Abstract
Plasmodium falciparum causes millions of malaria infections and hundreds of thousands of deaths annually. These parasites avoid the adaptive immune response by systematically cycling through a limited repertoire of variant surface antigens after which the number of circulating parasites drops to extremely low levels, coinciding with a loss of symptoms and eventual clearance of the infection. However, in regions with extended dry seasons or in individuals who no longer reside in endemic areas, asymptomatic infections have been observed to persist for many months or years, potentially serving as reservoirs for transmission. Recent work suggests the possibility that parasites can assume a state in which no variant surface antigens are expressed, thus rendering them virtually invisible to the immune system and enabling them to persist at low levels indefinitely.
Keywords: asymptomatic infection, malaria transmission, cytoadherence, var genes, immune evasion
Introduction
Malaria continues to be a major cause of morbidity and mortality throughout equatorial regions of the world and remains a primary cause of death among African children. While five Plasmodium species are known to infect humans, Plasmodium falciparum is responsible for the Lion’s share of infections and deaths globally [1]. The exceptional virulence of this species is due in part to extensive modifications the parasites make to their host red blood cells (RBC) over the course of the 48-hour asexual replication cycle. These include significant alterations to the RBC cytoskeleton resulting in substantially reduced deformability of the infected cell [2]. This disrupts the movement of infected RBCs through the narrow capillaries of the peripheral circulation and can also result in their filtration by the spleen. To avoid this fate, parasites anchor a hypervariable adhesive protein in the RBC membrane, extending into the extracellular space where it can bind to a variety of endothelial receptors displayed on the surface of the vasculature (Figure 1A) [3]. This results in the cytoadherence of infected RBCs to the blood vessel walls and sequestration of parasites in the deep tissue capillary beds (Figure 1B). The adherence of infected RBCs to the vascular endothelium results in obstructed blood flow, hypoxia and local inflammation, consequences that underlie many of the severe syndromes associated with P. falciparum infection, including cerebral malaria and pregnancy associated malaria [4]. This type of cytoadhesion is unique to P. falciparum and is thought to be a primary virulence determinant of this parasite species.
Figure 1.
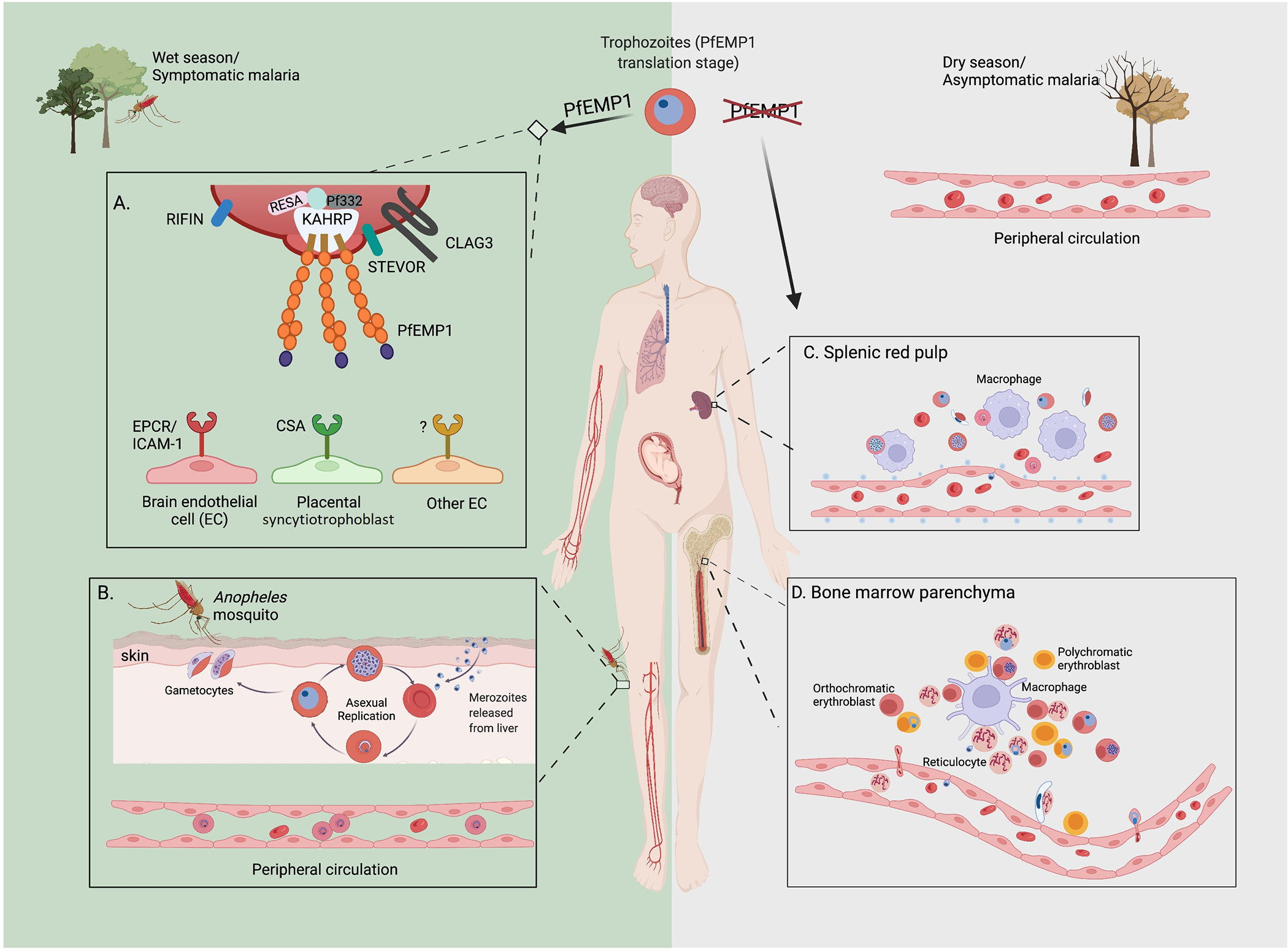
Schematic representation of the roles of PfEMP1, cytoadherence and sequestration of infected RBCs in symptomatic and asymptomatic malaria. Left green box: Symptomatic malaria cases during a rainy/transmission season or any symptomatic malaria cases characterized by high parasitemia: in the latter half of 48-hour replicative cycle, parasite-infected RBCs display the surface protein PfEMP1, the primary component mediating adhesion to variable receptors on the endothelial surface of the vasculature within different organs. This leads to sequestration of parasites in the deep tissue capillary beds, thereby avoiding filtration by the spleen. A. Display of PfEMP1 on the surface of infected RBC involves multiple other parasite-produced proteins, including knob associated histidine rich protein (KAHRP), Plasmodium falciparum 332 kd protein (Pf332), and ring expressed surface antigen (RESA). Other proteins displayed on the infected RBC surface include Rifins and Clag3. The specific PfEMP1 receptors on human endothelial cells associated with cerebral malaria include endothelial protein complex receptor (EPCR) and intracellular adhesion molecule 1 (ICAM-1), and pregnancy-associated malaria has been linked to the epithelial receptor chondroitin sulfate A (CSA) displayed on the surface of syncytiotrophoblasts, however receptors related to other malaria manifestations remain