Abstract
Introduction:
Hypertriglyceridemia, a component of the metabolic syndrome, is a known independent predictor of albuminuria and chronic kidney disease (CKD) in the general population. Previous studies have shown that the relationship of triglycerides with outcomes changes across stages of CKD. Our objective is to examine the association of triglycerides independent of other metabolic syndrome components with renal outcomes in diabetic patients with or without CKD.
Methods:
This retrospective cohort study included diabetic US veteran patients with valid data on triglycerides (TG), estimated glomerular filtration rate (eGFR) and albuminuria (UACR), between fiscal years 2004 to 2006. Using Cox models adjusted for clinical characteristics and laboratory markers, we evaluated the relationship of TG with incident albuminuria (stratified by eGFR category) and based on eGFR (stratified by baseline albuminuria categories). To evaluate the relationship of TG with time to end-stage renal disease (ESRD), we stratified models by baseline CKD stage (eGFR category) and baseline albuminuria stage ascertained at time of TG measurement.
Results:
In a cohort of 138,675 diabetic veterans, the mean±SD age was 65±11 years old, and included 3% females and 14% African Americans. The cohort also included 28% of patients with non-dialysis dependent CKD (eGFR<60 mL/min/173m2), as well as 28% of patients with albuminuria (≥30 mg/g). The median (IQR) of serum TG was 148(100, 222) mg/dL. We observed a slight positive linear association between TG and incident CKD after adjustment for case-mix and laboratory variables among non-albuminuric and microalbuminuric patients. High TG levels were associated with ESRD in CKD 3A non-albuminuric patients and in CKD 3A and 4/5 in patients with microalbuminuria.
Discussion/Conclusion:
In a large cohort, we have shown that elevated TG are associated with all kidney outcomes tested independently of other metabolic syndrome components in diabetic patients with normal eGFR and normal albumin excretion rate, but the association is weaker in some groups of diabetic patients with pre-existing renal complications.
Keywords: Chronic Kidney Disease, Albuminuria, renal outcomes, triglycerides, metabolic syndrome
Introduction
Approximately 40% of patients with type II diabetes develop diabetic kidney disease, the leading cause of chronic kidney disease (CKD) and end stage renal disease (ESRD) in the US [1]. Whereas not all patients with type II diabetes develop CKD or ESRD, albuminuria, a main feature of diabetic kidney disease, can serve as a key factor associated with CKD, and is a preferred method for kidney disease staging and management [2]. Microalbuminuria and macroalbuminuria are defined as having a urinary albumin/creatinine ratio (UACR) of 30–300 mg/g and >300 mg/g, respectively. Both are shown to be early factors associated with kidney and cardiovascular disease in the general population [3]. The exact mechanism of action is not completely understood, however is believed to be due to endothelial damage of the renal glomeruli [4].
Hypertriglyceridemia, a component of metabolic syndrome, is also a known independent risk factor for albuminuria and kidney disease in the general population [5,6]. Compounding the situation further, hypertriglyceridemia is associated with obesity and insulin resistance, both of which contribute to the progression of kidney disease [7]. Previous studies have shown associations between each component of metabolic syndrome, including triglycerides, and renal outcomes, indicating the need to study this relationship in diabetic patients [6]. Studies evaluating the association between hypertriglyceridemia and albuminuria in patients with type II diabetes are scarce, while others have been conducted on patients with type I diabetes which is less associated with metabolic syndrome and insulin resistance. Additionally, studies on type II diabetes patients did not report microalbuminuria and macroalbuminuria values separately [8,9].
In this study, we sought to examine the relationship of triglycerides with incident microalbuminuria and macroalbuminuria, separately, among patients with UACR <30 mg/g, with consideration for other metabolic syndrome components. In addition, we evaluate the association of serum triglycerides and time to ESRD or incident CKD among and across different stages of kidney function and albuminuria.
Methods
Primary Study Population
The source population comprised patients who received at least one serum lipid measurement at any US Veterans Affairs (VA) Medical Center between fiscal years 2004 to 2006. The construction of LIPROVET (lipid profiles and management in veterans in CKD) has been previously described elsewhere [10]. Given the large sample size, non-intrusive nature, and patient anonymity, required written consent was waived. This study was approved by the institutional review board of the Tibor Rubin VA Medical Center of Long Beach, CA.
For this study, we excluded patients for missing a serum TG measurement during the study period, with ESRD, missing an estimated glomerular filtration rate (eGFR) measurement prior to the TG measurement, invalid censoring information, missing data on metabolic syndrome components, being non-diabetic and missing an UACR measurement prior to the TG measurement. The final study cohort included 138,675 diabetic patients with valid data on TG, eGFR and UACR (Supplemental Figure 1). We examined three outcomes: I) time to ESRD, II) time to incident CKD among 99,705 non-CKD patients, and III) time to incident albuminuria (Alb) among 100,129 Alb A1 patients.
Demographics and Clinical Measurements
Clinical records from the VA, Centers for Medicare and Medicaid Services (CMS), and the United States Renal Data System (USRDS) were combined for the ascertainment and measurement of demographic characteristics [10]. Data on select lipid, hypertension and diabetes medication at the time of the TG measurement were obtained from VA and CMS databases only. Combined VA and CMS databases sourced data on chronic conditions using ICD-9 codes recorded within two years prior to the TG measurement. Data on indications of ever being a smoker or ever being an alcoholic were measured by the VA databases only [11]. Patients with either a recent comorbid diagnosis or medication prescription for diabetes were included in this cohort [12].
Data on laboratory measurements, including TG and eGFR were only sourced from VA records. All low-density lipoprotein cholesterol values were calculated with the Martin-Hopkins equation, while all eGFR values were calculated with the CKD-EPI equation [13,14]. Laboratory measurements of UACR or divided measurements of urine albumin and urine creatinine recorded on the same day were used in analyses. Measurements indicating urine protein or proteinuria were not included as UACR. eGFR values were used for CKD staging: non-CKD, 3A, 3B, and combined 4/5 (>60, 45-<60, 30-<45 and <30 mL/min/1.73m2, respectively) [15]. UACR values were used for albuminuria staging as: Alb A1, A2 and A3 (<30, 30–300 and >300 mg/g, respectively) [16]. Metabolic syndrome components were defined by laboratory values, medications or chronic conditions, as applicable (Supplemental Table 1) [17]. Measurements of waist circumference were highly missing in the database and elevated body mass index was used as a proxy measure.
Exposure and Outcome Assessment
The earliest TG measurement within the study period were categorized into the following: <80, 80-<120, 120-<160 (reference), 160-<200, 200-<240 and ≥240 mg/dL [10]. We then explored the relationship between TG and the following outcomes: ESRD with the receipt of renal replacement therapy, incident CKD and incident albuminuria. Transition to ESRD was defined by the date of first service from USRDS records. Incident CKD among non-CKD patients was defined by having at least two eGFR values measured at least 90 days apart, where the eGFR was <60 mL/min/1.73m2 and never rose above this threshold over follow-up [15].
