SUMMARY
An organism’s metabolic activity leaves an extracellular footprint and dynamic changes in this exometabolome inform about nutrient uptake, waste disposal and signaling activities. Using non-targeted mass spectrometry, we report exometabolome dynamics of hypoxia-induced, non-replicating mycobacteria that are thought to play a role in latent tuberculosis. Despite evidence of active metabolism, little is known about the mechanisms enabling obligate aerobic mycobacteria to cope with hypoxia, resulting in long-term survival and increased chemotherapeutic tolerance. The dynamics of 379 extracellular compounds of Mycobacterium smegmatis were deconvoluted with a genome-scale metabolic reaction-pair network to generate hypotheses about intracellular pathway usage. Time-resolved 13C-tracing and mutant experiments then demonstrated a crucial, energy-generating role of asparagine utilization and non-generic usage of the glyoxylate shunt for hypoxic fitness. Experiments with M. bovis and M. tuberculosis revealed the general relevance of asparagine fermentation and a variable contribution of the glyoxylate shunt to non-replicative, hypoxic survival between the three species.
INTRODUCTION
The nutritional environment of bacterial species colonizing other organisms in a commensal or pathogenic fashion is complex (Hooper et al., 2002; Brown et al., 2008). Bacteria not only exploit nutrients in their surrounding for proliferation and energy generation but also modulate the environment by disposing metabolic byproducts or secreting signaling molecules. The sum of such metabolite uptake and secretion leads to an altered composition of the exometabolome – the ensemble of metabolites in the extracellular space, sometimes also referred to as the metabolic footprint (Kell et al., 2005). Among the protocols to analyze the exometabolome, Fourier transform-infrared spectroscopy, nuclear magnetic resonance spectroscopy and mass spectrometry-based techniques have been reported (Allen et al., 2003; Kaderbhai et al., 2003; Pope et al., 2009; Behrends et al., 2013). Interpretation of the data, however, has been restricted either to statistical comparison of static exometabolome measurements with growth phenotypes or to intuitive reasoning from a rather limited list of pre-defined metabolites (Allen et al., 2003; Paczia et al., 2012; Jain et al., 2012).
We were interested in the transition from exponential growth to the hypoxia-triggered, non-replicative state of mycobacteria, often associated with clinically latent tuberculosis (Boshoff and Barry, 2005; Gengenbacher and Kaufmann, 2012). Such asymptomatic infections with tubercle bacilli are estimated to affect about a third of the human population world-wide and increased tolerance for chemotherapeutics during this stage is a major hurdle for the eradication of the pathogen (Chao and Rubin, 2010). A better understanding of the mechanisms underlying bacterial survival of up to several decades in the host is therefore a prerequisite to treat latent tuberculosis (Esmail et al., 2012). One of the in vitro models to study these mechanisms exploits gradual oxygen depletion to shift mycobacterial cultures into a state of halted replication due to the obligate aerobic nature of these species. Studies of such Wayne cultures demonstrated the need to maintain a polarized membrane for the regeneration of ATP in the absence of growth (Rao et al., 2008). Analysis of the transcriptome, enzyme activities and qualitative carbon fluxes suggested remodeling of metabolism and some basic fermentative activity as a response to oxygen limitation (Watanabe et al., 2011). Complex culture broth containing amino acids, bovine serum albumine, lipids and glucose is routinely used in Wayne experiments because it improves hypoxic survival (Wayne and Lin, 1982). This suggests the occurrence of additional metabolic processes, beyond the described fermentation of glucose to succinate (Watanabe et al., 2011).
We here used Mycobacterium smegmatis under Wayne conditions to probe the culture supernatant of the complex broth at high temporal resolution, adding a dynamic component to the acquired data. High-resolution mass spectrometry allowed us to annotate several hundred extracellular metabolites, of which more than 200 significantly altered their concentration in an oxygen-dependent manner. Both the dynamic nature and the broad coverage of the metabolite measurements enabled us to apply a genome-scale metabolic network model to infer the involvement of intracellular metabolic pathways in the utilization of substrates and secretion of reaction products. The generated hypotheses were validated using 13C-tracing and genetic knock-out strains and findings from the non-pathogenic model organism were tested for their relevance in the pathogen M. tuberculosis and its vaccination strain M. bovis BCG.
RESULTS
Dynamic Exometabolome Measurement in Wayne Setup
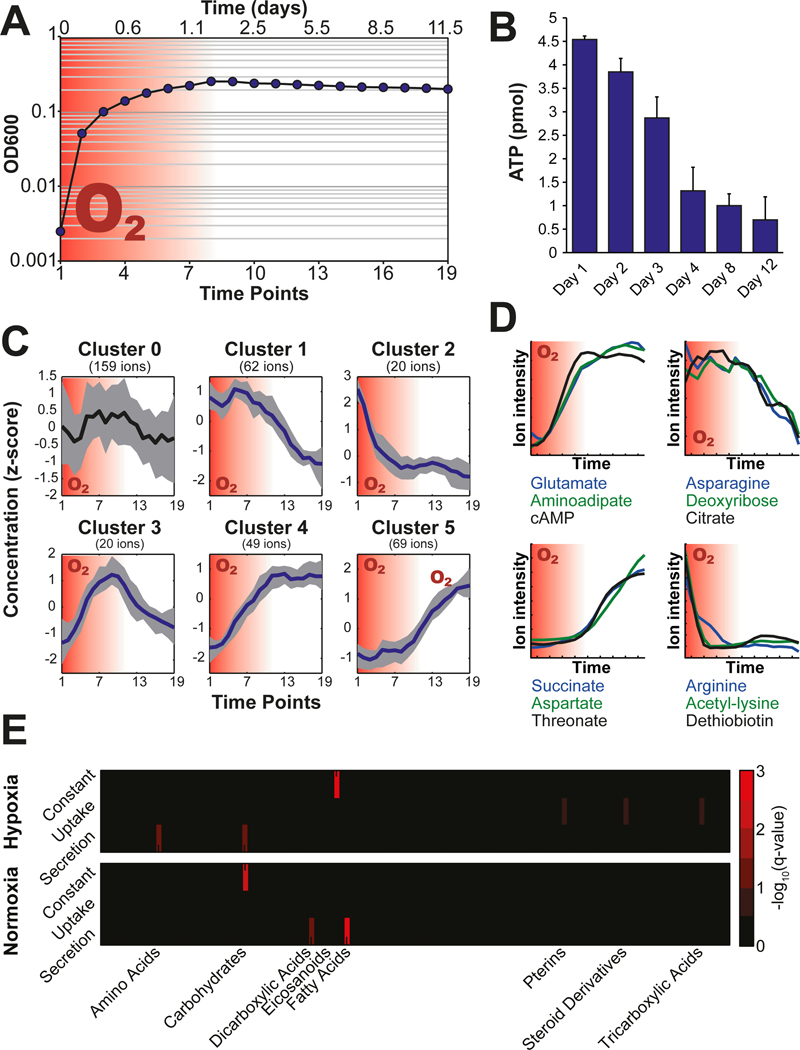
Shifts from exponential growth to the non-replicating state of mycobacteria can be achieved through gradual depletion of the available oxygen in the so-called Wayne setup (Wayne and Sohaskey, 2001). We set up cultures in serum bottles, whose septum enables multiple sampling from the same culture vessel throughout the entire course of the experiment. We validated this modified setup by demonstrating oxygen depletion through fading of methylene blue upon stalling growth as described earlier (Fig. 1A) (Wayne and Hayes, 1996). The intracellular ATP concentration dropped by roughly 80% and the NADH to NAD+ ratio increased drastically upon depletion of oxygen, which are both prominent characteristics of Wayne conditions (Rao et al., 2008; Watanabe et al., 2011) (Fig. 1B, Fig. S1). Based on these results we conclude that the modified experimental setup reproduces classical Wayne conditions.
