In this open-label treatment period of a phase 2 randomized clinical trial comprising 96 patients with refractory hypercholesterolemia, evinacumab, 15 mg/kg, administered intravenously once every 4 weeks provided sustained and clinically meaningful reductions in LDL-C level over time, with a mean reduction of 45.5% at week 72 from baseline.
Meaning
This study demonstrates the potential utility of evinacumab as an effective and generally well-tolerated LLT for patients with refractory hypercholesterolemia.
Abstract
Importance
Patients with refractory hypercholesterolemia who do not achieve their guideline-defined low-density lipoprotein cholesterol (LDL-C) thresholds despite treatment with maximally tolerated combinations of lipid-lowering therapies (LLTs) have an increased risk of atherosclerotic cardiovascular disease (ASCVD).
Objective
To evaluate longer-term efficacy and safety of evinacumab in patients with refractory hypercholesterolemia.
Design, Setting, and Participants
This randomized clinical trial included a 2-week screening period followed by a 16-week double-blind treatment period (DBTP) for subcutaneous regimens (evinacumab, 450 mg, once weekly [QW]; evinacumab, 300 mg, QW; evinacumab, 300 mg, every 2 weeks; or placebo QW) or a 24-week DBTP for intravenous regimens (evinacumab, 15 mg/kg, every 4 weeks [Q4W]; evinacumab, 5 mg/kg, Q4W; or placebo Q4W); a 48-week open-label treatment period (OLTP) for intravenous treatment only; and a 24-week follow-up period. Patients from 85 sites across 20 countries were recruited for the study; patients with primary hypercholesterolemia (defined as heterozygous familial hypercholesterolemia or established clinical ASCVD without familial hypercholesterolemia) who entered the 48-week OLTP were included. In addition, the patients’ hypercholesterolemia was refractory to maximally tolerated LLTs.
Interventions
All patients entering the OLTP received evinacumab, 15 mg/kg, intravenously Q4W.
Main Outcomes and Measures
Efficacy outcomes included change in LDL-C level and other lipid/lipoprotein parameters from baseline to week 72 (end of the OLTP). Safety outcomes included assessment of treatment-emergent adverse events (TEAEs).
Results
A total of 96 patients (mean [SD] age, 54.4 [11.3] years; 52 female [54.2%]) entered the OLTP, of whom 88 (91.7%) completed the OLTP. Mean (SD) baseline LDL-C level was 145.9 (55.2) mg/dL. At week 72, evinacumab, 15 mg/kg, reduced mean (SD) LDL-C level from baseline by 45.5% (28.7%) in the overall cohort. Evinacumab, 15 mg/kg, reduced mean (SD) apolipoprotein B (38.0% [22.1%]), non–high density lipoprotein cholesterol (48.4% [23.2%]), total cholesterol (42.6% [17.5%]), and median (IQR) fasting triglyceride (57.2% [65.4%-44.4%]) levels at week 72 from baseline in the overall cohort. TEAEs occurred in 78 of 96 patients (81.3%). Serious TEAEs occurred in 9 of 96 patients (9.4%); all were considered unrelated to study treatment.
Conclusions and Relevance
In patients with refractory hypercholesterolemia, evinacumab provided sustained reductions in LDL-C level and was generally well tolerated.
Trial Registration
ClinicalTrials.gov Identifier: NCT03175367
Introduction
Cardiovascular disease (CVD) is the leading cause of global mortality and a significant contributor to disability.1 The association between elevated low-density lipoprotein cholesterol (LDL-C) levels and increased risk of atherosclerotic CVD (ASCVD) has been established by various genetic, epidemiologic, and randomized interventional clinical trials.2
In individuals with severe hypercholesterolemia (LDL-C ≥190 mg/dL; to convert to millimoles per liter, multiply by 0.0259), an important diagnosis to consider is familial hypercholesterolemia (FH).3 Heterozygous FH (HeFH) is a common yet underdiagnosed genetic disorder.4 More than 90% of patients with genetically confirmed HeFH have a pathogenic loss-of-function gene variation in the LDL-receptor (LDLR) gene, leading to diminished or absent hepatic uptake and clearance of circulating LDL-C.5,6 Despite treatment with maximally tolerated combinations of available lipid-lowering therapies (LLTs), some patients with HeFH do not achieve recommended LDL-C treatment thresholds.7,8,9
Angiopoietinlike protein 3 (ANGPTL3) plays an important role in regulating lipoprotein metabolism via inhibition of lipoprotein lipase and endothelial lipase.10 Evinacumab is a fully human monoclonal antibody that inhibits ANGPTL3, promoting extensive remodeling of very low-density lipoprotein and leading to reduced LDL-C levels.11 During the double-blind treatment period (DBTP) of a phase 2 randomized clinical trial, evinacumab significantly reduced LDL-C levels in patients with and without HeFH and refractory hypercholesterolemia.11 Here, we report results from the subsequent open-label treatment period (OLTP) of this trial.
