Abstract
Background
The effect of HBV on neonatal and maternal outcomes can create a basis for more accurate clinical decision-making. So, the aim of this meta-analysis is to detrmine the effect of chronic hepatitis B virus on the risk of pregnancy outcomes by combining cohort studies.
Methods
International databases in this meta-analysis included the Cumulated Index to Nursing and Allied Health Literature (CINAHL), SPORT Discuss via the EBSCO interface, PubMed (Medline), Scopus, Web of Science, Embase, which were searched up to April 2023. All cohort studies reporting the risk ratio (RR) with a 95% confidence interval (CI) were included in the study. The quality assessment was done based on the Newcastle–Ottawa Scale (NOS).
Results
Finally, thirty-five cohort studies were selected for meta-analysis. Outcomes of interest included pre-eclampsia, gestational diabetes, abortion, preterm birth, infant death, and other related outcomes. Results showed that the pooled RR for incident gestational diabetes in pregnant women with choronic hepatitis B infection was 1.16 (RR: 1.16; 95% CI 1.13–1.18; I-square: 92.89%; P value: 0.00). Similarly, the association between the presence of hepatitis B infection in pregnant women and the occurrence of pre-eclampsia was 1.10 (RR: 1.10; 95% CI 1.04–1.16; I-square: 92.06%; P value: 0.00). The risk of preterm delivery in pregnant women with hepatitis B infection was 1.17 times that of pregnant women without hepatitis B infection (RR: 1.17; 95% CI 1.14–1.20; I-squared: 94.32%; P value: 0.00).
Conclusion
This meta-analysis found that hepatitis B infection during pregnancy may be associated with an increased risk of gestational diabetes, preterm delivery, pre-eclampsia, and eclampsia. However, confirmation of this association, as well as the specific biological pathways involved in the association between HBV infection and pregnancy outcomes, requires further investigation.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12985-023-02182-0.
Keywords: Hepatitis B virus, Pregnancy outcomes, Pre-eclampsia, Gestational diabetes, Abortion, Premature birth, Infant death
Introduction
A double-stranded DNA virus belonging to the hepadnaviridae family, hepatitis B virus (HBV). The virus is enveloped and has a core with a viral DNA genome of approximately 3200 base pairs. In patient blood, the virus was initially identified as “Australia antigen,” subsequently known as hepatitis B surface antigen (HBsAg). Later on, as a marker for those at high risk of transmitting the disease, the hepatitis B e antigen (HBeAg) was discovered. The virus penetrates a hepatocyte, delivers its genome to the nucleus, and changes the relaxed circular DNA into covalently closed circular DNA (cccDNA) [1–3]. Significant human morbidity and death are brought on by HBV infection, mostly as a result of the effects of chronic infection. According to recent estimates of chronically infected people ranging from 240 to 350 million, more than two billion people have ever been infected with HBV [4–6]. Around 0.5–1.2 million people die annually on average [7]. There are three geographic regions where the prevalence of HBV infection is highest: East Asia and Africa (> 8%), the Mediterranean region (2–8%), and Eastern Europe (2%) [8]. More than half of the 350 million HBV carriers worldwide acquire the virus during pregnancy; rates of mother-to-child transmission also differ dramatically depending on the mother’s hepatitis B e antigen (HBeAg) status [9, 10]. Some mechanisms explain how HBV infection affects pregnancy. Reduced CD8 T cells and increased viral activity are caused by inhibiting the Th1 immune response and simulating the Th2 immune response, impairing the immunological response to HBV [11]. Due to the cross-reaction, increased regulatory T cells, and malfunctioning CD8 T cells, the exposure of the fetus to HBeAg may generate fetal T helper cell tolerance to HBeAg and HBcAg, which rasie HBV DNA levels during pregnancy [12, 13]. Studies have shown an increased risk of both newborn and maternal morbidity associated with HBV infection, including fetal distress, gestational diabetes mellitus, preterm delivery, and meconium peritonitis[14–18]. Also, antepartum hemorrhage causing placental abruption and placenta previa can increase. A lower Apgar score is the only perinatal complication [14, 18]. However, there isn’t much research on the mechanisms underlying these results [19].
The effect of HBV on neonatal and maternal outcomes can create a basis for more accurate clinical decision-making. By identifying the specific connection between HBV and pregnancy outcomes, clinicians and specialists can reduce the impacts and enhance the quality of life for HBV patients. The research may aid in updating clinical guidelines and improving the care of HBV patients, which may aid in the early detection and prevention of pregnancy outcomes. Thus, by combining cohort data, we aimed to systematically review and meta-analyze the relationship between HBV and pregnancy outcomes.
Methods
The current study was a systematic review and meta-analysis, which was conducted to determine the exact relationship between the presence of infection and the chronic HBV and the occurrence of maternal outcomes such as pre-eclampsia, gestational diabetes, abortion, premature birth, infant death, and other related results. The primary studies in this meta-analysis were prospective or retrospective cohort studies. All the steps of this study were developed and carried out based on the structure of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline [20].
At first, the search strategy aimed at retrieving cohort studies published in the target field in international databases such as the Cumulated Index to Nursing and Allied Health Literature (CINAHL), SPORT Discuss via the EBSCO interface, PubMed (Medline), Scopus, Web of Science, and Embase. Then the retrieved articles were screened in Endnote version 8. Keywords were included: “Hepatitis B”, “Hepatitis B Virus”, “Pregnancy Outcome”, “Maternal Morbidity”, “Maternal Death”, “Pre-Eclampsia”, “Premature Birth”, and “Gestational Diabetes”, along with their synonyms in the Mesh database. To perform the screening, first, the duplicates of retrieved articles in the software were removed, and then, in the first step, screening based on the title; in the second step, screening based on the subject; and in the final step, screening based on the full text of the articles was done. And after these steps, the final articles were selected. The time frame for searching international databases and screening was from January 1990 to February 2023. Also, all the screening steps were done independently by authors (MA and MA), and disputes were resolved by the third researcher (YM), who was an expert. To carry out a comprehensive and detailed search, or in other words, to carry out gray literature to complete the search strategy, the first ten pages of Google Scholar along with a manual search (checking the sources and references of the final selected studies) were also done by the authors. The inclusion criteria and the final selection of studies in this meta-analysis were based on the PECOT structure, the specifics of which are detailed in Table 1. The studies that had all the characteristics listed in Table 2 were included in the present meta-analysis. After the screening, to extract the desired information from these articles based on the purpose of the study, a checklist prepared with the opinion of experts was used. Checklist elements included authors’ names, type of study, year of publication, total sample size, country of study, type of population, age, and effect size reported in the studies. Two authors (MA and MA/KZ) carried out data extraction independently, and the third researcher (YM), an expert, resolved disputes.
Table 1.
Selection criteria for primary studies to enter the present meta-analysis
| P (Population) | E (Exposure) | C (Comparison) | O (Outcomes) | T (Type of Studies) |
|---|---|---|---|---|
| The target population included all pregnant women without any restrictions | The intended exposure or cause was the presence of infection or hepatitis B virus in pregnant women | The comparison group included healthy pregnant women without hepatitis B infection | The intended Outcomes included neonatal death, miscarriage, eclampsia or preeclampsia, preterm birth, gestational diabetes, and other outcomes reported in selected cohort studies | The type of studies included only cohort studies |
Table 2.
