Summary
Background
Recurrences of herpes simplex virus (HSV) in the orofacial region (herpes labialis or cold sores) impact quality-of-life. We aimed to study whether the bacille Calmette-Guérin (BCG) vaccine can attenuate cold sore recurrences through off-target immunomodulatory effects.
Methods
In this nested randomised controlled trial within the multicentre, phase 3 BRACE trial, 6828 healthcare workers were randomised in 36 sites in Australia, the Netherlands, Spain, the United Kingdom and Brazil, to receive BCG-Denmark or no BCG (1:1 ratio using a web-based procedure) and followed for 12 months with 3-monthly questionnaires. Exclusion criteria included contraindication to BCG vaccine or previous vaccination with BCG within the past year, any other live-attenuated vaccine within the last month, or any COVID-specific vaccine. The intervention group received one intradermal dose of 0.1 mL of BCG-Denmark corresponding to 2−8 x 105 colony forming units of Mycobacterium bovis, Danish strain 1331. The primary outcome was the difference in restricted mean survival time (i.e., time to first cold-sore recurrence), in participants with frequent recurrent herpes labialis (≥4 recurrences/year), analysed by intention-to-treat. Secondary outcomes addressed additional questions, including analyses in other sub-populations. Adverse events were monitored closely during the first 3 months and were reported in all participants who received one dose of study drug according to intervention received. The BRACE trial is registered with ClinicalTrials.gov, NCT04327206.
Findings
Between March 30, 2020 and February 18, 2021, 84 individuals with frequent recurrent cold sores were randomly assigned to BCG (n = 38) or control (n = 46). The average time to first cold-sore recurrence was 1.55 months longer in the BCG group (95% CI 0.27–2.82, p = 0.02) than the control group (hazard ratio 0.54, 95% CI 0.32–0.91; intention-to-treat). The beneficial effect of BCG was greater in the as-treated population (difference 1.91 months, 95% CI 0.69–3.12, p = 0.003; hazard ratio 0.45, 95% CI 0.26–0.76). In prespecified subgroup analyses, only sex modified the treatment effect (interaction p = 0.007), with benefit restricted to males. Over 12 months, a greater proportion of participants in the BCG group compared with the control group reported a decrease in duration (61% vs 21%), severity (74% vs 21%), frequency (55% vs 21%), and impact on quality of life (42% vs 15%) of cold sore recurrences. In participants who had ever had a cold sore, there was also a decrease in self-reported burden of recurrences in the BCG group. In participants who had never had a cold sore, there was an increased risk of a first episode in the BCG group (risk difference 1.4%; 95% CI 0.3–2.6%, p = 0.02). There were no safety concerns.
Interpretation
BCG-Denmark vaccination had a beneficial effect on herpes labialis, particularly in males with frequent recurrences, but may increase the risk of a first cold sore.
Funding
Bill & Melinda Gates Foundation, the Minderoo Foundation, Sarah and Lachlan Murdoch, the Royal Children's Hospital Foundation, Health Services Union NSW, the Peter Sowerby Foundation, SA Health, the Insurance Advisernet Foundation, the NAB Foundation, the Calvert-Jones Foundation, the Modara Pines Charitable Foundation, the UHG Foundation Pty Ltd, Epworth Healthcare, and individual donors.
Keywords: Herpes simplex virus, Cold sore, Herpes labialis, Bacille Calmette-Guérin, Mycobacterium bovis, Prevention, Secondary prophylaxis, Off-target effects, Non-specific effects, Heterologous immunity
Research in context.
Evidence before this study
In a recent systematic review, updated in April 2023, vaccination with bacille Calmette-Guérin (BCG) was found to be beneficial in 78% of individuals with recurrent herpes labialis or genitalis. Albeit promising, these results were mainly from observational studies done in the 1970s and have never been confirmed in a randomised controlled trial. One previous trial, restricted to patients with herpes genitalis, had a number of limitations, in particular the use of a potentially beneficial intervention in the control group.
Added value of this study
In a randomised controlled trial to assess whether BCG vaccination protects against COVID-19, we did a nested sub-study to determine the effect of BCG vaccination on attenuating cold sore (herpes labialis) recurrences in individuals with frequent recurrent cold sore episodes. We found a 53% increase in the mean time spent without a first recurrence over 12 months following BCG-Denmark vaccination, and a significant decrease in the self-reported burden of cold sore recurrences. We also observed a small increased risk of reporting a first cold sore episode in the BCG group.
Implications of all the available evidence
BCG-Denmark vaccination attenuates recurrences of herpes labialis, particularly in males and in those with frequent recurrences. Future studies should focus on other populations in which herpes recurrence has a significant impact, including children (in whom cold sore recurrences impact feeding and schooling) and individuals with recurrent herpes genitalis.
Introduction
Recurrent herpes labialis (‘cold sore’) is a common condition caused by reactivation of the herpes simplex virus (HSV) in the orofacial region.1 HSV-1 is endemic worldwide, infecting up to 95% of individuals,2 of whom between 14 and 40% have frequent recurrent cold sores.3 Recurrences can be debilitating and significantly reduce quality of life. Long-term suppressive therapy with an oral antiviral is somewhat effective in reducing the burden of HSV recurrences, but is costly, and involves the risk of adverse events.4, 5, 6 There is still no vaccine available against HSV.7
In addition to its intended effects, the anti-tuberculosis vaccine, bacille Calmette-Guérin (BCG), induces off-target (‘non-specific’) effects that confer additional benefits.8 Examples of these immunomodulatory effects include reduction in all-cause mortality in infants,9 prevention and treatment of certain cancers,10,11 protection against progression of autoimmune diseases,12 and protection against a variety of viral infections.13,14 Observational studies suggest this includes the prevention of HSV recurrences.14 In a systematic review of observational studies, BCG vaccination was found to be beneficial in 78% of individuals with recurrent herpes labialis or genitalis, with 37% being recurrence-free for an extended period, 41% experiencing less frequent or severe episodes, and only 22% reporting no change.14 Albeit promising, these results have never been confirmed in a randomised controlled trial (RCT). The only RCT published to date, restricted to patients with herpes genitalis, had a number of limitations, in particular the use of a potentially beneficial intervention in the control group.15
This study aimed to determine in a subgroup of adults with recurrent cold sores whether BCG vaccination compared with placebo reduces HSV recurrences in the BRACE RCT (BCG vaccination to reduce the impact of COVID-19 in healthcare workers).16 Secondary aims addressed a wider range of objectives, including the impact of BCG on the burden of recurrences and the risk of a first cold sore episode in sub-groups defined by differing frequencies of HSV recurrence.
Methods
Trial design, setting, participants and intervention
The BRACE trial is a multicentre phase 3 RCT that enrolled healthcare workers in 36 sites in Australia, the Netherlands, Spain, the United Kingdom and Brazil, which assessed whether BCG vaccination protects against COVID-19. The BRACE trial primary outcomes and protocol have been previously published (ClinicalTrials.gov NCT04327206).16,17 Briefly, exclusion criteria included contraindication to BCG vaccine or previous vaccination with BCG within the last year, any other live-attenuated vaccine within the last month, or any COVID-specific vaccine. Participants were randomised in a 1:1 ratio using a web-based procedure (REDCap®).18 Those in the intervention group received one intradermal injection of 0.1 mL of BCG-Denmark (AJ Vaccines, Copenhagen; corresponding to 2−8 x 105 colony forming units of Mycobacterium bovis, Danish strain 1331; batch numbers detailed in the Appendix). Participants randomised to the control group received either no intervention (defined as BRACE stage 1, recruited before May 2020) or placebo saline intradermal injection (defined as BRACE stage 2, recruited after May 2020).16 Participants were followed up for 12 months, and adverse events monitored closely during the first 3 months.16,19
Ethics
The study was approved by the Royal Children's Hospital Melbourne Human Research Ethics Committee (No. 62586); the protocol was approved by the ethics committee at each site and all participants provided informed consent.
