Abstract
Background/Aim
Diabetes mellitus is a risk for subsequent nephrogenic anemia due to accelerated decline in renal function, and the global diabetic population continues to grow exponentially. In clinical studies, sodium-glucose co-transporter 2 (SGLT2) inhibitors, one of the drugs used to treat diabetes, have recently attracted attention as anemia suppressors, but there is still lack of evidence on this matter. The present study aimed to investigate the effects of SGLT2 inhibitor administration on anemia suppression using hemoglobin (Hb) levels as an indicator.
Patients and Methods
We conducted a longitudinal study to evaluate and compare the changes in Hb levels in diabetes patients treated with SGLT2 inhibitors (n=48) and those treated with DPP-4 inhibitors (n=48). Study participants were stratified into sub-cohorts based on sex, and the Hb level trajectory in the participants was observed for 90 days.
Results
We evaluated the use of SGLT2 inhibitors as a prophylactic factor for the decline in Hb levels and compared it to that of DPP-4 inhibitors [odds ratio (OR)=3.40, 95% confidence interval (CI)=1.93-6.00]. Administration of SGLT2 inhibitors and DPP-4 inhibitors resulted in decline of 14.4±0.34 and 12.4±0.31 g/dl (p<0.001), respectively, in male Hb levels from baseline to 90 days. Notably, the prophylactic effect of SGLT2 inhibitors on the reduction in Hb levels was independent of renal function and sex.
Conclusion
SGLT2 inhibitors prevent the reduction in Hb levels and exhibit anti-anemic effects.
Keywords: Diabetes, SGLT2 inhibitor, hemoglobin, retrospective analysis, observational study
In recent years, diabetes mellitus has been one of the most common lifestyle-related diseases, and its incidence is increasing. According to the International Diabetes Federation (an umbrella organization of over 230 national diabetes associations in more than 160 countries and territories), the global diabetic population continues to grow exponentially (1). Globally, the prevalence of diabetes in 2019 was 9.3% (463 million people) and is projected to increase to 10.2% (578 million people) by 2030 and 10.9% (700 million people) by 2045 (1).
Type 2 diabetes is a prevalent illness that causes major vascular, renal, and neurological complications (2). Diabetic nephropathy or diabetic kidney disease is the leading cause of end-stage renal disease (ESRD), with more than half of all cases being attributed to these metabolic disorders (3). The kidney is responsible for filtering all blood glucose, and under conditions of hyperglycemia, the increased filtered load induces oxidative damage and injury to the delicate vasculature and surrounding tubules (3). This leads to diabetic nephropathy and ESRD. Patients with ESRD must undergo dialysis or kidney transplantation to prolong survival, which increases family and social burden (4). Decreased renal function increases the risk of secondary renal anemia (5).
In addition to exhibiting hypoglycemic effects through urinary glucose excretion, sodium-glucose co-transporter 2 (SGLT2) inhibitors show various other effects, such as weight loss, improvement of blood lipid levels, and lowering blood pressure. Recent antidiabetic drugs have been found to exert other actions besides their hypoglycemic effects. SGLT2 inhibitors promote urinary glucose excretion by inhibiting the activity of SGLT2, a transporter responsible for transporting glucose in tubules. SGLT2 inhibitors have been indicated for the treatment of renal insufficiency. The CREDENCE study evaluated the effect of canagliflozin on renal function in patients with type 2 diabetes and proteinuric chronic kidney disease (6). Renal and cardiovascular events were significantly lower in the canagliflozin group than in the placebo group (6). Currently, seven SGLT2 inhibitors (ipragliflozin, dapagliflozin, luseogliflozin, tofogliflozin, canagliflozin, and empagliflozin) are in clinical use. In addition to their cardio- and renoprotective effects, hematopoietic effects of SGLT2 inhibitors have also been reported (7,8). David et al. found a significant difference in hemoglobin (Hb) concentrations between patients with type 2 diabetes mellitus with coronary artery disease treated with 10 mg empagliflozin and placebo at six months-13.9 (g/dl) in the placebo group and 14.6 (g/dl) in the empagliflozin group (9). The results indicates a long-term increase in Hb levels, suggesting anemia suppression (10). In other clinical studies, the dapagliflozin group showed higher hemoglobin and hematocrit levels than in the placebo group (11). However, the mechanism underlying the anemia suppression effect of SGLT2 inhibitors remains unelucidated.
