Key Points
Question
What is the prevalence of sexual dysfunction in individuals with schizophrenia and the factors associated with heterogeneity of dysfunction?
Findings
In this systematic review and meta-analysis of 72 studies including 21 076 participants with schizophrenia, prevalence and heterogeneity of global sexual dysfunction were high. Study design, time and location, sociodemographic data, alcohol use disorder, psychiatric diagnosis, illness severity, and the use of antidepressants and anxiolytics were associated with heterogeneity; associations were also found between lower prevalence of sexual dysfunction and use of antidepressant medication.
Meaning
The findings in this study suggest that prevalence of sexual dysfunction remains high in people with schizophrenia with no obvious improvement over time or better tolerance of second-generation antipsychotics; treating depression may be a key point to reduce sexual dysfunction in individuals with schizophrenia.
This systematic review and meta-analysis evaluates the prevalence of and factors associated with sexual dysfunction in individuals with schizophrenia-spectrum disorders.
Abstract
Importance
In individuals with schizophrenia, antipsychotic-induced dysfunctions are frequent but often underexplored in clinical practice.
Objective
To synthetize the data of observational studies exploring the prevalence of sexual dysfunction in individuals with schizophrenia-spectrum disorders as well as associated factors.
Data Sources
A systematic literature search without language or time restrictions was conducted in Google, Google Scholar, PubMed/MEDLINE, Science Direct, and Université Sorbonne Paris Cité for studies published up to June 8, 2022.
Study Selection
All observational studies reporting a prevalence of sexual dysfunction in schizophrenia-spectrum disorder were included.
Data Extraction and Synthesis
The MOOSE guidelines with independent extraction by 2 observers and random-effects models were used.
Main Outcomes and Measures
The prevalence of sexual dysfunction and each specific dysfunction.
Results
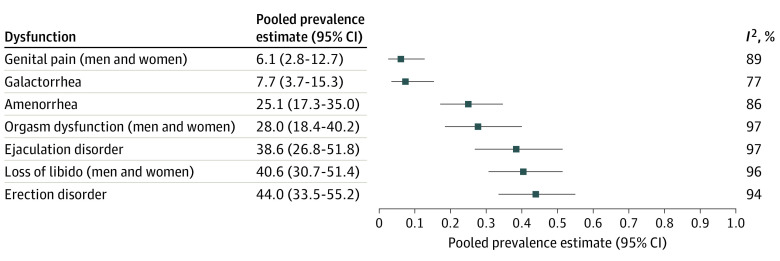
A total of 72 of 1119 studies from 33 countries on 6 continents published from inception to June 2022 were included with a total of 21 076 participants with schizophrenia. The pooled global prevalence of sexual dysfunctions was 56.4% (95% CI, 50.5-62.2), with a prevalence of 55.7% (95% CI, 48.1-63.1) for men and 60.0% (95% CI, 48.0-70.8) for women. The most frequent sexual dysfunction was erectile dysfunction in men (44%; 95% CI, 33.5-55.2), followed by loss of libido in men (41%; 95% CI, 30.7-51.4), ejaculation dysfunction in men (39%; 95% CI, 26.8-51.8), orgasm dysfunction in women (28%; 95% CI, 18.4-40.2), and amenorrhea in women (25%; 95% CI, 17.3-35.0). Factors associated with heterogeneity were study design, time and location, sociodemographic data, alcohol use disorder, psychiatric diagnosis, illness severity, and the use of antidepressants and anxiolytics. Sexual dysfunctions were more frequent in schizophrenia vs schizoaffective disorders, and erectile disorders were less frequent in individuals with longer illness duration. Antidepressant and mood stabilizer prescriptions were associated with lower rates of erection disorders (β, −6.30; 95% CI, −10.82 to −1.78); P = .006 and −13.21; 95% CI, −17.59 to −8.83; P < .001, respectively) and ejaculation disorders (β, −6.10; 95% CI, −10.68 to −1.53; P = .009 and β, −11.57; 95% CI, −16.34 to −6.80; P < .001, respectively). No obvious improvements in the rates of sexual dysfunction at other times were found, and there were conflicting results regarding antipsychotic classes.
Conclusions and Relevance
This systematic review and meta-analysis found a high prevalence of sexual dysfunction among individuals with schizophrenia, with considerable heterogeneity in associated factors. The findings also suggest that some dysfunctions may be explained by schizophrenia. The association between lower rates of dysfunction and antidepressant use suggests that treating comorbid depression could be an effective strategy to improve sexual health. A lack of data on metabolic parameters and physical health in general was also noted, while these issues are frequent in the care of schizophrenia.
Introduction
The attention given to sexual health in schizophrenia has increased since the end of the 20th century.1 However, schizophrenia has been associated with increased sexual dysfunction only in a small meta-analysis2 of 10 observational studies (3 case-control studies and 7 cross-sectional studies) published in 2020. An meta-analysis3 of sexual dysfunction in psychiatric patients taking antipsychotics and limited to studies with sexual dysfunction as the primary objective and using validated scales concluded that antipsychotics were a major source of heterogeneity in the prevalence of sexual dysfunction in schizophrenia, with quetiapine, ziprasidone, perphenazine, aripiprazole, olanzapine, risperidone, haloperidol, clozapine, and thioridazine having an increasing impact on sexual function ranging from 16% (quetiapine) to 60% (thioridazine). One of the major pathophysiological mechanisms is probably the inhibition of dopamine D2 receptors. This inhibition in the tuberoinfundibular pathway is associated with increased serum prolactin levels.4 A dose of more than 200 ng/mL of risperidone5 induces amenorrhea, breast tenderness, and galactorrhea in women and hypogonadism in men by disrupting the secretion of GnRH by the hypothalamus.6,7 Antipsychotic-induced sexual dysfunctions may also occur independently of increased prolactinemia due to increased antiparkinsonian adverse effects, including blunted affect and anhedonia.4 Anti–α1-adrenergic receptor inhibition may also induce sexual dysfunctions through relaxation of the intracavernosal smooth muscle fibers, inducing erection and ejaculation dysfunctions in men.8 Anti–H1 receptor inhibition induces sedation, which may alter satisfactory sexual activity.4
Antipsychotic-induced sexual dysfunctions thus appear to be heterogeneous by their type and pathophysiological mechanism. Distinguishing each specific sexual dysfunction may increase the accuracy of interventions. A recent systematic review9 found that the most frequent disorders in schizophrenia were loss of libido and erectile dysfunction in men. Decreased libido may be the result of the negative syndrome of the illness associated with anhedonia and social withdrawal.10 Major depression is a frequent comorbidity of schizophrenia found in approximately one-third of patients.11 It is underscreened and undertreated due to confusion with the negative symptoms of schizophrenia.11 Several potential risk factors for sexual dysfunction in schizophrenia have been identified, including comorbid major depression, unemployment, singlehood, tobacco smoking, and cannabis and alcohol use disorders.9 In summary, we need to update the data on sexual dysfunction prevalence, as the only meta-analysis on this topic was published more than 10 years ago, and we need a quantitative analysis of associated factors that may explain the heterogeneity of sexual dysfunctions in schizophrenia with a focus on each sexual dysfunction (in each and both sexes) resulting from different pathophysiological mechanisms. The objectives of the present study were to determine the prevalence of sexual dysfunction in schizophrenia and of each specific dysfunction (sexual dysfunctions in each sex, loss of libido, orgasm dysfunction, genital pain, erectile and ejaculation dysfunctions, amenorrhea, and galactorrhea) and to identify factors associated with heterogeneity.
Methods
Search Strategy and Sources of Information
The study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline.12 We performed a literature review on 5 digital databases: PubMed/MEDLINE, Science Direct, Google Scholar, Google, and Université Sorbonne Paris Cité from inception to June 8, 2022. The search paradigm is presented in eAppendix 1 in Supplement 1. The protocol was registered in PROSPERO (CRD42022355766). Ethical approval was not sought or required, as the study involved no individual patient data.
Eligibility Criteria
All observational studies reporting a prevalence of sexual dysfunction (by any tool of measure, which includes nonvalidated tools, such as binary questions and unstructured clinical interviews) in patients with schizophrenia or schizoaffective disorder were included in the present work. In the case of longitudinal studies, cross-sectional baseline data were included. While English search words were used for PubMed/MEDLINE, Science Direct, Google Scholar, Google and French words for Université Sorbonne Paris Cité, there was no language limitation to ensure the comprehensiveness of the data, and the articles were translated as needed to extract relevant data.
All interventional studies were excluded to eliminate a participation bias and because randomized clinical trials overselect participants.13 Studies including inpatients were excluded, assuming that the stress of the hospitalization and the short-term phase of the disease would have a major impact on sexual dysfunction. Studies assessing only treatment-resistant patients were not included, as we hypothesized an overestimation bias in these patients, who usually receive high doses of treatments with persistent psychotic symptomatology. In summary, the aim of the present work was to assess sexual dysfunction in the daily life of treated patients, not in resistant or hospitalized patients.