Outcome measures
Questionnaires were completed by participants at baseline and at 3, 6, 9, and 12-months of follow-up. In the baseline questionnaire, participants were asked demographic questions (including sex) and details about their previous history of cold sores, including age at first episode, number of cold sore episodes (overall and in the last year), use of treatment for cold sores, and impact of cold sores on quality of life. In the follow-up questionnaires, participants were asked whether they had had a cold sore recurrence since their last completed questionnaire, the frequency of episodes, when the first recurrence began, any treatment used for cold sores, and whether they noticed a change in cold sore recurrences in terms of frequency, duration, severity, and impact on quality of life.
The primary outcome for this nested study was the time from randomisation to first self-reported cold sore recurrence in the subset of participants with frequent recurrent herpes labialis, defined as reporting four or more cold sore recurrences in the preceding year at the baseline questionnaire. Secondary outcomes included the number of cold sore recurrences, proportion of participants with a cold sore recurrence, and proportion of participants with a perceived change in duration, severity, frequency, or impact on quality of life of cold sore recurrences (see Appendix).
Statistical analysis
The statistical analysis plan was finalised before unblinding (see Appendix). Sample size calculations were based on the primary outcomes of incidence of severe and symptomatic COVID-19 in the BRACE trial.17 Participants were included under their randomised treatment group, as per the intention-to-treat principle. Our target estimate was the difference in restricted mean survival time (RMST, i.e., time free from first cold sore recurrence) over 12-months of follow-up and 95% confidence intervals (CI), estimated using a flexible parametric survival (Royston-Parmar) model on the log cumulative hazard scale, standardised for stratification factors used during randomisation (age group, presence of comorbidity, geographical location, and study stage).20 Assuming the proportional hazards assumption was found to be reasonable, we also presented hazard ratios (HR) and their corresponding 95% CI. Participant data were censored at 12-months of follow-up; otherwise, data were censored at loss to follow-up or at the time of latest survey completion date without missing outcome data. Therefore, RMST can be interpreted as the average time before first cold sore recurrence restricted to 12 months follow-up (or to 12 months follow-up in those without recurrence). For clarity, this will be referred to as the ‘time to first cold sore recurrence’.
Binary outcomes were summarised as between group model-fitted marginal risk differences (RD) and 95% CI estimated using binomial regression models adjusted for stratification factors. The frequency of cold sore episodes over 12 months was summarised as an incidence rate ratio (IRR) and 95% CI estimated using a negative binomial regression model adjusted for stratification factors.
Supplementary analyses were done in different subsets of participants: (i) in participants who ever had a cold sore (i.e., who reported having any number of previous cold sore episodes at baseline); (ii) in participants who reported 1 or more cold sore episodes in the preceding year; (iii) in participants who reported 2 or more cold sore episodes in the preceding year; and (iv) in participants who previously never had a cold sore. Sensitivity analyses were done: (i) limiting data to that ascertained through 3-month recall; (ii) in hypothetical scenarios in which suppressive therapy was not available (censoring data at initiation of suppressive therapy); and (iii) in the as-treated population, with participants analysed according to the intervention they received.
Subgroup analyses were done for the primary outcome, including by: (i) sex (male/female); (ii) history of previous BCG (BCG-naïve/previous BCG); (iii) geographical location (Australia/Brazil/Europe); (iv) age (<40 years/40–59 years/≥60 years); (v) age at first cold sore episode (0 to 5/6 to 12/13 to 18/>18 years-old); and (vi) study stage (stage 1/stage 2). Heterogeneity of the treatment effect by subgroup was assessed through inclusion of an interaction term (treatment x subgroup) within the flexible parametric survival model.
Multiple imputation was used for the binary and count outcomes when >10% of participants were missing (see Appendix). All p values are two-sided. All analyses were done using Stata v16.1.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
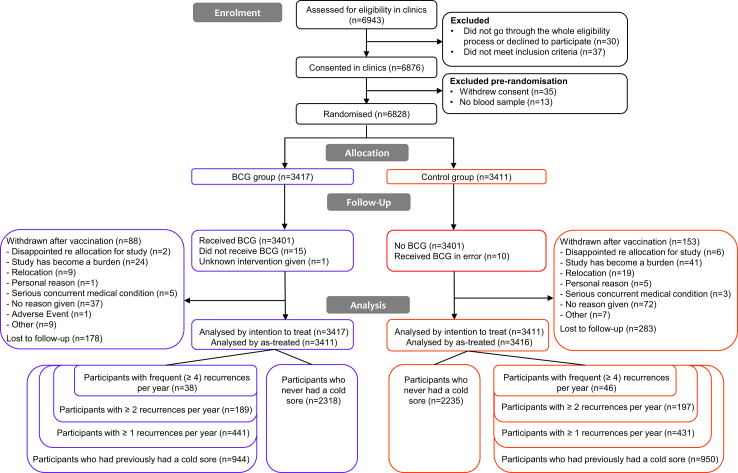
A total of 6828 participants were included in the BRACE trial between March 30, 2020 and April 1, 2021, 3417 in the BCG group and 3411 in the control group (Fig. 1). They had a mean age of 42 years (standard deviation 12 years) and were predominately female (75%). Their characteristics are detailed in the Appendix.
Fig. 1.
Trial profile.
Of the 6447 participants who answered the baseline HSV questions, 1894 (29.4%) participants reported having had at least one cold sore episode in their life and, of these, 84/1633 (5.1%) (1.4% of all participants) reported frequent episodes with four or more recurrences in the year preceding inclusion. Age at first episode, number of cold sore episodes in the last year, and self-reported impact of cold sores on quality of life are detailed in Table 1. During follow up, 30 participants with frequent recurrent cold sores reported using oral therapy for cold sores: 19 in the BCG group and 11 in the control group (including 4/19 (21%) and 5/11 (45%) using long-term therapy to prevent recurrences, respectively).
Table 1.
Participants characteristics at baseline and during the trial.
| Participants with frequent recurrent cold sores |
||
|---|---|---|
| Control | BCG | |
| Baseline characteristics | ||
| Participants | 46 | 38 |
| Sex, female | 41/46 (89%) | 34/38 (89%) |
| Age, years | 43.6 (11.8) | 41.8 (10.6) |
| Presence of comorbidities | 10/46 (22%) | 5/38 (13%) |
| Geographical location | ||
| Australia | 30/46 (65%) | 26/38 (68%) |
| Europe | 10/46 (22%) | 7/38 (18%) |
| South America | 6/46 (13%) | 5/38 (13%) |
| Personal history of BCG or tuberculosis | ||
| BCG in the past | 22/46 (52%) | 20/38 (53%) |
| Lived in tuberculosis endemic country | 5/46 (11%) | 3/38 (8%) |
| Previous known latent tuberculosis infection | 0/46 (0%) | 0/38 (0%) |
| Previous positive tuberculin skin test (>5 mm) | 4/46 (9%) | 5/38 (13%) |
| Personal history of cold sores | ||
| Ever had a cold sore | 46/46 (100%) | 38/38 (100%) |
| Age at first episode of cold sore | ||
| <1 year old | 1/43 (2%) | 1/38 (3%) |
| 1–5 years old | 10/43 (23%) | 9/38 (24%) |
| 6–12 years old | 11/43 (26%) | 8/38 (21%) |
| 13–18 years old | 9/43 (21%) | 12/38 (32%) |
| 19–35 years old | 10/43 (23%) | 7/38 (18%) |
| 36–50 years old | 1/43 (2%) | 1/38 (3%) |
| >50 years old | 1/43 (2%) | 0/38 (0%) |
| Recurrence frequency in past year | ||
| 4–6 episodes | 36/46 (78%) | 33/38 (87%) |
| 7–12 episodes | 10/46 (22%) | 4/38 (11%) |
| ≥13 episodes | 0/46 (0%) | 1/38 (3%) |
| Impact of cold sores on quality of life | ||
| Does not impact quality of life | 15/46 (33%) | 9/38 (24%) |
| Painful | 21/46 (46%) | 19/38 (50%) |
| Aesthetically displeasing | 15/46 (33%) | 15/38 (39%) |
| Impact social life | 10/46 (22%) | 12/38 (32%) |
| Eating/drinking painful | 9/46 (20%) | 7/38 (18%) |
| Associated with bad mood | 11/46 (24%) | 7/38 (18%) |
| Impact work | 7/46 (15%) | 7/38 (18%) |
| Other impact | 1/46 (2%) | 1/38 (3%) |
| On-study data | ||
| Participants with 12 months of survey dataa | 37/46 (80%) | 37/38 (97%) |
| Completed survey with 3-month recall lengthb | 146/152 (96%) | 141/144 (98%) |
| On-study use of oral therapy for cold sores | 11/27 (41%) | 19/27 (70%) |
| To treat active cold sores | 6/11 (55%) | 15/19 (79%) |
| To prevent cold soresc | 0/11 (0%) | 0/19 (0%) |
| Both (treat and prevent)c | 5/11 (45%) | 4/19 (21%) |
This is surveys completed and occurrence of HSV reported but is not indicative of complete data for all outcomes (e.g. individual items on survey could be missed).