Generally, metformin is used as the first-line drug for patients with type 2 diabetes, while SGLT2 inhibitors or dipeptidyl peptidase 4 (DPP-4) inhibitors are often second-choice drugs (11). However, the superiority of the benefits of these two drugs remains controversial.
As noted above, it has been suggested that SGLT2 inhibitors have unique renoprotective effects as well as protective effects against the decline in Hb levels. However, it is inconclusive whether the contribution of SGLT2 inhibitors to Hb lowering is due to its renoprotective effect or secondary to glycemic control. Therefore, in this study, we observed the trajectory of changes in Hb levels with SGLT2 inhibitors, using DPP-4 inhibitors (oral drugs intended only for glycemic control and assumed not to affect renal function) as controls.
Patients and Methods
Study participants and design. Japanese outpatients and inpatients taking SGLT2 and DPP-4 inhibitors at the International University of Health and Welfare Hospital were enrolled between April 2018 and March 2020. Candidate patients who met the following exclusion criteria were excluded; Hb levels were not measured within 60 days, the patients used anti-anemia agents, their Hb levels were outside our reference values before initiating SGLT2 inhibitor or DPP-4 inhibitor therapy, or their Hb levels could not be measured over time. Finally, the SGLT2 inhibitor and DPP-4 inhibitor user groups were adjusted by propensity score matching to achieve a 1:1 ratio (see statistical analyses section for details).
This retrospective observational study evaluated changes in Hb levels during observation periods as the primary outcome. Hb levels were measured at baseline and 1-30, 31-60, and 61-90 days after the initiation of SGLT2 inhibitor or DPP-4 inhibitor administration. In addition, a trend toward increasing Hb levels was established as the secondary outcome. We defined the increasing tendency as an increase of at least 1 g/dl during this observation (within 90 days) (12). To assess background factors, we also evaluated sex, age, HbA1c levels, renal function, and use of medication preparations. The evaluation of renal function was based on the estimated glomerular filtration rate (eGFR), which was calculated using the following formula: 194× [serum creatinine level (SCr)]–1.094× age–0.287 (×0.739 if female) (13).
All four SGLT2 inhibitors [empagliflozin (Jadiance® tablet), canagliflozin (Canaglu® tablet), dapagliflozin (Fosiga® tablet), and luseogliflozin (Rocefi® tablet)] were acceptable, as were the other oral hypoglycemic agents, insulin, and GLP-1 receptor agonists. Five DPP-4 inhibitors [vildagliptin (Equa® tablet), sitagliptin (Glactive® tablet), teneligliptin (Tenelia® tablet), linagliptin (Trazenta® tablet) and alogliptin (Nesina® tablet)] were used as the control group.
Statistical analyses. Continuous and categorical variables were presented as mean±standard deviation (SD) and proportions. Welch’s t-test was used to compare outcomes between the SGLT2 inhibitor and DPP-4 inhibitor groups. Logistic regression analysis was performed to determine the relationship between the medications (SGLT2 inhibitors or DPP-4 inhibitors) and the adjusted increasing trend in Hb levels, defined as secondary outcomes. As the population characteristics (e.g., male proportion) differed significantly between the SGLT2 inhibitors and DPP-4 inhibitors groups, we performed propensity score matching to adjust the effect modifiers (age) between the two groups and based on the sample size of the smallest subgroup.
All calculations were performed using BellCurve for Excel version 3.22 (Social Research and Information, Inc., Tokyo, Japan), and the significance level (α) was set at 5%.
Research ethics compliance. This study was conducted following the Declaration of Helsinki. It was approved by the institutional review board (IRB)/ethics committee of the International University of Health and Welfare (approval number: 21-Ig-124). The study received ethical approval for using an opt-out methodology based on the low risk to the patients and the potential benefits of anticipated results from this study for the patients.
