Abstract
The efficacy of doxycycline treatment (10 mg/kg of body weight every 24 h for 42 days) in eliminating Ehrlichia canis from four subclinically infected dogs was evaluated. One dog remained PCR positive, suggesting that 6 weeks of doxycycline treatment may not be sufficient to clear E. canis parasites from all subclinically infected dogs. Serology (indirect immunofluorescent antibody assay) was shown to be unreliable in assessing recovery from the carrier state, as anti-E. canis antibodies persisted after elimination of the parasite. Our findings suggest that an increase in the platelet count may be an important indicator for dogs that recover from subclinical ehrlichiosis.
Canine monocytic ehrlichiosis (CME), a tick-borne disease caused by the rickettsia Ehrlichia canis, was first recognized as a distinct clinical entity in Algeria in 1935 (6). Since then, it has been acknowledged worldwide as an important infectious disease of dogs and other canids (11).
The pathogenesis of CME involves an incubation period of 8 to 20 days, followed by acute, subclinical, and sometimes chronic phases (8). Mild hematological abnormalities in experimentally infected dogs in the subclinical phase have been reported. These abnormalities include thrombocytopenia (which is the most consistent finding) and a significant decrease in leukocytes due to a reduction in the neutrophil counts (4, 13).
The persistent elevation of anti-E. canis antibody titers for extended periods after the acute phase of CME, as well as the continued presence of these antibodies after treatment of acute CME, is poorly understood (8, 11). Recent demonstration of E. canis DNA by PCR in dogs 34 months after experimental infection suggested that dogs suffering from subclinical CME harbor the rickettsia (7). Treatment of these dogs should therefore be undertaken in order to prevent them from developing the chronic phase of the disease. In this study we evaluated the efficacy of doxycycline treatment in eliminating E. canis from subclinically infected beagles. PCR was used in order to determine whether clinically healthy dogs experimentally infected with E. canis remained E. canis carriers after 42 days of treatment with doxycycline (10 mg/kg of body weight every 24 h [q24h]). To this end PCR was performed on blood samples, bone marrow aspirates, and splenic aspirates, pre- and posttreatment, and results were compared to results by indirect immunofluorescent antibody (IFA) testing and to the hematological profile.
Six clinically healthy beagle dogs (Harlan Laboratories, Indianapolis, Ind.), ranging from 8 to 12 months old, were used in this study. All dogs were seronegative for E. canis antibodies as determined by IFA testing, and their hematological and biochemical parameters were within normal ranges. Dogs were inoculated intravenously with 5 ml of heparinized blood from a beagle infected with the Israeli strain (strain 611) of E. canis, which has been isolated and genetically characterized (9). Rectal temperature, food consumption, and clinical signs were monitored every other day, and blood samples for hematology and serology were collected twice weekly for the first 60 days postinoculation and at least once a month for an additional 6 months thereafter. Clinical signs, hematology, biochemistry, and serology of these dogs during the acute and subclinical stages of the disease have been previously described (9, 13). The dogs were dipped regularly against ectoparasites (Paramite-Vetkem).
For this study, blood, bone marrow, and splenic aspirates were collected from the dogs 34 months postinfection. Complete hematological examination and serology were carried out on all blood and serum samples, respectively, while PCR and microscopic evaluation were performed on the blood, bone marrow, and splenic aspirates. All dogs were treated with doxycycline (10 mg/kg per os q24h) for 42 days. Four and 6 weeks after commencement of therapy, blood was redrawn for hematology, microscopic evaluation, serology, and PCR. At the end of the therapeutic regimen (6 weeks), bone marrow and splenic aspirates in addition to blood were collected for PCR and microscopic evaluation. Two of the six dogs (dogs 5 and 6) whose blood, bone marrow, and splenic aspirates were found to be negative by PCR at the beginning of the trial (34 months postinfection) served as controls for this study (the therapy portion of the study) and were treated in the same manner as all other dogs.
Blood samples were collected in plastic tubes with no anticoagulant, and serum was separated by centrifugation 2 h after blood was drawn and stored at −70°C until serological tests were carried out. The IFA test was performed as previously described (9, 12). Blood for hematology was collected in plastic tubes containing EDTA. Hematological assays were performed within 2 h of blood collection with an automated cell counter (Minos ST; Vet, Montpellier, France), calibrated for canine blood. The following parameters were determined: hematocrit, hemoglobin concentration, total erythrocyte count, mean corpuscular volume, mean corpuscular hemoglobin concentration, total leukocyte count, platelet count, and mean platelet volume.