The charactristics of included studies related to effect of HBV on the risk of maternal outcomes
| References | Authors (Years) Country |
Type of study | Study population | Sample size | Neonatal death | Eclampsia | Number of Pre-Eclampsia (Positive pregnancies) |
Maternal age (Yrs, mean ± SD) |
Gestational age (weeks, mean ± SD) | Gestational hypertension | Parity | GDM | Preterm birth |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [38] |
Zhang (2020) China |
Retrospective cohort |
HBsAg-positive and HBsAg-negative pregnancies |
HBsAg-positive = 9699 HBsAg-negative = 73,076 |
NR | NR |
HBsAg-positive = 139 (1.43%) HBsAg-negative = 1302 (1.78%) |
HBsAg-positive = 30.33 ± 4.50 HBsAg-negative = 30.28 ± 4.45 |
HBsAg-positive = 38.18 ± 2.96 HBsAg-negative = 38.17 ± 3.51 |
HBsAg-positive = 157 HBsAg-negative = 1421 |
1 = HBsAg-positive = 82 (0.85%) HBsAg-negative = 760 (1.04%) > 1 = HBsAg-positive = 9617 (99.15%) HBsAg-negative = 72,316 (98.96%) |
HBsAg-positive = 1663 HBsAg-negative = 11,982 |
NR |
| [22] |
Tan (2016) China |
Retrospective cohort |
HBsAg-positive and HBsAg-negative pregnancies |
HBsAg-positive = 948 HBsAg-negative = 21,426 |
NR | NR |
HBsAg-positive = 33 (3.5%) HBsAg-negative = 523 (2.4%) |
< 35 = HBsAg-positive = 813 (85.8) HBsAg-negative = 18 876 (88.1) HBsAg-positive = 135 (14.2) HBsAg-negative = 2550 (11.9) |
39 (38–39) |
HBsAg-positive = 9 HBsAg-negative = 288 |
Nulliparity = HBsAg-positive = 713 (75.2) HBsAg-negative = 16 489 (77.0) Multiparity = HBsAg-positive = 235 (24.8) HBsAg-negative = 4937 (23.0) |
HBsAg-positive = 112 HBsAg-negative = 1758 |
NR |
| [21] |
Stokkeland (2017) Sweden |
Retrospective cohort |
HBsAg-positive and HBsAg-negative pregnancies |
HBsAg-positive = 2990 HBsAg-negative = 1,090,979 |
NR | NR |
HBsAg-positive = 59 (1.97) HBsAg-negative = 30,030 (2.75) |
≤ 24 = HBsAg-positive = 506 HBsAg-negative = 156,906 25–34 = HBsAg-positive = 1780 HBsAg-negative = 709,970 ≥ 35 = HBsAg-positive = 7.4 HBsAg-negative = 226,587 |
NR | NR |
1 = HBsAg-positive = 1014 HBsAg-negative = 488,887 2 = HBsAg-positive = 989 HBsAg-negative = 396,034 + 3 = HBsAg-positive = 987 HBsAg-negative = 206,058 |
HBsAg-positive = 68 HBsAg-negative = 11,262 |
HBsAg-positive = 175 HBsAg-negative = 53,452 |
| [29] |
Lok (2021) Hong Kong |
Retrospective cohort | HBV negative = 79487HBV positive = 8402 |
HBV negative = 105 HBV positive = 11 |
HBV negative = 37 HBV positive = 5 |
HBsAg-positive = 66 (0.78%) HBsAg-negative = 1103 (1.25%) |
NR | NR |
HBsAg-positive = 185 HBsAg-negative = 2366 |
Nulliparity = HBsAg-positive = 4082 HBsAg-negative = 39,178 Multiparity = HBsAg-positive = 4320 HBsAg-negative = 40,309 |
HBsAg-positive = 763 HBsAg-negative = 6877 |
HBsAg-positive = 522 HBsAg-negative = 5299 |
|
| [25] |
Chen (2022) United States |
Retrospective cohort study |
HBsAg-positive and HBsAg-negative pregnancies |
Control = 28,499,085 HBV = 51,200 | NR |
Control = 5.28% HBV = 4.48% |
NR | NR | NR | NR |
Control = 6.94% HBV = 12.94% |
Control = 6.27% HBV = 5.52% |
|
| [37] |
Yin (2021) China |
Retrospective cohort study |
HBsAg-positive and HBsAg-negative pregnancies |
HBV negative = 36,500 HBV positive = 3039 |
NR | NR |
HBV negative = 924 HBV positive = 103 |
NR | 38.55 ± 2.17 |
HBV negative = 242 HBV positive = 18 |
NR |
HBV negative = 3529 HBV positive = 366 |
HBV negative = 1720 HBV positive = 119 |
| [23] |
Bajema (2018) United States |
Retrospective cohort study |
HBsAg-positive and HBsAg-negative pregnancies |
HBV negative = 22 410 HBV positive = 4391 |
NR |
HBV negative = 25 HBV positive = 9 |
HBV negative = 1299 HBV positive = 177 |
NR | NR | NR |
0 = HBsAg-positive = 1935 HBsAg-negative = 9249 > 1 = HBsAg-positive = 2388 HBsAg-negative = 12,790 |
HBV negative = 1109 HBV positive = 389 |
NR |
| [36] |
Xiong (2021) China |
Retrospective cohort study |
HBsAg-positive and HBsAg-negative pregnancies |
HBV negative = 6,216 HBV positive = 795 |
NR | NR |
HBV negative = 132 HBV positive = 16 |
HBV negative = 31 (28–34) HBV positive = 31 (28–34) |
NR | NR | NR |
HBV negative = 1585 HBV positive = 221 |
HBV negative = 967 HBV positive = 141 |
| [27] |
Connell (2011) USA |
Retrospective cohort study |
HBsAg-positive and HBsAg-negative pregnancies |
HBV negative = 1 668 911 HBV positive = 1458 |
NR | NR |
HBV negative = 4.46% HBV positive = 4.25% |
NR | NR |
HBsAg-negative = 57,744 HBsAg-positive = 40 |
NR |
HBsAg-negative = 11,849 HBsAg-positive = 29 |
HBsAg-negative = 147,532 HBsAg-positive = 132 |
| [18] |
Lao (2007) China |
Retrospective cohort study |
HBsAg-positive and HBsAg-negative pregnancies |
HBV negative = 12,547 HBV positive = 1138 |
NR | NR |
HBV negative = 2.8% HBV positive = 1.8% |
HBsAg-negative = 30.3 ± 5.3 HBsAg-positive = 30.2 ± 5.2 |
HBsAg-negative = 39.0 ± 1.8 HBsAg-positive = 38.9 ± 1.8 |
NR | NR |
HBsAg-negative = 1279 HBsAg-positive = 141 |
|
| [28] |
Lao (2013) Hong Kong |
Retrospective cohort study |
HBsAg-positive and HBsAg-negative pregnancies |
HBV negative = 77,936 HBV positive = 8636 |
NR | NR |
HBV negative = 1.1% HBV positive = 0.8% |
HBsAg-negative = 29.9 ± 5.2 HBsAg-positive = 29.8 ± 5.1 |
HBsAg-negative = 39.0 ± 1.8 HBsAg-positive = 38.9 ± 1.8 |
HBsAg-negative = 545 HBsAg-positive = 52 |
NR |
HBsAg-negative = 5221 HBsAg-positive = 553 |
HBsAg-negative = 4988 HBsAg-positive = 526 |
| [50] |
Lobstein (2011) Germany |
Retrospectively |
HBsAg-positive and HBsAg-negative pregnancies |
HBV negative = 8,154 HBV positive = 39 |
NR |
HBV negative = 26 HBV positive = 0 |
HBV negative = 160 HBV positive = 1 |
NR | NR | NR | NR |
HBsAg-negative = 36 HBsAg-positive = 0 |
HBsAg-negative = 922 HBsAg-positive = 8 |
| [31] |
Reddick (2011) United States |
Retrospective cohort study |
HBsAg-positive and HBsAg-negative pregnancies |
HBV negative = 296,773 HBV positive = 891 |
NR | NR |
HBV negative = 9729 HBV positive = 33 |
NR | NR | NR | NR |
HBV negative = 7464 HBV positive = 35 |
HBsAg-negative = 35,991 HBsAg-positive = 197 |
| [32] |
Sirilert (2014) Thailand |
Retrospective cohort study |
HBsAg-positive and HBsAg-negative pregnancies |
HBsAg-negative = 22,331 HBsAg-positive = 1472 |
NR | NR |
HBsAg-negative = 1496 HBsAg-positive = 91 |
HBV negative = 27.82 ± 7.36 HBV positive = 27.69 ± 5.67 |
HBV negative = 37.74 ± 3.22 HBV positive = 37.37 ± 2.91 |
NR | NR |
HBsAg-negative = 1290 HBsAg-positive = 98 |
HBsAg-negative = 2181 HBsAg-positive = 171 |
| [34] |
To (2003) Hong Kong |
Retrospective |
HBsAg-positive and HBsAg-negative pregnancies |
HBsAg-negative = 12,452 HBsAg-positive = 1340 |
HBsAg-negative = 18 HBsAg-positive = 1 |
HBsAg-negative = 6 HBsAg-positive = 0 |
HBsAg-negative = 99 HBsAg-positive = 3 |
NR |
HBsAg-negative = 38.9 ± 2.91 HBsAg-positive = 38.9 ± 2.61 |
HBsAg-negative = 447 HBsAg-positive = 27 |
Nulliparity = HBsAg-positive = 622 (46.4) HBsAg-negative = 5952 (47.8) Multiparity = HBsAg-positive = 718 (53.5) HBsAg-negative = 6500 (52.2) |
HBsAg-negative = 478 HBsAg-positive = 42 |
NR |
| [30] |
Mak (2013) Hong Kong |
Retrospective cohort |
HBsAg-positive and HBsAg-negative pregnancies |
HBsAg-negative = 8778 HBsAg-positive = 784 |
NR |
HBsAg-negative = 0 HBsAg-positive = 3 |
HBsAg-negative = 142 HBsAg-positive = 9 |
NR | NR |
HBsAg-negative = 114 HBsAg-positive = 7 |
NR |
HBsAg-negative = 710 HBsAg-positive = 59 |
HBsAg-negative = 205 HBsAg-positive = 19 |
| [49] |
Huang (2014) China |
Cohort |
HBsAg-positive and HBsAg-negative pregnancies |
HBsAg-negative = 5734 HBsAg-positive = 461 |
NR | NR |
HBsAg-negative = 79 HBsAg-positive = 5 |
NR | NR |
HBsAg-negative = 336 HBsAg-positive = 35 |
NR | NR | NR |
| [40] |
Zhuang (2017) China |
Prospective cohort |
HBsAg-positive and HBsAg-negative pregnancies |
HBsAg-negative = 35,642 HBsAg-positive = 1113 |
NR |
HBsAg-negative = 1114 HBsAg-positive = 40 |
NR | NR | NR | NR |
HBsAg-negative = 2605 HBsAg-positive = 98 |
HBsAg-negative = 2767 HBsAg-positive = 108 |
|
| [48] |
Zheng (2021) China |
Retrospective cohort |
HBsAg-positive and HBsAg-negative pregnancies |
