Abstract
Background:
Dedicator of cytokinesis 8 (DOCK8) deficiency is a combined immunodeficiency caused by autosomal recessive loss-of-function mutations in the DOCK8 gene. This disorder, often referred to as autosomal recessive hyper IgE syndrome, is characterised by recurrent cutaneous infections, elevated levels of serum IgE, and severe atopic disease including anaphylaxis to certain foods. However, the contribution of defects in CD4+ T cells to disease pathogenesis in these patients has not been thoroughly investigated.
Objective:
To investigate the phenotype and function of the peripheral CD4+ T cell compartment in DOCK8-deficient patients and compare it to normal healthy donors to determine (1) intrinsic and extrinsic defects in CD4+ T cells and (2) how these defects account for the characteristic clinical features of DOCK8 deficiency.
Methods:
We performed indepth analysis of the CD4+ T cell compartment of DOCK8-deficient patients and normal healthy controls. We enumerated naïve, central memory and effector memory CD4+ T cells as well as regulatory T cells, T follicular helper cells, Th1, Th2 and Th17 subsets, and assessed cytokine production and transcription factor expression by CD4+ T cell subsets in vitro following non-polarising as well as Th1-, Th2- and Th17-polarising conditions. Finally, using the ImmunoCAP assay, plasma from DOCK8-deficient patients and normal healthy controls was investigated for IgE specific for staple food (egg white, milk, codfish, wheat, peanut, soyabean) and house dust mite allergens.
Results:
DOCK8-deficient memory CD4+ T cells were biased towards a Th2 type, characterised by high production of IL-4, IL-5 and IL-13. This was at the expense of other subsets as evidenced by defects in IFNγ-expressing Th1 cells and IL-17A-, IL-17F-, and IL-22 expressing Th17 cells when compared to normal memory CD4+ T cells. Examination of allergen specific IgE revealed that plasma IgE from DOCK8-deficient patients was directed against staple food antigens, but not house dust mites.
Conclusion:
Investigations into the CD4+ T cell compartment in DOCK8 deficient patients provided an explanation for some of the clinical signs of this disorder. On one hand, the Th2 bias is likely to contribute to atopic disease, such as eczema and food allergies, which are characteristic to DOCK8 deficiency. On the other hand, defects in Th1 and Th17 cells compromise anti-viral and anti-fungal immunity, respectively explaining the characteristic infectious susceptibility of DOCK8-deficient patients.
Keywords: Dedicator of cytokinesis 8, CD4+ T cell differentiation, Th2 skewing, allergy, atopic disease, chronic mucocutaneous candidiasis, viral immunity
CAPSULE SUMMARY
DOCK8-deficient CD4+ T cells exhibit dysregulated cytokine responses, with exaggerated production of Th2 cytokines, and impaired production of Th1 and Th17 cytokines. Collectively these findings provide explanations for some of the clinical features of DOCK8 deficiency, such as eczema and food allergies, and recurrent viral and microbial infections.
INTRODUCTION
Bi-allelic loss-of-function mutations in dedicator of cytokinesis 8 (DOCK8) cause a combined immunodeficiency also known as an autosomal recessive form of hyper IgE syndrome (AR-HIES)1, 2. Affected patients typically present with recurrent Staphylococcus aureus skin infections, recurrent and severe cutaneous viral infections (herpes simplex virus, human papillomavirus, Molluscum contagiosum virus), elevated serum IgE levels, lymphopenia, eosinophilia and an increased risk of malignancy1–3. DOCK8-deficient patients also exhibit impaired humoral immune responses against protein and polysaccharide antigens following natural infection or vaccination. Strikingly, DOCK8 deficiency predisposes most affected patients to developing asthma and severe allergies against food and environmental antigens1–5. However, the mechanisms underlying severe allergy are currently unknown.
DOCK8 functions as a guanine nucleotide exchange factor to activate Rho-family GTPases such as CDC42, which in turn mediate downstream events including cell activation, division, survival, differentiation, adhesion, and migration6–8. Despite this, it is not immediately clear how mutations in DOCK8 result in the devastating immune abnormalities characteristic of patients with AR-HIES. However, as DOCK8 is predominantly expressed by hematopoietic cells, it is likely to play critical lymphocyte-intrinsic roles in generating appropriate cellular and humoral immune responses against infectious diseases. Consistent with this hypothesis, allogeneic hematopoietic stem cell transplant (HSCT) of DOCK8-deficient patients overcomes recurrent cutaneous viral infections, eczematous rash, and reduces IgE levels and eosinophilia9–14. In regards to food allergies in DOCK8 deficiency, some reports have documented improvement post-HSCT10, 11, 14, while others reported amelioration to symptoms13 or no change9, 15.
Ex vivo and in vitro analyses of lymphocyte subsets from DOCK8-deficient patients have shed some light on disease pathogenesis. For instance, DOCK8-deficient patients have normal to increased numbers of total B cells but further analysis revealed a decrease in circulating memory (CD27+) B cells5, 16. Functionally, compared with normal B cells, DOCK8-deficient B cells exhibit poor responses to the TLR9 ligand CpG, although responses following CD40 engagement are largely intact5. In B cells, DOCK8 acts as an adaptor protein connecting the TLR9-MYD88 pathway to STAT3 signalling, which is required for B cell proliferation and differentiation, as evidenced by defective function of STAT3-deficient human B cells in vivo and in vitro17–20. These defects are likely to underlie poor humoral immunity in DOCK8-deficiency. Paradoxically, an increase in autoantibodies directed against nuclear, cytoplasmic and extracellular matrix antigens has been detected in DOCK8-deficient patients, possibly due to decreased regulatory T cells (Tregs) in these patients21.
Previously, when we examined the T cell compartment in DOCK8-deficient individuals, we found a severe reduction in naïve, central memory (CD45RA−CCR7+) and effector memory (CD45RA−CCR7−) CD8+ T cells but a marked accumulation of CD45RA+CCR7− terminally differentiated (i.e. “exhausted”) effector memory cells22. Strikingly, central and effector memory CD8+ T cells from DOCK8-deficient individuals displayed phenotypic features of T cell exhaustion, with increased expression of CD57, 2B4 and CD95, and accelerated loss of CD28 and CD127 (IL-7Rα)22. Furthermore, DOCK8-deficient naïve and memory CD8+ T cells failed to proliferate in vitro in response to T cell receptor (TCR) stimulation 22. More recently, DOCK8-deficient CD8+ T cells were reported to undergo “cytothripsis”, a form of cell death associated with defects in cell survival, morphology and trafficking23. These defects prevented the generation of long-lived resident memory CD8+ T cells in the skin and subsequently impaired the immune response to herpes virus infection at this site23. Taken together, these defects in the CD8+ T cell compartment provide a plausible explanation for viral susceptibility in DOCK8-deficient patients. DOCK8-deficient patients have also been found to have defects in the development of NKT cells and function of NK cells24, 25. These defects may contribute to clinical features of DOCK8-deficeint individuals, such as increased susceptibility to viral infections and malignancies.
In contrast to these established defects in B cells, Tregs, CD8+ T cells, NK cells and NKT cells, much less is known about the consequences of DOCK8 mutations in other human CD4+ T helper cells. While it has been reported that the frequencies of naïve and memory CD4+ T cells in DOCK8-deficient patients are normal, DOCK8-deficient naïve and memory CD4+ T cells do have a defect in TCR-induced proliferation; however this is less severe than that observed for DOCK8-deficient CD8+ T cells22. Consequently, this deficit is unlikely to cause clinical features such as atopic disease (dermatitis, severe food allergies) and increased IgE production in DOCK8 deficiency. For this reason, we have undertaken a detailed analysis of the CD4+ T cell compartment in DOCK8-deficient patients. We found that DOCK8-deficient memory CD4+ T cells have a bias towards Th2 cytokine expression (ie IL-4, IL-5, IL-13) and concomitant defective production of Th1 (IFNγ) and Th17 (IL-17A, IL-17F, IL-22) cytokines. Furthermore, this Th2 cytokine bias, as well as impaired Th17 immunity, in the absence of DOCK8 expression was T cell intrinsic and independent of defects in proliferation. This intrinsic Th2 bias by DOCK8-deficient CD4+ T cells may underlie atopic disease and hyper-IgE displayed by DOCK8-deficient patients. Additionally, impaired Th1 and Th17 cell responses are likely to account for impaired viral immunity and fungal infections such as chronic mucocutaneous candidiasis, respectively in DOCK8-deficient patients.
METHODS
Human samples
PBMCs and/or plasma were isolated from normal healthy donors (Australian Red Cross) and patients with DOCK8 deficiency (Table 1). The genotype of some of these patients have been previously reported1, 2, 15, 22, 24. DOCK8 expression in PBMCs from controls and patients was determined by flow cytometry, as previously described (Supplementary Fig 1)26. All studies were approved by Institutional Human Research Ethics Committees and written informed consent was obtained from patients.