Data Extraction
Two investigators (M.F. and V.A.) independently performed the literature review and extracted data. Controversial articles were discussed in a meeting between the 2 investigators, and in case of persistent disagreement, a third investigator (G.F.) was consulted and made the final decision. The extracted data are presented in eAppendix 2 in Supplement 1.
Assessment of Methodological Quality
The study quality assessment was carried out independently by 2 investigators (M.F. and V.A.) using the modified version of the Newcastle-Ottawa Scale (NOS).14 Studies were scored from 0 (poor quality) to 10 points (excellent quality). Studies scoring 7 or more points were considered good quality studies.
Statistical Analysis
A random logistic regression model was used to calculate the pooled prevalence estimates of sexual dysfunction and its 95% CI.15 A sensitivity analysis was performed with the inverse-variance random-effects model, confirming the robustness of the findings. Heterogeneity between studies was quantified using the I2 statistic.16 Sensitivity analyses were performed using the leave-one-out method. Publication bias was assessed graphically with a funnel plot and statistically with the Thompson-Sharp test when the number of studies exceeded 10.17 Subgroup analyses for binary variables and univariate meta-regressions for quantitative variables were used to assess factors moderating the pooled prevalence estimate of sexual dysfunction from individual studies. All analyses were performed using the meta package in R version 4.1.3 (R Foundation).18
Results
Study Characteristics
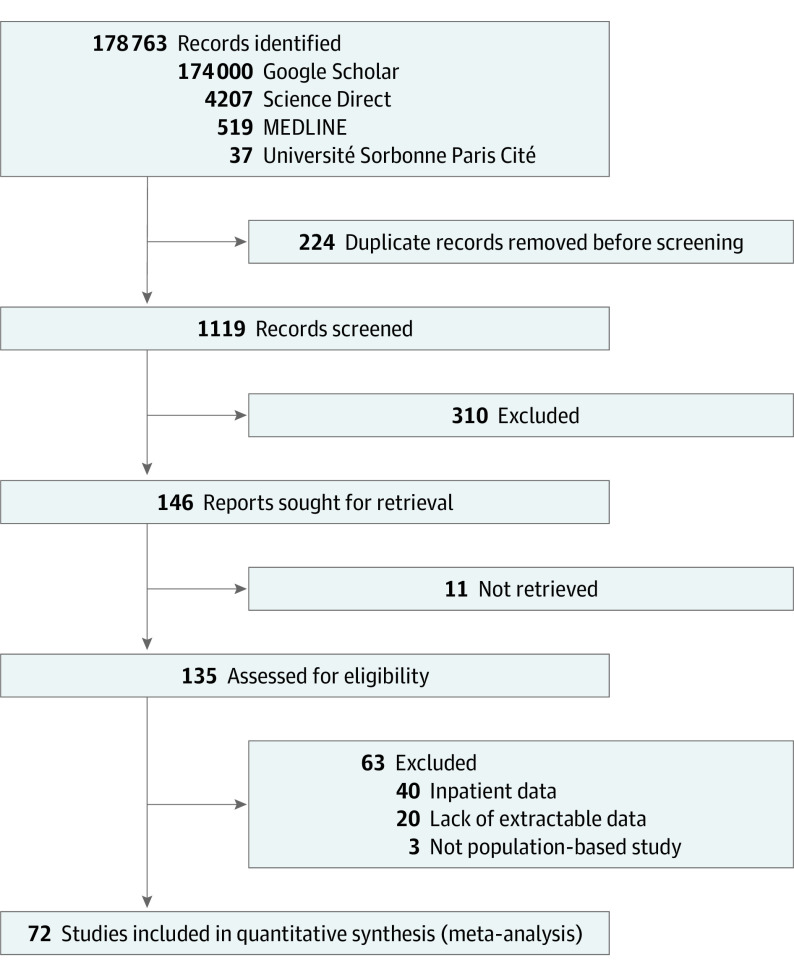
Seventy-two studies published between 1979 and 2021 were included in the random-effects model.6,7,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88 The flow diagram is presented in Figure 1. Among those, 35 studies included data on the prevalence of orgasm dysfunction, 34 on loss of libido, 10 on genital pain, 33 on erectile dysfunction, 19 on ejaculation dysfunction, 6 on amenorrhea, and 5 on galactorrhea. The 63 excluded studies and the reason for exclusion are presented in eAppendix 3 in Supplement 1.
Figure 1. PRISMA Flow Diagram.
Flow diagram for new systematic reviews, which included searches of databases and registers only.
The selected articles included 21 076 patients from 33 countries on 6 continents. The study characteristics are reported in eAppendices 4-6 in Supplement 1. The study quality assessment is presented in eAppendix 7 in Supplement 1. Overall, 30 studies (41.7%) were classified in the good quality group. A total of 59 studies (81.9%) used a standardized questionnaire to assess sexual dysfunctions (eAppendices 4-6 in Supplement 1). The most frequent sexual dysfunction scales were the Arizona Sexual Experience scale89 (N = 19), Changes in Sexual Functioning Questionnaire90 (N = 7), Sexual Functioning Questionnaire91 (N = 6), Female Sexual Function Index92 (N = 6), Udvalg for Kliniske Undersogelser93 (N = 4), and Psychotropic-Related Sexual Dysfunction Questionnaire94 (N = 4). Thirteen studies (18.1%) used a clinical semistructured interview, 6 (8.3%) used an original questionnaire, and 1 (1.4%) used medical records.
Pooled Prevalence Estimate of Global Sexual Dysfunctions
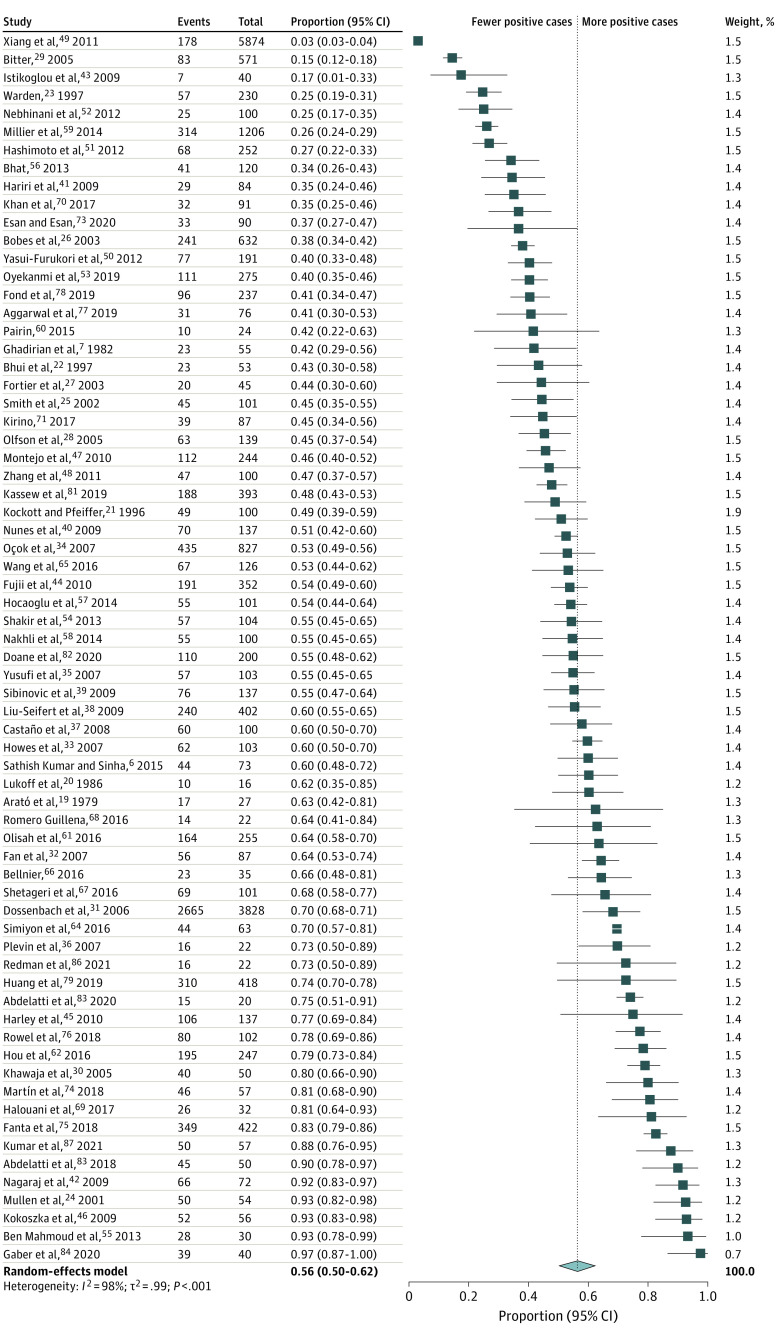
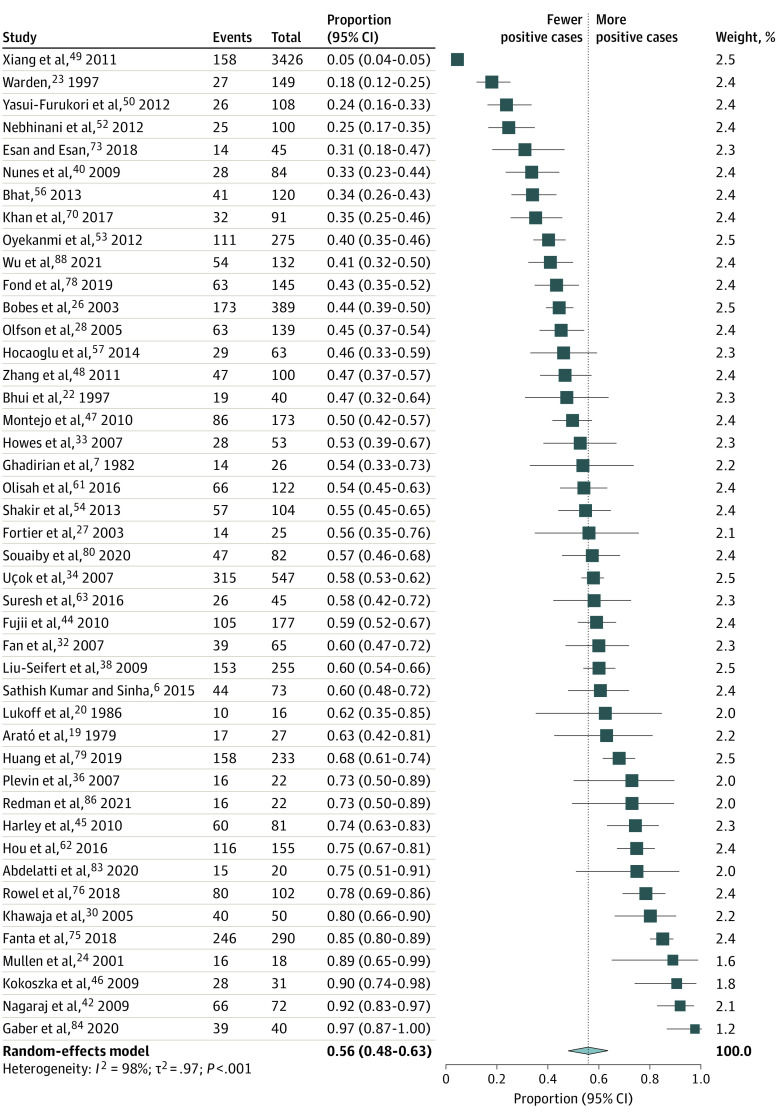
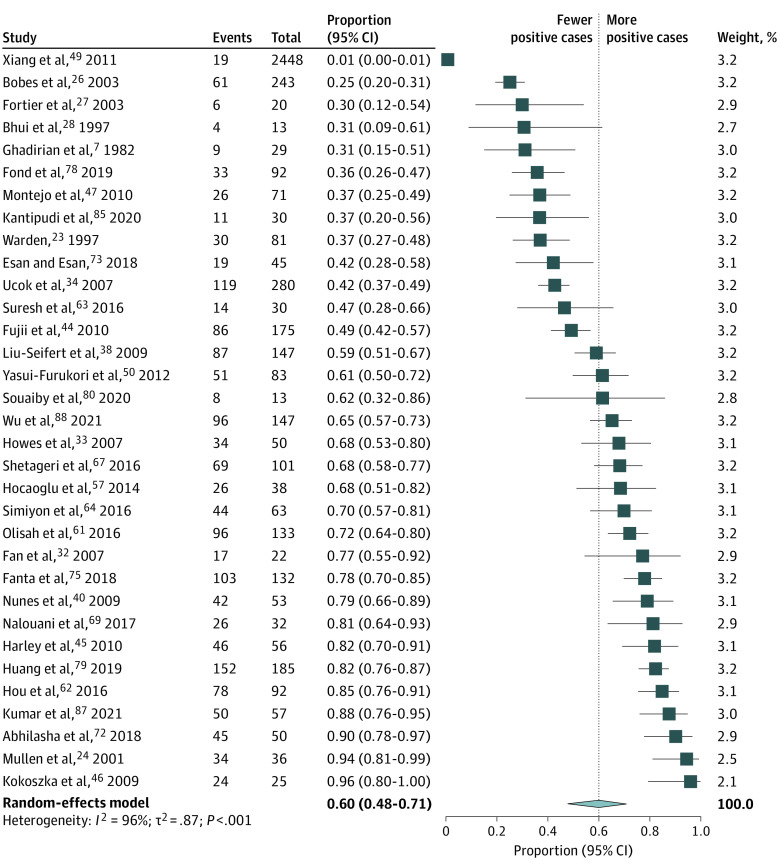