HBsAg-negative = 12,813 HBsAg-positive = 1302 |
NR | NR |
HBsAg-negative = 255 HBsAg-positive = 35 |
39.17 ± 2.06 | NR | NR |
HBsAg-negative = 1857 HBsAg-positive = 210 |
HBsAg-negative = 1857 HBsAg-positive = 210 |
|
| [46] |
Zhao (2020) China |
Retrospective |
HBsAg-positive and HBsAg-negative pregnancies |
HBsAg-negative = 29,648 HBsAg-positive = 3789 |
NR | NR | NR | NR | NR | NR |
1 = HBsAg-negative = 11,821 HBsAg-positive = 1394 2 < = HBsAg-negative = 17,805 HBsAg-positive = 2391 |
HBsAg-negative = 5263 HBsAg-positive = 757 |
HBsAg-negative = 1336 HBsAg-positive = 190 |
| [39] |
Zhao (2022) China |
HBsAg-positive and HBsAg-negative pregnancies |
HBsAg-negative = 89,686 HBsAg-positive = 10,355 |
NR | NR | NR | NR | NR | NR | NR |
HBsAg-negative = 13,048 HBsAg-positive = 1691 |
||
| [35] | Wu (2020) China | Retrospective cohort |
HBsAg-positive and HBsAg-negative pregnancies |
HBsAg-negative = 18,354 HBsAg-positive = 1146 |
NR | NR | NR | NR |
HBsAg-negative = 38.69 ± 3.56 HBsAg-positive = 37.16 ± 3.92 |
NR | NR |
HBsAg-negative = 3289 HBsAg-positive = 260 |
HBsAg-negative = 1502 HBsAg-positive = 128 |
| [33] | Sun (2021) China | Cohort |
HBsAg-positive and HBsAg-negative pregnancies |
HBsAg-negative = 47,855 HBsAg-positive = 1624 |
HBsAg-negative = 0.5 HBsAg-positive = 0.4 |
HBsAg-negative = 2.8 HBsAg-positive = 2.7 |
NR |
HBsAg-negative = 38.9 ± 1.9 HBsAg-positive = 38.9 ± 1.6 |
HBsAg-negative = 1.9 HBsAg-positive = 1.6 |
NR |
HBsAg-negative = 15.5 HBsAg-positive = 13.9 |
HBsAg-negative = 7.8 HBsAg-positive = 8.1 |
|
| [24] |
Bierhoff (2019) Myanmar-Thailand |
Retrospective Cohort |
HBsAg-positive and HBsAg-negative pregnancies |
HBsAg-negative = 10,338 HBsAg-positive = 687 |
HBsAg-negative = 44 HBsAg-positive = 3 |
HBsAg-negative = 1000 HBsAg-positive = 65 |
NR |
HBsAg-negative = 39.0 ± 1.7 HBsAg-positive = 39.1 ± 1.7 |
HBsAg-negative = 834 HBsAg-positive = 55 |
NR |
HBsAg-negative = 539 HBsAg-positive = 27 |
NR | |
| [26] |
Chen (2022) China |
Retrospective Cohort |
HBsAg-positive and HBsAg-negative pregnancies |
HBsAg-negative = 18,693 HBsAg-positive = 735 |
HBsAg-negative = 1906 HBsAg-positive = 60 |
NR | NR |
HBsAg-negative = 28.97 (23.43–34.43) HBsAg-positive = 29.72 (23.94–34.91) |
NR |
HBsAg-negative = 755 HBsAg-positive = 27 |
NR |
HBsAg-negative = 1959 HBsAg-positive = 63 |
NR |
| [42] |
Cui (2016) China |
Prospective cohort |
HBsAg-positive and HBsAg-negative pregnancies |
HBsAg-negative = 20,491 HBsAg-positive = 513 |
NR | NR |
HBsAg-negative = 216 HBsAg-positive = 4 |
HBsAg-negative = 27.03 ± 4.19 HBsAg-positive = 27.59 ± 4.02 |
NR | NR | NR |
HBsAg-negative = 232 HBsAg-positive = 6 |
HBsAg-negative = 1,718 HBsAg-positive = 49 |
| [45] |
Wang, Li (2019) China |
Retrospective Cohort |
HBsAg-positive and HBsAg-negative pregnancies |
HBsAg-negative = 7656 HBsAg-positive = 894 |
NR | NR | NR | NR | NR |
HBsAg-negative = 93 HBsAg-positive = 10 |
HBsAg-negative = 358 HBsAg-positive = 33 |
HBsAg-negative = 874 HBsAg-positive = 108 |
|
| [51] |
Chen (2015) China |
Retrospective Cohort |
HBsAg-positive and HBsAg-negative pregnancies |
HBsAg-negative = 428 HBsAg-positive = 380 |
HBsAg-negative = 2 HBsAg-positive = 2 |
NR | NR | NR |
HBsAg-negative = 39.9 ± 1.7 HBsAg-positive = 39.8 ± 1.9 |
NR | NR | NR |
HBsAg-negative = 6 HBsAg-positive = 11 |
| [41] |
Cheung (2022) Hong Kong |
Retrospective |
HBsAg-positive and HBsAg-negative pregnancies |
HBsAg-negative = 521 HBsAg-positive = 158 |
NR | NR |
HBsAg-negative = 11 HBsAg-positive = 5 |
NR | NR |
HBsAg-negative = 18 HBsAg-positive = 3 |
NR |
HBsAg-negative = 123 HBsAg-positive = 27 |
HBsAg-negative = 31 HBsAg-positive = 9 |
| [47] |
Peng (2019) china |
Retrospective Cohort |
HBsAg-positive and HBsAg-negative pregnancies |
HBsAg-negative = 964 HBsAg-positive = 964 |
NR | NR | NR | NR | NR | NR | NR |
HBsAg-negative = 16.5% HBsAg-positive = 10.5% |
NR |
| [52] |
Liu (2017) china |
Retrospective cohort |
HBsAg-positive and HBsAg-negative pregnancies |
HBsAg-negative = 469,138 HBsAg-positive = 20,827 |
NR | NR | NR | NR | NR | NR | NR | NR |
HBsAg-negative = 24,422 HBsAg-positive = 1344 |
| [44] |
Thungsuk (2008) Thailand |
Retrospective |
HBsAg-positive and HBsAg-negative pregnancies |
HBsAg-negative = 170 HBsAg-positive = 154 |
NR | NR |
HBsAg-negative = 0 HBsAg-positive = 2 |
NR | NR |
HBsAg-negative = 3 HBsAg-positive = 3 |
NR |
HBsAg-negative = 4 HBsAg-positive = 5 |
HBsAg-negative = 10 HBsAg-positive = 19 |
| [43] |
Tan (2017) China |
Retrospective cohort |
HBsAg-positive and HBsAg-negative pregnancies |
HBsAg-negative = 21,024 HBsAg-positive = 923 |
NR | NR | NR | NR |
HBsAg-negative = 38.5 HBsAg-positive = 38.3 |
NR |
Nulliparity HBsAg-negative = 16,172 HBsAg-positive = 695 Multiparity HBsAg-negative = 4852 HBsAg-positive = 228 |
HBsAg-negative = 1696 HBsAg-positive = 109 |
HBsAg-negative = 1600 HBsAg-positive = 84 |
| [54] | Xu (2021) China | Retrospective cohort |
HBsAg-positive and HBsAg-negative pregnancies |
HBsAg-negative = 52,094 HBsAg-positive = 2151 |
NR | NR | NR | NR | NR | NR |
0 = HBsAg-negative = 40,464 HBsAg-positive = 1561 1 = HBsAg-negative = 11,337 HBsAg-positive = 565 2 > = HBsAg-negative = 284 HBsAg-positive = 4 |
NR |
HBsAg-negative = 2319 HBsAg-positive = 129 |
| [53] | Salemi (2014) | Retrospective cohort |
HBsAg-positive and HBsAg-negative pregnancies |
HBsAg-negative = 2,213,722 HBsAg-positive = 3513 |
NR | NR | NR | NR | NR | NR |
Nulliparity HBsAg-negative = 935,227 HBsAg-positive = 1296 Multiparity HBsAg-negative = 1,274,813 HBsAg-positive = 2206 |
NR |
HBsAg-negative = 879,034 HBsAg-positive = 1492 |
NR Not Reported; GDM: Gastetional Diabetes; R: References, Yrs: Years
Evaluating the risk of bias
The NOS (Newcastle–Ottawa Quality Assessment Scale) checklist was used to evaluate the quality of the articles. This checklist is designed to assess the quality of cross-sectional studies. Each of these items is given a score of 1 if they are observed in the studies. And the maximum score for each study is 9 points. This step was done independently by two authors (MA and KZ), and in case of disagreement, the cases were referred to the third researcher (YM).
Statistical analysis
The intended effect size in this meta-analysis was the risk ratio (RR). First, the effect size and the confidence interval were extracted from the studies to perform the analysis. Then, in the desired software for analysis, the logarithm and the standard deviation (SD) of the RR logarithm were calculated, and by combining the logarithm and the standard deviation of the RR logarithm, meta-analysis was conducted. To check the heterogeneity and variance between the selected studies, Cochran’s Q and I2 tests were used. Statistical analysis was performed using STATA 17, and the P-value was considered lower than 0.05. Subgroup analyses were performed to determine the main source of heterogeneity in the current meta-analysis based on gestational age, the continent or country of study, and mothers’ age.
Results
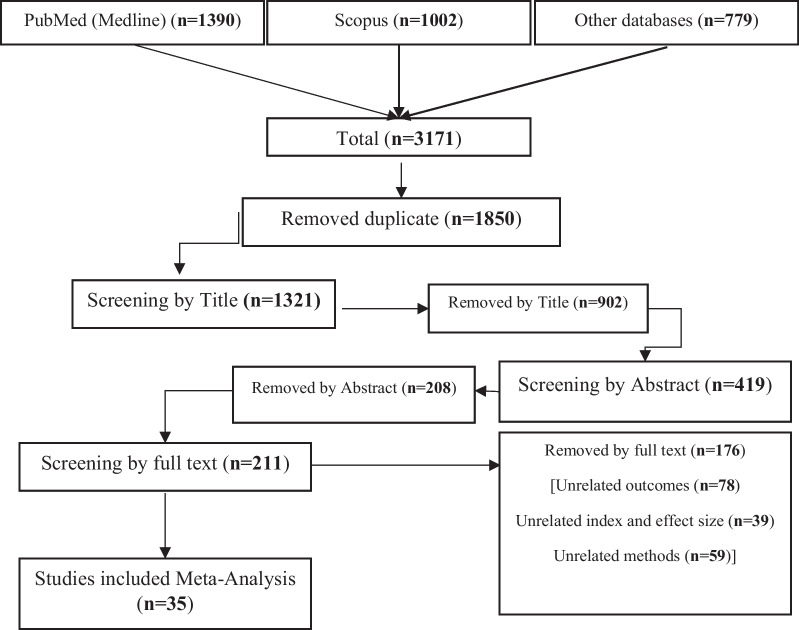
In this meta-analysis, after searching and retrieving all articles, 1390 articles in the PubMed database, 1002 articles in the Scopus database, and 779 articles in other relevant databases, including the Cumulated Index to Nursing and Allied Health Literature (CINAHL), SPORT Discuss via the EBSCO interface, Web of Science, and Embase, were retrieved. After removing the duplicates that included 1850 articles, 1321 articles were screened based on the title. In this stage, 902 articles were removed based on the title, and 419 articles entered the screening stage based on the abstract and then the full text. Finally, a total of 384 articles were removed in these steps. Thirty-five cohort studies were selected for meta-analysis and the present study (Table 2) (Fig. 1). The main point was that all selected cohort studies considered chronic HBV and examined its association with the occurrence of pregnancy outcomes.