Incident albuminuria was similarly defined among Alb A1 patients using UACR measurements. Albuminuria categories for outcomes included ≥30, 30–300, and >300 mg/g. Patients were followed from the date of their TG measurement and were censored for lost to follow-up, death, ESRD, December 31, 2014 (administrative) or incidence of CKD or albuminuria.
Statistical Analysis
Clinical characteristics for the cohort and stratified by TG group are presented in Table 1. Data are presented as mean±standard deviation, median (interquartile range) or percentage.
Table 1.
Patient Characteristics Stratified by Serum Triglycerides Group
| Serum Triglycerides (mg/dL) | |||||||
|---|---|---|---|---|---|---|---|
| Total | <80 | 80-<120 | 120-<160 | 160-<200 | 200-<240 | ≥240 | |
| N(%) | 138,675 | 19,435(14%) | 29,922(22%) | 26,715(19%) | 19,631(14%) | 13,420(10%) | 29,552(21%) |
| eGFR(mL/min/1.73m2) | 73 [58,88] | 75 [60,90] | 73[58,88] | 72[57,87] | 72[57,87] | 72[57,88] | 74[58,91] |
| eGFR category (%) | |||||||
| G1/G2 | 72 | 75 | 72 | 71 | 70 | 71 | 72 |
| G3a | 18 | 16 | 18 | 19 | 19 | 19 | 17 |
| G3b | 8 | 7 | 8 | 9 | 9 | 9 | 9 |
| G4/G5 | 2 | 1 | 2 | 2 | 2 | 2 | 2 |
| Albuminuria Stage (%) | |||||||
| Al | 72 | 75 | 74 | 73 | 72 | 72 | 68 |
| A2 | 24 | 22 | 23 | 23 | 24 | 24 | 27 |
| A3 | 4 | 3 | 4 | 4 | 4 | 4 | 5 |
| Age (years) | 65±11 | 67±11 | 67±11 | 66±11 | 66±10 | 65±10 | 62±10 |
| Gender (% Female) | 3 | 2 | 2 | 3 | 3 | 3 | 3 |
| Race (%) | |||||||
| White | 81 | 70 | 78 | 82 | 84 | 85 | 86 |
| African American | 14 | 26 | 18 | 14 | 11 | 10 | 8 |
| Other | 5 | 4 | 5 | 4 | 5 | 5 | 5 |
| Hispanic Ethnicity (%) | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| CCI | 2[1,3] | 2[1,3] | 2[1,3] | 2[1,3] | 2[1,3] | 2[1,3] | 2[1,3] |
| Comorbid Conditions (%) | |||||||
| MI | 7 | 6 | 8 | 8 | 8 | 8 | 7 |
| CHF | 12 | 12 | 13 | 13 | 12 | 12 | 11 |
| PVD | 12 | 12 | 13 | 13 | 12 | 12 | 11 |
| Cerebrovascular Disease | 9 | 9 | 10 | 10 | 10 | 9 | 8 |
| Dementia | 2 | 2 | 2 | 1 | 2 | 2 | 1 |
| COPD | 17 | 16 | 17 | 17 | 17 | 17 | 17 |
| Liver Disease | 3 | 3 | 3 | 3 | 3 | 2 | 3 |
| Cancer | 11 | 12 | 12 | 12 | 11 | 11 | 9 |
| Anemia | 7 | 7 | 7 | 7 | 7 | 7 | 7 |
| Atrial Fibrillation | 7 | 7 | 7 | 7 | 7 | 6 | 6 |
| Hypertension | 85 | 82 | 85 | 85 | 85 | 85 | 85 |
| ISHD | 35 | 32 | 36 | 36 | 36 | 37 | 35 |
| Depression | 18 | 14 | 15 | 17 | 18 | 19 | 23 |
| Substance Abuse | 4 | 5 | 4 | 4 | 4 | 4 | 5 |
| Ever Smoking | 64 | 60 | 63 | 64 | 65 | 66 | 68 |
| Ever Alcoholism | 15 | 17 | 15 | 15 | 14 | 14 | 14 |
| Laboratory Measurements | |||||||
| Albumin (g/dL) | 4.0±0.4 | 3.9±0.4 | 4.0±0.4 | 4.0±0.4 | 4.1±0.4 | 4.1±0.4 | 4.1±0.4 |
| ALP (U/L) | 70[57,87] | 69[56,86] | 70[57,86] | 70[57,86] | 70[57,86] | 70[57,86] | 72[58,89] |
| AST (U/L) | 22[18,27] | 22[18,27] | 22[18,27] | 22 [18,27] | 22 [18,27] | 22[18,27] | 23[18,29] |
| ALT (U/L) | 25[18,35] | 22[17,32] | 23[17,33] | 24[18,34] | 25[18,35] | 25 [19,36] | 27[20,39] |
| Glucose (mg/dL) | 150.2±61.8 | 136.1±53.9 | 140.5±54.2 | 145.3±55.7 | 150.4±58.7 | 154.3±60.8 | 172.1±74.4 |
| Hemoglobin Ale (%) | 7.4±1.6 | 7.2±1.5 | 7.2±1.4 | 7.3±1.5 | 7.4±1.5 | 7.4±1.6 | 7.8±1.8 |
| Hemoglobin (g/dL) | 14.1±1.6 | 13.7±1.6 | 13.9±1.6 | 14.1±1.6 | 14.3±1.6 | 14.3±1.6 | 14.4±1.6 |
| WBC (x 103/mm3) | 7.3±2.7 | 6.8±2.5 | 7.2±2.7 | 7.3±2.7 | 7.5±2.9 | 7.5±2.3 | 7.6±2.6 |
| SBP (mmHg) | 136±19 | 135±19 | 136±19 | 136±19 | 136±19 | 136±19 | 137±19 |
| DBP (mmHg) | 74±11 | 72±11 | 73±11 | 73±11 | 74±11 | 74±11 | 75±11 |
| BMI (kg/m2) | 32±6 | 29±6 | 31±6 | 32±6 | 32±6 | 33±6 | 33±6 |
| Lipid Panel (mg/dL) | |||||||
| Triglycerides | 148[100,222] | 64[54,72] | 100[90,110] | 138[129,148] | 178[168,188] | 217[208,228] | 321 [271,416] |
| HDL | 38[33,46] | 46[39,56] | 41 [35,49] | 39[33,45] | 37[32,43] | 36[31,42] | 34[30,40] |
| Cholesterol | 164[142,190] | 147[128,169] | 153 [134,175] | 160[140,183] | 166[146,190] | 172[151,197] | 189[164,220] |
| LDL | 96[79,117] | 85[69,102] | 91 [76,110] | 96 [79,116] | 100[82,120] | 101 [85,124] | 105[86,129] |
| Medications (%) | |||||||
| Statin | 50 | 47 | 52 | 52 | 52 | 52 | 48 |
| Ezetimibe | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Non-statin | 12 | 7 | 8 | 11 | 12 | 14 | 19 |
| Fibrate | 8 | 3 | 5 | 7 | 8 | 10 | 14 |
| Niacin | 3 | 3 | 3 | 3 | 3 | 4 | 4 |
| Fish Oil | 0.3 | 0.2 | 0.2 | 0.3 | 0.3 | 0.5 | 0.6 |
| Bile Acid Sequestrants | 0.6 | 0.5 | 0.5 | 0.7 | 0.7 | 0.7 | 0.8 |
| RAAS inhibitor | 56 | 54 | 56 | 56 | 57 | 57 | 57 |
Data presented as mean ± standard deviation, median[interquartile range], or percentage, as appropriate.
