Abstract
Background/Aim
As the largest organ of the human body, the skin serves as a critical barrier against environmental damage. However, many factors, such as genetics, sun exposure, and lifestyle choices can lead to skin damage creating wrinkles, sagging, and loss of elasticity. The use of skincare products containing natural ingredients has become increasingly popular as a way to combat the signs of aging. Caviar oil is one such ingredient that has gained attention due to its rich composition of fatty acids, vitamins, and minerals. The objective of this study was to investigate the potential anti-aging effects of caviar oil and to develop a product, Cavi Balm, which could potentially reduce wrinkles and skin sagging.
Materials and Methods
An in vitro model using the 3T3-L1 cell line was employed to assess the effect of caviar oil on adipocyte differentiation. An ex vivo study using human skin tissue was conducted to investigate the impact of caviar oil on collagen and elastin formation and the expression of matrix metalloproteinase-1,2,9 (MMP-1, MMP-2, MMP-9). Furthermore, 102 participants were enrolled in five clinical studies to evaluate the anti-aging efficacy of our product, “Cavi Balm”, in facial and neck wrinkles, facial and eye area lifting, and various skin parameters, such as skin moisture, skin elasticity, skin density, skin tightening relief, skin clarity, and skin turnover.
Results
In vitro, caviar oil enhanced adipocyte differentiation, and increased lipid accumulation inside the cells. The ex vivo analysis revealed that caviar oil reduced the expression levels of MMP-1, MMP-2, and MMP-9, and increased the formation of elastin and collagen I, III. Moreover, in the clinical study, Cavi Balm improved skin parameters after one-time use, with more significant effects observed after four weeks of usage.
Conclusion
Caviar oil has a substantial impact on mitigating skin aging and holds potential for application in anti-aging products.
Keywords: Caviar oil, anti-aging, anti-wrinkle, clinical human skin, ex vivo human skin tissue model
The skin is a barrier to protect our bodies from ultraviolet (UV) radiation and external infections, maintain homeostasis, and regulate our body’s temperature and immunological activity. Moreover, the skin holds significant aesthetic importance (1). Aging-associated morphological alterations, such as increased wrinkles, laxity, elastosis, telangiectasia, and dyspigmentation, can negatively impact an individual’s quality of life. The skin, unlike other body organs, is subject to a variety of extrinsic environmental factors, including exposure to chemicals, smoking, or UV radiation. Furthermore, endogenous factors, such as nutritional and hormonal imbalances, inevitably influence skin health (2,3).
Skin aging is a multifaceted, intricate physiological process that impacts the various layers of the skin and their supporting structures. Keratinocyte proliferation diminishes, cell renewal declines, and the epidermis is refined. Dermo-epidermal junction integrity is compromised, while extracellular matrix (ECM) composition, cellularity, and dermal vascularization all decrease, leading to collagen fiber disorganization, fragmentation, and reduction. Concurrently, muscle mass, subcutaneous adipose tissue, and atrophy undergo senescence, lipolysis, and visceral redistribution, weakening the adipose-muscular support of the skin and reducing skin thickness, resulting in sagging (4). The loss of skin tissue, particularly at the dermal and hypodermic levels, contributes to visible signs of aging, such as wrinkles (4). The deterioration of collagen and elastin, the skin’s primary structural components, is a known consequence of aging. Thus, preserving these crucial constituents is vital in delaying or mitigating skin aging. Consequently, most anti-aging approaches and products, including cosmetics, functional foods, topical agents, and surgical interventions (e.g., collagen injections), aim to maintain at least one of these fundamental skin components. Surgery, though costly and potentially painful with a risk of edema, offers immediate results (5). Various laser and light therapies have been proposed to address facial aging and dry skin, with varying degrees of success (6,7). In this study, we aimed to develop a product utilizing caviar oil for its anti-aging properties, moisturizing capabilities, and collagen-boosting potential as a viable strategy to improve skin wrinkles.
The Cavi Balm from KOREATECH Co. Ltd. in Korea contains 15% caviar oil, as well as other ingredients like Vaccinium Macrocarpon (Cranberry) fruit extract, ceramide, and adenosine to enhance skin hydration and mitigate wrinkles. Caviar oil is composed of Candida Bombicola/Glucose/Methyl Rapeseedate ferment, Macadamia ternifolia seed oil, and caviar extract. This oil is a rich source of essential fatty acids, such as docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), as well as amino acids, vitamins, and minerals. It is widely recognized for its health benefits and has been incorporated into various cosmetic products (8-10). The omega-3 polyunsaturated fatty acids (PUFAs), one of the typical fatty acid compositions of the fish oil extract, can prohibit UV-induced keratinocyte damage by regulating cyclooxygenase-2 (COX-2), nuclear factor kappa-light-chain-enhancer of activated B cell (NF-ĸB), and mitogen-activated protein kinase (MAPK)/extracellular-signal-regulated kinase (ERK) pathways (11). EPA, a ω-3 fatty acid, also can suppress UV-induced MMP1 expression via the inhibition of the mitogen-activated ERK kinase 1 (MEK1)/ERK/C-Fos and stress-activated kinase 1 (SEK1)/Jun-N-terminal kinase (JNK)/C-Jun pathways (12). In addition, DHA from caviar was proven to induce adipocyte differentiation and adiponectin production, thereby inhibiting collagen degradation and wrinkle formation (13). Therefore, it has assumed a high potential in anti-aging skin care. In this study, we employed in vitro cell culture models to evaluate caviar oil’s effects and used ex vivo human skin models to investigate its impact on protein expression related to anti-aging. We also assessed the product’s clinical efficacy on facial wrinkles to demonstrate caviar oil’s anti-aging properties. The findings suggest that Cavi Balm, containing 15% caviar oil, holds promise as a product capable of increasing skin hydration, elasticity, and rejuvenation while offering anti-wrinkle advantages and counteracting the “bulldog cheek” (marionette lines) appearance.
Materials and Methods
In vitro and ex vivo analyses. Cell viability. 3T3-L1 cells were seeded into a 24-well plate and treated with different concentrations of caviar oil from 156.25 ppm to 20,000 ppm for 24 h. After removing the medium, 200 μl of 0.5 mg/ml 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was added and incubated at 37˚C, 5% CO2 for 4 h. Then, the agent was removed, and 200 μl of DMSO were added. Purple cell pellets were dissolved in dimethyl sulfoxide (DMSO) at room temperature for 10 min. Cell viability was assessed by measuring the optical density at 570 nm.
Cell culture and adipocyte differentiation. The mouse preadipocyte 3T3-L1 cell lines were seeded in a 6-well plate at a density of 2.5×105 cells per well and cultured for 72 h in a Dulbecco’s Modified Essential Medium (DMEM) culture medium containing 10% bovine calf serum (BCS) and 1% Penicillin/Streptomycin (P/S) at 37˚C in 5% CO2 to reach 100% confluence. The culture medium was then removed, and adipocyte differentiation was initiated using DMEM medium supplemented with 10% fetal bovine serum (FBS), 1% P/S, 0.5 mM 3-Isobutyl-1-methylxanthine (IBMX), 1 µM dexamethasone, and 10 μg/ml insulin for two days at 37˚C in 5% CO2. During this period, the 3T3-L1 cell line was treated with various concentrations of caviar oil, including 625 ppm, 1,250 ppm, 2,500 ppm, 5,000 ppm, and 10,000 ppm. After the cells were transferred to a new culture in differentiation DMEM medium containing 10% FBS, 1% P/S, and 10 μg/ml insulin with different concentrations of caviar oil and incubated at 37˚C in 5% CO2 for an additional two days. The cells were then cultured at 37˚C in 5% CO2 for six days using normal DMEM medium supplemented with 10% FBS, and 1% P/S containing different concentrations of caviar oil. After that, the 3T3-L1 cell lines were washed three times with 1x PBS solution and fixed with 10% formal dehydrate at room temperature for 30 min. Following, the cells were rinsed three times with 1x PBS, and stained with Oil Red O working solution (ORO, Sigma Aldrich, Saint Louis, MO, USA) at room temperature for 20 min. Finally, cells were washed three times with 1x PBS solution, and the adipocytes were photographed at 200× magnification using an optical microscope Olympus CKX53 (Olympus, Tokyo, Japan) and dissolved lipid droplets in DMSO and measured absorbance at 520 nm with a Microplate spectrophotometer (BioTek, Winooski, VT, USA).