Denominator is the total number of surveys completed with HSV occurrence question answered.
Prevention therapy defined as a daily oral anti-viral treatment lasting more than 1 month.
Primary outcome
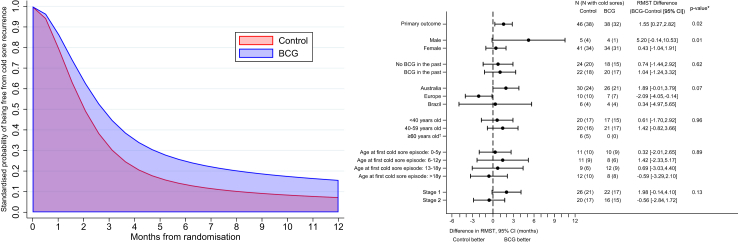
Of the 84 individuals with frequent recurrent cold sores, 70 reported a recurrence (32/38 (84%) in the BCG group and 38/46 (83%) in the control group) with a median time to recurrence of 2.2 (IQR 1.6, 3.5) months after randomisation in the BCG group, compared with 1.8 (IQR 1.1, 4.2) months in the control group. The time to first cold sore recurrence was 1.55 months greater in the BCG group (95% CI 0.27–2.82, p 0.02) with a HR of 0.54 (95% CI 0.32–0.91; Fig. 2, Table 2). The beneficial effect of BCG on the time to first cold sore recurrence was greater in the as-treated population (difference 1.91 months, 95% CI 0.69–3.12, p 0.003; HR 0.45, 95% CI 0.26–0.76; Table 2), and persisted in all the other sensitivity and supplementary analyses (Supplementary Tables S1–S3). In prespecified subgroup analyses (Fig. 2), only sex was associated with the treatment effect (p for treatment arm × sex interaction 0.007), with a difference in time to first cold sore recurrence of 5.20 (95% CI −0.14 to 10.53) months in males (HR 0.19, 95% CI 0.02–1.71), compared with 0.43 (95% CI −1.04 to 1.91) months in females (HR 0.87, 95% CI 0.53–1.41).
Fig. 2.
Primary outcome: time to cold sore recurrence by treatment arm, and in subgroup analyses. Forest plots show the subgroup analyses of the primary outcome, time to cold sore recurrence and 95% confidence intervals. ∗p-value for subgroup x treatment arm interaction, adjusted for randomisation strata (age group, presence of comorbidity, geographical location, and study stage). In stage 1, participants randomised to the control group received no intervention; in stage 2, participants randomised to the control group received placebo saline intradermal injection. †Few participants prevented the estimation of the treatment effect in this stratum.
Table 2.
Primary and secondary outcomes.
| Control | BCG | Difference |
p-value | |
|---|---|---|---|---|
| BCG–control | ||||
| Primary outcome: Time to first cold sore recurrence, in participants with ≥4 cold sore recurrences per year | ||||
| Intention to treat population | N = 46 | N = 38 | ||
| Participants with cold sore occurrence by 12 months | 38 | 32 | ||
| Median time to first recurrence (months)a | 1.8 (IQR 1.1, 4.2) | 2.2 (IQR 1.6, 3.5) | ||
| Event rate (per 100 person years) | 320 (233, 440) | 241 (170, 341) | ||
| RMST (months) | 3.0 (2.4, 3.8) | 4.6 (3.6, 5.8) | 1.6 (0.3, 2.8) | 0.02 |
| As treated population (sensitivity analysis) | N = 44 | N = 40 | ||
| Participants with cold sore occurrence by 12 months | 36 | 34 | ||
| Median time to first recurrence (months)a | 1.7 (IQR 1.1, 3.2) | 2.3 (IQR 1.6, 3.9) | ||
| Event rate (per 100 person years) | 329 (237, 456) | 239 (171, 335) | ||
| RMST (months) | 2.8 (2.2, 3.6) | 4.7 (3.8, 5.9) | 1.9 (0.7, 3.1) | 0.003 |
| Secondary outcomes by 12 months (analysed in ITT) | ||||
| Participants with ≥4 cold sore recurrences per year | N = 46 | N = 38 | ||
| Proportion of participants with cold sores recurrence | 38/41 (92.7%) | 32/38 (84.2%) | RD: −9.8% (−25.1, 5.5)b | 0.2 |
| Mean number of cold sores recurrences (months) | 3.9 (SD 3.8) | 4.0 (SD 3.6) | IRR: 0.9 (0.6, 1.5)b | 0.8 |
| Decrease in duration of cold sores recurrencesa | 7/34 (20.6%) | 19/31 (61.3%) | RD: 41.9% (20.4, 63.5) | <0.001 |
| Decrease in severity of cold sores recurrencesa | 7/34 (20.6%) | 23/31 (74.2%) | RD: 54.2% (33.7, 74.7) | <0.001 |
| Decrease in frequency of cold sores recurrencesa | 7/34 (20.6%) | 17/31 (54.8%) | RD: 34.7% (12.6, 56.8) | 0.002 |
| Decrease in impact on QoL of cold sores recurrencesa | 5/34 (14.7%) | 13/31 (41.9%) | RD: 25.4% (4.4, 46.5) | 0.02 |
| Participants with ≥2 cold sore recurrences per year | N = 197 | N = 189 | ||
| Time to first cold sore occurrence, RMST (months) | 6.2 (5.6, 6.8) | 6.1 (5.5, 6.7) | −0.1 (−1.0, 0.8) | 0.8 |
| Median time to first recurrence (months)a | 2.7 (IQR 1.6, 5.9) | 3.3 (IQR 1.9, 5.8) | ||
| Proportion of participants with cold sores recurrence | 135/182 (74.2%) | 131/176 (74.4%) | RD: −0.2% (−8.9, 8.6)b | 1.0 |
| Mean number of cold sores recurrences | 2.6 (SD 3.2) | 2.4 (SD 2.7) | IRR: 0.9 (0.7, 1.2)b | 0.5 |
| Decrease in duration of cold sores recurrencesa | 21/115 (18.3%) | 53/119 (44.5%) | RD: 26.0% (14.6, 37.4) | <0.001 |
| Decrease in severity of cold sores recurrencesa | 21/115 (18.3%) | 62/119 (52.1%) | RD: 32.7% (21.2, 44.2) | <0.001 |
| Decrease in frequency of cold sores recurrencesa | 20/115 (17.4%) | 52/119 (43.7%) | RD: 25.2% (13.9, 36.6) | <0.001 |
| Decrease in impact on QoL of cold sores recurrencesa | 14/115 (12.2%) | 39/119 (32.8%) | RD: 19.2% (8.9, 29.6) | <0.001 |
| Participants with ≥1 cold sore recurrence per year | N = 431 | N = 441 | ||
| Time to first cold sore occurrence, RMST (months) | 7.4 (7.0, 7.8) | 7.2 (6.8, 7.7) | −0.2 (−0.8, 0.4) | 0.6 |
| Median time to first recurrence (months)a | 3.3 (IQR 1.9, 6.7) | 3.1 (IQR 2.0, 5.7) | ||
| Proportion of participants with cold sores recurrence | 247/390 (63.3%) | 256/403 (63.5%) | RD: −0.1% (−6.6, 6.5)b | 1.0 |
| Mean number of cold sores recurrences | 1.8 (SD 2.8) | 1.8 (SD 2.5) | IRR: 1.0 (0.8, 1.2)b | 0.9 |
| Decrease in duration of cold sores recurrencesa | 37/213 (17.4%) | 89/229 (38.9%) | RD: 21.2% (13.1, 29.3) | <0.001 |
| Decrease in severity of cold sores recurrencesa | 36/213 (16.9%) | 101/229 (44.1%) | RD: 26.6% (18.4, 34.8) | <0.001 |
| Decrease in frequency of cold sores recurrencesa | 31/213 (14.6%) | 83/229 (36.2%) | RD: 21.4% (13.6, 29.2) | <0.001 |
| Decrease in impact on QoL of cold sores recurrencesa | 24/213 (11.3%) | 66/229 (28.8%) | RD: 16.9% (9.8, 24.1) | <0.001 |