Methods
The study protocol (Supplement 1) and all amendments were approved by the appropriate institutional review board or independent ethics committee at each participating study site. All patients provided written informed consent before enrollment. Full details of the study design have been published previously.11 The study included a run-in period, followed by a 2-week screening period; a 16-week (subcutaneous groups) or 24-week (intravenous groups) DBTP; a 48-week OLTP (intravenous groups only); and a 24-week follow-up period (eFigure 1 in Supplement 2). The study was reported in accordance with Consolidated Standards of Reporting Trials (CONSORT) reporting guidelines.
Patients (aged 18-80 years) with primary hypercholesterolemia (defined as HeFH or non-FH with a history of clinical ASCVD) and refractory hypercholesterolemia, despite use of a PCSK9 inhibitor and maximally tolerated statin with or without ezetimibe, were eligible. Diagnosis of HeFH was made by genotyping or clinical criteria (eAppendix in Supplement 2).11 Refractory hypercholesterolemia was defined as an LDL-C level of 70 mg/dL or greater, or 100 mg/dL or greater, for patients with or without clinical ASCVD, respectively. A complete list of eligibility criteria is provided in eAppendix in Supplement 2. Patients from the following self-reported race and ethnicity groups were included in the study: African American or Black, Asian, Hispanic or Latino, not Hispanic or Latino, White, and other (included those who did not self-report as African American or Black, American Indian or Alaska Native, Asian, Native Hawaiian or Other Pacific Islander, or White).
During the DBTP, patients were randomly assigned to subcutaneous (1:1:1:1) or intravenous (1:1:1) treatment regimens. Randomization was stratified 2:1 (HeFH:non-FH). Subcutaneous regimens included evinacumab, 450 mg, once weekly (QW); evinacumab, 300 mg, QW; evinacumab, 300 mg, once every 2 weeks; or placebo QW. Intravenous regimens included evinacumab, 15 mg/kg, every 4 weeks (Q4W); evinacumab, 5 mg/kg, Q4W; or placebo Q4W. All patients in the intravenous groups entering the OLTP received evinacumab, 15 mg/kg, intravenous Q4W.
The objective of the OLTP was to evaluate the longer-term safety and tolerability of evinacumab, 15 mg/kg, given intravenously Q4W. We also evaluated the longer-term efficacy of evinacumab, 15 mg/kg, administered intravenously Q4W on lipid/lipoprotein parameters. LDL-C level was calculated using the Friedewald formula.11 This analysis was performed on the OL safety population comprising patients who received 1 or more doses of OL evinacumab. Formal statistical testing was not performed; all analyses were evaluated through descriptive statistics.
Results
A total of 96 patients (mean [SD] age, 54.4 [11.3] years; 52 female [54.2%]; 44 male [45.8%]) entered the OLTP and received evinacumab, 15 mg/kg, intravenously Q4W, with 88 (91.7%) completing the 48-week OLTP (eFigure 2 in Supplement 2). Eight patients (8.3%) discontinued early from the OLTP (withdrawal of consent, 4 patients; investigator/sponsor decisions, 3 patients; noncompliance with protocol, 1 patient). Patients self-identified with the following race and ethnicity categories: 2 African American or Black (2.1%), 1 Asian (1.0%), 10 Hispanic or Latino (10.4%), 86 not Hispanic or Latino (89.6%), 86 White (89.6%), and 7 other (7.3%). Mean (SD) duration of evinacumab exposure was 45.8 (6.4) weeks. Seventy-nine patients (82.3%) had a diagnosis of HeFH; 17 (17.7%) patients had a history of clinical ASCVD without FH (Table 1).9 Mean (SD) baseline (week 0) calculated LDL-C level was 145.9 (55.2) mg/dL. Most patients (92 of 96 [95.8%]) were taking a PSCK9 inhibitor, 81 (84.4%) were receiving a statin, with 51 (53.1%) receiving a high-intensity statin (all patients were receiving maximally tolerated doses of statin), and 36 patients (37.5%) were taking ezetimibe. The primary reason for patients not receiving a high-intensity statin was due to tolerability issues related to adverse muscle symptoms.