Results
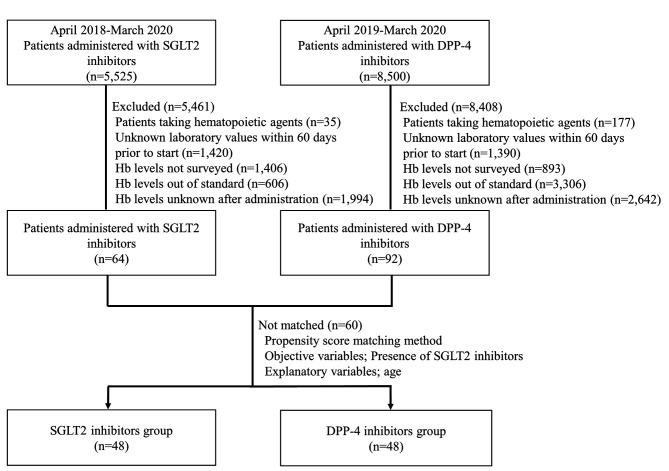
Between April 2018 and March 2020, 5,525 patients used SGLT2 inhibitors and 8,500 used DPP-4 inhibitors. Based on exclusion criteria, the number of patients in the SGLT2 inhibitor and DPP-4 inhibitor groups was 64 and 92, respectively. Therefore, we analyzed 96 backward cases using the propensity score matching method. Figure 1 shows the characteristics of the patients evaluated prior to the administration of SGLT2 inhibitors.
Figure 1. Flowchart of the study presenting the process for extraction and exclusion of patient data in this retrospective survey.
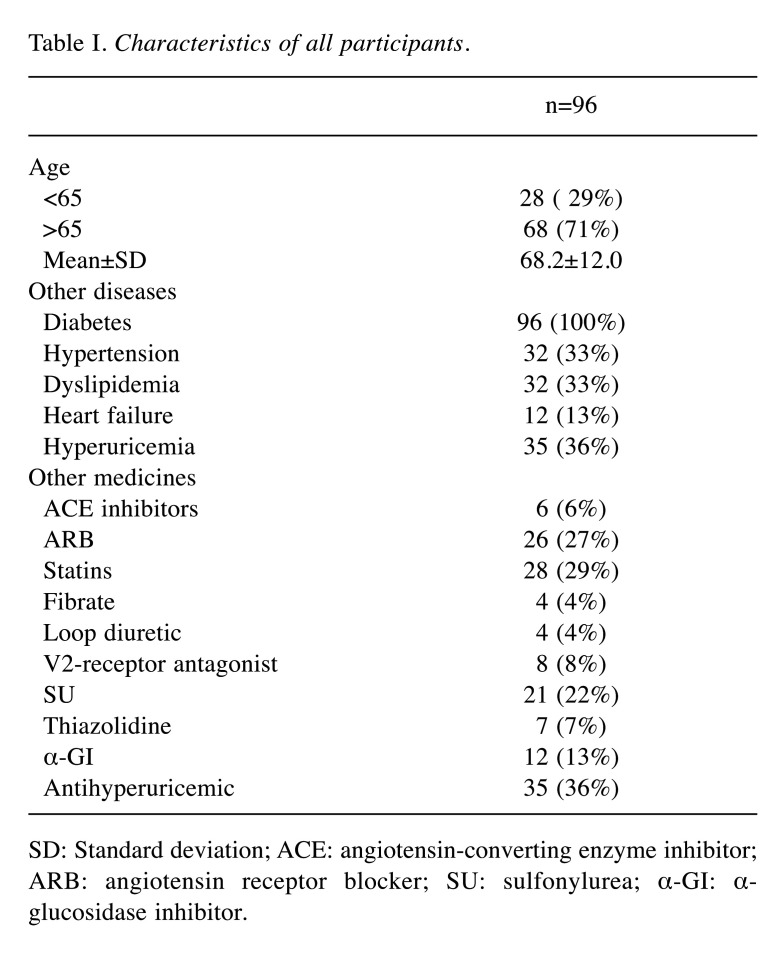
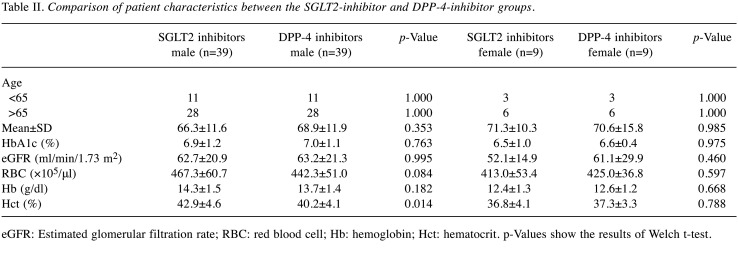
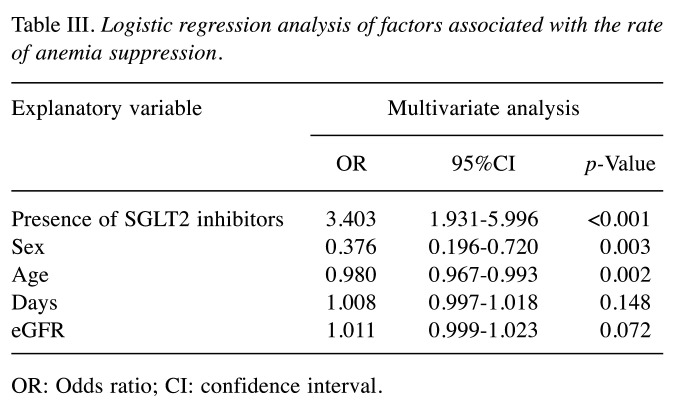
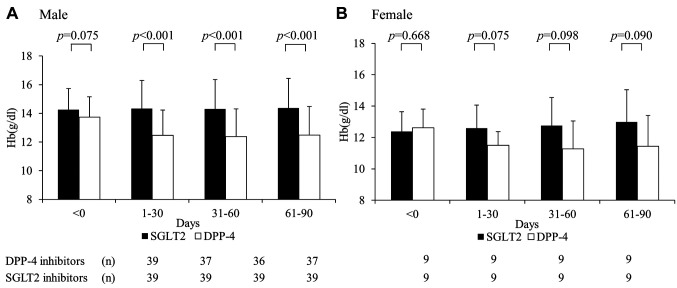
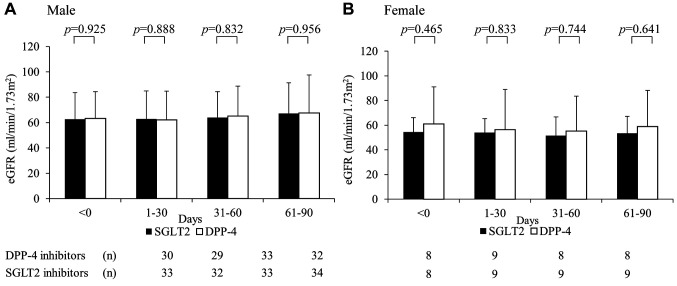
The following SGLT2 inhibitors were administered: empagliflozin (Jadiance® tablet, Boehringer Ingelheim, Tokyo, Japan) in 24 patients, canagliflozin (Canaglu® tablet, Mitsubishi Tanabe, Osaka, Japan) in 15 patients, dapagliflozin (Fosiga® tablet, AstraZeneca, Osaka, Japan) to 7 patients, and ruseogliflozin (Rocef® tablet, TAISHO, Tokyo, Japan) to 2 patients. The following DPP-4 inhibitors were administered: vildagliptin (Equa® tablet, NOVARTIS, Tokyo, Japan) to 2 patients, sitagliptin (Glactive® tablet, Ono, Osaka, Japan) to 20 patients, teneligliptin (Tenelia® tablet, Mitsubishi Tanabe) to 5 patients, linagliptin (Trazenta® tablet, Boehringer Ingelheim) to 17 patients, and alogliptin (Nesina® tablet, Takeda, Osaka, Japan) to 4 patients. The mean age of patients was 68.2 (±12.0) years, with 81.3% (78/96) being men (Table I). Male Hb levels in the SGLT2 and DPP-4 groups were 14.3 (±1.5) and 13.7 (±1.4) g/dl, respectively, before the start of treatment (Table II). Logistic regression analysis showed that the odds ratio for Hb anemia suppression effect was 3.403 (p<0.001) for men using SGLT2 inhibitors (Table III). Figure 2 shows the trend of Hb levels over time from before initiation of SGLT2 and DPP-4 inhibitor therapy. The Hb levels of men in the SGLT2 inhibitor group differed significantly from those of men in the DPP-4 inhibitor group at 1-30, 31-60, and 61-90 days (p<0.001), whereas the Hb levels of women in the SGLT2 inhibitor group were not significantly different from those in the DPP-4 inhibitor group during the entire period. Figure 3 shows the trend of eGFR over time from before the start of SGLT2 inhibitor and DPP-4 inhibitor treatment. Both men and women were not significantly different from the inhibitor group throughout the entire period.