Abbreviations: ALP; Alkaline Phosphatase, ALT; Alanine Aminotransferase, AST; Aspartate Aminotransferase, BMI; Body Mass Index, BUN; Blood Urea Nitrogen, CCI; Charlson Comorbidity Index, CHF; Congestive Heart Failure, CKD; Chronic Kidney Disease, COPD; Chronic Obstructive Pulmonary Disorder, DBP; Diastolic Blood Pressure, eGFR; estimated glomerular filtration rate, ESRD; end-stage renal disease, HDL; High Density Lipoprotein, ISHD; Ischemic Heart Disease, LDL; Low Density Lipoprotein, MI; Myocardial Infarction, PTSD; Post-traumatic Stress Disorder, PVD; Peripheral Vascular Disease, RAAS; renin-angiotensin aldosterone system, SBP; Systolic Blood Pressure, WBC; White Blood Cells Count
We used Cox proportional hazards models to evaluate the association of TG and our renal outcomes. Analyses were stratified by both CKD (eGFR category) and albuminuria stage, as applicable. Levels of covariate adjustments were the following: 1) unadjusted 2) age, 3) Case-Mix adjusted, which included age, gender, race, ethnicity, ever smoker, ever alcoholic, and the following comorbid conditions: Charlson comorbidity index, myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, chronic obstructive pulmonary disorder, dementia, liver disease, cancer, atrial fibrillation, hypertension, depression, ischemic heart disease, and prescription of statins, non-statins and renin-angiotensin-aldosterone system inhibitors (RAASi), 4) Case-Mix+Lab adjusted, which included the same variables as the Case-Mix model, as well as albumin, eGFR, logged UACR, BMI, hemoglobin A1c (HgbA1c) and HDL. We performed tests of trend to evaluate the linear relationship between very low (<80 mg/dL) and very high (≥240 mg/dL) levels of TG and outcomes, across ordinal levels of CKD stage. In sensitivity analyses, we also examined the fold-change in UACR measured at baseline and three years among N=79,413 Alb A1 patients with available data. Case-Mix+Lab adjusted logistic regressions were performed to evaluate the odds of a fold-change >1.5 (vs. ≤1.5), and stratified by baseline CKD stage as another evaluation of changes in albuminuria.
Data on clinical characteristics, smoking and alcoholism were missing for <0.4%, 3% and 15% of the cohort, respectively and were imputed using a missing category. Albumin, HgbA1c and HDL were missing for 30%, 18%, 0.4% of the cohort, respectively and were imputed by means. All analyses were performed using SAS Enterprise Guide (7.1)(Cary, NC).
Results
In a cohort of 138,675 diabetic veterans, the mean±SD was 65±11 years old, and included 3% females and 14% African Americans (Table 1). The cohort also included 28% of patients with non-dialysis dependent CKD, as well as 28% of patients with albuminuria (≥30 mg/g). The median(IQR) of serum TG was 148(100, 222) mg/dL. The patients with elevated TG were more likely to be younger, white, depressed, a smoker, obese and be prescribed a non-statin. Yet, these patients were also less likely to have had cancer and be an alcoholic. Similarly, we also stratified clinical characteristics by albuminuria stage (Supplemental Table 2). Patients with the highest level of albuminuria were less likely to be white, but have a higher prevalence of chronic conditions including cardiovascular disease subtypes.
Serum Triglycerides and Time to ESRD stratified by CKD (eGFR Category) and Albuminuria Stages
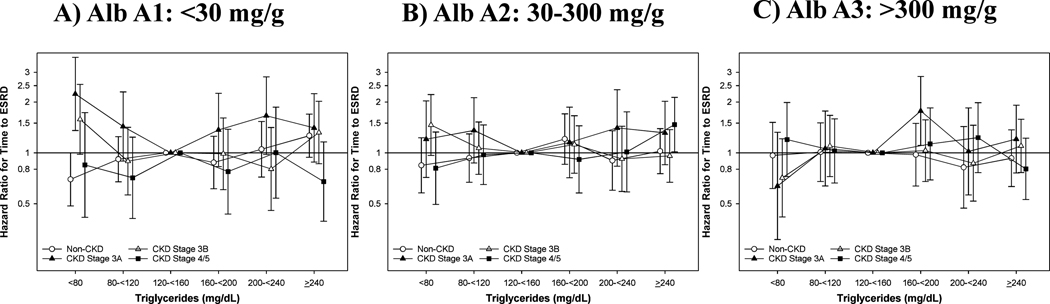
We observed 3,219 veterans who transition to ESRD over a follow-up of 9.6(7.2, 10.0) years, resulting in a crude rate of 2.82(2.72, 2.91) events per 1000 person-years (Supplemental Table 3A). In unadjusted analyses, patients with Alb A1 and non-CKD had a linear relationship between TG and transition to ESRD, while this relationship was U-shaped for those with CKD stage 3A-3B. Additional adjustments for Case-Mix and Lab covariates reflected a similar pattern for Alb A1 patients (Figure 1A, Supplemental Table 4A). Compared to the reference TG 120-<160 mg/dL, the positive relationship between TG ≥ 240 mg/dL and risk of ESRD transition diminished across CKD stage, where the relationship was attenuated for CKD stage 4/5 patients (p-trend =0.047). In Alb A1 patients, low TG <80 mg/dL were associated with a lower risk of ESRD transition in non-CKD patients, yet a higher risk in CKD stage 3A-3B patients.
Figure 1.
Association of Serum Triglycerides and Time to ESRD in Case-Mix+Lab Adjustment Across Stages of CKD and Albuminuria: A) Al, B) A2 and C) A3.
Case-Mix+Lab: age, gender, race, ethnicity, ever smoking, ever alcoholic, Charlson Comorbidity Index, myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, chronic pulmonary disease, dementia, liver disease, cancer, atrial fibrillation, hypertension, depression and ischemic heart disease, prescription of statins, non-statins and RAASi and albumin, eGFR, logged UACR, BMI, HgbAlc and HDL.