Table 1. Demographic and Baseline Characteristics of Patients Who Entered the Open-Label Treatment Period (OLTP), Both Overall and According to Double-Blind (DB) Treatment Group.
| Characteristic | DB placebo IV Q4W (n = 31) | DB evinacumab, 5 mg/kg IV Q4W (n = 32) | DB evinacumab, 15 mg/kg IV Q4W (n = 33) | Total (n = 96) |
|---|---|---|---|---|
| Age, mean (SD), y | 55.7 (10.7) | 56.7 (10.0) | 51.1 (12.6) | 54.4 (11.3) |
| Sex, No. (%) | ||||
| Female | 17 (54.8) | 20 (62.5) | 15 (45.5) | 52 (54.2) |
| Male | 14 (45.2) | 12 (37.5) | 18 (54.5) | 44 (45.8) |
| Race, No. (%)a | ||||
| African American or Black | 2 (6.5) | 0 | 0 | 2 (2.1) |
| Asian | 1 (3.2) | 0 | 0 | 1 (1.0) |
| White | 25 (80.6) | 30 (93.8) | 31 (93.9) | 86 (89.6) |
| Otherb | 3 (9.7) | 2 (6.3) | 2 (6.1) | 7 (7.3) |
| Ethnicity, No. (%)a | ||||
| Hispanic or Latino | 2 (6.5) | 7 (21.9) | 1 (3.0) | 10 (10.4) |
| Not Hispanic or Latino | 29 (93.5) | 25 (78.1) | 32 (97.0) | 86 (89.6) |
| BMI, mean (SD)c | 29.0 (5.2) | 28.4 (3.9) | 28.7 (4.8) | 28.7 (4.6) |
| HeFH, No. (%) | 25 (80.6) | 26 (81.3) | 28 (84.8) | 79 (82.3) |
| By genotyping | 11 (35.5) | 10 (31.3) | 9 (27.3) | 30 (31.3) |
| By clinical diagnosis | 14 (45.2) | 16 (50.0) | 19 (57.6) | 49 (51.0) |
| Non-FH, No. (%) | 6 (19.4) | 6 (18.8) | 5 (15.2) | 17 (17.7) |
| CHD, No. (%) | 24 (77.4) | 17 (53.1) | 19 (57.6) | 60 (62.5) |
| Calculated LDL-C level, mean (SD), mg/dL | 147.8 (45.5) | 143.3 (63.1) | 146.6 (56.9) | 145.9 (55.2) |
| Total cholesterol level, mean (SD), mg/dL | 234.3 (50.5) | 226.1 (61.8) | 222.4 (59.1) | 227.5 (57.0) |
| Non–HDL-C level, mean (SD), mg/dL | 179.0 (48.1) | 167.3 (64.1) | 172.5 (55.5) | 172.8 (55.9) |
| HDL-C level, mean (SD), mg/dL | 55.3 (18.4) | 58.9 (19.7) | 49.9 (14.1) | 54.6 (17.8) |
| Fasting triglyceride level, median (IQR), mg/dL | 147.0 (104.0-200.0) | 101.5 (86.5-148.0) | 120.0 (91.0-166.0) | 122.0 (92.5-171.0) |
| ApoA1, mean (SD), mg/dL | 157.9 (29.2) | 163.3 (32.0) | 146.3 (26.5) | 155.8 (29.9) |
| ApoB, mean (SD), mg/dL | 118.3 (27.0) | 111.8 (35.7) | 114.6 (32.9) | 114.9 (31.9) |
| ApoCIII, mean (SD), mg/dL | 11.3 (3.0) | 10.4 (3.3) | 9.5 (3.2) | 10.4 (3.2) |
| Lp(a), median (IQR), nmol/L | 31.0 (16.0-154.0) | 22.5 (18.0-66.0) | 35.0 (15.0-157.0) | 29.0 (16.5-120.0) |
| Any CV history/risk factors, No. (%) | 29 (93.5) | 28 (87.5) | 30 (90.9) | 87 (90.6) |
| CHD | 24 (77.4) | 17 (53.1) | 19 (57.6) | 60 (62.5) |
| Acute MI | 13 (41.9) | 8 (25.0) | 13 (39.4) | 34 (35.4%) |
| Silent MI | 0 | 0 | 0 | 0 |
| Angina (chronic stable/unstable) | 16 (51.6) | 13 (40.6) | 13 (39.4) | 42 (43.8) |
| Coronary revascularization procedure | 19 (61.3) | 12 (37.5) | 17 (51.5) | 48 (50.0) |
| CHD risk equivalents | 7 (22.6) | 5 (15.6) | 8 (24.2) | 20 (20.8) |
| Peripheral arterial disease | 1 (3.2) | 3 (9.4) | 6 (18.2) | 10 (10.4) |