Table I. Characteristics of all participants.
SD: Standard deviation; ACE: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker; SU: sulfonylurea; α-GI: α-glucosidase inhibitor.
Table II. Comparison of patient characteristics between the SGLT2-inhibitor and DPP-4-inhibitor groups.
eGFR: Estimated glomerular filtration rate; RBC: red blood cell; Hb: hemoglobin; Hct: hematocrit. p-Values show the results of Welch t-test.
Table III. Logistic regression analysis of factors associated with the rate of anemia suppression.
OR: Odds ratio; CI: confidence interval.
Figure 2. Changes in Hb levels at the start of SGLT2 inhibitor administration and at 1-30, 31-60, and 61-90 days. (A, B) Hb levels in patients receiving SGLT2 and DPP-4 inhibitors. p-Values are results of the Welch t-test.
Figure 3. Changes in the eGFR at the start of SGLT2 inhibitor administration and at 1-30, 31-60, and 61-90 days. (A, B) eGFR in patients receiving SGLT2 and DPP-4 inhibitors. p-Values are results of the Welch t-test.
Discussion
In this study, we found that the anemia suppression effects of SGLT2 inhibitors were not affected by the number of days of treatment or renal function status and were correlated with the use of SGLT2 inhibitors. Preservation of Hb levels with SGLT2 inhibitors was also observed in patients with impaired renal function and did not correlate with a decrease in the eGFR. A significant preservation of Hb levels was observed in the SGLT2 inhibitor group compared to that in the DPP-4 inhibitor group. Although dehydration was considered to be associated with cerebral infarction due to an increase in Hematocrit (Hct) levels caused by fluid volume concentration (14), Hct levels [42.9% (38.3-47.5) before treatment, 41.9% (36.0-47.9) at 1-30 days, 42.3% (36.7-47.9) at 31-60 days, and 42.3% (36.9-47.8) for 61-90 days] in the group treated with SGLT2 inhibitors were not elevated. Hct enrichment did not increase the incidence of cerebral infarction. There were no significant differences in Hct levels; only slight variations were observed during each period. This suggests that hemoconcentration due to dehydration is unlikely. Although there was a temporary increase in the BUN/CRE ratio and Hct, an apparent increase in Hb levels due to dehydration was not considered.