In Alb A2 patients, we observed an attenuated relationship between low TG<80 mg/dL and time to ESRD after adjustment for Case-Mix and Lab covariates (Figure 1B, Supplemental Table 4B). Likewise, we observed a U-shaped relationship with TG in CKD stage 3A patients only. The elevated risk with TG ≥ 240 mg/dL was observed in patients with CKD 3A and 4/5. Both non-CKD and CKD stage 3B patients with TG ≥ 240 mg/dL had a null risk with ESRD. Finally, patients with Alb A3 had a higher crude rate of transition to ESRD, yet relationships were less clear in this group. The relationship of low TG trended towards a lower risk of ESRD among CKD stage 3A and 3B patients compared to the reference (Figure 1C, Supplemental Table 4C). However, Alb A3 patients with elevated TG did not have a clear pattern in risk of ESRD across CKD stages (p-trend= 0.73). Notably, patients with both Alb A3 and CKD stage 3A, and TG 160-<200 mg/dL had a higher adjusted risk of ESRD compared to the reference.
Serum Triglycerides and Time to Incident CKD in Non-CKD patients stratified by Albuminuria Stage
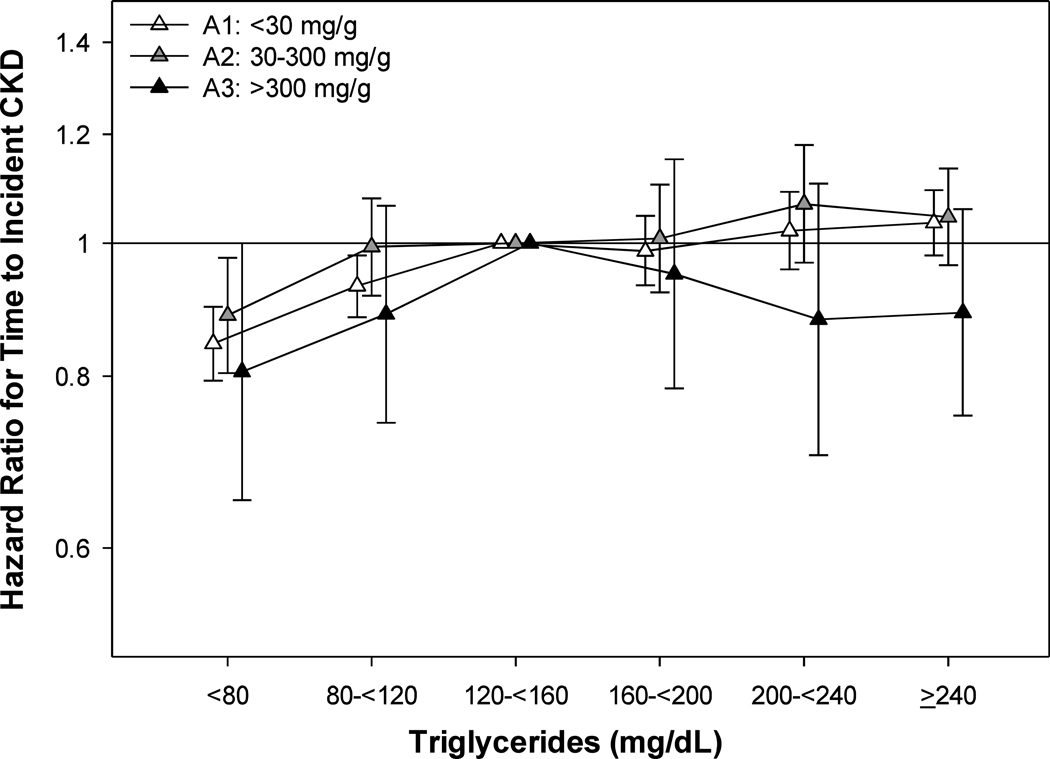
Among the 99,705 patients with non-CKD, we observed a crude rate of 26.7(26.4, 27.1) incident CKD events per 1000 person-years over follow-up (Supplemental Table 3B). In age-adjusted analyses, we observed a slight positive linear relationship between TG and time to incident CKD in Alb A1 and A2 patients, where those with high TG had a higher risk (Supplemental Table 5). Conversely, patients with Alb A3 yet elevated TG had a lower risk of incident CKD in age-adjusted analyses. These relationships were similar yet attenuated after further adjustment for Case-Mix and Lab covariates (Figure 2). Patients with low TG had a lower risk of incident CKD across all Alb stages, while the high risk for incident CKD and elevated TG were attenuated for Alb A1 and A2 patients. Patients with Alb A3 trended towards a lower risk of incident CKD for all TG levels.
Figure 2.
Association of Serum Triglycerides and Time to Incident CKD in Case-Mix+Lab Adjustment Among Non-CKD Patients and Across Albuminuria Stages.
Case-Mix+Lab: age, gender, race, ethnicity, ever smoking, ever alcoholic, Charlson Comorbidity Index, myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, chronic pulmonary disease, dementia, liver disease, cancer, atrial fibrillation, hypertension, depression and ischemic heart disease, prescription of statins, non-statins and RAASi and albumin, eGFR, logged UACR, BMI, HgbAlc and HDL.
Serum Triglycerides and Time to Incident Albuminuria in Alb A1 patients stratified by CKD Stage
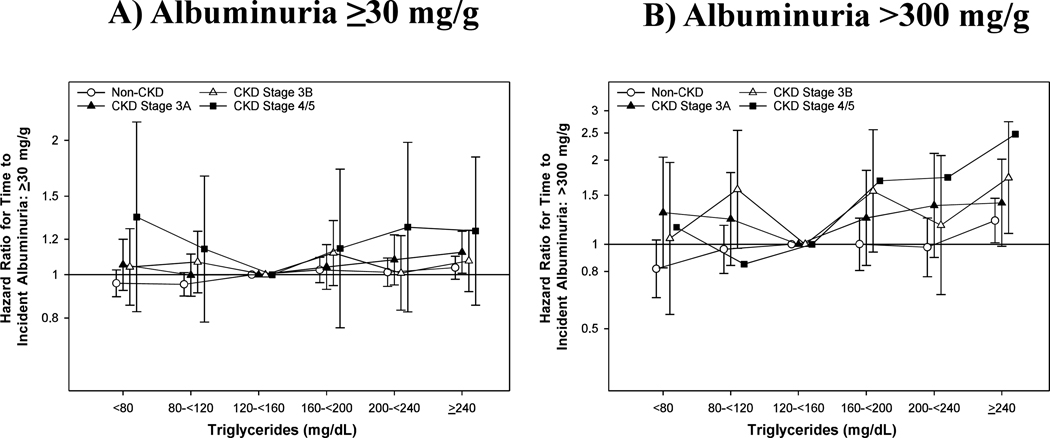
Finally in 100,129 patients with Alb A1, we observed a crude rate of 21.1(20.7, 21.4) incident albuminuria ≥ 30 mg/g per 1000 person-years (Supplemental Table 3). In unadjusted analyses, we observed a linear relationship between TG and time to incident albuminuria ≥ 30 mg/g in non-CKD, CKD stage 3A-3B patients, while the relationship for patients with CKD stage 4/5 was less clear (Supplemental Table 6). Case-mix and Lab adjustment identified a flat relationship between TG and incident albuminuria ≥ 30 mg/g for lower CKD stages (Figure 3A). Moreover, we observed a more pronounced U-shaped relationship with TG among CKD stage 4/5 patients, albeit with large confidence intervals. In CKD stage 4/5 patients, TG < 80 and ≥ 240 mg/dL were associated with a 1.35 and 1.25 times risk compared to the reference group. We observed a noticeable upward trend between high TG and incident albuminuria in increasing CKD stages (p-trend < 0.0001). Across levels of adjustment, the relationship with TG and incident albuminuria 30–300 mg/g were similar (Supplemental Figure 2).