largely unknown. B. In the human host, P. falciparum asexual replication leads to exponential growth of infected erythrocytes, while less than 20% of parasites commit to sexual development generating female and male gametocytes. During blood meals, female Anopheles mosquitos ingest male and female gametocytes, where they mate, develop and produce sporozoites that will be inoculated into a new human host, thereby perpetuating the parasite’s life cycle. Due to sequestration and splenic filtration, only the early stages of the asexual cycle (ring stages) are observed in blood smears of symptomatic malaria cases. Right gray box: Asymptomatic malaria cases during a dry season or after a lengthy infection: cases manifest as extremely low parasitemias and routine detection methods (blood smears and PCR) often fail to detect the parasites. In this review, we present a model that parasites not expressing PfEMP1 are primarily responsible for chronic, asymptomatic infections. Non-PfEMP1 expressing parasites lose the ability to cytoadhere, hence parasites numbers are held in check and infected humans don’t suffer from severe symptoms. C. The vast majority of infected RBCs are filtered and pitted by the spleen. There is evidence that some parasites can complete both sexual and asexual development inside the red pulp, however only infected RBCs that remain deformable, including ring stages and stage V gametocytes, can travel back to the circulation (the function of spleen during malaria infections was comprehensively discussed by Henry et al [54]. D. The bone marrow is another possible reservoir for parasites escaping both immune elimination and spleen clearance. Merozoites are likely the only form that can travel from the sinusoids to the bone marrow parenchyma. In the parenchyma, both asexual and sexually committed merozoites could invade and develop in erythrocyte precursor cells within erythroblastic islands. While orthochromatic cells can support merozoite entry, only enucleating reticulocytes support intracellular parasites growth [43]. Due the loss of deformability, only ring-infected or stage V gametocyte-infected reticulocytes can return to the peripheral blood. Neveu et al. [40] have shown that infection of erythroblasts by gametocytes and parasite-derived extracellular vesicles delay erythroid differentiation, enabling the stage V gametocyte-infected reticulocytes to successfully leave the bone marrow parenchyma, however it is unknown if asexual parasites have a similar mechanism. It is possible that asexual parasites instead tend to stay in the parenchyma where the bone marrow environment promotes sexual commitment. Thus, the bone marrow could serve as a reservoir for gametocytes, with occasional release of ring-infected reticulocytes.
The adhesive protein expressed on the surface of infected RBCs is called Plasmodium falciparum erythrocyte membrane protein one (PfEMP1) [5–7]. This protein contains a single transmembrane domain which spans the membrane of the infected RBC, thus positioning the cytoadhesive domains of the protein extracellularly where they can mediate cytoadherence. By residing within the RBC, the parasite is largely hidden from the immune system. However, the placement of PfEMP1 on the RBC surface stimulates production of a robust antibody response. As antibody titers rise, infected RBCs are eliminated and the parasitemia falls accordingly. However, subpopulations of parasites arise that express alternative, antigenically distinct forms of PfEMP1 and thus avoid antibody recognition, thereby reestablishing high parasitemias and the associated symptoms. This concept of sequential recognition of variant surface antigens represents the current model of antigenic variation that leads to the cyclical waves of parasitemia that are characteristic of infections by P. falciparum (Figure 2A) [8]. Experimental validation of this model in natural infections is difficult for both ethical and logistical reasons, however field studies have provided important data in support of the model [9,10].
