Abstract
Recent high-profile infectious disease outbreaks illustrate the importance of selecting appropriate control measures to protect a wider range of employees, other than those in healthcare settings. In such settings, where routine exposure risks are often high, control measures may be more available, routinely implemented, and studied for effectiveness. In the absence of evidence-based guidelines or established best practices for selecting appropriate control measures, employers may unduly rely on personal protective equipment (PPE) because of its wide availability and pervasiveness as a control measure, circumventing other effective options for protection. Control banding is one approach that may be used to assign job tasks into risk categories and prioritize the application of controls. This article proposes an initial control banding framework for workers at all levels of risk and incorporates a range of control options, including PPE. Using the National Institutes of Health (NIH) risk groups as a surrogate for toxicity and combining the exposure duration with the exposure likelihood, we can generate the risk of a job task to the worker.
Keywords: Infectious diseases, Pandemic preparedness, Aerosol transmissible disease
Recent high-profile infectious disease outbreaks illustrate the importance of selecting appropriate control measures to protect a wider range of employees than those in healthcare settings, where exposure risks are often high and control measures may be more available, routinely implemented, and studied for effectiveness.1–3 Guidance from government agencies and professional organizations tends to focus on workers in healthcare settings with direct patient care responsibilities, because they are in the highest risk categories, sometimes resulting in less emphasis on recommendations for lower risk workers.4
At the start of a novel infectious disease outbreak, there is often limited information about modes of transmission, pathogenicity, and other key variables important to making informed decisions about measures that will be needed to protect people who may be exposed. When such information becomes available, it is difficult to justify modifications to earlier decisions if there is no preestablished strategy or decision logic for selecting control measures. For the purposes of this article, we use the World Health Organization (WHO) definition of an outbreak as “an increase, often the occurrence of cases of disease in excess of what would normally be expected in a defined community, geographical area, or season.”5 We define a pandemic as an “epidemic, a sudden outbreak that becomes very widespread and affects a whole region, a continent, or the world due to a susceptible population.”6
In the absence of evidence-based guidelines and established best practices for selecting appropriate control measures, employers may unduly rely on personal protective equipment (PPE) because of its wide availability and pervasiveness as a control measure, circumventing other effective options for protection. Because demand for PPE may be significantly greater than supply—as demonstrated by recent public health emergencies such as the 2009 H1N1 influenza pandemic and the 2003 SARS epidemic, and as predicted in some outbreak models7–9—strategies for prioritization should be considered to ensure availability for US workers who need it most. By implementing other control strategies first, fewer workers would require PPE and more supplies would be available for workers in the highest risk categories.
Control banding is one approach for distributing workers into risk categories. Control banding models, first developed for pharmaceutical and chemical hazards,10 use toxicity and exposure information to qualitatively categorize risk into several levels or “bands,” which are then used to guide the selection of appropriate “controls,” or methods for eliminating or minimizing risk.11 Toxicity and exposure levels are relatively easy to predict or measure for chemical exposures, but ascribing them to biological exposures is more difficult.
For biological organisms, several factors contribute to the hazard, including virulence, infectivity, and pathogenicity. Virulence is a measure of the severity of the consequences of infection; infectivity is measured by the number of organisms required to induce infection; and pathogenicity is the ability of an organism to cause disease. For many human pathogens, we do not fully understand and cannot easily measure the virulence, infectious dose, pathogenicity, and/or route(s) of transmission.
Exposure to a disease spread through the respiratory route is a function of concentration of infectious organisms in the air and the frequency and duration of contact. Unfortunately, the few biological aerosol sampling devices available for personal exposure assessment have limited capability for detecting specific organisms, determining viability, or enumerating airborne concentrations.12,13
Thus, there is a range of challenges to developing a quantitative risk analysis for protecting workers during respiratory infectious disease outbreaks, including pandemics. Many key factors, including primary transmission routes, incubation periods, infectious doses, and the frequency of infections without symptoms (ie, asymptomatic infections) are often incompletely understood or difficult to discern precisely. Reed et al14 describe a risk assessment approach that broadly classifies severity level of a new influenza organism in the context of clinical manifestations and transmission using previous outbreaks as comparators, but they do not provide a method for determining individual-level risk based on varying levels of exposure to the emerging virus. For novel infectious disease outbreaks in which the organism’s epidemiology is evolving or not yet fully understood, a qualitative approach is needed to systematically analyze workers’ risks and identify appropriate controls.
This article sets forth a framework that outlines the concepts of control banding for workers in settings other than health care who are exposed to individuals who have active respiratory diseases that are known to be transmitted from person-to-person by aerosols. For example, the framework addresses diseases where aerosols are the primary form of transmission, such as tuberculosis (Mycobacterium tuberculosis) and rubeola (measles) virus, and diseases that are known or suspected to have a component of aerosol transmission, such as chickenpox (varicella) virus and influenza virus.