| Participants who ever had a cold sore | N = 950 | N = 944 | ||
| Time to first cold sore occurrence, RMST (months) | 9.0 (8.8, 9.3) | 8.9 (8.7, 9.2) | −0.1 (−0.4, 0.3) | 0.7 |
| Median time to first recurrence (months)a | 3.9 (IQR 2.0, 7.3) | 3.8 (IQR 2.2, 7.1) | ||
| Proportion of participants with cold sores recurrence | 369/832 (44.4%) | 385/866 (44.5%) | RD: 0% (−4.7, 4.6)b | 1.0 |
| Mean number of cold sores recurrences | 1.1 (SD 2.2) | 1.1 (SD 2.0) | IRR: 1.0 (0.8, 1.2)b | 0.9 |
| Decrease in duration of cold sores recurrencesa | 54/328 (16.5%) | 112/349 (32.1%) | RD: 15.2% (8.9, 21.6) | <0.001 |
| Decrease in severity of cold sores recurrencesa | 51/328 (15.5%) | 127/349 (36.4%) | RD: 20.5% (14.1, 26.9) | <0.001 |
| Decrease in frequency of cold sores recurrencesa | 46/328 (14.0%) | 103/349 (29.5%) | RD: 15.2% (9.2, 21.3) | <0.001 |
| Decrease in impact on QoL of cold sores recurrencesa | 36/328 (11.0%) | 81/349 (23.2%) | RD: 11.8% (6.3, 17.4) | <0.001 |
| Participants who never had cold sores | N = 2235 | N = 2318 | ||
| Time to first cold sore occurrence, RMST (months) | 11.8 (11.8, 11.9) | 11.8 (11.7, 11.8) | −0.1 (−0.1, 0.0) | 0.06 |
| Median time to first recurrence (months)a | 5.2 (IQR 2.7, 8.0) | 5.8 (IQR 2.8, 8.4) | ||
| Proportion of participants with cold sores recurrence | 59/1861 (3.2%) | 90/2007 (4.5%) | RD: 1.4% (0.3, 2.6)b | 0.02 |
| Mean number of cold sores recurrences | 0.0 (SD 0.3) | 0.1 (SD 0.4) | IRR: 1.5 (1.0, 2.3)b | 0.04 |
Numbers in parentheses represent 95% confidence intervals where not otherwise specified. All analyses are adjusted for stratification factors used at randomisation (age, geographical location, presence of comorbidity, study stage).
IRR: incidence rate ratio; RD: risk difference; QoL: quality of life; ITT: intention to treat; RMST: restricted mean survival time; SD: standard deviation; IQR: interquartile range.
Among participants with a cold sore recurrence during the study.
Multiple imputation was required due to >10% of missing data.
Secondary outcomes
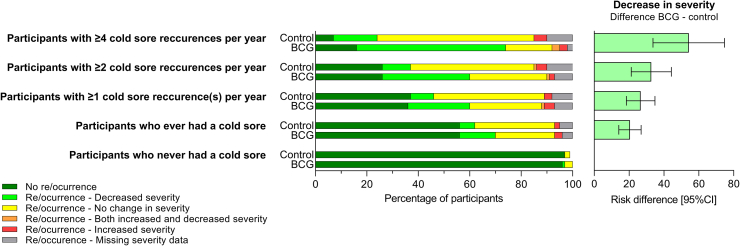
During the 12-month follow-up, a greater proportion of participants with frequent recurrent cold sores in the BCG group compared with the control group reported a decrease in duration (61% vs 21%; RD 42%, 95% CI 20–64%), severity (74% vs 21%; RD 54%, 95% CI 34–75%) and perceived frequency (55% vs 21%; RD 35%, 95% CI 13–57%) of cold sore recurrences (Table 2, Fig. 3). In addition, a greater proportion of participants in the BCG group compared with the control group reported a reduction in the impact of cold sores on their quality of life (42% vs 15%; RD 25%, 95% CI 4–47%; Table 2). There was no difference between the groups in the proportion of participants reporting any cold sore recurrence, or the total number of cold sore recurrences reported (Table 2).
Fig. 3.
Secondary outcome: cold sore re/occurrence and perceived difference in severity of cold sores episodes at 12 months after randomisation, by treatment arm and reported cold sore frequency at baseline.
Supplementary analyses in all participants who previously ever had a cold sore
In the analyses done in the 1894 participants who ever had a cold sore, there were differences in the proportion of participants reporting a perceived decrease in duration (32% vs 17%; RD 15%, 95% CI 9–22%), severity (36% vs 16%; RD 21%, 95% CI 14–27%), frequency (30% vs 14%; RD 15%, 95% CI 9–21%), and impact on quality of life (23% vs 11%; RD 12%, 95% CI 6–17%) of cold sore episodes in the BCG group compared with the control group (Table 2, Fig. 3). The magnitude of decrease in severity was proportional to the frequency of cold sore recurrences in the year preceding inclusion (Table 2, Fig. 3). There was no difference between the two groups in the time to first recurrence, the proportion of participants reporting a cold sore recurrence, or the number of cold sore recurrences (Table 2).
Supplementary analyses in participants who never previously had a cold sore
In the 4553 participants who reported no prior cold sore occurrences in the baseline questionnaire, the proportion of participants reporting a first cold sore episode during the 12 months of follow-up was greater in the BCG group (90/2007, 4.5%) compared with the control group (59/1861, 3.2%), with a RD of 1.4% (95% CI 0.3–2.6%, p 0.02; Table 2, Fig. 3). The time to first cold sore occurrence was also shorter (difference −0.07 months, 95% CI −0.14 to 0.00, p 0.06; Table 2) and the number of cold sore episodes over 12 months greater (IRR 1.5, 95% CI 1.0–2.3, p 0.04; Table 2) in the BCG group compared to the control group.
As previously reported, there were no safety concerns following BCG vaccination in the BRACE trial (see Appendix).17,19
Discussion
This is the first RCT in which the efficacy of BCG vaccination to reduce cold sore recurrence is assessed. In individuals with frequent recurrent cold sore episodes, we found a 53% increase in the mean time spent without a recurrence over the 12 months following BCG vaccination, together with a decrease in the self-reported burden of cold sore recurrences. Although, participants in the BCG group were more likely to use oral therapy during follow up, a higher proportion of participants in the control group reported using it for a longer period, to prevent cold sores. BCG vaccination had the highest impact on individuals with frequent recurrent cold sore episodes. A decrease in cold sore burden was observed in participants with less frequent cold sore recurrences, but of lower magnitude. The effect of BCG on delaying time to first cold sore recurrence was greater in the as-treated population, supporting this being a true beneficial effect of the intervention.