| Ischemic stroke | 2 (6.5) | 1 (3.1) | 2 (6.1) | 5 (5.2) |
| Chronic kidney disease | 1 (3.2) | 0 | 0 | 1 (1.0) |
| Known history of diabetes (type 1 or 2) and 2 or more additional risk factorsd | 4 (12.9) | 2 (6.3) | 2 (6.1) | 8 (8.3) |
| Categorization of CV risk factors, No. (%)e | ||||
| Very high CV risk | 26 (83.9) | 19 (59.4) | 21 (63.6) | 66 (68.8) |
| High CV risk | 5 (16.1) | 13 (40.6) | 12 (36.4) | 30 (31.3) |
| LLTs, No. (%) | ||||
| Any statin | 26 (83.9) | 25 (78.1) | 30 (90.9) | 81 (84.4) |
| High-intensity statinf | 14 (45.2) | 17 (53.1) | 20 (60.6) | 51 (53.1) |
| Ezetimibe | 11 (35.5) | 14 (43.8) | 11 (33.3) | 36 (37.5) |
| PCSK9 inhibitor | 30 (96.8) | 30 (93.8) | 32 (97.0) | 92 (95.8) |
Abbreviations: Apo, apolipoprotein; BMI, body mass index; CHD, coronary heart disease (including acute or stable MI, chronic stable or unstable angina, or coronary revascularization); CV, cardiovascular; FH, familial hypercholesterolemia; HeFH, heterozygous familial hypercholesterolemia; HDL-C, high-density lipoprotein cholesterol; IV, intravenous; LDL-C, low-density lipoprotein cholesterol; Lp(a), lipoprotein(a); LLT, lipid-lowering therapy; MI, myocardial infarction; PCSK9, proprotein convertase subtilisin/kexin type 9; Q4W, every 4 weeks.
SI conversion factor: To convert ApoA1 to grams per liter, multiply by 0.01; to convert ApoB to grams per liter, multiply by 0.01; to convert HDL-C to millimoles per liter, multiply by 0.0259; to convert LDL-C to millimoles per liter, multiply by 0.0259; to convert total cholesterol to millimoles per liter, multiply by 0.0259.
Race and ethnicity were determined based on self-report to the treating physician.
Patients were categorized as other if they did not self-report as African American or Black, American Indian or Alaska Native, Asian, Native Hawaiian or Other Pacific Islander, or White.
Calculated as weight in kilograms divided by height in meters squared.
Risk factors include history of ankle-brachial index 0.9 or less, hypertension, microalbuminuria or macroalbuminuria, proliferative diabetic retinopathy, and known family history of CHD.
Patients with very-high CV risk were defined as patients with CHD or CHD risk equivalents. Patients with high CV risk were defined as patients without very-high CV risk. To note, the definition of very-high CV risk in this study differs from the 2022 American College of Cardiology expert consensus decision pathway on the role of nonstatin therapies for LDL-C lowering in the management of ASCVD risk, which defines patients with very-high risk as those with a history of multiple major ASCVD events or 1 major ASCVD event and multiple high-risk conditions.9
High-intensity statin defined as rosuvastatin, 20 mg or 40 mg, daily or atorvastatin, 40 mg or 80 mg, daily.
