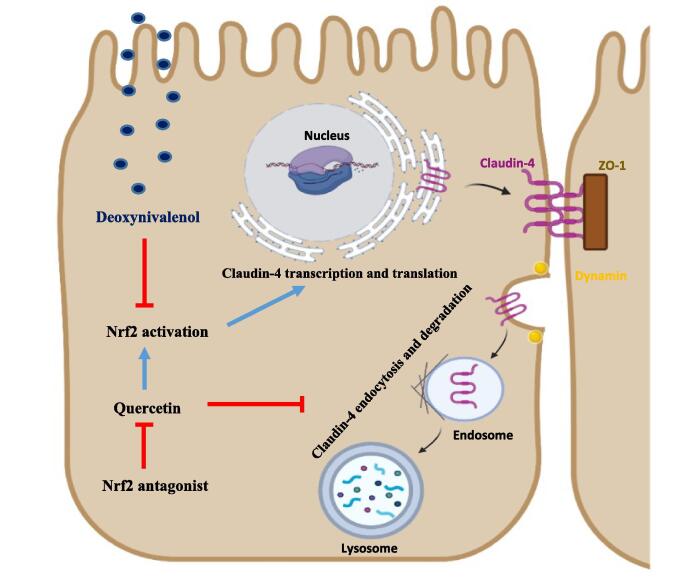
Graphical abstract
Keywords: Deoxynivalenol, Endocytosis, Nrf2, Quercetin, Tight junction
Highlights
-
•
Quercetin induces activation of Nrf2 signaling pathway and selectively upregulates claudin-4 expression.
-
•
Deoxynivalenol exposure inhibits Nrf2 transcriptional activity and decreases tight junction protein expression.
-
•
Quercetin supplementation ameliorates deoxynivalenol-induced endocytosis of claudin-4.
-
•
Protective effect of quercetin against deoxynivalenol-induced barrier loss is through Nrf2-dependent mechanism.
Abstract
The presence of deoxynivalenol (DON), one of the most frequently occurring mycotoxin, in food and feed has been considered a risk factor to both human and animal health. Molecular mechanisms that regulate DON effects in tissues are still poorly understood. However, recent evidence suggests that nuclear factor erythroid 2-like 2 (Nrf2) may be a major target during mycotoxin-induced intestinal barrier dysfunction. Although quercetin, a plant-derived flavonoid, is known to induce the activation of Nrf2 signaling pathway, its potential to mitigate effects of DON and the implication of Nrf2 in its physiological effects is poorly understood. Therefore, this study was conducted to investigate the protective effects of quercetin in alleviating the DON-induced barrier loss and intestinal injuries in IPEC-J2 cells and weaned piglets and determine the potential role of Nrf2. Quercetin treatment dose-dependently increased mRNA expression of Nrf2 target gene, NQO-1, and concomitantly increased the expression of claudin-4 at both mRNA and protein levels. Quercetin supplementation also reversed the reduction of claudin-4 caused by DON exposure in vivo and in vitro. The decreased membrane presence of claudin-4 and ZO-1 induced by DON was also blocked by quercetin. Furthermore, quercetin attenuated the endocytosis and degradation of claudin-4 caused by DON exposure. The effects of quercetin also included the restoration of transepithelial electrical resistance (TEER) and reduction of FITC-dextran permeability that have been perturbed by DON. However, the protective effects of quercetin against DON exposure were abolished by a specific Nrf2 inhibitor (brusatol), confirming the importance of Nrf2 in the regulation of TJP expression and barrier function by quercetin. In vivo study in weaned pigs showed that DON exposure impaired villus-crypt morphology as indicated by diffuse apical villus necrosis, villus atrophy and fusion. Notably, intestinal injuries caused by DON administration were partly mitigated by quercetin supplementation. Collectively, this study shows that quercetin could be used to prevent the DON-induced gut barrier dysfunction in humans and animals and the protective effects of quercetin against DON-induced intestinal barrier disruption is partly through Nrf2-dependent signaling pathway.
Introduction
The presence of trichothecene mycotoxins in food and feed has emerged as a major threat to both human and animal health due to their frequent occurrence. The intestine is an organ that is routinely exposed to high concentrations of mycotoxins (Maresca et al., 2002, Wang et al., 2021). Intestinal exposure to deoxynivalenol (DON) impaired the intestinal barrier function evidenced by the increased endocytosis and degradation of TJP (Li et al., 2021). Very importantly, it has been suggested that nuclear factor erythroid 2-like 2 (Nrf2) might play a major role in the response to mycotoxins exposure (Kozieł et al., 2021). Recent studies revealed that DON exposure suppressed Nrf2 signaling pathway and downregulated the expression of Nrf2 target genes in human hepatocellular carcinoma cells (Ndlovu et al., 2021). Given the demonstrated protective effect of Nrf2 activation in several conditions (Zhang et al., 2018, Zhou et al., 2021), we hypothesized that activation of Nrf2 might exert a protective effect against DON-induced intestinal barrier dysfunction.
Nrf2 plays a critical role in maintaining the redox balance in response to oxidative stress which occurs in almost all cell types (Kozieł et al., 2021). At steady state, Nrf2 forms a heterodimer with Keap1 (Kelch-like ECH associating protein 1) and locates in the cytoplasm as an inactive complex. Under stress conditions, Nrf2 dissociates from Keap1 and translocates into nucleus where it binds to antioxidant responsive element (ARE) and initiates the transcription of target genes involved in detoxification and antioxidant enzyme production (Kozieł et al., 2021). Recent evidence revealed a functional role of Nrf2 in the regulation of blood–brain barrier function in rats (Zhao et al., 2007). Studies using Nrf2-knockout mice also showed that Nrf2 deficiency compromised barrier function in the esophageal epithelium (Chen et al., 2014). The intestinal epithelium, as the largest physical barrier that separates the internal and external environments, maintains barrier function mainly through the modulation of tight junction protein (TJP) abundance (Groschwitz and Hogan, 2009). Nevertheless, the functional role of Nrf2 in the regulation of intestinal barrier function still remains unclear.
Quercetin, a plant-derived flavonoid, has been reported to exhibit a wide range of biological functions such as antioxidant, antimutagenic, anti-inflammatory and anticarcinogenic properties (Camuesco et al., 2004, Moskaug et al., 2005). Quercetin treatment also induced the activation of Nrf2 signaling pathway and increased Nrf2-mediated transcription activity (Tanigawa et al., 2007). In Caco-2 cells, quercetin treatment improved epithelial barrier function and increased claudin-4 expression (Amasheh et al. 2008). However, the connection between regulation of Nrf2 activity by quercetin and its protective effect is unknown. Therefore, we hypothesized that Nrf2 activation by quercetin plays a key role in protecting against DON-induced intestinal barrier dysfunction. Thus, the objective of this study was to investigate the protective effects of quercetin against DON-induced intestinal barrier loss and the role of Nrf2 in this process.
Materials and methods
Cell culture
IPEC-J2 cells, originally isolated from the mid-jejunum of a neonatal unsuckled piglet, were cultured as previously described (Li and Ajuwon, 2021). Briefly, cells were cultured in Dulbecco’s modified Eagle’s medium: Nutrient Mixture F-12 (DMEM/F12) supplemented with 5% fetal bovine serum, 1% penicillin–streptomycin mixture, 1% insulin-transferrin-selenium premix, and 5 ng/ml epidermal growth factor.
Cell treatment
Reagents (Deoxynivalenol (DON); quercetin and brusatol, a Nrf2 inhibitor) were purchased from Cayman Chemical (Ann Arbor, MI). All chemicals were first dissolved in dimethyl sulfoxide (DMSO) and then further diluted in cell culture medium. The final concentration of DMSO was 0.1% and was included in appropriate vehicle controls.