Fig. 1. Wayne cultures and exometabolome measurement.
(A) Culture density profile and the depletion of oxygen (red background). (B) Decrease of intracellular ATP concentrations upon transition into hypoxia-triggered non-replicating phase (ATP amount normalized to 1 mL culture at an optical density at 600 nm of 1.0). (C) K-means clustering of temporal profiles of exometabolites. (D) Schematic temporal profiles of exometabolites. Examples are given below the profiles. (E) Metabolite class enrichment analysis of concentration dynamics shows differences between the first, normoxic and the second, hypoxic phase of the Wayne cultures. All plots resulted from four independent biological replicate cultures and two technical replicate measurements.
For analysis of the exometabolome, 1% of the culture volume was sampled at 19 time points spread over 12 days. The sample volume was replaced by nitrogen to avoid disturbing the slow oxygen depletion caused by bacterial respiration (Fig. 1A). Bacteria were separated from the culture medium by centrifugation as previously validated for exometabolome analysis (Paczia et al., 2012). Metabolites in the culture supernatant were analyzed using flow injection on a quadrupole-time of flight mass spectrometer, which resulted in a total of 5’796 detected ions (Fuhrer et al., 2011). As previously reported (Fuhrer et al., 2011), we assigned 379 ions to 850 metabolites with a precision of 0.001 Da based on the KEGG metabolite repository specific for M. smegmatis (msm) (Kanehisa, 2000) including isomers (Table S1). As expected, glucose, citrate and asparagine were identified, which were added to the Tween®-albumine culture broth as pure chemicals. Further metabolites, such as amino acids, nucleotides and organic acids were likely derived from the supplemented casamino acid digest and bovine serum albumin, or were produced by the culture.
Filtering and Clustering of Dynamic Metabolite Profiles
To discover general metabolic trends associated with metabolite uptake or secretion, we filtered the metabolite concentration profiles for the ones showing significant changes over time. In a first step, we estimated the variances for each metabolite time course by summing up the standard deviation (SDsum) of a sliding time window spanning two neighboring time points, including all replicate measurements (Fig. S2A). In a second step, we calculated the SDsum for a thousand permutations of the time axis. The SDsum of the measured time course data was compared to this SDsum and filtered out, if not passing the set quantile cut-off (Fig. S2A). The cut-off criteria were optimized in silico using a synthetic dataset generated by a random function generator and eight 12-fold dilution series of a mixture of 59 chemical standard compounds quantified by the same mass spectrometry protocol as described. Time traces were compiled by random selection of the measured dilutions following 18 base-functions representing constant and significantly changing temporal patterns (Fig. S2B). We optimized the quantile cut-off based on 1620 different test runs with varying number of simulated time points, amplitude of the metabolite signal and quantile cut-offs. The optimization indicated an optimal quantile cut-off of 0.15 for 12–20 time points based on a combined sensitivity and selectivity criteria (Fig. S2B). The permutation filtering resulted in 267 annotated ions showing time-dependent concentration changes, statistically different from random measurement noise. These ion traces were subjected to k-means clustering, which led to the identification of five main temporal patterns with dependency on the presence of oxygen in the culture media (Fig. 1C). Clusters one and two contain metabolites that are depleted from the media specifically in the absence and presence of oxygen, respectively. Analogous behavior was observed for accumulating metabolites depicted by cluster four and five. Cluster three showed metabolites that accumulated during the first aerobic phase to be again reduced upon oxygen depletion. The concentration time courses of metabolites that did not meet the cut-off criteria of the permutation filter did not show any significant temporal trend, serving as validation for the applied filtering process. Representative metabolites for each cluster are shown in figure 1D (Fig. 1D).
Enrichment Analysis of Exometabolome Trends
Next, we asked whether the exometabolome changes were enriched for distinct types of metabolites, hinting at a particular metabolic pathway or subnetwork activity. To simplify the temporal metabolite patterns, we separated the first (aerobic) from the second (hypoxic) phase. This allowed us to bin metabolites into three response modes according to their concentration changes in either phase: i) increasing, ii) decreasing and iii) stable concentrations over time (Fig. S3). These groups were subjected to an enrichment analysis using metabolite classifiers (Wishart et al., 2013), which confirmed clear differences between the aerobic and the hypoxic cultivation phase with respect to decreasing and increasing exometabolite levels (Fig. 1E). While M. smegmatis did not seems to prefer specific substrates under aerobic conditions, primarily tricarboxylic acids and steroids were utilized during hypoxia, which were likely attached to bovine serum albumin in the medium. Similarly, different metabolites accumulated during the two phases. While dicarboxylic acid and long-chain fatty acids increased during the aerobic phase, amino acids and carbohydrates accumulated during hypoxia. The accumulation of fatty acids during the aerobic phase - when cells are still dividing - is likely due to the release of envelope lipids into the culture broth caused by the presence of detergents (Tween-80) in the culture medium. There are not obvious explanations for the change in carboxylic and amino acid levels. We excluded protein degradation as the cause for amino acid secretion, because they did not accumulate intracellularly (Fig. S4).
Exometabolome Interpretation Using a Genome-wide Metabolic Reaction-pair Network
The clustering of exometabolome time courses revealed clear differences in metabolic activity between the aerobic and the hypoxic phase of the experiment, but this statistical analysis could not reveal the underlying intracellular pathways. To identify active metabolic pathways under non-replicating conditions, we focused on exometabolome changes during the hypoxic phase to integrate them with the KEGG genome-wide reaction-pair network of M. smegmatis (Kanehisa, 2000). Under hypoxia, metabolic activity of obligate aerobic mycobacteria primarily serves to ensure survival. Since the lack of oxygen hinders complete combustion of nutrients to CO2, substrate uptake inevitably leads to metabolite secretion. Therefore, we linked the 85 increasing to the 82 decreasing ions using the KEGG genome-wide reaction-pair network of M. smegmatis resulting in a total of 4489 potentially connecting metabolic paths after data curation (exact procedure described in supporting information and Fig. S5).