The pooled prevalence estimates of global sexual dysfunctions and sexual dysfunctions in men and women with schizophrenia are presented in Figure 2. The pooled estimates and their 95% CIs were as follows: global prevalence of sexual dysfunctions, 56.4% (95% CI, 50.5-62.2), with values ranging from 3% to 98%; loss of libido, 40.6% (95% CI, 30.7-51.4); orgasm dysfunction, 28.0% (95% CI, 18.4-40.2); and genital pain, 6.1% (95% CI, 2.8-12.7). There was significant heterogeneity within the results for global dysfunction and sexual dysfunctions (I2 for men, 98% [P < .001]; I2 for women, 96% [P < .01]). The forest plots are presented in Figure 3 and eAppendices 8-10 in Supplement 1.
Figure 2. Studies Exploring the Prevalence of Sexual Dysfunctions in Schizophrenia (Random-Effects Model).
Figure 3. Pooled Prevalence Estimates of the Specific Dysfunctions (Random-Effects Model).
Pooled Prevalence Estimates of Sexual Dysfunctions in Men With Schizophrenia
The pooled estimates and their 95% CIs were as follows: male sexual dysfunction, 55.7% (95% CI, 48.1-63.1); erectile dysfunction, 44.0% (95% CI, 33.5-55.2); and ejaculation dysfunction, 38.6% (95% CI, 26.8-51.8). The forest plots are presented in Figure 3, Figure 4, and eAppendices 11-12 in Supplement 1.
Figure 4. Studies Exploring the Prevalence of Sexual Dysfunctions in Men With Schizophrenia (Random-Effects Model).
Pooled Prevalence Estimates of Sexual Dysfunctions in Women With Schizophrenia
The pooled estimates and their 95% CIs were as follows: female sexual dysfunction, 60.0% (95% CI, 48.0-70.8); amenorrhea, 25.1% (95% CI, 17.3-35.0); and galactorrhea, 7.7% (95% CI, 3.7-15.3). The forest plots are presented in Figure 3, Figure 5, and eAppendices 13-14 in Supplement 1.
Figure 5. Studies Exploring the Prevalence of Sexual Dysfunctions in Women With Schizophrenia (Random-Effects Model).
Leave-One-Out Analyses and Publication Bias
The leave-one-out analyses are presented in eAppendices 15-17 in Supplement 1. No study had a disproportionate effect on the pooled prevalence estimate of sexual dysfunction or on heterogeneity. The funnel plots are presented in eAppendices 18-20 in Supplement 1. Visual inspection of the forest plots did not identify substantial asymmetrical shape. The Thompson-Sharp test P values were significant for global sexual dysfunctions (t, 5.63; P < .001), sexual dysfunctions among men (t, 4.58; P < .001), sexual dysfunctions among women (t, 2.25; P = .03), orgasm dysfunction (t, −3.85; P < .001), and ejaculation disorder (t, −2.33; P = .03). The results were not significant for libido dysfunction or erection disorder.
Subgroup and Meta-Regression Analyses
Subgroup and meta-regression analyses are presented in eAppendices 21-23 (global dysfunctions) and 27-29 (specific dysfunctions) in Supplement 1, and meta-regression analyses are in eAppendices 24-26 (global dysfunctions) and 30-32 (specific dysfunctions) in Supplement 1. The significant associations are summarized as follows:
Study Design, Time, and Location
Compared to cohort studies, cross-sectional studies reported significantly higher rates of sexual, orgasm, and male sexual dysfunctions. Studies including consecutive participants reported significantly lower rates of loss of libido, male sexual dysfunction, and erectile dysfunction. Studies including sexual dysfunctions in their primary objective reported significantly higher rates of sexual dysfunctions. Studies including a validated tool for diagnosis reported higher rates of loss of libido. Studies including patient-reported diagnoses reported significantly higher rates of genital pain. Studies including clinical interview diagnosis reported lower rates of loss of libido and orgasm dysfunction. Studies including a clinician-rated tool diagnosis reported significantly higher rates of loss of libido. No significant association was found with study quality. We found that time and location were associated with higher rates of sexual dysfunction in more recent studies and lower rates in North America and Europe compared to the rest of the world.