For investigation of dose-dependent effects of these chemicals, cells were exposed to various concentrations of quercetin, brusatol or DON for 24 h. To investigate the protective effect of quercetin against DON exposure, cells were pretreated with quercetin (40 μM) for 2 h prior to DON exposure (2 μM) for another 24 h. For investigating the functional role of Nrf2 in the observed effects from quercetin, cells were pretreated with brusatol (100 nM) for 2 h before exposure to quercetin and DON for another 24 h. All final concentrations used were determined after extensive preliminary experiments (lactate dehydrogenase assay) to determine optimum concentrations that did not elicit cellular toxicity (Fig S1).
Animals, experimental design, and sample collection
All animal procedures were approved by the Purdue University Animal Care and Use Committee (Approval # 1112000441). All experimental procedures adhered to the highest standards of animal care and welfare. Nine male weaned piglets with an initial body weight of 6.8 ± 0.29 kg were randomly allocated to one of three treatments. The three treatment groups were: (1) Control, piglets fed a basal diet followed by oral administration of ethanol (CON group); (2) piglets fed a basal diet followed by oral administration of DON (DON group); (3) piglets fed a basal diet supplemented with 1 g quercetin/kg feed followed by oral administration of DON (Quer + DON group). The basal diet (Supplementary Table 1) was formulated to meet or exceed the nutrient requirements recommended by the National Research Council (2012) for pigs in this age group. Piglets were fed with experimental diets for 14 days and then fasted overnight (12 h) followed by oral administration of a single bolus of 0 or 0.2 mg DON/kg BW, respectively. DON was first dissolved in ethanol and further diluted with pure water up to a final volume of 10 ml. After 24 h following DON administration, all piglets were administered an analgesic prior to euthanasia with CO2 and intestinal mucosal samples were collected and stored at 80 °C until further analysis. Intestinal segments were also collected and fixed for later histological analysis.
Histopathological analysis
Histomorphology analysis of intestinal tissue samples was conducted as previously described (Oladele et al., 2021). Briefly, intestinal segments of duodenum and jejunum were collected and fixed in 10% neutral-buffered formalin at RT for 24 h, embedded into paraffin, and then cut into sections. Slides were deparaffinized and incubated with xylene, ethanol, and deionized water sequentially for rehydration. Tissue sections were then stained with hematoxylin and eosin. After staining, slides were dehydrated in graded ethanol as well as xylene and mounted in a xylene-based mounting medium.
RNA extraction and qRT-PCR
Total RNA was extracted from the cultured cells using TRIzol® reagent (Invitrogen, Carlsbad, CA, USA). RNA concentration was measured on Nanodrop ND-1000 spectrophotometer. 1 μg of RNA was reverse transcribed into cDNA using Moloney Murine Leukemia Virus (MMLV) reverse transcriptase, followed by a 10-fold dilution in nuclease-free water. Amplifications were conducted by quantitative real-time PCR (qRT-PCR) using SYBR green 2 × RT-PCR mix. Abundance of specific mRNA transcripts was calculated and normalized to 18S ribosomal RNA using delta Ct method (Pfaffl, 2001). The primers for PCR amplification were listed below: claudin-1 (Forward: 5′-TTTCCTCAATACAGGAGGGAAGC-3′, Reverse: 5′-CCCTCTCCCCACATTCGAG-3′); claudin-3 (Forward: 5′-GCCAAGATCCTCTACTCCGC-3′, Reverse: 5′-GAGAGCTGCCTAGCATCTGG-3′); claudin-4 (Forward: 5′-CTCTCGGACACCTTCCCAAG-3′, Reverse: 5′-GCAGTGGGAAGGTCAAAGG-3′); Occludin (Forward: 5′-CTACTCGTCCAACGGGAAAG-3′, Reverse: 5′-ACGCCTCCAAGTTACCACTG-3′); ZO-1 (Forward: 5′-AAGCCCTAAGTTCAATCACAATCT-3′, Reverse: 5′-ATCAAACTCAGGAGGCGGC-3′); NRF2 (Forward: 5′- TGTCTTTGGATTTAGCGTTTCGG-3′, Reverse: 5′-TCCATGTCCCTTGACAGCAA-3′); NQO1 (Forward: 5′-CCTCTGGCCAATTCAGAGTGG-3′, Reverse: 5′- TGTGCCCAATGCTGTACGTC-3′); 18S (Forward: 5′- ATCCCTGAGAAGTTCCAGCA-3′, Reverse: 5′- CCTCCTGGTGAGGTCGATGT-3′).
Western blotting
Western blot analysis of samples from cells as well as intestinal tissues was conducted as previously described (Li and Ajuwon, 2021). Briefly, samples were lysed in 1 × radio-immunoprecipitation assay (RIPA) buffer supplemented with 1% phosphatase and protease inhibitor cocktail (Thermofisher, Waltham, MA, USA). Proteins were resolved on a 10% SDS polyacrylamide gel and transferred onto nitrocellulose membranes (Bio-Rad, Hercules, CA, USA) Membranes were cut into sections, blocked, and then incubated with corresponding primary antibodies at a dilution of 1:1000 overnight at 4 °C. The following primary antibodies were used: Anti-claudin-1 and 4 (Catalog No. 519000 and 329400, respectively; Life Technologies, Carlsbad, CA, USA); anti-claudin-3 and occludin (Catalog No. ab15102 and ab31721, respectively; Abcam, Cambridge, MA, USA); Nrf2 (Catalog No. 66504-1-Ig, Proteintech Group, Rosemont, IL, USA); β-actin (Catalog No. 8457, respectively; Cell Signaling Technology, Danvers, MA, USA). Membranes were then incubated against corresponding secondary antibodies (anti-rabbit or anti-mouse IgG-horseradish peroxidase (HRP); Cell Signaling Technology, Danvers, MA, USA). Protein bands were visualized on FluorChem imager (Proteinsimple, San Jose, CA, USA) using immobilon chemiluminescent HRP substrate (Millipore, Billerica, MA, USA). Band densities were quantified using a Kodak EDAS290 imaging software (Kodak, New Haven, CT, USA).
Immunofluorescence
Immunofluorescence analysis of samples from cells was conducted as previously described (Li and Ajuwon, 2021). Briefly, cells on coverslips were rinsed with PBS, and fixed with 4% paraformaldehyde (PFA). Glycine was then added to neutralize the remaining PFA in each well. Cells were blocked and incubated with corresponding primary antibodies at a dilution of 1:500 overnight at 4℃. The following primary antibodies were used for immunofluorescence. Anti-claudin-4, and ZO-1 (Catalog No. 329400 and 339100, respectively; Life Technologies, Carlsbad, CA, USA); anti-EEA1 and Rab7 (Catalog No. 3288 and 9367, respectively; Cell Signaling Technology, Danvers, MA, USA). Cells were then incubated with DAPI (Cayman chemical, Ann Arbor, Michigan) and Alexa flour dye-conjugated secondary antibodies (Thermofisher, Waltham, MA, USA). Fluorescence signal was visualized using a Leica imaging software (Leica Microsystems Buffalo Grove, IL, USA).
Measurement of epithelial barrier function
Intestinal epithelial cell barrier function was assessed by measuring trans-epithelial electrical resistance (TEER) as previously described (Li and Ajuwon, 2021). Briefly, IPEC-J2 cells were seeded onto transwell inserts and grown as confluent monolayers. The monolayers were then pretreated with quercetin for 2 h at both apical and basolateral side, followed by DON exposure for another 24 h. TEER values were determined at indicated time points by EVOM2 Voltohmmeter (World Precision Instruments, Sarasota, FL, USA).