Evaluation of caviar oil absorption on human skin tissue. Human skin tissues (Dermalab™ FULL SKIN 2 cm×2 cm) were treated with saline (control group) and caviar oil (test group) and then recovered after 24 h and frozen. The recovered tissues were cryosectioned to a thickness of 8-10 μm and stained with Oil Red O solution, followed by nuclear staining using hematoxylin. Subsequently, the cross-sections of human skin tissue were observed at 200× magnification using an optical microscope (Zeiss Axio Observer 7, Oberkochen, Germany). The captured visual images were analyzed using Image J software (The National Institutes of Health, Bethesda, MD, USA). The stained skin area ratio was analyzed.
Preparation of treatment of human skin tissues with caviar oil. Human skin tissues collected after surgery of Korean women between the ages of 50 and 70 were used in the study with the approval of the KSRC Korea Skin Clinical Research Center IRB (IRB approval number: HBABN01-220801-HR-E0145-01). The human-derived skin tissues were washed twice with 1x PBS solution and then placed in a sterilized Petri dish. Human-derived skin tissues were punched by a Biopsy Punch (KAI Medical, Tokyo, Japan), and the skin tissues of all groups except for the untreated group were irradiated with an intensity of 200 mJ of UVB (BLX-LMC, Vilber Loumart, Collégien, France). After UVB irradiation, Transwell inserts (Corning, Corning, NY, USA) were attached to each 6-well plate, and skin tissues were placed on the Transwell insert. At this time, the positive control group (Ascorbic acid 1.00%), caviar oil groups at different concentrations of 1,000 ppm, 5,000 ppm, and 10,000 ppm were added to the DMEM culture medium containing 10% FBS, 1% P/S for 48 h.
Real time-PCR. After 48 h of cultivation, the crushed skin tissue was treated with Trizol and then reacted. Total RNA was extracted from the skin tissue using Total RNA extraction reagent (Takara Bio, Shiga, Japan) according to the manufacturer’s instructions. cDNA synthesis was performed from approximately 1 μg of total RNA according to the protocol provided by the RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, Waltham, MA, USA). Specific primer pairs analyzed the expression of MMP-1, MMP-2, and MMP-9 were as follows: MMP-1 forward 5’-ACA GCC CAG TAC TTA TTC CCT TTG -3’, MMP-1 reverse 5’-GGG CTT GAA GCT GCT TAC GA -3’, MMP-2 forward 5’- CCC CAA AAC GGA CAA AGA G -3’, MMP-2 reverse 5’- CAC GAG CAA AGG CAT CAT CC -3’, MMP-9 forward 5’- CAC TGT CCA CCC CTC AGA GC -3’, MMP-9 reverse 5’- GCC ACT TGT CGG CGA TAA GG -3’. For quantitative PCR, TB Green® Premix Ex Taq™ II (Takara Bio) was used on the ViiA™ Real-Time System (Thermo Fisher Scientific) with PCR condition following denaturation at 95˚C for 30 s, then 45 cycles of amplification at 95˚C for 5 s and 60˚C for 34 s. Relative mRNA expression values were determined using the comparative CT method, the 2-ΔΔCt method.
Preparation of tissues for histological analysis. The tissue samples were prepared by fixing and embedding in paraffin blocks. Tissue slides were then obtained by sectioning the blocks at a thickness of 3 μm. These section slides were used for further histological examinations.
Hematoxylin and eosin (H&E) staining. After the hydration process, tissue slides were stained with Hematoxylin and Eosin solutions, and the results were quantified by examining the stained slides under a microscope.
Collagen I and III staining. After hydration, the antigen in samples was retrieved with 10 mM Sodium Citrate Buffer, and then an antibody (collagen type I and III) was treated. Afterward, coloring was performed using ABC reagent and DAB solution, and counterstaining was performed with hematoxylin.
Victoria Blue staining. After the hydration process, staining was performed using Potassium Permanganate-Sulfuric Acid Working Solution, followed by a reaction with Sodium Bisulfite 1%. Afterward, color development was induced using 70% alcohol, and counterstaining was performed with Nuclear Fast Red stain.
Clinical study. There were 5 clinical studies conducted following Good Clinical Practice (GCP), Ministry of Food and Drug Safety (MFDS) related regulations, and Korea Skin Clinical Research Center’s standard operating instructions and was registered with IRB approval number at HBABN01-220502-HR-E0101-01, HBABN01-220502-HR-E0102-01, HBABN01-220502-HR-E0104-01, HBABN01-220502-HR-E0105-01, and HBABN01-220502-HR-E0106-01. Each clinical study recruited over 20 participants in accordance with the recommendations of MFDS Guideline. Potential participants who meet the inclusion criteria and not the exclusion criteria were selected and provided with an explanation of the study purpose, methods, expected efficacy, and potential side-effects. Participants who expressed their intent to participate were required to complete a consent form for research participation and joined the study. For the test after four weeks of product use, subjects applied the product three times a day for four weeks (once in the morning, evening, and afternoon). During the study period, the researchers observed the skin condition of the participants, and in case of serious adverse reactions other than predictable ones, prompt and appropriate measures were taken to minimize possible adverse effects. In the event of an adverse reaction, the research director determined the relevance to the test product, and if the problem was due to the test product, the sponsoring institution assumed full responsibility and ensured the safety of the research subjects by providing appropriate compensation.
Study procedure. All evaluations in this study were conducted at the same measurement site. Study participants washed the test site during the visit and then stabilized for 30 min in a room with constant temperature and humidity (22 ± 2˚C, 50±5%). For the test after using the product once, participants washed their face, and then the researcher rolled the product onto the entire face and neck of the subjects and tapped lightly to absorb.
Measurement of six types of deep wrinkles. The selected forehead, glabellar, nasolabial, marionette, lip, and neck were assessed by topographic via Antera 3D® CS (Miravex Limited, Dublin, Ireland) before and after using the product once and after four weeks of using the product. The wrinkle parameter values were analyzed using an analysis program.
Evaluation facial pillow marks relief effect. The effect of relieving facial pillow marks was assessed before, after a single product use, and after four weeks of product use. Pillow marks were artificially induced for 10 min on the selected cheek area at each time point. The Antera 3D® CS (Miravex Limited) was utilized, and volume parameter values were analyzed.
Measurement of transepidermal water loss. Transepidermal water loss in the selected cheek area was measured three times before, after using the product once, and after four weeks of product use, and the average values were calculated. Transepidermal water loss was measured using Tewameter® TM HEX (C+K, Köln, Germany).
Measurement of skin moisture. The skin moisture level of the selected cheek area was measured three times using Corneometer® CM 825 (C+K) before, after one use of the product, and after four weeks of product use, and then the average value was analyzed.
Measurement of skin clarity (transparency). The skin clarity (transparency, surface reflectance value) of the selected cheek area was measured three times using Lumiscan™ (True systems, Anyang-si, Gyeonggi-do, Republic of Korea) at the time points before product use, after one use of the product, and after four weeks of product use, and the average value was calculated and analyzed.