Sociodemographic Variables
Lower rates of loss of libido were reported in studies including a higher proportion of men. Higher rates of genital pain were reported in studies including more male and single participants, and higher rates of loss of libido were reported in studies including more unemployed participants.
Physical Health and Addictions
There was a significant association between the percentage of patients with alcohol use disorders and lower rates of male sexual dysfunction. No other significant associations were found.
Psychiatric Diagnosis and Illness Severity
Globally, we found that higher loss of libido, orgasm dysfunction, and erection disorder were reported in studies including more patients with schizophrenia vs schizoaffective disorder. The rate of erectile dysfunction decreased with the mean duration of illness. Higher rates of female sexual dysfunction were significantly associated with positive scores on the Positive and Negative Syndrome Scale.
Antipsychotic Classes and Daily Dose (Chlorpromazine Equivalents)
We found contradictory results concerning antipsychotic classes (first generation vs second generation) and sexual dysfunctions, and it was not possible to identify a class or specific antipsychotic associated with a higher or lower prevalence of sexual dysfunction. Sexual dysfunction prevalence was mostly not reported according to the administered antipsychotic, and there were not enough studies to determine the sexual dysfunction prevalence in samples treated with homogenous antipsychotic treatment.
Other Psychotropic Drugs
Given that these were observational studies, patients were often administered multiple treatments other than antipsychotics. Compared to other studies, erectile and ejaculation dysfunctions and loss of libido were significantly lower in studies including participants treated with antidepressants, and erectile and ejaculation dysfunctions decreased with the percentage of patients treated with antidepressants and mood stabilizers. Sexual dysfunctions in general among men and erectile and ejaculation dysfunctions were significantly lower in studies including patients with anxiolytics. Antidepressant and mood stabilizer prescriptions were associated with lower rates of erection disorders (β, −6.30; 95% CI, −10.82 to −1.78); P = .006 and −13.21; 95% CI, −17.59 to −8.83; P < .001, respectively) and ejaculation disorders (β, −6.10; 95% CI, −10.68 to −1.53; P = .009 and β, −11.57; 95% CI, −16.34 to −6.80; P < .001, respectively).
Comparative Pooled Prevalence Estimates of Sexual Dysfunction of the Inverse Variance Method vs Random Intercept Logistic Regression Model
The comparative pooled prevalence estimates of sexual dysfunction and 95% CIs for the inverse variance method vs random intercept logistic regression model are presented in eAppendix 33 in Supplement 1.
Discussion
This systematic review and meta-analysis synthesized a high number of observational studies including quantitative data on the prevalence of sexual dysfunctions in schizophrenia published over 4 decades and 6 continents. The prevalence of sexual dysfunction in schizophrenia was found to be high and heterogeneous. Our results provided important data on the prevalence of each sexual dysfunction. The most frequent sexual dysfunction was erectile dysfunction (44% of men), followed by loss of libido (41%), ejaculation dysfunction (39% of men), orgasm dysfunction (28%), and amenorrhea (25% of women). Galactorrhea and genital pain were less explored and less frequent.
These rates are extremely high. For comparison, erectile dysfunction affects an estimated 30 million men in the US according to the National Institute of Diabetes and Digestive and Kidney Diseases.95 The prevalence of erectile dysfunction increases with age, with approximately 12% of men younger than 60 years experiencing erectile dysfunction compared to 30% of men aged 70 years and older.95 The prevalence of loss of libido, also known as hypoactive sexual desire disorder, is estimated to be approximately 10% in women and 5% in men in the US according to the American Psychiatric Association’s DSM-5.10
We found multiple factors associated with this heterogeneity beyond the expected association with antipsychotics. Among these factors, study design (recruitment and tools but not quality) are classical factors inducing heterogeneity in prevalence studies. As expected, studies including sexual dysfunction in their primary objective reported a higher prevalence of sexual dysfunction. This may be explained by a selection bias.96,97 Our results suggest that studies including nonconsecutive participants may have included a selection bias overestimating the findings. Cross-sectional studies reported higher rates than cohort studies. Except for population-based studies (which were not available for sexual dysfunctions), cohort studies may induce a selection bias at inclusion. For clinical trials, patients to be included in a follow-up cohort may not be representative of all real-life patients, with better compliance, lower addictions, and other clinical setting–related factors explaining lower sexual dysfunctions in cohort studies. Similar to other comorbidities of schizophrenia,11 we found that some sexual dysfunctions were more frequently reported when a validated tool and a self-reported tool were used (compared to clinical interviews or nonvalidated tools). This may be particularly true if some patients are reluctant to answer or ashamed of answering questions about intimate areas during a clinical interview, which may also be modulated by age, sex of the patient, sex of the clinician, and cultural factors. The use of a self-reported validated tool could be actively promoted to improve screening in daily practice. This could be automated with online Patient-Reported Outcomes Measures (PROMs).98 We found that the Arizona Sexual Experience scale was the most frequently used questionnaire in the studies included in this work that could be used as a PROM in clinical practice. Importantly, we found that limiting the results to high-quality studies did not change our results, which reinforces their robustness and the confidence we can have in them.
We found significant associations between the prevalence of sexual dysfunctions and time and location. We found that most sexual dysfunctions had a higher prevalence in recent studies than in ancient studies and no evidence of an improvement in sexual dysfunction prevalence with the release of second-generation antipsychotics. Time and location may also intersect with other factors, such as study design, as discussed above. Geographical discrepancies could also be explained by differences in prescription habits and general social and health factors (including prevention, lifestyle and health care systems). Of note, we found no significant difference in sexual dysfunction prevalence in schizophrenia between high-income countries and low- to middle-income countries, but data in Africa and Polynesia were limited.
Our purpose was to provide a picture of observational studies of real-world stabilized outpatients with schizophrenia. Stabilization does not mean remission, and we found that 3 studies including patients with higher psychotic symptomatology and higher positive and negative symptoms also reported more frequent orgasm dysfunction and loss of libido. However, these few data call for additional studies including participants with high positive or negative symptom levels to confirm these associations. It is probably impossible to discriminate the part of the mental illness itself in the onset of sexual dysfunctions. For example, we found that a lower prevalence of sexual dysfunction was reported in studies including more patients with schizoaffective disorders. We do not know if the results found for antidepressants are explained by an indication bias. A strikingly missing clinical factor is comorbid major depressive disorder. The association of major depressive disorder with sexual dysfunction has been robustly demonstrated in other psychiatric populations and in the general population.99,100 A meta-analysis11 of observational studies recently concluded that approximately one-third of individuals with stabilized schizophrenia are identified with comorbid major depressive disorder, that is, 3 to 4 times the prevalence of the general population. We found that the studies with the highest proportion of antidepressant, mood stabilizer, and anxiolytic prescriptions also reported lower rates of sexual dysfunctions compared to other studies, which is a compelling reason to investigate the role of major depression in the high prevalence of sexual dysfunctions in schizophrenia and their treatment. Thus, the systematic coscreening of sexual dysfunctions combined with major depression may be an effective strategy to improve the care of schizophrenia.
Limitations
This study has limitations. More than 80% of the included studies used validated tools, and their absence was only associated with a higher prevalence of loss of libido. Most factors known to increase sexual dysfunction in the general population (eg, hypertension, diabetes, overweight and obesity, tobacco smoking, and sleep disorders) were not identified as risk factors in our results; however, they were poorly explored in the included studies. Tobacco smoking and overweight or obesity are 2 major sources of sexual dysfunctions that are frequently present in people with schizophrenia, and their role should be better delimited. It is currently impossible to capture diet parameters, as no questionnaire has been specifically designed for this purpose. This issue will probably be addressed in the coming years.101 These results may not be extrapolated to some continents, such as Africa and Polynesia, which were underrepresented. Additionally, the presence of publication bias in our meta-analysis cannot be entirely ruled out, as indicated by the statistical significance of the Thompson-Sharp test. However, the absence of a clear asymmetrical pattern in the funnel plots suggests that the likelihood of publication bias is relatively low. Heterogeneity or methodological differences may also contribute to the observed results.17
Conclusions
Four decades of studies have reported sexual dysfunction as extremely frequent in schizophrenia. Beyond methodological discrepancies partially explaining heterogeneity, we found important evidence in observational studies suggesting that improving the screening and treatment of depression may be an effective strategy to improve sexual health in patients with schizophrenia. Promoting systematic health assessment in sexual dysfunction studies could also help better understand associations between sexual dysfunction and metabolic parameters.