At the end of TEER measurement, fluorescein isothiocyanate-labeled dextran (FITC-dextran, 4-kDa) was added to the apical side of transwell inserts to measure the paracellular permeability. The concentration of FITC-dextran at the basolateral side was measured using fluorometry (Tecan Group Ltd., Switzerland) with excitation and emission wavelengths of 485 and 530 nm, respectively.
Statistical analysis
Data were analyzed through one-way ANOVA and presented as means ± SEM using SAS (version 9.4). Tukey multiple comparison analysis was performed to determine significance of differences among treatment means. Unpaired t-test was conducted to determine the difference between two treatment groups. P < 0.05 was considered significant, whereas P-values between 0.05 and 0.1 was considered a tendency. Results are illustrated in graphs using the GraphPad Prism (GraphPad Software Inc, San Diego, CA).
Results
Quercetin increased Nrf2 target gene expression and selectively regulated expression of TJP
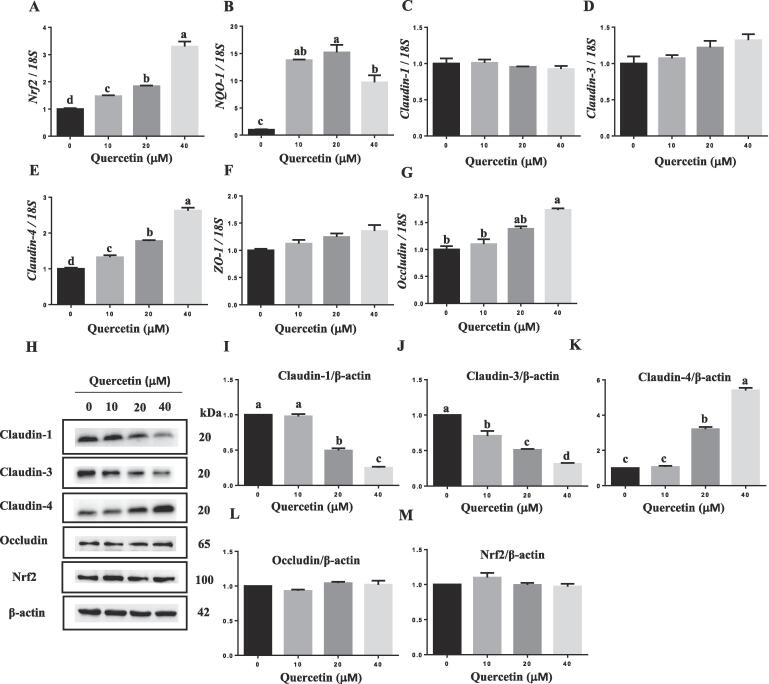
Quercetin supplementation significantly increased the mRNA expression of NQO-1 (Fig. 1B), a well-recognized Nrf2 target gene, suggesting that Nrf2 transcriptional activity was induced by quercetin in IPEC-J2 cells. Treatment of cells with quercetin also dose-dependently increased the mRNA expression of NRF2 (Fig. 1A). However, the protein abundance of Nrf2 was not affected by quercetin supplementation (Fig. 1M). Additionally, quercetin treatment selectively increased mRNA expression of claudin-4 (Fig. 1E), whereas that of claudin-1 (Fig. 1C), claudin-3 (Fig. 1D) and ZO-1 (Fig. 1F) were not affected. At the protein level, quercetin increased the expression of claudin-4 (Fig. 1K) in a dose-dependent manner but decreased that of claudin-1 (Fig. 1I) and claudin-3 (Fig. 1J). These results suggest that quercetin selectively regulates the expression of TJP in IPEC-J2 cells.
Fig. 1.
Quercetin increased Nrf2 transcriptional activity and selectively regulated TJP expression. IPEC-J2 cells were treated with various concentrations of quercetin (0–40 μM) for 24 h. The mRNA expression of NRF2 (A), NQO-1 (B), claudin-1 (C), claudin-3 (D), claudin-4 (E), ZO-1 (F) and occludin (G) was determined by real-time PCR. The expression levels of mRNA are expressed relative to the values in the control. The protein expression of claudin-1, 3, 4, occludin and Nrf2 was measured by western blot (H) and quantified (I-M). Band densities are expressed relative to the values in the control. Different superscript lowercase letters within each group indicate significant difference (p < 0.05). n = 3.
DON suppressed Nrf2 target gene and TJP expression
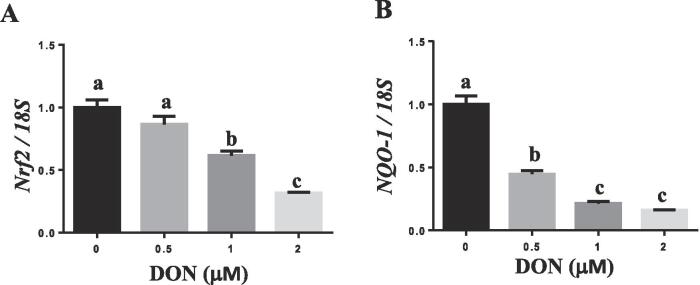
DON exposure dose-dependently decreased the mRNA expression of NRF2 (Fig. 2A) and NQO-1 (Fig. 2B), indicating that Nrf2 transcriptional activity was suppressed by DON exposure. Treatment of cells with DON also decreased the protein abundance of claudin-1, 3 and 4 (Fig. 3A).
Fig. 2.
DON exposure decreased Nrf2 transcriptional activity. IPEC-J2 cells were treated with different concentrations of DON (0–2 μM) for 24 h. The mRNA expression of NRF2 (A) and NQO-1 (B) was determined by real-time PCR. The expression levels of mRNA are expressed relative to the values in the control. Different superscript lowercase letters within each group indicate significant difference (p < 0.05). n = 3.
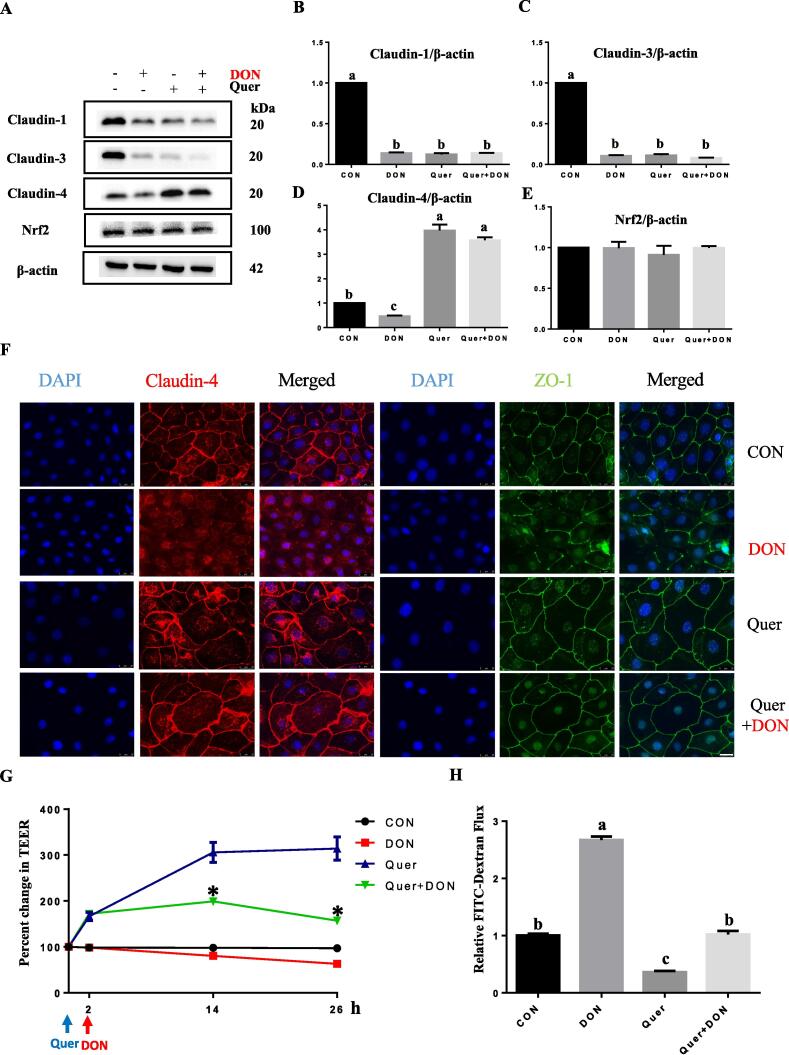
Fig. 3.