Table 1:
DOCK8 deficient patients
| DOCK8-deficient patients | Mutation | Gender | Age at study | IgE (IU/ml) | Allergies/atopic disease | Infections | Other |
|---|---|---|---|---|---|---|---|
| #1 | Homozygous 114 kb deletion spanning exons 4 – 26 | female | 14 | 4,864 – 10,000 | - No known allergies - Eczema - Hypereosinophilia without lymphopenia |
Pneumonia, cutaneous lesions and abscesses, fungal infections, lymphadenitis, cheilitis, Chrysosporium parvum. | Chronic diarrhea, rectal prolapse, bronchiectasis, tolerated BCG vaccine. Deceased. |
| #2 | Homoz A->T; position 70 exon 7; K271X | female | 12 | 10,000 | - No known allergies - Eczema |
Severe M. contagiosum, pneumonia, meningitis. | |
| #3 | Homozygous 400 kb deletion (totality of DOCK8 + 5’ of KANK1) | female | 12 | >5,000 | - Multiple food, environmental, and drug allergies - Severe eczema (lichenification) - Hypereosinophilia (>3000/mm3) |
Stomatitis, M. contagiosum, respiratory syncytial virus, HSV1, Candida sp, H. influenza, P. jirovecii. | Abdominal vasculitis, lymphadenopathy, splenomegaly, CD3+ lymphopenia. Successful HSCT. |
| #4 | Homozygous 114 kb deletion spanning exons 4 – 26 | male | 10 | 1,552 | - No known allergies - Eczema - Hypereosinophilia (7800/mm3). |
Recurrent otitis media, herpes labialis, HPV, disseminated plain warts, onychomycosis, Salmonella sp. | Arthritis, uveitis, interstitial lung disease, inflammatory bowel disease, mesenteric vasculitis. Tolerated BCG vaccine. Deceased. |
| #5 | Homozygous 114 kb deletion spanning exons 4 – 26 | female | 12 | 19,302 | - No known allergies - Eosinophilia (5,000/mm3). |
Recurrent upper respiratory tract infection, HPV, flat warts, herpetic stomatitis, Giardia lamblia, Salmonella enterica, E. coli. | Uncomplicated chickenpox. Inflammatory bowel disease, abdominal vasculitis, thrombocytosis. Tolerated BCG vaccine. Deceased. |
| #6 | c.3733_3734del AG; p.R1245EfsX5 | male | 12 | 1,500 | - Multiple food allergies (egg, cow’s milk) - Peanut sensitised (tolerant) - Environmental allergies (house dust mite, rye grass, bermuda grass). Previous allergic rhinitis - Infrequent episodic asthma (viral induced) in childhood - Eczema |
Methicillin-resistant S. aureus infection, M. contagiosum, recurrent otitis media. | |
| #7 | Homozygous deletion 9p24.3 323,819-324,708 | female | 8 | 9,196 | - Food allergies - Diffuse colonic and esophageal eosinophilia - Eczema - Asthma |
CMV, BK virus, chronic Salmonella, recurrent sinopulmonary infections, skin abscesses. | Sclerosing cholangitis. |
| #8 | heterozygous deletions involving exons 22–25 and 3–32 | female | 14 | 6,270 | - Food allergies - Environmental allergies - Rhinitis - Asthma - Allergic conjunctivitis - Eczema |
HPV, M. contagiosum, meningitis, bacteremia, fungal skin infections. | Vasculopathy. Allergic symptoms improved after transplant. |
| #9 | • Large heterozygous. deletion (~200kb) • 2bp heterozygous deletion in exon 41 (c.5307–5308 del AC, pL1770fsX1783 |
female | 7 | >6,000 | - No known allergies - Severe eczema (lichenification) - Eosinophilia (>3,000/mm3) |
Skin abscesses, M. contagiosum, recurrent respiratory tract infection, chronic otitis, maxillary sinusitis, bronchiectasis, HPV warts, HSV, H. influenza, Salmonella spp. | ↑ IgG, ↓ IgM, ↑IgA, CD4+ lymphopenia. |
| #10 | • Large heterozygous. deletion (~200kb) • 2bp heterozygous deletion in exon 41 (c.5307–5308 del AC, pL1770fsX1783 |
male | 10 | >4,400 | - No known allergies - Moderate eczema - Eosinophilia |
Skin abscess, M. contagiosum, recurrent upper respiratory tract infection, HPV disseminated warts, HSV stomatitis, S. aureus, S. pyrogenes. | ↑ IgG, ↓ IgM, ↑ IgA, CD4+ lymphopenia. |
| #11 | heterozygous large deletions one deletion involving the two gene copies of 80kb in 5’ part of the gene and a deletion of one copy of 320kb encompassing the 2/3rd of the 3’ region of DOCK8 gene and the 5’ part of the KANK1 gene | male | 13 | >1,100 | - No known allergies - Severe eczema (lichenification) - Eosinophilia (>700/mm3) |
Chronic otitis, clavicle osteomyelitis, bronchitis, pneumonia, bronchiectasis, Morganella spp., P. aeruginosa, Proteus mirabillis, H. influenza, Giardia intestinalis. | Sclerosing cholangitis, ↑ IgA, ↓ IgM, lymphopenia. Died of post-HSCT complications. |
| #12 | splice site mutation (exon 11) > frame shift, homozygous | male | 17 | 17,045 | - Food allergies (pork, peanut, chocolate, dairy, egg) - Severe eczema |
Chronic cutaneous and ocular HSV, M. contagiosum, warts, S. aureus skin infections, cutaneous dermatophyte infection. | Chronic liver disease with vanishing bile ducts on biopsy of unclear etiology. Calficied dilated aorta. |
| #13 | Exon 41: c5182C>T homozygous p.R1728X. | male | 3 | 24,893 | - Food allergies (milk, egg, tree nuts, peanut) - Severe eczema - Asthma |
S. aureus skin infections, Herpetic keratitis, warts, onchycomycosis, bacterial, viral and Pneumocystis pneumonia. | |
| #14 | Large deletion + stop codon (exon 11) | male | 16 | 51,010 | - Eczema - Asthma |
Sinopulmonary infections, Neisseria meningitides arthritis, M. contagiosum and warts. | |
| #15 | Unknown (lack DOCK8 protein; see Supplementary Fig 1) | male | 5 | 17,300 | - Food allergies (milk, egg, cashew, pistachio, almond, beef, lamb) - Eczema - Asthma - Bronchiectasis |
HSV, S. pyrogenes, H. influenzae, C. albicans, Adenovirus, Norovirus, HHV6, EBV, CMV, VZV, Aspergillus Niger, Cladosporium. | |
| #16 | Unknown (lack DOCK8 protein; see Supplementary Fig 1) | female | 4 | 8,100 | - Food allergies (egg, milk, macadamia) - Environmental allergies (house dust mites) - Eczema - Asthma - Allergic rhinitis |
Ocular herpes, recurrent lower respiratory tract infection, chronic ear infections. | Bell’s Palsy. |
| #17 | Homozygous deletion spanning exon 15–48 | female | 4 | 2,294 | - Food allergies (peanut cashew, pistachio, sesame) - Sensitization to walnut and egg - Drug allergy (Propofol) - Mild Eczema |
Cryptosporidial cholangitis, chronic adenoviral carriage, mild M. contagiosum, Giardia, non-typhi Salmonella, low level CMV viraemia, otitis externa. | |
| #18 | c.12114A>G: p. K405R | female | 18, | >10,000 | - Food allergies (beans, beef, chicken, cow’s milk, egg, fish, peanut, pork, tree nuts, tomato) - Environmental allergies (dust, dog, grasses, mold) - Drug allergies (Cefipime, Lactinex, Propofol) - Eczema (herpeticum) |
S. aureus, H. influenzae, Cryptococcal meningitis, Acinetobacter baumannii sepsis, HSV keratitis, herpes zoster virus. | Delayed puberty. Deceased. |
| #19 | Homozygous for a deletion of Exons 28–35 | female | 17 | 8,031 | - Food allergies (lentils) - Severe eczema |
Chronic oral HSV, sinopulmonary infections, onychomycosis and thrush, S. aureus skin infections. | |
| #20 | Homozygous nonsense mutations Exon19: c.2044G>T, p.E682X | female | 11 | 6,690 | - Food allergies eggs, milk, nuts, soy, wheat) - Severe eczema |
S. aureus skin infections, HSV keratitis. | |
| #21 | Large deletion (exon 21 to end of gene) + small indel with frameshift mutation (exon 12) | male | 25 | 1,162 | - Food allergies (nuts) - Eczema |
HSV keratitis, sinopulmonary infections, extensive warts. | Squamous cell carcinoma pre-HSCT. |
| #22 | Large deletion (exon 21 to end of gene) + small indel with frameshift mutation (exon 12) | female | 22 | 39 | - No known allergies | Extensive warts, sinopulmonary infections. | Severe bronchiectasis. |
| #23 | Nonsense mutation (exon 17) + small indel with frameshift mutation (exon 36) | female | 16 | 180 | - Mild eczema | M. contagiosum, warts, sinopulmonary infections. | EBV-B cell lymphoma. |
| #24 | Large deletion (exons 13 to 26) + splicing mutation (intron 5) | male | 12 | 1,563 | - Food allergies (tree nuts) - Mild eczema |
Extensive warts, sinopulmonary infections, S. aureus osteomyelitis. | |
| #25 | Large deletion (promoter to exon 17) + nonsense mutation (exon 8) | female | 19 | 5,604 | - Food allergies (milk, egg, wheat, nuts) - Asthma - Moderate eczema |
Sinopulmonary infections, warts and M. contagiosum, Pneumocystis pneumonia, S. aureus skin infections, mucosal candidiasis. | Burkitt’s lymphoma (EBV negative), vasculopathy of mid-aorta with bilateral renal artery stenosis, heart failure, improved post HSCT. |
| #26 | Homozygous deletion of at least exons 4–13 | female | 9 | 2 | - Asthma, - Mild eczema |
Sinopulmonary infections, warts. | |
| #27 | Homozygous deletion of exon 36 | female | 20 | >6,000 | - Food allergies (milk, kiwi) - Asthma - Moderate eczema |
Sinopulmonary infections, warts, chronic cutaneous HSV. | Cerebral vasculopathy with stroke and aortic vasculopathy. |
| #28 | large homozygous deletion of more than 174 kb affecting most of DOCK8 (260876_43519 0) from intron 1 to exon 39 | female | 12 | 1,855-8,460 | - Food allergies (egg and lentils) - Eczema - Eosinophilia (1,532/mm3) |
Diarrhea, upper respiratory infections, recurrent meningoencephalitis, chronic otitis media, esophageal candidiasis, lower urinary tract infection, pyelonephritis (twice), Pseudomonas sp (ear), E. coli. | Failure to thrive (short stature), mild scoliosis, seronegative hepatitis, liver steatosis, mild hepatosplenomegaly, extensive abdominal vasculitis, elevated liver enzymes, ↑IgA ↑ IgG, ↑IgM, CD3+ lymphopenia (600/ml). |
The following patients were used in these experiments:
• phenotyping (#1–18);
• ex vivo cytokine and in vitro differentiation (#1, #2, #6, #7, #9, #10, #15, #17, #18);
• plasma IgE (#6, #12, #14, #15, #17, #19–28)
Antibodies and Reagents
Alexa488-conjugated anti-GATA3, Alexa647-conjugated anti-CXCR5, APC-Cy7-conjugated anti-CD4, BUV395-conjugated anti-IFNγ, BV711-conjugated anti-IL-2, Pe-Cy7-conjugated anti-CD25, PE-conjugated anti-CCR6 and anti-mouse IgG1, and PerCpCy5.5-conjugated anti-CD127 and anti-Tbet were purchased from Becton Dickinson. Alexa488-conjugated anti-IL-10, eFluor660-conjugated anti-IL-21, FITC-conjugated anti-CD45RA, PE-conjugated IL-22, Pe-Cy7-conjugated anti-IL-4 and mouse IgG1 were purchased from eBiosciences. APC-Cy7-conjugated anti-IL-17A, BV421-conjugated anti-CXCR3, and BV605-conjugated anti-TNFα was purchased from Biolegend. FITC-conjugated anti-CCR7 and recombinant human IL-12 was purchased from R&D Systems. Anti-DOCK8 mAb was purchased from Santa Cruz Biotechnology. Recombinant human TGFβ, IL-1β, IL-6, IL-21 and IL-23 were from Peprotech. Prostaglandin E2, PMA, calcium ionophore (ionomycin), Brefeldin A, and saponin were purchased from Sigma-Aldrich and recombinant human IL-4 was provided by Dr Rene de Waal Malefyt (DNAX Research Institute, Palo Alto, CA). T cell activation and expansion (TAE) beads (anti-CD2/CD3/CD28) were purchased from Miltenyi Biotec and CFSE was purchased from Invitrogen.