For the purposes of this article, aerosol transmissible diseases are defined using the terminology first presented by the Centers for Disease Control and Prevention (CDC) in an influenza transmission workshop summary14 and later incorporated into a report by the Institute of Medicine (now the National Academy of Medicine).15 Aerosol transmission involves person-to-person spread through the air by infectious aerosols ranging in diameter from a few nanometers to 100 μm, which are small enough to be in-haled and deposited in the respiratory system.15
This article proposes an initial framework for non-healthcare workers at all levels of risk and incorporates a range of control options, including PPE. We do not consider, however, the level or nature of PPE in detail; other available resources review PPE features and selection.16–18 In general, healthcare workers are in the highest risk category and are addressed elsewhere.4
When prioritizing controls during an infectious disease outbreak, all modes of transmission should be considered in determining how to protect workers. This article applies to all diseases with potential to spread via aerosols (obligate, preferential, or opportunistic), even if aerosol transmission is not the dominant mode of transmission.19 To determine the likelihood of aerosol transmission, we suggest using a systematic approach that considers all relevant experimental, toxicological, biological, and epidemiologic evidence, such as that described by Jones and Brosseau,20 especially during the early stages, when the epidemiology is incompletely defined, to avoid mischaracterization.
The primary audiences for this article are leaders in clinical medicine, public health, occupational health and safety, health policy, and infection control and other organizational decision makers who are involved in epidemic or pandemic preparedness. The resulting recommendations will be useful to employers, organizational leaders, and policymakers for emergency planning and preparedness well ahead of an outbreak or pandemic and should limit reliance on PPE.
We present here a framework on which disease-specific models and more precise decision algorithms may be added; we do not intend to provide guidance for public health actions or recommendations for individual patient management. To facilitate understanding by all readers and to avoid confusion, we use nomenclature that is not discipline specific. The proposed concepts could be used iteratively, periodically revisited before, during, and after an outbreak if the epidemiology, clinical manifestations, and ramifications become increasingly evident (Figure 1).
Figure 1.
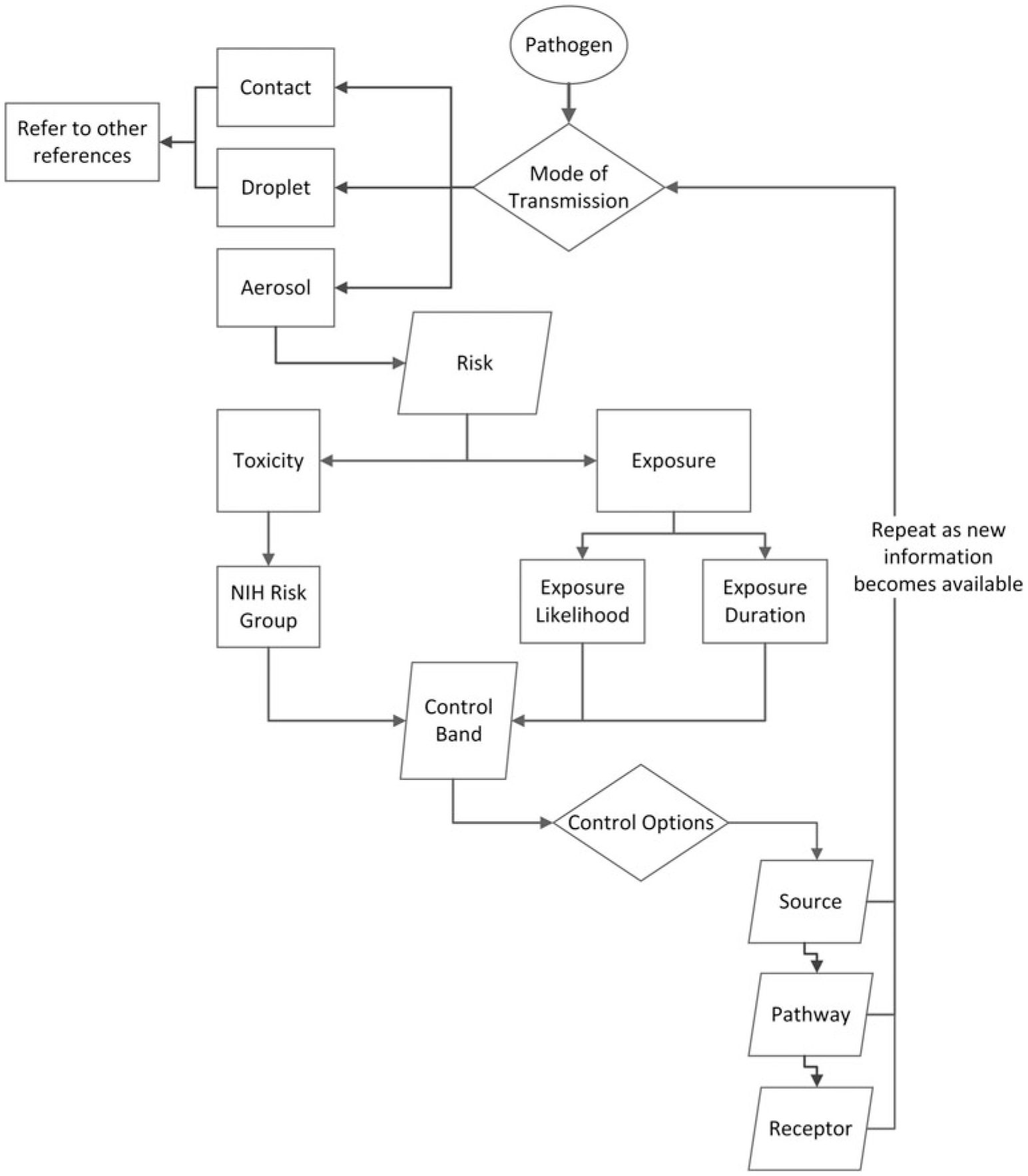
Aerosol control band process
Methods
Toxicity
Because quantitative values for microbial pathogen toxicity are generally not available, we use the 4 NIH risk groups as surrogates for infectious human pathogens (Table 1). These were originally developed to guide the selection of appropriate control options for laboratory research involving biological organisms.21 The NIH risk groups take into consideration both the degree of harm and the availability of preventive and treatment measures. They are tailored to the epidemiology of the US population and may be less applicable to populations in other geographic locations.22–26
Table 1.
Risk Group Definitionsa
| Risk Group | Definition |
|---|---|
| R1 | Agents not associated with disease in healthy adult humans (eg, asporogenic Bacillus subtilis or Escherichia coli if it does not possess a complete lipopolysaccharide) |
| R2 | Agents associated with human disease that is rarely serious and for which preventive or therapeutic interventions are often available (eg, Adenoviruses or Listeria) |