Overall, the benefit of BCG vaccination was mainly qualitative (e.g. decreased severity of episode, lower impact of episode on quality of life), rather than quantitative (e.g. proportion of participant with a recurrence, number of recurrences). This highlights the need to include outcome measures that are clinically relevant in clinical trials, as even small changes, which may be difficult to capture with quantitative measures alone, can significantly impact individuals’ everyday lives.
Previous observational studies reporting the impact of BCG vaccination on HSV have involved a total of 127 individuals with recurrent cold sores and 162 with recurrent herpes genitalis.14 In the two studies restricted to individuals with recurrent cold sores, nearly all participants benefitted from BCG vaccination, with 48% remaining recurrence-free and 45% clearly improving, with fewer or less severe recurrences.14,21
The only RCT addressing the use of BCG for HSV recurrence was restricted to adults with active herpes genitalis (first episode or recurrence). In that trial, a total of 155 adults were randomised to BCG-Glaxo vaccination or to an intradermal injection of Candida sp. antigen. The mean frequency of recurrence was unchanged after BCG vaccination, but was lower among those with recurrent herpes genitalis who received intradermal Candida.15 However, interpretation of this finding is complicated by potential methodological limitations, the most important being the control group receiving a potentially beneficial intervention, as Candida sp. skin test antigen is also known to induce non-specific immunomodulation.22,23
We observed a sex-differential effect, with BCG vaccination benefitting males with frequently recurring cold sores more than females. Although our study was underpowered to detect subgroup effects, it is consistent with previous studies reporting an influence of sex on the immune response to infections and vaccines,24 as well as on off-target effects of vaccination.25
A previous study suggests that repeated doses of BCG might be more effective than a single dose to reduce herpes genitalis recurrences. In this prospective study of 38 adults with severe and recalcitrant recurrent episodes, BCG-Glaxo vaccination was given once a month for a maximum of 6 total doses. The intervention was beneficial for 63% of the individuals, with 21% remaining recurrence-free and 42% experiencing fewer or milder episodes.26 In our study, there was no additional benefit in those who had previously received BCG, however the prior dose was generally given decades earlier, often in the neonatal period.
The mechanisms underlying predisposition to recurrent HSV are uncertain. Host genetics and minor variations in the immune system may play a role. Several genes associated with HSV infection and their recurrence have been identified,27, 28, 29, 30, 31 but these findings have not been replicated. Imbalance in lymphocyte T subsets has been reported in individuals with recurrent herpes labialis32 or genitalis.33 In one study, normalisation of the ratio following BCG vaccination paralleled clinical improvement.33 In a case report, defective interferon-γ production due to specific defects in double-stranded RNA recognition were identified in three patients with severe recurrent HSV-2 skin eruptions, who subsequently got better after interferon-γ treatment.29 Finally, in individuals with recurrent cold sores, clinical improvement following BCG vaccination has also been associated with significant changes in in-vitro HSV-Ag-induced leukocyte migration inhibition, which remained unchanged in two non-responders.21
BCG vaccine has well-documented effects on both the innate and the adaptive immune system.34,35 However, the specific immunological mechanisms that underlie the off-target effect of BCG vaccination are not yet fully understood. In those with recurrent cold sores, a BCG-induced change in the balance of lymphocytes subsets and/or enhancement of interferon-γ production might play a role in the observed clinical effect.29,35, 36, 37
In our study, despite a beneficial effect on HSV recurrences, BCG vaccination did not reduce the risk of a first cold sore episode. The opposite was in fact observed, with a slightly increased risk in the BCG group. This might be explained by different immunological mechanisms underlying susceptibility to initial infection compared with the mechanisms controlling latent infection and suppressing reactivation. This provides an interesting insight on the immunomodulation induced by BCG vaccination. It is noteworthy that this finding is consistent with the results or the BRACE trial which found that the risk of COVID-19 was higher in the BCG group when compared with the placebo group.17 We plan further exploration of these mechanisms using blood samples collected from participants at baseline and during follow-up.
The small number of participants with frequent recurrent cold sores included in this analysis and the inability to ensure complete blinding in trials of BCG are potential limitations of this study. In addition, the episodes were self-reported, without virological confirmation. However, this is the first RCT to report the effect of BCG vaccination on cold sore recurrence.
In summary, BCG-Denmark vaccination had a beneficial effect on herpes labialis, with an observed increase in the time spent without a recurrence amongst individuals previously reporting frequent recurrences. The self-reported burden of cold sore recurrences generally decreased following vaccination, especially in those with the most frequent recurrences. Interestingly, in contrast, we observed a small increased risk of reporting a first cold sore episode in the BCG group. Future studies should focus on other populations in which herpes recurrence has a significant impact, including children (in whom cold sore recurrences impact feeding and schooling) and amongst individuals with recurrent herpes genitalis.
Contributors
All authors contributed substantially to the BRACE trial. NC led the BRACE trial. LFP designed the cold sore questionnaires for the nested study. LFP and EMD cleaned the data. CLM wrote the statistical analysis plan with input from LFP, and did all the statistical analysis. LFP wrote the first draft of the report with input for NC and CLM, and all authors critically reviewed it. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication. LFP and CLM have directly accessed and verified the underlying data reported in the manuscript.
Data sharing statement
Deidentified participant data and data dictionary are available to others on request and on completion of a signed data access agreement. Requests can be made in writing to braceresearch@mcri.edu.au.
Declaration of interests
We declare no competing interests.
Acknowledgements
We thank the trial participants and the site personnel who recruited participants and assisted with the trial (detailed in the appendix); AJ Vaccines (Copenhagen) for facilitating BCG vaccine supplies; Devon Freewheelers (UK) for UK transport; Catalent (UK) for drug management; Fiocruz Clinical Research Platform for coordination and monitoring of sites in Brazil; the Pharmacy, Pathology and Immunisation Service teams from The Royal Children's Hospital Melbourne, and the Orygen Group for their support.
The trial is supported by the Bill & Melinda Gates Foundation [INV- 017302], the Minderoo Foundation [COV-001], Sarah and Lachlan Murdoch, the Royal Children’s Hospital Foundation [2020-1263 BRACE Trial], Health Services Union NSW, the Peter Sowerby Foundation, SA Health, the Insurance Advisernet Foundation, the NAB Foundation, the Calvert-Jones Foundation, the Modara Pines Charitable Foundation, the UHG Foundation Pty Ltd, Epworth Healthcare and individual donors. The funders had no role in the collection, analysis and interpretation of data or in the preparation, review or approval of the manuscript. The Murdoch Children's Research Institute (MCRI) leads the BRACE trial across 36 sites in five countries. It is supported by the Victorian Government’s Operational Infrastructure Support Programme. NC is supported by a National Health and Medical Research Council (NHMRC) Investigator Grant (GNT1197117). LFP is supported by the Swiss National Science Foundation (Early Postdoc Mobility Grant, P2GEP3_178155).
Footnotes
Translation: For Spanish, Portugese, Dutch, and French translations of the abstract see Supplementary Materials section.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.102203.