Quercetin reversed the epithelial barrier loss caused by DON.IPEC-J2 cells were incubated in the presence or absence of DON (2 μM) for 24 h with or without pretreatment with quercetin (40 μM) for 2 h. The protein expression of claudin-1, 3, 4 and Nrf2 was measured by western blot (A) and quantified (B-E). Band densities are expressed relative to the control. Representative immunofluorescence of claudin-4 and ZO-1 (F) following 24 h of DON (2 μM) exposure with or without pretreatment of quercetin (40 μM). DAPI (blue), Claudin-4 (red), and ZO-1 (green) staining were shown. Scale bars, 25 μm. Cell monolayers grown on transwells were pretreated with or without quercetin (40 μM) prior to DON exposure (2 μM) and TEER (G) was measured over a 26-h period (*P < 0.05, DON vs. Quer + DON). The relative paracellular permeability of FITC-dextran (H) was measured at the end of TEER measurement. Different superscript lowercase letters within each group indicate significant difference (P < 0.05). n = 3. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Quercetin protected against DON-induced intestinal barrier dysfunction
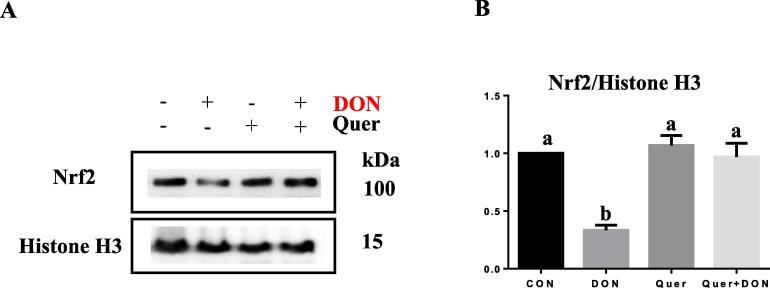
To investigate the protective effect of quercetin against DON-induced intestinal barrier dysfunction, cells were pretreated with quercetin prior to DON exposure. Results showed quercetin supplementation reversed the reduction of claudin-4 (Fig. 3D) caused by DON exposure. Immunofluorescence also showed that the decreased membrane presence of claudin-4 and ZO-1 (Fig. 3F) caused by DON was reversed by quercetin. Effect of quercetin supplementation also included the restoration of transepithelial electrical resistance (TEER) and reduction of FITC-dextran permeability (Fig. 3G and 3H) that have been perturbed by DON. Although, the protein abundance of Nrf2 was not affected by DON or quercetin supplementation, cell fractionation analysis showed that DON exposure reduced the nuclear presence of Nrf2 and this was blocked by quercetin (Fig. 4A, B).
Fig. 4.
Quercetin supplementation reversed the decreased nuclear presence of Nrf2 caused by DON.IPEC-J2 cells were incubated in the presence of DON (2 μM) for 24 h with or without pretreatment with quercetin (40 μM) for 2 h. The protein expression of Nrf2 and histone H3 was measured by western blot (A) and quantified (B). Band densities are expressed relative to the control.
Quercetin attenuated the endocytosis of claudin-4 caused by DON
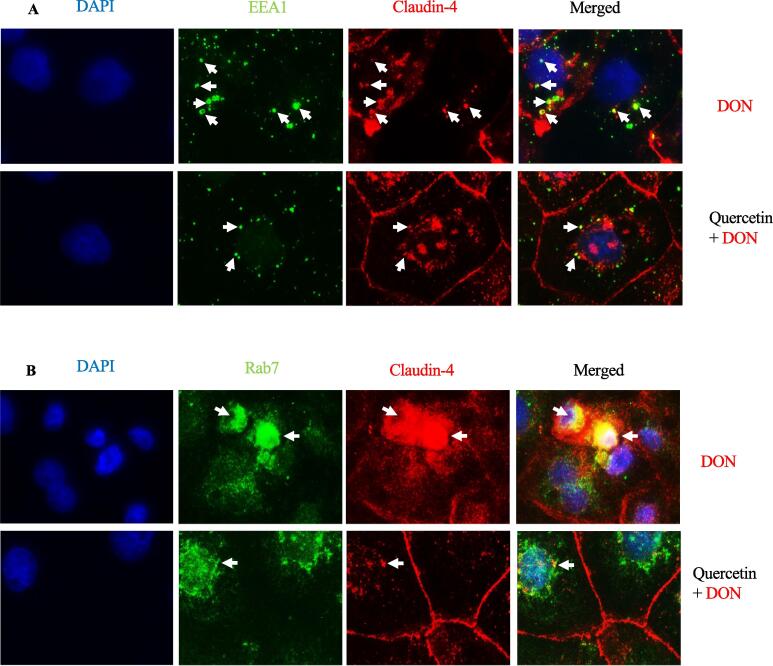
We had earlier shown that DON exposure increased the endocytosis and degradation of claudin-4, a process dependent on both dynamin and actin activity (Li et al., 2021). Consistent with our previous findings, treatment of cells with DON induced the co-localization of the early and late endosomal markers (EEA1, Rab7) with claudin-4 (Fig. 5). However, this co-localization was partly blocked by quercetin supplementation, indicating that quercetin decelerated the endocytosis of claudin-4 caused by DON exposure.
Fig. 5.