Figure 2.
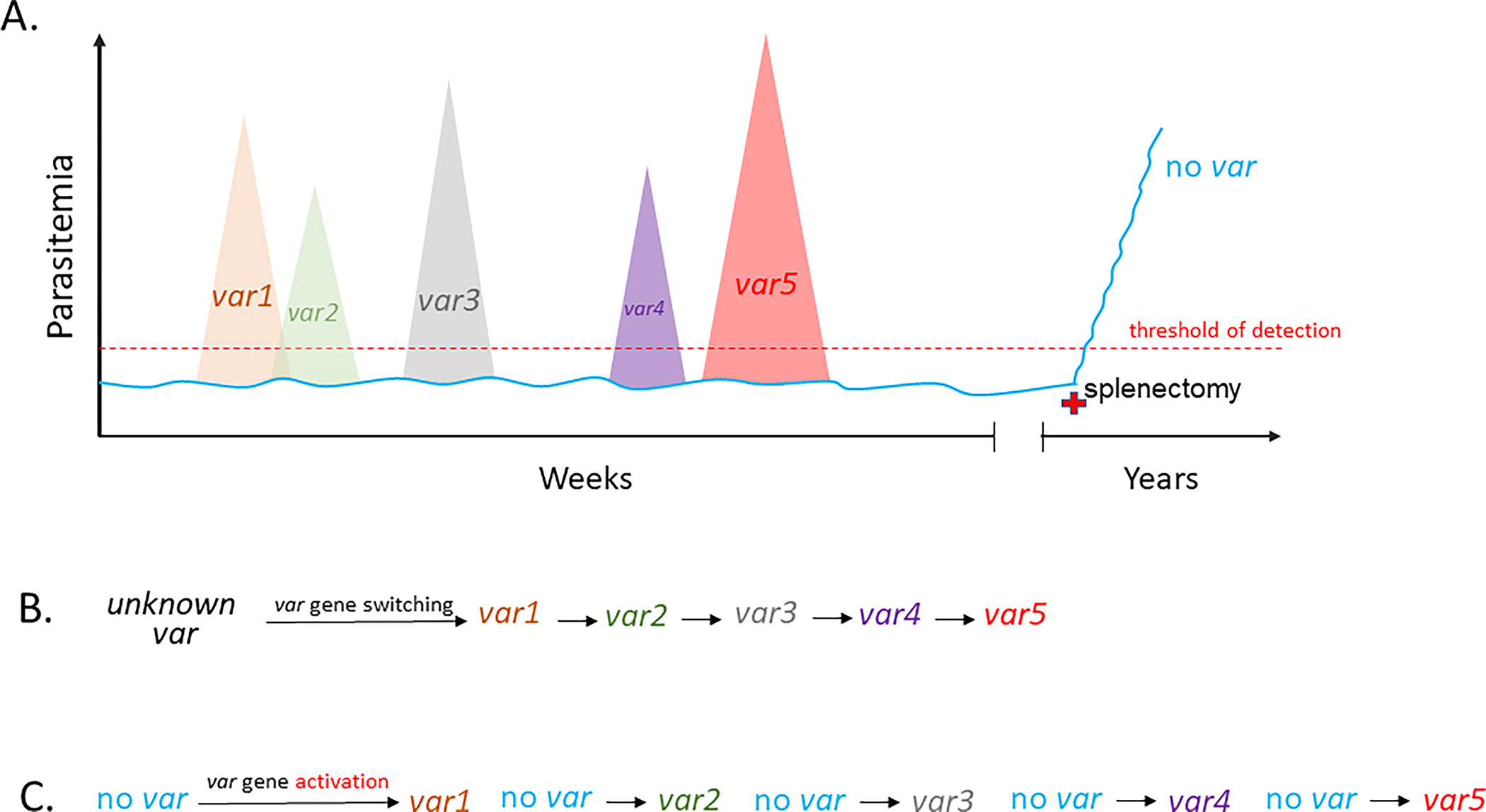
Changes in parasitemia over the course of a hypothetical Plasmodium falciparum infection. A. Levels of parasitemia are shown on the vertical axis while time is shown horizontally. Large waves of parasitemia are indicative of activation of specific var genes, leading to surface display of distinct forms of PfEMP1 and cytoadhesion of the infected RBCs, thereby avoiding clearance by the spleen. Expansion of these parasite populations is countered by the production of anti-PfEMP1 antibodies, resulting in rapid reductions in parasitemia. Activation of alternative var genes leads to new waves of parasitemia, shown with different colors, until the var repertoire is exhausted. Low levels of non-cytoadherent, non-PfEMP1 expressing parasites (blue line) that do not express var genes can persist below the threshold of detection (red dashed line). These parasites can serve as a reservoir for each new wave of parasitemia while avoiding the antibody response and extending the infection indefinitely. This population of parasites is prevented from expanding due to filtration by the spleen, however splenectomy (red cross) can lead to rapid expansion of this non-cytoadherent population. B and C. Switches in var gene expression underlying the waves of parasitemia shown in A. B displays switching directly from one var to the next while C displays independent activation of each var gene from a low level, persistent population that does not express var genes. The “no var” state could be identical to or derived from parasites that express a very low level of numerous var genes, referred to as the “many” state, that has been observed in cultured parasites.