Contributor Information
Nigel Curtis, Email: nigel.curtis@rch.org.au.
the BRACE Trial Consortium Group:
Nigel Curtis, Andrew Davidson, Kaya Gardiner, Amanda Gwee, Tenaya Jamieson, Nicole Messina, Thilanka Morawakage, Susan Perlen, Kirsten Perrett, Laure Pittet, Amber Sastry, Jia Wei Teo, Francesca Orsini, Katherine Lee, Cecilia Moore, Suzanna Vidmar, Laure Pittet, Rashida Ali, Ross Dunn, Peta Edler, Grace Gell, Casey Goodall, Richard Hall, Ann Krastev, Nathan La, Ellie McDonald, Nick McPhate, Thao Nguyen, Jack Ren, Luke Stevens, Nicole Messina, Ahmed Alamrousi, Rhian Bonnici, Thanh Dang, Susie Germano, Jenny Hua, Rebecca McElroy, Monica Razmovska, Scott Reddiex, Xiaofang Wang, Jeremy Anderson, Kristy Azzopardi, Vicki Bennett-Wood, Anna Czajko, Nadia Mazarakis, Conor McCafferty, Frances Oppedisano, Belinda Ortika, Casey Pell, Leena Spry, Ryan Toh, Sunitha Velagapudi, Amanda Vlahos, Ashleigh Wee-Hee, Pedro Ramos, Karina De La Cruz, Dinusha Gamage, Anushka Karunanayake, Isabella Mezzetti, Benjamin Ong, Ronita Singh, Enoshini Sooriyarachchi, Suellen Nicholson, Natalie Cain, Rianne Brizuela, Han Huang, Veronica Abruzzo, Morgan Bealing, Patricia Bimboese, Kirsty Bowes, Emma Burrell, Joyce Chan, Jac Cushnahan, Hannah Elborough, Olivia Elkington, Kieran Fahey, Monique Fernandez, Catherine Flynn, Sarah Fowler, Marie Gentile Andrit, Bojana Gladanac, Catherine Hammond, Norine Ma, Sam Macalister, Emmah Milojevic, Jesutofunmi Mojeed, Jill Nguyen, Liz O'Donnell, Nadia Olivier, Isabelle Ooi, Stephanie Reynolds, Lisa Shen, Barb Sherry, Judith Spotswood, Jamie Wedderburn, Angela Younes, Donna Legge, Jason Bell, Jo Cheah, Annie Cobbledick, Kee Lim, Sonja Elia, Lynne Addlem, Anna Bourke, Clare Brophy, Nadine Henare, Narelle Jenkins, Francesca Machingaifa, Skye Miller, Kirsten Mitchell, Sigrid Pitkin, Kate Wall, Paola Villanueva, Nigel Crawford, Laure Pittet, Wendy Norton, Niki Tan, Thilakavathi Chengodu, Diane Dawson, Victoria Gordon, Tony Korman, Jess O'Bryan, Veronica Abruzzo, Sophie Agius, Samantha Bannister, Jess Bucholc, Alison Burns, Beatriz Camesella, John Carlin, Marianna Ciaverella, Maxwell Curtis, Stephanie Firth, Christina Guo, Matthew Hannan, Erin Hill, Sri Joshi, Katherine Lieschke, Megan Mathers, Sasha Odoi, Ashleigh Rak, Chris Richards, Leah Steve, Carolyn Stewart, Eva Sudbury, Helen Thomson, Emma Watts, Fiona Williams, Angela Young, Penny Glenn, Andrew Kaynes, Amandine Philippart De Floy, Sandy Buchanan, Thijs Sondag, Ivy Xie, Harriet Edmund, Bridie Byrne, Tom Keeble, Belle Ngien, Fran Noonan, Michelle Wearing-Smith, Alison Clarke, Pemma Davies, Oliver Eastwood, Alric Ellinghaus, Rachid Ghieh, Zahra Hilton, Emma Jennings, Athina Kakkos, Iris Liang, Katie Nicol, Sally O'Callaghan, Helen Osman, Gowri Rajaram, Sophia Ratcliffe, Victoria Rayner, Ashleigh Salmon, Angela Scheppokat, Aimee Stevens, Rebekah Street, Nicholas Toogood, Nicholas Wood, Twinkle Bahaduri, Therese Baulman, Jennifer Byrne, Candace Carter, Mary Corbett, Aiken Dao, Maria Desylva, Andrew Dunn, Evangeline Gardiner, Rosemary Joyce, Rama Kandasamy, Craig Munns, Lisa Pelayo, Ketaki Sharma, Katrina Sterling, Caitlin Uren, Clinton Colaco, Mark Douglas, Kate Hamilton, Adam Bartlett, Brendan McMullan, Pamela Palasanthiran, Phoebe Williams, Justin Beardsley, Nikki Bergant, Renier Lagunday, Kristen Overton, Jeffrey Post, Yasmeen Al-Hindawi, Sarah Barney, Anthony Byrne, Lee Mead, Marshall Plit, David Lynn, Saoirse Benson, Stephen Blake, Rochelle Botten, Tee Yee Chern, Georgina Eden, Liddy Griffith, Jane James, Miriam Lynn, Angela Markow, Domenic Sacca, Natalie Stevens, Steve Wesselingh, Catriona Doran, Simone Barry, Alice Sawka, Sue Evans, Louise Goodchild, Christine Heath, Meredith Krieg, Helen Marshall, Mark McMillan, Mary Walker, Peter Richmond, Nelly Amenyogbe, Christina Anthony, Annabelle Arnold, Beth Arrowsmith, Rym Ben-Othman, Sharon Clark, Jemma Dunnill, Nat Eiffler, Krist Ewe, Carolyn Finucane, Lorraine Flynn, Camille Gibson, Lucy Hartnell, Elysia Hollams, Heidi Hutton, Lance Jarvis, Jane Jones, Jan Jones, Karen Jones, Jennifer Kent, Tobias Kollmann, Debbie Lalich, Wenna Lee, Rachel Lim, Sonia McAlister, Fiona McDonald, Andrea Meehan, Asma Minhaj, Lisa Montgomery, Melissa O'Donnell, Jaslyn Ong, Joanne Ong, Kimberley Parkin, Glady Perez, Catherine Power, Shadie Rezazadeh, Holly Richmond, Sally Rogers, Nikki Schultz, Margaret Shave, Patrycja Skut, Lisa Stiglmayer, Alexandra Truelove, Ushma Wadia, Rachael Wallace, Justin Waring, Michelle England, Erin Latkovic, Laurens Manning, Susan Herrmann, Michaela Lucas, Marcus Lacerda, Paulo Henrique Andrade, Fabiane Bianca Barbosa, Dayanne Barros, Larissa Brasil, Ana Greyce Capella, Ramon Castro, Erlane Costa, Dilcimar de Souza, Maianne Dias, José Dias, Klenilson Ferreira, Paula Figueiredo, Thamires Freitas, Ana Carolina Furtado, Larissa Gama, Vanessa Godinho, Cintia Gouy, Daniele Hinojosa, Bruno Jardim, Tyane Jardim, Joel Junior, Augustto Lima, Bernardo Maia, Adriana Marins, Kelry Mazurega, Tercilene Medeiros, Rosangela Melo, Marinete Moraes, Elizandra Nascimento, Juliana Neves, Maria Gabriela Oliveira, Thais Oliveira, Ingrid Oliveira, Arthur Otsuka, Rayssa Paes, Handerson Pereira, Gabrielle Pereira, Christiane Prado, Evelyn Queiroz, Laleyska Rodrigues, Bebeto Rodrigues, Vanderson Sampaio, Anna Gabriela Santos, Daniel Santos, Tilza Santos, Evelyn Santos, Ariandra Sartim, Ana Beatriz Silva, Juliana Silva, Emanuelle Silva, Mariana Simão, Caroline Soares, Antonny Sousa, Alexandre Trindade, Fernando Val, Adria Vasconcelos, Heline Vasconcelos, Julio Croda, Carolinne Abreu, Katya Martinez Almeida, Camila Bitencourt de Andrade, Jhenyfer Thalyta Campos Angelo, Ghislaine Gonçalvez de Araújo Arcanjo, Bianca Maria Silva Menezes Arruda, Wellyngthon Espindola Ayala, Adelita Agripina Refosco Barbosa, Felipe Zampieri Vieira Batista, Fabiani de Morais Batista, Miriam de Jesus Costa, Mariana Garcia Croda, Lais Alves da Cruz, Roberta Carolina Pereira Diogo, Rodrigo Cezar Dutra Escobar, Iara Rodrigues Fernandes, Leticia Ramires Figueiredo, Leandro Galdino Cavalcanti Gonçalves, Sarita Lahdo, Joyce dos Santos Lencina, Guilherme Teodoro de Lima, Larissa Santos Matos, Bruna Tayara Leopoldina Meireles, Debora Quadros Moreira, Lilian Batista Silva Muranaka, Adriely de Oliveira, Karla Regina Warszawski de Oliveira, Matheus Vieira de Oliveira, Roberto Dias de Oliveira, Andrea Antonia Souza de Almeida dos Reis Pereira, Marco Puga, Caroliny Veron