Abstract
Background/Aim
Urinary bladder cancer has various etiologies and tends to recur and then progress to a higher grade. When muscles are invaded, the response to conventional therapy is poor and the quality of life deteriorates rapidly. Here, we summarize and compare two representative methods used to create the syngeneic mouse models required for immunological research.
Materials and Methods
In this study, we utilized six-week-old female C3H/HeNCrl mice and the mouse bladder tumor cell line MBT-2. The first method involved transurethral catheterization with poly-L-lysine pretreatment (catheter group), while the second method involved transperitoneal incision and direct injection of tumor cells into the bladder wall (open group). Mouse postoperative status was monitored on a weekly basis using magnetic resonance imaging (MRI).
Results
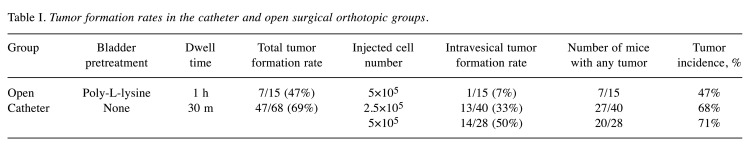
The catheter group had a tumor development rate of 47% (7 out of 15 mice), with only 1 mouse developing an intravesical tumor. In contrast, the open group had a higher tumor formation rate of 69% (47 out of 68 mice), with 27 mice showing intravesical tumor formation. Notably, with a lower cell count, urinary obstruction events were observed 2 weeks post-inoculation, which is one week later than the higher cell count group.
Conclusion
In this study, we conducted a comparative analysis between the transurethral catheterization method and the transperitoneal incision and direct injection method in animal bladder tumor models. Our findings provide evidence of the consistent effectiveness in constructing a stable model within the open group. Well-designed orthotopic animal models are essential.
Keywords: Urinary bladder neoplasms, non-muscle invasive bladder neoplasms, animal models, immunotherapy, therapeutics
Bladder cancer is the 10th most common disease worldwide. The major risk factors include a genetic predisposition, medication, radiation, infection, tobacco smoke exposure, and occupational exposure to toxins (1,2). Cancer may present either as a low-grade papillary neoplasm or as a high-grade papillary carcinoma/carcinoma in-situ; muscle invasion greatly affects treatment and prognosis (3). Bacillus Calmette-Guerin (BCG) treatment and chemotherapy have long been favored (4). As targeted and immune therapies are now becoming the mainstay treatments of advanced cancers (5), a biologically relevant mouse model is required for the preclinical evaluation of such drugs. Several orthotopic bladder models have been described, but they are technically demanding, and the reproducibility is relatively low (6-9). Here, we compared bladder wall tumor and intravesical tumor formation rates between groups subjected to catheter inoculation and open surgery. We identified the optimal number and growth rate of cells. We evaluated tumor formation, size, and depth of invasion using magnetic resonance imaging (MRI) and light microscopy.
Materials and Methods
Mice. Six-week-old female C3H/HeNCrl mice were purchased from OrientBio (Seongnam, Republic of Korea) and maintained in an animal house with free access to autoclaved pelleted food and water. The Chonnam National University Medical School Research Institutional Animal Care and Use Committee approved the experimental protocol (CNU IACUC-H-2022-31). Animal maintenance and all in vivo experiments were conducted in accordance with recognized principles for animal management and use (DHU publication, NIH 80-23).
Cell line and cell culture. The mouse bladder tumor cell line MBT-2 was kindly provided by Professor Chaeyong Jung (Chonnam National University Medical School). Cells were cultured in Roswell Park Memorial Institute (RPMI)-1640 medium (Gibco Thermo Scientific, Waltham, MA, USA) with 10% (v/v) fetal bovine serum (FBS, Gibco), 1% (w/v) penicillin/streptomycin (Gibco), 2 mM of L-glutamine (Lonza Ltd., Basel, Switzerland), and 1% (w/v) N-(2-hydroxyethyl) piperazine-N’-(2-ethane sulfonic acid) (HEPES, Sigma-Aldrich Korea, Seoul, Republic of Korea). Cultures were maintained in a humidified atmosphere under 5% (v/v) CO2 at 37˚C and the medium changed every 2 days.