Ultrasound-guided fine-needle aspirates of the spleen were collected from the dogs. For this purpose the dogs were anesthetized with a mixture of ketamine and xylazine (2 mg of each/kg). At the same time, bone marrow aspirates were taken from the iliac crests of all dogs. Samples were collected in plastic tubes containing EDTA and kept frozen at −20°C until DNA was extracted. Giemsa-stained smears, prepared from the blood, bone marrow, and splenic aspirates, were microscopically evaluated for the presence of E. canis morulae. DNA was extracted as previously described (1, 7). PCR was performed in two rounds as previously described (5) with a model PTC-100 thermocycler (MJ Research). The products were visualized on a 1.5% agarose gel with ethidium bromide and UV light. Positive and negative controls were used for the PCR test, positive controls were retrieved from E. canis-infected DH82 cells, and negative controls were retrieved from noninfected DH82 cells.
All dogs seroconverted and showed clinical signs consistent with acute CME by day 15 postinoculation. They were not treated medically, and by day 30 to 40 all had recovered and were clinically healthy. They remained so throughout the remainder of the study period.
Pretreatment IFA titers (34 months postinoculation) and 4- and 6-week posttreatment titers are presented in Table 1. The five pretreated seropositive dogs (dogs 1 to 5) remained seropositive throughout the treatment course.
TABLE 1.
Anti-E. canis IFA titers in 6 subclinically infected beagle dogs before and during doxycycline treatment
| Dog | Titer of IFA
|
||
|---|---|---|---|
| Pretreatment | After 4 weeks of treatment | After 6 weeks of treatment | |
| 1 | 1:5,120 | 1:1,280 | 1:5,120 |
| 2 | 1:1,280 | 1:1,280 | 1:1,280 |
| 3 | 1:2,560 | 1:2,560 | 1:2,560 |
| 4 | 1:5,120 | 1:5,120 | 1:5,120 |
| 5 | 1:640 | 1:1,280 | 1:1,280 |
| 6 | Negative | Negative | Negative |
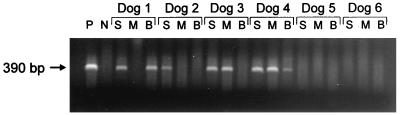
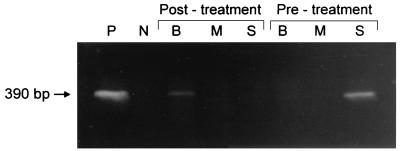
Pretreatment, all the organ samples tested from four dogs (dogs 1 to 4) were PCR positive while those from two dogs (dogs 5 and 6) were PCR negative (Fig. 1). Splenic samples from all four positive dogs were positive, while bone marrow aspirates and blood samples from only two of the positive dogs were positive. Four weeks after treatment, all blood samples were PCR negative. Six weeks after treatment, PCR results were negative for all splenic and bone marrow aspirates and for all but one blood sample (dog 2) (Fig. 2).
FIG. 1.
Pretreatment PCR results of six beagles 34 months postinoculation with E. canis. S, splenic aspirate; M, bone marrow aspirate; B, blood sample; P, positive control retrieved from E. canis-infected DH82 cells; N, negative control retrieved from noninfected DH82 cells.
FIG. 2.
PCR results of dog 2 (the only dog that remained PCR positive posttreatment) before treatment and after 42 days of doxycycline (10 mg/kg per os q24h) treatment. S, splenic aspirate; M, bone marrow aspirate; B, blood sample; P, positive control retrieved from E. canis-infected DH82 cells; N, negative control retrieved from noninfected DH82 cells.
No E. canis morulae were observed microscopically on Giemsa-stained smears in any of the blood samples, bone marrow aspirates, or splenic aspirates from all dogs pre- and posttreatment. Three of the four PCR-positive dogs (dogs 2 to 4) were mildly thrombocytopenic (<200,000 platelets μl−1) pretreatment. However, at the posttreatment evaluations (after 4 and 6 weeks of treatment), all dogs had platelet counts within the reference range (200,000 to 500,000 platelets μl−1) (Table 2).
TABLE 2.
