Abstract
Background/Aim
This study aimed to investigate the effects of acupuncture treatment through the ear acupoints on transport stress in experimental microminipigs.
Materials and Methods
Experiment 1: Six animals were equally divided into two groups (Control and Treatment). In the treatment group, before transportation (6 h; vehicle and plane), short, ultrathin circular transdermal needles were applied to locations corresponding to the acupoints on the apical area of both ears. Peripheral blood samples were collected from the cranial vena cava 2 days before and immediately after transportation. Blood stress markers, biochemistry indicators, and oxidative stress levels were examined. Experiment 2 (follow-up study: diarrhea incidence after transportation): Diarrhea incidence after transportation in the control and treatment groups was investigated.
Results
Experiment 1: Transport stress induced an increase in blood cortisol, serum amyloid A (SAA), glucose, non-esterified fatty acid, and derivatives of reactive oxygen metabolites (d-ROMs) and decreased the biological antioxidant potential (BAP)/d-ROMs ratio yet did not affect BAP. Acupuncture suppressed the increases in SAA and d-ROMs values and the decrease in BAP/d-ROMs ratio. Experiment 2: The total diarrhea incidence was 25% in the control group, whereas diarrhea was not observed in the treatment group.
Conclusion
Acupuncture treatment suppresses hypothalamic–pituitary–adrenal function and, as a result, reduces transport stress without affecting the suppression of the central catecholaminergic system. Acupuncture treatment for transport stress can improve animal welfare.
Keywords: Acupuncture, animal welfare, swine, transport stress
In recent decades, there has been growing concern regarding stress among pigs because of the undesirable consequences that stress imposes on the normal physiology of pigs, their welfare, and general productive performance (1). It is well known that pigs are exposed to stress during transportation. An increase in plasma cortisol concentration has been found in connection with transport (2). The active response to stress is mainly associated with adrenal medulla and sympathetic nervous system (catecholamine) activation, whereas the passive response is hypothesized to be related to stimulation of the pituitary–adrenocortical system (3). Moreover, transport stress may be more or less severe depending on several factors, including crowding, temperature, and duration. However, measures against transport stress in pigs are insufficient and require further investigation.
Oriental medicines, including acupuncture, generally have fewer side effects than Western medicines. Therefore, acupuncture is useful for avoiding drug residue and side effect issues in laboratory animals and livestock. In a prior study, we developed transport stress-reducing acupuncture in dogs. Transdermal needles were applied to acupoints on both ears and successfully suppressed symptoms such as drooling and vomiting (4).
In this study, we applied the canine transport stress-reducing acupuncture treatment on experimental microminipigs and investigated the effect of acupuncture treatment on transport stress to further animal welfare.
Materials and Methods
Animals. All microminipigs were obtained from one breeder (Fuji Micra Inc., Shizuoka, Japan). This research was performed in accordance with the Institutional Guidelines for Animal Experiments and in compliance with the Japanese Act on Welfare and Management of Animals (Act No. 105 and Notification No. 6).
Experiment 1. Blood stress marker examination. Six mature male microminipigs were used in this study. The animals were divided into two groups: control (n=3) and treatment (acupuncture treatment, n=3). In the treatment group, short, ultra-thin circular transdermal needles (Pyonex 1.5 mm, SEIRIN, Shizuoka, Japan, diameter×length=0.2×1.5 mm) were applied to locations corresponding to the acupoints on the apical area of both ears, which are identified as Erjian Points one to three (“Jisen” in Japanese), approximately 30-60 min before transportation. All animals were transported from the production farm to the research facility in individual cages using transport vehicles and planes for approximately 6 h.