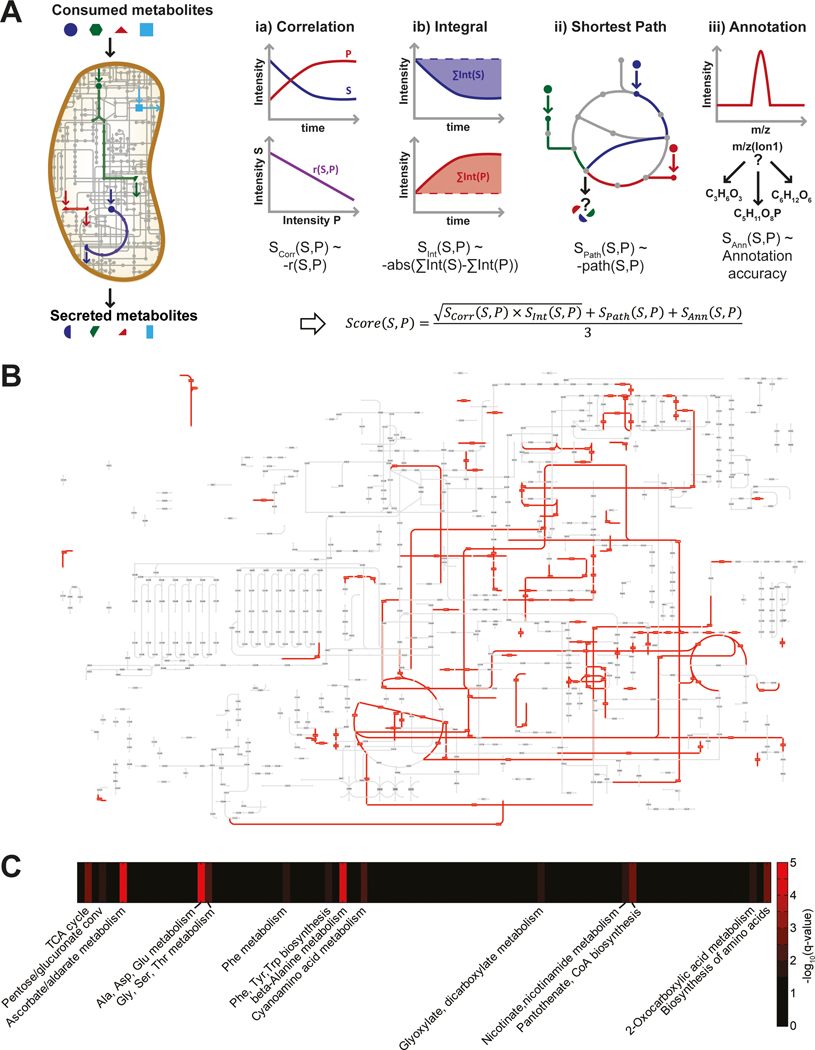
To identify the most probable metabolic pathway through which a product is formed from a substrate, we used three scoring criteria to estimate the likelihood for each of the 4489 pairwise connections (Fig. 2A). First, we scored the temporal similarity of metabolite profiles by weighting the correlation and magnitude of the temporal exometabolite profiles of the putative substrate and product. Perfectly anti-correlating pairs of metabolite traces were given highest scores, as a secreted metabolite is expected to accumulate, when its precursor substrate is simultaneously consumed. The temporal integral of the ion intensity served as a surrogate for the change in concentration of metabolites. Metabolite pairs showing comparable temporal intensity integrals were scored highest, as neither combustion nor formation of biomass is expected, which should lead to equimolar metabolite uptake and secretion. Second, we performed a degree-weighted shortest path search, similar to optimization for minimal sum of fluxes in flux balance analysis to score the most probable pathway connecting a given pair of substrate and product (Croes et al., 2006; Blum and Kohlbacher, 2008; Orth et al., 2010). Third, we scored the confidence in annotation to account for the degeneracy of ion annotation based on accurate mass. The average of these three sub-scores was calculated for each of the 4489 potential substrate-product pairs (Fig. 2, Table S2). Ranking these pairs according to their score, hence assigns a probability to potential metabolic fluxes between a consumed and secreted metabolite.
Fig. 2. Identification of the most probable carbon fluxes using a genome-scale metabolic model.
(A) Scheme of the algorithm scoring metabolic pathways converting consumed substrates into secreted metabolites. (B) KEGG metabolic pathway map overlaid with the 250 top pathway hits showing a coverage of 12.1%. (C) Enrichment analysis of the top one percent hits for their metabolic pathway definition.
To illustrate the established link between exometabolome data and the underlying metabolic activity, we compared all KEGG reaction pairs with the ones used to link consumed and secreted metabolites. The coverage of our evaluated pathways (4489) covered 37.3% of the entire KEGG reaction network, whereas 12.1% and 2.8% were covered by the top 250 and 50 hits, respectively (Fig. 2B and Fig. S6). To learn about general metabolic activities from these ranked metabolite pairs, we performed pathway enrichment of reactions involved in the top 50, representing about 1%, of suggested metabolic routes compared to the rest (Fig. 2C, Table S2). Enrichment of several amino acid related pathways suggested a role of amino acid metabolism under hypoxia-triggered non-replicating conditions, as earlier demonstrated for some amino acids (Hutter and Dick, 1998; Tang et al., 2009; Shi et al., 2010; Berney et al., 2012). Exemplary is the secretion of glutamate, which is in line with its previously suggested hypoxic production, based on enzyme activity and transcript data (Shi et al., 2010). Furthermore, reactions of tricarboxylic acid (TCA) cycle, glyoxylate, coenzyme A and nicotinate cofactor metabolism and carbohydrate oxidation were overrepresented in the top hits of all potential substrate-product connections (Fig 2B). While TCA cycle, glyoxylate metabolism and nicotine-derived cofactors have previously been linked to the non-replicating stage of mycobacteria (Wayne and Lin, 1982; Shi et al., 2010; Watanabe et al., 2011), oxidation of sugars and coenzyme A have not yet been linked to Wayne culture metabolism. Such oxidation reactions could contribute to the bacteria’s redox maintenance during hypoxia, as they potentially supply NADPH required for anabolic and repair reactions. Integration of dynamic exometabolome data with a genome-wide metabolic model using our algorithm locates areas of metabolic activity in non-replicating mycobacteria. Based on the scores, we chose to investigate functionality of metabolic flux to the two major products aspartate and succinate by 13C-tracing and genetics in the following two sections.