Figure 3.
Association of Serum Triglycerides and Time to Incident Albuminuria of A) ≥30 mg/g and B) >300 mg/g in Case-Mix+Lab Adjustment Among Albuminuria Al Patients.
Case-Mix+Lab: age, gender, race, ethnicity, ever smoking, ever alcoholic, Charlson Comorbidity Index, myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, chronic pulmonary disease, dementia, liver disease, cancer, atrial fibrillation, hypertension, depression and ischemic heart disease, prescription of statins, non-statins and RAASi and albumin, eGFR, logged UACR, BMI, HgbAlc and HDL.
Finally, we explored the relationship between TG and incident albuminuria >300 mg/g. We observed a far lower crude rate of 1.95(1.85, 2.04) events per 1000 person-years. Like previous analyses, the relationship between TG and incident albuminuria >300 mg/g in non-CKD patients was linear after adjustment for Case-mix and Lab covariates (Figure 3B, Supplemental Table 7). The relationship for CKD stage 3A and 3B patients was somewhat attenuated, however we observed a higher risk of albuminuria >300 mg/g with high TG. The HR(95%CI) for TG ≥ 240 mg/dL for CKD stage 3A and 3B were 1.41(0.98, 2.21) and 1.73(1.09, 2.74), respectively. We were unable to sufficiently evaluate this relationship among CKD stage 4/5 patients given low power, yet also observed a similar increasing trend with advancing CKD stages(p-trend <0.0001).
Sensitivity Analyses
Among 79,413 patients with baseline Alb A1 and a subsequent UACR measurement within three years to the start of the study, we calculated a fold change to characterize the difference in UACR levels relative to a patient’s baseline value. For Alb Al patients, the odds ratio of an elevated fold >1.5 demonstrated a similar relationship to our incident albuminuria analyses (Supplemental Figure 3). Non-CKD and CKD stage 3A-3B patients demonstrated a flat relationship between TG and an elevated fold change, where non-CKD and CKD stage 3A patients had a higher odds. While the relationship among CKD stage 4/5 patients was largely attenuated, it similarly trended towards a higher odds of an elevated UACR change.
Discussion
The main finding of our study is that in patients with diabetes, normal eGFR and normal albumin excretion, triglycerides are positively associated with the time to proteinuria, onset of CKD defined as eGFR<60 mL/min/1.73m2, and ESRD, and that this relationship can be extended to some of the patients with CKD. In our previous study using data from the same cohort, higher triglycerides have also been identified as a risk factor for deterioration of eGFR and shortened time to renal replacement therapy [18]. However, the present study in patients with diabetes further defines the association by also examining albumin excretion data and shows this association seems to be limited to patients with normal baseline eGFR and albumin excretion rate and certain groups of patients with microalbuminuria and advanced CKD.
Our study on US veterans with diabetes confirms the findings from the general population where hypertriglyceridemia as a component of the metabolic syndrome has been shown to be independently associated with incident albuminuria. This was reported in Chinese [19], Korean [20], Japanese [21], Italian [22], and Native American [23] populations. The Chinese medical literature describes a risk factor for kidney disease and cardiovascular outcomes labeled “hypertriglyceridemic waist”, emphasizing the role of hypertriglyceridemia within the features of metabolic syndrome [24].
Type II diabetes studies have shown a similar association but most of the studies did not separate microalbuminuria from proteinuria. In the Italian Association of Clinical Diabetologists Study [9], which aimed to look at predictors of individual components of diabetic kidney disease and their relationship with traditional risk factors, for every 10 mg/dl higher triglyceride level, the risk of increased albumin excretion was 1% higher. Similarly the increase in risk was reported to be 12% per mmol in the Swedish National Diabetes Register [8], and 10% per mmol in a Finnish study [25]. In a single center study in Asian patients, the association was reported to be 39% higher per mmol [26], suggesting a stronger relationship in Asian patients. In another study, the time to onset of microalbuminuria was higher if more than 50% of triglyceride levels were above 150 mg/dl [27]. Similar studies were reported in patients with type I diabetes, where, as opposed to type II diabetes, metabolic syndrome and insulin resistance is not the main feature of the disease [28–29].
In our study, we separated microalbuminuria onset from proteinuria onset and we used time to onset as a measure of the effect. There was a shorter time to onset of proteinuria, while microalbuminuria was not associated with elevated triglycerides. This was true for all stages of CKD. In our study, we also reported that in patients without CKD (defined by eGFR≥60 ml/min/1.72m2) at baseline, low triglycerides were associated with a lower risk of development of proteinuria, but not microalbuminuria. Patients with diabetes and decreased eGFR have two distinct phenotypes: albuminuric and nonalbuminuric [30]. The albuminuric diabetic nephropathy is more rapidly progressing [31], and increases in UACR over time in this group is associated with subsequent decline of eGFR [32]. The transition from nonalbuminuric to albuminuric phenotype is insufficiently studied. In our study, 22.7% of the CKD patients with nonalbuminuric phenotype at baseline developed UACR>30 mg/g. We believe that this group of patients who switched phenotype will need to be studied in observational studies and possibly targeted for triglyceride lowering therapies in randomized clinical trials.
Microalbuminuria is associated with an inflammatory process[33], and thus a reflection of a range of inflammatory states such as metabolic syndrome (e.g. hypertension, hyperlipidemia, obesity, insulin resistance), infections, or rheumatologic diseases[33]. The content of triglycerides in the blood is largely influenced by dietary carbohydrate content and obesity[34]. Patients with a diet high in carbohydrates also tend to have higher levels of inflammation either through diet alone, being obese, or poor glycemic control, which are all related to a pro-inflammatory state. Hypertriglyceridemia may be a proxy of high inflammation, where hyperglyceridemia may be mediating the relationship between inflammation and renal outcomes. Unfortunately, data are limited in that reliable information on inflammatory markers to conduct a mediation analyses were not available, but future studies should study this important research question.