Measurement of skin density. The dermal density of the cheek of participants was measured using the Ultrasound Probe of DermaLab® Series SkinLab Combo (Cortex Technology, Aalborg, Denmark) before and after using the product for 4 weeks. The device displayed low density as a dark color and high density as a bright color through the signal strength.
Measurement of skin elasticity. The skin elasticity of the selected cheek area was measured using the Cutometer® MPA580 (C+K) before, after one use of the product, and after four weeks of product use. The same posture and distance were maintained throughout the measurement process to ensure consistent measurements.
Measurement of five types of facial gloss (diamond zone radiance). The forehead left and right eye areas, and left and right mouth areas were selected for measurement before and after product use. The skin’s gloss was measured three times using the Skin-Glossymeter GL 200 (C+K), and the average value was analyzed. This device operates on the reflection principle. The measured value is expressed in Glossymeter units (GU).
Measurement of three types of eye area lifting. The selected eyelids, bags under the eyes, crow’s feet, and the bulldog area were photographed using Antera 3D® CS (Miravex Limited) before using the product, after using the product once, and after four weeks of using the product. Lifting (skin filling) parameter values were analyzed using an analysis program.
Measurement of three-dimensional (3D) facial lifting. A 3D image of the subject’s face was captured using VECTRA H2 (Canfield, Parsippany-Troy Hills, NJ, USA) under the controlled conditions (consistent posture and distance) before, after product use one time, and after four weeks of product use. The length of each study area was analyzed using the captured 3D image and analysis software.
Evaluation of makeup entrapment relief effect. The participants’ facial areas were classified into 2 groups: a control group (which did not use Cavi Balm) and a test group (which used Cavi Balm). A makeup product (foundation) was applied to the test area. Antera 3D® CS (Miravex Limited) was used to photograph the test area before and after a single use of the product of both the control group and the test product group. The skin volume parameter was measured using an analysis program.
Evaluation of skin tightening relief. Skin tightening relief was carried out by participants based on the degree of skin tightening before and after a single use of the product, as well as after four weeks of product use. The evaluation criteria involved measuring the degree of skin tightening (Grade 1: no tightening at all, ~Grade 7: severe tightening), with the average value of the calculated scale being analyzed.
Measurement of skin moisture under hot and cold air environmental conditions. The skin moisture content was measured on the forearm area (3×3 cm2) and compared between the control group (no application of Cavi Balm) and the Cavi Balm applied group. The hot/cold air environmental conditions (warm air: 40±3˚C, cold air: 20±3˚C) were achieved by using an electric heater (combined fan) from Cixi Pengxiang Electric Appliance Co., LTD, Zhejiang, PR China. The posture and distance of the participants were fixed to ensure consistency across participants. Warm air and cold air were applied to the forearm for 5 minutes each, and the total exposure time was 10 min. The moisture level of the skin in the selected forearm was measured three times using the Corneometer® CM 825 (C+K) before, after using the product once, and after applying hot/cold air conditions, and the average value was analyzed.
Measurement of skin turnover. The control group (no application of Cavi Balm) and a Cavi Balm applied group were selected in the left or right forearm (1.5×1.5 cm2) through randomization and proceeded respectively. A total of 20 μl of 5% Dansyl chloride dissolved in Squalene was first applied to a 1.5×1.5 cm2 cotton pad, Tegaderm (3M, Saint Paul, MN, USA). Subsequently, the cotton pads were applied to the control group (without the application of the product) and Cavi Balm applied group. Skin staining was induced by using an occlusive patch for 24 h. UV images were taken before and after dansyl chloride staining and after ten days of product use by Ghost In The Mirror (PSI Plus, Suwon-si, Gyeonggi-do, Republic of Korea). The analysis was based on the intensity value of the measurement area using the Image-pro® 10 (Media Cybernetics, Rockville, MD, USA) program. It was determined that a greater change in intensity value indicates a higher effect of improving turnover.
Statistical analysis. All data were analyzed for statistical significance using the SPSS Package Program (IBM, Armonk, NY, USA). Statistical analysis of variables for parametric values was performed using the paired t-test and RM-ANOVA. *p<0.05, **p<0.01 and ***p<0.001 were considered statistically significant. All figures were drawn using Graph Prism 9 Software (Graphpad Software, San Diego, CA, USA).
Results
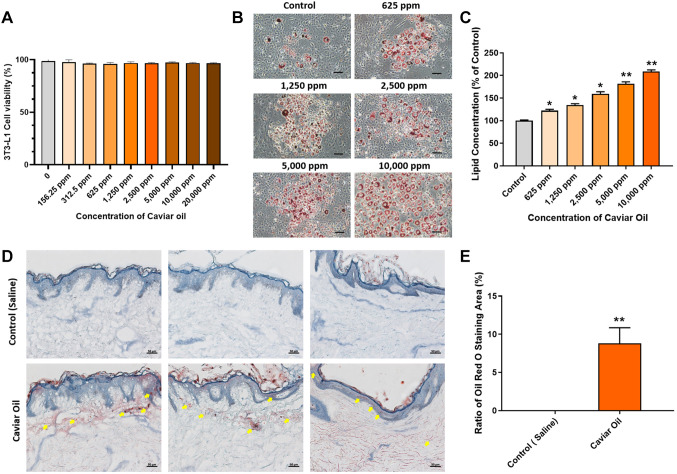
Effect of caviar oil on cell viability. The effect of caviar oil on cell viability of 3T3-L1 cells was evaluated by the MTT assay. 3T3-L1 cells were incubated with different concentrations of caviar oil from 156.25 ppm to 20,000 ppm for 24 h. Caviar oil did not exhibit any significant reduction in cell viability among different concentrations of caviar oil (Figure 1A). This result indicates that caviar oil is non-toxic and safe for cells.
Figure 1. Caviar oil enhanced adipocyte differentiation. (A) Cell viability was confirmed by the MTT assay after treatment with of caviar oil at different concentrations. (B). The morphology of 3T3-L1 cells was observed by Oil Red O staining captured under a microscope (the lipid droplets are displayed red round). Scale bar=100 μm. (C) The adipocyte differentiation efficacy at different caviar oil concentrations. (D) Histological evaluation of caviar oil on ex vivo human skin tissue by Oil Red O staining. Scale bar=50 μm. (E) The analysis of Oil Red O staining is in ex vivo human skin tissues at 24 h. Data are shown as the mean±SEM. *p<0.05, **p<0.01.
Effect of caviar oil on adipocyte differentiation. To evaluate the effect of caviar oil on adipocyte differentiation. 3T3-L1 cells were treated with different concentrations of caviar oil ranging from 625 to 10,000 ppm for ten days. Cells were performed Oil Red O staining assay to identify intracellular lipids in adipocyte differentiation. The results showed that the caviar oil gradually increased the adipocyte differentiation, and depending on the concentration of caviar oil, the lipid accumulation inside the cells increased from 122.14% to 208.56% compared to the control group. At a dose of 10,000 ppm, the lipid accumulation displayed the highest among other groups (Figure 1B and C). These results indicate that caviar oil is considered to have an adipocyte differentiation effect at the in vitro level.
Evaluation of caviar oil absorption in human skin tissue. To evaluate absorption ex vivo, human skin tissue was stained with Oil Red O following 24 h treatment with caviar oil. Absorption of caviar oil was observed in the epidermal and dermal layers, compared to the control group that was treated with saline (Figures 1D and E). Absorption in the caviar oil group was 8.81%, compared to 0.0% in the control group. Overall, caviar oil was found to be sufficiently absorbed into the epidermal and dermal layers of human skin tissue.