| R3 | Agents associated with serious or lethal human disease for which preventive or therapeutic interventions may be available (eg, SARS-CoV or Mycobacterium tuberculosis) |
| R4 | Agents likely to cause serious or lethal human disease for which preventive or therapeutic interventions are not usually available (eg, Ebola virus or Herpes B virus) |
Modified from NIH21
For novel or newly emerging organisms, the NIH risk group is often 1 or more levels higher than the endemic organism. For instance, seasonal influenza is in risk group 2, while novel influenza is in risk group 3. When the risk group for a novel organism has not yet been determined, it is generally appropriate to use the risk group of a similar organism. For example, before Middle East respiratory syndrome (MERS) was well characterized and assigned a risk group, it was typically assigned to the same category as other coronaviruses (risk group 2). It was reassigned to a higher level, risk group 3, after it was observed to have several characteristics in common with severe acute respiratory syndrome (SARS).27
Identifying the NIH risk group represents a disease-specific starting point. If an organism is novel or its reservoir is unknown, we suggest consulting with infectious disease peer-reviewed literature or subject matter experts to determine whether upward adjustment of the risk group may be appropriate. We propose using a precautionary approach and not lowering the risk group without formal guidance from a public health agency or other authority, because categorizing the risk group is a starting point for determining needed control banding measures, and it may result in increased adverse health outcomes when risks are underestimated.28
Exposure
The following exposure estimates assume an affected community is experiencing an infectious disease outbreak. For aerosol transmissible organisms, exposure depends on the likelihood of exposure (L) and the duration (D) of exposure to potentially infectious individuals:11
We suggest characterizing likelihood of exposure as unlikely (L1) for a worker who does not come into close contact with others who are possibly infectious, such as office workers who do not interact with many people or truck drivers who are alone in their cabs. A possible exposure (L2) would describe a worker who has numerous close contacts with many individuals whose infectious status is not known, but there are mitigating circumstances that would suggest that they have some potential to be infected (eg, they reside and circulate in a community where the outbreak is active). These workers may include those who interact with a large number of people every day, such as those in retail sales. A likely exposure (L3) would describe workers who are directly interacting with individuals who are known or likely to be infectious, such as a paramedic or healthcare provider.
The likelihood of infection would be increased for activities involving generation of or interactions with infectious aerosols. Respiratory viruses may be aerosolized via coughing, sneezing, vomiting, diarrhea, and, in rare cases, hemorrhage.12,13,19 Infectious aerosols may also be created during medical procedures (eg, intubation), laboratory manipulations (eg, centrifugation), and, in some circumstances, environmental cleaning of surfaces contaminated with infectious body fluids.13 Using the precautionary principle,29 if workers are likely to interact with potentially infectious fluids, such as cleaning vomit or soiled toilets, their work tasks may generate aerosols, increasing the likelihood for exposure. For example, a bus driver may be responsible for cleaning after a passenger vomits, increasing the exposure likelihood from L2 to L3. By assigning the highest level of likelihood of infection, one can implement control strategies for the worst-case scenario and scale back if warranted.