eAppendix 1. Search paradigm
eAppendix 2. Extracted data
eAppendix 3. Excluded studies and reason for exclusion
eAppendix 4. Characteristics of the included studies reporting a prevalence of global sexual dysfunction in schizophrenia
eAppendix 5. Characteristics of the included studies reporting a prevalence of sexual dysfunction in men with schizophrenia
eAppendix 6. Characteristics of the included studies reporting a prevalence of sexual dysfunction in women with schizophrenia
eAppendix 7. Study quality
eAppendix 8. Forest plot of studies exploring the prevalence of loss of libido in schizophrenia
eAppendix 9. Forest plot of studies exploring the prevalence of orgasm dysfunction in schizophrenia
eAppendix 10. Forest plot of studies exploring the prevalence of genital pain in schizophrenia
eAppendix 11. Forest plot of studies exploring the prevalence of erection disorder in schizophrenia
eAppendix 12. Forest plot of studies exploring the prevalence of ejaculation disorder in schizophrenia
eAppendix 13. Forest plot of studies exploring the prevalence of amenorrhea in schizophrenia
eAppendix 14. Forest plot of studies exploring the prevalence of galactorrhea in schizophrenia
eAppendix 15. Leave-one-out analyses
eAppendix 16. Leave-one-out analyses, men
eAppendix 17. Leave-one-out analyses, women
eAppendix 18. Funnel plot
eAppendix 19. Funnel plot, men
eAppendix 20. Funnel plot, women
eAppendix 21. Factors associated with the global prevalence of sexual dysfunctions in schizophrenia: subgroup analyses
eAppendix 22. Factors associated with the prevalence of sexual dysfunctions in men with schizophrenia: subgroup analyses
eAppendix 23. Factors associated with the prevalence of sexual dysfunctions in women with schizophrenia: subgroup analyses
eAppendix 24. Factors associated with the global prevalence of sexual dysfunctions in schizophrenia: metaregression analyses
eAppendix 25. Factors associated with the prevalence of sexual dysfunctions in men with schizophrenia: metaregression analyses
eAppendix 26. Factors associated with the prevalence of sexual dysfunctions in women with schizophrenia: metaregression analyses
eAppendix 27. Factors associated with the prevalence of loss of libido, orgasm dysfunction, genital pain and sex specific dysfunctions in schizophrenia: subgroup analyses
eAppendix 28. Factors associated with the prevalence of specific dysfunctions in men with schizophrenia: subgroup analyses
eAppendix 29. Factors associated with the prevalence of specific dysfunctions in women with schizophrenia: subgroup analyses
eAppendix 30. Factors associated with the prevalence of loss of libido, orgasm dysfunction and genital pain in schizophrenia: meta-regression analyses
eAppendix 31. Factors associated with the prevalence of sex specific dysfunctions in men with schizophrenia: meta-regression analyses
eAppendix 32. Factors associated with the prevalence of sex specific dysfunctions in women with schizophrenia: meta-regression analyses
eAppendix 33. Comparative pooled prevalence estimates of sexual dysfunction and its 95% confidence interval of the Inverse variance method vs. Random intercept logistic regression model
Data sharing statement
References
- 1.Verhulst J, Schneidman B. Schizophrenia and sexual functioning. Hosp Community Psychiatry. 1981;32(4):259-262. doi: 10.1176/ps.32.4.259 [DOI] [PubMed] [Google Scholar]
- 2.Zhao S, Wang X, Qiang X, et al. Is there an association between schizophrenia and sexual dysfunction in both sexes? a systematic review and meta-analysis. J Sex Med. 2020;17(8):1476-1488. doi: 10.1016/j.jsxm.2020.03.005 [DOI] [PubMed] [Google Scholar]
- 3.Serretti A, Chiesa A. A meta-analysis of sexual dysfunction in psychiatric patients taking antipsychotics. Int Clin Psychopharmacol. 2011;26(3):130-140. doi: 10.1097/YIC.0b013e328341e434 [DOI] [PubMed] [Google Scholar]
- 4.Huhn M, Nikolakopoulou A, Schneider-Thoma J, et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet. 2019;394(10202):939-951. doi: 10.1016/S0140-6736(19)31135-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kleinberg DL, Davis JM, de Coster R, Van Baelen B, Brecher M. Prolactin levels and adverse events in patients treated with risperidone. J Clin Psychopharmacol. 1999;19(1):57-61. doi: 10.1097/00004714-199902000-00011 [DOI] [PubMed] [Google Scholar]
- 6.Sathish Kumar SV, Sinha VK. Comparative study of sexual dysfunction and serum prolactin level associated with olanzapine, risperidone, and clozapine in patients with remitted schizophrenia. Indian J Psychiatry. 2015;57(4):386-391. doi: 10.4103/0019-5545.171856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghadirian AM, Chouinard G, Annable L. Sexual dysfunction and plasma prolactin levels in neuroleptic-treated schizophrenic outpatients. J Nerv Ment Dis. 1982;170(8):463-467. doi: 10.1097/00005053-198208000-00004 [DOI] [PubMed] [Google Scholar]
- 8.Traish A, Kim NN, Moreland RB, Goldstein I. Role of alpha adrenergic receptors in erectile function. Int J Impot Res. 2000;12(S1):S48-S63. doi: 10.1038/sj.ijir.3900506 [DOI] [PubMed] [Google Scholar]
- 9.Dumontaud M, Korchia T, Khouani J, et al. Sexual dysfunctions in schizophrenia: beyond antipsychotics. a systematic review. Prog Neuropsychopharmacol Biol Psychiatry. 2020;98:109804. doi: 10.1016/j.pnpbp.2019.109804 [DOI] [PubMed] [Google Scholar]
- 10.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013, doi: 10.1176/appi.books.9780890425596. [DOI] [Google Scholar]
- 11.Etchecopar-Etchart D, Korchia T, Loundou A, et al. Comorbid major depressive disorder in schizophrenia: a systematic review and meta-analysis. Schizophr Bull. 2021;47(2):298-308. doi: 10.1093/schbul/sbaa153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee SW, Koo MJ. PRISMA 2020 statement and guidelines for systematic review and meta-analysis articles, and their underlying mathematics: Life Cycle committee recommendations. Life Cycle. 2022;2. doi: 10.54724/lc.2022.e9 [DOI] [Google Scholar]
- 13.Taipale H, Schneider-Thoma J, Pinzón-Espinosa J, et al. Representation and outcomes of individuals with schizophrenia seen in everyday practice who are ineligible for randomized clinical trials. JAMA Psychiatry. 2022;79(3):210-218. doi: 10.1001/jamapsychiatry.2021.3990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herzog R, Álvarez-Pasquin MJ, Díaz C, Del Barrio JL, Estrada JM, Gil Á. Are healthcare workers’ intentions to vaccinate related to their knowledge, beliefs and attitudes? a systematic review. BMC Public Health. 2013;13:154. doi: 10.1186/1471-2458-13-154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stijnen T, Hamza TH, Özdemir P. Random effects meta-analysis of event outcome in the framework of the generalized linear mixed model with applications in sparse data. Stat Med. 2010;29(29):3046-3067. doi: 10.1002/sim.4040 [DOI] [PubMed] [Google Scholar]
- 16.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sterne JAC, Sutton AJ, Ioannidis JPA, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. doi: 10.1136/bmj.d4002 [DOI] [PubMed] [Google Scholar]
- 18.Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22(4):153-160. doi: 10.1136/ebmental-2019-300117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arató M, Erdös A, Polgár M. Endocrinological changes in patients with sexual dysfunction under long-term neuroleptic treatment. Pharmakopsychiatr Neuropsychopharmakol. 1979;12(6):426-431. doi: 10.1055/s-0028-1094639 [DOI] [PubMed] [Google Scholar]
- 20.Lukoff D, Gioia-Hasick D, Sullivan G, Golden JS, Nuechterlein KH. Sex education and rehabilitation with schizophrenic male outpatients. Schizophr Bull. 1986;12(4):669-677. doi: 10.1093/schbul/12.4.669 [DOI] [PubMed] [Google Scholar]
- 21.Kockott G, Pfeiffer W. Sexual disorders in nonacute psychiatric outpatients. Compr Psychiatry. 1996;37(1):56-61. doi: 10.1016/S0010-440X(96)90052-8 [DOI] [PubMed] [Google Scholar]