Different forms of PfEMP1 are encoded by members of the multicopy var gene family [5–7]. Parasites express only one var gene at a time and switching which var gene is transcriptionally active leads to the antigenic variation described above. This leads to lengthy, chronic infections that can last well over a year [11,12]; however, it is thought that parasites could eventually exhaust their var repertoire, leading to clearance of the infection [12–14]. The genome of any given parasite encodes between 45–90 var genes and different parasites isolates differ greatly in their var repertoires, thus there are likely thousands of PfEMP1 variants circulating in any geographical area [15–17]. The antigenic diversity displayed by different parasites enables people to be infected repeatedly, although overtime individuals ultimately develop sufficiently broad antibody protection to prevent high parasitemias and severe illness [18]. The acquisition of this type of semi-sterile immunity occurs progressively as individuals age within endemic regions and is both age and exposure dependent [19], consistent with accumulation of antibody responses that recognize a broad range of PfEMP1 types. Protection appears to depend on the generation of antibodies against the more pathogenic forms of PfEMP1, specifically what are called type A variants that bind to the endothelial receptor EPCR [20]. Additional evidence for the key role of anti-PfEMP1 antibodies in naturally acquired immunity is provided by the sudden vulnerability of women to severe malaria during their first pregnancy [21]. This results from expression a form of PfEMP1 that binds exclusively to a placental-specific receptor [22] and is only expressed by parasites when they infected pregnant women. After exposure to this form of PfEMP1 and the production of corresponding antibodies, women are protected from subsequent bouts of pregnancy associated malaria and regain the semi-sterile immunity associated with age [23–25], thus strongly implicating anti-PfEMP1 antibodies as the primary factor limiting disease in asymptomatic individuals.
Examples of lengthy asymptomatic infections
Chronic malaria infections provide parasites with prolonged opportunities for transmission to their definitive host, the female Anopheles mosquito. This is particularly important in geographical regions with extended dry seasons where the mosquito vector can be absent for up to six months at a time. Given that P. falciparum does not have a dormant liver stage like some other Plasmodium species, it must maintain continuous asexual replication and gametocytogenesis within the human host to bridge periods of zero transmission. Thus for P. falciparum to remain endemic in regions of seasonal transmission, the ability to maintain a chronic infection is paramount. Investigations into infections that persist over the course of a dry season have found that they are generally asymptomatic and consequently the infected individuals do not seek treatment [26–29]. Such infections could therefore function as important parasite reservoirs that serve as a source of new infections upon the return of the mosquito vectors. This concern is reinforced by recent studies indicating that in some areas the majority of transmission is driven by asymptomatic carriers [30]. Extreme examples of chronic infection that persist in individuals who no longer reside within areas of active transmission, sometimes lasting over a decade, have also been described and confirmed [12]. How parasites can maintain continuous infections for such extended time periods without exhausting their repertoire of variant antigens is not understood, however recent reports from a study in Mali have found that these infections are characterized by parasites that remain in the circulation longer over the course of the asexual cycle, suggesting reduced cytoadhesive capacity, resulting in increased splenic clearance and lower parasitemias [26,31,32]. This could implicate a change in PfEMP1 expression, although the details remain obscure.
Asymptomatic P. falciparum infections that have remained undetected for years are often detected upon splenectomy, after which parasitemias rapidly rise thus revealing previously undiagnosed infections [33–35]. This provides additional evidence that the persisting parasites are poorly cytoadherent and thus the population is largely controlled through filtration by the spleen. These observations suggest that when an infecting parasite population has exhausted its variant antigen repertoire, parasites can stop expressing PfEMP1, enabling them to avoid recognition by PfEMP1-specific antibodies and thus allowing them to persist indefinitely despite a robust antibody response. This hypothetical situation would mostly likely happen in older individuals who had been infected repeatedly and thus have a high titer of broadly reactive anti-PfEMP1 antibodies. Such parasites would not be cytoadherent and thus would be very efficiently cleared by the spleen (Figure 1C), explaining both the asymptomatic nature of the infections as well as the rapid increase in parasitemias upon splenectomy. A detailed case study by Bachmann and colleagues provides strong evidence for this model [33]. They described a patient in Germany who tested negative for parasites by examination of thick and thin blood films as well as by malaria antigen tests. However, upon splenectomy, the patient developed a high parasitemia that included late trophozoites and schizonts in the peripheral circulation, stages that are typically cytoadherent and thus not observed on blood films. Interestingly, immature gametocytes, which are typically sequestered in the bone marrow, also were observed in the blood smears, suggesting that the spleen similarly selects for sequestration of these stages. Closer examination of samples obtained directly from the patient found that the infected RBCs were not cytoadherent and var gene expression was not detectable. Remarkably, after in vitro culture of the parasites for several weeks, the infected cells regained cytoadherent properties and var gene transcripts were easily detected, indicating that the non-cytoadhesive phenotype was reversible. This example suggests that in addition to antigenic variation through var gene switching, parasites can also assume a state in which var genes are not expressed, thus rendering the infected RBCs non-cytoadherent and invulnerable to anti-PfEMP1 antibodies. Such populations of parasites would be largely controlled through splenic clearance but could potentially persist at low levels for extended periods and thereby contribute to transmission. Similarly, non-PfEMP1 parasites might also be favored at the end of a dry season when only parasites that have persisted through a lengthy infection remain and which will have cycled through most or all of their variant repertoire.