We define exposure duration (D) as the number of hours in an 8-hour workday an employee is likely to be in contact with potentially infectious individuals, with 3 categories ranging from low (D1 ≤ 3 hours) to medium (D2 = 3 to 6 hours) to high (D3 ≥ 6 hours). Since we are not aware of data specifically supporting this or other categorizations, this approach is intended to be a rational foundation subject to modification and future validation. For example, if workdays are longer than 8 hours, the percent time may be a more appropriate metric (eg, 40%, 75%, >75%).
We suggest treating exposure (E) as a multiplicative function of exposure likelihood (L) and duration (D) (Table 2).
Table 2.
Exposure
| Daily Duration a | |||
|---|---|---|---|
| D3 (>6 hours) | |||
| L1 (unlikely exposure) | E1 | E1 | E1 |
| L2 (possible exposure) | E2 | E2 | E3 |
| L3 (likely exposure) | E2 | E3 | E3 |
Number of potential exposure hours per 8-hour workday.
Control Band
The control banding approach presented here is intended to apply to healthy workers who do not have preexisting health conditions that may impair immune function or other host defenses against aerosol transmissible infectious diseases. Using the matrix shown in Table 3, the appropriate control band may be selected based on the risk group (R1 to R4) and exposure (E1 to E3). Special accommodations or higher levels of control may be necessary for workers who are at increased risk of acquiring infections or developing complications from infections.
Table 3.
Control Band
| Risk | ||||
|---|---|---|---|---|
| Exposure | R1 | R2 | R3 | R4 |
| E1 | A | A | B | B |
| E2 | A | B | B | C |
| E3 | B | B | C | C |
Controls
We propose using a hierarchy of controls that focuses first on source, followed by pathway, and finally receptor control.30,31 This approach conceptually simplifies methods of exposure control and may be used to select a control or set of controls, starting with options that eliminate aerosols at the source (Table 4). Subsequently, controls that interrupt the pathway from the infectious source to the receptor (ie, worker) may be selected. The epidemiology of pathogens may change over time due to ecological pressures, such as antimicrobial resistance or genetic drift; however, these pathogen-specific changes are typically identified by clinical and public health laboratories weeks or months after they begin to occur. In the meantime, employers may need to make immediate or rapid decisions to protect workers based on available data. In such instances, for all control bands in our proposed framework, a key goal is to reduce the exposure level as much as possible, preferably to E1, using available control options.
Table 4.
Selection of Controls
| Banda | Control Options |
|---|---|
| A | Source first |
| Pathway second—generally prudent | |
| Receptor controls—generally not necessary | |
| B | Source first—may require multiple options |
| Pathway second—may require multiple options | |
| Receptor controls—if source and pathway are not effective | |
| C | Source first—may require multiple options |
| Pathway second—may require multiple options | |
| Receptor controls—generally prudent |
If higher precautions are desired, such as during periods of high uncertainty, the control band may be voluntarily elevated.
Source Control
Source control may include removal or reduction of exposure at the source (infected person), such as precluding those who are ill from attendance at social gatherings, equipping ill individuals with facemasks to block exhaled particles or expelled secretions, administering vaccinations to those who may become sources, and cohorting or isolating symptomatic people in separate locations.
Pathway Control
These controls interrupt the pathway between the source (infected individuals) and the receptor (workers). They may include constructing barriers between the source and receptor, requiring symptomatic individuals to reside in well-ventilated spaces, or changing policies, procedures, or work practices.
Receptor Control
These controls prevent exposure at the receptor (worker) and may include changing work practices or task design to prevent or minimize close interactions with the source, placing the employee in a physical enclosure, or requiring the employee to wear PPE.
Other types of controls should be applied before selecting those that prevent exposure at the receptor (worker). Consistent with previously published guidance,32,33 selecting PPE is last in our proposed hierarchy, primarily because it requires user acceptance and proper use to be effective and may introduce other hazards by interfering with job performance.34 For aerosol exposures, the most applicable PPE will likely be a respirator, although other types of PPE such as surgical masks, face shields, and eye protection may also be appropriate, especially if droplet or contact modes of transmission are known or suspected. Other resources are available for selecting the correct respirator for a particular infectious disease exposure.16,18
The control band should be used to identify workers in the highest risk categories. Employers with workers in control band A may be able to reduce exposure levels primarily using source controls and, when necessary, some pathway controls. Employers with workers in control band B may be able to reduce exposure levels using source and pathway controls, although some workers may require the use of multiple control options or, as a last resort, receptor controls. Employers with workers in control band C may aim to reduce exposure as much as possible using source and pathway controls, although receptor controls are likely to be necessary.