- 22.Bhui K, Puffet A, Strathdee G. Sexual and relationship problems amongst patients with severe chronic psychoses. Soc Psychiatry Psychiatr Epidemiol. 1997;32(8):459-467. doi: 10.1007/BF00789140 [DOI] [PubMed] [Google Scholar]
- 23.Warden SJ. Sex Differences in Response to Treatment With Risperidone. Master’s thesis. University of Calgary; 1997. Accessed March 13, 2022. doi: 10.11575/PRISM/12036 [DOI]
- 24.Mullen B, Brar JS, Vagnucci AH, Ganguli R. Frequency of sexual dysfunctions in patients with schizophrenia on haloperidol, clozapine or risperidone. Schizophr Res. 2001;48(1):155-158. doi: 10.1016/S0920-9964(00)00061-X [DOI] [PubMed] [Google Scholar]
- 25.Smith SM, O’Keane V, Murray R. Sexual dysfunction in patients taking conventional antipsychotic medication. Br J Psychiatry. 2002;181:49-55. doi: 10.1192/bjp.181.1.49 [DOI] [PubMed] [Google Scholar]
- 26.Bobes J, Garc A-Portilla MP, Rejas J, et al. Frequency of sexual dysfunction and other reproductive side-effects in patients with schizophrenia treated with risperidone, olanzapine, quetiapine, or haloperidol: the results of the EIRE study. J Sex Marital Ther. 2003;29(2):125-147. doi: 10.1080/713847170 [DOI] [PubMed] [Google Scholar]
- 27.Fortier P, Mottard JP, Trudel G, Even S. Study of sexuality-related characteristics in young adults with schizophrenia treated with novel neuroleptics and in a comparison group of young adults. Schizophr Bull. 2003;29(3):559-572. doi: 10.1093/oxfordjournals.schbul.a007028 [DOI] [PubMed] [Google Scholar]
- 28.Olfson M, Uttaro T, Carson WH, Tafesse E. Male sexual dysfunction and quality of life in schizophrenia. J Clin Psychiatry. 2005;66(3):331-338. doi: 10.4088/JCP.v66n0309 [DOI] [PubMed] [Google Scholar]
- 29.Bitter I, Basson BR, Dossenbach MR. Antipsychotic treatment and sexual functioning in first-time neuroleptic-treated schizophrenic patients. Int Clin Psychopharmacol. 2005;20(1):19-21. doi: 10.1097/00004850-200501000-00004 [DOI] [PubMed] [Google Scholar]
- 30.Khawaja MY. Sexual dysfunction in male patients taking antipsychotics. J Ayub Med Coll Abbottabad. 2005;17(3):73-75. [PubMed] [Google Scholar]
- 31.Dossenbach M, Dyachkova Y, Pirildar S, et al. Effects of atypical and typical antipsychotic treatments on sexual function in patients with schizophrenia: 12-month results from the Intercontinental Schizophrenia Outpatient Health Outcomes (IC-SOHO) study. Eur Psychiatry. 2006;21(4):251-258. doi: 10.1016/j.eurpsy.2005.12.005 [DOI] [PubMed] [Google Scholar]
- 32.Fan X, Henderson DC, Chiang E, et al. Sexual functioning, psychopathology and quality of life in patients with schizophrenia. Schizophr Res. 2007;94(1-3):119-127. doi: 10.1016/j.schres.2007.04.033 [DOI] [PubMed] [Google Scholar]
- 33.Howes OD, Wheeler MJ, Pilowsky LS, Landau S, Murray RM, Smith S. Sexual function and gonadal hormones in patients taking antipsychotic treatment for schizophrenia or schizoaffective disorder. J Clin Psychiatry. 2007;68(3):361-367. doi: 10.4088/JCP.v68n0302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uçok A, Incesu C, Aker T, Erkoç S. Sexual dysfunction in patients with schizophrenia on antipsychotic medication. Eur Psychiatry. 2007;22(5):328-333. doi: 10.1016/j.eurpsy.2007.01.001 [DOI] [PubMed] [Google Scholar]
- 35.Yusufi B, Mukherjee S, Flanagan R, et al. Prevalence and nature of side effects during clozapine maintenance treatment and the relationship with clozapine dose and plasma concentration. Int Clin Psychopharmacol. 2007;22(4):238-243. doi: 10.1097/YIC.0b013e32819f8f17 [DOI] [PubMed] [Google Scholar]
- 36.Plevin D, Galletly C, Roughan P. Sexual dysfunction in men treated with depot antipsychotic drugs: a pilot study. Sex Health. 2007;4(4):269-271. doi: 10.1071/SH07012 [DOI] [PubMed] [Google Scholar]
- 37.Castaño J, Garnier C, Portillo F, et al. P.3.c.039 Sexual dysfunction in patients with schizophrenia on antipsychotic medication. Eur Neuropsychopharmacol. 2008;18:S426. doi: 10.1016/S0924-977X(08)70623-0 [DOI] [Google Scholar]
- 38.Liu-Seifert H, Kinon BJ, Tennant CJ, Sniadecki J, Volavka J. Sexual dysfunction in patients with schizophrenia treated with conventional antipsychotics or risperidone. Neuropsychiatr Dis Treat. 2009;5:47-54. doi: 10.2147/NDT.S4766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sibinovic V. Sexual dysfunction in patients on antipsychotic therapy Vladica Sibinovic Psychiatry Clinic CC NIS. Eur Psychiatry. 2009;24(S1):1. doi: 10.1016/S0924-9338(09)71430-018926669 [DOI] [Google Scholar]
- 40.Nunes LVA, Dieckmann LHJ, Lacaz FS, Bressan R, Matsuo T, de Jesus Mari J. The accuracy of the Arizona Sexual Experience Scale (ASEX) to identify sexual dysfunction in patients of the schizophrenia spectrum. A acuracia da Escala de Experiencia Sexual do Arizona (ASEX) para identificar disfuncao sexual em pacientes do espectro da esquizofrenia. Arch Clin Psychiatry. 2009;36(5):189. doi: 10.1590/S0101-60832009000500002 [DOI] [Google Scholar]
- 41.Hariri AG, Karadag F, Gurol DT, Aksoy UM, Tezcan AE. Sexual problems in a sample of the Turkish psychiatric population. Compr Psychiatry. 2009;50(4):353-360. doi: 10.1016/j.comppsych.2008.09.009 [DOI] [PubMed] [Google Scholar]
- 42.Nagaraj AKM, Pai NB, Rao S. A comparative study of sexual dysfunction involving risperidone, quetiapine, and olanzapine. Indian J Psychiatry. 2009;51(4):265-271. doi: 10.4103/0019-5545.58291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Istikoglou C, Vlissides D, Michelidakis K, Mikirditsian OP. 3.c.001 Quality of life: sexual dysfunction in young people with schizophrenia treated with ziprasidone. Eur Neuropsychopharmacol. 2009;19:S511. doi: 10.1016/S0924-977X(09)70808-9 [DOI] [Google Scholar]
- 44.Fujii A, Yasui-Furukori N, Sugawara N, et al. Sexual dysfunction in Japanese patients with schizophrenia treated with antipsychotics. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(2):288-293. doi: 10.1016/j.pnpbp.2009.11.022 [DOI] [PubMed] [Google Scholar]
- 45.Harley EWY, Boardman J, Craig T. Sexual problems in schizophrenia: prevalence and characteristics. a cross sectional survey. Soc Psychiatry Psychiatr Epidemiol. 2010;45(7):759-766. doi: 10.1007/s00127-009-0119-0 [DOI] [PubMed] [Google Scholar]
- 46.Kokoszka A, Abd El Aal M, Jodko A, Kwiatkowska A. Frequency of subjectively assessed symptoms of sexual dysfunction and sexual disorders. Article in Polish. Psychiatr Pol. 2009;43(6):705-718. [PubMed] [Google Scholar]
- 47.Montejo AL, Majadas S, Rico-Villademoros F, et al. ; Spanish Working Group for the Study of Psychotropic-Related Sexual Dysfunction . Frequency of sexual dysfunction in patients with a psychotic disorder receiving antipsychotics. J Sex Med. 2010;7(10):3404-3413. doi: 10.1111/j.1743-6109.2010.01709.x [DOI] [PubMed] [Google Scholar]
- 48.Zhang XR, Zhang ZJ, Zhu RX, Yuan YG, Jenkins TA, Reynolds GP. Sexual dysfunction in male schizophrenia: influence of antipsychotic drugs, prolactin and polymorphisms of the dopamine D2 receptor genes. Pharmacogenomics. 2011;12(8):1127-1136. doi: 10.2217/pgs.11.46 [DOI] [PubMed] [Google Scholar]
- 49.Xiang YT, Wang CY, Si TM, et al. The low frequency of reported sexual dysfunction in Asian patients with schizophrenia (2001-2009): low occurrence or ignored side effect? Hum Psychopharmacol. 2011;26(4-5):352-357. doi: 10.1002/hup.1213 [DOI] [PubMed] [Google Scholar]
- 50.Yasui-Furukori N, Fujii A, Sugawara N, et al. No association between hormonal abnormality and sexual dysfunction in Japanese schizophrenia patients treated with antipsychotics. Hum Psychopharmacol. 2012;27(1):82-89. doi: 10.1002/hup.1275 [DOI] [PubMed] [Google Scholar]
- 51.Hashimoto Y, Uno J, Miwa T, Kurihara M, Tanifuji H, Tensho M. Effects of antipsychotic polypharmacy on side-effects and concurrent use of medications in schizophrenic outpatients. Psychiatry Clin Neurosci. 2012;66(5):405-410. doi: 10.1111/j.1440-1819.2012.02376.x [DOI] [PubMed] [Google Scholar]