A var gene switch-intermediate state
The possibility that parasites can enter a state in which they do not express var genes is supported by recent studies on var gene transcriptional switching. For example, it has been observed that in cultured parasites the level of var gene expression can vary dramatically, including populations where var gene expression is exceptionally low [36]. More recently, it was shown that in addition to switching between var genes, parasites can also enter a state where total var gene expression is very low and heterogeneous (X. Zhang et al., unpublished; X. Zhang et al., abstract 49, and F. Florini et al., abstract 212, 32nd Molecular Parasitology Meeting, Woods Hole, MA, October, 2021), a state that was previously proposed to explain var switching patterns observed in cultured parasites and suggested to possibly represent a switching-intermediate state [37]. The similarity of such cultured parasites to the long-term persistent parasites recovered from splenectomized patients suggests that this state is not simply something observed in culture, but could represent the non-PfEMP1 expressing, non-cytoadherent parasites detected in long-term asymptomatic infections.
The possibility that parasites don’t always express PfEMP1 and that non-PfEMP1 expressing parasites can persist in infected individuals changes our understanding of antigenic variation in P. falciparum. It was previously assumed that the waves of parasitemia observed over the course of an infection result from coordinated transcriptional switching from one var gene to the next (Figure 2A and B), thus leading to rising and falling parasite populations, each expressing a single or very small number of var genes. However, this model requires coordination of var gene switching to ensure that all surviving parasites switch to the same gene, a formidable challenge considering that the number of individual parasites in an infected individual can number in the billions. In contrast, if a small population of non-PfEMP1 expressing parasites exists within an infected individual, it could serve as a reservoir that seeds each wave of parasitemia independently. Specifically, while a population of non-PfEMP1 expressing parasites would presumably be held in check by splenic clearance, any parasites that activated expression of a var would become cytoadhesive, thus avoiding removal by the spleen, leading to rapid proliferation and resulting in a wave of nearly homogenous parasites. Subsequent waves of parasitemia could arise from similar, independent switching events, all originating from the same, low-level non-PfEMP1 expressing population (Figure 2A and C). No coordination of var gene switching is required. This model also provides an explanation for the rapid proliferation of non-cytoadherent parasites upon splenectomy.
Conclusions: Survival and function of non-cytoadherent parasites
P. falciparum infected RBCs are known to employ cytoadhesion to avoid filtration and destruction by the spleen. However, the rapid proliferation of non-cytoadhesive parasites after splenectomy in asymptomatic patients suggests that non-PfEMP1 expressing parasites can persist in vivo at very low levels, possibly below the level of detection by PCR (~1 parasite/μl) or rapid diagnostic tests. If true, where are these parasites hiding? Examination of the removed spleens in the examples of long-term symptomatic infections mentioned above identified significant amounts of hemozoin, suggesting that most parasites are in fact successfully removed from the circulation and destroyed [33,38]. Any persisting parasites are therefore likely to be avoiding the peripheral circulation through retention in an anatomical region with reduced blood flow. The bone marrow is one such place (Figure 1D) and has been shown to be the site of retention of gametocyte infected RBCs [39,40] which sequester through changes in RBC deformability rather than cytoadhesion [41]. Examination of bone marrow samples also consistently detect asexual stages, consistent with this hypothesis [39,42,43]. Future studies, for example employing rapidly advancing single cell technologies, could be used to test this hypothesis by examining var gene expression in asexual parasites obtained from bone marrow samples, or to identify the rare late-stage parasites in the peripheral circulation of asymptomatic individuals. Such studies could also investigate the other variant gene families (rif, stevor, Pfmc-2TM), whose potential role in chronic infections remains essentially unstudied.
The ability of malaria parasites to maintain chronic infections is important for their ability to successfully transmit between hosts. This is particularly vital in geographical regions that experience extended periods when the mosquito vector is absent, for example in places that have lengthy dry seasons. Infections must be maintained at least long enough to bridge the transmission seasons, and even lengthier infections are likely to be advantageous. While antigenic variation can extend the length of infections in individuals with little acquired immunity, individuals living in highly endemic regions generally develop broad antibody recognition of variant surface antigens [44–46], thus limiting this strategy for extending infections. In P. vivax [47] and possibly P. ovale [48,49], this function can be fulfilled through the formation of dormant liver stages called hypnozoites which can reactivate after extended time periods, thus bridging periods when transmission is absent. However, P. falciparum does not form hypnozoites and thus must maintain continuous asexual replication throughout time periods when the vector is absent. The persistence of non-PfEMP1 expressing parasites in asymptomatic individuals could fulfill this evolutionarily important function. Other mechanisms likely also contribute to chronic infections, including mitotic recombination generating new var genes [50–52] or a possible dormant population of non-replicating parasites [53]. Importantly, given that asymptomatic carriers seldom seek treatment, such infections could prove to be a significant obstacle to malaria elimination and eventual eradication.