Quercetin reduced the colocalization of internalized claudin-4 with endosome markers induced by DON. IPEC-J2 cells were incubated in the presence of DON (2 μM) for 24 h with or without pretreatment of quercetin (40 μM) for 2 h. Cells were then double-labeled for claudin-4 and markers for early (EEA1) and late endosomes (Rab7). Internalized claudin-4 induced by DON colocalizes with EEA1 and Rab7 (arrowheads). DAPI (blue), claudin-4 (red), EEA1 (green) and Rab7 (green) staining are shown. Scale bars, 5 μm; n = 3. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
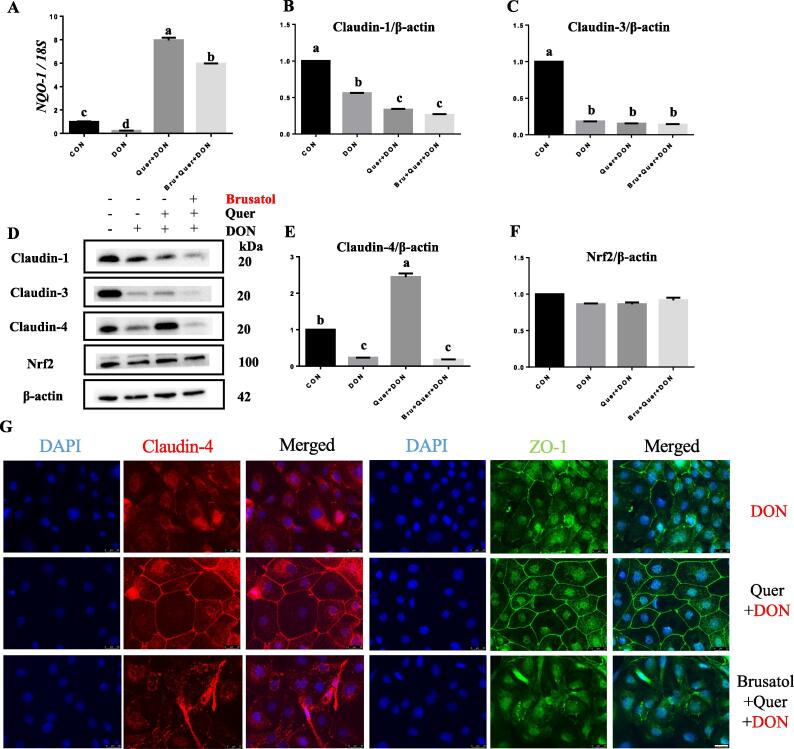
The protective effect of quercetin against DON exposure was regulated by Nrf2 pathway
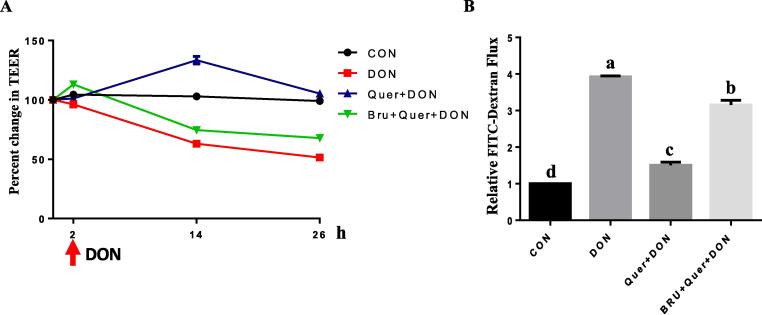
To further confirm the role of Nrf2 in the observed effects of quercetin against DON exposure and exclude the potential involvement of Nrf2-independent mechanisms, cells were pretreated with the specific Nrf2 inhibitor, brusatol, before exposure to quercetin and DON. As expected, the observed quercetin-induced upregulation of claudin-4 (Fig. 6E) in the presence of DON was abolished by brusatol. Pretreatment of cells with the Nrf2 inhibitor also prevented the quercetin-induced protection of membrane presence of claudin-4 and ZO-1 (Fig. 6G) during DON exposure. The effectiveness of quercetin in opposing DON-induced decrease in TEER values (Fig. 7A) and the increase in FITC-dextran permeability (Fig. 7B) was also abrogated by Nrf2 inhibitor, confirming the involvement of Nrf2 in the protective effects of quercetin against DON exposure. These results suggest that the protective effect of quercetin in mitigating DON-induced intestinal barrier dysfunction potentially involves Nrf2 signaling pathway.
Fig. 6.
The protective effect of quercetin against DON-induced TJP reduction was abolished by Nrf2 antagonist.IPEC-J2 cells were first pretreated with or without brusatol (100 nM) for 2 h, and then incubated in the presence or absence of quercetin (40 μM) for another 2 h prior to DON (2 μM) exposure. The mRNA expression of NQO-1 (A) was determined by real-time PCR. The expression levels of mRNA are expressed relative to the values in the control. The protein expression of claudin-1, 3, 4 and Nrf2 was measured by western blot and quantified (B-F). The band densities are expressed relative to the control. Different superscript lowercase letters within each group indicate significant difference (P < 0.05). Representative immunofluorescence of claudin-4 and ZO-1 (G) following 24 h of DON (2 μM) exposure with or without pretreatment of brusatol (100 nM) and quercetin (40 μM). DAPI (blue), Claudin-4 (red), and ZO-1 (green) staining are shown. Scale bars, 25 μm. n = 3. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 7.
The protective effect of quercetin against DON-induced barrier loss was abrogated by Nrf2 antagonist. Cell monolayers grown on transwells were first pretreated with or without brusatol (100 nM) for 2 h, and then incubated in the presence or absence of quercetin (40 μM) and DON (2 μM) and TEER (A) was measured over a 26-h period. The relative paracellular permeability of FITC-dextran (B) was measured at the end of TEER measurement. Different superscript lowercase letters within each group indicate significant difference (P < 0.05). n = 3.
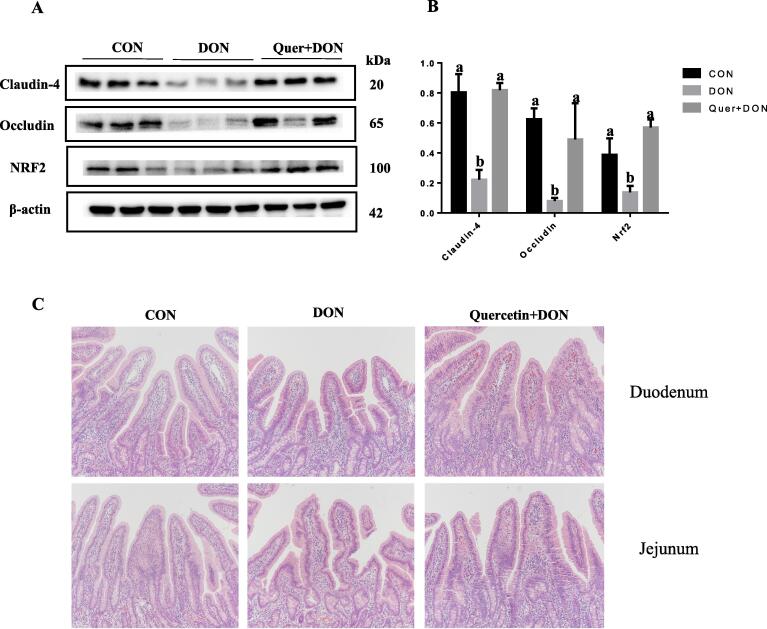
Quercetin protected against DON-induced reduction of TJP and intestinal injuries in weaned piglets
To evaluate the protective effect of quercetin against DON-induced stress in vivo, weaned piglets were fed with a basal diet supplemented with quercetin prior to DON exposure. Consistent with in vitro data, DON administration decreased the protein expression of claudin-4 and occludin (Fig. 8A). Quercetin supplementation reversed the reduction of TJP induced by DON exposure (Fig. 8B). Intestinal histology analysis showed that DON exposure negatively affected villus-crypt morphology as indicated by diffuse apical villus necrosis, villus atrophy and fusion. Notably, intestinal injuries caused by DON administration were partly mitigated in the Quer + DON group (Fig. 8C). Compared with control group, piglets exposed to DON alone showed lower villus height in duodenum and jejunum. Interestingly, the shortened villus height caused by DON exposure was also ameliorated by quercetin supplementation.
Fig. 8.
Quercetin supplementation reversed the TJP reduction and intestinal injuries caused by DON in weaned piglets. Weaned piglets were fed with a basal diet supplemented with or without quercetin (40 μM) prior to DON (2 μM) exposure. The protein expression of claudin-4, occludin and Nrf2 in the duodenum of piglets from different groups was measured by western blot (A) and quantified (B). Representative HE staining (C) in the duodenum and jejunum of piglets from different groups. n = 3.