CD4+ T cell phenotyping
To identify naïve, central memory (TCM) and effector memory (TEM) CD4+ T cell populations, PBMCs were incubated with mAbs to CD4, CCR7 and CD45RA and the frequency of CD4+CCR7+CD45RA+ (naïve), CD4+CCR7+CD45RA− (TCM), and CD4+CCR7−CD45RA− (TEM) populations determined by flow cytometry. To identify CD4+ T helper cell populations, PBMCs were incubated with mAbs to CD4, CD25, CD127, CXCR5, CD45RA, CCR6 and CXCR3, and the frequency of Tregs (CD4+CD25hiCD127lo), Tfh (CD4+CD25loCD127hi CD45RA−CXCR5+), Th1 (CD4+CD25loCD127hiCD45RA−CXCR5−CXCR3+CCR6−), Th2 (CD4+ CD25loCD127hiCD45RA−CXCR5−CXCR3−CCR6−) and Th17 (CD4+CD25loCD127hiCD45RA−CXCR5−CXCR3−CCR6+) subsets determined as previously described20.
Analysis of cytokine expression/secretion by CD4+ and CD8+ T cells
Naive and memory CD4+ T cells or naïve, memory and TEMRA CD8+ T cells22 were isolated by cell sorting on a FACS ARIA (Becton Dickinson; > 98% purity) and cultured with T cell activation and expansion (TAE) beads (anti-CD2/CD3/CD28; Miltenyi Biotech) in 96 well round bottomed well plates. After 5 days, supernatants were harvested and production of IL-2, IL-4, IL-5, IL-6, IL-10, IL-13, IL-17A, IL-17F, IFNγ and TNFα determined by cytometric bead arrays (CBA; Becton Dickinson). For cytokine expression, activated T cells were re-stimulated with PMA (100 ng/ml) and ionomycin (750 ng/ml) for 6 hours, with Brefeldin A (10 μg/ml) added after 2 hours. Cells were then fixed with formaldehyde and expression of IFNγ, IL-4, IL-17A, IL-22, IL-21, IL-10, TNFα and IL-2 detected by intracellular staining20, 27–29.
Analysis of transcription factor expression by CD4+ T cells
Expression of TBET and GATA3 protein was assessed by intracellular staining using a Fix/Perm kit from eBioscience. Expression of RORC was determined by QPCR as previously described29.
Analysis of DOCK8 expression
To determine intracellular DOCK8 expression, PBMCs were fixed with formaldehyde and stained with an unconjugated DOCK8 or an isotype control mouse IgG1 mAb. A secondary PE conjugated rat anti-mouse IgG1 was then used with saponin as the permeablising agent.
Analysis of CD4+ T cell proliferation
To investigate proliferation, naïve and memory CD4+ T cells were isolated by cell sorting and then labeled with CFSE. Their proliferation status was determined by assessing dilution of CFSE after 5 days of in vitro culture28, 29.
In vitro Th1, Th2, Th17 cell differentiation
Naive and memory CD4+ T cells were isolated by cell sorting and cultured under neutral/Th0 (TAE beads alone), or under Th1 (50 ng/ml IL-12), Th2 (100 U/ml, IL-4) or Th17 (2.5 ng/mL TGFβ, 50 ng/mL IL-1β, 50 ng/mL IL-6, 50 ng/mL IL-21, 50 ng/mL IL-23, 50 ng/mL PGE2) polarising conditions. After 5 days cytokine secretion was analysed by CBA and intracellular staining27, 29, 30.
ImmunoCAP assay
Plasma from normal donors and DOCK8-deficient patients was analysed for allergen specific IgE Abs by the Sydney South West Pathology Service (Royal Prince Alfred Hospital, Sydney Australia) using the Phadia 250 ImmunoCAP platform (Thermo Scientific). IgE specific for a staple food mix (FX5; egg white, milk, codfish, wheat, peanut and soyabean) or house dust mite mix was determined.
Statistical analysis
Significant differences were determined using either a Students t-test, multiple t-tests, one-way or two-way ANOVA (Prism; GraphPad Software).
RESULTS
Effects of DOCK8 deficiency on the generation of effector CD4+ T cell subsets in vivo.
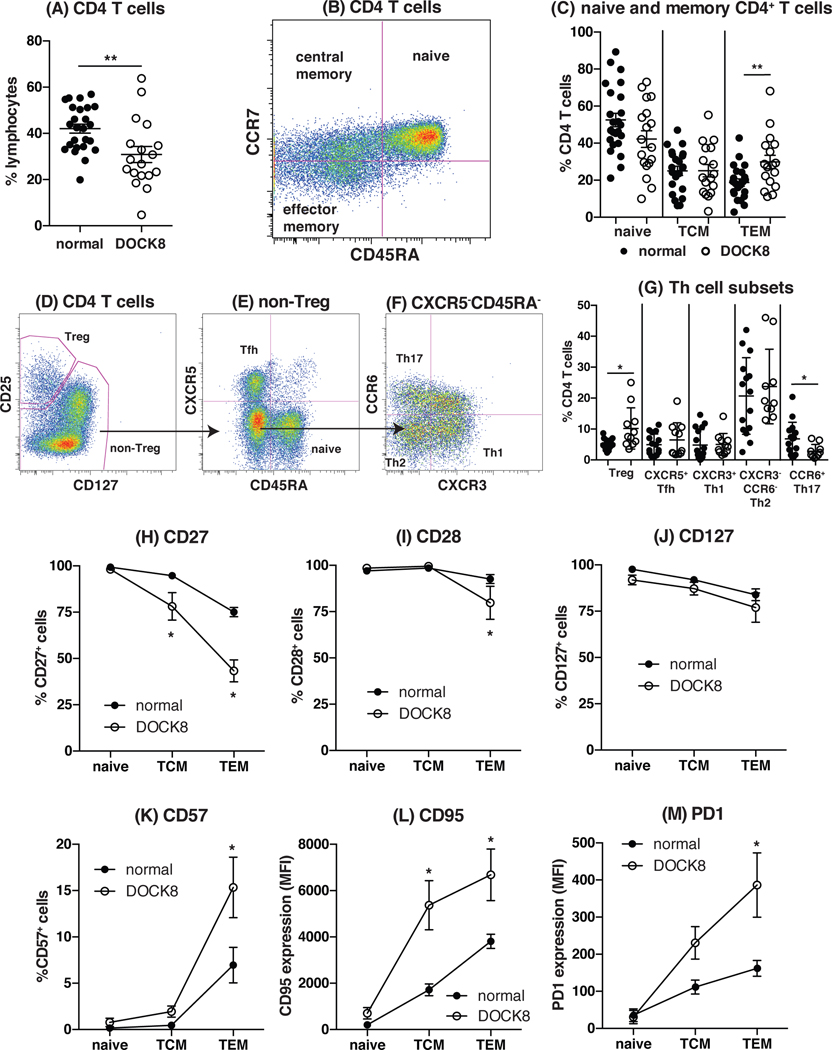
As an initial step in investigating CD4+ Th cell function in the absence of DOCK8, we assessed the CD4+ T cell compartment in DOCK8-deficient patients to determine whether the generation and differentiation of CD4+ T cells was affected and whether this could contribute to the combined immunodeficiency typical of these individuals. We previously investigated the peripheral T cell compartment in a small cohort (n = 6) of DOCK8-deficient patients22. We have now increased our cohort to comprise 18 individuals from 15 unrelated families and have extended our analysis to include additional surface markers to further distinguish different subsets within the CD4+ T cell population (Fig 1). Lack of DOCK8 expression in lymphocytes and monocytes from a representative healthy control, one unaffected sibling and 4 DOCK8-deficient patients is depicted in Supplementary Fig 1. Analysis of this larger cohort of DOCK8-deficient patients confirmed a statistically significant reduction in CD4+ T cells compared to normal donors (Fig 1A, normal: 42 ± 2%, n = 25; DOCK8: 31 ± 3.5%, n = 18; p = 0.0045). Naïve, central memory (TCM) and effector memory (TEM) CD4+ T cells can be resolved according to the differential expression of CD45RA and CCR731 (Fig 1B). This analysis revealed that the naïve and TCM compartments in DOCK8-deficient patients are comparable to normal individuals, but TEM CD4+ T cells were significantly increased in DOCK8-deficient patients (Fig 1C). Hence, despite the reduction in total CD4+ T cells, DOCK8-deficient CD4+ T cells differentiate normally into naïve and TCM cells; this is accompanied by a mild increase in TEM cells.
Figure 1: Phenotype of the peripheral CD4+ T cell compartment in DOCK8-deficient patients.
(A) The frequency of CD4+ T cells in normal donors and DOCK8-deficient patients. (B, C) Naïve (CD45RA+CCR7+), central memory (TCM; CD45RA−CCR7+) and effector memory (TEM; CD45RA−CCR7−) populations in normal donors (closed symbol; n = 25) and DOCK8-deficient patients (open symbol; n = 18) were enumerated based on expression of CD45RA and CCR7. (D-G) PBMCs were labelled with mAbs against CD4, CD45RA, CD25, CD127, CXCR5, CXCR3 and CCR6. (D) Treg cells were identified as CD25hiCD127lo. (E) Amongst the non-Treg population naïve and Tfh cells were identified as CXCR5−CD45RA+ and CXCR5+CD45RA−, respectively. (F) Th1, Th2 and Th17 populations were identified within the population of CXCR5−CD45RA− memory CD4+ T cells as CXCR3+ CCR6−, CCR6−CXCR3− and CCR6+CXCR3− cells, respectively. (G) Using this gating the frequency of Tregs, Tfh, Th1, Th2 and Th17 cells within the CD4+ T cell compartment was determined in normal individuals (closed symbol; n = 15 or 16) and in DOCK8-deficient patients (open symbol; n = 10 or 11). Each point represents an individual donor or patient. Statistics performed with Prism using Student t-test. (H-M) Naïve (CD45RA+CCR7+), central memory (TCM; CD45RA−CCR7+) and effector memory (TEM; CD45RA−CCR7−) populations in normal donors (closed symbol) and DOCK8-deficient patients (open symbol) were identified and assessed for expression of (H) CD27, (I) CD28, (J) CD127, (K) CD57, (L) CD95 and (M) PD1. Each point corresponds to the mean ± SEM % of cells expressing the indicated surface receptor, or MFI (mean fluorescence intensity) of expression (n = 4 – 12 normal donors or DOCK8-deficient individuals). Statistics performed with Prism using t-test.
Using a recently described gating strategy20, 32, we next examined the CD4+ T cell compartment for additional effector subsets, namely CD25hiCD127lo Tregs (Fig 1D, G)33, CXCR5+CD45RA− T follicular helper (Tfh) cells (Fig 1E, G), CD45RA−CXCR5− CXCR3+CCR6− Th1 (Fig 1F, G), CD45RA−CXCR5− CXCR3−CCR6− Th2 (Fig 1F, G), and CD45RA−CXCR5− CXCR3−CCR6+ Th17 (Fig 1F, G) cells. DOCK8-deficient patients had an increased frequency of Tregs (Fig 1D, G, normal: 5.3 ± 0.43%, n = 16; DOCK8: 10 ± 2%, n = 11; p = 0.011) but decreased frequency of Th17 cells (Fig 1F, G, normal: 6.8 ± 1.4%, n = 15; DOCK8: 2.8 ± 0.7%, n = 10; p = 0.037), while frequencies of Tfh, Th1 and Th2 cells according to this phenotypic delineation in DOCK8-deficient patients were similar to normal donors (Fig 1D – G). Thus, there appears to be selective paucity of Th17 cells due to DOCK8 mutations.