Asparagine to aspartate conversion supports survival under hypoxic conditions
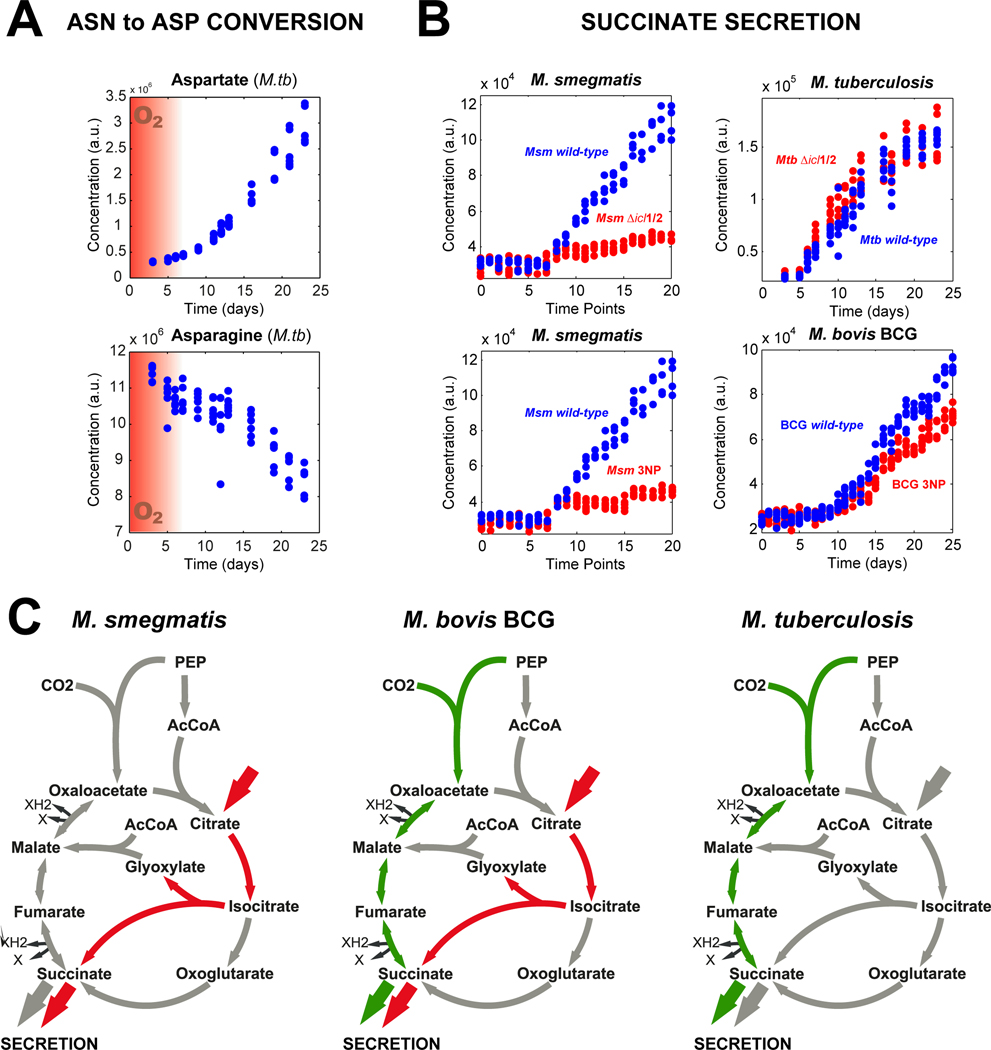
Consumption of asparagine and secretion of aspartate represent the general trend of predicted flux through reactions in amino acids metabolism during non-replication, and the deamination of asparagine to aspartate was among the reactions with the highest overall score (Fig. 3A, Table S2). The fact that 2 g/L asparagine are present in the empirically developed growth media used for Wayne conditions further motivated us to investigate its utilization in the absence of growth. Using LC-MS/MS we quantified asparagine consumption during the nine days of hypoxia-induced non-replication to 476 ± 135 mg/L and aspartate formation to 505 ± 19 mg/L (Buescher et al., 2010). Notably, this mass turnover correspond to more than seven times the entire bacterial dry mass of the culture (68.7 ± 3.5 mg/L). To corroborate the hypothesis of asparagine to aspartate conversion (Fig 3A), we supplemented the culture broth with uniformly 13C-labeled asparagine. Expectedly, we found only fully 13C-labeled aspartate in the medium during the hypoxic phase by specific measurements using ultra high-pressure chromatography-coupled tandem mass spectrometry (Fig. 3B) (Rühl et al., 2011). Further metabolism of asparagine did not occur because we did not find any 13C-labeling in extra- or intracellular succinate or intracellular malate (Fig. 3B). This absence of hypoxic TCA cycle fluxes is in contrast to M. tuberculosis, for which 13C-label progression from asparagine into the reductive branch of the TCA cycle was demonstrated and associated with a fermentative metabolism mediated by fumarate reductase activity under hypoxic conditions (Watanabe et al., 2011). The lack of such an enzyme in M. smegmatis further supports our finding of asparagine not being incorporated into the TCA cycle.
Fig. 3. Asparagine to aspartate conversion promotes survival under non-replicating conditions.
(A) 10 top hits of predicted metabolic pathways using consumed asparagine. The numbers depict the rank of the probability scores of shown metabolic pathways using asparagine as a substrate. The absolute score is represented by the arrow size (Table S2). (B) 13C-asparagine feeding experiment and tracing of the labeling in the endo- and exometabolome. Fully 13C-labeled extracellular metabolites are shown in yellow, non-labeled extracellular metabolites are shown in blue. The fraction of each mass isomer in the total pool of intracellular metabolites is indicated by different colors. (C) Extracellular aspartate levels at different time points of Wayne cultures at the time of sample collection (left side) and after cell-free incubation until day 12 of the experiment (right side). (D) Extracellular aspartate levels at different time points of lysed Wayne cultures at the time of sample collection (left side) and after cell-free incubation until day 12 (right side) (E) Fitness during non-replication in the presence and absence of asparagine in the media. Colony forming units (cfu) were calculated from four independent cultures. Plots in subfigure C, D, and E resulted from four independent biological replicate cultures and two technical replicate measurements (including plating for cfu determination), whereas 13C-labeling was performed in two independent biological experiments (B).
To find out whether the conversion of asparagine to aspartate occurs by an intra- or extracellular enzyme, we first demonstrated the absence of spontaneous asparagine to aspartate conversion in non-inoculated culture medium incubated for 12 days (Fig. 3C). When incubating sterile-filtered culture supernatant collected at day three and six until day 12, we observed low but significant aspartate accumulation that must result from extracellular enzyme activity (Fig. 3C). To test the intracellular capacity of non-growing Wayne cultures for aspartate production, the same experiment was repeated by incubating sterile-filtered supernatants taken from sonicated cells. While intracellular aspartate levels were negligible, incubation of sterile-filtered cell lysates collected at day 3 and 6 led to an accumulation of aspartate that was larger than in the experiment with culture supernatant (Fig. 3D). Since extracellularly catalyzed aspartate accumulation is less than the accumulation that occurs in Wayne cultures, we conclude that extracellular enzyme activity plays only a partial role in the conversion of asparagine to aspartate. The inability of lysates from aerobically grown bacteria (day 1) to convert asparagine to aspartate demonstrates that the required enzyme is only expressed (or activated) under hypoxic conditions.