Fig. 1.
A flow diagram demonstrating the study selection process
Gestational diabetes
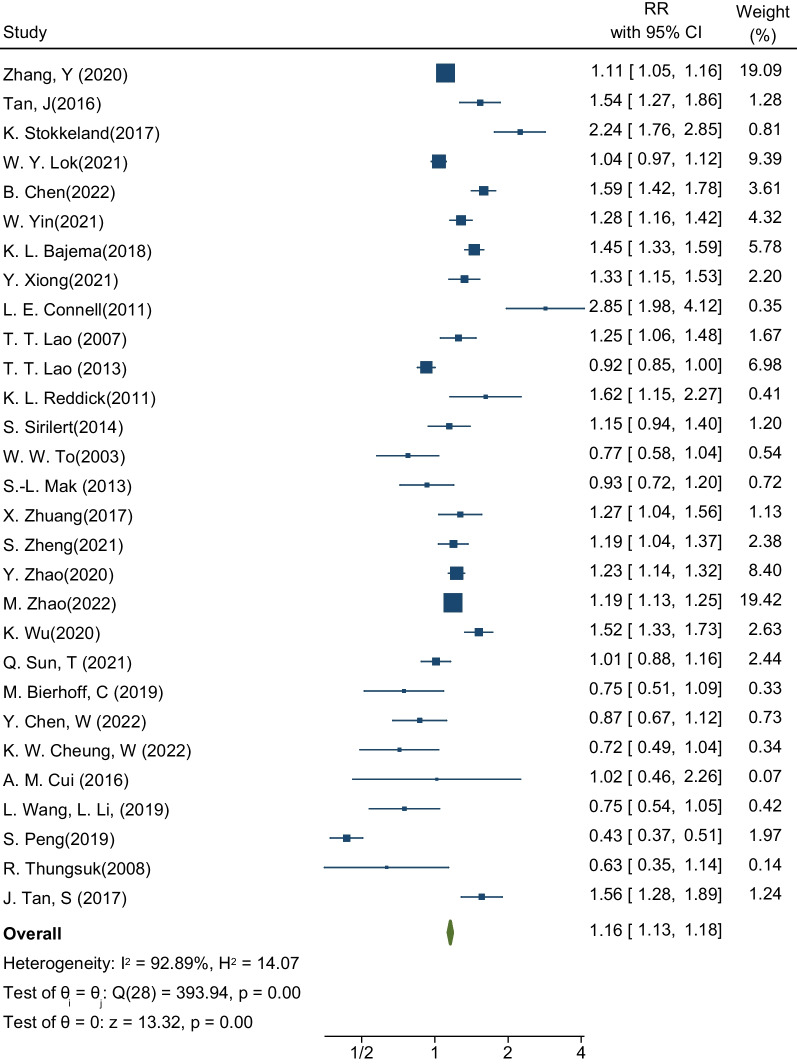
The first desired outcome in this meta-analysis was to estimate the incidence of GDM in pregnant women with HBV. The sample size was equal to 32,370,174 pregnant women in a total of 29 studies, of which 121,737 pregnant women were infected with HBV [18, 21–48]. These 29 studies determined the relationship between the presence of HBV infection and the occurrence of GDM. The highest and lowest effect sizes reported in these studies were related to the study by L.E. Connell et al. and the study by S. Peng et al. After pooling the studies, the pooled RR for incident GDM was 1.16. This means that the risk of developing GDM in pregnant women with HBV infection is 1.16 times that of healthy pregnant women (RR: 1.16; 95% CI 1.13–1.18; I square: 92.89%; P value: 0.00) (Fig. 2). The analysis of publication bias in this meta-analysis was performed using the Eggers test and reported in Table 3. Based on the results of this test, diffusion bias did not occur in the analysis and combination of studies to investigate the relationship between the presence of HBV infection and the occurrence of GDM (B: -0.89; SE: 0.979; P-value: 0.361).
Fig. 2.
Forest plot of the effect of Hepatitis B Virus on the risk of GDM in pregnant women
Table 3.
Meta-analysis of the effect of HBV on the risk of maternal outcomes based on continents, age, and gestational diabetes
| Variables | Categories | No. study | Pooled RR (% 95 CI) | Heterogenity Assesment between sudies | Heterogenity Assesment between subgroup | Publication bias assesments | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| I2 (%) | P value | Q | Q | Pvalue | B | SE | P value | ||||
| PreEclampsia | |||||||||||
| Overall pooled estimate | 20 | 1.10 (1.04–1.16) | 92.06 | 0.00 | 239.28 | – | – | − 0.94 | 0.661 | 0.156 | |
| Continent | Europe | 2 | 0.71 (0.55–0.92) | 0.00 | 0.89 | 0.02 | 53.44 | 0.00 | |||
| Asia | 15 | 1.24 (1.17–1.32) | 92.14 | 0.00 | 178.05 | ||||||
| American | 3 | 0.80 (0.72–0.90) | 74.28 | 0.02 | 7.78 | ||||||
| Age | < 30 | 7 | 1.21 (1.10–1.34) | 43.54 | 0.10 | 10.63 | 19.45 | 0.00 | |||
| > 30 | 4 | 0.82 (0.72–0.95) | 11.69 | 0.33 | 3.40 | ||||||
| Gestational age | < 38 | 2 | 0.91 (0.75–1.12) | 0.00 | 0.67 | 0.18 | 2.57 | 0.11 | |||
| > 38 | 8 | 1.09 (1.00–1.20) | 80.20 | 0.00 | 35.35 | ||||||
| GDM | |||||||||||
| Overall pooled estimate | 29 | 1.16 (1.13–1.18) | 92.89 | 0.00 | 393.94 | – | – | − 0.89 | 0.979 | 0.361 | |
| Continent | Europe | 1 | 2.24 (1.76–2.85) | – | – | – | 108.08 | 0.00 | |||
| Asia | 24 | 1.11 (1.09–1.14) | 91.58 | 0.00 | 273.13 | ||||||
| American | 4 | 1.54 (1.44–1.65) | 76.44 | 0.01 | 12.73 | ||||||
| Age | < 30 | 13 | 1.12 (1.08–1.16) | 94.80 | 0.00 | 249.95 | 1.29 | 0.26 | |||
| > 30 | 7 | 1.16 (1.11–1.21) | 86.03 | 0.00 | 35.79 | ||||||
| Gestational age | < 38 | 3 | 1.38 (1.24–1.54) | 65.84 | 0.05 | 5.85 | 14.62 | 0.00 | |||
| > 38 | 11 | 1.11 (1.07–1.14) | 85.62 | 0.00 | 69.53 | ||||||
| Preterm birth | |||||||||||
| Overall pooled estimate | 27 | 1.17 (1.14–1.20) | 94.32 | 0.00 | 457.57 | – | – | − 0.72 | 0.883 | 0.417 | |
| Continent | Europe | 2 | 1.27 (1.09–1.48) | 0.00 | 0.76 | 0.09 | 125.25 | 0.00 | |||
| Asia | 20 | 1.07 (1.04–1.10) | 85.47 | 0.00 | 130.79 | ||||||
| American | 5 | 1.48 (1.41–1.55) | 98.01 | 0.00 | 201.43 | ||||||
| Age | < 30 | 12 | 1.02 (0.97–1.06) | 79.03 | 0.00 | 52.46 | 0.02 | 0.90 | |||
| > 30 | 6 | 1.02 (0.96–1.09) | 85.73 | 0.00 | 35.03 | ||||||
| Gestational age | < 38 | 3 | 1.30 (1.17–1.45) | 0.00 | 0.52 | 1.33 | 27.62 | 0.00 | |||
| > 38 | 8 | 0.95 (0.91–1.00) | 74.05 | 0.00 | 26.97 | ||||||
| Eclampsia | |||||||||||
| Overall pooled estimate | 3 | 1.48 (0.95–2.29) | 0.00 | 0.87 | 0.28 | – | – | − 0.58 | 1.749 | 0.7387 | |
| Gestationalhypertension | |||||||||||
| Overall pooled estimate | 15 | 0.83 (0.77–0.90) | 12.78 | 0.31 | 16.05 | – | – | 0.00 | 0.604 | 0.997 | |
| Eclampsia + PreEclampsia | |||||||||||
| Overall pooled estimate | 4 | 0.85 (0.82–0.89) | 48.41 | 0.12 | 5.82 | – | – | 1.60 | 0.700 | 0.0220 | |
| Abortion | |||||||||||
| Overall pooled estimate | 2 | 0.97 (0.71–1.33) | 78.96 | 0.03 | 4.75 | – | – | – | – | – | |
| Neonatal death | |||||||||||
| Overall pooled estimate | 6 | 0.83 (0.67–1.03) | 0.00 | 0.95 | 1.18 | – | – | 0.22 | 0.711 | 0.755 | |
Subgroup analyses by continent, age, and gestational age are reported in Table 3. The results showed that the relationship between the presence of HBV infection and the occurrence of GDM in pregnant women living in Europe is higher than in pregnant women living in Asia and America. But the significant point is that one study is in the European subgroup, which makes the possibility of comparison challenging. If this subgroup analysis is not taken into account and the Asian and American regions are considered, the results show that pregnant women living in the United States (RR: 1.54; 95% CI 1.44–1.65; I square: 76.44%; P value: 0.01) have a higher risk of developing HBV infection compared to pregnant women living in Asia (RR: 1.11; 95% CI 1.09–1.14; I square: 91.58%; P value: 0.00) (Table 3). Based on maternal age and gestational age by week, the results showed that age over 30 years and gestational age below 38 weeks aggravate the effect of HBV infection on the occurrence of GDM, and the risk of developing GDM is higher in these women (Table 3).