Assessment of expression of additional surface markers associated with CD4+ T cell differentiation indicated that the naïve, TCM and TEM CD4+ T cell populations from DOCK8-deficient patients had undergone greater activation and terminal differentiation than corresponding CD4+ T cell subsets isolated from normal donors (Fig 1H–M). Specifically, the loss of expression of CD27 (Fig 1H), CD28 (Fig 1I) and CD127 (Fig 1J) and acquisition of CD57 (Fig 1K), CD95 (Fig 1L) and PD-1 (Fig 1M) by CD4+ TCM and TEM cells was exaggerated for DOCK8-deficient patients compared to controls. Collectively, DOCK8 deficiency compromises the generation of Th17 cells, and results in the premature terminal differentiation of memory cells such that they acquire a senescent/exhausted phenotype.
DOCK8 deficient memory CD4+ T cells are biased towards Th2 cytokines.
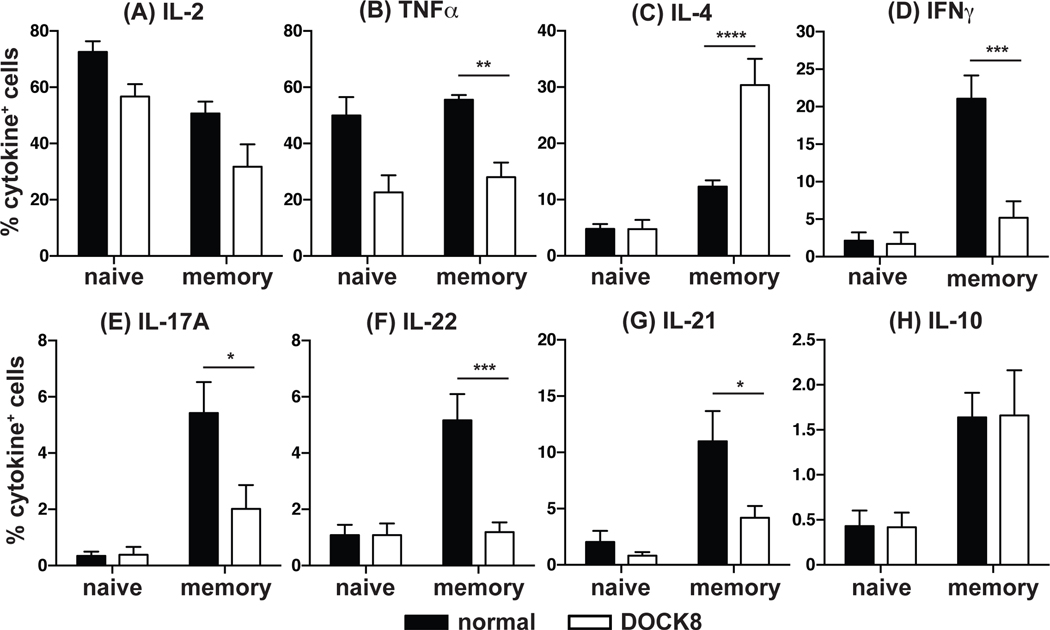
Given the decrease in CCR6+CXCR3− cells – which are enriched for Th17-cytokine producing cells in healthy donors20, 34–36 – in DOCK8-deficient patients, we investigated cytokine expression by naïve and memory CD4+ T cells (Fig 2). Naïve (CD45RA+CCR7+) and total memory (CD45RA−CCR7+/−) CD4+ T cells were sort-purified from normal donors and DOCK8-deficient patients and then cultured with T cell activation and expansion (TAE) beads conjugated to mAbs specific for CD3, CD28 and CD2 for 5 days. After this time cells were restimulated with PMA and ionomycin in the presence of Brefeldin A and intracellular expression of IFNγ, IL-4, IL-17A, IL-22, IL-21, IL-10, TNFα and IL-2 determined (Fig 2). Apart from IL-2 (Fig 2A) and TNFα (Fig 2B), which are expressed by 40–80% of normal naïve cells, only a small proportion of naïve cells (ie <5%) expressed any of the other cytokines examined. DOCK8-deficient naïve CD4+ T cells expressed a comparable level of IL-2 (Fig 2A) and TNFα (Fig 2B) to that of normal naïve CD4+ T cells. However, analysis of the memory CD4+ T cell compartment in DOCK8-deficient patients revealed marked perturbations in differentiation in vivo. A significantly greater proportion of DOCK8-deficient memory CD4+ T cells expressed IL-4 compared to normal memory CD4+ T cells (Fig 2C), suggesting a skewing to the Th2 effector lineage. Examination of mean fluorescence intensity of IL-4+ cells in DOCK8-deficient and normal memory CD4+ T cells revealed no significant differences (data not shown), suggesting there is an increase in the frequency of IL-4 expressing cells in the DOCK8 memory CD4+ T cell compartment, but a comparable amount of IL-4 is produced on a per cell basis. Furthermore, this increase in IL-4+ cells in DOCK8-deficient memory CD4+ T cells was accompanied by significant reductions in expression of Th1 cytokines IFNγ (Fig 2D) and TNFα (Fig 2B), the Th17 cytokines IL-17A (Fig 2E) and IL-22 (Fig 2F), and the Tfh cytokine IL-21 (Fig 2G). In contrast to these defects, expression of IL-10 (Fig 2H) and IL-2 (Fig 2A) by memory CD4+ T cells was unaffected by DOCK8 deficiency.
Figure 2: DOCK8-deficient memory CD4+ T cells display a Th2 cytokine expression bias.
Naïve (CD45RA+CCR7+) and memory (CD45RA−CCR7+/−) CD4+ T cells were isolated from normal donors and DOCK8-deficient patients and cultured with TAE beads for 5 days. Cells were then re-stimulated with PMA/ionomycin for 6 hours in the presence of Brefeldin A for the last 4 hours. Intracellular expression of (A) IL-2, (B) TNFα, (C) IL-4, (D) IFNγ, (E) IL-17A, (F) IL-22, (G) IL-21 and (H) IL-10 was determined using saponin as the permeabilising agent followed by flow cytometric analysis. Data represent the mean ± SEM of 8 normal donors or 8 DOCK8-deficient patients. Statistics performed with Prism using One-way ANOVA.
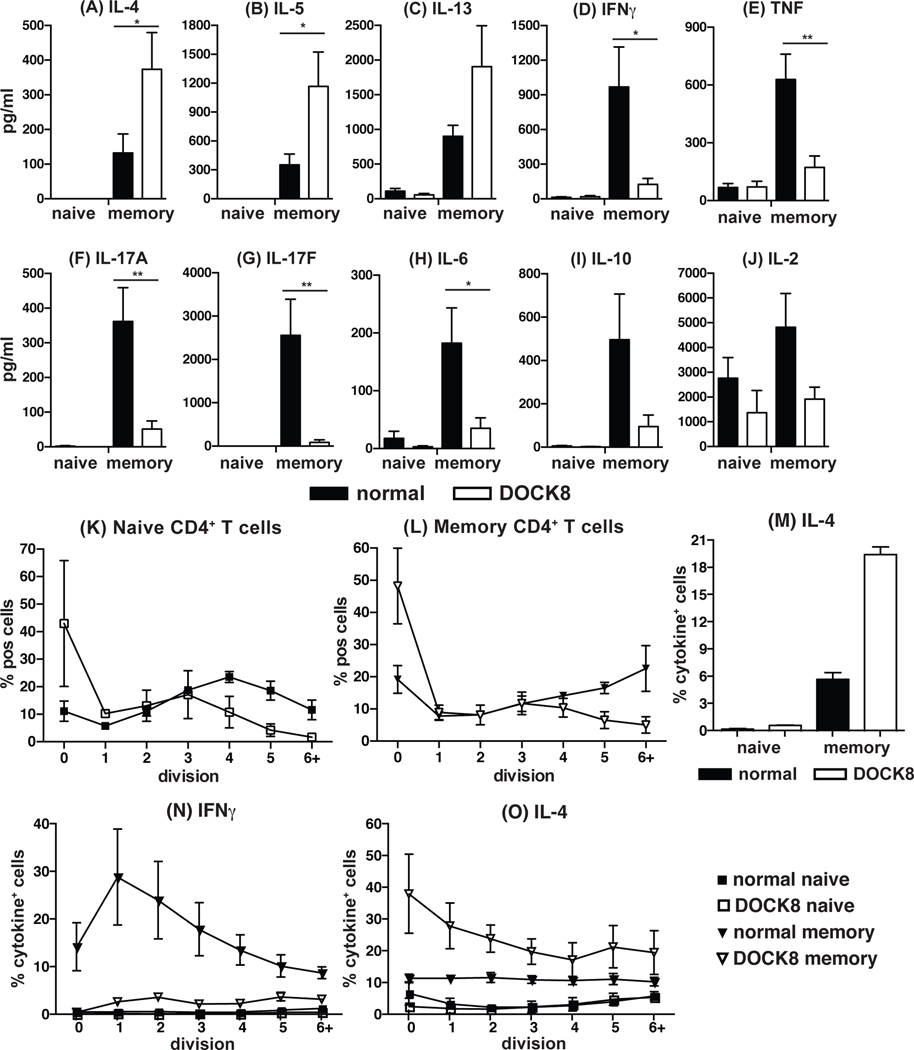
The Th2 skewing by DOCK8-deficient memory CD4+ T cells was also assessed by measuring cytokine secretion during the 5-day activation period (Fig 3). This indicated concordance between expression and secretion of cytokines when assessed by intracellular staining and flow cytometry or cytometric bead array, respectively. Analysis of an extended panel of cytokines showed that DOCK8-deficient memory T cells secreted not only more IL-4 than normal memory CD4+ T cells, but also more of the Th2 cytokines IL-5 and IL-13 (Fig 3A–C) and less Th1 (IFNγ and TNFα; Fig 3D, E) and Th17 (IL-17A and IL-17F; Fig 3F, G) cytokines. Production of IL-6 (Fig 3H) by DOCK8-deficient memory CD4+ T cells was also significantly less than normal memory cells. There were trends for reduced production of IL-10 and IL-2 by DOCK8-deficient memory CD4+ T cells, however these values were not significantly less than controls (Fig 3I, J). Lastly, production of TNFα and IL-2 by DOCK8-deficient naïve CD4+ T cells was normal (Fig 3E, J). Taken together, these results indicate that memory CD4+ T cells from DOCK8-deficient patients display a Th2 bias in that they primarily express IL-4, IL-5 and IL-13 and notably lower levels of cytokines characteristic of other T helper subsets.
Figure 3: DOCK8-deficient memory CD4+ T cells secrete elevated quantities of the Th2 cytokines IL-4, IL-5 and IL-13 independently of differences in cell proliferation.