Discussion
The presence of mycotoxins in food and feed has been considered a major risk factor for human and animal health, as up to 25% of the world’s cereal production could be contaminated (Pinton and Oswald, 2014). The intestine has been shown as the first physical barrier against external contaminants, as well as the major target organ for these mycotoxins (Maresca et al., 2002, Wang et al., 2020). We have shown previously that intestinal exposure of DON increased endocytosis and degradation of TJP, thereby compromising intestinal barrier function (Li et al., 2023). However, the underlying mechanism by which DON induces intestinal barrier loss still remains unclear. Recent evidence suggests that Nrf2 may play an important role in response to mycotoxin exposure (Kozieł et al., 2021). Zhang et al. (2018) showed that T-2 toxin, type B of the trichothecenes family of mycotoxin exposure induced oxidative stress and decreased the expression of Nrf2 and HO-1 at both mRNA and protein levels in N2a cells. Interestingly, pretreatment of cells with Nrf2 activator mitigated the toxic effects caused by T-2 toxin, indicating that Nrf2 signaling pathway may serve as the main response element to T-2 toxin-induced stress (Kozieł et al., 2021). In another in vivo study, Nrf2 signaling pathway was suppressed in mice exposed to T-2 toxin (Chaudhary and Lakshmana, 2010). Consistent with previous findings, data from current study shows that DON exposure dose-dependently decreased the mRNA expression of NRF2 and NQO-1, suggesting that DON suppressed the transcription activity of Nrf2 in IPEC-J2 cells. Previously, we showed that DON exposure induced the activation of MAPK signaling pathway (Li et al., 2021). Sun et al. (2009) reported that Nrf2 can be phosphorylated at multiple sites by MAPK which may inhibit Nrf2-mediated transcription activity. Therefore, it is possible that DON-induced MAPK activation suppresses Nrf2 signaling pathway. Another possible explanation is that DON increased the expression of Keap1 and reduced the nuclear translocation of Nrf2, thus suppressing the transcription of Nrf2 target genes (Zhou et al., 2021). Given the protective effect of Nrf2 activation in other conditions (Zhang et al., 2018, Zhou et al., 2021), we hypothesized that Nrf2 activation by quercetin could exert beneficial effect against DON exposure. As expected, quercetin supplementation attenuated DON-induced barrier loss and intestinal injuries at both in vivo and in vitro conditions in this study. In addition to the potential functional role of Nrf2 on claudin-4 transcription, we also found that quercetin treatment attenuated the endocytosis of claudin-4 caused by DON exposure. Huang et al. (2010) reported that quercetin inhibited the endocytic activity of dendritic cells by measuring FITC-dextran uptake. Another study conducted by Ganesan et al. (2012) also showed that quercetin treatment inhibited rhinovirus endocytosis in airway epithelial cells. Quercetin has been reported to bind with actin filaments and inhibit actin polymerization, a process required for the invagination, maturation and scission of endocytic vesicles (Böhl et al., 2007, Li et al., 2021). It is, therefore, possible that quercetin blocks the cytoskeleton reorganization during endocytosis of claudin-4, thus increasing its membrane presence compared to the cells exposed to DON alone (Böhl et al., 2007).
Quercetin is a widely distributed bioflavonoid extracted from various food resources such as grapes, onions, wine, berries, seeds and vegetables (Oboh et al., 2016). It also exhibits a versatile biological and pharmacological activity as well as therapeutic efficacy. Oral administration of quercetin decreased pro-inflammatory cytokines and nitric oxide derived from macrophages, suggesting that quercetin is a potent anti-inflammatory agent targeting the inflammatory response caused by experimental arthritis (Mamani-Matsuda et al., 2006). Quercetin also exhibits antibacterial effects against various strains of bacteria, particularly those infecting the respiratory, urinary and gastrointestinal system (Ramos et al., 2006). Oxidative stress is characterized as an imbalance between pro-oxidants and antioxidant, with a shift toward the pro-oxidant side (Meyers et al., 2008). The most extensively studied biological function of quercetin is its antioxidant property wherein it protects cells from cellular damage induced by free radicals (David et al., 2016). It has been shown that quercetin can act as hydrogen atoms donor to scavenge free radicals through the reaction R• + ROO• → ROOR (Halvorsen et al., 2002).
The intestinal epithelium is lined with a single layer of epithelial cells which are tightly connected with one another by the formation of tight junction, adherens junction, and desmosomes. The integrity of the intercellular tight junction is the major determinant of barrier function of intestinal epithelium (Groschwitz and Hogan, 2009, Turner, 2009). Recent evidence revealed that quercetin enhanced intestinal barrier function in both in vivo and in vitro models (Amasheh et al., 2008, Fuentes et al., 2022). However, the effects of quercetin in the regulation of TJP expression and barrier function and the underlying mechanism of such effect are still poorly understood. Herein, we present data that confirm a direct regulation of intestinal TJP by quercetin. Specifically, we showed that quercetin treatment dose-dependently increased the expression of claudin-4 at both mRNA and protein levels which was consistent with previous findings (Amasheh et al., 2008). Indeed, a direct upregulation of claudin-4 promoter by quercetin has been confirmed in Caco-2 cells (Amasheh et al., 2008). However, details of the transcriptional regulation of claudin-4 by quercetin is still unclear with regard to the identity of the transcription factors involved.
Interestingly, recent evidence showed that quercetin increased the expression of Nrf2 and stabilized Nrf2 protein by inhibiting its proteasomal degradation, thereby enhancing Nrf2 mediated transcription activity (Tanigawa et al., 2007). Data from the present study also found that treatment of cells with quercetin increased the mRNA expression of Nrf2 as well as its target gene, NQO-1, suggesting that quercetin increased Nrf2 transcription activity in this study. Chen et al. (2014) reported that Nrf2 deficiency in esophageal epithelium decreased expression of claudin-4 and compromised barrier function. Previously, ChIP analysis has revealed that Nrf2 could directly bind the claudin-4 promotor (Chen et al., 2014), suggesting that the increased claudin-4 expression induced by quercetin could be regulated by Nrf2-dependent signaling pathway. Intriguingly, quercetin treatment decreased the expression of claudin-1 and 3 at the protein, but not mRNA level, indicating the potential involvement of post-translational modification of TJP induced by quercetin (Van Itallie et al., 2004, Li et al., 2022). A recent study conducted by Shashikanth et al. (2022) reported that interclaudin interference affected claudin channel function formed by claudin-2 in MDCK cells. Further analysis showed claudin-4 expression increased the mobile fraction of claudin-2, thereby decreasing claudin-2 anchoring at tight junctions. Mechanistically, the expression of claudin-4 interrupted claudin-2 polymers and reduced the number of claudin-2 strands by increasing its endocytosis and degradation in the lysosome (Shashikanth et al., 2022). It is, therefore, possible that the increased expression of claudin-4 induced by quercetin reduces the anchoring of claudin-1 and 3 at tight junctions, hence increasing their degradation, in a similar manner.
Quercetin as one of the most potent antioxidants could exert its beneficial effects through multiple intracellular signaling pathways. A study conducted by Kim et al. (2010) reported that quercetin treatment induced apoptosis through activation of AMPK signaling pathway in HT-29 colon cancer cells. Oral administration of quercetin also demonstrated anti-tumor effect by inhibition of JAK2/STAT3 signaling pathway (Wu et al., 2019). Shan et al. (2009) also found that inhibited human SW480 colon cancer growth via suppressing Wnt/β-Catenin signaling pathway. Therefore, quercetin could function independently of Nrf2 pathway. To confirm whether Nrf2 is required in mediating the protective effect of quercetin against DON exposure. Cells were pretreated with brusatol, a well-known specific Nrf2 inhibitor (Cai et al., 2019) prior to quercetin and DON exposure. As expected, brusatol pretreatment abolished the observed beneficial effect of quercetin against DON exposure, indicating that quercetin improved intestinal barrier function through Nrf2-dependent mechanism in this study (Fig. 9).