Peripheral blood samples were collected from the cranial vena cava 2 days before and immediately after transportation. The following stress markers were assessed in the blood: concentrations of cortisol using electrochemiluminescence immunoassay and catecholamines (including adrenaline and noradrenaline) using high-performance liquid chromatography. In the biochemical examination, the concentrations of serum amyloid A (SAA) and glucose were measured using a simple blood glucose meter (Nipro Stat Strip XP2, Nipro, Osaka, Japan) and non-esterified fatty acids (NEFA) were measured using NEFA C-Test Wako (Fujifilm Wako Pure Chemical Corporation, Osaka, Japan). Furthermore, blood oxidative stress, derivatives of reactive oxygen metabolites (d-ROMs) concentrations, biological antioxidant potential (BAP), and the BAP/d-ROMs ratio were measured. The SAA concentrations and blood oxidative stress were measured as described below.
Measurement of SAA concentration. SAA concentration was measured using a Pentra C200 automated biochemical analyzer (HORIBA ABX SAS, Montpellier, France) with an SAA reagent specialized for animal serum or plasma (VET-SAA “Eiken” Reagent; Eiken Chemical Co., Ltd., Tokyo, Japan). Furthermore, SAA concentration was calculated based on a standard curve generated using a VET-SAA Calibrator Set (Eiken Chemical Co., Ltd.).
Measurement of blood oxidative stress. Serum d-ROMs and BAP levels represent reactive oxygen metabolites and antioxidant capacity, respectively. Serum levels of these two markers were measured using a FREE Carrio Duo Redox Analyzer system (Wismerll, Tokyo, Japan), which included a photometer and thermostatically regulated mini-centrifuge. Both d-ROMs and BAP tests were conducted according to manufacturer instructions.
Experiment 2. Follow-up study: Incidence of diarrhea after transportation. The incidence of diarrhea in microminipigs after transportation was investigated. Twenty animals were divided into four groups. The control (no treatment, n=2) and treatment groups (n=6) were subjected to a short duration of transportation (approximately 1 h using transport vehicle), and the other control (no treatment, n=6) and treatment groups (n=6) were subjected to a longer duration of transportation (approximately 6 h using transport vehicle and plane). Animals in both treatment groups were treated with acupuncture as in experiment 1. In addition to these four groups, 161 animals treated with acupuncture, as in experiment 1, were observed for diarrhea incidence after prolonged transportation (approximately 17 h using transport vehicle).
Statistical analysis. Blood examination data are expressed as mean±standard deviation. Statistical analysis of the differences before and after treatment was performed using a paired t-test and that of the incidence was performed using Fisher’s exact test. All statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria) (5). Values of p<0.05 were considered statistically significant.
Results
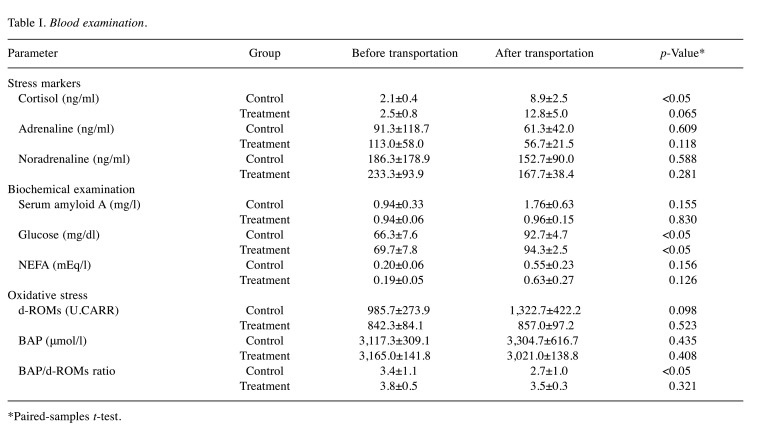
Experiment 1. Blood stress marker examination. Data from blood examinations before and after transportation are shown in Table I. Regarding blood stress markers, the blood cortisol concentrations in the control and treatment groups after transportation showed higher values and tendencies compared with those before transportation. Blood adrenaline and noradrenaline concentrations did not increase compared with those before transportation.
Table I. Blood examination.
*Paired-samples t-test.