- 52.Nebhinani N, Grover S, Avasthi A. Sexual dysfunction in male subjects receiving trifluoperazine, risperidone, or olanzapine: rates vary with assessment questionnaire. Prim Care Companion CNS Disord. 2012;14(2):PCC.11m01199. doi: 10.4088/PCC.11m01199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oyekanmi AK, Adelufosi AO, Abayomi O, Adebowale TO. Demographic and clinical correlates of sexual dysfunction among Nigerian male outpatients on conventional antipsychotic medications. BMC Res Notes. 2012;5:267. doi: 10.1186/1756-0500-5-267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shakir AS, Marzook AA, Kadim J, Khalid H, Jassam MD. Sexual dysfunctions among male schizophrenic patients attending Al-Rashad Sex Clinic. AL-Kindy Coll Med J. 2013;9(2):21-24. [Google Scholar]
- 55.Ben Mahmoud S, Zouari L, Dammak M, Ben Thabet J, Zouari N, Maâlej M. Évaluation de la sexualité d’une série de 61 sujets atteints de psychose chronique. Evaluation of sexuality in 61 subjects suffering from chronic psychosis. Sexologies. 2013;22(2):90-96. doi: 10.1016/j.sexol.2012.08.001 [DOI] [Google Scholar]
- 56.Bhat MY, Abbas Z, Farhat S, Shoid S. A comparative study of sexual dysfunction associated with typical and atypical anti-psychotics in outdoor patients. Int J Health Sci Res. 2013;3(4):42-49. https://www.ijhsr.org/IJHSR_Vol.3_Issue.4_April2013/7.pdf [Google Scholar]
- 57.Hocaoglu C, Celik FH, Kandemir G, Guveli H, Bahceci B. Sexual dysfunction in outpatients with schizophrenia in Turkey: a cross-sectional study. Shanghai Arch Psychiatry. 2014;26(6):347-356. doi: 10.11919/j.issn.1002-0829.214101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nakhli J, El Kissi Y, Bouhlel S, et al. Reliability and validity of the Arizona sexual experiences scale-Arabic version in Tunisian patients with schizophrenia. Compr Psychiatry. 2014;55(6):1473-1477. doi: 10.1016/j.comppsych.2014.04.006 [DOI] [PubMed] [Google Scholar]
- 59.Millier A, Amri I, Boyer L, Auquier P, Toumi M. Utility decrements associated with side effects in schizophrenia. J Med Econ. 2014;17(12):853-861. doi: 10.3111/13696998.2014.964405 [DOI] [PubMed] [Google Scholar]
- 60.Pairin JM. Evaluation de la Qualité de Vie Sexuelle Chez les Patients Schizophrènes: Etude Observationnelle a Travers la Passation de l’Échelle Arizona Sexual Experience Scale. Doctoral thesis. Université d’Angers; 2015. https://dune.univ-angers.fr/fichiers/20117155/2015MCEM5010/fichier/5010F.pdf
- 61.Olisah VO, Sheikh TL, Abah ER, Mahmud-Ajeigbe AF. Sociodemographic and clinical correlates of sexual dysfunction among psychiatric outpatients receiving common psychotropic medications in a Neuropsychiatric Hospital in Northern Nigeria. Niger J Clin Pract. 2016;19(6):799-806. doi: 10.4103/1119-3077.180063 [DOI] [PubMed] [Google Scholar]
- 62.Hou CL, Zang Y, Rosen RC, et al. Sexual dysfunction and its impact on quality of life in Chinese patients with schizophrenia treated in primary care. Compr Psychiatry. 2016;65:116-121. doi: 10.1016/j.comppsych.2015.11.002 [DOI] [PubMed] [Google Scholar]
- 63.Suresh Babu P. A Comparative Study on Evaluation of Sexual Dysfunction in Patients Treated With Atypical Antipsychotics Involving Risperidone, Olanzapine, and Quetiapine. Master’s thesis. Madras Medical College; 2016. https://www.iosrjournals.org/iosr-jdms/papers/Vol20-issue8/Ser-4/G2008043337.pdf
- 64.Simiyon M, Chandra PS, Desai G. Sexual dysfunction among women with schizophrenia—a cross sectional study from India. Asian J Psychiatr. 2016;24:93-98. doi: 10.1016/j.ajp.2016.08.022 [DOI] [PubMed] [Google Scholar]
- 65.Wang YX, Zhang P, Xin LM, et al. Chinese version of the Psychotropic-Related Sexual Dysfunction Questionnaire (PRSexDQ-SALSEX): validity and reliability for schizophrenic patients taking antipsychotics. Psychiatry Res. 2016;246:303-307. doi: 10.1016/j.psychres.2016.05.063 [DOI] [PubMed] [Google Scholar]
- 66.Bellnier TJ. Collee of psychiatric and neurologic pharmacists 2016 poster abstracts. J Pharm Pract. 2016;29(3):270-341. doi: 10.1177/0897190016645328 [DOI] [Google Scholar]
- 67.Shetageri VN, Bhogale GS, Patil NM, Nayak RB, Chate SS. Sexual dysfunction among females receiving psychotropic medication: a hospital-based cross-sectional study. Indian J Psychol Med. 2016;38(5):447-454. doi: 10.4103/0253-7176.191379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Romero Guillena SL, Plasencia Garcia de Diego B, Garcia Salguero, M. Sexual dysfunction and functionality. assessment of a sexual dysfunction intervention program for patients with schizophrenia. Eur Neuropsychopharmacol. 2016;26(2):S500. doi: 10.1016/S0924-977X(16)31517-6 [DOI] [Google Scholar]
- 69.Halouani N, Ellouze S, Aloulou J, Charfeddine F, Aribi L, Amami O. Sexualité de la femme schizophrène en Tunisie: étude cas–témoins. Sexologies. 2017;27(4):211-216. doi: 10.1016/j.sexol.2017.12.003 [DOI] [Google Scholar]
- 70.Khan A, Nawaz H, Nazneen Z, Yousafzai A. Anti-psychotics induced sexual dysfunction. Pak J Physiol. 2017;13(3). http://pjp.pps.org.pk/index.php/PJP/article/view/69 [Google Scholar]
- 71.Kirino E. Serum prolactin levels and sexual dysfunction in patients with schizophrenia treated with antipsychotics: comparison between aripiprazole and other atypical antipsychotics. Ann Gen Psychiatry. 2017;16:43. doi: 10.1186/s12991-017-0166-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Abhilasha P. Prevalence of sexual dysfunction in females with and without schizophrenia. Am J Psychiatry Neurosci. 2018;6(3):56. doi: 10.11648/j.ajpn.20180603.11 [DOI] [Google Scholar]
- 73.Esan O, Esan A. Sexual dysfunction among patients with schizophrenia in southwest Nigeria. J Sex Marital Ther. 2018;44(7):657-666. doi: 10.1080/0092623X.2018.1447055 [DOI] [PubMed] [Google Scholar]
- 74.Martín JC, Acuña MJ, Labrador J, Blanco M, Casas C. Sexual dysfunction factors in patients with schizophrenia treated with second generation antipsychotics: not only prolactin. Actas Esp Psiquiatr. 2018;46(6):217-225. [PubMed] [Google Scholar]
- 75.Fanta T, Haile K, Abebaw D, Assefa D, Hibdye G. Assessment of sexual dysfunction and associated factors among patients with schizophrenia in Ethiopia, 2017. BMC Psychiatry. 2018;18(1):158. doi: 10.1186/s12888-018-1738-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rowel WWJSM, Liyange ULNS, Hewawitharana UH, Dayabandara M, Rodrigo A. Erectile dysfunction among male patients diagnosed with schizophrenia being treated with antipsychotic medication, and the impact on quality of life. Sri Lanka J Psychiatry. 2018;9(2):10. doi: 10.4038/sljpsyc.v9i2.8185 [DOI] [Google Scholar]
- 77.Aggarwal S, Grover S, Chakrabarti S. A comparative study evaluating the marital and sexual functioning in patients with schizophrenia and depressive disorders. Asian J Psychiatr. 2019;39:128-134. doi: 10.1016/j.ajp.2018.12.021 [DOI] [PubMed] [Google Scholar]
- 78.Fond G, Godin O, Dumontaud M, et al. ; FACE-SZ (FondaMental Academic Centers of Expertise for Schizophrenia) group . Sexual dysfunctions are associated with major depression, chronic inflammation and anticholinergic consumption in the real-world schizophrenia FACE-SZ national cohort. Prog Neuropsychopharmacol Biol Psychiatry. 2019;94:109654. doi: 10.1016/j.pnpbp.2019.109654 [DOI] [PubMed] [Google Scholar]
- 79.Huang YH, Hou CL, Ng CH, et al. Sexual dysfunction in Chinese rural patients with schizophrenia. BMC Psychiatry. 2019;19(1):218. doi: 10.1186/s12888-019-2205-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Souaiby L, Kazour F, Zoghbi M, Bou Khalil R, Richa S. Sexual dysfunction in patients with schizophrenia and schizoaffective disorder and its association with adherence to antipsychotic medication. J Ment Health. 2020;29(6):623-630. doi: 10.1080/09638237.2019.1581333 [DOI] [PubMed] [Google Scholar]