Highlights.
Malaria caused by P. falciparum can result in chronic infections lasting years.
Loss of surface antigen expression enables parasites to avoid immune detection.
Long-term, persistent infections are typically asymptomatic and left untreated.
Asymptomatic infections can span dry seasons and serve as a transmission reservoir.
Funding:
This work was supported by National Institutes of Health grants AI52390 and AI99327 to KWD. The Department of Microbiology and Immunology at Weill Medical College of Cornell University acknowledges the support of the William Randolph Hearst Foundation.
Footnotes
Declarations of interest: none.
References
* of special interest
** of outstanding interest
- 1.WHO: World Malaria Report 2021. 2021.
- 2.Moxon CA, Grau GE, Craig AG: Malaria: modification of the red blood cell and consequences in the human host. Br. J. Haematol 2011, 154:670–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deitsch KW, Dzikowski R: Variant Gene Expression and Antigenic Variation by Malaria Parasites. Annu. Rev. Microbiol 2017, 71:625–641. [DOI] [PubMed] [Google Scholar]
- 4.Bernabeu M, Smith JD: EPCR and Malaria Severity: The Center of a Perfect Storm. Trends Parasitol 2017, 33:295–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baruch DI, Pasloske BL, Singh HB, Bi X, Ma XC, Feldman M, Taraschi TF, Howard RJ: Cloning the P. falciparum gene encoding PfEMP1, a malarial variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell 1995, 82:77–87. [DOI] [PubMed] [Google Scholar]
- 6.Smith JD, Chitnis CE, Craig AG, Roberts DJ, Hudson-Taylor DE, Peterson DS, Pinches R, Newbold CI, Miller LH: Switches in expression of Plasmodium falciparum var genes correlate with changes in antigenic and cytoadherent phenotypes of infected erythrocytes. Cell 1995, 82:101–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Su X, Heatwole VM, Wertheimer SP, Guinet F, Herrfeldt JV, Peterson DS, Ravetch JV, Wellems TE: A large and diverse gene family (var) encodes 200–350 kD proteins implicated in the antigenic variation and cytoadherence of Plasmodium falciparum-infected erythrocytes. Cell 1995, 82:89–100. [DOI] [PubMed] [Google Scholar]
- 8.Miller LH, Baruch DI, Marsh K, Doumbo OK: The pathogenic basis of malaria. Nature 2002, 415:673–679. [DOI] [PubMed] [Google Scholar]
- 9.Staalsoe T, Hamad AA, Hviid L, Elhassan IM, Arnot DE, Theander TG: In vivo switching between variant surface antigens in human Plasmodium falciparum infection. Journal of Infectious Diseases 2002, 186:719–722. [DOI] [PubMed] [Google Scholar]
- 10.Warimwe GM, Recker M, Kiragu EW, Buckee CO, Wambua J, Musyoki JN, Marsh K, Bull PC: Plasmodium falciparum var gene expression homogeneity as a marker of the host-parasite relationship under different levels of naturally acquired immunity to malaria. PLoS One 2013, 8:e70467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller LH, Good MF, Milon G: Malaria Pathogenesis. Science 1994, 264:1878–1883. [DOI] [PubMed] [Google Scholar]
- 12.Ashley EA, White NJ: The duration of Plasmodium falciparum infections. Malar J 2014, 13:500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roe MS, O’Flaherty K, Fowkes FJI: Can malaria parasites be spontaneously cleared? Trends Parasitol 2022. [DOI] [PubMed] [Google Scholar]
- 14.Childs LM, Buckee CO: Dissecting the determinants of malaria chronicity: why within-host models struggle to reproduce infection dynamics. J R Soc Interface 2015, 12:20141379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Otto TD, Bohme U, Sanders M, Reid A, Bruske EI, Duffy CW, Bull PC, Pearson RD, Abdi A, Dimonte S, et al. : Long read assemblies of geographically dispersed Plasmodium falciparum isolates reveal highly structured subtelomeres. Wellcome. Open. Res 2018, 3:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Otto T, Assefa S, Böhme U, Sanders M, Kwiatkowski D, Berriman M, Newbold D: Evolutionary analysis of the most polymorphic gene family in falciparum malaria. Wellcome Open Res 2019, 4:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Day KP, Artzy-Randrup Y, Tiedje KE, Rougeron V, Chen DS, Rask TS, Rorick MM, Migot-Nabias F, Deloron P, Luty AJF, et al. : Evidence of strain structure in Plasmodium falciparum var gene repertoires in children from Gabon, West Africa. Proc Natl Acad Sci U S A 2017, 114:E4103–E4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Flaherty K, Roe M, Fowkes FJI: The role of naturally acquired antimalarial antibodies in subclinical Plasmodium spp. infection. J Leukoc Biol 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trape J-T, Rogier C: Combating malaria morbidity and mortality by reducing transmission. Parasitology Today 1996, 12:236–240. [DOI] [PubMed] [Google Scholar]