Preeclampsia
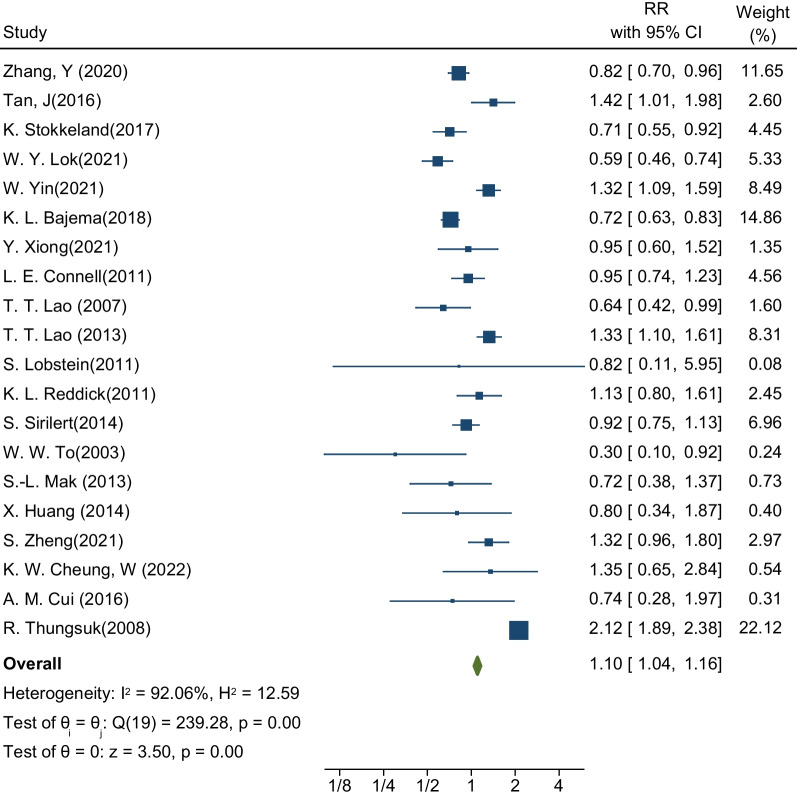
The second desired outcome in this meta-analysis was to estimate the risk of preeclampsia in pregnant women with HBV infection. The sample size was equal to 3,217,1538 pregnant women in a total of 20 studies, of which 103,392 were infected with hepatitis [18, 21–23, 27–32, 34, 36–38, 41, 42, 44, 48–50]. These 20 studies determined the relationship between the presence of HBV infection and the occurrence of preeclampsia. The highest and lowest effect sizes reported in these studies, respectively, are related to the study by R. Thungsuk and colleagues (RR: 2.12; % 95 CI 1.89–2.38) and the study by W.W. To et al. (RR: 0.30; % 95 CI 0.10–0.92). After pooling the studies, the pooled RR for preeclampsia was 1.10. This means that the risk of preeclampsia in pregnant women with HBV infection is 1.10 times that of healthy pregnant women (RR: 1.10; % 95 CI 1.04–1.16; I square: 92.06%; P value: 0.00) (Fig. 3). The analysis of publication bias in this meta-analysis was performed using the Eggers test and reported in Table 3. Based on the results of this test, diffusion bias did not occur in the analysis and combination of studies to investigate the relationship between the presence of HBV infection and the occurrence of preeclampsia (B: − 0.94; SE: 0.661; P value: 0.156).
Fig. 3.
Forest plot of the effect of Hepatitis B Virus on the risk of preeclampsia in pregnant women
The results of the subgroup analysis in Table 3 showed a relationship between the presence of HBV infection and the occurrence of preeclampsia in pregnant women living in Asia (RR: 1.24; 95% CI 1.17–1.32; I square: 92.14%; P value: 0.00). More than pregnant women living in Europe (RR: 0.71; % 95 CI 0.55–0.92; I square: 0.00%; P value: 0.89) and America (RR: 0.80; % 95 CI 0.72–0.90; I square: 74.28%; P value: 0.02). The important point is that HBV in pregnant Asian women has a positive and significant association with the occurrence of preeclampsia, while in European and American pregnant women, this relationship is inverse and protective. Based on maternal age and gestational age based on weeks, the results showed that an age below 30 years and a gestational age above 38 weeks aggravate the effect of HBV infection on the occurrence of preeclampsia, and the risk of preeclampsia is higher in these women (Table 3).
Preterm delivery
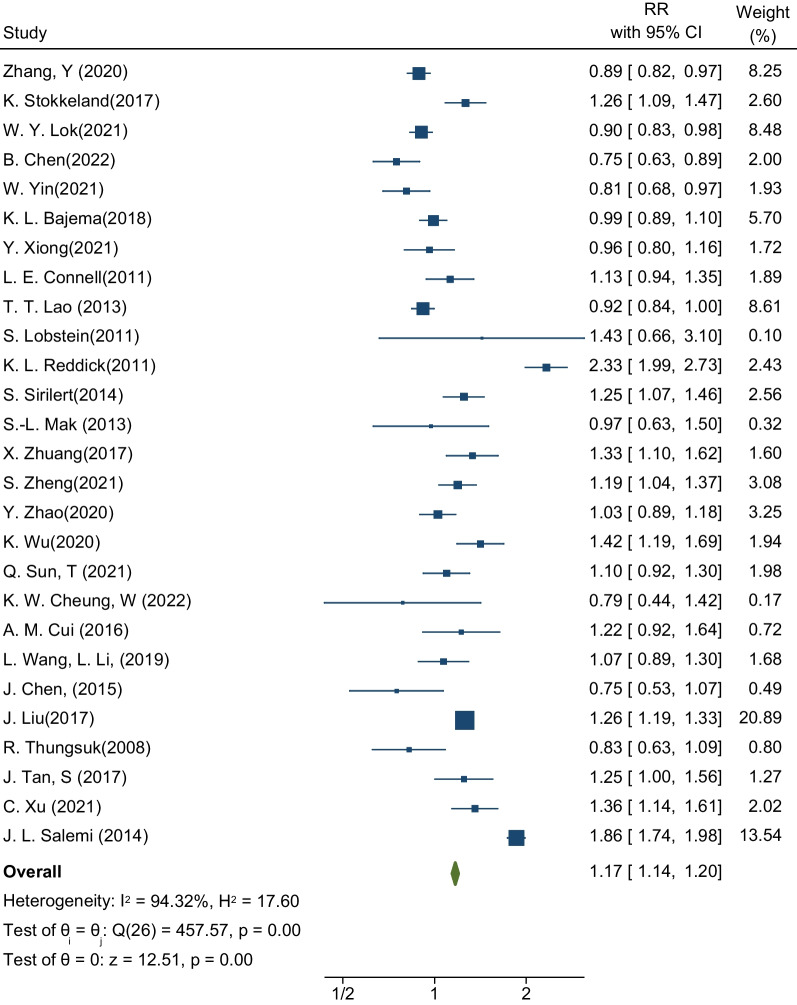
The third desired outcome in this meta-analysis was the estimation of the risk of premature delivery in pregnant women with HBV infection. The sample size was equal to 34,950,154 pregnant women in a total of 27 studies, of which 132,441 pregnant women were infected with hepatitis [21, 23, 25, 27–33, 35–38, 40–46, 48, 50–54]. These 27 studies determined the relationship between the presence of HBV infection and the occurrence of premature birth. The highest and lowest effect sizes reported in these studies are respectively related to the study of L. Reddick K. et al. (RR: 2.33; 95% CI 1.99–2.73) and the study of B. Chen et al. (RR: 0.75; % 95 CI 0.63–0.89). After pooling the studies, the pooled RR for preterm delivery was 1.17. This means that the risk of premature delivery in pregnant women with HBV infection is 1.17 times that of healthy pregnant women (RR: 1.17; % 95 CI 1.14–1.20; I square: 94.32%; P value: 0.00) (Fig. 4). The analysis of publication bias in this meta-analysis was performed using the Eggers test and reported in Table 3. Based on the results of this test, diffusion bias did not occur in the analysis and combination of studies to investigate the relationship between the presence of HBV infection and the occurrence of premature birth (B: − 0.72; SE: 0.883; P value: 0.417).
Fig. 4.
Forest plot of the effect of Hepatitis B Virus on the risk of preterm birth in pregnant women
The results of the subgroup analysis in Table 3 showed that the relationship between the presence of HBV infection and the occurrence of premature birth in pregnant women living in the United States (RR: 1.48; 95% CI 1.41–1.55; I square: 98.01%; P value: 0.00) is greater than that in pregnant women living in Europe (RR: 1.27; % 95 CI 1.09–1.48; I square: 94.12%; P value: 0.00) and Asia (RR: 1.07; % 95 CI 1.04–1.10; I square: 85.47%; P value: 0.00). Based on maternal age and gestational age by week, the results showed that gestational age lower than 38 weeks aggravates the effect of HBV infection on the occurrence of premature birth, and the risk of premature birth is higher in these women (Table 3).
Other outcomes
Other outcomes examined in this meta-analysis included eclampsia, gestational hypertension, miscarriage, and neonatal death. The meta-analysis results showed that the risk of eclampsia in pregnant women with HBV infection was equal to 1.48 (RR: 1.48; 95% CI 0.95–2.29; I square: 0.00%; P value: 0.87), but it was not statistically significant. If the risk of gestational hypertension (RR: 0.83; % 95 CI 0.77–0.90; I square: 12.78%; P value: 0.31), miscarriage (RR: 0.97; % 95 CI 0.71–1.33; I square): 78.96%; P value: 0.03), and neonatal death (RR: 0.83; % 95 CI 0.67–1.03; I square: 0.00%; P value: 0.95) in pregnant women with HBV infection was less than one. These results are reported in the Additional file 1.
Discussion
The purpose of the present meta-analysis was to determine the relationship between HBV infection during pregnancy and the occurrence of pregnancy outcomes such as preeclampsia, premature birth, gestational diabetes, abortion, eclampsia, and hypertension during pregnancy. The results showed that the presence of HBV infection could increase the risk of adverse pregnancy outcomes such as preeclampsia, premature birth, gestational diabetes, abortion, eclampsia, and hypertension.