At week 72 (the end of the OLTP), evinacumab, 15 mg/kg, reduced LDL-C level from baseline by a mean (SD) percentage change of 45.5% (28.7%) in the overall cohort, with mean (SD) reductions of 43.0% (29.5%), 49.9% (23.3%), and 43.8% (32.9%) in patients who had received evinacumab, 15 mg/kg, evinacumab, 5 mg/kg, and placebo, respectively, during the DBTP (Figure; eTable 1 in Supplement 2). Corresponding mean (SD) absolute reductions in LDL-C level at week 72 from baseline were 72.2 (55.4) mg/dL, 70.3 (63.8) mg/dL, 79.1 (55.6) mg/dL, and 67.7 (48.0) mg/dL for the overall, DB evinacumab, 15 mg/kg, DB evinacumab, 5 mg/kg, and DB placebo groups, respectively. Overall, a similar decrease in LDL-C level was observed in patients with predicted loss-of-function gene variations in LDLR, gain-of-function gene variations in PCSK9, or missense variations in LDLR and/or apolipoprotein (Apo) B (eFigure 3 in Supplement 2). At week 72, the proportion of patients achieving LDL-C level targets of less than 55 mg/dL, less than 70 mg/dL, and less than 100 mg/dL with OL evinacumab was 31.3% (25 of 80), 52.5% (42 of 80), and 82.5% (66 of 80), respectively (eTable 2 in Supplement 2).
Figure. Mean (SE) Percentage Change in Calculated Low-Density Lipoprotein Cholesterol Level From Baseline Over Time by Double-Blind (DB) Treatment Group.
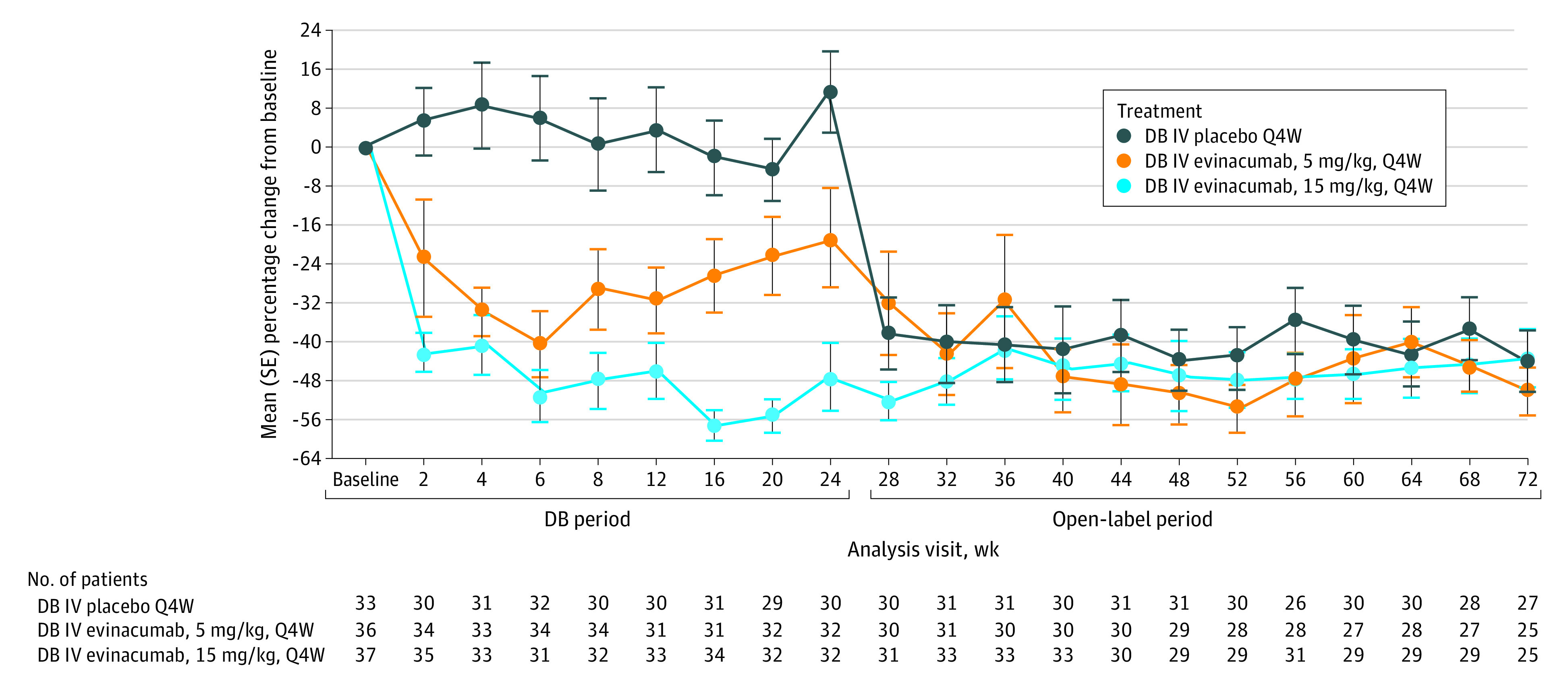
The receiving-treatment period ends at the last infusion date plus 35 days. IV indicates intravenous; Q4W, every 4 weeks.