Naïve and memory CD4+ T cells were sorted from normal donors and DOCK8-deficient patients and cultured with TAE beads for 5 days. After this time, culture supernatants were examined for secretion of (A) IL-4 (B) IL-5, (C) IL-13, (D) IFNγ, (E) TNF, (F) IL-17A, (G) IL-17F, (H) IL-6, (I) IL-10, (J) IL-2, using a custom designed cytometric bead array (CBA; BD biosciences). Data represent the mean ± SEM of experiments using cells from 9 normal donors or DOCK8-deficient patients. Statistics performed with Prism using One-way ANOVA. (K-L) Naive (K) and memory (L) CD4+ T cells were isolated from normal donors (n = 4) and DOCK8-deficient patients (n = 4), labelled with CFSE and cultured with TAE beads for 5 days. After this time, the frequency of cells in each division was determined by dilution of CFSE. (M) Sorted naïve and memory CD4+ were immediately restimulated with PMA/ionomycin for 6 hours in the presence of Brefeldin A and IL-4 expression determined by intracellular staining and flow cytometry. (N, O) Naive and memory CD4+ T cells were labelled with CFSE, cultured with TAE beads for 5 days, and the proportion of cells expressing (L) IFNγ or (M) IL-4 was determined for each division interval by dilution of CFSE. Data represent the mean ± SEM of 2 – 4 normal donors and DOCK8-deficient patients.
Th2 cytokine bias by DOCK8-deficient memory CD4+ T cells is independent of defects in cell proliferation.
Previous work has shown that several features of lymphocyte differentiation such as Ig class switching and antibody secretion by naïve B cells, and cytokine production and cell surface phenotype expression by naïve T cells are regulated by cell division28, 37–39. DOCK8-deficient naïve (Fig 3K) and memory (Fig 3L) CD4+ T cells were found to have impaired cell division in vitro, consistent with our previous findings22. Thus, it was possible that the perturbed cytokine profile reflected reduced proliferation by DOCK8-deficient memory CD4+ T cells. However, the Th2 bias of DOCK8-deficient memory CD4+ T cells was not due to a proliferative defect as evidenced by two important and related findings. First, when memory cells were isolated and restimulated immediately for analysis of cytokine expression, the preferential production of IL-4 by DOCK8-deficient over normal memory CD4+ T cells was still observed in the absence of cell proliferation (Fig 3M). Similarly, the poor production of Th1 and Th17 cytokines by DOCK8-deficient memory CD4+ T cells did not result from impaired proliferation because reductions in expression of IFNγ (normal: 17.7%, DOCK8: 6.9%) and IL-22 (normal: 3.7%, DOCK8: 1.8%) respectively were also observed when assessed under these ex vivo stimulatory conditions. Second, analysis of cells that had undergone different rounds of divisions in vitro revealed that the decrease in IFNγ (Fig 3N) and increase in IL-4 (Fig 3O) expression displayed by DOCK8-deficient versus normal memory CD4+ T cells was evident for all division intervals examined. Thus, the preference of DOCK8-deficient memory CD4+ T cells to produce Th2, but not Th1, cytokines is independent of any proliferative defects in these cells.
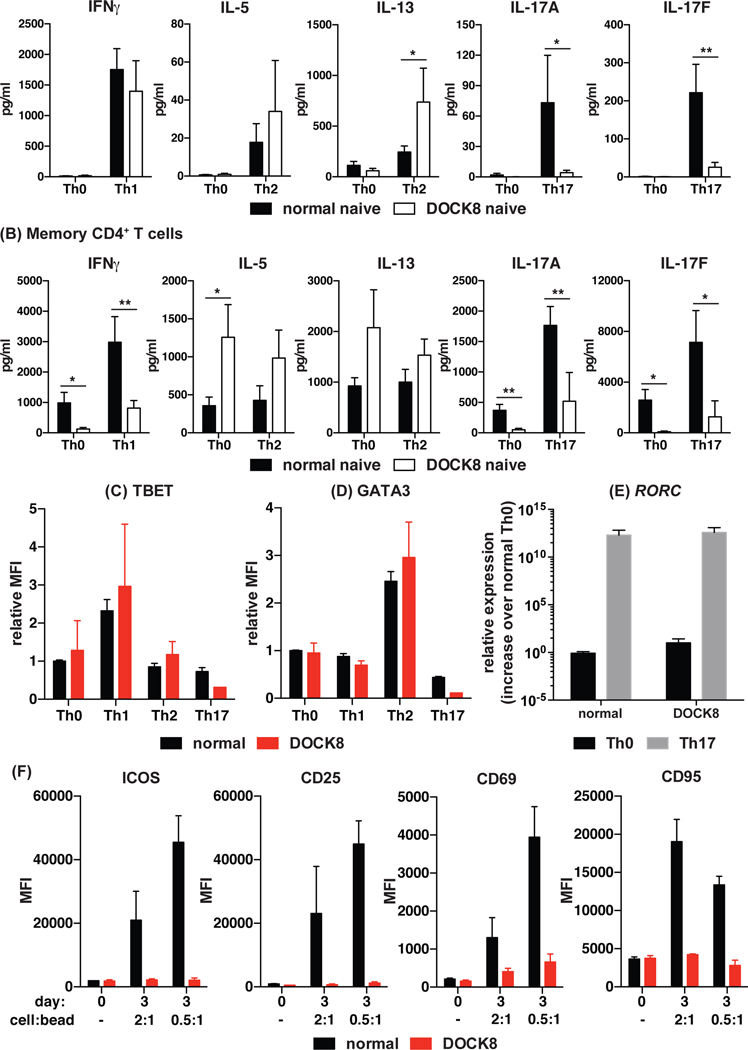
Naive DOCK8-deficient CD4+ T cells can differentiate into effector cells producing Th1 and Th2, but not Th17, cytokines in vitro.
To determine if the defects in cytokine production displayed by DOCK8-deficient memory CD4+ T cells are cell-intrinsic or due to extrinsic factors, we isolated naïve CD4+ T cells from normal donors and DOCK8-deficient patients and subjected them to in vitro culture under Th0, Th1, Th2 or Th17 polarising conditions. Interestingly, DOCK8-deficient naïve CD4+ T cells were able to differentiate into Th1 cells (IFNγ and TNFα) to the same extent as normal naïve CD4+ T cells (Fig 4A, left panel). Consistent with the data for memory CD4+ T cells ex vivo, DOCK8-deficient naïve CD4+ T cells produced greater amounts of Th2 (IL-5 and IL-13) cytokines than control naïve CD4+ T cells under Th2-polarising conditions (Fig 4A, middle panels). Similarly, DOCK8-deficient naïve CD4+ T cells failed to differentiate into IL-17A- and IL-17F-secreting cells when subjected to Th17 polarising conditions in vitro (Fig 4A, right panels). Notably, DOCK8-deficient naïve CD4+ T cells were capable of responding to the Th17 culture as shown by the reduction in basal levels of secretion of IL-5 and IL-13 compared to the Th0 culture (data not shown). When we examined memory CD4+ T cells from healthy donors, we found that production of IFNγ and IL-17A/F could be increased approximately 2–4 fold by Th1 and Th17 culture conditions, respectively, compared to Th0 conditions (Fig 4B). The net increase in production of these cytokines by DOCK8-deficient memory CD4+ T cells under Th1 and Th17 conditions compared to Th0 conditions was also ~2–6 fold. Despite this, the levels of IFNγ and IL-17A/F secreted by Th1- and Th17-stimulated DOCK8-deficient memory CD4+ T cells were substantially less than not only Th1- and Th17-stimulated normal memory CD4+ T cells, but also Th0-stimulated normal memory CD4+ T cells (Fig 4B). This likely reflects expansion of the few Th1 and Th17 cells present in the DOCK8 memory CD4+ T cell compartment rather than de novo differentiation into these effector subsets in vitro.
Figure 4: Intrinsic defects in CD4+ T cell cytokine secretion due to DOCK8 mutations.
(A) Naïve and (B) memory CD4+ T cells were isolated from normal donors and DOCK8-deficient patients and activated under neutral conditions (Th0; TAE only), or Th1- (+ IL-12), Th2- (+ IL-4), or Th17- (+ IL-1β, IL-6, IL-21, IL-23, TGFβ, PG) polarising conditions. After 5 days, secretion of Th1 (IFNγ), Th2 (IL-5, IL-13) and Th17 (IL-17A, IL-17F) cytokines was determined by CBA. The data represent the mean ± SEM of experiments using cells from 5 – 9 normal donors and DOCK8-deficient patients. Expression of (C) TBET and (D) GATA3 was determined by flow cytometry; the data represent the fold change (mean ± sem) in expression of the indicated transcription factor relative to Th0 culture of the normal control. (E) expression of RORC was determined by QPCR. Data represent the mean and SEM of 2 – 3 normal donors and DOCK8-deficient patients. Statistics performed with Prism using two-way ANOVA
Consistent with the data for cytokine secretion, DOCK8-deficient naïve CD4+ T cells that were polarised towards Th1 and Th2 fates upregulated TBET (Fig 4C) and GATA3 (Fig 4D), respectively, to the same extent as normal naïve CD4+ T cells. Furthermore, compared to the Th0 culture, TBET and GATA3 expression was decreased in DOCK8-deficient naïve CD4+ T cells cultured under Th17 polarising conditions, demonstrating responsive of these cells to this culture (Fig 4C, D). In our hands, detection of RORγt expression by flow cytometry was not particularly sensitive, as we found that only a small proportion of naïve CD4+ T cells (~5%) expressed RORγt in Th17 compared to Th0 activated cultures (data not shown). To overcome this, RORC expression was determined by QPCR. When RORC expression was examined, it was not expressed by naive CD4+ T cells activated under Th0 conditions, but was up-regulated in both normal and DOCK8-deficient naïve CD4+ T cells cultured under Th17 polarising conditions (Fig 4E). Taken together, these data indicate the Th17 cytokine defect in DOCK8 deficiency is T cell intrinsic, and cannot be restored by Th17 polarising conditions for either naïve or memory cells. Furthermore, the ability of Th17 culture conditions to induce RORC expression in the absence of DOCK8 indicates that the defect in Th17 differentiation is downstream of RORC. In contrast, DOCK8-deficient naïve CD4+ T cells can differentiate normally into Th1 cells, and exhibit exaggerated Th2 differentiation, when provided with the appropriate stimuli in vitro.