As for biochemical examination, the SAA values in the control group after transportation increased approximately two-fold (rate of increase: 2.00±0.71-fold) compared with those before transportation; concentrations in the treatment group did not increase (rate of increase: 1.04±0.21-fold). Blood glucose concentrations were significantly higher in both groups after transportation than those before. Blood NEFA concentrations were higher after transportation than those before in all animals, and the mean value increased approximately three-fold after transportation.
Regarding oxidative stress, the blood concentrations of d-ROMs and BAP in both groups showed no significant changes before and after transportation. However, the d-ROMs values in the control group after transportation increased about 1.3-fold (rate of increase: 1.32±0.42-fold) compared with those before transport; values in the treatment group did not increase (rate of increase: 1.02±0.04-fold). The BAP/d-ROMs ratio in the control group after transportation was lower than that before transportation, whereas that in the treatment group showed no significant change.
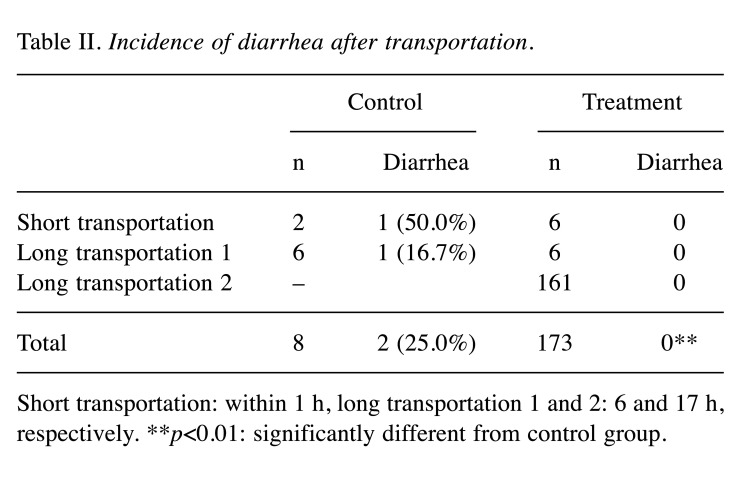
Experiment 2. Follow-up study: Incidence of diarrhea after transplantation. As shown in Table II, diarrhea incidence was 25% in the control group (total short and long transportation), whereas diarrhea was not observed during both short and long transportation in the treatment group.
Table II. Incidence of diarrhea after transportation.
Short transportation: within 1 h, long transportation 1 and 2: 6 and 17 h, respectively. **p<0.01: significantly different from control group.
Discussion
To the best of our knowledge, this study represents the first evaluation of acupuncture treatment as a method of reducing transportation stress in experimental microminipigs. In experiment 1, transport stress induced an increase in blood cortisol and glucose concentrations and a decrease in the BAP/d-ROMs ratio. Acupuncture suppressed the increase in blood SAA concentration and decreased the BAP/d-ROMs ratio.
In this study, acupuncture was performed at the acupoints “Jisen”, which are one or three points on the outer blood vessel of the auricle apex and are anatomically located in the region innervated by the trigeminal nerve (4). Treatment stimulation also affects the center of the hypothalamus and vagus nerve and is thought to be affected by somatic autonomic reflexes, such as suppression and enhancement of gastric acid secretion. Generally, treatment of the apex of the ear is effective against common cold, moderate heat, poisonings, febrile disease, indigestion, and shock.
Data on cortisol, a typical stress marker in pigs, suggest that acupuncture treatment has a stress-relieving effect. Cortisol is the major glucocorticoid in pigs and increases in relation to the hypothalamic–pituitary–adrenal (HPA) axis activation (1). Results of previous studies assessing cortisol effectiveness in pigs as a parameter for stress quantification during transportation show controversial findings. For example, Piñeiro et al. reported that cortisol concentrations were not modified after 24 and 48 h of transport, indicating that cortisol levels may have returned to baseline during the long transportation (6). In the 6-h transportation conducted in this study, all individuals in both groups had elevated blood cortisol concentrations after transportation. Furthermore, a statistical comparison of the pre- and post-transportation values for each group showed significant differences only for the control group. However, this is insufficient to confirm the inhibitory effects of acupuncture on elevated blood cortisol levels; further research is required to confirm these effects.