Preeclampsia can be considered one of the most common pregnancy complications in the last months of pregnancy (usually from the 20th week of pregnancy to 7 days after delivery), which is observed in 5% of pregnant women. Preeclampsia has no specific symptoms and is dangerous for the fetus and the mother. The contraction of blood vessels causes this disease and, as a result, leads to an increase in blood pressure and a decrease in blood flow in fetal organs such as the liver, kidney, and brain. This reduction in blood flow in the uterus leads to problems for the fetus, such as reduced growth, reduced amniotic fluid, etc. The current meta-analysis showed that the presence of HBV infection could increase the risk of preeclampsia in pregnant women by 10% compared to pregnant women without HBV infection. Meta-analysis studies are conducted to determine the more accurate and error-free effect and relationship between two important factors. These studies can control many possible errors in the relationship between exposure and the desired outcome. The important point in studying the relationship between the presence of HBV infection and the occurrence of preeclampsia in pregnant women was the difference in the pathophysiology and epidemiology of preeclampsia and HBV infection in early studies conducted in the world. In addition, the statistical population and method of care for each of these conditions differed in these studies. Other infections, such as parasitic, viral, and bacterial infections, may have a significant impact on the incidence and prevalence of pre-eclampsia in pregnant women, and the presence of other blood-borne and sexually transmitted infections, particularly hepatitis C and HIV/AIDS, may also increase susceptibility to HBV infection. As a result, it is likely that the presence of these conditions and other infections predisposes women to pre-eclampsia, with HBV acting only as an aggravating or enabling factor [23, 25, 28, 55, 56]. This factor was one of the main reasons for the high heterogeneity in the analysis of the relationship between the presence of HBV infection and the occurrence of preeclampsia.
Subgroup analyses in determining the relationship between HBV infection and the occurrence of preeclampsia in pregnant women showed that the risk of infection is higher in Asian women. This result confirmed the difference in the effect of HBV on the occurrence of preeclampsia in different geographical regions, because in other regions such as Europe or America, the relationship between HBV infection and the occurrence of preeclampsia was an inverse or protective relationship. The main reason for this is the difference in culture, the way services related to prenatal care before, during, and after pregnancy are provided and received, and, most importantly, the prevalence of other infections that are effective in Asian countries or other locations. Finally, there are plausible reasons in clinical texts for the association between HBV and the development of pre-eclampsia. In general, HBV infection has been associated with an increased risk of atherosclerosis in pregnant women [56–60]. Furthermore, preeclampsia (marked by maternal endothelial dysfunction) may result in an imbalance of angiogenic, anti-angiogenic, and proangiogenic substances such as vascular endothelial growth factor [55, 61–65]. According to previous research, there is a considerable link between HBV infection and insulin resistance, thrombocytopenia, obesity, and kidney damage or proteinuria [66–68]. The interplay of these illnesses and HBV could explain the link between HBV and preeclampsia.
In the present meta-analysis, results showed that the presence of HBV infection increases the risk of gestational diabetes in pregnant women by 16% compared to women without HBV infection. Also, the risk of developing gestational diabetes in Asian women with HBV infection was lower than that of American women. This difference can be attributed to the importance of the issue of pregnancy and the difference in receiving services related to gestational diabetes screening in the Asian region and Asian countries. In addition, the different diet (especially in Southeast Asian regions) and the way of doing physical activity can be considered other reasons for this difference [69, 70]. Another point that can justify this relationship between Asian pregnant women is the difference in the prevalence of HBV infection in different regions, especially in different Asian and American countries [71, 72]. Of course, the various studies conducted in this field and the results of this meta-analysis confirm the fact that to more accurately determine the relationship between HBV infection and the occurrence of gestational diabetes, there is a need to conduct more studies taking into account the prevalence of HBV, the presence of chronic disease, and the background another is genetic factors and environmental factors such as nutritional and non-nutritive behaviors (smoking, alcohol, unprotected sex, etc.) [37, 73, 74]. An important factor that needs to be investigated in this connection is the body mass index of pregnant women, which can disrupt the relationship between HBV infection and the occurrence of gestational diabetes as an important confounding factor. In addition, the presence of other factors, such as high blood pressure, can also be one of the other factors influencing the relationship between HBV infection and the occurrence of gestational diabetes. These causes can be related to the occurrence of pre-eclampsia, metabolic syndrome, and then GDM [18, 28, 75, 76]. The current meta-analysis used a search technique that lasted through February 2023, and all retrieved studies were examined and screened. The inclusion of a defined selection of cohort studies, as well as the consideration of a specific time span for analysis and reporting, distinguishes this meta-analysis from review studies. Because the goal of this meta-analysis was to look at the relationship between hepatitis B infection and pregnancy outcomes, cohort studies were deemed the best primary research design for determining the relationship without taking into account interventions. This was one of the most significant distinctions between this meta-analysis and earlier research. In contrast, previous meta-analyses did not include all pregnancy outcomes. The outcomes of gestational diabetes and preterm birth were not included in the study by Karamati et al. [77], and the outcomes of gestational diabetes, pre-eclampsia, preterm birth, and miscarriage were not included in the analysis and review by Oliviera et al. [78] These outcomes were evaluated and analyzed in the current meta-analysis.
The important point of this meta-analysis was to estimate the effect size with high accuracy. Although the degree of heterogeneity in the estimated effect size was high, this degree of heterogeneity was indicative of statistical heterogeneity as determined by the I square index and Cochrane’s Q test. The important point was the absence of clinical heterogeneity or its presence at an acceptable level, which is interpreted by the estimated confidence intervals. All the confidence intervals obtained for the desired relationships in the present meta-analysis were narrow, which indicated high precision in the analysis. On the other hand, the narrow confidence interval is one of most important items in clinical interpretation and clinical justification of communication. For example, to determine the relationship between HBV infection and gestational diabetes, the estimated effect size was 1.16 with a confidence interval of 1.13 to 1.18. Despite the heterogeneity rate of 92.7%, the calculated confidence interval is very narrow, which indicates the existence of a sufficient sample and number of studies to determine the relationship and confirms the accuracy of the calculated relationship. Another strength of the present meta-analysis was the subgroup analysis based on the variables of geographic regions, gestational age, and the age of the pregnant mother, which to some extent, shows the role of confounding and other influencing variables in the relationship between HBV infection and pregnancy outcomes. The results of this meta-analysis can be very effective in developing or updating clinical guidelines.
One of the weaknesses or limitations of the present meta-analysis is the failure to perform subgroup analyses based on important variables such as body mass index, the presence of other infections (HIV/AIDS, hepatitis C infection), other underlying diseases (hypertension, genetics) pointed out that due to the lack of reporting of primary studies in their results, they were not included in the results of the present meta-analysis. We suggest that future studies be conducted to determine the relationship between HBV infection and the occurrence of pregnancy outcomes by considering these variables.
Conclusion
This meta-analysis found that hepatitis B infection during pregnancy may be associated with an increased risk of gestational diabetes, preterm delivery, pre-eclampsia, and eclampsia. However, confirmation of this association, as well as the specific biological pathways involved in the association between HBV infection and pregnancy outcomes, requires further investigation. As a result, it is crucial to enhance programs and healthcare services for women in society, focusing on the promotion of screening, care, and treatment programs for infectious diseases, particularly HBV. These efforts should be implemented across various communities, with particular emphasis on developing societies.
Supplementary Information
Additional file 1. Supplementary Figures.
Acknowledgements
We would like to thank all the authors whose articles have been used in this meta-analysis.
Abbreviations
- CI
Confidence interval
- I2
I square
- RR
Risk ratio
- PRISMA
Preferred reporting items for systematic reviews and meta-analyses
- GDM
Gastational diabetes
- CINAHL
Cumulated index to nursing and allied health literature
- HBeAg
Hepatitis B e antigen
- CccDNA
Covalently closed circular DNA
- HBV
Hepatitis B virus
Author contributions
YM: concept development (provided idea for the research). MA, GHM and MA: search strategy. MA and YM: data extraction. YM: supervision. KZ, MA, and YM: analysis/interpretation. All authors: writing (responsible for writing a substantive part of the manuscript).
Funding
None.