We also reported that for the onset of CKD, low triglycerides are associated with a lower risk of the outcome irrespective of UACR, while high triglycerides are associated with a higher risk of the outcome in patients with normal UACR or with microalbuminuria, but not with proteinuria. The relationship between triglyceride level and time to onset of CKD in the former groups is inverse. An increase in risk of CKD with elevation of triglycerides was reported in the Swedish National Diabetes Register with 20% increased risk per mmol triglyceride concentration [8]. Other studies reported the association between incidence of CKD and hypertriglyceridemia but with no decrease with increasing albumin excretion rate [9,33] . In the Italian Association of Clinical Diabetologists Study [9], every 10 mg/dl higher triglyceride level, the risk of decreased eGFR (<60 mL/min/1.73m2) was 2% in patients with normal albumin excretion rate and 3% in patients with increased albumin excretion rate. Moreover, the Renal Insufficiency And Cardiovascular Events Study [35] reported that in patients with type II diabetes, higher triglyceride concentration is associated with a progressive increase in the risk of CKD and that the relationship was stronger with increasing severity of albuminuria. In a small Japanese study, Kitaoka et. al reported post-meal triglyceridemia was associated with higher odds of nephropathy progression (similar to our cohort defined as changes in UACR) in multivariable adjusted models among 161 patients with normoalbuminuria (67.7%), microalbuminuria (28%), or macroalbuminuria (4.3%) [36].
Lipotoxicity can contribute to kidney disease by inducing glomerulosclerosis, tubulointerstitial injury, and cellular lipid loading in both glomeruli and tubules [37]. Increased glomerular uptake of circulating lipids and tubular reabsorption of albumin-bound fatty acids result in the increased deposition of triglycerides in the renal parenchymal cells. These lipids undergo chemical modification, leading to the formation of metabolites and free radicals that are damaging to the endothelium of renal microvessels [38,39]. This can trigger the transforming growth factor (TGF) expression in human glomerular epithelial cells, resulting in the accumulation of abnormal lipid and, eventually, glomerulosclerosis [40]. Previous studies have shown that the impact of triglycerides on CKD is evident only in CKD stage 3 but not in CKD stage 4 and 5 [41], suggesting that intervention in correcting hypertriglyceridemia in patients with CKD should be performed as early as possible.
There are several limitations to these analyses. First, we excluded a large proportion of diabetic patients who were without a valid UACR measurement within the VA. The presence of diabetes is a clinical indication for UACR collection, and thus this select population may be representative of diabetic veteran patients who are more regular users of the VA medical system and under closer specialized VA care. Given the observational nature of this study, our analyses may be subjected to residual confounding such as diet and other adiposity related factors. Our study population was largely comprised of males versus females (97.0 versus 3.0%, respectively), which may limit generalizability to female patients, who may have differences in lipid metabolism and renal disease development or progression due to sex hormones, sex chromosomes, sex hormone and sex chromosome interactions, or other genetic and epigenetic factors [42,43]. Finally, while waist circumference is a standard measurement for metabolic syndrome, only 49 patients had available data, and so we used BMI ≥30 kg/m2 as a surrogate marker [44]. Previous studies, however, reported a strong correlation between BMI and waist circumference (rho = 0.78) [45].
Yet, strengths of this study include the large number of diabetic veteran patients, with sufficient data to evaluate strata of CKD and albuminuria. Furthermore, the covariate rich datasets of the VA and CMS allowed for the detailed measurements of medication and laboratory data. Moreover, we required a strict definition in the evaluation of incident CKD and albuminuria to more conservatively identify renal outcomes.
Conclusion
In a large cohort, we have shown that elevated triglycerides are associated with all kidney outcomes in patients with normal eGFR and normal albumin excretion rate but the association is weaker in patients with preexisting microvascular renal complications. Interventions addressing triglyceride lowering have been shown to delay the onset or worsening of kidney outcomes for fenofibrate [4–48], niacin [49], and Omega 3 fatty acids [50–52]. Clinical evidence has indicated that agents such SGLT2 inhibitors (glucosuria effects) [53], thiazolidinediones, and some DPP-4 inhibitors can reduce the risk of development or worsening of albuminuria through a range of anti-inflammatory mechanisms and their effect on other conditions (e.g. obesity) that can have profound impact on the kidneys [53,54]. Clinical trials should be conducted in patients with diabetes before the onset of microvascular complications to test the hypothesis that targeting triglycerides concentration may prevent the development of kidney outcomes, which in turn are predictors of cardiovascular outcomes.
Supplementary Material
Acknowledgment
Opinions expressed in this presentation are those of the authors and do not represent the official opinion of the US Department of Veterans Affairs or the US Government.
Funding Source
KKZ has been supported by the NIH/NIDDK mid-career award K24-DK091419. ES is supported by a career development award from the Office of Research and Development of the Department of Veterans Affairs (IK2- CX 001266-01). KS acknowledges funding from NIH R01-DK125586. The data reported here have been supplied by the US Veterans Administration. Support for VA/CMS data is provided by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Health Services Research and Development, VA Information Resource Center (Project Numbers SDR 02-237 and 98-004).
Footnotes
Statement of Ethics
Study approval statement: This study was approved by the institutional review board of the Tibor Rubin VA Medical Center of Long Beach, CA (Decision reference number: 1406), which waived the requirement of informed consent because of the anonymous characteristics of the data.