In renal anemia due to end-stage renal failure, anemia progresses when the eGFR falls below 60 ml/min/1.73 m2, and the risk of anemic complications rapidly increases even if the subsequent eGFR decline is minor (15). A study by Lambers et al. reported a 10% decrease in the eGFR after 12 weeks of treatment with dapagliflozin. However, despite the decrease in renal function, an increase in erythropoietin (EPO) and reticulocytes was observed, with a peak after four weeks (16). In the present study, we observed no correlation between the anemia suppression effect of Hb and renal function. Including patients with end-stage renal failure may have affected the anemia suppression effects of Hb. Further studies are needed because we did not analyze the eGFR by classification. SGLT2 inhibitors are also known to have renoprotective effects (17). In the EMPA-REG OUTCOME study, it was reported that the entire empagliflozin group showed a decrease in the eGFR in the early stage of treatment but no significant decrease thereafter, and the final eGFR decrease was less than that in the placebo group (18,19). In the CANVAS study, the suppression of progression and regression of the albuminuria stage, a marker of renal function, was significantly higher in the canagliflozin group (20). As a mechanism of anemia inhibition, SGLT2 consumes ATP, and urinary glucose is excreted to the blood by Na/glucose co-transporters (21). The proximal peritubule is hypoxic owing to the reabsorption of urinary glucose by SGLT2. SGLT2 inhibitors reduce hypoxia by suppressing the function of these transporters and restores the function of erythropoietin-producing cells in the interstitium (22). In addition, renal mitochondrial function improves in response to SGLT2 inhibitors, and experimental studies suggest the possibility of improved tubular autophagy (23). An increase in Hb levels is also observed in patients with impaired renal function, which may be related to the renoprotective effect of SGLT2 inhibitors. Yamazaki et al. administered dapagliflozin (10 mg/kg) to non-diabetic Wistar–Kyoto and Wistar rats (male, 5 weeks old) and investigated its anemia suppression effects (24). No increase in Hb and Hct levels was observed in renal anemia. These results suggested that the mechanism of action of SGLT2 inhibitors involved in increasing Hb levels may be related to their half-life, metabolism, and selectivity for SGLT2. Recent randomized controlled studies have shown that type 2 diabetes is a pro-inflammatory condition and hepcidin, a known inhibitor of erythropoiesis, is increased in pro-inflammatory conditions. Other mechanisms of anemia inhibition suggest that dapagliflozin suppresses hepcidin levels, improves iron kinetics, and increases erythropoiesis (25). Various papers have reported the anemia-suppressing effects of SGLT2 inhibitors, but have yet to provide a clear mechanism. The logistic regression analysis results suggested that there may be a correlation between sex and anemia suppression. It has also been reported that testosterone and SGLT2 inhibitor-induced erythropoiesis can be attributed to increased erythrocytes (26). Men may also be more affected by the anemia suppression effects of SGLT2 inhibitors than women.
However, in addition to their clinical benefits for diabetes patients with renal anemia, as described above, it is also necessary to examine the adverse effects of polycythemia vera. If an increase of >18 g/dl in Hb levels in men and >16 g/dl in women is the criteria for polycythemia vera, one man (0.02%) had Hb >18 g/dl and one woman (0.05%) had Hb >16 g/dl after SGLT2 inhibitor treatment. The frequency of occurrence was less than 1% in both sexes and was not considered high. This may improve the symptoms of anemia. However, caution should be exercised because blood clots are caused by polycythemia vera. Severe cases of polycythemia associated with SGLT2 inhibitors have been reported in a few case reports (27,28). The present study suggests that it may be a widespread phenomenon, with certain patients potentially falling into the category of drug-induced polycythemia vera and may partly be caused by unrecognized effects of SGLT2 inhibitors. These findings suggest that differences in the efficacy of SGLT2 inhibitors may lead to future differentiation of their use. The preservation of Hb levels in men taking SGLT2 inhibitors was significantly greater than that in men taking DPP-4 inhibitors. An increasing trend was also observed among women, although the difference was not statistically significant. The multiple regression analysis showed a significant difference between the two groups, suggesting that sex may influence the results.
This study had several limitations. First, it was a single-center, retrospective study. Given the fact that only Japanese patients were included in the study, there may be racial differences when compared to the results of other studies. To extrapolate the results of this study from the Japanese population to the global population, it will be necessary to account for population differences to validate generalizability. Second, anemia suppression effects may be due to the increased EPO induced by SGLT2 inhibitors. However, EPO levels were not measured in this study. Thus, given the limitations of the present study design, future studies should be more extensive and should focus on changes in EPO when examining the effects of SGLT2 inhibitors on Hb levels in patients with diabetes.
Conclusion
The results of the study suggest that patients receiving SGLT2 inhibitors may have the benefit of anemia suppression compared to patients taking DPP-4 inhibitors.
Conflicts of Interest
The Authors have no conflicts of interest with any companies related to this study that should be disclosed.