Surgical techniques. MBT-2 cells were injected in two ways into the urinary bladder of C3H/HeNCrl mice. The first method involved the use of a transurethral catheter (catheter group) and the second involved transperitoneal incision and direct injection of tumor cells into the bladder wall (open group). Mice in the catheter group were anesthetized via intramuscular injection of 2 ml of Zoletil 50 (tiletamine and zolazepam; Virbac Co., Westlake, TX, USA) and 1 ml of Rompun (xylazine; Bayer Animal Health, Leverkusen, Germany), along with 17 ml of phosphate-buffered saline (PBS) (100 μl/10 g body weight). A 24-G catheter was lubricated with concentrated glycerin and transurethrally inserted into the urinary bladder; all urine was removed. Fifty microliters of 0.1% (w/v) poly-L-lysine (PLL; Sigma-Aldrich Korea) was then infused via the catheter and retained in the bladder for 20 min. The PLL was then drained away using a syringe. Only the cylinder was removed; the catheter remained in place. Fifty microliters of a suspension of 5×105 MBT-2 cells in PBS were instilled and left in place for 40 min without removing the syringe. After 1 h, the catheter was removed and the mice were allowed to recover from anesthesia. Water was withheld for 16 h to reduce post-surgical urination and thus increase the opportunity for tumor cells to interact with the bladder mucosa.
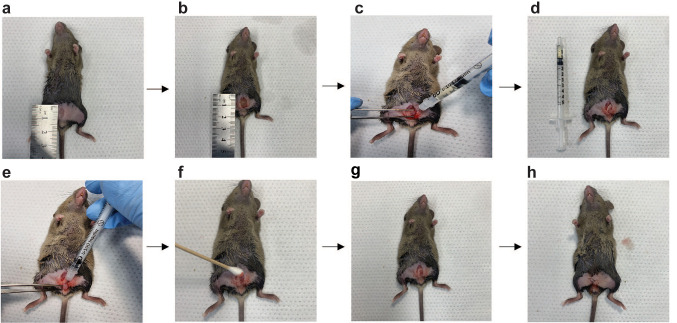
The mice in the open group were anesthetized via intramuscular injection of the combination described above. The skin of the surgical site in the lower abdomen was cleared of hair using depilatory cream and disinfected with iodine. After creating a 1-cm incision on the abdominopelvic midline, the bladder was pulled out of the abdominal cavity and urine was drained away using a 31-G Ultra-Fine II needle (BD Biosciences, San Jose, CA, USA). The bladder was maintained outside and the dome was pierced intramurally with a 31-G needle as parallel as possible to the muscle layer. Cell suspensions were prepared with either 2.5×105 or 5×105 MBT-2 cells that were injected slowly and carefully. The bladder peritoneal surface was wiped with a cotton swab and the bladder was returned to the abdominopelvic cavity. After a 30-min dwell time, the incised abdominal muscle and skin were sutured and re-disinfected with iodine. After recovering from anesthesia, the mice were deprived of water for 16 h to minimize the loss of tumor cells via post-surgical urination (Figure 1).
Figure 1. Surgical orthotopic inoculation of bladder cancer cells (MBT-2 cells) into a C3H/HeNCrl mouse. (a) After removing the hair from the surgical plane and disinfecting the plane with alcohol, a 1-cm incision was created in the skin and the abdominopelvic wall. (b) The bladder was externalized to facilitate access. (c) All urine was aspirated using a syringe. (d) To determine the approximate bladder volume, the amount of urine aspirated was measured and the bladder condition was checked. (e) After inserting the needle into an appropriate location on the bladder wall, tumor cells were slowly injected. (f) The area around the injection site was cleaned using a cotton swab. (g) The bladder was returned to the abdominal cavity. (h) The skin was closed with sutures.