Since we cannot typically change the properties of the organism or the severity of disease that it may cause, it may not be possible to reduce the risk band of an organism. We emphasize reduction of risk via a decrease in the likelihood or duration of exposure. The principal goal of our framework is to reduce exposure to E1 levels, by selecting additional control strategies from the source and pathway categories and reducing reliance on PPE.
Case Examples
Our proposed approach to control banding is intended to serve as an initial framework for future development of decision algorithms and validation studies. To begin advancing this construct, we offer the following hypothetical case examples, drawn from past events. The following examples do not necessarily represent official US government policies or positions; rather, they are presented as a basis for further discussion and deliberation.
Example 1. A package delivery person during a human coronavirus outbreak
A delivery person would be expected to interact with few people per day, mostly leaving packages on porches or entryways, and occasionally requesting a signature from an intended receiver to verify successful delivery. Early information received about the disease suggests it is less toxic than SARS or MERS and less likely to be aerosol transmissible.
Control Band Calculation
Risk Group:
R2—Human coronavirus is in risk group 2.21
Exposure Likelihood:
L1—The delivery person interacts with few people each workday, most of whom are expected to be healthy.
Duration:
D3—We assume the delivery person works for 10 hours per day, placing him or her in D3 (high duration).
Exposure:
E1—Combining the exposure likelihood (L1, unlikely exposure) and exposure duration (D3, high duration) results in an E1 exposure (low exposure) (see Table 2).
Control Band:
A—Risk group R2 and exposure E1 result in control band A (Table 3). This may require pathway controls, but receptor controls are generally not necessary.
Goal:
Exposure level is already in the lowest category and controls might not be necessary; however, employers can choose to implement additional measures. A possible source control would be reducing interactions with package receivers when possible (eg, leave package near door, ring bell to notify recipient, leave before recipient arrives at door). A possible pathway control would use the door as a barrier between the recipient and the deliverer and hand off the signature tablet without coming face-to-face with the recipient.
Example 2. A maintenance worker in a primary school (K-5) during a novel influenza outbreak
A maintenance worker typically interacts with small numbers of people throughout the workday but may interact with 1 or more children who are ill with respiratory infection. Ill children might have a productive cough or vomit, producing aerosols. Additionally, cleaning methods could re-aerosolize the organism.
Control Band Calculation
Risk Group:
R3—Novel influenza is in risk group 3.21
Exposure Likelihood:
L2—Although a janitor would be expected to interact with few people throughout the day (L1), he or she may assist (or clean soiled areas contaminated by) ill individuals, raising the level to L2.
Duration:
D1—We assume a janitor works about 8 hours per day but might be exposed to aerosolized particles for ≤1 hour per day, placing him or her in D1 (low duration).
Exposure:
E2—Combining the exposure likelihood (L2, possible exposure) and exposure duration (D1, low duration) results in an E2 exposure (medium exposure) (Table 2).
Control Band:
B—Risk group R3 and exposure E2 result in control band B (Table 3). This may require multiple control options; receptor controls are required only if source and pathway controls are not adequate.
Goal:
The goal is to reduce the exposure level from E2 to E1.
Selecting Controls
Source Controls:
Excluding symptomatic students from school or screening students before school entry might reduce a maintenance worker’s likelihood of interacting with infectious aerosols.
Effect of Source Control:
If most infectious students are successfully excluded from entering the school, the exposure likelihood can be reduced from L2 (possible exposure) to L1 (unlikely exposure).
Has exposure been reduced to the lowest level, E1?
Combining exposure likelihood of L1 (unlikely exposure) and exposure duration of D1 (low duration) should result in exposure of E1 (low exposure). Pathway and receptor controls may not be necessary.
Pathway Controls:
If concerns remain about the number of ill students who continue to attend school, revised cleaning methods that lower opportunities for interaction with aerosols could be considered. In addition to revision of cleaning methods to ensure the vomit is covered, ventilation in the area could be increased for some period before the janitor cleans to reduce the presence of aerosols.
Effect of Pathway Control:
Revised cleaning methods and ventilation might reduce the exposure likelihood, from L2 (possible exposure) to L1 (unlikely exposure).
Has exposure been reduced to the lowest level (E1)?
Combining exposure likelihood L1 (unlikely exposure) with exposure duration D3 (high duration) reduces exposure from E3 to E1.
Receptor Controls:
Receptor controls might not be necessary.
Example 3. During a tuberculosis outbreak, a correctional officer working full-time in a prison is closely interacting with infected prisoners who are housed in isolation rooms
A correctional officer is expected to interact with many people throughout the workday, only a small number of whom might be infectious during a tuberculosis outbreak in the facility.