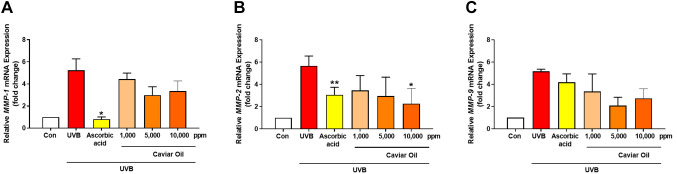
Evaluation of caviar oil’s effect on wrinkle-related mRNA expression level in human skin tissue model. mRNA expression levels of MMP-1, MMP-2, and MMP-9 were analyzed using Realtime-PCR under the same experimental conditions. As Figure 2A displays a significant reduction in MMP-1 mRNA expression levels in UVB-irradiated human tissues treated with 1,000, 5,000, and 10,000 ppm of caviar oil compared to the control group, with decreases of 17.71%, 74.96%, and 55.49%, respectively. Similar trends were observed in the mRNA expression levels of MMP-2 and MMP-9 (Figure 2B and C). These findings suggest that caviar oil may serve as a potent anti-aging factor for skin by inhibiting MMP-1, MMP-2, and MMP-9 gene expression.
Figure 2. The expression mRNA level of (A) MMP-1, (B) MMP-2, (C) MMP-9 were measured by RT-PCR. The means of SDs are the average values of three independent experiments, *p<0.05, **p<0.01. MMP-1: Matrix metalloproteinase-1; MMP-2: matrix metalloproteinase-2; MMP-9: matrix metalloproteinase-9; UVB: ultraviolet B; Con: control group.
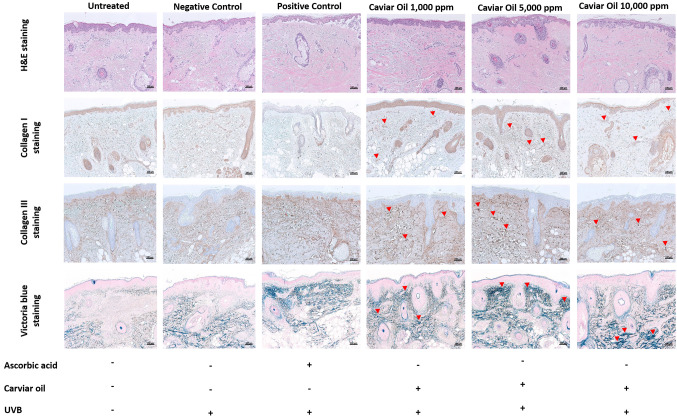
Histological evaluation of caviar oil on ex vivo human skin tissue. H&E staining results revealed no significant structural changes in skin tissue treated with 1,000, 5,000, and 10,000 ppm of caviar oil compared to normal skin tissue, indicating that caviar oil did not damage the skin structure. Collagen I and Collagen III staining demonstrated a gradual increase in collagen across all caviar oil-treated groups. Elastic fiber observations, as measured by Victoria blue staining, showed continuous enhancement with increasing concentrations of caviar oil. Collectively, these observations suggest that caviar oil may possess anti-wrinkle and elastin-enhancing effects (Figure 3).
Figure 3. Histological evaluation of anti-winkle effect in an ex vivo human tissue model by hematoxylin and eosin staining, Collagen I, III staining, Victoria blue staining. Scale bar=100 μm. UVB: Ultraviolet B.
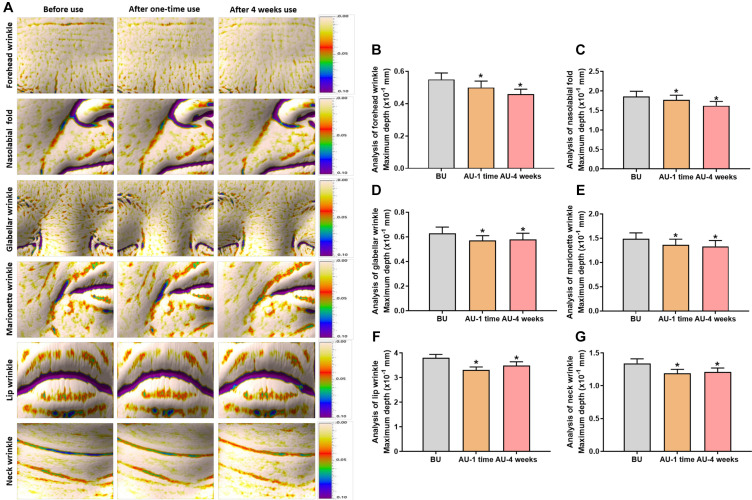
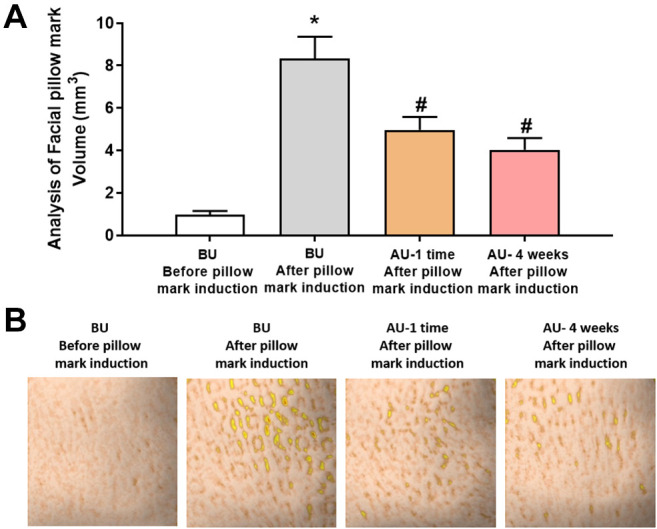
Evaluation of Cavi Balm’s effect on human skin improvement. The effectiveness of Cavi Balm, containing 15% caviar oil, on human skin was assessed through clinical investigation, analyzing six types of deep wrinkles, such as forehead, glabellar, nasolabial folds, marionette, lips, and neck wrinkles, after one-time use and four weeks of use (Figure 4A). Figure 4B demonstrates a significant reduction in forehead wrinkles after the first use (9.09%) and four weeks after use (16.36%), compared to before use. Similarly, nasolabial fold analysis showed remarkable decreases of 4.84% and 12.90% after one-time use and four weeks of use, respectively (Figure 4C). Furthermore, Figure 4D, E, F, and G show a similar trend in glabellar wrinkles, marionette wrinkles, lip wrinkles, and neck wrinkles, and it demonstrated a more effective reduction of wrinkles after four weeks of use of the product. In terms of facial pillow mark relief analysis, the parameter value for pillow marks significantly decreased after using the product compared to before the induction of pillow marks, with rates of change observed at 40.41% after one-time use and 51.56% after four weeks of use, respectively (Figure 5).
Figure 4. The improvement effect evaluation of Cavi Balm on human skin before and after one-time use, four weeks after using the product, by analyzing six types of deep wrinkles. (A). Image of six types of deep wrinkles. (B) Forehead wrinkle analysis. (C) Nasolabial fold analysis. (D) Glabellar wrinkle analysis. (E) Marionette wrinkle analysis. (F) Lip wrinkle analysis. (G) Neck wrinkle analysis. Data are shown as the mean±SEM. *p<0.05. BU: Before use; AU: after use.
Figure 5. Effect evaluation of Cavi Balm on reducing facial pillow mark. (A) Analysis of facial pillow mark; (B) Image of the pillow mark induction before and after use Cavi Balm. Data are shown as the mean±SEM. *p<0.05 vs. before pillow mark induction, #p<0.05 vs. before using the product.