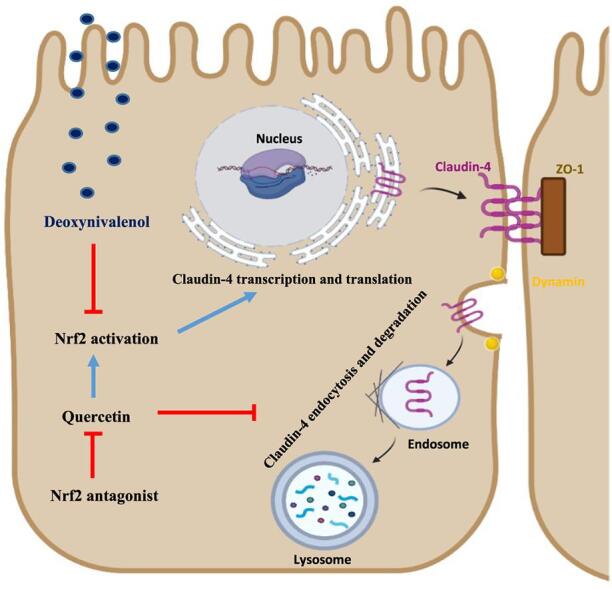
Fig. 9.
A proposed mechanism for the turnover of claudin-4 regulated by Quercetin. DON exposure suppressed Nrf2 transcriptional activity together with reduced claudin-4 expression. This process can be reversed by quercetin supplementation through the activation of Nrf2 signaling pathway. Quercetin can also decelerate the endocytosis and degradation of claudin-4 caused by DON exposure. However, the protective effects of quercetin against DON exposure can be abolished by Nrf2 antagonist.
In sum, this study demonstrates a protective effect of quercetin against DON-induced epithelial barrier loss and intestinal injuries through Nrf2-dependent mechanisms. This suggests that quercetin supplementation could be protective, especially against DON-induced gut barrier dysfunction.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was financially sponsored by a fellowship from the Department of Animal Sciences, Purdue University, and support from United Animal Health Inc.
Author contributions
KM Ajuwon and N Horn obtained financial support. E Li and KM Ajuwon conceived and designed the study. E Li performed the experiments and analyzed the data. E Li and KM Ajuwon wrote the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crtox.2023.100122.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Effects of quercetin supplementation on LDH release in IPEC-J2 cells. IPEC-J2 cells were treated with various concentrations of quercetin (0-40 μM) for 24 h. LDH release into the cell culture medium was used for the quantitative assessment of cell viability. Cells treated with Triton X-100 were used for maximum (100%) LDH release. n = 3. Different lowercase superscript letters within each group indicate significant difference (P < 0.05).
Data availability
Data will be made available on request.
References
- Amasheh M., Schlichter S., Amasheh S., et al. Quercetin enhances epithelial barrier function and increases claudin-4 expression in Caco-2 cells. J. Nutr. 2008;138(6):1067–1073. doi: 10.1093/jn/138.6.1067. [DOI] [PubMed] [Google Scholar]
- Böhl M., Tietze S., Sokoll A., Madathil S., Pfennig F., Apostolakis J., Fahmy K., Gutzeit H.O. Flavonoids affect actin functions in cytoplasm and nucleus. Biophys. J. 2007;93(8):2767–2780. doi: 10.1529/biophysj.107.107813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai S.J., Liu Y., Han S., et al. Brusatol, an NRF2 inhibitor for future cancer therapeutic. Cell Biosci. 2019;9(1):1–3. doi: 10.1186/s13578-019-0309-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camuesco D., Comalada M., Rodríguez-Cabezas M.E., Nieto A., Lorente M.D., Concha A., Zarzuelo A., Gálvez J. The intestinal anti-inflammatory effect of quercitrin is associated with an inhibition in iNOS expression. Br. J. Pharmacol. 2004;143(7):908–918. doi: 10.1038/sj.bjp.0705941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary M., Lakshmana Rao P.V. Brain oxidative stress after dermal and subcutaneous exposure of T-2 toxin in mice. Food Chem. Toxicol. 2010;48:3436–3442. doi: 10.1016/J.FCT.2010.09.018. [DOI] [PubMed] [Google Scholar]
- Chen H., Hu Y., Fang Y.u., Djukic Z., Yamamoto M., Shaheen N.J., Orlando R.C., Chen X. Nrf2 deficiency impairs the barrier function of mouse oesophageal epithelium. Gut. 2014;63(5):711–719. doi: 10.1136/gutjnl-2012-303731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Council, N. R. (2012). Nutrient requirements of swine. Natl. Acad. Press, Washington, DC.
- David A.V.A., Arulmoli R., Parasuraman S. Overviews of biological importance of quercetin: A bioactive flavonoid. Phcog. Rev. 2016;10(20):84. doi: 10.4103/0973-7847.194044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes J., Brunser O., Atala E., Herranz J., de Camargo A.C., Zbinden-Foncea H., Speisky H. Protection against indomethacin-induced loss of intestinal epithelial barrier function by a quercetin oxidation metabolite present in onion peel: In vitro and in vivo studies. J. Nutr. Biochem. 2022;100:108886. doi: 10.1016/j.jnutbio.2021.108886. [DOI] [PubMed] [Google Scholar]
- Ganesan S., Faris A.N., Comstock A.T., Wang Q., Nanua S., Hershenson M.B., Sajjan U.S. (2012) Quercetin inhibits rhinovirus replication in vitro and in vivo. Antiviral Res. 2012;94(3):258–271. doi: 10.1016/j.antiviral.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groschwitz K.R., Hogan S.P. Intestinal barrier function: molecular regulation and disease pathogenesis. J. Allergy Clin. Immunol. 2009;124(1):3–20. doi: 10.1016/j.jaci.2009.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halvorsen B.L., Holte K., Myhrstad M.C.W., Barikmo I., Hvattum E., Remberg S.F., Wold A.-B., Haffner K., Baugerød H., Andersen L.F., Moskaug Ø., Jacobs D.R., Blomhoff R. A systematic screening of total antioxidants in dietary plants. J. Nutr. 2002;132(3):461–471. doi: 10.1093/jn/132.3.461. [DOI] [PubMed] [Google Scholar]
- Huang R Y, Yu Y L, Cheng W C, et al. (2010) Immunosuppressive effect of quercetin on dendritic cell activation and function. The Journal of Immunology, 184(12): 6815-6821. https://doi.org/10.4049/jimmunol.0903991. [DOI] [PubMed]
- Kim H.-J., Kim S.-K., Kim B.-S., Lee S.-H., Park Y.-S., Park B.-K., Kim S.-J., Kim J., Choi C., Kim J.-S., Cho S.-D., Jung J.-W., Roh K.-H., Kang K.-S., Jung J.-Y. Apoptotic effect of quercetin on HT-29 colon cancer cells via the AMPK signaling pathway. J. Agric. Food Chem. 2010;58(15):8643–8650. doi: 10.1021/jf101510z. [DOI] [PubMed] [Google Scholar]
- Kozieł M.J., Kowalska K., Piastowska-Ciesielska A.W. Nrf2: a main responsive element in cells to mycotoxin-induced toxicity. Arch. Toxicol. 2021;95(5):1521–1533. doi: 10.1007/s00204-021-02995-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li E., Ajuwon K.M. Mechanism of endocytic regulation of intestinal tight junction remodeling during nutrient starvation in jejunal IPEC-J2 cells. FASEB J. 2021;35(2):e21356. doi: 10.1096/fj.202002098R. [DOI] [PubMed] [Google Scholar]
- Li E., Horn N., Ajuwon K.M. Mechanisms of deoxynivalenol-induced endocytosis and degradation of tight junction proteins in jejunal IPEC-J2 cells involve selective activation of the MAPK pathways. Arch. Toxicol. 2021;95(6):2065–2079. doi: 10.1007/s00204-021-03044-w. [DOI] [PubMed] [Google Scholar]
- Li E., Horn N., Ajuwon K.M. EPA and DHA inhibit endocytosis of claudin-4 and protect against deoxynivalenol-induced intestinal barrier dysfunction through PPARγ dependent and independent pathways in jejunal IPEC-J2 cells. Food Res. Int. 2022;2022 doi: 10.1016/j.foodres.2022.111420. [DOI] [PubMed] [Google Scholar]