An increase in peripheral catecholamines by elevated sympathetic nervous activity causes not only physiological stress responses but also deterioration of meat quality in domestic swine (1,7). Additionally, elevated central noradrenergic activity causes psychological and emotional stress responses, such as anxiety, fear, and exaggerated aggressiveness (8). Because peripheral noradrenaline levels are known to correlate with central noradrenaline levels (9,10), acupuncture treatment may also suppress transportation-induced increases in central noradrenaline levels. Several studies have indicated that acupuncture modulates the activity of the locus coeruleus, which is the primary source of central noradrenergic neurons (11). We hypothesized that acupuncture treatment during transportation may relieve the extensive stress responses caused by the catecholaminergic system and contribute to animal well-being. However, no statistically significant differences in the peripheral catecholamine levels were observed before and after transportation in this study, and the effects of acupuncture could not be confirmed.
The transport stress phenomenon in this study was further investigated by analyzing blood SAA, glucose, and NEFA concentrations. SAA is an acute-phase protein (APP) identified as a positive acute-phase reactant (3,12). APPs, such as SAA, haptoglobin, and C-reactive protein, are recognized markers of inflammation and have also been proposed as indicators of stress in cattle and pigs (6,13). Glucose and its alternative fuels, NEFA, beta-hydroxybutyrate, and glycerol, can be used as indicators of metabolic stress induced by food and water restriction (1). Glucose is also affected by the sympathetic nervous system, and the HPA axis and could be an indicator of these responses (1). In this study, transportation stress induced an increase in blood SAA, glucose, and NEFA values, and acupuncture treatment alone suppressed the increase in blood SAA values. The increase in blood glucose and NEFA values was also considered to reflect transportation stress because of the increase in cortisol via HPA axis activation, whereas acupuncture treatment did not affect these changes. It has been reported that APPs, including SAA, increase after prolonged transportation of pigs for 12 h or longer (11). Longer transportation times and continuous post-transportation sampling may show an increase in SAA due to transportation stress and the effect of acupuncture treatment, warranting further study in the future.
The novel findings regarding blood oxidative stress markers in this study are of considerable interest. Oxidative stress may be involved in various human disorders and pathogeneses (14-16). The d-ROMs are among the blood markers used to evaluate oxidative stress, and elevated levels have been reported in pigs reared under chronic heat stress (12). However, it is not necessarily accompanied by an increase in common stress markers, such as cortisol, and the causal relationship has not been clarified. Furthermore, the BAP/d-ROMs ratio is important because antioxidant reactions occur in vivo to resist oxidative stress. In humans, obesity and metabolic syndrome are associated with low blood BAP/d-ROMs ratio (17). BAP and d-ROMs also serve as promising lines of investigation as markers of stress in animals but have only been evaluated in a limited number of studies in swine (18-20). In this study, transportation stress induced a decrease in the BAP/d-ROMs ratio, while acupuncture treatment reversed this change. The decrease in the BAP/d-ROMs ratio owing to transportation stress was caused by an increase in the d-ROMs and remaining BAP values after transportation. Therefore, it was thought that acupuncture treatment reduced transportation stress and, as a result, improved blood d-ROMs levels and the BAP/d-ROMs ratio.
Transportation stress is associated with increased gastrointestinal permeability, bacterial translocation, toxin production, and tissue damage as well as low immunity (21,22). In experiment 2, diarrhea occurred in microminipigs without acupuncture treatment after transportation, considered to be because of transport stress. Pig transportation induces oxidative damage to the intestines, resulting in the destruction of intestinal integrity (21). Because acupuncture treatment suppressed diarrhea occurrence in microminipigs, it has been suggested that acupuncture suppresses diarrhea in other pig breeds. Taken together with the results of experiment 1, the effects of transportation and acupuncture on blood SAA levels and oxidative stress may be related to intestinal oxidative stress. Although the effect of the autonomic nervous system on transport stress was not determined from the present data, it is well known that intestinal function and the autonomic nervous system are closely interrelated. Further research is required to clarify the mechanism of diarrhea induced by transport stress and validate the effectiveness of acupuncture treatment.