Results have been presented at the virtual 2020 American Society of Nephrology Kidney Week
Conflicts of Interest/Disclosures:
KKZ has received honoraria and/or support from Abbott, Abbvie, Alexion, Amgen, American Society of Nephrology, Astra-Zeneca, AVEO Oncology, Chugai, DaVita, Fresenius, Genentech, Haymarket Media, Hofstra Medical School, International Federation of Kidney Foundations, International Society of Hemodialysis, International Society of Renal Nutrition & Metabolism, Japanese Society of Dialysis Therapy, Hospira, Kabi, Keryx, Novartis, National Institutes of Health, National Kidney Foundation, OPKO, Pfizer, Relypsa, Resverlogix, Sandoz, Sanofi, Shire, Vifor, UpToDate, and ZSPharma. CPK has received honoraria from Akebia, Ardelyx, Astra-Zeneca, Bayer, Cara Therapeutics, Reata and Tricida. Other authors do not have a conflict of interest.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
References
- 1.Saran R, Li Y, Robinson B, et al. US Renal Data System 2015 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 2016. doi: 10.1053/j.ajkd.2015.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coresh J Aligning albuminuria and proteinuria measurements. J Am Soc Nephrol. 2020. doi: 10.1681/ASN.2020010049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stephen R, Jolly SE, Nally JV., Navaneethan SD. Albuminuria: When urine predicts kidney and cardiovascular disease. Cleve Clin J Med. 2014. doi: 10.3949/ccjm.81a.13040 [DOI] [PubMed] [Google Scholar]
- 4.Seliger SL, Salimi S, Pierre V, Giffuni J, Katzel L, Parsa A. Microvascular endothelial dysfunction is associated with albuminuria and CKD in older adults. BMC Nephrol. 2016. doi: 10.1186/s12882-016-0303-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas G, Sehgal AR, Kashyap SR, Srinivas TR, Kirwan JP, Navaneethan SD. Metabolic syndrome and kidney disease: A systematic review and meta-analysis. Clin J Am Soc Nephrol. 2011. doi: 10.2215/CJN.02180311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Navaneethan SD, Schold JD, Kirwan JP, et al. Metabolic syndrome, ESRD, and death in CKD. Clin J Am Soc Nephrol. 2013. doi: 10.2215/CJN.09870912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knight SF, Imig JD. Obesity, insulin resistance, and renal function. Microcirculation. 2007. doi: 10.1080/10739680701283018 [DOI] [PubMed] [Google Scholar]
- 8.Afghahi H, Cederholm J, Eliasson B, et al. Risk factors for the development of albuminuria and renal impairment in type 2 diabetesthe Swedish National Diabetes Register (NDR). Nephrol Dial Transplant. 2011. doi: 10.1093/ndt/gfq535 [DOI] [PubMed] [Google Scholar]
- 9.De Cosmo S, Viazzi F, Pacilli A, et al. Predictors of chronic kidney disease in type 2 diabetes: A longitudinal study from the AMD Annals initiative. Med (United States). 2016. doi: 10.1097/MD.0000000000004007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soohoo M, Moradi H, Obi Y, Kovesdy CP, Kalantar-Zadeh K, Streja E. Serum triglycerides and mortality risk across stages of chronic kidney disease in 2 million U.S. veterans. J Clin Lipidol. 2019. doi: 10.1016/j.jacl.2019.08.001 [DOI] [PubMed] [Google Scholar]
- 11.McGinnis KA, Brandt CA, Skanderson M, et al. Validating smoking data from the Veteran’s affairs health factors dataset, an electronic data source. Nicotine Tob Res. 2011. doi: 10.1093/ntr/ntr206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller DR, Safford MM, Pogach LM. Who Has Diabetes? Best Estimates of Diabetes Prevalence in the Department of Veterans Affairs Based on Computerized Patient Data. Diabetes Care. 2004. doi: 10.2337/diacare.27.suppl_2.B10 [DOI] [PubMed] [Google Scholar]
- 13.Martin SS, Blaha MJ, Elshazly MB, et al. Comparison of a novel method vs the Friedewald equation for estimating low-density lipoprotein cholesterol levels from the standard lipid profile. JAMA - J Am Med Assoc. 2013. doi: 10.1001/jama.2013.280532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levey AS, Stevens LA, Schmid CH, et al. A New Equation to Estimate Glomerular Filtration Rate Disclosure of conflicts of interest: We have received confirmation from Drs. Ann Intern Med. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amdur RL, Chawla LS, Amodeo S, Kimmel PL, Palant CE. Outcomes following diagnosis of acute renal failure in U.S. veterans: Focus on acute tubular necrosis. Kidney Int. 2009. doi: 10.1038/ki.2009.332 [DOI] [PubMed] [Google Scholar]
- 16.Levey AS, De Jong PE, Coresh J, et al. The definition, classification, and prognosis of chronic kidney disease: A KDIGO Controversies Conference report. Kidney Int.2011;80(1): 17–28. doi: 10.1038/ki.2010.483 [DOI] [PubMed] [Google Scholar]
- 17.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation. 2005. doi: 10.1161/CIRCULATI0NAHA.105.169404 [DOI] [PubMed] [Google Scholar]
- 18.Soohoo M, C-EK HM, et al. Triglyceride and Time to ESRD Across CKD Stages With Adjustment for Other Components of Metabolic Syndrome Among 2.1 Million US Veterans. Circulation. 2018;138:A13276. [Google Scholar]
- 19.Lee YY, Yang CK, Weng YM, Chuang CH, Yu W, Chen JC. All components of metabolic syndrome are associated with microalbuminuria in a Chinese population. PLoS One. 2016. doi: 10.1371/journal.pone.0157303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hyo SC, Seung HR, Lee KB. The relationship of microalbuminuria with metabolic syndrome. Nephron - Clin Pract. 2006. doi: 10.1159/000093995 [DOI] [PubMed] [Google Scholar]
- 21.Watanabe H, Obata H, Watanabe T, Sasaki S, Nagai K, Aizawa Y. Metabolic syndrome and risk of development of chronic kidney disease: The Niigata preventive medicine study. Diabetes Metab Res Rev. 2010. doi: 10.1002/dmrr.1058 [DOI] [PubMed] [Google Scholar]
- 22.Franciosi M, Pellegrini F, Sacco M, et al. Identifying patients at risk for microalbuminuria via interaction of the components of the metabolic syndrome: A cross-sectional analytic study. Clin J Am Soc Nephrol. 2007. doi: 10.2215/CJN.01190307 [DOI] [PubMed] [Google Scholar]
- 23.Lucove J, Vupputuri S, Heiss G, North K, Russell M. Metabolic Syndrome and the Development of CKD in American Indians: The Strong Heart Study. Am J Kidney Dis. 2008. doi: 10.1053/j.ajkd.2007.09.014 [DOI] [PubMed] [Google Scholar]
- 24.Su W, Wang J, Mu Y. Association between hypertriglyceridemic waist phenotype and increased urinary albumin- creatinine ratio in Chinese adults: The reaction study.Diabetes, Metab Syndr Obes Targets Ther. 2020. doi: 10.2147/DMSO.S257736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stehouwer CDA, Gall MA, Twisk JWR, Knudsen E, Emeis JJ, Parving HH. Increased urinary albumin excretion, endothelial dysfunction, and chronic low-grade inflammation in type 2 diabetes: Progressive, interrelated, and independently associated with risk of death. Diabetes. 2002. doi: 10.2337/diabetes.51.4.1157 [DOI] [PubMed] [Google Scholar]