Authors’ Contributions
Conceptualization: J. Sato; Methodology: R. Hashimoto, T. Oya, J. Sato; Formal analysis: Y. Yoshida; Investigation; Y. Yoshida, T. Oya; Data curation: Y. Yoshida, T. Oya; Writing - original draft; Y. Yoshida; Writing - review & editing: Y. Yoshida, K, Takahashi, R. Hashimoto, T. Oya, J. Sato. Project administration; J. Sato.
Acknowledgements
The Authors would like to thank their colleagues at the Department of Pharmacy, International University of Health, and Welfare Hospital.
References
- 1.Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova K, Shaw JE, Bright D, Williams R. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 2.Vijan S. Type 2 diabetes. Ann Intern Med. 2019;171(9):ITC65–ITC80. doi: 10.7326/AITC201911050. [DOI] [PubMed] [Google Scholar]
- 3.Bjornstad P, Cherney DZ. Renal hyperfiltration in adolescents with type 2 diabetes: physiology, sex differences, and implications for diabetic kidney disease. Curr Diab Rep. 2018;18(5):22. doi: 10.1007/s11892-018-0996-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Defronzo RA, Norton L, Abdul-ghani M. Renal, metabolic and cardiovascular considerations of SGLT2 inhibition. Nat Rev Nephrol. 2017;13(1):11–26. doi: 10.1038/nrneph.2016.170. [DOI] [PubMed] [Google Scholar]
- 5.Mikhail A, Brown C, Williams JA, Mathrani V, Shrivastava R, Evans J, Isaac H, Bhandari S. Renal association clinical practice guideline on Anaemia of Chronic Kidney Disease. BMC Nephrol. 2017;18(1):345. doi: 10.1186/s12882-017-0688-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJ, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Cannon CP, Capuano G, Chu P, De Zeeuw D, Greene T, Levin A, Pollock C, Wheeler DC, Yavin Y, Zhang H, Zinman B, Meininger G, Brenner BM, Mahaffey KW. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295–2306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 7.Petrie MC, Verma S, Docherty KF, Inzucchi SE, Anand I, Belohlávek J, Böhm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett J, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O’Meara E, Vinh PN, Schou M, Tereshchenko S, Køber L, Kosiborod MN, Langkilde AM, Martinez FA, Ponikowski P, Sabatine MS, Sjöstrand M, Solomon SD, Johanson P, Greasley PJ, Boulton D, Bengtsson O, Jhund PS, McMurray JJV. Effect of dapagliflozin on worsening heart failure and cardiovascular death in patients with heart failure with and without diabetes. JAMA. 2020;323(14):1353–1368. doi: 10.1001/jama.2020.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vallon V, Verma S. Effects of SGLT2 inhibitors on kidney and cardiovascular function. Annu Rev Physiol. 2021;83:503–528. doi: 10.1146/annurev-physiol-031620-095920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mazer CD, Hare GM, Connelly PW, Gilbert RE, Shehata N, Quan A, Teoh H, Leiter LA, Zinman B, Jüni P, Zuo F, Mistry N, Thorpe KE, Goldenberg RM, Yan AT, Connelly KA, Verma S. Effect of empagliflozin on erythropoietin levels, iron stores, and red blood cell morphology in patients with type 2 diabetes mellitus and coronary artery disease. Circulation. 2020;141(8):704–707. doi: 10.1161/CIRCULATIONAHA.119.044235. [DOI] [PubMed] [Google Scholar]
- 10.Zinman B, Lachin JM, Inzucchi SE. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2016;374(11):1094. doi: 10.1056/NEJMc1600827. [DOI] [PubMed] [Google Scholar]
- 11.Ahrén B. DPP-4 inhibitors. Best Pract Res Clin Endocrinol Metab. 2007;21(4):517–533. doi: 10.1016/j.beem.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 12.Shepshelovich D, Rozen-zvi B, Avni T, Gafter U, Gafter-gvili A. Intravenous versus oral iron supplementation for the treatment of anemia in CKD: An updated systematic review and meta-analysis. Am J Kidney Dis. 2016;68(5):677–690. doi: 10.1053/j.ajkd.2016.04.018. [DOI] [PubMed] [Google Scholar]