At week 72, mean (SD) percentage changes with evinacumab, 15 mg/kg, from baseline in the overall cohort were −42.6% (17.5%) for total cholesterol, −23.8% (19.8%) for high-density lipoprotein cholesterol (HDL-C), −48.4% (23.2%) for non–HDL-C, −28.0% (13.2%) for ApoA1, −38.0% (22.1%) for ApoB, and −70.9% (20.0%) for ApoCIII levels; median (IQR) changes were −57.2% (−65.4% to −44.4%) for fasting triglyceride level and −12.7% (−34.3% to 5.1%) for lipoprotein(a) level (eTable 1 in Supplement 2).
Overall, 78 of 96 patients (81.3%) experienced at least 1 treatment-emergent adverse event (TEAE) during the OLTP (Table 2; eTable 3 in Supplement 2). The most common TEAE with evinacumab, 15 mg/kg, given intravenously Q4W was nasopharyngitis (12.5% [12 of 96]), similar to the DBTP. Exposure-adjusted TEAEs for the OLTP (along with corresponding data for placebo-treated patients in the DBTP) are presented in Table 2. A comprehensive summary of exposure-adjusted TEAEs occurring during the DBTP for all intravenous treatment groups is presented in eTable 4 in Supplement 2. Four of 96 patients (4.2%) presented with laboratory abnormalities pertaining to liver function, one of whom had TEAEs of increased alanine aminotransferase and increased aspartate aminotransferase that resolved completely and were considered unrelated to study treatment by the investigator (eTable 5 in Supplement 2). Four of 96 patients (4.2%) experienced at least 1 TEAE considered related to study treatment by the investigator; these were an increase in LDL-C level of moderate severity (n = 1), soft feces and frequent bowel movements of mild severity (n = 1), malaise (considered an infusion-related reaction; n = 1), and influenzalike illness of mild severity (n = 1). In all 4 patients, there was complete resolution of TEAEs, and no changes to study treatment were made.
Table 2. Summary of Treatment-Emergent Adverse Events (TEAEs) for All Patients During the Open-Label Treatment Period (OLTP) and During the Double-Blind Treatment Period (DBTP) for Patients Who Received DB Placeboa.
| TEAE type | TEAEs during the OLTP for all patients who received evinacumab 15 mg/kg IV Q4W (n = 96; PY = 122.8) | TEAEs during the DBTP for patients who received placebo IV Q4W in the DBTP and entered the OLTP (n = 31; PY = 14.4) | ||
|---|---|---|---|---|
| No. (%) of patients | No./100 PY | No. (%) of patients | No./100 PY | |
| Any TEAE | 78 (81.3) | 159.0 | 22 (71.0) | 319.2 |
| TE serious AEs | 9 (9.4) | 7.8 | 1 (3.2) | 7.0 |
| TEAEs leading to treatment discontinuation | 0 | 0 | 0 | 0 |
| TEAEs leading to death | 0 | 0 | 0 | 0 |
| TEAEs occurring in >5% of patients during the OLTPb,c | ||||
| Nasopharyngitis | 12 (12.5) | 10.6 | 2 (6.5) | 14.2 |
| Urinary tract infection | 11 (11.5) | 9.4 | 0 | 0 |
| Headache | 10 (10.4) | 8.9 | 5 (16.1) | 39.4 |
| Influenzalike illness | 8 (8.3) | 7.0 | 3 (9.7) | 22.0 |
| Upper respiratory tract infection | 8 (8.3) | 6.8 | 0 | 0 |
| Arthralgia | 5 (5.2) | 4.1 | 2 (6.5) | 14.7 |
| Back pain | 5 (5.2) | 4.2 | 2 (6.5) | 14.6 |
| Chest pain | 5 (5.2) | 4.2 | 0 | 0 |
| Fatigue | 5 (5.2) | 4.2 | 2 (6.5) | 13.9 |
Abbreviations: DB, double blind; IV, intravenous; OL, open label; PY, patient-years; Q4W, every 4 weeks.