Ramos, Thaynara Haynara Souza da Rosa, Karla Lopes dos Santos, Claudinalva Ribeiro dos Santos, Dyenyffer Stéffany Leopoldina dos Santos, Karina Marques Santos, Paulo César Pereira da Silva, Paulo Victor Rocha da Silva, Débora dos Santos Silva, Patricia Vieira da Silva, Bruno Freitas da Rosa Soares, Mariana Gazzoni Sperotto, Mariana Mayumi Tadokoro, Daniel Tsuha, Hugo Miguel Ramos Vieira, Margareth Maria Pretti Dalcolmo, Cíntia Maria Lopes Alves da Paixão, Gabriela Corrêa E Castro, Simone Silva Collopy, Renato da Costa Silva, Samyra Almeida da Silveira, Alda Maria Da-Cruz, Alessandra Maria da Silva Passos de Carvalho, Rita de Cássia Batista, Maria Luciana Silva De Freitas, Aline Gerhardt de Oliveira Ferreira, Ana Paula Conceição de Souza, Paola Cerbino Doblas, Ayla Alcoforado da Silva dos Santos, Vanessa Cristine de Moraes dos Santos, Dayane Alves dos Santos Gomes, Anderson Lage Fortunato, Adriano Gomes-Silva, Monique Pinto Gonçalves, Paulo Leandro Garcia Meireless Junior, Estela Martins da Costa Carvalho, Fernando do Couto Motta, Ligia Maria Olivo de Mendonça, Girlene dos Santos Pandine, Rosa Maria Plácido Pereira, Ivan Ramos Maia, Jorge Luiz da Rocha, João Victor Paiva Romano, Glauce dos Santos, Erica Fernandes da Silva, Marilda Agudo Mendonça Teixeira de Siqueira, Ágatha Cristinne Prudêncio Soares, Marc Bonten, Sandra Franch Arroyo, Henny Ophorst-den Besten, Anna Boon, Karin M. Brakke, Axel Janssen, Marijke A.H. Koopmans, Toos Lemmens, Titia Leurink, Cristina Prat-Aymerich, Engelien Septer-Bijleveld, Kimberly Stadhouders, Darren Troeman, Marije van der Waal, Marjoleine van Opdorp, Nicolette van Sluis, Beatrijs Wolters, Jan Kluytmans, Jannie Romme, Wouter van den Bijllaardt, Linda van Mook, M.M.L (Miranda) van Rijen, P.M.G. Filius, Jet Gisolf, Frances Greven, Danique Huijbens, Robert Jan Hassing, R.C. Pon, Lieke Preijers, J.H. van Leusen, Harald Verheij, Wim Boersma, Evelien Brans, Paul Kloeg, Kitty Molenaar-Groot, Nhat Khanh Nguyen, Nienke Paternotte, Anke Rol, Lida Stooper, Helga Dijkstra, Esther Eggenhuizen, Lucas Huijs, Simone Moorlag, Mihai Netea, Eva Pranger, Esther Taks, Jaap ten Oever, Rob ter Heine, Kitty Blauwendraat, Bob Meek, Isil Erkaya, Houda Harbech, Nienke Roescher, Rifka Peeters, Menno te Riele, Carmen Zhou, Esther Calbo, Cristina Badia Marti, Emma Triviño Palomares, Tomás Perez Porcuna, Anabel Barriocanal, Ana Maria Barriocanal, Irma Casas, Jose Dominguez, Maria Esteve, Alicia Lacoma, Irene Latorre, Gemma Molina, Barbara Molina, Antoni Rosell, Sandra Vidal, Lydia Barrera, Natalia Bustos, Ines Portillo Calderón, David Gutierrez Campos, Jose Manuel Carretero, Angel Dominguez Castellano, Renato Compagnone, Encarnacion Ramirez de Arellano, Almudena de la Serna, Maria Dolores del Toro Lopez, Marie-Alix Clement Espindola, Ana Belen Martin Gutierrez, Alvaro Pascual Hernandez, Virginia Palomo Jiménez, Elisa Moreno, Nicolas Navarrete, Teresa Rodriguez Paño, Jesús Rodríguez-Baño, Enriqueta Tristán, Maria Jose Rios Villegas, Atsegiñe Canga Garces, Erika Castro Amo, Raquel Coya Guerrero, Josune Goikoetxea, Leticia Jorge, Cristina Perez, María Carmen Fariñas Álvarez, Manuel Gutierrez Cuadra, Francisco Arnaiz de las Revillas Almajano, Pilar Bohedo Garcia, Teresa Giménez Poderos, Claudia González Rico, Blanca Sanchez, Olga Valero, Noelia Vega, John Campbell, Anna Barnes, Helen Catterick, Tim Cranston, Phoebe Dawe, Emily Fletcher, Liam Fouracre, Alison Gifford, John Kirkwood, Christopher Martin, Amy McAnew, Marcus Mitchell, Georgina Newman, Abby O'Connell, Jakob Onysk, Lynne Quinn, Shelley Rhodes, Samuel Stone, Lorrie Symons, Harry Tripp, Adilia Warris, Darcy Watkins, Bethany Whale, Alex Harding, Gemma Lockhart, Kate Sidaway-Lee, John Campbell, Sam Hilton, Sarah Manton, Daniel Webber-Rookes, Rachel Winder, James Moore, Freya Bateman, Michael Gibbons, Bridget Knight, Julie Moss, Sarah Statton, Josephine Studham, Lydia Hall, Will Moyle, and Tamsin Venton
Appendix A. Supplementary data
References
- 1.Whitley R.J., Roizman B. Herpes simplex virus infections. Lancet. 2001;357(9267):1513–1518. doi: 10.1016/S0140-6736(00)04638-9. [DOI] [PubMed] [Google Scholar]
- 2.Looker K.J., Magaret A.S., May M.T., et al. Global and regional estimates of prevalent and incident herpes simplex virus type 1 infections in 2012. PLoS One. 2015;10(10) doi: 10.1371/journal.pone.0140765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Embil J.A., Stephens R.G., Manuel F.R. Prevalence of recurrent herpes labialis and aphthous ulcers among young adults on six continents. Can Med Assoc J. 1975;113(7):627–630. [PMC free article] [PubMed] [Google Scholar]
- 4.Chi C.C., Wang S.H., Delamere F.M., Wojnarowska F., Peters M.C., Kanjirath P.P. Interventions for prevention of herpes simplex labialis (cold sores on the lips) Cochrane Database Syst Rev. 2015;8:CD010095. doi: 10.1002/14651858.CD010095.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le Cleach L., Trinquart L., Do G., et al. Oral antiviral therapy for prevention of genital herpes outbreaks in immunocompetent and nonpregnant patients. Cochrane Database Syst Rev. 2014;8 doi: 10.1002/14651858.CD009036.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pittet L.F., Curtis N. Does oral antiviral suppressive therapy prevent recurrent herpes labialis in children? Arch Dis Child. 2019;104(9):916–919. doi: 10.1136/archdischild-2019-317249. [DOI] [PubMed] [Google Scholar]
- 7.Johnston C., Gottlieb S.L., Wald A. Status of vaccine research and development of vaccines for herpes simplex virus. Vaccine. 2016;34(26):2948–2952. doi: 10.1016/j.vaccine.2015.12.076. [DOI] [PubMed] [Google Scholar]
- 8.Pollard A.J., Finn A., Curtis N. Non-specific effects of vaccines: plausible and potentially important, but implications uncertain. Arch Dis Child. 2017;102(11):1077–1081. doi: 10.1136/archdischild-2015-310282. [DOI] [PubMed] [Google Scholar]
- 9.Higgins J.P., Soares-Weiser K., Lopez-Lopez J.A., et al. Association of BCG, DTP, and measles containing vaccines with childhood mortality: systematic review. BMJ. 2016;355:i5170. doi: 10.1136/bmj.i5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alexandroff A.B., Jackson A.M., O'Donnell M.A., James K. BCG immunotherapy of bladder cancer: 20 years on. Lancet. 1999;353(9165):1689–1694. doi: 10.1016/S0140-6736(98)07422-4. [DOI] [PubMed] [Google Scholar]