Specific sensitisation of DOCK8-deficient patients to food allergens
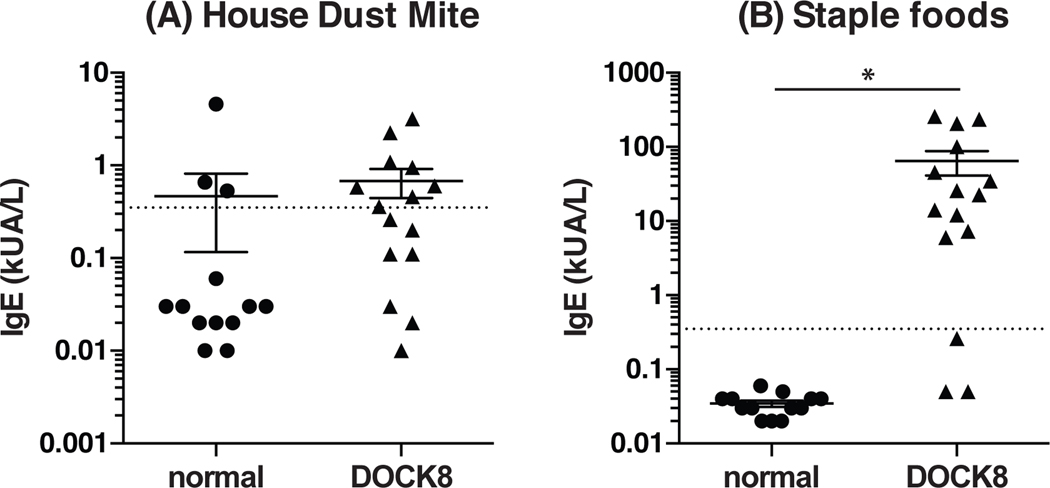
Exaggerated Th2 immune responses have traditionally been associated with allergy and atopic disease40. It was thus intriguing to note that CD4+ T cells from DOCK8-deficient patients were biased towards production of Th2 cytokines, and that these patients have severe allergies. To determine if the Th2 bias in DOCK8-deficient human CD4+ T cells is related to their increased susceptibility to food allergies we examined the specificity of IgE in serum samples from DOCK8-deficient patients and normal healthy donors to staple foods (i.e. egg white, milk, codfish, wheat, peanut, soyabean), as well as to non-food allergens such as house dust mites. We found that a comparable frequency of normal individuals and DOCK8-deficient patients had IgE specific to house dust mites (Fig 5A). Strikingly, the majority of plasma samples from DOCK8-deficient patients (80%; 12/15), but none of the normal controls tested, had IgE that was specific for the staple food mix (Fig 5B). Thus, DOCK8-deficient patients have a Th2 bias that manifest clinically as specific sensitisation to oral allergens and this may explain the marked propensity of these immunodeficient patients to develop food allergies.
Figure 5: IgE in DOCK8 deficient patients is specific for staple foods and not other Ags such as house dust mites.
Plasma from normal donors and DOCK8-deficient patients was analysed for IgE specific for (A) a staple food mix (egg white, milk, codfish, wheat, peanut and soyabean) and (B) a house dust mite mix by ImmunoCAP. The data represent the mean ± SEM of 13 normal donors and 15 DOCK8-deficient patients. The dotted line refers to the upper limit of the negative reference interval (0.35 kUA/L).
DISCUSSION
Identifying defects in lymphocyte development or function in primary immunodeficiencies provides the opportunity to elucidate the cellular and molecular basis for the clinical features of the disease. Studies of DOCK8-deficient humans and mice have indeed revealed critical cell-intrinsic roles for DOCK8 in the generation of B-cell memory and long-lived humoral immunity5, 41, CD8+ T cell differentiation and anti-viral responses22, 23, 42, 43, NK cell cytotoxic function24 and NKT cell development25. Collectively, these defects underlie poor Ab responses to T-dependent and T-independent Ags, and impaired cell-mediated immunity to pathogens including herpes viruses, human papilloma virus (HPV) and Molluscum contagiosum virus. We have now investigated CD4+ T cell differentiation in DOCK8-deficient patients in an attempt to understand other aspects of AR-HIES, such as susceptibility to bacterial and fungal infections, atopic disease, food allergies and hyper-IgE.
Our data revealed that DOCK8-deficient CD4+ T cells have dysregulated expression of surface molecules including CD27, CD57, CD95 and PD-1. This likely results from chronic infection with pathogens, such as herpes viruses (HSV, CMV, VZV), HPV and Molluscum contagiosum virus, akin to what has been described for CD8+ T cells in not only DOCK8 deficiency22, but other primary immunodeficiencies such as XLP44, 45, autosomal dominant HIES (STAT3 deficiency46) and PIK3CD gain of function mutations47, which are characterised by chronic exposure to infectious pathogens. Furthermore, in the absence of DOCK8, memory CD4+ T cells are polarised towards a Th2 cytokine phenotype at the expense of Th1 and Th17 cytokines. The reduction in Th17 cells was apparent not only from the lack of cells producing IL-17A, IL-17F and IL-22, but also the reduction in CCR6+ memory CD4+ T cells. This is consistent with our previous studies which revealed parallel reductions in cells secreting IL-17A/IL-17F and CCR6+ CD4+ T cells in patients with STAT3 loss-of function or STAT1gain-of function mutations17, 20, 29, indicating that flow cytometric analysis of CCR6+ memory CD4+ T cells is a reliable and rapid means of quantifying Th17 cells. Interestingly, DOCK8-deficient naïve CD4+ T cells were able to differentiate into TBET-expressing and Th1-cytokine secreting cells when provided with exogenous signals in vitro. This suggests that defects in IFNγ production by DOCK8-deficient memory CD4+ T cells ex vivo are extrinsic, and possibly result from suboptimal priming by Ag-presenting cells and provision of IL-12 in vivo. Consistent with this, DOCK8-deficient murine dendritic cells failed to accumulate in the parenchyma of lymph nodes where they are required for T cell priming during immune responses48. This defect was attributed to compromised Cdc42 function in the absence of DOCK8 expression48. Another possibility is that excessive production of IL-4, which restrains differentiation of human naïve CD4+ T cells into Th1 cells49, is responsible for impairing IFNγ production by DOCK8-deficient memory CD4+ T cells. This would be consistent with our recent observations of heightened production of Th2 cytokines and corresponding reductions in IFNγ production ex vivo by memory CD4+ T cells from individuals with loss-of function mutations in STAT3, IL21R, IL12RB1, TYK2 or RORC20, 50. While DOCK8-deficient naïve CD4+ T cells could express RORC in vitro following activation under Th17-polarisng conditions, IL-17A/F cytokine secretion remained greatly impaired. Thus, an intrinsic defect distal to induction of RORC expression underlies the inability of DOCK8-deficient CD4+ T cells to become Th17 cells. Although Th1- and Th17-polarising conditions did increase IFNγ and IL-17A/F production by DOCK8-deficient memory CD4+ T cells, these cells continued to produce lower levels of these cytokines than normal cells under similar culture conditions. Interestingly, CD4+ T cells from DOCK8-deficient mice expressed normal levels of TBET and GATA3 when activated under Th1 and Th2 polarising conditions, respectively, in vitro42. Furthermore, while IFNγ expression by in vitro-derived murine DOCK8-deficient Th1 cells was normal, DOCK8-deficient CD4+ T cells cultured under Th2 conditions showed an increase in IL-4-expressing cells42, suggesting that consistent with human DOCK8 deficiency, murine DOCK8 deficient CD4+ T cells also display a Th2 bias under certain settings.
These findings provide a potential explanation for some of the clinical features of DOCK8 deficiency, such as infectious susceptibility, severe food allergies, hyper IgE and eosinophilia. First, the lack of Th17 cells would predispose DOCK8-deficient individuals to mucocutaneous infections with Candida albicans. This is reminiscent of other monogenic primary immunodeficiencies characterised by impaired Th17/IL-17-mediated immunity and the high incidence of chronic mucocutaneous candidiasis (CMC) in affected individuals ie loss-of-function mutations in STAT3, IL17RA, IL17RC, IL17F, ACT1 and RORC, and gain-of-function mutations in STAT120, 29, 50–55. Compared to other primary immunodeficiencies with defects in Th17 cytokines, IL-17A/IL-17F production by DOCK8-deficient memory CD4+ T was less than that observed for RORC- or STAT3-deficient memory CD4+ T cells20, 50. Remarkably, the quantitative impact of specific gene mutations on the generation of Th17 cells correlates with, or predicts, the incidence of fungal infections such as CMC in these individuals. Thus, ~85% of patients with mutations in STAT3 or RORC develop CMC 50, 56, but fungal infections is observed in only ~40–60% of DOCK8-deficient patients, as shown for the cohort studied here (Table 1), and in a larger study of 57 patients57. Thus, it is likely that there is a direct association between levels of IL-17A/IL-17F production in different PID patients and incidence of CMC. Second, the predominance of memory CD4+ T cells producing high levels of IL-4, IL-5 and IL-13 could contribute to the characteristic pathophysiological Th2 features of AR-HIES: severe allergy, eosinophilia and hyper-IgE58. This exaggerated Th2 response may also reduce Th17 differentiation59, thereby further compromising Th17-mediated anti-fungal immune responses. Although memory CD4+ T cells displayed reduced IFNγ production ex vivo, DOCK8-deficient naïve CD4+ T cells could differentiate into Th1 cells in vitro. Thus, Th1-mediated immunity, while reduced, may be sufficient in these individuals to elicit protective immunity. Indeed, this would be consistent with a lack of disease caused by poorly virulent mycobacteria, such as BCG vaccines and environmental species - which require IFNγ-mediated immunity for protection60 - in DOCK8 deficiency. In the scenario of anti-viral immunity, the increased Th2-cytokine environment within the memory CD4+ T cell compartment may also inhibit IFNγ production by CD8+ T cells. Consistent with this, analysis of DOCK8-deficient memory CD8+ T cells ex vivo revealed a defect in IFNγ expression and secretion compared to memory cells from healthy donors (Supplementary Fig 2A, B)1. Thus, by diminishing Th1 responses, a Th2 bias could contribute to persistent viral infections displayed by DOCK8-deficient patients. Third, beyond Th1, Th2 and Th17 cytokines, we also noted reduced production of IL-6 by DOCK8-deficient memory CD4+ T cells. While there have been no genetic studies linking impaired IL-6 production with infection with specific pathogens, autoantibodies against IL-6 were reported in an individual with recurrent staphylococcal infection (Puel et al J Immunol 2008). Thus it is possible that poor IL-6-mediated immunity in DOCK8 deficiency underlies staphylococcal infection in affected patients. Fourth, while previous work demonstrated that DOCK8 functions intrinsically in B cells to regulate differentiation, reduced production of IL-21 (and potentially IL-10) by DOCK8-deficient memory CD4+ T cells may also contribute to impaired humoral immune responses in AR-HIES, as these cytokines are the main drivers of human B cell activation, proliferation and differentiation61. This is supported by our observation that DOCK8-deficient memory CD4+ T cells present with defects in IL-21 expression ex vivo (Figure 2) and that naïve DOCK8-deficient CD4+ T cells failed to differentiate into IL-21-expressing cells as efficiently as normal naïve CD4+ T cells when cultured under Tfh cell polarising conditions (Supplementary Fig 2C).