Availability of data and materials
Data and materials are available within the complementary materials, and further information can be available by request to the corresponding author.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Summers J, Mason WS. Replication of the genome of a hepatitis B-like virus by reverse transcription of an RNA intermediate. Cell. 1982;29(2):403–415. doi: 10.1016/0092-8674(82)90157-x. [DOI] [PubMed] [Google Scholar]
- 2.Kao J-H. Role of viral factors in the natural course and therapy of chronic hepatitis B. Hep Intl. 2007;1:415–430. doi: 10.1007/s12072-007-9033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tong S, Kim K-H, Chante C, Wands J, Li J. Hepatitis B virus e antigen variants. Int J Med Sci. 2005;2(1):2. doi: 10.7150/ijms.2.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ott JJ, Stevens GA, Groeger J, Wiersma ST. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine. 2012;30(12):2212–2219. doi: 10.1016/j.vaccine.2011.12.116. [DOI] [PubMed] [Google Scholar]
- 5.Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepatitis. 2004;11(2):97–107. doi: 10.1046/j.1365-2893.2003.00487.x. [DOI] [PubMed] [Google Scholar]
- 6.MacLachlan JH, Cowie BC. Hepatitis B virus epidemiology. Cold Spring Harb Perspect Med. 2015;5(5):a021410. doi: 10.1101/cshperspect.a021410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liaw Y-F, Chu C-M. Hepatitis B virus infection. The lancet. 2009;373(9663):582–592. doi: 10.1016/S0140-6736(09)60207-5. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen MH, Wong G, Gane E, Kao J-H, Dusheiko G. Hepatitis B virus: advances in prevention, diagnosis, and therapy. Clin Microbiol Rev. 2020;33(2):e00046–e119. doi: 10.1128/CMR.00046-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pan CQ, Duan ZP, Bhamidimarri KR, Zou HB, Liang XF, Li JIE, et al. An algorithm for risk assessment and intervention of mother to child transmission of hepatitis B virus. Clin Gastroenterol Hepatol. 2012;10(5):452–459. doi: 10.1016/j.cgh.2011.10.041. [DOI] [PubMed] [Google Scholar]
- 10.Jonas MM. Hepatitis B and pregnancy: an underestimated issue. Liver Int. 2009;29:133–139. doi: 10.1111/j.1478-3231.2008.01933.x. [DOI] [PubMed] [Google Scholar]
- 11.Thimme R, Wieland S, Steiger C, Ghrayeb J, Reimann KA, Purcell RH, et al. CD8+ T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J Virol. 2003;77(1):68–76. doi: 10.1128/JVI.77.1.68-76.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Milich DR, Jones JE, Hughes JL, Price J, Raney AK, McLachlan A. Is a function of the secreted hepatitis B e antigen to induce immunologic tolerance in utero? Proc Natl Acad Sci. 1990;87(17):6599–6603. doi: 10.1073/pnas.87.17.6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trehanpati N, Hissar S, Shrivastav S, Sarin SK. Immunological mechanisms of hepatitis B virus persistence in newborns. Indian J Med Res. 2013;138(5):700. [PMC free article] [PubMed] [Google Scholar]
- 14.Tse KY, Ho LF, Lao T. The impact of maternal HBsAg carrier status on pregnancy outcomes: a case–control study. J Hepatol. 2005;43(5):771–775. doi: 10.1016/j.jhep.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 15.Pavel A, Tîrşia E, Maior E, Cristea A. Detrimental effects of hepatitis B virus infection on the development of the product of conception. Virologie. 1983;34(1):35–40. [PubMed] [Google Scholar]
- 16.Hak SD, Do YS, Kyun RL. The influence of hepatitis B virus on the fetus in pregnancy. Pediatr Int. 1987;29(3):449–454. doi: 10.1111/j.1442-200x.1987.tb00344.x. [DOI] [PubMed] [Google Scholar]
- 17.Su WH, Wang PH, Yuan CC, Chang SP. Fetal meconium peritonitis in the infant of a woman with fulminant hepatitis B. A case report. J Reprod Med. 2002;47(11):952–954. [PubMed] [Google Scholar]
- 18.Lao TT, Chan BCP, Leung W-C, Ho L-F, Tse K-Y. Maternal hepatitis B infection and gestational diabetes mellitus. J Hepatol. 2007;47(1):46–50. doi: 10.1016/j.jhep.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 19.Sirilert S, Tongsong T. Hepatitis B virus infection in pregnancy: immunological response, natural course and pregnancy outcomes. J Clin Med. 2021;10(13):2926. doi: 10.3390/jcm10132926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knobloch K, Yoon U, Vogt PM. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement and publication bias. J Cranio-Maxillofac Surg. 2011;39(2):91–92. doi: 10.1016/j.jcms.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Stokkeland K, Ludvigsson JF, Hultcrantz R, Ekbom A, Höijer J, Bottai M, et al. Pregnancy outcome in more than 5000 births to women with viral hepatitis: a population-based cohort study in Sweden. Eur J Epidemiol. 2017;32(7):617–625. doi: 10.1007/s10654-017-0261-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan J, Liu X, Mao X, Yu J, Chen M, Li Y, et al. HBsAg positivity during pregnancy and adverse maternal outcomes: a retrospective cohort analysis. J Viral Hepat. 2016;23(10):812–819. doi: 10.1111/jvh.12545. [DOI] [PubMed] [Google Scholar]
- 23.Bajema KL, Stankiewicz Karita HC, Tenforde MW, Hawes SE, Heffron R. Maternal hepatitis B infection and pregnancy outcomes in the United States: a population-based cohort study. Open Forum Infectious Diseases. 2018;5(6) [DOI] [PMC free article] [PubMed]
- 24.Bierhoff M, Angkurawaranon C, Myat Min A, Gilder ME, Win Tun N, Keereevijitt A, et al. Maternal hepatitis B infection burden, comorbidity and pregnancy outcome in a low-income population on the Myanmar-Thailand border: a retrospective cohort study. J Pregnancy. 2019;2019:8435019. doi: 10.1155/2019/8435019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen B, Wang Y, Lange M, Kushner T. Hepatitis C is associated with more adverse pregnancy outcomes than hepatitis B: A 7-year national inpatient sample study. Hepatol Commun. 2022;6(9):2465–2473. doi: 10.1002/hep4.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Y, Ning W, Wang X, Chen Y, Wu B, Tao J. Maternal hepatitis B surface antigen carrier status and pregnancy outcome: a retrospective cohort study. Epidemiol Infect. 2022;150:1–22. doi: 10.1017/S0950268822000681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Connell LE, Salihu HM, Salemi JL, August EM, Weldeselasse H, Mbah AK. Maternal hepatitis B and hepatitis C carrier status and perinatal outcomes. Liver Int. 2011;31(8):1163–1170. doi: 10.1111/j.1478-3231.2011.02556.x. [DOI] [PubMed] [Google Scholar]
- 28.Lao TT, Sahota DS, Cheng YK, Law LW, Leung TY. Maternal hepatitis B surface antigen status and incidence of pre-eclampsia. J Viral Hepat. 2013;20(5):343–349. doi: 10.1111/jvh.12037. [DOI] [PubMed] [Google Scholar]
- 29.Lok WY, Kong CW, To WWK. Prevalence of Hepatitis B Carrier Status and Its Negative Association with Hypertensive Disorders in Pregnancy. Obstetr Gynecol Int. 2021;2021. [DOI] [PMC free article] [PubMed]
- 30.Mak SL, Leung KY. Hepatitis B carriers in Hong Kong: prevalence and pregnancy outcomes. HKJGOM. 2013;13(1):67–73. [Google Scholar]
- 31.Reddick KL, Jhaveri R, Gandhi M, James AH, Swamy GK. Pregnancy outcomes associated with viral hepatitis. J Viral Hepat. 2011;18(7):e394–e398. doi: 10.1111/j.1365-2893.2011.01436.x. [DOI] [PubMed] [Google Scholar]
- 32.Sirilert S, Traisrisilp K, Sirivatanapa P, Tongsong T. Pregnancy outcomes among chronic carriers of hepatitis B virus. Int J Gynaecol Obstet. 2014;126(2):106–110. doi: 10.1016/j.ijgo.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 33.Sun Q, Lao TT, Du M, Xie M, Sun Y, Bai B, et al. Chronic maternal hepatitis B virus infection and pregnancy outcome- a single center study in Kunming, China. BMC Infect Dis. 2021;21(1):253. doi: 10.1186/s12879-021-05946-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.To WW, Cheung W, Mok KM. Hepatitis B surface antigen carrier status and its correlation to gestational hypertension. Aust N Z J Obstet Gynaecol. 2003;43(2):119–122. doi: 10.1046/j.0004-8666.2003.00029.x. [DOI] [PubMed] [Google Scholar]
- 35.Wu K, Wang H, Li S, Zhang H, Zhu B. Maternal hepatitis B infection status and adverse pregnancy outcomes: a retrospective cohort analysis. Arch Gynecol Obstet. 2020;302(3):595–602. doi: 10.1007/s00404-020-05630-2. [DOI] [PubMed] [Google Scholar]
- 36.Xiong Y, Liu C, Huang S, Wang J, Qi Y, Yao G, et al. Impact of maternal infection with hepatitis B virus on pregnancy complications and neonatal outcomes for women undergoing assisted reproductive technology treatment: A population-based study. J Viral Hepat. 2021;28(4):613–620. doi: 10.1111/jvh.13472. [DOI] [PubMed] [Google Scholar]
- 37.Yin W, Chen B, Yang Y, Li X, Li R, Xie J, et al. Association between maternal hepatitis B virus carrier and gestational diabetes mellitus: a retrospective cohort analysis. Virol J. 2021;18(1):226. doi: 10.1186/s12985-021-01691-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Y, Chen J, Liao T, Chen S, Yan J, Lin X. Maternal HBsAg carriers and pregnancy outcomes: a retrospective cohort analysis of 85,190 pregnancies. BMC Pregnancy Childbirth. 2020;20(1) [DOI] [PMC free article] [PubMed]
- 39.Zhao M, Yang S, Su X, Hung TC, Liu Y, Zheng W. Hepatitis B virus infection and increased risk of gestational diabetes regardless of liver function status: a xiamen area population-based Study. Front Physiol. 2022;13:938149. doi: 10.3389/fphys.2022.938149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhuang X, Cui AM, Wang Q, Cheng XY, Shen Y, Cai WH, et al. Liver Dysfunction during pregnancy and Its association of with preterm birth in China: a prospective cohort study. EBioMedicine. 2017;26:152–156. doi: 10.1016/j.ebiom.2017.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheung KW, Wang WL, So PL, Wong DN, Mak ASL, Hui WN, et al. Original relationship between viral load and pregnancy outcomes among hepatitis B carriers. Taiwan J Obstet Gynecol. 2022;61(4):630–633. doi: 10.1016/j.tjog.2021.08.006. [DOI] [PubMed] [Google Scholar]