Platelet counts in six subclinically infected beagle dogs before and during doxycycline treatment
| Dog | No. of platelets (μl−1)
|
||
|---|---|---|---|
| Pretreatment | After 4 weeks of treatment | After 6 weeks of treatment | |
| 1 | 260,000 | 268,000 | 260,000 |
| 2 | 157,000 | 391,000 | 307,000 |
| 3 | 184,000 | 244,000 | 201,000 |
| 4 | 140,000 | 244,000 | 210,000 |
| 5 | 317,000 | 503,000 | 380,000 |
| 6 | 470,000 | 387,000 | 235,000 |
A state of premunition has been reported to occur in dogs subclinically infected with E. canis and also in infected dogs after short-term treatment with oxytetracycline (2, 10, 11). Studies carried out with untreated experimentally infected dogs have shown that dogs in the subclinical phase of the disease carry the parasite for years after infection (2, 7), the consequence of which may be the risk of developing the severe, life-threatening chronic phase of the disease (8, 13). In order to prevent subclinically infected dogs from developing the chronic disease, we propose identification and treatment of these dogs. Our recommended treatment regimen for acute CME includes the combination of doxycycline and imidocarb dipropionate (8). A previous study concluded that a 14-day treatment with doxycycline (5 mg/kg q12h) eliminated acute E. canis infection (3). In the present study, we evaluated the efficacy of doxycycline treatment (10 mg/kg q24h for 6 weeks) in eliminating E. canis from subclinically infected dogs. We selected doxycycline, as this drug is the most widely available drug used in the treatment of CME, while imidocarb is unavailable or not approved for use in many countries.
Nested PCR with primers specific for E. canis was previously shown to be highly sensitive and specific for detection of E. canis (5, 14); it could detect as little as 0.2 pg of purified E. canis DNA (14). The nested PCR for E. canis was proposed to be useful for assessing clearance of the organisms after antibiotic therapy (14). The efficacy of 6-week treatment with doxycycline in our study as determined by nested PCR was 75%, that is, three of four dogs were cleared of the parasite. Therefore, our results suggest that in order to achieve higher efficacy in clearing the rickettsia from subclinically infected dogs when treating them with doxycycline only, a long duration of doxycycline treatment may be needed. Our results also suggest that PCR may be a useful tool in determining whether dogs are in the subclinical phase of CME and whether dogs treated for CME recover from the disease and eliminate the rickettsia.
Blood PCR results were all negative after 4 weeks of treatment; however, the blood sample of one dog was found to be PCR positive 6 weeks posttreatment. The fact that the blood sample of dog 2 was PCR negative at 4 weeks and PCR positive at 6 weeks suggests the possibility of cyclic parasitemia during recovery from CME. It also suggests that the organism may “hide” in other organs such as spleen and bone marrow. However, as neither splenic nor bone marrow aspirates were drawn 4 weeks after treatment, this hypothesis remains speculative.
All of the pretreatment splenic samples of the 4 PCR-positive subclinically infected dogs were found to be PCR positive (as determined by a positive PCR result for at least one of three different organ samples: blood, splenic aspirate, or bone marrow aspirate). This result suggested that the spleen is the most likely organ in which the parasite is harbored in subclinically infected dogs (7) or that it contains higher concentrations of the rickettsia and therefore ehrlichial DNA was more easily detectable in these samples. The fact that the splenic sample of dog 2 was PCR negative but that its blood sample 6 weeks after treatment was PCR positive implies that the spleen may not be as significant in recovering dogs treated with doxycycline. Our findings also emphasize the need for samples from all three sources (spleen, bone marrow, and blood) for PCR testing in determining infection with E. canis and the fact that one source may not be sufficient.
Clinicians often face the problem of determining the efficacy of treatment of CME without having any immediately available test to confirm success or failure. Our results show that the IFA assay is not a reliable method to judge the success of treatment during or shortly after treatment of subclinical CME, as anti-E. canis antibody titers did not change significantly during the 6-week treatment period and persisted after the treatment. It was interesting that even though dog 5 was PCR negative at the beginning of the treatment, it was found to be seropositive and its IFA titers did not change significantly during and after treatment, suggesting an aberrant continuous humoral response caused by the earlier E. canis infection (prior to elimination).
As opposed to E. canis IFA titers, platelet counts increased 4 weeks after the commencement of treatment and reached counts within the reference range in all pretreated thrombocytopenic PCR-positive subclinically infected dogs. These findings suggest that an increase in platelet counts may be an important indicator for dogs recovering from subclinical CME.