A characteristic and perhaps unique feature of DOCK8 deficiency compared to other primary immunodeficiencies (including primary immunodeficiencies in which there are high levels of IgE such as dominant negative mutations in STAT3) is the very high incidence of food allergies1–5. When we examined allergen-specific IgE from DOCK8-deficient patients we found it was mostly directed towards staple foods rather than non-food allergens such as house dust mites. This is consistent with a recent report which also showed that this pattern of allergen-specific IgE is unique to DOCK8 deficiency62, inasmuch that DOCK8 deficient patients had IgE directed towards food Ags, while patients with atopic dermatitis tended to have IgE specific for aeroallergens, yet the reactivity of IgE in STAT3-deficient individuals against specific allergens was comparable to that of normal donors62. Since food allergies are more common in children who often outgrow them once they reach adolescence, IgE sensitisation to food Ags and not house dust mites in DOCK8 deficiency could be attributable to the younger age of our DOCK8 cohort compared to our normal controls. However, this is unlikely as 9 of the 12 DOCK8 deficient patients that still had IgE specific to food Ags were adolescents or adults. In the scenario of STAT3 deficiency, the reduced level of IgE specific for food allergens when compared to patients with atopic dermatitis has been attributed to a defect in basophil activation and mast cell degranulation, with the latter process found to be STAT3-dependent63. This is interesting because although patients with mutations in DOCK8 or STAT3, or individuals with atopic dermatitis, all display increased serum IgE, eczema and atopic disease, DOCK8 deficiency specifically predisposes to food allergies. The mechanism whereby this occurs is unclear, but it is tempting to speculate that it is related to the Th2 bias of DOCK8-deficient memory CD4+ T cells. While Th2 skewing has been reported in DOCK8-deficient mice following Th2 polarisation in vitro42, to our knowledge, IgE responses following exposure to food allergens has not been investigated in mice, but may provide invaluable insights into whether exposure to food allergens is the driver of IgE production in DOCK8 deficiency. Nevertheless, our findings reinforce the value of direct interrogation of patient cells and highlight the need to be cognisant of species-specific differences that impact translation of murine studies to humans.
The underlying cause for the biased Th2 nature of memory CD4+ T cells in DOCK8-deficient patients remains to be determined. Examination of the TCR Vβ repertoire in the CD4+ T cell compartment of DOCK8 deficient patients and healthy normal donors did not reveal any substantial differences (data not shown). However, there is evidence showing that the strength of the signal received through the TCR greatly influences differentiation of CD4+ T cells towards specific subtypes. Specifically, low doses of Ag/low level TCR signalling favour humoral or IL-4-mediated Th2 immune responses while high doses of Ag/strong TCR signalling favour cellular or IFNγ-mediated Th1 immune responses64–66. This is also supported genetically, with the finding that murine CD4+ T cells with a hypomorphic mutation in Card11 reduces TCR-mediated signal strength resulting in exaggerated Th2 differentiation, allergic disease, dermatitis and hyper-IgE67. Based on this, we hypothesise that in the absence of DOCK8 CD4+ T cells receive a qualitatively weaker TCR signal, potential due to defective immunological synapse formation41, which favors their preferential differentiation into Th2 cells at the expense of other Th cell subsets. The original studies on strength of TCR signals influencing murine Th cell differentiation predated the discovery of Th17 cells. However, studies in mice and humans have since demonstrated a requirement for sustained and robust TCR signalling in naïve CD4+ T cells for commitment to a Th17 functional phenotype in vitro and in vivo68, 69. Thus, we would also hypothesise that reduced TCR signal strength in DOCK8-deficient CD4+ T cells impairs their differentiation into Th17 cells.
In conclusion we reveal that the CD4+ T cell compartment is greatly altered in the absence of DOCK8. Specifically, DOCK8-deficient patients present with an increase in Th2 cells and defects in Th1 and Th17 cell differentiation. This skewing of CD4+ T helper subsets is likely to account for some of the clinical manifestations in DOCK8-deficient individuals. On one hand, defects in Th17 cells explain susceptibility of DOCK8-deficient patients to mucocutaneous candidiasis. On the other hand, increased Th2 cells may contribute to the allergic phenotype and hyper-IgE displayed by AR-HIES patients. Strikingly, within our DOCK8 cohort, all the patients investigated had IgE that was specific for at least one of the following foods - egg white, milk, codfish, wheat, peanut and soyabean-, but not non-food allergens. These results indicate that the detection of high titers of IgE specific for food but not to other allergens is predictive of DOCK8 deficiency. Thus, future studies to identify signalling pathways and cellular processes affected by DOCK8 deficiency in CD4+ T cells will not only improve our understanding of disease pathogenesis in affected DOCK8-deficient individuals, but also patients with atopic disease.
Supplementary Material
KEY MESSAGES.
DOCK8-deficient CD4+ T cells present with a Th2 cytokine bias, but also defects in Th1 and Th17 cells
The Th2 cytokine bias by DOCK8-deficient Th2 cells contributes to atopic disease such as eczema and food allergies in DOCK8 deficiency
Th17 cell defect is T cell intrinsic and contributes to compromised anti-fungal immunity in DOCK8-deficient patients.
Acknowledgements
We would like to thank Rob Salomon, Eric Lam, and Vitri Dewi from the Garvan Institute Flow facility for cell sorting, Helen Matthews (NIAID, NIH) for clinical support, Kathryn Payne (Garvan Institute) for technical support, and all of the patients and their families for participating in this project. This work was supported by research grants awarded by the National Health and Medical Research Council (NHMRC) of Australia (to CSM and SGT, 1060303, 596813, 1016953; to KLR, 1022922), and Rockefeller University Center for 541 Clinical and Translational Science (5UL1RR024143, to JLC). This research was supported in part by the Intramural Research Program of the NIH, NIAID. CSM is a recipient of a Career Development Fellowship (1008820) and SGT a Principal Research Fellowship (1042925) from the NHMRC of Australia, and a Fulbright Senior Scholar.
ABBREVIATIONS USED:
- AR-HIES
autosomal recessive hyper IgE syndrome
- BCG
Bacille Calmette-Guerin
- CMC
chronic mucocutaneous candidiasis
- CMV
cytomegalovirus
- DOCK8
Dedicator of cytokinesis 8
- EBV
Epstein-Barr virus
- HHV6
human herpes virus 6
- HPV
human papilloma virus
- HSCT
Hematopoietic stem cell transplant
- HSV
herpes simplex virus
- STAT
signal transducer and activator of transcription
- TAE
T cell activation and expansion
- TCM
central memory T cell
- TCR
T cell receptor
- TEM
effector memory T cell
- Tfh
T follicular helper
- Tregs
regulatory T cells
- VZV
Varicella-zoster virus
- XLP
X-liked lymphoproliferative disease
REFERENCES
- 1.Zhang Q, Davis JC, Lamborn IT, Freeman AF, Jing H, Favreau AJ, et al. Combined immunodeficiency associated with DOCK8 mutations. N Engl J Med, 2009:2046–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Engelhardt KR, McGhee S, Winkler S, Sassi A, Woellner C, Lopez-Herrera G, et al. Large deletions and point mutations involving the dedicator of cytokinesis 8 (DOCK8) in the autosomal-recessive form of hyper-IgE syndrome. J Allergy Clin Immunol 2009; 124:1289–302.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Su HC, Jing H, Zhang Q. DOCK8 deficiency. Annals of the New York Academy of Sciences, 2011:26–33. [DOI] [PubMed] [Google Scholar]
- 4.Dasouki M, Okonkwo KC, Ray A, Folmsbeel CK, Gozales D, Keles S, et al. Deficient T Cell Receptor Excision Circles (TRECs) in autosomal recessive hyper IgE syndrome caused by DOCK8 mutation: implications for pathogenesis and potential detection by newborn screening. Clin Immunol, 2011:128–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jabara HH, Mcdonald DR, Janssen E, Massaad MJ, Ramesh N, Borzutzky A, et al. DOCK8 functions as an adaptor that links TLR-MyD88 signaling to B cell activation. Nature Immunology, 2012:612–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruusala A, Aspenström P. Isolation and characterisation of DOCK8, a member of the DOCK180-related regulators of cell morphology. FEBS Lett, 2004:159–66. [DOI] [PubMed] [Google Scholar]
- 7.Tybulewicz VLJ, Henderson RB. Rho family GTPases and their regulators in lymphocytes. Nat Rev Immunol, 2009:630–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Côté J-F, Vuori K. GEF what? Dock180 and related proteins help Rac to polarize cells in new ways. Trends in Cell Biology, 2007:383–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bittner TC, Pannicke U, Renner ED, Notheis G, Hoffmann F, Belohradsky BH, et al. Successful long-term correction of autosomal recessive hyper-IgE syndrome due to DOCK8 deficiency by hematopoietic stem cell transplantation. Klin Padiatr, 2010:351–5. [DOI] [PubMed] [Google Scholar]
- 10.Boztug H, Karitnig-Weiß C, Ausserer B, Renner ED, Albert MH, Sawalle-Belohradsky J, et al. Clinical and immunological correction of DOCK8 deficiency by allogeneic hematopoietic stem cell transplantation following a reduced toxicity conditioning regimen. Pediatr Hematol Oncol, 2012:585–94. [DOI] [PubMed] [Google Scholar]
- 11.Barlogis V, Galambrun C, Chambost H, Lamoureux-Toth S, Petit P, Stephan J-L, et al. Successful allogeneic hematopoietic stem cell transplantation for DOCK8 deficiency. J Allergy Clin Immunol, 2011:420–22.e2. [DOI] [PubMed] [Google Scholar]
- 12.Gatz SA, Benninghoff U, Schütz C, Schulz A, Hönig M, Pannicke U, et al. Curative treatment of autosomal-recessive hyper-IgE syndrome by hematopoietic cell transplantation. Bone Marrow Transplant, 2011:552–6. [DOI] [PubMed] [Google Scholar]
- 13.Metin A, Tavil B, Azık F, Azkur D, Ok-Bozkaya I, Kocabas C, et al. Successful bone marrow transplantation for DOCK8 deficient hyper IgE syndrome. Pediatr Transplant, 2012:398–9. [DOI] [PubMed] [Google Scholar]
- 14.Azık F, Azkur D, Avcı Z, Vezir E, Işık P, Tunç B, et al. Resolution of food-induced anaphylaxis in DOCK8-deficient patients following bone marrow transplantation. Turk J Pediatr, 2015:112–5. [PubMed] [Google Scholar]