- 42.Cui AM, Cheng XY, Shao JG, Li HB, Wang XL, Shen Y, et al. Maternal hepatitis B virus carrier status and pregnancy outcomes: a prospective cohort study. BMC Pregnancy Childbirth. 2016;16:87. doi: 10.1186/s12884-016-0884-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tan J, Huang S, He G, Tang L, Ren Y, Zheng J, et al. Maternal hepatitis B surface antigen carrier status and its impact on neonatal outcomes: a cohort study of 21 947 singleton newborns in China. J Matern Fetal Neonatal Med. 2017;30(18):2219–2224. doi: 10.1080/14767058.2016.1243098. [DOI] [PubMed] [Google Scholar]
- 44.Thungsuk R. Maternal hepatitis B infection and pregnacy outcomes. J Prapokklao Hospital Clin Med Educ Center. 2008;25(3):246–254. [Google Scholar]
- 45.Wang L, Li L, Huang C, Diao L, Lian R, Li Y, et al. Maternal chronic hepatitis B virus infection does not affect pregnancy outcomes in infertile patients receiving first in vitro fertilization treatment. Fertil Steril. 2019;112(2):250–7.e1. doi: 10.1016/j.fertnstert.2019.03.039. [DOI] [PubMed] [Google Scholar]
- 46.Zhao Y, Chen YL, Song HQ, Huang PY, Wang LY, Liu W, et al. Effects of maternal hepatitis B surface antigen positive status on the pregnancy outcomes: a retrospective study in Xiamen, China, 2011–2018. PLoS ONE. 2020;15(3):e0229732. doi: 10.1371/journal.pone.0229732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peng S, Wan Z, Lin X, Li X, Du Y. Maternal hepatitis B surface antigen carrier status increased the incidence of gestational diabetes mellitus. BMC Infect Dis. 2019;19(1):147. doi: 10.1186/s12879-019-3749-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zheng S, Zhang H, Chen R, Yan J, Han Q. Pregnancy complicated with hepatitis B virus infection and preterm birth: a retrospective cohort study. BMC Pregnancy Childbirth. 2021;21(1):513. doi: 10.1186/s12884-021-03978-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang X, Tan H, Li X, Zhou S, Wen SW, Luo M. Maternal chronic HBV infection would not increase the risk of pregnancy-induced hypertension–results from pregnancy cohort in Liuyang rural China. PLoS ONE. 2014;9(12):e114248. doi: 10.1371/journal.pone.0114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lobstein S, Faber R, Tillmann HL. Prevalence of hepatitis B among pregnant women and its impact on pregnancy and newborn complications at a tertiary hospital in the eastern part of Germany. Digestion. 2011;83(1–2):76–82. doi: 10.1159/000320455. [DOI] [PubMed] [Google Scholar]
- 51.Chen J, Zhang S, Zhou YH, Xu BY, Hu YL. Minimal adverse influence of maternal hepatitis B carrier status on perinatal outcomes and child’s growth. J Maternal-Fetal Neonatal Med. 2015;28(18):2192–2196. doi: 10.3109/14767058.2014.981805. [DOI] [PubMed] [Google Scholar]
- 52.Liu J, Zhang SK, Liu M, Wang QM, Shen HP, Zhang YP. Maternal pre-pregnancy infection with hepatitis B virus and the risk of preterm birth: a population-based cohort study. Lancet Global Health. 2017;5(6):E624–E632. doi: 10.1016/S2214-109X(17)30142-0. [DOI] [PubMed] [Google Scholar]
- 53.Salemi JL, Whiteman VE, August EM, Chandler K, Mbah AK, Salihu HM. Maternal hepatitis B and hepatitis C infection and neonatal neurological outcomes. J Viral Hepat. 2014;21(11):e144–e153. doi: 10.1111/jvh.12250. [DOI] [PubMed] [Google Scholar]
- 54.Xu C, Bao Y, Zuo J, Li Y, Tang Y, Qu X, et al. Maternal chronic hepatitis B virus infection and the risk of preterm birth: A retrospective cohort analysis in Chinese women. J Viral Hepat. 2021;28(10):1422–1430. doi: 10.1111/jvh.13585. [DOI] [PubMed] [Google Scholar]
- 55.Ahmed MA, Sharif ME, Rayis DA, Nasr AM, Adam I. Hepatitis B infection and preeclampsia among pregnant Sudanese women. Virology journal. 2018;15:1–4. doi: 10.1186/s12985-018-0927-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang QT, Chen JH, Zhong M, Hang LL, Wei SS, Yu YH. Chronic hepatitis b infection is associated with decreased risk of preeclampsia: a meta-analysis of observational studies. Cell Physiol Biochem. 2016;38(5):1860–1868. doi: 10.1159/000445548. [DOI] [PubMed] [Google Scholar]
- 57.Huang QT, Wei SS, Zhong M, Hang LL, Xu YY, Cai GX, et al. Chronic hepatitis B infection and risk of preterm labor: a meta-analysis of observational studies. J Clin Virol. 2014;61(1):3–8. doi: 10.1016/j.jcv.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 58.Ishizaka N, Ishizaka Y, Takahashi E, Toda EI, Hashimoto H, Ohno M, et al. Increased prevalence of carotid atherosclerosis in hepatitis B virus carriers. Circulation. 2002;105(9):1028–1030. doi: 10.1161/hc0902.105718. [DOI] [PubMed] [Google Scholar]
- 59.Jiang R, Wang T, Yao Y, Zhou F, Huang X. Hepatitis B infection and intrahepatic cholestasis of pregnancy: a systematic review and meta-analysis. Medicine. 2020;99(31). [DOI] [PMC free article] [PubMed]
- 60.Ma X, Sun D, Li C, Ying J, Yan Y. Chronic hepatitis B virus infection and preterm labor(birth) in pregnant women-an updated systematic review and meta-analysis. J Med Virol. 2018;90(1):93–100. doi: 10.1002/jmv.24927. [DOI] [PubMed] [Google Scholar]
- 61.Asaye Z, Aferu T, Asefa A, Feyissa D, Regasa T, Kebede O, et al. Prevalence of hepatitis b virus among pregnant women on antenatal care follow-up at mizan-tepi university teaching hospital and mizan health center southwest ethiopia. Int J General Med. 2021;195–200. [DOI] [PMC free article] [PubMed]
- 62.Carpio GCA, Taguba AQ, Tan LCQ, Ong JP, Daez MLO. Prevalence and risk factors of hepatitis B infection in pregnant women at the prenatal clinic of the university of the Philippines-Philippine general hospital. Clin Gastroenterol Hepatol. 2015;13(7):e83. [Google Scholar]
- 63.Hirokoshi K, Maeshima Y, Kobayashi K, Matsuura E, Sugiyama H, Yamasaki Y, et al. Increase of serum angiopoietin-2 during pregnancy is suppressed in women with preeclampsia. Am J Hypertens. 2005;18(9):1181–1188. doi: 10.1016/j.amjhyper.2005.03.745. [DOI] [PubMed] [Google Scholar]
- 64.Khakhkhar VM, Bhuva PJ, Bhuva SP, Patel CP, Cholera MS. Sero-prevalence of hepatitis B amongst pregnant women attending the antenatal clinic of a tertiary care hospital, Jamnagar (Gujarat) Natl J Med Res. 2012;2(03):362–365. [Google Scholar]
- 65.Yakasai IA, Ayyuba Ru, Abubakar IS, Ibrahim SA. Sero-prevalence of hepatitis B virus infection and its risk factors among pregnant women attending antenatal clinic at Aminu Kano Teaching Hospital, Kano, Nigeria. J Basic Clin Reprod Sci. 2012;1(1–2):49–55. [Google Scholar]
- 66.Chien C-H, Chen L-W, Lin C-L, Chang S-W, Shyu Y-C, Chen K-F, et al. Unawareness of hepatitis B virus infection confers on higher rate of metabolic syndrome: a community-based study. Sci Rep. 2017;7(1):9869. doi: 10.1038/s41598-017-10029-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Joo EJ, Chang Y, Yeom JS, Lee YG, Ryu S. Hepatitis B infection is associated with an increased incidence of thrombocytopenia in healthy adults without cirrhosis. J Viral Hepatitis. 2017;24(3):253–258. doi: 10.1111/jvh.12642. [DOI] [PubMed] [Google Scholar]
- 68.Shah AS, Amarapurkar DN. Spectrum of hepatitis B and renal involvement. Liver Int. 2018;38(1):23–32. doi: 10.1111/liv.13498. [DOI] [PubMed] [Google Scholar]
- 69.Chen DS. Public health measures to control hepatitis B virus infection in the developing countries of the Asia-Pacific region. J Gastroenterol Hepatol. 2000;15:E7–E10. doi: 10.1046/j.1440-1746.2000.02095.x. [DOI] [PubMed] [Google Scholar]
- 70.Chen CJ, Wang LY, Yu MW. Epidemiology of hepatitis B virus infection in the Asia-Pacific region. J Gastroenterol Hepatol. 2000;15:E3–E6. doi: 10.1046/j.1440-1746.2000.02124.x. [DOI] [PubMed] [Google Scholar]
- 71.Ma J, Bauman A. Obstetric profiles and pregnancy outcomes of immigrant women in New South Wales, 1990–1992. Aust N Z J Obstet Gynaecol. 1996;36(2):119–125. doi: 10.1111/j.1479-828x.1996.tb03265.x. [DOI] [PubMed] [Google Scholar]
- 72.Kawsar M, Goh B. Hepatitis B virus infection among Chinese residents in the United Kingdom. Sexually transmitted infections. 2002;78(3):166–168. doi: 10.1136/sti.78.3.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen YL, Peng L, He J, Wu JX, Tian RX, Xu JQ, et al. Impact of hepatitis B virus infection on maternal and infant outcomes of women with gestational diabetes mellitus: a three-year retrospective study. J Diabetes Compl. 2022;36(6). [DOI] [PubMed]
- 74.Kong D, Liu H, Wei S, Wang Y, Hu A, Han W, et al. A meta-analysis of the association between gestational diabetes mellitus and chronic hepatitis B infection during pregnancy. BMC Res Notes. 2014;7:139. doi: 10.1186/1756-0500-7-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Beischer NA, Oats JN, Henry OA, Sheedy MT, Walstab JE. Incidence and severity of gestational diabetes mellitus according to country of birth in women living in Australia. Diabetes. 1991;40(Supplement_2):35–38. doi: 10.2337/diab.40.2.s35. [DOI] [PubMed] [Google Scholar]
- 76.Yue D, Molyneaux L, Ross G, Constantino M, Child A, Turtle J. Why does ethnicity affect prevalence of gestational diabetes? The underwater volcano theory. Diabet Med. 1996;13(8):748–752. doi: 10.1002/(SICI)1096-9136(199608)13:8<748::AID-DIA164>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 77.Keramat A, Younesian M, Fesharaki MG, Hasani M, Mirzaei S, Ebrahimi E, et al. Inactive hepatitis B carrier and pregnancy outcomes: a systematic review and meta–analysis. Iran J Public Health. 2017;46(4):468. [PMC free article] [PubMed] [Google Scholar]
- 78.Oliveira D, Pereira F, do Rosário Martins M, Castro R, Cordeiro L, Fronteira I. A systematic review of the maternal and neonatal complications in hepatitis B infection. J Clin Virol. 2020;133:104680. doi: 10.1016/j.jcv.2020.104680. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Supplementary Figures.
Data Availability Statement
Data and materials are available within the complementary materials, and further information can be available by request to the corresponding author.