Magnetic resonance imaging. Mouse postoperative status was checked by MRI each week until sacrifice. Prior to MRI, the mice were anesthetized with Zoletil 50 and Rompun as described above, and placed in the prone position in an MRI knee coil. For MRI, an M-series compact M7 whole-body scanner (Aspect Imaging, Shoham, Israel) was used. The T2-weighted image repetition time was 3,250 ms, the echo time was 73.2 ms, the field of view was 100 mm, the matrix was 128×128, the slice thickness was 1.5 mm, and there were 15 slices. Images were reconstructed with Bee DICOM viewer software (SinoUnion Healthcare Inc. Beijing, PR China).
Results
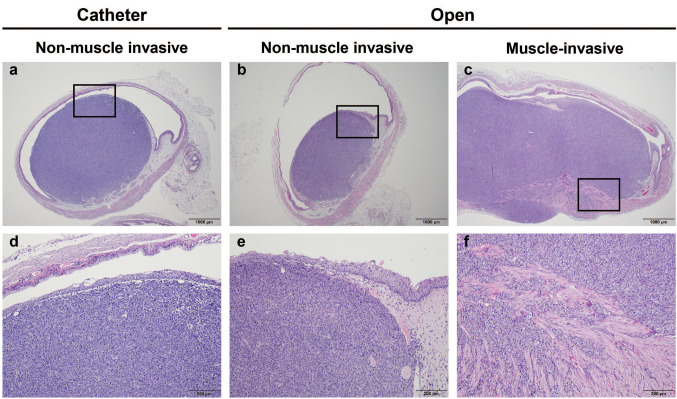
Tumor formation rate. In the catheter group, tumors formed in 7 of 15 mice, and an intravesical tumor formed in 1 mouse. Most mice only had extravesical tumors or extravesical tumors and smaller intravesical masses. In the open group, tumors formed in 47 of the 68 mice; the tumor formation rate (69%) was higher than in the catheter group (47%) (Table I). The intravesical tumor formation rate was higher when 5.0×105 tumor cells rather than 2.5×105 cells were injected (50 vs. 33%). When 5.0×105 tumor cells were administered, tumors were evident within 1 week and urination problems developed before 3 weeks. When 2.5×105 tumor cells were administered, tumors were observed from 2 weeks after implantation. Histopathologically, intravesical tumors were polypoid or sessile masses with broad bases. In both groups, tumors were covered by non-neoplastic urothelium. Muscle invasion was commonly observed 3 weeks after tumor cell inoculation (Figure 2), but it remains unclear whether this indicated true invasion or injection tract seeding.
Table I. Tumor formation rates in the catheter and open surgical orthotopic groups.
Figure 2. Representative histopathological features of the orthotopic bladder cancer models: catheter and open surgery models. (a) Intravesical tumor formation below the intact urothelium was observed in one mouse in the catheter group. (b) Non-muscle invasive intravesical tumor formation was evident when the open surgery method was used. (c) One stable tumor with muscle invasion was generated using the open surgical method. (d) The catheter method yielded tumors covered with non-neoplastic urothelium. (e) Similarly, the open group had tumors covered with intact urothelium. (f) In the open surgical group, the area of muscle invasion is shown under high magnification.
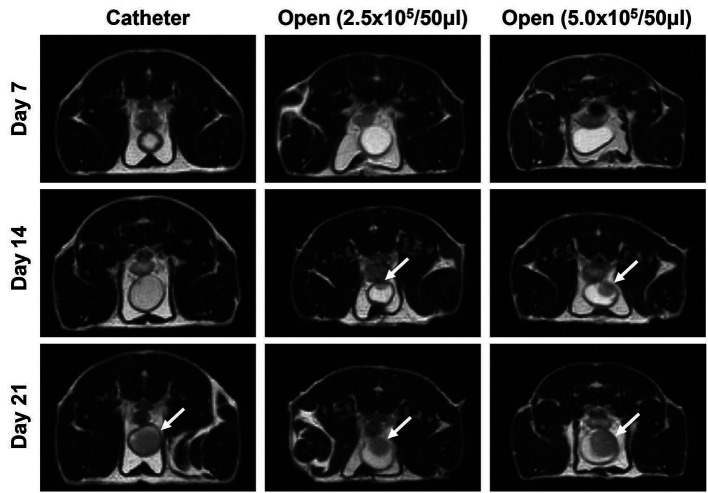
Tumor imaging. Tumors were detected by MRI as early as 1 week after cell inoculation. Most tumors were found on day 14 and confirmed on day 21 (Figure 3). Tumors within bladder walls evidenced filling defects on MRI. Extravesical masses presented as enhancing or mass-forming lesions around the bladder.