- 20.Obeng-Adjei N, Larremore DB, Turner L, Ongoiba A, Li S, Doumbo S, Yazew TB, Kayentao K, Miller LH, Traore B, et al. : Longitudinal analysis of naturally acquired PfEMP1 CIDR domain variant antibodies identifies associations with malaria protection. JCI Insight 2020, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Desai M, ter Kuile FO, Nosten F, McGready R, Asamoa K, Brabin B, Newman RD: Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis 2007, 7:93–104. [DOI] [PubMed] [Google Scholar]
- 22.Salanti A, Staalsoe T, Lavstsen T, Jensen ATR, Sowa MPK, Arnot DE, Hviid L, Theander TG: Selective upregulation of a single distinctly structured var gene in chondroitin sulphate A-adhering Plasmodium falciparum involved in pregnancy-associated malaria. Molecular Microbiology 2003, 49:179–191. [DOI] [PubMed] [Google Scholar]
- 23.Maubert B, Fievet N, Tami G, Cot M, Boudin C, Deloron P: Development of antibodies against chondroitin sulfate A-adherent Plasmodium falciparum in pregnant women. Infect. Immun 1999, 67:5367–5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oleinikov AV, Rossnagle E, Francis S, Mutabingwa TK, Fried M, Duffy PE: Effects of sex, parity, and sequence variation on seroreactivity to candidate pregnancy malaria vaccine antigens. J. Infect. Dis 2007, 196:155–164. [DOI] [PubMed] [Google Scholar]
- 25.Salanti A, Dahlback M, Turner L, Nielsen MA, Barfod L, Magistrado P, Jensen AT, Lavstsen T, Ofori MF, Marsh K, et al. : Evidence for the involvement of VAR2CSA in pregnancy-associated malaria. J. Exp. Med 2004, 200:1197–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.**.Andrade CM, Fleckenstein H, Thomson-Luque R, Doumbo S, Lima NF, Anderson C, Hibbert J, Hopp CS, Tran TM, Li S, et al. : Increased circulation time of Plasmodium falciparum underlies persistent asymptomatic infection in the dry season. Nat Med 2020, 26:1929–1940. [DOI] [PubMed] [Google Scholar]
- 27.Cohee LM, Valim C, Coalson JE, Nyambalo A, Chilombe M, Ngwira A, Bauleni A, Seydel KB, Wilson ML, Taylor TE, et al. : School-based screening and treatment may reduce P. falciparum transmission. Sci Rep 2021, 11:6905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bejon P: Malaria parasites hide in plain sight in the dry season. Nat Med 2020, 26:1816–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Collins KA, Ceesay S, Drammeh S, Jaiteh FK, Guery MA, Lanke K, Grignard L, Stone W, Conway DJ, D’Alessandro U, et al. : A cohort study on the duration of Plasmodium falciparum infections during the dry season in The Gambia. J Infect Dis 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.**.Andolina C, Rek JC, Briggs J, Okoth J, Musiime A, Ramjith J, Teyssier N, Conrad M, Nankabirwa JI, Lanke K, et al. : Sources of persistent malaria transmission in a setting with effective malaria control in eastern Uganda: a longitudinal, observational cohort study. Lancet Infect Dis 2021, 21:1568–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.**.Thomson-Luque R, Votborg-Novel L, Ndovie W, Andrade CM, Niangaly M, Attipa C, Lima NF, Coulibaly D, Doumtabe D, Guindo B, et al. : Plasmodium falciparum transcription in different clinical presentations of malaria associates with circulation time of infected erythrocytes. Nat Commun 2021, 12:4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.*.Nyarko PB, Claessens A: Understanding Host-Pathogen-Vector Interactions with Chronic Asymptomatic Malaria Infections. Trends Parasitol 2021, 37:195–204. [DOI] [PubMed] [Google Scholar]
- 33.Bachmann A, Esser C, Petter M, Predehl S, von K, V, Schmiedel S, Bruchhaus I, Tannich E: Absence of erythrocyte sequestration and lack of multicopy gene family expression in Plasmodium falciparum from a splenectomized malaria patient. PLoS. ONE 2009, 4:e7459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Howden BP, Vaddadi G, Manitta J, Grayson ML: Chronic falciparum malaria causing massive splenomegaly 9 years after leaving an endemic area. Med J Aust 2005, 182:186–188. [DOI] [PubMed] [Google Scholar]
- 35.Bidegain F, Berry A, Alvarez M, Verhille O, Huguet F, Brousset P, Pris J, Marchou B, Magnaval JF: Acute Plasmodium falciparum malaria following splenectomy for suspected lymphoma in 2 patients. Clin Infect Dis 2005, 40:e97–100. [DOI] [PubMed] [Google Scholar]