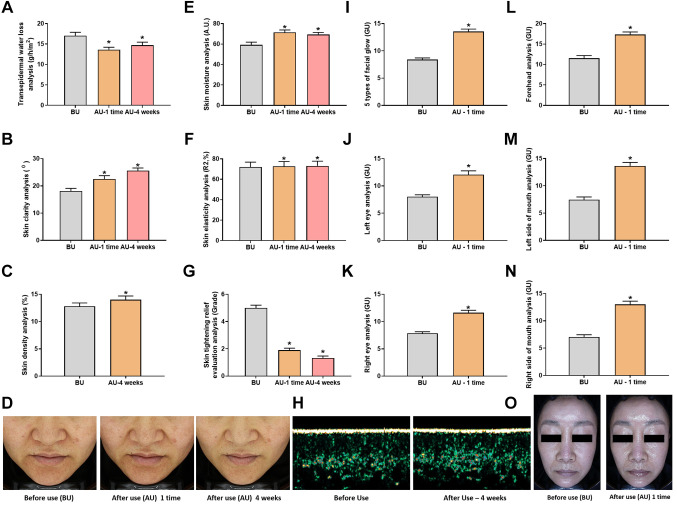
The effect of Cavi Balm on human skin was further assessed through various parameters, including transepidermal water loss, skin moisture, skin elasticity, skin clarity, skin density, and skin tightening relief, following Cavi Balm application. The analysis results revealed that compared to before product use, the transepidermal water loss was significantly reduced after one-time use and after four weeks of product use, with change rates of 20.01% and 13.73%, respectively (Figure 6A). The moisturizing properties of Cavi Balm are attributed to its ability to supply water to the skin directly, enhance occlusion to prevent transepidermal water loss, promote skin hydration, and bolster the water content of the stratum barrier. Additionally, the product fills minor skin fissures, forms a gentle protective layer, and defends the skin against friction. Skin moisture analysis revealed a significant increase after one-time and four-week product use compared to before use, with change rates of 20.57% and 17.15%, respectively (Figure 6E). Skin density also increased significantly by 9.38% after four weeks of product use compared to before product use. Skin elasticity analysis and skin clarity analysis were conducted, comparing the results before product use, after one-time use, and after four weeks of product use (Figure 6B, D, and F). All metrics displayed a similar trend, with gradual improvement and the most significant effect after four weeks of product use. The skin tightening relief evaluation analysis demonstrated a notable decrease in skin tightening scores after one-time use and four weeks of product use compared to before using the product, with change rates of 62.00% and 73.40%, respectively (Figure 6G). In addition, the assessment of facial gloss (diamond zone radiance) displayed substantial improvement after a single product use compared to before use, exhibiting a change rate of 61.38% (Figure 6I and O). As a result of the analysis, the gloss (diamond zone radiance) of the forehead, left eye area, right eye area, left mouth area, and right mouth area significantly increased after product use compared to before product use. These rates were 50.39%, 50.31%, 47.84%, 83.22%, and 84.30%, respectively (Figure 6J, K, L, M, and N). Collectively, these findings suggest that Cavi Balm effectively provides hydration and moisture to the skin.
Figure 6. Human skin improvement effect evaluation test on Cavi Balm. (A) Transepidermal water loss. (B), (D) Skin clarity (transparency) analysis. (C), (H) Skin density analysis. (E) Skin moisture analysis. (F) Skin elasticity analysis. (G) Skin tightening relief evaluation analysis. (I) Five types of facial gloss (diamond zone gloss) (J) Facial gloss analysis (left eye). (K) Facial gloss analysis (right eye). (L) Facial gloss analysis (forehead). (M) Facial gloss analysis (left side of mouth). (N) Facial gloss analysis (right side of mouth). Data are shown as the mean±SEM. *p<0.05. BU: Before use; AU: after use.
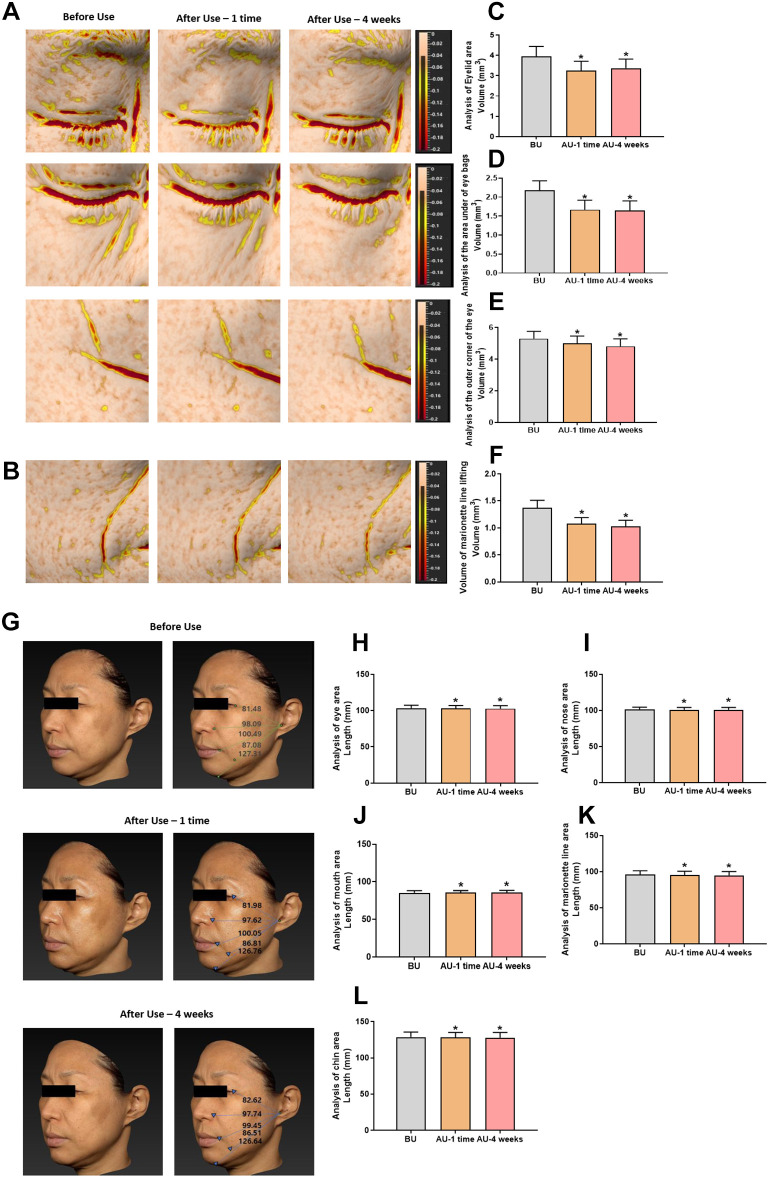
Furthermore, the effects of Cavi Balm were evaluated through analysis of three types of eye area lifting (skin filling), including the eyelid area, the area under eye bags, and the outer corner of the eye, as well as the analysis of marionette lines lifting. The results showed significant improvement compared to before using the product (Figure 7A, B, C, D, E, and F). More evaluation of the Cavi Balm effect was performed by analysis of five types of 3D facial lifting, including the eye, nose, mouth, marionette lines, and chin areas. The product’s effects were immediately apparent after one-time use compared to before using the product (Figure 7G, H, I, J, K, and L).