- 15.Happel CS, Stone KD, Freeman AF, Shah NN, Wang A, Lyons JJ, et al. Food Allergies Can Persist After Myeloablative Hematopoietic Stem Cell Transplantation in DOCK8-Deficient Patients. J Allergy Clin Immunol 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janssen E, Tsitsikov E, Al-Herz W, Lefranc G, Megarbane A, Dasouki M, et al. Flow cytometry biomarkers distinguish DOCK8 deficiency from severe atopic dermatitis. Clin Immunol, 2014:220–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Avery DT, Deenick EK, Ma CS, Suryani S, Simpson N, Chew GY, et al. B cell-intrinsic signaling through IL-21 receptor and STAT3 is required for establishing long-lived antibody responses in humans. J Exp Med 2010; 207:155–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deenick EK, Avery DT, Chan A, Berglund LJ, Ives ML, Moens L, et al. Naive and memory human B cells have distinct requirements for STAT3 activation to differentiate into antibody-secreting plasma cells. J Exp Med 2013; 210:2739–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berglund LJ, Avery DT, Ma CS, Moens L, Deenick EK, Bustamante J, et al. IL-21 signalling via STAT3 primes human naive B cells to respond to IL-2 to enhance their differentiation into plasmablasts. Blood 2013; 122:3940–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma CS, Wong N, Rao G, Avery DT, Torpy J, Hambridge T, et al. Monogenic mutations differentially affect the quantity and quality of T follicular helper cells in patients with human primary immunodeficiencies. J Allergy Clin Immunol 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janssen E, Morbach H, Ullas S, Bannock JM, Massad C, Menard L, et al. Dedicator of cytokinesis 8-deficient patients have a breakdown in peripheral B-cell tolerance and defective regulatory T cells. J Allergy Clin Immunol, 2014:1365–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Randall KL, Chan SS-Y, Ma CS, Fung I, Mei Y, Yabas M, et al. DOCK8 deficiency impairs CD8 T cell survival and function in humans and mice. J Exp Med, 2011:2305–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Q, Dove CG, Hor JL, Murdock HM, Strauss-Albee DM, Garcia JA, et al. DOCK8 regulates lymphocyte shape integrity for skin antiviral immunity. J Exp Med, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mizesko MC, Banerjee PP, Monaco-Shawver L, Mace EM, Bernal WE, Sawalle-Belohradsky J, et al. Defective actin accumulation impairs human natural killer cell function in patients with dedicator of cytokinesis 8 deficiency. Journal of Allergy and Clinical Immunology, 2013:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crawford G, Enders A, Gileadi U, Stankovic S, Zhang Q, Lambe T, et al. DOCK8 is critical for the survival and function of NKT cells. Blood, 2013:2052–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jing H, Zhang Q, Zhang Y, Hill BJ, Dove CG, Gelfand EW, et al. Somatic reversion in dedicator of cytokinesis 8 immunodeficiency modulates disease phenotype. J Allergy Clin Immunol, 2014:1667–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma CS, Avery DT, Chan A, Batten M, Bustamante J, Boisson-Dupuis S, et al. Functional STAT3 deficiency compromises the generation of human T follicular helper cells. Blood 2012; 119:3997–4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma CS, Hare NJ, Nichols KE, Dupré L, Andolfi G, Roncarolo M-G, et al. Impaired humoral immunity in X-linked lymphoproliferative disease is associated with defective IL-10 production by CD4+ T cells. J Clin Invest 2005; 115:1049–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma CS, Chew GYJ, Simpson N, Priyadarshi A, Wong M, Grimbacher B, et al. Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3. J Exp Med 2008; 205:1551–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma CS, Suryani S, Avery DT, Chan A, Nanan R, Santner-Nanan B, et al. Early commitment of naïve human CD4+ T cells to the T follicular helper (TFH) cell lineage is induced by IL-12. Immunol Cell Biol 2009; 87:590–600. [DOI] [PubMed] [Google Scholar]
- 31.Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature, 1999:708–12. [DOI] [PubMed] [Google Scholar]
- 32.Morita R, Schmitt N, Bentebibel S-E, Ranganathan R, Bourdery L, Zurawski G, et al. Human Blood CXCR5(+)CD4(+) T Cells Are Counterparts of T Follicular Cells and Contain Specific Subsets that Differentially Support Antibody Secretion. Immunity, 2011:108–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A, et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med, 2006:1693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh SP, Zhang HH, Foley JF, Hedrick MN, Farber JM. Human T cells that are able to produce IL-17 express the chemokine receptor CCR6. J Immunol, 2008:214–21. [DOI] [PubMed] [Google Scholar]
- 35.Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, et al. Surface phenotype and antigenic specificity of human interleukin 17–producing T helper memory cells. Nat Immunol, 2007:639–46. [DOI] [PubMed] [Google Scholar]
- 36.Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, et al. Phenotypic and functional features of human Th17 cells. Journal of Experimental Medicine, 2007:1849–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gett AV, Hodgkin PD. Cell division regulates the T cell cytokine repertoire, revealing a mechanism underlying immune class regulation. Proc Natl Acad Sci USA, 1998:9488–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tangye SG, Ferguson A, Avery DT, Ma CS, Hodgkin PD. Isotype switching by human B cells is division-associated and regulated by cytokines. J Immunol, 2002:4298–306. [DOI] [PubMed] [Google Scholar]
- 39.Ma CS, Hodgkin PD, Tangye SG. Automatic generation of lymphocyte heterogeneity: Division-dependent changes in the expression of CD27, CCR7 and CD45 by activated human naive CD4+ T cells are independently regulated. Immunology and Cell Biology, 2004:67–74. [DOI] [PubMed] [Google Scholar]
- 40.Maggi E. The TH1/TH2 paradigm in allergy. Immunotechnology 1998; 3:233–44. [DOI] [PubMed] [Google Scholar]
- 41.Randall KL, Lambe T, Johnson A, Treanor B, Kucharska E, Domaschenz H, et al. Dock8 mutations cripple B cell immunological synapses, germinal centers and long-lived antibody production. Nat Immunol, 2009:1283–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lambe T, Crawford G, Johnson AL, Crockford TL, Bouriez-Jones T, Smyth AM, et al. DOCK8 is essential for T-cell survival and the maintenance of CD8+ T-cell memory. Eur J Immunol, 2011:3423–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Flesch IEA, Randall KL, Hollett NA, Di Law H, Miosge LA, Sontani Y, et al. Delayed control of herpes simplex virus infection and impaired CD4(+) T-cell migration to the skin in mouse models of DOCK8 deficiency. Immunology and Cell Biology, 2015:517–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Palendira U, Low C, Bell AI, Ma CS, Abbott RJM, Phan TG, et al. Expansion of somatically reverted memory CD8+ T cells in patients with X-linked lymphoproliferative disease caused by selective pressure from Epstein-Barr virus. J Exp Med 2012; 209:913–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Plunkett FJ, Franzese O, Belaramani LL, Fletcher JM, Gilmour KC, Sharifi R, et al. The impact of telomere erosion on memory CD8+ T cells in patients with X-linked lymphoproliferative syndrome. Mech Ageing Dev 2005; 126:855–65. [DOI] [PubMed] [Google Scholar]
- 46.Ives ML, Ma CS, Palendira U, Chan A, Bustamante J, Boisson-Dupuis S, et al. Signal transducer and activator of transcription 3 (STAT3) mutations underlying autosomal dominant hyper-IgE syndrome impair human CD8(+) T-cell memory formation and function. J Allergy Clin Immunol 2013; 132:400–11.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lucas CL, Kuehn HS, Zhao F, Niemela JE, Deenick EK, Palendira U, et al. Dominant-activating germline mutations in the gene encoding the PI(3)K catalytic subunit p110δ result in T cell senescence and human immunodeficiency. Nature Immunology 2014; 15:88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harada Y, Tanaka Y, Terasawa M, Pieczyk M, Habiro K, Katakai T, et al. DOCK8 is a Cdc42 activator critical for interstitial dendritic cell migration during immune responses. Blood, 2012:4451–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sornasse T, Larenas PV, Davis KA, de Vries JE, Yssel H. Differentiation and stability of T helper 1 and 2 cells derived from naive human neonatal CD4+ T cells, analyzed at the single-cell level. J Exp Med, 1996:473–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Okada S, Markle JG, Deenick EK, Mele F, Averbuch D, Lagos M, et al. IMMUNODEFICIENCIES. Impairment of immunity to Candida and Mycobacterium in humans with bi-allelic RORC mutations. Science, 2015:606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Milner JD, Brenchley JM, Laurence A, Freeman AF, Hill BJ, Elias KM, et al. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature 2008; 452:773–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de Beaucoudrey L, Puel A, Filipe-Santos O, Cobat A, Ghandil P, Chrabieh M, et al. Mutations in STAT3 and IL12RB1 impair the development of human IL-17-producing T cells. J Exp Med 2008; 205:1543–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Puel A, Cypowyj S, Bustamante J, Wright JF, Liu L, Lim HK, et al. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science, 2011:65–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boisson B, Wang C, Pedergnana V, Wu L, Cypowyj S, Rybojad M, et al. An ACT1 mutation selectively abolishes interleukin-17 responses in humans with chronic mucocutaneous candidiasis. Immunity 2013; 39:676–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ling Y, Cypowyj S, Aytekin C, Galicchio M, Camcioglu Y, Nepesov S, et al. Inherited IL-17RC deficiency in patients with chronic mucocutaneous candidiasis. J Exp Med 2015; 212:619–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grimbacher B, Holland SM, Puck JM. Hyper-IgE syndromes. Immunol Rev, 2005:244–50. [DOI] [PubMed] [Google Scholar]
- 57.Engelhardt KR, Gertz ME, Keles S, Schäffer AA, Sigmund EC, Glocker C, et al. The extended clinical phenotype of 64 patients with dedicator of cytokinesis 8 deficiency. J Allergy Clin Immunol, 2015:402–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takatsu K, Nakajima H. IL-5 and eosinophilia. Current Opinion in Immunology, 2008:288–94. [DOI] [PubMed] [Google Scholar]
- 59.Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol, 2007:950–7. [DOI] [PubMed] [Google Scholar]
- 60.Boisson-Dupuis S, Bustamante J, El-Baghdadi J, Camcioglu Y, Parvaneh N, El Azbaoui S, et al. Inherited and acquired immunodeficiencies underlying tuberculosis in childhood. Immunol Rev, 2015:103–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moens L, Tangye SG. Cytokine-Mediated Regulation of Plasma Cell Generation: IL-21 Takes Center Stage. Front Immunol 2014; 5:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Boos AC, Hagl B, Schlesinger A, Halm BE, Ballenberger N, Pinarci M, et al. Atopic dermatitis, STAT3- and DOCK8-hyper-IgE syndromes differ in IgE-based sensitization pattern. Allergy, 2014:943–53. [DOI] [PubMed] [Google Scholar]
- 63.Siegel AM, Stone KD, Cruse G, Lawrence MG, Olivera A, Jung M-y, et al. Diminished allergic disease in patients with STAT3 mutations reveals a role for STAT3 signaling in mast cell degranulation. J Allergy Clin Immunol, 2013:1388–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Parish CR, Liew FY. Immune response to chemically modified flagellin. 3. Enhanced cell-mediated immunity during high and low zone antibody tolerance to flagellin. J Exp Med, 1972:298–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Constant S, Pfeiffer C, Woodard A, Pasqualini T, Bottomly K. Extent of T cell receptor ligation can determine the functional differentiation of naive CD4+ T cells. J Exp Med, 1995:1591–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brandt K, van der Bosch J, Fliegert R, Gehring S. TSST-1 induces Th1 or Th2 differentiation in naïve CD4+ T cells in a dose- and APC-dependent manner. Scand J Immunol, 2002:572–9. [DOI] [PubMed] [Google Scholar]
- 67.Altin JA, Tian L, Liston A, Bertram EM, Goodnow CC, Cook MC. Decreased T-cell receptor signaling through CARD11 differentially compromises forkhead box protein 3-positive regulatory versus T(H)2 effector cells to cause allergy. J Allergy Clin Immunol 2011; 127:1277–85.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kastirr I, Crosti M, Maglie S, Paroni M, Steckel B, Moro M, et al. Signal Strength and Metabolic Requirements Control Cytokine-Induced Th17 Differentiation of Uncommitted Human T Cells. J Immunol, 2015:3617–27. [DOI] [PubMed] [Google Scholar]
- 69.Gomez-Rodriguez J, Sahu N, Handon R, Davidson TS, Anderson SM, Kirby MR, et al. Differential expression of interleukin-17A and −17F is coupled to T cell receptor signaling via inducible T cell kinase. Immunity 2009; 31:587–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.