- 26.Low S, Tai ES, Yeoh LY, et al. Onset and progression of kidney disease in type 2 diabetes among multi-ethnic Asian population. J Diabetes Complications. 2016;30(7):1248–1254. doi: 10.1016/j.jdiacomp.2016.05.020 [DOI] [PubMed] [Google Scholar]
- 27.Bardini G, Innocenti M, Rotella CM, Giannini S, Mannucci E. Variability of triglyceride levels and incidence of microalbuminuria in type 2 diabetes. J Clin Lipidol. 2016. doi: 10.1016/j.jacl.2015.10.001 [DOI] [PubMed] [Google Scholar]
- 28.Bjornstad P Insulin sensitivity and complications in type 1 diabetes: New insights. World J Diabetes. 2015. doi: 10.4239/wjd.v6.i1.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tolonen N, Forsblom C, Thorn L, et al. Lipid abnormalities predict progression of renal disease in patients with type 1 diabetes. Diabetologia. 2009. doi: 10.1007/s00125-009-1541-2 [DOI] [PubMed] [Google Scholar]
- 30.Thomas MC, MacIsaac RJ, Jerums G, et al. Nonalbuminuric renal impairment in type 2 diabetic patients and in the general population (National Evaluation of the Frequency of Renal Impairment cO-existing with NIDDM [NEFRON] 11). Diabetes Care. 2009. doi: 10.2337/dc08-2186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pugliese G Updating the natural history of diabetic nephropathy. Acta Diabetol. 2014. doi: 10.1007/s00592-014-0650-7 [DOI] [PubMed] [Google Scholar]
- 32.Sumida K, Molnar MZ, Potukuchi PK, et al. Changes in albuminuria and subsequent risk of incident kidney disease. Clin J Am Soc Nephrol. 2017. doi: 10.2215/CJN.02720317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bakris GL, Molitch M. Microalbuminuria as a risk predictor in diabetes: The continuing saga. Diabetes Care. 2014. doi: 10.2337/dc13-1870 [DOI] [PubMed] [Google Scholar]
- 34.Parks EJ. Effect of dietary carbohydrate on triglyceride metabolism in humans. In: Journal of Nutrition. ; 2001. doi: 10.1093/jn/131.10.2772s [DOI] [PubMed] [Google Scholar]
- 35.Penno G, Solini A, Zoppini G, et al. Hypertriglyceridemia is independently associated with renal, but not retinal complications in subjects with type 2 diabetes: A cross-sectional analysis of the Renal Insufficiency and Cardiovascular Events (RIACE) Italian Multicenter Study. PLoS One. 2015. doi: 10.1371/journal.pone.0125512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kitaoka K, Takenouchi A, Tsuboi A, Fukuo K, Kazumi T. Association of Postbreakfast Triglyceride and Visit-to-Visit Annual Variation of Fasting Plasma Glucose with Progression of Diabetic Nephropathy in Patients with Type 2 Diabetes. J Diabetes Res. 2016. doi: 10.1155/2016/4351376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weinberg JM. Lipotoxicity. Kidney Int. 2006;70(9):1560–1566. doi: 10.1038/sj.ki.5001834. [DOI] [PubMed] [Google Scholar]
- 38.Iacobini C, Menini S, Ricci C, et al. Advanced lipoxidation end-products mediate lipid-induced glomerular injury: Role of receptor-mediated mechanisms. J Pathol. 2009. doi: 10.1002/path.2536 [DOI] [PubMed] [Google Scholar]
- 39.Losada M, Alio JL. Malondialdehyde serum concentration in type 1 diabetic with and without retinopaty. Doc Ophthalmol. 1997. doi: 10.1007/BF02569062 [DOI] [PubMed] [Google Scholar]
- 40.Ding G, Van Goor H, Ricardo SD, Orlowski JM, Diamond JR. Oxidized LDL stimulates the expression of TGF-β and fibronectin in human glomerular epithelial cells. Kidney Int. 1997. doi: 10.1038/ki.1997.18 [DOI] [PubMed] [Google Scholar]
- 41.Lee PH, Chang HY, Tung CW, et al. Hypertriglyceridemia: An independent risk factor of chronic kidney disease in Taiwanese adults. Am J Med Sci. 2009. doi: 10.1097/MAJ.0b013e3181a92804 [DOI] [PubMed] [Google Scholar]
- 42.Bairey Merz CN, Dember LM, Ingelfinger JR, et al. Sex and the kidneys: current understanding and research opportunities. Nat Rev Nephrol. 2019. doi: 10.1038/s41581-019-0208-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palmisano BT, Zhu L, Eckel RH, Stafford JM. Sex differences in lipid and lipoprotein metabolism. Mol Metab. 2018. doi: 10.1016/j.molmet.2018.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nilsson PM, Tuomilehto J, Rydén L. The metabolic syndrome - What is it and how should it be managed? Eur JPrev Cardiol. 2019. doi: 10.1177/2047487319886404 [DOI] [PubMed] [Google Scholar]
- 45.Soohoo M, Hashemi L, Hsiung JT, Moradi H, Budoff MJ, Kovesdy CP, Kalantar-Zadeh K, & Streja E (2022). Association of Serum Triglycerides and Renal Outcomes among 1.6 Million US Veterans. Nephron, 146(5), 457–468. https://doi.org/10.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ansquer JC, Foucher C, Rattier S, Taskinen MR, Steiner G. Fenofibrate reduces progression to microalbuminuria over 3 years in a placebo-controlled study in type 2 diabetes: Results from the Diabetes Atherosclerosis Intervention Study (DAIS). Am J Kidney Dis. 2005. doi: 10.1053/j.ajkd.2004.11.004 [DOI] [PubMed] [Google Scholar]
- 47.Frazier R, Mehta R, Cai X, et al. Associations of Fenofibrate Therapy With Incidence and Progression of CKD in Patients With Type 2 Diabetes. Kidney Int Reports. 2019. doi: 10.1016/j.ekir.2018.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yen CL, Fan PC, Lin MS, et al. Fenofibrate Delays the Need for Dialysis and Reduces Cardiovascular Risk among Patients with Advanced CKD. J Clin Endocrinol Metab.2021. doi: 10.1210/clinem/dgab137 [DOI] [PubMed] [Google Scholar]
- 49.Streja E, Kovesdy CP, Streja DA, Moradi H, Kalantar-Zadeh K, Kashyap ML. Niacin and Progression of CKD. Am J Kidney Dis. 2015. doi: 10.1053/j.ajkd.2014.11.033 [DOI] [PubMed] [Google Scholar]
- 50.Chung HF, Long KZ, Hsu CC, et al. Association of n-3 polyunsaturated fatty acids and inflammatory indicators with renal function decline in type 2 diabetes. Clin Nutr. 2015. doi: 10.1016/j.clnu.2014.02.009 [DOI] [PubMed] [Google Scholar]
- 51.Miller ER, Juraschek SP, Appel LJ, et al. The effect of n-3 long-chain polyunsaturated fatty acid supplementation on urine protein excretion and kidney function: Meta-analysis of clinical trials. Am J Clin Nutr. 2009. doi: 10.3945/ajcn.2008.26867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shimizu H, Ohtani K ichi, Tanaka Y, Sato N, Mori M, Shimomura Y. Long-term effect of eicosapentaenoic acid ethyl (EPA-E) on albuminuria of non-insulin dependent diabetic patients. Diabetes Res Clin Pract. 1995. doi: 10.1016/0168-8227(95)01056-J [DOI] [PubMed] [Google Scholar]
- 53.Skrabic R, Kumric M, Vrdoljak J, Rusic D, Skrabic I, Vilovic M, Martinovic D, Duplancic V, Ticinovic Kurir T, Bozic J. SGLT2 Inhibitors in Chronic Kidney Disease: From Mechanisms to Clinical Practice. Biomedicines. 2022. Oct 1;10(10):2458. doi: 10.3390/bio. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tong L, Adler S. Glycemic control of type 2 diabetes mellitus across stages of renal impairment: information for primary care providers. Postgrad Med. 2018. doi: 10.1080/00325481.2018.1457397 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.