Figure 7. Analysis of lifting effect before and after using Cavi Balm. (A) Image of 3 types of eye area lifting. (B) Image of marionette line lifting. (C) Analysis of 3 types of eye area lifting (eyelid area). (D) Analysis of 3 types of eye area lifting (the area under the eye bags). (E) Analysis of 3 types of eye area lifting (the outer corner of the eyes). (F) Analysis of marionette line lifting. (G) Image of five types of 3D facial lifting. (H) Analysis of five types of 3D facial lifting (eye area). (I) Analysis of five types of 3D facial lifting (nose area). (J) Analysis of five types of 3D facial lifting (mouth area). (K) Analysis of five types of 3D facial lifting (marionette line area). (L) Analysis of five types of 3D facial lifting (chin area). Data are shown as the mean±SEM. *p<0.05. BU: Before use; AU: after use.
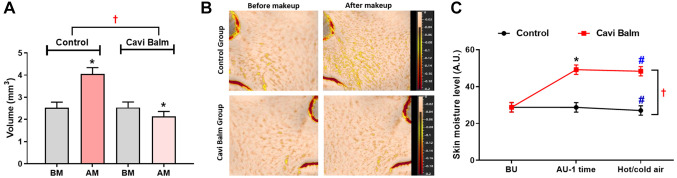
The evaluation of Cavi Balm’s effectiveness in minimizing makeup entrapment, which can result in clogged pores and acne, was conducted by comparing the control group (not using Cavi Balm) and the Cavi Balm applied group before using makeup products (foundation). The analysis results showed that compared to before and after applying foundation, the skin volume parameter value significantly increased in the control group while notably decreasing in the Cavi Balm applied group (Figure 8A). Furthermore, the comparative analysis between the two groups revealed that the skin volume parameter value of the Cavi Balm applied group was significantly lower than the control group (not using Cavi Balm) after makeup application (Figure 8A and B). These findings suggest that Cavi Balm aids in reducing makeup entrapment on the skin.
Figure 8. Evaluation of the effect of Cavi Balm on reducing makeup entrapment and maintaining skin’s moisture under hot/cold air environment conditions. (A) Analysis of makeup entrapment mitigation ability of Cavi balm. (B) Image of makeup entrapment mitigation effect. (C) Evaluation of skin moisture retain ability under hot/cold air conditions. Data are shown as mean±SEM. *p<0.05 vs. before using product, #p<0.05 vs. after product use once, †p<0.05 vs. control group. BM: Before makeup; AM: after makeup.
Furthermore, evaluating the long-lasting moisturizing effect of Cavi Balm on human skin under hot and cold air environmental conditions was conduct. As Figure 8C demonstrates, the skin moisture level significantly increased in the Cavi Balm applied group, with change rate was 70.92%. In contrast, the control group (no application) did not show a significant difference. Both the control group and the Cavi Balm applied group exhibited a decrease in skin moisture in a hot/cold wind environment. However, the skin moisture level of the Cavi Balm applied group had a change rate of 1.73%, which was significantly better maintained than the control group at 6.02% (Figure 8C). This result indicates that Cavi Balm helps maintain skin moisture in hot/cold air conditions.
Evaluation of skin turnover. To evaluate the effect of Cavi Balm on human skin turnover improvement, this investigation was performed by induction of dansyl chloride skin pigmentation. The control group (no application of Cavi Balm) and a Cavi Balm applied group were selected in the left or right forearm and were photographed in UV mode before, right after dansyl chloride staining and ten days after staining after a use of a Cavi Balm. As a result of the analysis, as shown in Figure 9A and B, at the time point after dansyl chloride staining, the skin intensity value of the Cavi Balm application group remarkably increased compared to the control group. This means that dansyl chloride staining became homogeneous in both groups. Also, ten days after staining, skin intensity values were significantly reduced in the control group, and Cavi Balm application group. The rate of change in the skin intensity value of the Cavi Balm application group was 57.85%, showing a greater reduction than that of the control group, (41.83%) (Figure 9A and B).
Figure 9. Evaluation of human skin turnover improvement effect of Cavi Balm. (A) Analysis of skin intensity. (B) Image of skin turnover. (C) Transepidermal water loss analysis. Data are shown as the mean±SEM. *p<0.05 vs. before staining, #p<0.05 vs. after staining, †p<0.05 vs. control group.
In addition, the evaluation of transepidermal water loss was measured between the two groups. The result of the analysis mentioned in Figure 9C, compared to after dansyl chloride staining, transepidermal water loss was significantly decreased in both the control and Cavi Balm applied groups at ten days after staining. The rate of change in transepidermal water loss in the Cavi Balm applied group was 29.75%, which was significantly more reduced than that of the control group, which was 20.24%. All these investigations may believe that Cavi Balm may improve skin turnover.
Discussion
Caviar oil is a distinctive, proprietary oil that contains alpha-lipoic acid (ALA), EPA, and DHA, which are the primary phospholipids’ primary elements and are rich in oleic and linoleic acid. Numerous studies have illustrated the impact of EPA and DHA in reducing inflammation by modulating cytokine synthesis, cellular metabolism, and gene expression in inflammation and angiogenesis (14,15). Various inflammatory disorders, such as cancer, neurological, cardiovascular, obesity, and diabetes have been linked to the anti-inflammatory effect of fatty acids (16-20). Additionally, fish oil comprises vitamin D, vitamin A, free fatty acids, phospholipids, and other nutrients known to increase adiponectin secretion in adipocytes (21-23). These components may serve as dietary supplements to mitigate the severity of several skin issues, such as melanogenesis, allergy, dermatitis, and wrinkles, and are considered to have beneficial effects on the skin (21,24,25). In this study, we investigated that caviar oil increased intracellular lipid accumulation and adipocyte differentiation. These findings provided evidence that caviar oil may play a crucial role in adiponectin production, a hormone capable of enhancing the production of collagen and elastin, reducing the differentiation of skin cells, supporting the process of skin repair, and combating inflammation.
The development of wrinkles on the skin is linked to degradation of the extracellular matrix (ECM), which can be caused by exposure to UV radiation (26). This breakdown is facilitated by matrix metalloproteinases (MMPs), with MMP-1 being specifically responsible for breaking down interstitial collagen, while MMP-2 and MMP-9 are known to break down denatured collagen and elastin. While these enzymes are important for tissue remodeling and repair, their activity can become dysregulated and contribute to skin aging and damage. MMP-1 release in irradiated skin is stimulated by direct damage within the irradiated cells or by cytokines and other factors produced in response to UV radiation, leading to ECM degradation (27). The goal of most bioassays for skin aging is to identify compounds that can prevent UV-induced MMP overproduction. The study revealed that caviar oil decreased mRNA expression levels of MMP-1, MMP-2, and MMP-9 following UVB exposure. Based on prior studies, we propose that caviar oil suppresses MMP-1, MMP-2, and MMP-9 gene expression by inducing differentiation and adiponectin production and decreasing pro-inflammatory cytokine production induced by UV radiation. This, in turn, can potentially inhibit premature skin aging caused by UVB radiation.
Moreover, as vital components of the extracellular matrix, which underpin bodily tissues, collagen, and elastin provide skin strength, moisture, and elasticity. A loss of collagen and elastin brings on wrinkles (28-30). Therefore, collagen I and III staining showed a significant increase in collagen and elastin in the sample treatment with caviar oil and Victoria blue staining, indicating that caviar oil is considered a potent factor of skin anti-aging and can rationally control skin moisture.