To test the functional importance of asparagine transformation to aspartate, we repeated the Wayne experiment without asparagine supplementation. As expected, there was no accumulation of aspartate in the culture supernatant (Fig. S7). Additionally, a significant drop of viability of the hypoxic bacteria was observed in the absence of asparagine, which we assayed by colony counts (Fig. 3E). After eight days of Wayne cultivation, the number of colonies dropped to half, compared to standard asparagine conditions. These data demonstrate that conversion of asparagine to aspartate helps survival during the hypoxia-triggered, non-replicating stage of M. smegmatis.
Succinate Secretion Caused by Isocitrate Lyase Activity Increases Non-replicating Survival
Secretion of succinate produced from glucose and asparagine plays an important role in hypoxic cultures of M. tuberculosis through reversion of the TCA cycle flux to confer basic fermentative activity (Watanabe et al., 2011). While we also observed such succinate secretion in hypoxic M. smegmatis, our scoring algorithm predicted aconitate and citrate as upstream substrates, suggesting flux through the TCA cycle or the glyoxylate shunt (Fig. 4A). The fact that asparagine labeling was not found in succinate of M. smegmatis further indicated different metabolic fluxes than previously described for M. tuberculosis (Watanabe et al., 2011). To validate this prediction, we replaced citrate in the culture medium by [U-13C]-citrate and consistently found [U-13C]-succinate (Fig. 4B). Intriguingly, we only detected incorporation of 13C-label into intracellular isocitrate but not into intracellular oxoglutarate or malate, indicating absence of fluxes through the TCA cycle, but rather unusual, non-anabolic flux through the glyoxylate shunt (Fig. 4B). The label propagation dynamics demonstrated that succinate was exclusively derived from isocitrate through the glyoxylate shunt enzyme isocitrate lyase, encoded by two isoenzymes (icl1, MSMEG_0911 and icl2, MSMEG_3706). This finding is consistent with increased isocitrate lyase expression and enzymatic activity during the transition to hypoxia (Wayne and Lin, 1982; Shi et al., 2010), but extracellular citrate is an unexpected substrate because it does not support aerobic growth of mycobacteria (Bowles and Segal, 1965).
Fig. 4. Succinate and glycine secretion caused by isocitrate lyase activity increases non-replicating survival.
(A) 10 top hits of predicted metabolic pathways leading to succinate secretion. The numbers depict the rank of the probability scores of shown metabolic pathways leading to secreted succinate. The absolute scores are represented by the arrow size (Table S2). (B) [U-13C]-citrate feeding experiment and tracing of the labeling in the endo- and exometabolome. The fraction of each mass isomer in the total pool of intracellular metabolites is indicated by different colors. (C) Disruption of the isocitrate lyase genes (Δicl1 Δicl2) impairs both uptake of citrate and secretion of succinate. (D) [U-13C]-glycine secretion upon [U-13C]-citrate feeding depends on isocitrate lyase activity (E) Fitness upon resuscitation of wild-type and the Δicl1/2 strain after different times under hypoxic conditions. A minimum of three independent biological experiments was performed for each plot and untargeted measurements were performed in two technical replicates.
To further validate citrate metabolism through the glyoxylate shunt to succinate, we repeated the Wayne experiment with a mutant lacking both isocitrate lyase isoenzymes (Upton and McKinney, 2007). Extracellular citrate levels remained constant throughout the entire course of cultivation, while succinate was barely secreted (Fig. 4C). Furthermore, hypoxic levels of intracellular succinate were much lower in the mutant than in the wild-type (Fig. S8). These results confirm our prediction and unambiguously link succinate secretion in M. smegmatis to the consumption of citrate and aconitate (Fig. S8). Although succinate secretion is common to both M. tuberculosis and M. smegmatis, the underlying metabolism is hence very different. In M. tuberculosis succinate formation occurs via reversion of the reductive TCA cycle and serves as a final electron acceptor to help regenerate redox cofactors (Watanabe et al., 2011). In hypoxic M. smegmatis, in contrast, succinate formation occurs via isocitrate lyase that does not involve cofactors and hence cannot fulfill fermentative functions.
In the canonical glyoxylate shunt, glyoxylate is produced at equimolar amounts to succinate and then fused with acetyl-CoA to malate by the malate synthase (glcB, MSMEG_3640) (Smith et al., 2003). Since deletion of glcB did not affect the hypoxic exometabolome, there is no carbon flux through malate synthase, as already suggested by the absence of 13C-labeling propagation from citrate to malate (Fig 4B, Fig. S9). These results demonstrate a further difference in glyoxylate shunt activity with carbon flux passing only through isocitrate lyase, but not through malate synthase. Our algorithm predicted the conversion of citrate to glycine as the most probable origin of secreted glycine under hypoxia (Table S2). Amongst all evaluated metabolic pathways, citrate to glycine conversion scored in the top 1.25% (number 56 of all 4489 evaluated pathways, Table S2). We tested this prediction experimentally by 13C-tracing of labeled citrate to secreted glycine under Wayne conditions. The fact that [U-13C]-glycine was only found in the supernatant of wild-type, but not of ICL-deficient (Δicl1 Δicl2) cultures clearly validated our computational prediction (Fig. 4D). Based on enzyme activity measurements in cell-free extracts, such reductive amination of glyoxylate with ammonia and NADH to glycine and NAD+ during hypoxia was proposed previously (Wayne and Lin, 1982). The reaction’s oxidation of NADH would convey a fermentative role in disposing of accumulated reduction equivalents to hypoxic mycobacteria (Boshoff and Barry, 2005). Functionally, isocitrate lyase flux supports survival of hypoxic M. smegmatis because the Δicl1 Δicl2 double mutant exhibited more than one third lower colony forming units during hypoxia and prolonged lag-phases after re-aeration (Fig. 4E, Fig. S10).