In conclusion, the results of this study suggest that 6 weeks of doxycycline treatment (10 mg/kg q24h) may not be sufficient to clear E. canis parasites from all subclinically infected dogs and that PCR with multiple organ sampling may be a useful tool in determining recovery from CME.
Acknowledgments
We thank Niels C. Pedersen, Janet E. Foley, and Amy M. Poland from the Center for Companion Animal Health at the University of California, Davis, for their professional assistance.
REFERENCES
- 1.Barlough J, Madigan E, DeRock J E, Bigornia L. Nested polymerase chain reaction for detection of Ehrlichia equi genomic DNA in horses and ticks (Ixodes pacificus) Vet Parasitol. 1996;63:319–329. doi: 10.1016/0304-4017(95)00904-3. [DOI] [PubMed] [Google Scholar]
- 2.Bool P H. Studies on Ehrlichia canis (syn. Rickettsia canis) Acta Trop. 1959;16:97–107. [PubMed] [Google Scholar]
- 3.Breitschwerdt E B, Hegarty B C, Hancock S I. Doxycycline treatment and challenge infection with two Ehrlichia canis strains. J Vet Intern Med. 1997;11:132. . (ACVIM abstract.) [Google Scholar]
- 4.Codner E C, Farris-Smith L. Characterization of the subclinical phase of ehrlichiosis in dogs. J Am Vet Med Assoc. 1986;189:47–50. [PubMed] [Google Scholar]
- 5.Dawson J E, Biggie K L, Warner C K, Cookson K, Jenkins S, Levine J F, Olson J G. Polymerase chain reaction evidence of Ehrlichia chaffeensis, an etiologic agent of human ehrlichiosis, in dogs from southern Virginia. Am J Vet Res. 1996;57:1175–1179. [PubMed] [Google Scholar]
- 6.Donatien A, Lestoquard A. Existence en Algerie d’une rickettsia du chien. Bull Soc Pathol Exot. 1935;28:418–419. [Google Scholar]
- 7.Harrus S, Waner T, Aizenberg I, Foley J E, Poland A M, Bark H. Amplification of ehrlichial DNA from dogs 34 months after infection with Ehrlichia canis. J Clin Microbiol. 1998;36:73–76. doi: 10.1128/jcm.36.1.73-76.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harrus S, Waner T, Bark H. Canine monocytic ehrlichiosis—an update. Compend Contin Educ Pract Vet. 1997;19:431–444. [Google Scholar]
- 9.Keysary A, Waner T, Rosner M, Warner C K, Dawson J E, Zass R, Biggie K L, Harrus S. The first isolation, in vitro propagation, and genetic characterization of Ehrlichia canis in Israel. Vet Parasitol. 1996;62:331–340. doi: 10.1016/0304-4017(95)00866-7. [DOI] [PubMed] [Google Scholar]
- 10.Leeflang P. Relation between carrier state oxytetracycline administration and immunity in Ehrlichia canis infection. Vet Rec. 1971;90:703–704. doi: 10.1136/vr.90.25.703. [DOI] [PubMed] [Google Scholar]
- 11.Ristic M, Holland C J. Canine ehrlichiosis. In: Woldehiwet Z, Ristic M, editors. Rickettsial and chlamydial diseases of domestic animals. Oxford, United Kingdom: Pergamon Press; 1993. pp. 169–186. [Google Scholar]
- 12.Ristic M, Huxsoll D L, Weisiger R M, Hildebrandt P K, Nyindo M B A. Serological diagnosis of tropical canine pancytopenia by indirect immunofluorescence. Infect Immun. 1972;6:226–231. doi: 10.1128/iai.6.3.226-231.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waner T, Harrus S, Bark H, Bogin E, Avidar Y, Keysary A. Characterization of the subclinical phase of canine ehrlichiosis in experimentally infected beagle dogs. Vet Parasitol. 1997;69:307–317. doi: 10.1016/s0304-4017(96)01130-2. [DOI] [PubMed] [Google Scholar]
- 14.Wen B, Rikihisa Y, Mott J M, Greene R, Kim H Y, Zhi N, Couto G C, Unver A, Bartsch R. Comparison of nested PCR with immunofluorescent-antibody assay for detection of Ehrlichia canis infection in dogs treated with doxycycline. J Clin Microbiol. 1997;35:1852–1855. doi: 10.1128/jcm.35.7.1852-1855.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]