The OL TEAE period was defined from the day of the first dose of OL study treatment administration to the day of the last dose of OL study treatment administration +168 days (24 weeks). The DB TEAE period was defined from the day of the first dose of DB study treatment administration to the day of the first dose of OL study treatment administration. PY for the OLTP and the DBTP is defined for patients with an event censored at the time of first event and for patients without an event. PY corresponds to the length of the TEAE period. Thus, the PY listed in the table headers are the maximum possible PY but will differ for each TEAE depending on how many patients experienced the TEAE (and thus had follow-up time censored).
The events are listed in descending order of frequency in the OLTP.
All TEAEs occurring in more than 5% of patients during the OLTP are listed. For comparative purposes, only corresponding TEAE data for the DBTP is provided for placebo-treated patients. A more comprehensive summary of TEAEs during the DBTP for all DB IV treatment groups is provided in eTable 4 in Supplement 2.
Serious TEAEs occurred in 9 of 96 patients (9.4%); these were carotid artery stenosis (n = 1), chest pain (n = 1), coronavirus infection (n = 1), gallbladder polyp (n = 1), gastrointestinal motility disorder (n = 1), joint effusion (n = 1), pulmonary embolism (n = 1), atrial fibrillation and transitional cell carcinoma (both in a single patient), and atrial fibrillation, palpitations, and dyspnea (all in a single patient). These serious TEAEs were not considered drug-related by the investigator. No deaths or TEAEs leading to discontinuation were reported.
Discussion
Results of the 48-week OLTP of this phase 2 randomized clinical trial demonstrate that evinacumab, 15 mg/kg, Q4W reduced LDL-C level by 45.5% in patients with refractory hypercholesterolemia, with reductions sustained through week 72. Patients who switched from DB evinacumab, 5 mg/kg, to evinacumab, 15 mg/kg, had an intensification of the LDL-C-lowering effect; patients who switched from DB placebo had similar efficacy on evinacumab, 15 mg/kg. Evinacumab also reduced other atherogenic lipoproteins during the 48-week OLTP, supporting the observed results from the previously published 24-week DBTP.11 The safety profile of evinacumab during the OLTP remained consistent with that observed during the DBTP.
The 2022 American College of Cardiology expert consensus decision pathway on the role of nonstatin therapies for LDL-C lowering in the management of ASCVD risk recommends LDL-C thresholds of less than 55 mg/dL for adults with ASCVD at very high risk, less than 70 mg/dL for adults with ASCVD not at very high risk, and less than 100 mg/dL for adults without clinical ASCVD and baseline LDL-C of 190 mg/dL or greater not due to secondary causes.9 To note, the definition of refractory hypercholesterolemia used in this study was formulated before these recently recommended LDL-C thresholds. Although each LLT considered standard of care that is used for the treatment of HeFH can contribute to the overall lowering of LDL-C level, a subset of patients do not achieve these recommended LDL-C thresholds, thus remaining at risk for ASCVD.7,8 In our study, which included a high proportion of patients with HeFH, 52.5% achieved LDL-C levels less than 70 mg/dL after treatment with OL evinacumab.
Limitations
Limitations of this study include the small sample size, relatively short treatment duration, and minimal diversity in the racial and ethnic backgrounds of study participants. Currently, evinacumab is approved in the US as an adjunct to other LDL-C–lowering therapies for patients (aged 5 years and older) with homozygous FH.12 Although acknowledging that long-term intravenous administration of evinacumab could be burdensome for a broader population with refractory hypercholesterolemia, standard-of-care LLTs are insufficient in reducing LDL-C level for a subset of patients, thus indicating a clinical need for novel agents.
Conclusions
Results of this randomized clinical trial demonstrate that, in patients with and without HeFH and refractory hypercholesterolemia, evinacumab provided clinically meaningful and sustained reductions in LDL-C level and was generally well tolerated. Larger studies in a broader population would be needed to define clinical efficacy and provide more robust long-term safety data.
Trial Protocol
eAppendix.