Figure 3. Representative MRI images in both groups. Intravesical tumors were observed after 3 weeks in the catheter group (left panel, white arrow) and after 2 weeks in the open surgery group (middle panel, white arrow). Larger tumors were observed after open surgery with injection of the higher number of tumor cells (5.0×105/50 μl) (right panel, white arrow).
Discussion
We sought to develop a bladder cancer model with high rates of bladder wall and intravesical tumor formation. Additionally, we sought to determine the optimal cell number and generation rate for in vivo work. We evaluated two techniques: catheter-based delivery of cells to the bladder wall after PLL pretreatment and direct injection of cells via a surgical incision. Although the catheter-based method is non-invasive, high-level catheterization skills are required and the rate of intravesical tumor formation is low. Although surgery is obviously invasive, it facilitates intravesical tumor formation because cells are injected directly into the bladder wall.
During catheter-based cell instillation, it is important to monitor bladder volume to prevent bladder overexpansion or regurgitation before injecting cells after PLL pretreatment. It is essential not to force catheter insertion if resistance is encountered; ultrasonography-assisted instillation is recommended to avoid extravesical tumor formation.
The surgical method, although invasive, afforded better reproducibility and a higher tumor formation rate. MRI was used to confirm tumor formation and monitor tumor growth; tumors were evident as early as 1 week after injection of 5×105 cells. However, if tumors grow for >2 weeks, serious urinary obstruction may develop. The lower cell concentration reduces the tumor growth rate, thus creating a window for in vivo experiments. MRI more accurately revealed tumor size, the extent of invasion and extravesical mass formation than ultrasonography.
No long slender mass formed after the use of either the catheter or surgical method, but if a large tumor was observed in the direction of the needle injection, or if there was a lump (i.e., a skip lesion) in extravesical fat, we considered that injection tract seeding had occurred. To prevent contamination and seeding in the needle tract, care must be taken not to generate negative pressure within the needle until it is completely removed from the body.
An ideal mouse model would recapitulate the tumorigenesis of human bladder cancer. However, both methods yielded tumors that were sessile masses with broad bases and luminal projections, covered with non-neoplastic urothelium. This does not mimic the papillary architecture of the noninvasive urothelial neoplasms seen in humans with bladder cancer. Although muscle invasion was not observed in mice, there was no contact with urine and the tumor microenvironment thus differed from that of humans. Neither model yielded non-muscle invasive urothelial neoplasms.
Conclusion
In summary, we sought to develop a highly reproducible mouse bladder cancer model with appropriately sized tumors growing at a rate that allows for in vivo experiments. The surgical method, although invasive, was more appropriate given the high rate of intravesical tumor formation and good reproducibility. The appropriate cell number will vary by the mouse strain and orthotopic model chosen. It is important to use tumor cells that exhibit an appropriate growth rate.
Conflicts of Interest
The Authors have declared that no competing interests exist.
Authors’ Contributions
KHL and SSK designed this study. JIN and EJA carried out the experiment. JIN and MGN drafted the manuscript. KSM and EC performed data analysis. SSK and CC carried out a pathological examination. KSM and EC gave a critical comment on animal modeling. KSM, KHL, and SSK assisted with the manuscript preparation and data analysis. All Authors read and approved the final manuscript.
Acknowledgements
This work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Minist (2022R1A2C1011889 for KHL, 2021R1F1A1062783 for SSK) and by Chonnam National University Hospital Biomedical Research Institute (BCRI23057 for KHL), and GIST Research Institute (GRI) research collaboration grant funded by GIST in 2023 (for EC). There was no role of the funding bodies in the design of the study, in the collection, analysis, and interpretation of data or in writing of the manuscript.