Conclusion
In conclusion, acupuncture treatment suppresses HPA axis activation, thereby reducing transport stress; however, it did not suppress central catecholaminergic or sympathetic activity in microminipigs. Acupuncture treatment for transport stress can improve animal welfare.
Funding
This research was partly supported by the Japan Society for the Promotion of Science (JSPS), Kakenhi, Grant Numbers 22K06006 (MI), 23380185, 16K08023, and 20K06395 (HK).
Conflicts of Interest
No conflicts of interest exist concerning this study.
Authors’ Contributions
M.I., K.A., and H.K. collected the sample materials. M.I. and H.K. planned the study; M.I., K.A., Y.F., O.Y., and H.K. performed the experiments and analyzed the data. M.I., T,K, H.M., H.K.O, R.T., T.M., O.Y., and H.K. drafted the manuscript. All Authors have read and approved the final version of the manuscript.
Acknowledgements
The Authors would like to thank all the staff members of Shin Nippon Biomedical Laboratories, Ltd. for technical assistance.
References
- 1.Martínez-Miró S, Tecles F, Ramón M, Escribano D, Hernández F, Madrid J, Orengo J, Martínez-Subiela S, Manteca X, Cerón JJ. Causes, consequences and biomarkers of stress in swine: an update. BMC Vet Res. 2016;12(1):171. doi: 10.1186/s12917-016-0791-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dalin AM, Magnusson U, Häggendal J, Nyberg L. The effect of transport stress on plasma levels of catecholamines, cortisol, corticosteroid-binding globulin, blood cell count, and lymphocyte proliferation in pigs. Acta Vet Scand. 1993;34(1):59–68. doi: 10.1186/BF03548224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cui Y, Wang C, Hao Y, Gu X, Wang H. Chronic heat stress induces acute phase responses and serum metabolome changes in finishing pigs. Animals (Basel) 2019;9(7):395. doi: 10.3390/ani9070395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawaguchi H, Sasatake Y, Noguchi M, Akioka K, Miura N, Takeishi K, Horiuchi M, Tanimoto A. Study of a novel acupuncture treatment for canine transportation stress. J Jpn Vet Med Assoc. 2016;69:143–146. [Google Scholar]
- 5.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piñeiro M, Piñeiro C, Carpintero R, Morales J, Campbell FM, Eckersall PD, Toussaint MJ, Lampreave F. Characterisation of the pig acute phase protein response to road transport. Vet J. 2007;173(3):669–674. doi: 10.1016/j.tvjl.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Foury A, Devillers N, Sanchez M, Griffon H, Le Roy P, Mormède P. Stress hormones, carcass composition and meat quality in Large White×Duroc pigs. Meat Sci. 2005;69(4):703–707. doi: 10.1016/j.meatsci.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto K, Shinba T, Yoshii M. Psychiatric symptoms of noradrenergic dysfunction: A pathophysiological view. Psychiatry Clin Neurosci. 2014;68(1):1–20. doi: 10.1111/pcn.12126. [DOI] [PubMed] [Google Scholar]
- 9.Roy A. Norepinephrine and its metabolites in cerebrospinal fluid, plasma, and urine. Arch Gen Psychiatry. 1988;45(9):849. doi: 10.1001/archpsyc.1988.01800330081010. [DOI] [PubMed] [Google Scholar]
- 10.Zegler MG, Lake CR, Wood JH, Brooks BR, Ebert MH. Relationship between norepinephrine in blood and cerebrospinal fluid in the presence of a blood cerebrospinal fluid barrier for norepinephrine. J Neurochem. 1977;28(3):677–679. doi: 10.1111/j.1471-4159.1977.tb10444.x. [DOI] [PubMed] [Google Scholar]