Aspartate and Succinate Secretion in M. tuberculosis and M. bovis BCG
Given the importance of aspartate secretion and non-canonical glyoxylate shunt activity for hypoxic survival of M. smegmatis, we set out to test whether these two metabolic activities also play a role in hypoxic M. tuberculosis Erdmann and the vaccination strain M. bovis BCG. Akin to M. smegmatis, both species secreted aspartate and succinate upon depletion of oxygen (Fig. 5A, Fig. S 11). To estimate the contribution of the glyoxylate shunt flux to hypoxic succinate secretion, we compared extracellular succinate accumulation in wild-type strains and upon blockage of the ICL activity (Δicl1 Δicl2 or 3-nitropropionate, 3NP). In contrast to M. smegmatis, blockage of ICL activity only led to a partial or no reduction of succinate secretion by M. bovis BCG and M. tuberculosis, respectively (Fig. 5B), suggesting low carbon flux through ICL in hypoxic M. bovis BCG and absence of ICL flux in hypoxic M. tuberculosis. This is in agreement with previous reports linking succinate secretion in M. tuberculosis to fermentative reversal of the reductive TCA cycle and the need to apply acetate conditions to force carbon flux through ICL under hypoxic conditions (Watanabe et al., 2011; Eoh and Rhee, 2013). These results revealed clear differences of hypoxic TCA cycle organization and ICL relevance between mycobacterial species. While extracellular succinate in hypoxic M. smegmatis is solely derived through ICL (non-canonical glyoxylate shunt), hypoxic M. bovis BCG additionally reverses the reductive TCA cycle to produce succinate, which is the sole pathway to form succinate in hypoxic M. tuberculosis (Fig. 5C). Should such metabolic variability occur also amongst clinical isolates of M. tuberculosis, it must be considered for the bacilli’s ability to deal with the host’s defense mechanisms and to chemotherapeutics that require metabolic rearrangements.
Fig. 5. Aspartate and succinate secretion by hypoxic M. tuberculosis and M. bovis BCG and M. smegmatis.
(A) Extracellular aspartate accumulation and simultaneous asparagine depletion in M. tuberculosis Wayne cultures. (B) Comparison of hypoxic succinate secretion between different mycobacterial species and its dependency on the glyoxylate shunt. Metabolic flux through the Isocitrate lyase was blocked either genetically (Δicl1/2) or chemically by 3-nitropropionate (3NP). (C) Hypoxic carbon fluxes in the TCA cycle and glyoxylate shunt vary between M. tuberculosis and its common non-pathogenic model organisms M. smegmatis and M. bovis BCG. A minimum of three independent biological experiments and two technical replicates measurements was used for each plot.
DISCUSSION
Semi-quantitative profiles of mycobacterial exometabolome dynamics resulting from cultivation in a nutritionally complex environment were systematically analyzed. Statistical analysis revealed consumed and secreted metabolites through their consistent time profiles, from which we inferred the underlying metabolism with a genome-scale metabolic reaction-pair model. A combination of statistics and network analysis yielded a likelihood score for intracellular pathways in explaining the transformation of consumed into secreted metabolites. Global assessment of the top hits of predicted metabolic routes suggested active TCA cycle, glyoxylate, coenzyme A, nicotinate and carbohydrate metabolism. Investigating two top-hit predictions through 13C-tracing and genetics, we demonstrated the relevance of asparagine to aspartate and citrate to succinate conversion and product secretion for survival of hypoxic M. smegmatis. While hypoxic production of aspartate and succinate were conserved among mycobacteria, the underlying pathways of succinate fermentation differed substantially between species. This emphasizes the need for a better understanding of the various mycobacterial models to select the most suitable experimental setup for the questions tackled.
By which metabolic mechanism contribute aspartate and succinate fluxes to hypoxic fitness? In acetate-grown M. tuberculosis, succinate secretion has been proposed to contribute to membrane polarization upon fast shift to hypoxia (Eoh and Rhee, 2013), in analogy to anaerobic E. coli where H+/succinate symport can generate −70 mV potential of de-energized membrane vesicles, which is comparable to the potential of anaerobic mycobacteria (Engel et al., 1994; Rao et al., 2008). Intriguingly, the Δicl1 Δicl2 double mutant exhibited increased secretion of aspartate, suggesting compensation for the reduced secretion of the structurally related succinate, both of which can be transported by the same protein (Fig. S12) (Kay and Kornberg, 1971). The roughly twenty times greater compensatory aspartate secretion in relation to the loss of succinate secretion indicates lower efficiency of aspartate symport and potential interference of produced ammonia through de-amination of asparagine. Considering symport of one proton per aspartate and two protons per succinate at a requirement of three protons for ADP phosphorylation to ATP (Tomashek and Brusilow, 2000), we estimated the hypoxic ATP generation rate by the two here identified fermentative pathways to about 10.3 μmol per gram dry weight (343.4 ± 17.5 ug mL-1 OD-1) and hour, with aspartate and succinate contributing about 74% and 26%, respectively. Such low energy production rate is in line with long-term survival of mycobacteria under nutrient-starved conditions (Gengenbacher et al., 2010). Thus, the here newly proposed energy-generating, fermentative function of hypoxic aspartate and succinate secretion is a likely key determinant of mycobacterial resistance to adverse environmental conditions in a pathogenic and non-pathogenic context.
Interestingly, asparagine has recently been suggested as a major nutrient of M. tuberculosis during early infection and its in vivo availability was directly demonstrated in macrophages (Gouzy et al., 2013; 2014). This work linked asparagine consumption with biomass generation and defense mechanisms of replicating bacteria against the macrophages’ innate immune response. In contrast, here we suggest an additional, fermentative role for asparagine in hypoxic, non-replicating populations of mycobacteria that is associated with later stage and in particular latent tuberculosis infections (Boshoff and Barry, 2005). Thus, it is conceivable that in particular the unexpected asparagine fermentation could be targeted for tackling latent M. tuberculosis infections when most cells reside in hypoxic granulomas.
The developed approach takes advantage of the easier accessibility of dynamic exometabolome data compared to intracellular metabolome data to quickly generate metabolic hypothesis, and hence to prioritize follow-up experiments. Ideally exometabolome data are interpreted with stoichiometric models using flux balance analysis, but the semi-quantitative nature of untargeted metabolomics data precludes such straightforward application. For the growing number of untargeted metabolomics data we present here a blueprint to prioritize active pathways, for example in situations with complex nutritional conditions or with limited understanding of an organism’s physiology under a given condition. Both problems are the norm in typical microbial communities, such as the human microbiota, various biofilm communities and others. Our approach should enable systematic investigations of the nutrient exchanges in such communities and help to disentangle the metabolic contribution of single species.
EXPERIMENTAL PROCEDURES
Chemicals, Strains and Culture Conditions
All chemicals were purchased from Sigma-Aldrich (Schnelldorf, Switzerland). M. smegmatis ΔglcB, Δicl1 Δicl2 and the parent strain were courteously received from John D. McKinney. Strains were plated on agar (15 g/L) based on 7H9 salts containing Middlebrook ADC supplements and incubated at 37°C. Liquid cultures were cultivated at 37°C under constant agitation in Dubos Tween-albumin broth, mixed from pure chemicals (Wayne and Hayes, 1996). 100 mL of culture medium was inoculated to an optical density at 600 nm of 0.005 in 150 mL serum bottles (VWR International & Omnilab AG) resulting in a head-space ratio of 0.5, as originally described for Wayne cultures (Wayne and Hayes, 1996). Cultures were incubated at 37°C and stirred with 400 rpm using a small cross-shaped stir bar (diameter = 2 cm). Methylene blue was added to a final concentration of 2.5 μg/mL. Samples to monitor the culture density, for agar plating or for metabolic analysis were collected through the rubber membrane of the serum bottle using a syringe under nitrogen protection gas.