- 13.Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53(6):982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 14.Tang J, Ye L, Yan Q, Zhang X, Wang L. Effects of sodium-glucose cotransporter 2 inhibitors on water and sodium metabolism. Front Pharmacol. 2022;13:800490. doi: 10.3389/fphar.2022.800490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Astor BC, Muntner P, Levin A, Eustace JA, Coresh J. Association of kidney function with anemia. Arch Intern Med. 2002;162(12):1401. doi: 10.1001/archinte.162.12.1401. [DOI] [PubMed] [Google Scholar]
- 16.Lambers Heerspink HJ, de Zeeuw D, Wie L, Leslie B, List J. Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab. 2013;15(9):853–862. doi: 10.1111/dom.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nespoux J, Vallon V. SGLT2 inhibition and kidney protection. Clin Sci (Lond) 2018;132(12):1329–1339. doi: 10.1042/CS20171298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wanner Ch, Inzucchi SE, Zinman B. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375(18):1801–1802. doi: 10.1056/NEJMc1611290. [DOI] [PubMed] [Google Scholar]
- 19.Cherney DZI, Zinman B, Inzucchi SE, Koitka-weber A, Mattheus M, Von Eynatten M, Wanner C. Effects of empagliflozin on the urinary albumin-to-creatinine ratio in patients with type 2 diabetes and established cardiovascular disease: an exploratory analysis from the EMPA-REG OUTCOME randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2017;5(8):610–621. doi: 10.1016/S2213-8587(17)30182-1. [DOI] [PubMed] [Google Scholar]
- 20.Neal B, Perkovic V, Mahaffey KW, De Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 21.Wright EM, Loo DDF, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev. 2011;91(2):733–794. doi: 10.1152/physrev.00055.2009. [DOI] [PubMed] [Google Scholar]
- 22.Lambers Heerspink HJ, de Zeeuw D, Wie L, Leslie B, List J. Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab. 2013;15(9):853–862. doi: 10.1111/dom.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nespoux J, Vallon V. Renal effects of SGLT2 inhibitors: an update. Curr Opin Nephrol Hypertens. 2020;29(2):190–198. doi: 10.1097/MNH.0000000000000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamazaki D, Konishi Y, Morikawa T, Kobara H, Masaki T, Hitomi H, Osafune K, Nakano D, Kittikulsuth W, Nishiyama A. Failure to confirm a sodium-glucose cotransporter2 inhibitor-induced hematopoietic effect in non-diabetic rats with renal anemia. J Diabetes Investig. 2020;11(4):834–843. doi: 10.1111/jdi.13205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghanim H, Abuaysheh S, Hejna J, Green K, Batra M, Makdissi A, Chaudhuri A, Dandona P. Dapagliflozin suppresses hepcidin and increases erythropoiesis. J Clin Endocrinol Metab. 2020;105(4):e1056–e1063. doi: 10.1210/clinem/dgaa057. [DOI] [PubMed] [Google Scholar]
- 26.Motta G, Zavattaro M, Romeo F, Lanfranco F, Broglio F. Risk of erythrocytosis during concomitant testosterone and SGLT2-inhibitor treatment: A Warning From Two Clinical Cases. J Clin Endocrinol Metab. 2019;104(3):819–822. doi: 10.1210/jc.2018-01702. [DOI] [PubMed] [Google Scholar]
- 27.Das L, Bhansali A, Walia R. Unmasking and aggravation of polycythemia vera by canagliflozin. Diabet Med. 2018;35(11):1613–1616. doi: 10.1111/dme.13706. [DOI] [PubMed] [Google Scholar]
- 28.Gupta R, Gupta A, Shrikhande M, Tyagi K, Ghosh A, Misra A. Marked erythrocytosis during treatment with sodium glucose cotransporter-2 inhibitors-report of two cases. Diabetes Res Clin Pract. 2020;162:108127. doi: 10.1016/j.diabres.2020.108127. [DOI] [PubMed] [Google Scholar]