- 11.Lee G, Kim W. The modulatory effect of acupuncture on the activity of locus coeruleus neuronal cells: a review. Evid Based Complement Alternat Med. 2017;2017:9785345. doi: 10.1155/2017/9785345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ijiri M, Odo K, Sato M, Kawaguchi M, Fujimoto Y, Miura N, Matsuo T, Hou DX, Yamato O, Tanabe T, Kawaguchi H. Potential biomarkers for chronic seasonal heat stress in Kagoshima Berkshire pigs reared in the subtropical region. J Vet Res. 2022;66(2):209–214. doi: 10.2478/jvetres-2022-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lomborg SR, Nielsen LR, Heegaard PMH, Jacobsen S. Acute phase proteins in cattle after exposure to complex stress. Vet Res Commun. 2008;32(7):575–582. doi: 10.1007/s11259-008-9057-7. [DOI] [PubMed] [Google Scholar]
- 14.Fukui T, Yamauchi K, Maruyama M, Yasuda T, Kohno M, Abe Y. Significance of measuring oxidative stress in lifestyle-related diseases from the viewpoint of correlation between d-ROMs and BAP in Japanese subjects. Hypertens Res. 2011;34(9):1041–1045. doi: 10.1038/hr.2011.76. [DOI] [PubMed] [Google Scholar]
- 15.Hirata Y, Yamamoto E, Tokitsu T, Fujisue K, Kurokawa H, Sugamura K, Sakamoto K, Tsujita K, Tanaka T, Kaikita K, Hokimoto S, Sugiyama S, Ogawa H. The pivotal role of a novel biomarker of reactive oxygen species in chronic kidney disease. Medicine (Baltimore) 2015;94(25):e1040. doi: 10.1097/MD.0000000000001040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirata Y, Yamamoto E, Tokitsu T, Kusaka H, Fujisue K, Kurokawa H, Sugamura K, Maeda H, Tsujita K, Kaikita K, Hokimoto S, Sugiyama S, Ogawa H. Reactive oxygen metabolites are closely associated with the diagnosis and prognosis of coronary artery disease. J Am Heart Assoc. 2015;4(2):e001451. doi: 10.1161/JAHA.114.001451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faienza MF, Francavilla R, Goffredo R, Ventura A, Marzano F, Panzarino G, Marinelli G, Cavallo L, Di Bitonto G. Oxidative stress in obesity and metabolic syndrome in children and adolescents. Horm Res Paediatr. 2012;78(3):158–164. doi: 10.1159/000342642. [DOI] [PubMed] [Google Scholar]
- 18.Sauerwein H, Schmitz S, Hiss S. The acute phase protein haptoglobin and its relation to oxidative status in piglets undergoing weaning-induced stress. Redox Rep. 2005;10(6):295–302. doi: 10.1179/135100005X83725. [DOI] [PubMed] [Google Scholar]
- 19.Shono S, Gin A, Minowa F, Okubo K, Mochizuki M. The oxidative stress markers of horses-the comparison with other animals and the influence of exercise and disease. Animals (Basel) 2020;10(4):617. doi: 10.3390/ani10040617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yun W, Song M, Lee J, Oh H, An J, Kim G, Lee S, Lee S, Kim HB, Cho J. Arginine addition in a diet for weaning pigs can improve the growth performance under heat stress. J Anim Sci Technol. 2020;62(4):460–467. doi: 10.5187/jast.2020.62.4.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He Y, Sang Z, Zhuo Y, Wang X, Guo Z, He L, Zeng C, Dai H. Transport stress induces pig jejunum tissue oxidative damage and results in autophagy/mitophagy activation. J Anim Physiol Anim Nutr (Berl) 2019;103(5):1521–1529. doi: 10.1111/jpn.13161. [DOI] [PubMed] [Google Scholar]
- 22.Zou Y, Wei HK, Xiang QH, Wang J, Zhou YF, Peng J. Protective effect of quercetin on pig intestinal integrity after transport stress is associated with regulation oxidative status and inflammation. J Vet Med Sci. 2016;78(9):1487–1494. doi: 10.1292/jvms.16-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]