Resucitation assays were performed in a plate reader (Biolector®, m2p-labs) using 48-well plates (FlowerPlate®, m2p-labs) and a culture volume of 1.5 mL. Wayne cultures were diluted 1:10 in fresh medium and incubated at 37°C, 800 rpm and 95% humidity. Bacterial growth was monitored every 10 min by light scattering using a gain of 30. Middlebrook 7H10 agar was used to determine colony forming units (cfu). Dry cell mass was determined by filtration, washing of cells and drying at 70°C until constant biomass of an equivalent of 25 mL culture volume at an optical density of 1.0 as previously described (Mallette, 1969).
Sampling for the Analysis of Exo- and Endometabolome
Culture samples were collected from Wayne bottles as described above. Samples for exometabolome analysis were prepared by centrifugation to separate cells form culture supernatant as previously described (Paczia et al., 2012). Quenching of metabolic processes, extraction of intracellular metabolites and sample preparation was performed by fast filtration as previously reported for M. smegmatis (Zimmermann et al., 2013).
Mass Spectrometry Analysis
Flow injection mass spectrometry for the untargeted analysis of compounds followed the protocol previously described using a quadrupole-coupled time of flight instrument (Agilent 6550) at low mass-range settings (Fuhrer et al., 2011). Extracellular samples were diluted 100 times in water and measured using the instrument’s dynamic range mode. The instrument’s high resolution mode was used for the measurement of intracellular metabolites (amino acids, NAD+, NADH). Ultra high-pressure chromatography-coupled tandem mass spectrometry using negative ionization was used for absolute quantification of selected metabolites (aspartate, asparagine and ATP) and measurement of mass isotopomer distribution of metabolites for 13C-tracing as previously described (Buescher et al., 2010; Rühl et al., 2011). Ultra high-pressure chromatography-coupled (Agilent 1200 Infinity) tandem mass spectrometry (AB SCIEX Qtrap 5500) using positive ionization was adapted from Jain et al. to measure the mass isotopomer distribution of glycine (Jain et al., 2012). In brief, 5 μL of supernatant were injected on an Agilent HILIC Plus RRHD column (100 × 2.1 mm x 1.8 μM) at 50°C using mobile phase A (10 mM ammonium formate, 0,1% (v/v) formic acid) and mobile phase B (acetonitrile, 0,1% (v/v) formic acid): initial conditions: 10% A, 400 μL / min; 2 min: 60% A, 400 μL / min; 3 min: 60% A, 400 μL / min; 4 min: 90% A, 300 μL / min; 5 min: 90% A, 300 μL / min; 7 min: 10% A, 400 μL / min; 9 min: 10% A, 400 μL / min. The MRM transition for glycine was 76 to 30 m/z and for [U-13C]-glycine 78 to 31 m/z. The other machine settings were optimized according to the manufacturer’s recommendations for single compound optimizations.
Supplementary Material
ACKNOWLEDGMENTS
John D McKinney for genetic deletion strains of M. smegmatis and M. tuberculosis. This research was, in part, funded by EU FP7 project SysteMTb (MZ and US) and by the Intramural Research Program of NIAID (HIB and CEB).
Footnotes
Computational Analysis
Computing was performed with MatLab (Mathworks, Natick, MA, United States) as further documented in the supporting information. All codes are publically available on the group’s website (http://www.imsb.ethz.ch/research/sauer.html).
SUPPORTING INFORMATION
Experimental procedures, Fig. S1 – 12 and Table S1 – 3. The reaction pair model, the codes used for computational analysis, and the measurement raw data can be downloaded from http://www.imsb.ethz.ch/research/sauer.html.
REFERENCES
- Allen J, Davey HM, Broadhurst D, Heald JK, Rowland JJ, Oliver SG, and Kell DB (2003) High-throughput classification of yeast mutants for functional genomics using metabolic footprinting. Nat Biotechnol 21: 692–696. [DOI] [PubMed] [Google Scholar]
- Behrends V, Bell TJ, Liebeke M, Cordes-Blauert A, Ashraf SN, Nair C, et al. (2013) Metabolite profiling to characterize disease-related bacteria: gluconate excretion by Pseudomonas aeruginosa mutants and clinical isolates from cystic fibrosis patients. J Biol Chem 288: 15098–15109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berney M, Weimar MR, Heikal A, and Cook GM (2012) Regulation of proline metabolism in mycobacteria and its role in carbon metabolism under hypoxia. Mol Microbiol 84: 664–681. [DOI] [PubMed] [Google Scholar]
- Blum T. and Kohlbacher O. (2008) Using atom mapping rules for an improved detection of relevant routes in weighted metabolic networks. J. Comput. Biol. 15: 565–576. [DOI] [PubMed] [Google Scholar]
- Boshoff HIM and Barry CE (2005) Tuberculosis - metabolism and respiration in the absence of growth. Nat Rev Microbiol 3: 70–80. [DOI] [PubMed] [Google Scholar]
- Bowles JA and Segal W. (1965) Kinetics of Utilization of Organic Compounds in the Growth of Mycobacterium tuberculosis. The Journal of Bacteriology 90: 157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SA, Palmer KL, and Whiteley M. (2008) Revisiting the host as a growth medium. Nat Rev Microbiol 6: 657–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buescher JM, Moco S, Sauer U, and Zamboni N. (2010) Ultrahigh performance liquid chromatography-tandem mass spectrometry method for fast and robust quantification of anionic and aromatic metabolites. Anal Chem 82: 4403–4412. [DOI] [PubMed] [Google Scholar]
- Chao MC and Rubin EJ (2010) Letting sleeping dos lie: does dormancy play a role in tuberculosis? Annu Rev Microbiol 64: 293–311. [DOI] [PubMed] [Google Scholar]
- Croes D, Couche F, Wodak SJ, and van Helden J. (2006) Inferring meaningful pathways in weighted metabolic networks. J Mol Biol 356: 222–236. [DOI] [PubMed] [Google Scholar]
- Engel P, Kramer R, and Unden G. (1994) Transport of C4-dicarboxylates by anaerobically grown Escherichia coli. Energetics and mechanism of exchange, uptake and efflux. Eur J Biochem 222: 605–614. [DOI] [PubMed] [Google Scholar]
- Eoh H. and Rhee KY (2013) Multifunctional essentiality of succinate metabolism in adaptation to hypoxia in Mycobacterium tuberculosis. Proceedings of the National Academy of Sciences 110: 6554–6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmail H, Barry CE III, and Wilkinson RJ (2012) Understanding latent tuberculosis: the key to improved diagnostic and novel treatment strategies. Drug Discov Today 17: 514–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrer T, Heer D, Begemann B, and Zamboni N. (2011) High-throughput, accurate mass metabolome profiling of cellular extracts by flow injection-time-of-flight mass spectrometry. Anal Chem 83: 7074–7080. [DOI] [PubMed] [Google Scholar]