- 81.Kassew T, Demilew D, Birhanu A, Wonde M, Liyew B, Shumet S. Attitude towards antipsychotic medications in patients diagnosed with schizophrenia: a cross-sectional study at Amanuel Mental Specialized Hospital, Addis Ababa, Ethiopia. Schizophr Res Treatment. 2019;2019:5094017. doi: 10.1155/2019/5094017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Doane MJ, Sajatovic M, Weiden PJ, et al. Antipsychotic treatment experiences of people with schizophrenia: patient perspectives from an online survey. Patient Prefer Adherence. 2020;14:2043-2054. doi: 10.2147/PPA.S270020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Abdelatti SI, Ismail RM, Hamed RA. Sexual dysfunctions in a sample of male psychiatric patients compared to medically ill patients. Middle East Curr Psychiatry. 2020;27(1):12. doi: 10.1186/s43045-020-00022-3 [DOI] [Google Scholar]
- 84.Gaber HD, El-Beeh KAM, Abd Al-Naser FAW, Hosny A. Erectile dysfunction in patients with first-episode psychosis. Andrologia. 2020;52(11):e13793. doi: 10.1111/and.13793 [DOI] [PubMed] [Google Scholar]
- 85.Kantipudi S, Suresh N, Ayyadurai P, Ramanathan S. Sexual dysfunction and marital relationship in women with schizophrenia in comparison with caregivers: a hospital-based study. J Psychosexual Health. 2020;2:87-92. doi: 10.1177/2631831820918133 [DOI] [Google Scholar]
- 86.Redman B, Kitchen C, Johnson KW, Bezwada P, Kelly DL. Levels of prolactin and testosterone and associated sexual dysfunction and breast abnormalities in men with schizophrenia treated with antipsychotic medications. J Psychiatr Res. 2021;143:50-53. doi: 10.1016/j.jpsychires.2021.08.022 [DOI] [PubMed] [Google Scholar]
- 87.Kumar PNS, Radhika MK, Suresh R, Uvais NA. Comparative study of sexual side effects in female patients with schizophrenia receiving risperidone or olanzapine. Prim Care Companion CNS Disord. 2021;23(4):20m02835. doi: 10.4088/PCC.20m02835 [DOI] [PubMed] [Google Scholar]
- 88.Wu TH, Lin CH, Goh KK, et al. The relationships between hyperprolactinemia, metabolic disturbance, and sexual dysfunction in patients with schizophrenia under olanzapine treatment. Front Pharmacol. 2021;12:718800. doi: 10.3389/fphar.2021.718800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.McGahuey CA, Gelenberg AJ, Laukes CA, et al. The Arizona Sexual Experience scale (ASEX): reliability and validity. J Sex Marital Ther. 2000;26(1):25-40. doi: 10.1080/009262300278623 [DOI] [PubMed] [Google Scholar]
- 90.Clayton AH, McGarvey EL, Clavet GJ. The Changes in Sexual Functioning Questionnaire (CSFQ): development, reliability, and validity. Psychopharmacol Bull. 1997;33(4):731-745. [PubMed] [Google Scholar]
- 91.Symonds T, Abraham L, Bushmakin AG, Williams K, Martin M, Cappelleri JC. Sexual function questionnaire: further refinement and validation. J Sex Med. 2012;9(10):2609-2616. doi: 10.1111/j.1743-6109.2011.02627.x [DOI] [PubMed] [Google Scholar]
- 92.Rosen R, Brown C, Heiman J, et al. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26(2):191-208. doi: 10.1080/009262300278597 [DOI] [PubMed] [Google Scholar]
- 93.Lingjaerde O, Ahlfors UG, Bech P, Dencker SJ, Elgen K. The UKU side effect rating scale. a new comprehensive rating scale for psychotropic drugs and a cross-sectional study of side effects in neuroleptic-treated patients. Acta Psychiatr Scand Suppl. 1987;334(s334):1-100. doi: 10.1111/j.1600-0447.1987.tb10566.x [DOI] [PubMed] [Google Scholar]
- 94.Montejo ÁL, Rico-Villademoros F. Psychometric properties of the Psychotropic-Related Sexual Dysfunction Questionnaire (PRSexDQ-SALSEX) in patients with schizophrenia and other psychotic disorders. J Sex Marital Ther. 2008;34(3):227-239. doi: 10.1080/00926230701866125 [DOI] [PubMed] [Google Scholar]
- 95.National Institute of Diabetes and Digestive and Kidney Diseases . Erectile dysfunction (ED). Accessed March 13, 2023. https://www.niddk.nih.gov/health-information/urologic-diseases/erectile-dysfunction
- 96.Mark DH. Interpreting the term selection bias in medical research. Fam Med. 1997;29(2):132-136. [PubMed] [Google Scholar]
- 97.Delgado-Rodríguez M, Llorca J. Bias. J Epidemiol Community Health. 2004;58(8):635-641. doi: 10.1136/jech.2003.008466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fernandes S, Fond G, Zendjidjian X, et al. The Patient-Reported Experience Measure for Improving Quality of Care in Mental Health (PREMIUM) project in France: study protocol for the development and implementation strategy. Patient Prefer Adherence. 2019;13:165-177. doi: 10.2147/PPA.S172100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Seidman SN, Roose SP. Sexual dysfunction and depression. Curr Psychiatry Rep. 2001;3(3):202-208. doi: 10.1007/s11920-001-0053-7 [DOI] [PubMed] [Google Scholar]
- 100.Laurent SM, Simons AD. Sexual dysfunction in depression and anxiety: conceptualizing sexual dysfunction as part of an internalizing dimension. Clin Psychol Rev. 2009;29(7):573-585. doi: 10.1016/j.cpr.2009.06.007 [DOI] [PubMed] [Google Scholar]
- 101.Achour Y, Mallet J, Korchia T, et al. Développer la psychonutrition dans la pratique clinique psychiatrique. Ann Méd-Psychol Rev Psychiatr. 2022;180(7):674-676. doi: 10.1016/j.amp.2022.07.018 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Search paradigm
eAppendix 2. Extracted data
eAppendix 3. Excluded studies and reason for exclusion
eAppendix 4. Characteristics of the included studies reporting a prevalence of global sexual dysfunction in schizophrenia
eAppendix 5. Characteristics of the included studies reporting a prevalence of sexual dysfunction in men with schizophrenia
eAppendix 6. Characteristics of the included studies reporting a prevalence of sexual dysfunction in women with schizophrenia
eAppendix 7. Study quality
eAppendix 8. Forest plot of studies exploring the prevalence of loss of libido in schizophrenia
eAppendix 9. Forest plot of studies exploring the prevalence of orgasm dysfunction in schizophrenia
eAppendix 10. Forest plot of studies exploring the prevalence of genital pain in schizophrenia
eAppendix 11. Forest plot of studies exploring the prevalence of erection disorder in schizophrenia
eAppendix 12. Forest plot of studies exploring the prevalence of ejaculation disorder in schizophrenia
eAppendix 13. Forest plot of studies exploring the prevalence of amenorrhea in schizophrenia
eAppendix 14. Forest plot of studies exploring the prevalence of galactorrhea in schizophrenia
eAppendix 15. Leave-one-out analyses
eAppendix 16. Leave-one-out analyses, men
eAppendix 17. Leave-one-out analyses, women
eAppendix 18. Funnel plot
eAppendix 19. Funnel plot, men
eAppendix 20. Funnel plot, women
eAppendix 21. Factors associated with the global prevalence of sexual dysfunctions in schizophrenia: subgroup analyses
eAppendix 22. Factors associated with the prevalence of sexual dysfunctions in men with schizophrenia: subgroup analyses
eAppendix 23. Factors associated with the prevalence of sexual dysfunctions in women with schizophrenia: subgroup analyses
eAppendix 24. Factors associated with the global prevalence of sexual dysfunctions in schizophrenia: metaregression analyses
eAppendix 25. Factors associated with the prevalence of sexual dysfunctions in men with schizophrenia: metaregression analyses
eAppendix 26. Factors associated with the prevalence of sexual dysfunctions in women with schizophrenia: metaregression analyses
eAppendix 27. Factors associated with the prevalence of loss of libido, orgasm dysfunction, genital pain and sex specific dysfunctions in schizophrenia: subgroup analyses
eAppendix 28. Factors associated with the prevalence of specific dysfunctions in men with schizophrenia: subgroup analyses
eAppendix 29. Factors associated with the prevalence of specific dysfunctions in women with schizophrenia: subgroup analyses
eAppendix 30. Factors associated with the prevalence of loss of libido, orgasm dysfunction and genital pain in schizophrenia: meta-regression analyses
eAppendix 31. Factors associated with the prevalence of sex specific dysfunctions in men with schizophrenia: meta-regression analyses
eAppendix 32. Factors associated with the prevalence of sex specific dysfunctions in women with schizophrenia: meta-regression analyses
eAppendix 33. Comparative pooled prevalence estimates of sexual dysfunction and its 95% confidence interval of the Inverse variance method vs. Random intercept logistic regression model
Data sharing statement