Based on the effect of caviar oil on ex vivo research, five clinical studies were performed with our product, “Cavi Balm” on 102 Korean women ages between 20-60 years. The evaluation of the product’s effect was compared before using the product, after one-time use, and four weeks of use of the product. The clinical results showed that Cavi Balm significantly improved skin moisture, elasticity, skin clarity, and skin tightening relief after one-time use. These finding on Cavi Balm are similar to several recent clinical investigations that have been conducted to explore the potential of natural compounds. For example, Bolke et al. performed a randomized, placebo-controlled, single-blind clinical study on 72 healthy women aged 35 to 55 years randomized to be treated with blend collagen or a placebo for 12 weeks. The result showed markedly improved skin moisture and elasticity after treatment (31). Another clinical investigation of oral ingestion of hydrolyzed collagen with plant extracts exhibited a significant improvement in skin elasticity (32), while Chang et al. investigated 50 participants treated with hydrolyzed collagen for four weeks and demonstrated significant improvement in skin hydration, elasticity, spot, gloss, wrinkle, harshness, smooth, pore, collagen, and erythema that may be obtained by the combination of green caviar, djulis, and collagen (33). Taken together, the effective evidence from clinical investigation of Cavi Balm suggests that Cavi Balm has the potential to become a valuable product for inhibiting skin aging.
Numerous factors can contribute to wrinkles, which are prominent signs of aging skin. Dehydration can lead to the degradation of elastin in the skin and the formation of wrinkles (34). Preservation and enhancement of skin moisture are frequently the goals of skin creams and cosmetic products (35). Most of the water in young skin is accurately referred to as “bound water” because it is attached to proteins. This is crucial for many proteins’ mechanical characteristics, structure, and interactions. Aged skin is frequently parched and worn. In addition, a more significant proportion of the water in aged skin exists structurally in a tetrahedron form. As a result, proteins interact less with water in aged skin because they are more hydrophobic and folded, and glycosaminoglycans (GAGs) are clustered on elastic material than water in older skin bonds to itself (32). Therefore, water has a vital role in the skin. Cavi Balm also improves skin turnover, helps maintain moisturizing in hot/cold air conditions and significantly reduces six types of deep wrinkles, including forehead, glabellar, nasolabial, marionette, lip, and neck wrinkles after not only one-time use, but also after four weeks of usage. This evidence suggests that Cavi Balm can effectively address various aged skin concerns and contribute to overall skin rejuvenation by promoting hydration, elasticity, and wrinkle reduction.
Conclusion
The present study investigated the effects of caviar oil on adipocyte differentiation, MMP expression, and skin parameters in vitro, ex vivo, and through clinical trials. The results demonstrated that caviar oil enhances adipocyte differentiation and increases lipid accumulation in vitro, reduces MMP expression and increases the formation of elastin and collagen I, III ex vivo, and improves skin moisture, elasticity, gloss, density, clarity, and reduces transepidermal water loss in clinical trials. These findings indicate that caviar oil has a significant impact on mitigating skin aging and has the potential to serve as a functional ingredient in effective anti-aging skincare products. The development of Cavi Balm, which contains 15% caviar oil, is a promising step in this direction, and its demonstrated ability to improve skin parameters highlights its potential contribution to anti-aging.
Conflicts of Interest
The Authors declare that there are no conflicts of interest.
Authors’ Contributions
Linh Thi Thuy Le, Baek Kyu Kim, Pham Ngoc Chien, Hyo Sun Han, Chan-Yeong Heo: conceptualization, methodology, writing original draft and editing. Linh Thi Thuy Le, Pham Ngoc Chien, Keonwoo Choi, Hyo Sun Han, Ui-Jae Hwang, Hongbin Kim: data curation, investigation, visualization. Hyo Sun Han, Chan-Yeong Heo: supervision, validation, project administration.
Acknowledgements
This research was funded by grant No. 14-2015-0008 from SNUBH Research Fund and by a grand of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HR22C1363).
References
- 1.Blanpain C, Fuchs E. Epidermal stem cells of the skin. Annu Rev Cell Dev Biol. 2006;22:339–373. doi: 10.1146/annurev.cellbio.22.010305.104357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krutmann J, Bouloc A, Sore G, Bernard BA, Passeron T. The skin aging exposome. J Dermatol Sci. 2017;85(3):152–161. doi: 10.1016/j.jdermsci.2016.09.015. [DOI] [PubMed] [Google Scholar]
- 3.Makrantonaki E, Adjaye J, Herwig R, Brink TC, Groth D, Hultschig C, Lehrach H, Zouboulis CC. Age-specific hormonal decline is accompanied by transcriptional changes in human sebocytes in vitro. Aging Cell. 2006;5(4):331–344. doi: 10.1111/j.1474-9726.2006.00223.x. [DOI] [PubMed] [Google Scholar]
- 4.Makrantonaki E, Zouboulis CC. Androgens and ageing of the skin. Curr Opin Endocrinol Diabetes Obes. 2009;16(3):240–245. doi: 10.1097/MED.0b013e32832b71dc. [DOI] [PubMed] [Google Scholar]
- 5.Haneke E. Adverse effects of fillers and their histopathology. Facial Plast Surg. 2014;30(06):599–614. doi: 10.1055/s-0034-1396755. [DOI] [PubMed] [Google Scholar]
- 6.Cannarozzo G, Fazia G, Bennardo L, Tamburi F, Amoruso GF, Del Duca E, Nistico SP. A new 675 nm laser device in the treatment of facial aging: A prospective observational study. Photobiomodul Photomed Laser Surg. 2021;39(2):118–122. doi: 10.1089/photob.2020.4908. [DOI] [PubMed] [Google Scholar]
- 7.Nistico SP, Silvestri M, Zingoni T, Tamburi F, Bennardo L, Cannarozzo G. Combination of fractional CO2 laser and rhodamine-intense pulsed light in facial rejuvenation: A randomized controlled trial. Photobiomodul Photomed Laser Surg. 2021;39(2):113–117. doi: 10.1089/photob.2020.4876. [DOI] [PubMed] [Google Scholar]
- 8.Xu X, Lu S, Li X, Bai F, Wang J, Zhou X, Gao R, Zeng M, Zhao Y. Effects of microbial diversity and phospholipids on flavor profile of caviar from hybrid sturgeon (Huso dauricus×Acipenser schrencki) Food Chem. 2022;377:131969. doi: 10.1016/j.foodchem.2021.131969. [DOI] [PubMed] [Google Scholar]
- 9.Vasconi M, Tirloni E, Stella S, Coppola C, Lopez A, Bellagamba F, Bernardi C, Moretti VM. Comparison of chemical composition and safety issues in fish roe products: Application of chemometrics to chemical data. Foods. 2020;9(5):540. doi: 10.3390/foods9050540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park J, Kim D, Lee M, Han S, Jun W, Jung HM, Koo YK, Na GH, Han SH, Han J, Kim OK. Enzyme-treated caviar prevents UVB irradiation-induced skin photoaging. Mar Drugs. 2022;20(11) doi: 10.3390/md20110685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pilkington SM, Watson REB, Nicolaou A, Rhodes LE. Omega-3 polyunsaturated fatty acids: photoprotective macronutrients. Exp Dermatol. 2011;20(7):537–543. doi: 10.1111/j.1600-0625.2011.01294.x. [DOI] [PubMed] [Google Scholar]