- Gengenbacher M. and Kaufmann SHE (2012) Mycobacterium tuberculosis: success through dormancy. FEMS Microbiology Reviews 36: 514–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gengenbacher M, Rao SPS, Pethe K, and Dick T. (2010) Nutrient-starved, non-replicating Mycobacterium tuberculosis requires respiration, ATP synthase and isocitrate lyase for maintenance of ATP homeostasis and viability. Microbiology (Reading, Engl) 156: 81–87. [DOI] [PubMed] [Google Scholar]
- Gouzy A, Larrouy-Maumus G, Bottai D, Levillain F, Dumas A, Wallach JB, et al. (2014) Mycobacterium tuberculosis exploits asparagine to assimilate nitrogen and resist acid stress during infection. PLoS Pathog 10: e1003928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouzy A, Larrouy-Maumus G, Wu T-D, Peixoto A, Levillain F, Lugo-Villarino G, et al. (2013) Mycobacterium tuberculosis nitrogen assimilation and host colonization require aspartate. Nat Chem Biol 9: 674–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper LV, Midtvedt T, and Gordon JI (2002) How host-microbial interactions shape the nutrient environment of the mammalian intestine. Annu Rev Nutr. [DOI] [PubMed] [Google Scholar]
- Hutter B. and Dick T. (1998) Increased alanine dehydrogenase activity during dormancy in Mycobacterium smegmatis. FEMS Microbiol Lett 167: 7–11. [DOI] [PubMed] [Google Scholar]
- Jain M, Nilsson R, Sharma S, Madhusudhan N, Kitami T, Souza AL, et al. (2012) Metabolite Profiling Identifies a Key Role for Glycine in Rapid Cancer Cell Proliferation. Science 336: 1040–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaderbhai NN, Broadhurst DI, Ellis DI, Goodacre R, and Kell DB (2003) Functional genomics via metabolic footprinting: monitoring metabolite secretion by Escherichia coli tryptophan metabolism mutants using FT-IR and direct injection electrospray mass spectrometry. Comp. Funct. Genomics 4: 376–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehisa M. (2000) KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res 28: 27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay WW and Kornberg HL (1971) The Uptake of C4-Dicarboxylic Acids by Escherichia coli. Eur J Biochem 18: 274–281. [DOI] [PubMed] [Google Scholar]
- Kell DB, Brown M, Davey HM, Dunn WB, Spasic I, and Oliver SG (2005) Metabolic footprinting and systems biology: the medium is the message. Nat Rev Microbiol 3: 557–565. [DOI] [PubMed] [Google Scholar]
- Mallette MF (1969) Chapter XV Evaluation of Growth by Physical and Chemical Means. Methods in microbiology. [Google Scholar]
- Orth JD, Thiele I, and Palsson BØ (2010) What is flux balance analysis? Nat Biotechnol 28: 245–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paczia N, Nilgen A, Lehmann T, Gätgens J, Wiechert W, and Noack S. (2012) Extensive exometabolome analysis reveals extended overflow metabolism in various microorganisms. Microb. Cell Fact. 11: 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope GA, MacKenzie DA, Defernez M, and Roberts IN (2009) Metabolic footprinting for the study of microbial biodiversity. Cold Spring Harb Protoc 2009: pdb.prot5222. [DOI] [PubMed] [Google Scholar]
- Rao SPS, Alonso S, Rand L, Dick T, and Pethe K. (2008) The protonmotive force is required for maintaining ATP homeostasis and viability of hypoxic, nonreplicating Mycobacterium tuberculosis. Proc Natl Acad Sci USA 105: 11945–11950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rühl M, Rupp B, Nöh K, Wiechert W, Sauer U, and Zamboni N. (2011) Collisional fragmentation of central carbon metabolites in LC-MS/MS increases precision of 13C metabolic flux analysis. Biotechnol Bioeng 109: 763–771. [DOI] [PubMed] [Google Scholar]
- Shi L, Sohaskey C, Pfeiffer C, Datta P, and Parks M. (2010) Carbon flux rerouting during Mycobacterium tuberculosis growth arrestmmi_7399. Wiley Online Library. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CV, Huang C-C, Miczak A, Russell DG, Sacchettini JC, and Honer Zu Bentrup K. (2003) Biochemical and structural studies of malate synthase from Mycobacterium tuberculosis. J Biol Chem 278: 1735–1743. [DOI] [PubMed] [Google Scholar]
- Tang YJ, Shui W, Myers S, Feng X, Bertozzi C, and Keasling JD (2009) Central metabolism in Mycobacterium smegmatis during the transition from O2-rich to O2-poor conditions as studied by isotopomer-assisted metabolite analysis. Biotechnol Lett 31: 1233–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomashek JJ and Brusilow WS (2000) Stoichiometry of energy coupling by proton-translocating ATPases: a history of variability. J. Bioenerg. Biomembr. 32: 493–500. [DOI] [PubMed] [Google Scholar]
- Upton AM and McKinney JD (2007) Role of the methylcitrate cycle in propionate metabolism and detoxification in Mycobacterium smegmatis. Microbiology (Reading, Engl) 153: 3973–3982. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Zimmermann M, Goodwin MB, Sauer U, Barry CE, and Boshoff HI (2011) Fumarate Reductase Activity Maintains an Energized Membrane in Anaerobic Mycobacterium tuberculosis. PLoS Pathog 7: e1002287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne LG and Hayes LG (1996) An in vitro model for sequential study of shiftdown of Mycobacterium tuberculosis through two stages of nonreplicating persistence. Infect Immun 64: 2062–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne LG and Lin KY (1982) Glyoxylate metabolism and adaptation of Mycobacterium tuberculosis to survival under anaerobic conditions. Infect Immun 37: 1042–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne LG and Sohaskey CD (2001) Nonreplicating persistence of mycobacterium tuberculosis. Annu Rev Microbiol 55: 139–163. [DOI] [PubMed] [Google Scholar]
- Wishart DS, Jewison T, Guo AC, Wilson M, Knox C, Liu Y, et al. (2013) HMDB 3.0--The Human Metabolome Database in 2013. Nucleic Acids Res 41: D801–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann M, Thormann V, Sauer U, and Zamboni N. (2013) Nontargeted profiling of coenzyme A thioesters in biological samples by tandem mass spectrometry. Anal Chem 85: 8284–8290. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.