Control Band Calculation
Risk Group:
R3—Tuberculosis is in risk group 3.21
Exposure Likelihood:
L2 (possible exposure)—The correctional officer’s responsibilities can include transporting inmates, which involves close interaction with inmates with known or suspected tuberculosis.
Duration:
D3—We assume a part-time prison guard works about 8 hours per day, placing him or her in category D3 (high duration).
Exposure:
E3—Combining an exposure likelihood of L2 (possible exposure) and a duration of D3 (high duration) results in an E3 exposure (medium exposure) (Table 2).
Control Band:
C—Risk group R3 and exposure E3 result in control band C (Table 3). Receptor controls are generally prudent.
Goal:
The goal is to reduce the exposure level from E3 to E1.
Selecting Controls
Source Controls:
The prison administration could consider locating prisoners with exposure to patients with known infection in a separate area until absence of infection is confirmed.
Effect of Source Control:
Isolation of the prisoner might reduce the exposure of other people at the prison but would not be expected to successfully reduce the exposure of a correctional officer who is required to interact with inmates while they are in an isolation room; the E3 stays at E3.
Pathway Controls:
Isolation in a separate and well-ventilated room may diminish distribution of infectious aerosols, protecting other inmates and workers.
Effect of Pathway Control:
Pathway controls will not reduce the exposure for a prison guard required to interact with inmates while they are in an isolation room; exposure remains at E3.
Receptor Controls:
The number of guards who are permitted to interact with infected inmates could be reduced by assigning a small group of them to work in the isolation ward. Guards who interact with infectious inmates in isolation rooms would have increased risk and would be required to wear a respirator.
Effect of Receptor Control:
Protecting the receptor could lower the exposure likelihood from L2 to L1 through controls that limit interaction with infectious individuals or through the applicable use of a respirator. An exposure likelihood of L1 combined with an exposure duration of D3 results in an exposure of E1.
Summary and Conclusions
In this article, we propose a systematic semi-quantitative procedure for risk analysis based on a control-banding framework. The process provides a means to analyze exposure risk from aerosol transmissible infectious pathogens. The process enables an initial assessment when various factors concerning an outbreak (ie, virulence, infectivity, and transmissibility) are poorly understood. It also could provide a transparent decision framework for recommending different levels of exposure control and changes to those levels, as new data become available.
Throughout we emphasize the importance of lowering workers’ exposure to infectious aerosols by diminishing the likelihood, frequency, and duration of their contacts with infectious individuals. While this framework may not satisfy all possible jobs and tasks, it does represent an initial systematic approach for risk assessors and risk managers that will be further refined with disease-specific algorithms and subjected to empiric validation in advance of the quantitative algorithms.
To demonstrate how this process is used, we present 3 case examples of hypothetical exposure scenarios. Users of the framework choose source, pathway, and receptor exposure conditions that best describe their employees and particular workplace situations: (1) a package deliverer during a coronavirus outbreak; (2) a school maintenance worker during novel influenza; and (3) a correctional officer during a tuberculosis outbreak at the facility.
Risk managers and other decision makers are encouraged to consider a priori source and pathway controls whenever possible during pandemic preparedness planning and whenever implementing a control banding process. Using control banding during planning activities should lead to implementation of source and pathway controls that lower job and task exposures during an outbreak, thus limiting the number of people required to use PPE.
We expect available information about the epidemiology of new or novel pathogens, including severity and disease transmission characteristics, to be limited at first, but rapidly evolving in the early stages of a contagious outbreak. Planning for the implementation of control banding may diminish the number of infected people and slow the progression of the spreading epidemic.35 As more is learned about infectivity, virulence, and transmission modes, the control banding process may be repeated iteratively, incorporating new and evolving information to refine and optimize control methods.
A control banding approach has both strengths and limitations. First, the process guides the risk assessor to think through all of the relevant factors that affect the risk of contracting the disease—a checklist approach. Second, it provides transparency and documentation on why a particular risk management strategy was chosen. Third, the process allows for decision making before an outbreak event occurs, or early in an outbreak situation, and facilitates rational resource allocation. Once a decision is made to use control banding, focusing on each aspect of exposure separately (frequency, duration, and concentration), a broader range of control options may be considered.
This proposed framework also has several limitations—primarily the lack of formal validation of our proposed framework. The qualitative criteria presented here need to be evaluated and perhaps adjusted so that the outcome control bands fit within norms learned from experience with past outbreaks. Another limitation is the challenge of tailoring control banding approaches to each setting, since generalizing an approach for one setting to another may not be appropriate. It will be important to test this approach with stake-holders to assess feasibility and acceptability of the framework. Other variables could affect the likelihood of exposure, such as contact with infectious people outside of the workplace, failure to account for work tasks or activities that involve interactions with infectious people, and failure of controls to operate as expected. Finally, we have not accounted for other modes of transmission; this would require a more complex model beyond the goals and timeframe of this project.