eTable 1. Percentage Change in Lipid/Lipoprotein Efficacy Parameters From Baselinea to Week 72 in the OLTP, Both Overall and According to DB Treatment Group
eTable 2. Proportion of Patients Achieving LDL-C Thresholds at Week 72 in the OLTP, Both Overall and According to DB Treatment Group
eTable 3. Summary of TEAEs During the OLTP, Both Overall and According to DB Treatment Groupa
eTable 4. Summary of TEAEs During the DBTP According to DB Treatment Groupsa
eTable 5. Laboratory Investigations for Liver Function During the OLTP, Both Overall and According to DB Treatment Groupa
eFigure 1. Study Design
eFigure 2. Patient Flow Diagram
eFigure 3. Percentage Change in LDL-C at Week 72 From Baseline Categorized by Genotypinga
Data Sharing Statement
References
- 1.Roth GA, Mensah GA, Johnson CO, et al. ; GBD-NHLBI-JACC Global Burden of Cardiovascular Diseases Writing Group . Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 Study. J Am Coll Cardiol. 2020;76(25):2982-3021. doi: 10.1016/j.jacc.2020.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ference BA, Ginsberg HN, Graham I, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38(32):2459-2472. doi: 10.1093/eurheartj/ehx144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol. 2019;73(24):3168-3209. doi: 10.1016/j.jacc.2018.11.002 [DOI] [PubMed] [Google Scholar]
- 4.McGowan MP, Hosseini Dehkordi SH, Moriarty PM, Duell PB. Diagnosis and treatment of heterozygous familial hypercholesterolemia. J Am Heart Assoc. 2019;8(24):e013225. doi: 10.1161/JAHA.119.013225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gidding SS, Champagne MA, de Ferranti SD, et al. ; American Heart Association Atherosclerosis, Hypertension, and Obesity in Young Committee of Council on Cardiovascular Disease in Young, Council on Cardiovascular and Stroke Nursing, Council on Functional Genomics and Translational Biology, and Council on Lifestyle and Cardiometabolic Health . The agenda for familial hypercholesterolemia: a scientific statement from the American Heart Association. Circulation. 2015;132(22):2167-2192. doi: 10.1161/CIR.0000000000000297 [DOI] [PubMed] [Google Scholar]
- 6.Nordestgaard BG, Chapman MJ, Humphries SE, et al. ; European Atherosclerosis Society Consensus Panel . Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. Eur Heart J. 2013;34(45):3478-3490a. doi: 10.1093/eurheartj/eht273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rallidis LS, Liberopoulos EN, Vlachopoulos C, et al. Very high-risk familial hypercholesterolemia patients in real life: the remaining gap in achieving the current LDL-C targets despite the use of PCSK9 inhibitors. Atherosclerosis. 2020;309:67-69. doi: 10.1016/j.atherosclerosis.2020.07.018 [DOI] [PubMed] [Google Scholar]
- 8.Gaudet D, López-Sendón JL, Averna M, et al. ; ODYSSEY APPRISE Study investigators . Safety and efficacy of alirocumab in a real-life setting: the ODYSSEY APPRISE study. Eur J Prev Cardiol. 2022;28(17):1864-1872. doi: 10.1093/eurjpc/zwaa097 [DOI] [PubMed] [Google Scholar]
- 9.Lloyd-Jones DM, Morris PB, Ballantyne CM, et al. ; Writing Committee . 2022 ACC expert consensus decision pathway on the role of nonstatin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2022;80(14):1366-1418. doi: 10.1016/j.jacc.2022.07.006 [DOI] [PubMed] [Google Scholar]
- 10.Rosenson RS. Existing and emerging therapies for the treatment of familial hypercholesterolemia. J Lipid Res. 2021;62:100060. doi: 10.1016/j.jlr.2021.100060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenson RS, Burgess LJ, Ebenbichler CF, et al. Evinacumab in patients with refractory hypercholesterolemia. N Engl J Med. 2020;383(24):2307-2319. doi: 10.1056/NEJMoa2031049 [DOI] [PubMed] [Google Scholar]
- 12.Regeneron Pharmaceuticals Inc . EVKEEZA (evinacumab-dgnb) injection, for intravenous use. Accessed April 9, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761181s000lbl.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eAppendix.
eTable 1. Percentage Change in Lipid/Lipoprotein Efficacy Parameters From Baselinea to Week 72 in the OLTP, Both Overall and According to DB Treatment Group
eTable 2. Proportion of Patients Achieving LDL-C Thresholds at Week 72 in the OLTP, Both Overall and According to DB Treatment Group
eTable 3. Summary of TEAEs During the OLTP, Both Overall and According to DB Treatment Groupa
eTable 4. Summary of TEAEs During the DBTP According to DB Treatment Groupsa
eTable 5. Laboratory Investigations for Liver Function During the OLTP, Both Overall and According to DB Treatment Groupa
eFigure 1. Study Design
eFigure 2. Patient Flow Diagram
eFigure 3. Percentage Change in LDL-C at Week 72 From Baseline Categorized by Genotypinga
Data Sharing Statement