- Li E., Li C., Horn N., Ajuwon K.M. PPARγ activation inhibits endocytosis of claudin-4 and protects against deoxynivalenol-induced intestinal barrier dysfunction in IPEC-J2 cells and weaned piglets. Toxicol. Lett. 2023;375:8–20. doi: 10.1016/j.toxlet.2022.12.015. [DOI] [PubMed] [Google Scholar]
- Mamani-Matsuda M., Kauss T., Al-Kharrat A., Rambert J., Fawaz F., Thiolat D., Moynet D., Coves S., Malvy D., Mossalayi M.D. Therapeutic and preventive properties of quercetin in experimental arthritis correlate with decreased macrophage inflammatory mediators. Biochem. Pharmacol. 2006;72(10):1304–1310. doi: 10.1016/j.bcp.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Maresca M., Mahfoud R., Garmy N., Fantini J. The mycotoxin deoxynivalenol affects nutrient absorption in human intestinal epithelial cells. J. Nutr. 2002;132(9):2723–2731. doi: 10.1093/jn/132.9.2723. [DOI] [PubMed] [Google Scholar]
- Meyers K.J., Rudolf J.L., Mitchell A.E. Influence of dietary quercetin on glutathione redox status in mice. J. Agric. Food Chem. 2008;56(3):830–836. doi: 10.1021/jf072358l. [DOI] [PubMed] [Google Scholar]
- Moskaug J.Ø., Carlsen H., Myhrstad M.C.W., et al. Polyphenols and glutathione synthesis regulation. Am. J. Clin. Nutr. 2005;81(1):277S–283S. doi: 10.1093/ajcn/81.1.277S. [DOI] [PubMed] [Google Scholar]
- Ndlovu S., Nagiah S., Abdul N.S., Ghazi T., Chuturgoon A.A. Deoxynivalenol downregulates NRF2-induced cytoprotective response in human hepatocellular carcinoma (HepG2) cells. Toxicon. 2021;193:4–12. doi: 10.1016/j.toxicon.2021.01.017. [DOI] [PubMed] [Google Scholar]
- Oboh G, Ademosun AO, Ogunsuyi OB. (2016) Quercetin and its role in chronic diseases. Drug discovery from mother nature, 377-387. https://doi.org/10.1007/978-3-319-41342-6_17. [DOI] [PubMed]
- Oladele P., Li E., Lu H., Cozannet P., Nakatsu C., Johnson T., Adeola O., Ajuwon K.M. Effect of a carbohydrase admixture in growing pigs fed wheat-based diets in thermoneutral and heat stress conditions. J. Anim. Sci. 2021;99(10) doi: 10.1093/jas/skab254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001;29(9):e45–e. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinton P., Oswald I.P. Effect of deoxynivalenol and other Type B trichothecenes on the intestine: a review. Toxins. 2014;6(5):1615–1643. doi: 10.3390/toxins6051615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos F.A., Takaishi Y., Shirotori M., Kawaguchi Y., Tsuchiya K., Shibata H., Higuti T., Tadokoro T., Takeuchi M. Antibacterial and antioxidant activities of quercetin oxidation products from yellow onion (Allium cepa) skin. J. Agric. Food Chem. 2006;54(10):3551–3557. doi: 10.1021/jf060251c. [DOI] [PubMed] [Google Scholar]
- Shan B.E., Wang M.X., Li R. Quercetin inhibit human SW480 colon cancer growth in association with inhibition of cyclin D1 and survivin expression through Wnt/β-catenin signaling pathway. Cancer Invest. 2009;27(6):604–612. doi: 10.1080/07357900802337191. [DOI] [PubMed] [Google Scholar]
- Shashikanth N., France M.M., Xiao R., Haest X., Rizzo H.E., Yeste J., Reiner J., Turner J.R. Tight junction channel regulation by interclaudin interference. Nat. Commun. 2022;13(1) doi: 10.1038/s41467-022-31587-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z., Huang Z., Zhang D.D., Morty R.E. Phosphorylation of Nrf2 at multiple sites by MAP kinases has a limited contribution in modulating the Nrf2-dependent antioxidant response. PLoS One. 2009;4(8):e6588. doi: 10.1371/journal.pone.0006588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanigawa S., Fujii M., Hou D.X. Action of Nrf2 and Keap1 in ARE-mediated NQO1 expression by quercetin. Free Radic. Biol. Med. 2007;42(11):1690–1703. doi: 10.1016/j.freeradbiomed.2007.02.017. [DOI] [PubMed] [Google Scholar]
- Turner J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009;9(11):799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- Van Itallie C.M., Colegio O.R., Anderson J.M. The cytoplasmic tails of claudins can influence tight junction barrier properties through effects on protein stability. J. Membr. Biol. 2004;199(1):29–38. doi: 10.1007/s00232-004-0673-z. [DOI] [PubMed] [Google Scholar]
- Wang S., Zhang C., Yang J., Wang X.u., Wu K., Zhang B., Zhang J., Yang A.o., Rajput S.A., Qi D. Sodium butyrate protects the intestinal barrier by modulating intestinal host defense peptide expression and gut microbiota after a challenge with deoxynivalenol in weaned piglets. J. Agric. Food Chem. 2020;68(15):4515–4527. doi: 10.1021/acs.jafc.0c00791. [DOI] [PubMed] [Google Scholar]
- Wang S., Wu K., Xue D., Zhang C., Rajput S.A., Qi D. (2021) Mechanism of deoxynivalenol mediated gastrointestinal toxicity: Insights from mitochondrial dysfunction. Food Chem. Toxicol. 2021;153:112214. doi: 10.1016/j.fct.2021.112214. [DOI] [PubMed] [Google Scholar]
- Wu L., Li J., Liu T., Li S., Feng J., Yu Q., Zhang J., Chen J., Zhou Y., Ji J., Chen K., Mao Y., Wang F., Dai W., Fan X., Wu J., Guo C. Quercetin shows anti-tumor effect in hepatocellular carcinoma LM3 cells by abrogating JAK2/STAT3 signaling pathway. Cancer Med. 2019;8(10):4806–4820. doi: 10.1002/cam4.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Wang Y., Velkov T., Tang S., Dai C. T-2 toxin-induced toxicity in neuroblastoma-2a cells involves the generation of reactive oxygen, mitochondrial dysfunction and inhibition of Nrf2/HO-1 pathway. Food Chem. Toxicol. 2018;114:88–97. doi: 10.1016/j.fct.2018.02.010. [DOI] [PubMed] [Google Scholar]
- Zhao J., Moore A.N., Redell J.B., Dash P.K. Enhancing expression of Nrf2-driven genes protects the blood–brain barrier after brain injury. J. Neurosci. 2007;27(38):10240–10248. doi: 10.1523/JNEUROSCI.1683-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Lin H., Qin Y., et al. L-Carnosine Protects Against Deoxynivalenol-Induced Oxidative Stress in Intestinal Stem Cells by Regulating the Keap1/Nrf2 Signaling Pathway. Mol. Nutr. Food Res. 2021;65(17):2100406. doi: 10.1002/mnfr.202100406. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effects of quercetin supplementation on LDH release in IPEC-J2 cells. IPEC-J2 cells were treated with various concentrations of quercetin (0-40 μM) for 24 h. LDH release into the cell culture medium was used for the quantitative assessment of cell viability. Cells treated with Triton X-100 were used for maximum (100%) LDH release. n = 3. Different lowercase superscript letters within each group indicate significant difference (P < 0.05).
Data Availability Statement
Data will be made available on request.