While the framework does not describe specific solutions for workplaces, it allows for identification of workers in the highest risk categories and for employers to be innovative in their selection of the best control measures for their organization. In some instances, especially when empiric data were lacking, we used professional judgment for ranking exposure variables. Further development of decision tools and scientific study are necessary to operationalize our proposed framework.
The demand for respiratory protective devices is likely to outstrip supply during a novel infectious disease outbreak. To conserve limited resources, a rational approach is needed for ensuring that all workers are protected. The process we have proposed is an initial framework for risk managers and other decision makers to employ in the context of preparedness and response.
Acknowledgments
This work was supported by NIOSH contract 200-2016-M-91200 and IPA 16IPA1616802. We acknowledge Harold Boyles for his review of the paper. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the National Institute for Occupational Safety and Health or the Centers for Disease Control and Prevention.
Contributor Information
Margaret Sietsema, Environmental and Occupational Health Sciences, School of Public Health, University of Illinois at Chicago..
Lew Radonovich, National Personal Protective Technology Laboratory, National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention, Pittsburgh, PA..
Frank J. Hearl, National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention, Washington, DC..
Edward M. Fisher, National Personal Protective Technology Laboratory, National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention, Pittsburgh, PA..
Lisa M. Brosseau, Environmental and Occupational Health Sciences, School of Public Health, University of Illinois at Chicago..
Ronald E. Shaffer, National Personal Protective Technology Laboratory, National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention, Pittsburgh, PA..
Lisa M. Koonin, Influenza Coordination Unit, National Center for Immunizations and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, GA..
References
- 1.Evans DK, Goldstein M, Popova A. Health-care worker mortality and the legacy of the Ebola epidemic. Lancet Glob Health 2015;3(8):e439–e440. [DOI] [PubMed] [Google Scholar]
- 2.Buxton Bridges C, Katz JM, Seto WH, et al. Risk of influenza A (H5N1) infection among health care workers exposed to patients with influenza A (H5N1), Hong Kong. J Infect Dis 2000;181(1):344–348. [DOI] [PubMed] [Google Scholar]
- 3.Thompson WW, Shay DK, Weintraub E, et al. Influenza-associated hospitalizations in the United States. JAMA 2004; 292(11):1333. [DOI] [PubMed] [Google Scholar]
- 4.Occupational Safety and Health Administration. Guidance on Preparing Workplaces for an Influenza Pandemic. 2009. https://www.osha.gov/Publications/OSHA3327pandemic.pdf. Accessed February 27, 2109.
- 5.World Health Organization. Disease outbreaks. http://www.who.int/topics/disease_outbreaks/en/. Accessed February 27, 2109.
- 6.Medicinenet.com. Definition of pandemic. Updated 2016. https://www.medicinenet.com/script/main/art.asp?articlekey=4751. Accessed February 27, 2109.
- 7.Beckman S, Materna B, Goldmacher S, et al. Evaluation of respiratory protection programs and practices in California hospitals during the 2009–2010 H1N1 influenza pandemic. Am J Infect Control 2013;41(11):1024–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carias C, Rainisch G, Shankar M, et al. Potential demand for respirators and surgical masks during a hypothetical influenza pandemic in the United States. Clin Infect Dis 2015; 60(Suppl 1):S42–S51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Srinivasan A, Jernign DB, Liedtke L, Strausbaugh L. Hospital preparedness for severe acute respiratory syndrome in the United States: views from a national survey of infectious diseases consultants. Clin Infect Dis 2004;39(2):272–274. [DOI] [PubMed] [Google Scholar]
- 10.Zalk DM, Nelson DI. History and evolution of control banding: a review. J Occup Environ Hyg 2008;5(5):330–346. [DOI] [PubMed] [Google Scholar]
- 11.Milz S, Conrad R, Soule R. Principles of evaluating worker exposure. In: Anna D, ed. The Occupational Environment: Its Evaluation, Control, and Management. 3rd ed. Fairfax, VA: AIHA Press; 2003:114–127. [Google Scholar]
- 12.Lindsley WG, Noti JD, Blachere FM, et al. Viable influenza A virus in airborne particles from human coughs. J Occup Environ Hyg 2015;12(2):107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burge HA, ed. Bioaerosols. Boca Raton, FL: CRC Press, Inc; 1995. [Google Scholar]
- 14.Reed C, Angulo FJ, Swerdlow DL, et al. Estimates of the prevalence of pandemic (H1N1) 2009, United States, April-July 2009. Emerg Infect Dis 2009;15(12):2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Snider DE, Bridges CB, Weissman DN. Meeting summary of the workshop “Approaches to better understand human influenza transmission.” November 4–5, 2010. https://www.cdc.gov/influenzatransmissionworkshop2010/pdf/Influenza_Transmission_Workshop_Summary_508.pdf. Accessed February 27, 2109.