- 36.Merrick CJ, Jiang RH, Skillman KM, Samarakoon U, Moore RM, Dzikowski R, Ferdig MT, Duraisingh MT: Functional analysis of sirtuin genes in multiple Plasmodium falciparum strains. PLoS. ONE 2015, 10:e0118865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Recker M, Buckee CO, Serazin A, Kyes S, Pinches R, Christodoulou Z, Springer AL, Gupta S, Newbold CI: Antigenic variation in Plasmodium falciparum malaria involves a highly structured switching pattern. PLoS. Pathog 2011, 7:e1001306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.*.Kho S, Qotrunnada L, Leonardo L, Andries B, Wardani PAI, Fricot A, Henry B, Hardy D, Margyaningsih NI, Apriyanti D, et al. : Hidden Biomass of Intact Malaria Parasites in the Human Spleen. N Engl J Med 2021, 384:2067–2069. [DOI] [PubMed] [Google Scholar]
- 39.Joice R, Nilsson SK, Montgomery J, Dankwa S, Egan E, Morahan B, Seydel KB, Bertuccini L, Alano P, Williamson KC, et al. : Plasmodium falciparum transmission stages accumulate in the human bone marrow. Sci. Transl. Med 2014, 6:244re245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neveu G, Richard C, Dupuy F, Behera P, Volpe F, Subramani PA, Marcel-Zerrougui B, Vallin P, Andrieu M, Minz AM, et al. : Plasmodium falciparum sexual parasites develop in human erythroblasts and affect erythropoiesis. Blood 2020, 136:1381–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tiburcio M, Niang M, Deplaine G, Perrot S, Bischoff E, Ndour PA, Silvestrini F, Khattab A, Milon G, David PH, et al. : A switch in infected erythrocyte deformability at the maturation and blood circulation of Plasmodium falciparum transmission stages. Blood 2012, 119:e172–e180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wickramasinghe SN, Phillips RE, Looareesuwan S, Warrell DA, Hughes M: The bone marrow in human cerebral malaria: parasite sequestration within sinusoids. Br J Haematol 1987, 66:295–306. [DOI] [PubMed] [Google Scholar]
- 43.Tamez PA, Liu H, Fernandez-Pol S, Haldar K, Wickrema A: Stage-specific susceptibility of human erythroblasts to Plasmodium falciparum malaria infection. Blood 2009, 114:3652–3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marsh K, Otoo L, Hayes RJ, Carson DC, Greenwood BM: Antibodies to blood stage antigens of Plasmodium falciparum in rural Gambians and their relation to protection against infection. Trans R Soc Trop Med Hyg 1989, 83:293–303. [DOI] [PubMed] [Google Scholar]
- 45.Bull PC, Lowe BS, Kortok M, Molyneux CS, Newbold CI, Marsh K: Parasite antigens on the infected red cell surface are targets for naturally acquired immunity to malaria. Nat. Med 1998, 4:358–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giha HA, Staalsoe T, Dodoo D, Roper C, Satti GM, Arnot DE, Hviid L, Theander TG: Antibodies to variable Plasmodium falciparum-infected erythrocyte surface antigens are associated with protection from novel malaria infections. Immunol. Lett 2000, 71:117–126. [DOI] [PubMed] [Google Scholar]
- 47.*.Angrisano F, Robinson LJ : Plasmodium vivax - How hidden reservoirs hinder global malaria elimination. Parasitol Int 2022, 87:102526. [DOI] [PubMed] [Google Scholar]
- 48.Groger M, Fischer HS, Veletzky L, Lalremruata A, Ramharter M: A systematic review of the clinical presentation, treatment and relapse characteristics of human Plasmodium ovale malaria. Malar J 2017, 16:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Richter J, Franken G, Mehlhorn H, Labisch A, Haussinger D: What is the evidence for the existence of Plasmodium ovale hypnozoites? Parasitol Res 2010, 107:1285–1290. [DOI] [PubMed] [Google Scholar]
- 50.Bopp SE, Manary MJ, Bright AT, Johnston GL, Dharia NV, Luna FL, McCormack S, Plouffe D, McNamara CW, Walker JR, et al. : Mitotic evolution of Plasmodium falciparum shows a stable core genome but recombination in antigen families. PLoS. Genet 2013, 9:e1003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Claessens A, Hamilton WL, Kekre M, Otto TD, Faizullabhoy A, Rayner JC, Kwiatkowski D: Generation of antigenic diversity in Plasmodium falciparum by structured rearrangement of Var genes during mitosis. PLoS. Genet 2014, 10:e1004812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang X, Alexander N, Leonardi I, Mason C, Kirkman LA, Deitsch KW: Rapid antigen diversification through mitotic recombination in the human malaria parasite Plasmodium falciparum. PLoS. Biol 2019, 17:e3000271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Codd A, Teuscher F, Kyle DE, Cheng Q, Gatton ML: Artemisinin-induced parasite dormancy: a plausible mechanism for treatment failure. Malar J 2011, 10:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Henry B, Roussel C, Carucci M, Brousse V, Ndour PA, Buffet P: The Human Spleen in Malaria: Filter or Shelter? Trends Parasitol 2020, 36:435–446. [DOI] [PubMed] [Google Scholar]