References
- 1.Moschini M, Zaffuto E, Karakiewicz PI, Andrea DD, Foerster B, Abufaraj M, Soria F, Mattei A, Montorsi F, Briganti A, Shariat SF. External beam radiotherapy increases the risk of bladder cancer when compared with radical prostatectomy in patients affected by prostate cancer: a population-based analysis. Eur Urol. 2019;75(2):319–328. doi: 10.1016/j.eururo.2018.09.034. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Randi G, Bosetti C, Levi F, Negri E, Boyle P, La Vecchia C. Declining mortality from bladder cancer in Europe. BJU Int. 2007;101(1):11–9. doi: 10.1111/j.1464-410X.2007.07239.x. [DOI] [PubMed] [Google Scholar]
- 3.Kamoun A, de Reyniès A, Allory Y, Sjödahl G, Robertson AG, Seiler R, Hoadley KA, Groeneveld CS, Al-Ahmadie H, Choi W, Castro MAA, Fontugne J, Eriksson P, Mo Q, Kardos J, Zlotta A, Hartmann A, Dinney CP, Bellmunt J, Powles T, Malats N, Chan KS, Kim WY, McConkey DJ, Black PC, Dyrskjøt L, Höglund M, Lerner SP, Real FX, Radvanyi F, Bladder Cancer Molecular Taxonomy Group A consensus molecular classification of muscle-invasive bladder cancer. Eur Urol. 2020;77(4):420–433. doi: 10.1016/j.eururo.2019.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flaig TW, Spiess PE, Abern M, Agarwal N, Bangs R, Boorjian SA, Buyyounouski MK, Chan K, Chang S, Friedlander T, Greenberg RE, Guru KA, Herr HW, Hoffman-Censits J, Kishan A, Kundu S, Lele SM, Mamtani R, Margulis V, Mian OY, Michalski J, Montgomery JS, Nandagopal L, Pagliaro LC, Parikh M, Patterson A, Plimack ER, Pohar KS, Preston MA, Richards K, Sexton WJ, Siefker-Radtke AO, Tollefson M, Tward J, Wright JL, Dwyer MA, Cassara CJ, Gurski LA. NCCN guidelines® insights: Bladder cancer, version 2.2022. J Natl Compr Canc Netw. 2022;20(8):866–878. doi: 10.6004/jnccn.2022.0041. [DOI] [PubMed] [Google Scholar]
- 5.Lenis AT, Lec PM, Chamie K, Mshs M. Bladder cancer. Jama. 2020;324(19):1980. doi: 10.1001/jama.2020.17598. [DOI] [PubMed] [Google Scholar]
- 6.Huebner D, Rieger C, Bergmann R, Ullrich M, Meister S, Toma M, Wiedemuth R, Temme A, Novotny V, Wirth MP, Bachmann M, Pietzsch J, Fuessel S. An orthotopic xenograft model for high-risk non-muscle invasive bladder cancer in mice: influence of mouse strain, tumor cell count, dwell time and bladder pretreatment. BMC Cancer. 2017;17(1):790. doi: 10.1186/s12885-017-3778-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jäger W, Moskalev I, Janssen C, Hayashi T, Awrey S, Gust KM, So AI, Zhang K, Fazli L, Li E, Thüroff JW, Lange D, Black PC. Ultrasound-guided intramural inoculation of orthotopic bladder cancer xenografts: a novel high-precision approach. PLoS One. 2013;8(3):e59536. doi: 10.1371/journal.pone.0059536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lorenzatti Hiles G, Cates AL, El-Sawy L, Day KC, Broses LJ, Han AL, Briggs HL, Emamdjomeh A, Chou A, Abel EV, Liebert M, Palmbos PL, Udager AM, Keller ET, Day ML. A surgical orthotopic approach for studying the invasive progression of human bladder cancer. Nat Protoc. 2019;14(3):738–755. doi: 10.1038/s41596-018-0112-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun YU, Nishino H, Zhao M, Miyake K, Sugisawa N, Yamamoto J, Tashiro Y, Inubushi S, Hamada K, Zhu G, Lim H, Hoffman RM. A Non-invasive Imageable GFP-expressing Mouse Model of Orthotopic Human Bladder Cancer. In Vivo. 2020;34(6):3225–3231. doi: 10.21873/invivo.12158. [DOI] [PMC free article] [PubMed] [Google Scholar]