- 16.Lavoie J, Cloutier Y, Lara J, Marchand G. Guide on Respiratory Protection Against Bioaerosols: Recommendations on Its Selection and Use. Montreal: IRSST Publications; 2007. [Google Scholar]
- 17.Lenhart SW, Seitz T, Trout D, Bollinger N. Issues affecting respirator selection for workers exposed to infectious aerosols: emphasis on healthcare settings. Appl Biosaf 2004;9(1):20–36. [Google Scholar]
- 18.McCullough NV, Brosseau LM. Selecting respirators for control of worker exposure to infectious aerosols. Infect Control Hosp Epidemiol 1999;20(2):136–144. [DOI] [PubMed] [Google Scholar]
- 19.Roy CJ, Milton DK. Airborne transmission of communicable infection—the elusive pathway. N Engl J Med 2004; 350(17):1710–1712. [DOI] [PubMed] [Google Scholar]
- 20.Jones RM, Brosseau LM. Aerosol transmission of infectious disease. J Occup Environ Med 2015;57(5):501–508. [DOI] [PubMed] [Google Scholar]
- 21.American Biological Safety Association. Risk group database. https://my.absa.org/Riskgroups. Accessed February 27, 2109.
- 22.European Agency for Safety and Health at Work. Directive 2000/54/EC biological agents at work: ‘On the protection of workers from risks related to exposure to biological agents at work.’ 2000. https://osha.europa.eu/en/legislation/directives/exposure-to-biological-agents/77. Accessed February 27, 2109.
- 23.Australian/New Zealand Standard. Safety in laboratories. Part 3: Microbiological aspects and containment facilities. 2010:AS/NZS 2243.3. https://www.saiglobal.com/pdftemp/previews/osh/as/as2000/2200/22433.pdf. Accessed February 27, 2109.
- 24.Biosafety and Biotechnology Unit. Belgian classifications for micro-organisms based on their biological risks. 2006. https://www.biosafety.be/content/tools-belgian-classification-micro-organisms-based-their-biological-risks. Accessed February 27, 2109.
- 25.Canadian Biosafety Standard. 2d ed. 2016. https://www.canada.ca/en/public-health/services/canadian-biosafety-standards-guidelines/second-edition.html. Accessed March 27, 2019. [Google Scholar]
- 26.World Health Organization. Laboratory Biosafety Manual. 2004. https://www.who.int/csr/resources/publications/biosafety/WHO_CDS_CSR_LYO_2004_11/en/. Accessed February 27, 2109.
- 27.US Department of Health and Human Services. Middle East respiratory syndrome cornoavirus (MERS-CoV). Federal Regist 2013;215:6675. [Google Scholar]
- 28.US Department of Health and Human Services, Centers for Disease Control and Prevention, National Institutes of Health. Biosafety in Microbiological and Biomedical Laboratories. 5th ed. Washington, DC: HHS; 2009. [Google Scholar]
- 29.Kriebel D, Tickner J, Epstein P, et al. The precautionary principle in environmental science. Environ Health Perspect 2001;109(9):871–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tielemans E, Schneider T, Goede H, et al. Conceptual model for assessment of inhalation exposure: defining modifying factors. Ann Occup Hyg 2008;52(7):577–586. [DOI] [PubMed] [Google Scholar]
- 31.Smith TJ, Hammond SK, Hallock M, Woskie SR. Exposure assessment for epidemiology: characteristics of exposure. Appl Occup Environ Hyg 1991;6(6):441–447. [Google Scholar]
- 32.Siegel JD, Rhinehart E, Jackson M, Chiarello L; Healthcare Infection Control Practices Advisory Committee. 2007 guidelines for isolation precautions: preventing transmission of infectious agents in healthcare settings. 2007. https://www.cdc.gov/niosh/docket/archive/pdfs/NIOSH-219/0219-010107-siegel.pdf. Accessed February 27, 2109. [DOI] [PMC free article] [PubMed]
- 33.Jensen PA, Lambert LA, Iademarco MF, Ridzon R. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep 2005;54(RR-17):1–141. [PubMed] [Google Scholar]
- 34.Centers for Disease Control and Prevention. Hierarchy of controls. Updated 2017. https://www.cdc.gov/niosh/topics/hierarchy/default.html. Accessed February 27, 2109.
- 35.Holloway R, Rasmussen SA, Zaza S, Cox NJ, Jernigan DB. Updated preparedness and response framework for influenza pandemics. MMWR Recomm Rep 2014;63(RR-06):1–18. [PubMed] [Google Scholar]