- 11.Kolmel K.F., Grange J.M., Krone B., et al. Prior immunisation of patients with malignant melanoma with vaccinia or BCG is associated with better survival. Eur J Cancer. 2005;41(1):118–125. doi: 10.1016/j.ejca.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 12.Ristori G., Faustman D., Matarese G., Romano S., Salvetti M. Bridging the gap between vaccination with Bacille Calmette-Guerin (BCG) and immunological tolerance: the cases of type 1 diabetes and multiple sclerosis. Curr Opin Immunol. 2018;55:89–96. doi: 10.1016/j.coi.2018.09.016. [DOI] [PubMed] [Google Scholar]
- 13.Moorlag S., Arts R.J.W., van Crevel R., Netea M.G. Non-specific effects of BCG vaccine on viral infections. Clin Microbiol Infect. 2019;25(12):1473–1478. doi: 10.1016/j.cmi.2019.04.020. [DOI] [PubMed] [Google Scholar]
- 14.Pittet L.F., Curtis N. Does bacillus Calmette-Guerin vaccine prevent herpes simplex virus recurrences? A systematic review. Rev Med Virol. 2021;31(1):1–9. doi: 10.1002/rmv.2151. [DOI] [PubMed] [Google Scholar]
- 15.Douglas J.M., Vontver L.A., Stamm W.E., et al. Ineffectiveness and toxicity of BCG vaccine for the prevention of recurrent genital herpes. Antimicrob Agents Chemother. 1985;27(2):203–206. doi: 10.1128/aac.27.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pittet L.F., Messina N.L., Gardiner K., et al. BCG vaccination to reduce the impact of COVID-19 in healthcare workers: protocol for a randomised controlled trial (BRACE trial) BMJ Open. 2021;11(10) doi: 10.1136/bmjopen-2021-052101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pittet L.F., Messina N.L., Orsini F., et al. Randomized trial of BCG vaccine to protect against Covid-19 in health care workers. N Engl J Med. 2023;388(17):1582–1596. doi: 10.1056/NEJMoa2212616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Villanueva P., Crawford N.W., Garcia Croda M., et al. Safety of BCG vaccination and revaccination in healthcare workers. Hum Vaccin Immunother. 2023;19(2) doi: 10.1080/21645515.2023.2239088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Royston P., Parmar M.K.B. Restricted mean survival time: an alternative to the hazard ratio for the design and analysis of randomized trials with a time-to-event outcome. BMC Med Res Methodol. 2013;13(1):152. doi: 10.1186/1471-2288-13-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jarisch R., Sandor I., Cerni C. [The leukocyte migration inhibition test (LMIT) in recurrent herpes simplex labialis. Comparison of the results of treatment with BCG and Levamisole (author's transl)] Arch Dermatol Res. 1979;265(1):15–22. doi: 10.1007/BF00412697. [DOI] [PubMed] [Google Scholar]
- 22.Nakagawa M., Coleman H.N., Wang X., Daniels J., Sikes J., Nagarajan U.M. IL-12 secretion by Langerhans cells stimulated with Candida skin test reagent is mediated by dectin-1 in some healthy individuals. Cytokine. 2014;65(2):202–209. doi: 10.1016/j.cyto.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X., Coleman H.N., Nagarajan U., Spencer H.J., Nakagawa M. Candida skin test reagent as a novel adjuvant for a human papillomavirus peptide-based therapeutic vaccine. Vaccine. 2013;31(49):5806–5813. doi: 10.1016/j.vaccine.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zimmermann P., Curtis N. Factors that influence the immune response to vaccination. Clin Microbiol Rev. 2019;32(2) doi: 10.1128/CMR.00084-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flanagan K.L., Plebanski M. Sex-differential heterologous (non-specific) effects of vaccines: an emerging public health issue that needs to be understood and exploited. Expert Rev Vaccines. 2017;16(1):5–13. doi: 10.1080/14760584.2016.1203260. [DOI] [PubMed] [Google Scholar]
- 26.Bierman S.M. BCG immunoprophylaxis of recurrent herpes progenitalis. Arch Dermatol. 1976;112(10):1410–1415. [PubMed] [Google Scholar]
- 27.Hobbs M.R., Jones B.B., Otterud B.E., Leppert M., Kriesel J.D. Identification of a herpes simplex labialis susceptibility region on human chromosome 21. J Infect Dis. 2008;197(3):340–346. doi: 10.1086/525540. [DOI] [PubMed] [Google Scholar]
- 28.Itzhaki R., Wozniak M. Susceptibility to herpes simplex labialis conferred by the gene encoding apolipoprotein E. J Infect Dis. 2008;198(4):624–625. doi: 10.1086/590213. author reply 5-6. [DOI] [PubMed] [Google Scholar]
- 29.Arts P., van de Veerdonk F.L., van der Lee R., et al. Immunologic defects in severe mucocutaneous HSV-2 infections: response to IFN-gamma therapy. J Allergy Clin Immunol. 2016;138(3):895–898. doi: 10.1016/j.jaci.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 30.Kriesel J.D., Jones B.B., Matsunami N., et al. C21orf91 genotypes correlate with herpes simplex labialis (cold sore) frequency: description of a cold sore susceptibility gene. J Infect Dis. 2011;204(11):1654–1662. doi: 10.1093/infdis/jir633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santamaria P., Bowyer R.C.E., Nibali L. Associations between host genetic variants and herpes simplex labialis in the twins UK cohort. Arch Oral Biol. 2023;145 doi: 10.1016/j.archoralbio.2022.105587. [DOI] [PubMed] [Google Scholar]
- 32.Mei X.X., Lei S.S., Xu L., et al. Herpes simplex virus type I-infected disorders alter the balance between Treg and Th17 cells in recurrent herpes labialis patients. Int J Immunopathol Pharmacol. 2020;34 doi: 10.1177/2058738420933099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deng Y.H., Chen X.P., Xu C.R., Chen Y.L., Wan M., Yang D.L. Enhancement of BCG-PSN on the cytokines in CD8+ T lymphocytes in the peripheral blood of patients with recurrent genital herpes. [Chinese] Chin J Microbiol Immunol. 2004;24(11):901–904. [Google Scholar]
- 34.Netea M.G., Quintin J., van der Meer J.W. Trained immunity: a memory for innate host defense. Cell Host Microbe. 2011;9(5):355–361. doi: 10.1016/j.chom.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 35.Netea M.G., Dominguez-Andres J., Barreiro L.B., et al. Defining trained immunity and its role in health and disease. Nat Rev Immunol. 2020;20(6):375–388. doi: 10.1038/s41577-020-0285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kleinnijenhuis J., Quintin J., Preijers F., et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci U S A. 2012;109(43):17537–17542. doi: 10.1073/pnas.1202870109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kandasamy R., Voysey M., McQuaid F., et al. Non-specific immunological effects of selected routine childhood immunisations: systematic review. BMJ. 2016;355:i5225. doi: 10.1136/bmj.i5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.