- 12.Kim HH, Shin CM, Park C, Kim KH, Cho KH, Eun HC, Chung JH. Eicosapentaenoic acid inhibits UV-induced MMP-1 expression in human dermal fibroblasts. J Lipid Res. 2005;46(8):1712–1720. doi: 10.1194/jlr.M500105-JLR200. [DOI] [PubMed] [Google Scholar]
- 13.Lee KE, Nho YH, Yun SK, Park SM, Kang S, Yeo H. Caviar extract and its constituent DHA inhibits UVB-irradiated skin aging by inducing adiponectin production. Int J Mol Sci. 2020;21(9) doi: 10.3390/ijms21093383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Popadić S, Ramić Z, Medenica L, Mostarica-Stojkovic M, Popadić D. Antiproliferative effect of docosahexaenoic acid on adult human keratinocytes in vitro / Antiproliferativni efekat dokosaheksanoične kiseline na adultne humane keratinocite in vitro. Serbian J Dermatology Venereol. 2009;1(2):61–67. doi: 10.2478/v10249-011-0005-0. [DOI] [Google Scholar]
- 15.Calder PC. Functional roles of fatty acids and their effects on human health. JPEN J Parenter Enteral Nutr. 2015;39(1_suppl):18S–32S. doi: 10.1177/0148607115595980. [DOI] [PubMed] [Google Scholar]
- 16.Pizato N, Luzete BC, Kiffer L, Correa LH, De Oliveira Santos I, Assumpcao JAF, Ito MK, Magalhaes KG. Omega-3 docosahexaenoic acid induces pyroptosis cell death in triple-negative breast cancer cells. Sci Rep. 2018;8(1):1952. doi: 10.1038/s41598-018-20422-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen X, Chen C, Fan S, Wu S, Yang F, Fang Z, Fu H, Li Y. Omega-3 polyunsaturated fatty acid attenuates the inflammatory response by modulating microglia polarization through SIRT1-mediated deacetylation of the HMGB1/NF-ĸB pathway following experimental traumatic brain injury. J Neuroinflammation. 2018;15(1):116. doi: 10.1186/s12974-018-1151-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oikonomou E, Vogiatzi G, Karlis D, Siasos G, Chrysohoou C, Zografos T, Lazaros G, Tsalamandris S, Mourouzis K, Georgiopoulos G, Toutouza M, Tousoulis D. Effects of omega-3 polyunsaturated fatty acids on fibrosis, endothelial function and myocardial performance, in ischemic heart failure patients. Clin Nutr. 2019;38(3):1188–1197. doi: 10.1016/j.clnu.2018.04.017. [DOI] [PubMed] [Google Scholar]
- 19.De Mello AH, Schraiber RDB, Goldim MPDS, Garcez ML, Gomes ML, De Bem Silveira G, Zaccaron RP, Schuck PF, Budni J, Silveira PCL, Petronilho F, Rezin GT. Omega-3 fatty acids attenuate brain alterations in high-fat diet-induced obesity model. Mol Neurobiol. 2019;56(1):513–524. doi: 10.1007/s12035-018-1097-6. [DOI] [PubMed] [Google Scholar]
- 20.Jung TW, Chung YH, Kim H, Abd El-aty AM, Jeong JH. Protectin DX attenuates LPS-induced inflammation and insulin resistance in adipocytes via AMPK-mediated suppression of the NF-ĸB pathway. Am J Physiol Endocrinol Metab. 2018;315(4):E543–E551. doi: 10.1152/ajpendo.00408.2017. [DOI] [PubMed] [Google Scholar]
- 21.Huang TH, Wang PW, Yang SC, Chou WL, Fang JY. Cosmetic and therapeutic applications of fish oil’s fatty acids on the skin. Mar Drugs. 2018;16(8):256. doi: 10.3390/md16080256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oster RT, Tishinsky JM, Yuan Z, Robinson LE. Docosahexaenoic acid increases cellular adiponectin mRNA and secreted adiponectin protein, as well as PPARγ mRNA, in 3T3-L1 adipocytes. Appl Physiol Nutr Metab. 2010;35(6):783–789. doi: 10.1139/H10-076. [DOI] [PubMed] [Google Scholar]
- 23.Itoh M, Suganami T, Satoh N, Tanimoto-koyama K, Yuan X, Tanaka M, Kawano H, Yano T, Aoe S, Takeya M, Shimatsu A, Kuzuya H, Kamei Y, Ogawa Y. Increased adiponectin secretion by highly purified eicosapentaenoic acid in rodent models of obesity and human obese subjects. Arterioscler Thromb Vasc Biol. 2007;27(9):1918–1925. doi: 10.1161/ATVBAHA.106.136853. [DOI] [PubMed] [Google Scholar]
- 24.Murali G, Desouza CV, Clevenger ME, Ramalingam R, Saraswathi V. Differential effects of eicosapentaenoic acid and docosahexaenoic acid in promoting the differentiation of 3T3-L1 preadipocytes. Prostaglandins Leukot Essent Fatty Acids. 2014;90(1):13–21. doi: 10.1016/j.plefa.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 25.Sawada Y, Saito-Sasaki N, Nakamura M. Omega 3 fatty acid and skin diseases. Front Immunol. 2021;11:623052. doi: 10.3389/fimmu.2020.623052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rittie L. UV-light-induced signal cascades and skin aging. Ageing Res Rev. 2002;1(4):705–720. doi: 10.1016/s1568-1637(02)00024-7. [DOI] [PubMed] [Google Scholar]
- 27.Antille C, Tran C, Sorg O, Saurat J. Penetration and metabolism of topical retinoids in ex vivo organ-cultured full-thickness human skin explants. Skin Pharmacol Physiol. 2004;17(3):124–128. doi: 10.1159/000077238. [DOI] [PubMed] [Google Scholar]
- 28.Perez-Rico C, Pascual G, Sotomayor S, Asunsolo A, Cifuentes A, Garcia-Honduvilla N, Bujan J. Elastin development-associated extracellular matrix constituents of subepithelial connective tissue in human pterygium. Invest Ophthalmol Vis Sci. 2014;55(10):6309–6318. doi: 10.1167/iovs.14-14214. [DOI] [PubMed] [Google Scholar]
- 29.Baumann L, Bernstein EF, Weiss AS, Bates D, Humphrey S, Silberberg M, Daniels R. Clinical relevance of elastin in the structure and function of skin. Aesthet Surg J Open Forum. 2021;3(3):ojab019. doi: 10.1093/asjof/ojab019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reilly DM, Lozano J. Skin collagen through the lifestages: importance for skin health and beauty. Plast Aesthet Res. 2021;8:2. doi: 10.20517/2347-9264.2020.153. [DOI] [Google Scholar]
- 31.Bolke L, Schlippe G, Gerß J, Voss W. A Collagen supplement improves skin hydration, elasticity, roughness, and density: Results of a randomized, placebo-controlled, blind study. Nutrients. 2019;11(10) doi: 10.3390/nu11102494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waller JM, Maibach HI. Age and skin structure and function, a quantitative approach (II): protein, glycosaminoglycan, water, and lipid content and structure. Skin Res Technol. 2006;12(3):145–154. doi: 10.1111/j.0909-752X.2006.00146.x. [DOI] [PubMed] [Google Scholar]
- 33.Chang H, Lin Y, Lin Y, Lin Y, Hu W, Chiang C. Hydrolyzed collagen combined with djulis and green caviar improve skin condition: A randomized, placebo-controlled trial. Curr Res Nutr Food Sci J. 2021;9(2):533–541. doi: 10.12944/crnfsj.9.2.16. [DOI] [Google Scholar]
- 34.Choi JW, Kwon SH, Huh CH, Park KC, Youn SW. The influences of skin visco-elasticity, hydration level and aging on the formation of wrinkles: a comprehensive and objective approach. Skin Res Technol. 2013;19(1):e349–e355. doi: 10.1111/j.1600-0846.2012.00650.x. [DOI] [PubMed] [Google Scholar]
- 35.Milani M, Sparavigna A. The 24-hour skin hydration and barrier function effects of a hyaluronic 1%, glycerin 5%, and Centella asiatica stem cells extract moisturizing fluid: an intra-subject, randomized, assessor-blinded study. Clin Cosmet Investig Dermatol. 2017;10:311–315. doi: 10.2147/CCID.S144180. [DOI] [PMC free article] [PubMed] [Google Scholar]